Polymicrobial infections in CF cases likely impact patient health, but the mechanism(s) underlying such interactions is poorly understood. Here, we show using an in vitro model system that interactions between Pseudomonas and Streptococcus are modulated by zinc availability, and clinical data are consistent with this model. Together with previous studies, our work supports a role for metal homeostasis as a key factor driving microbial interactions.
KEYWORDS: Pseudomonas aeruginosa, Streptococcus, cystic fibrosis, biofilm, polymicrobial, zinc
ABSTRACT
Airway infections associated with cystic fibrosis (CF) are polymicrobial. We reported previously that clinical isolates of Pseudomonas aeruginosa promote the growth of a variety of streptococcal species. To explore the mechanistic basis of this interaction, we performed a genetic screen to identify mutants of Streptococcus sanginuis SK36 whose growth was no longer enhanced by P. aeruginosa PAO1. Mutations in the zinc uptake systems of S. sanguinis SK36 reduced growth of these strains by 1 to 3 logs compared to that of wild-type S. sanguinis SK36 when grown in coculture with P. aeruginosa PAO1, and exogenous zinc (0.1 to 10 μM) rescued the coculture defect of zinc uptake mutants of S. sanguinis SK36. Zinc uptake mutants of S. sanguinis SK36 had no obvious growth defect in monoculture. Consistent with competition for zinc driving coculture dynamics, S. sanguinis SK36 grown in coculture with P. aeruginosa showed increased expression of zinc uptake genes compared to that of S. sanguinis grown alone. Strains of P. aeruginosa PAO1 defective in zinc transport also supported ∼2-fold more growth by S. sanguinis compared to that in coculture with wild-type P. aeruginosa PAO1. An analysis of 118 CF sputum samples revealed that total zinc levels varied from ∼5 to 145 μM. At relatively low zinc levels, Pseudomonas and Streptococcus spp. were found in approximately equal abundance; at higher zinc levels, we observed a decline in relative abundance of Streptococcus spp., perhaps as a result of increasing zinc toxicity. Together, our data indicate that the relative abundances of these microbes in the CF airway may be impacted by zinc levels.
IMPORTANCE Polymicrobial infections in CF cases likely impact patient health, but the mechanism(s) underlying such interactions is poorly understood. Here, we show using an in vitro model system that interactions between Pseudomonas and Streptococcus are modulated by zinc availability, and clinical data are consistent with this model. Together with previous studies, our work supports a role for metal homeostasis as a key factor driving microbial interactions.
INTRODUCTION
Cystic fibrosis (CF) is a monogenic autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (1). It is estimated that ∼70,000 individuals in the world are affected by CF, and the most common mutation, caused by a deletion of phenylalanine at the 508th amino acid within the CFTR protein (ΔF508), is found in approximately 70% of this population (2, 3). CFTR dysfunction affects several body systems, and progressive lung disease due to chronic and recurrent microbial infections is the leading cause of morbidity and mortality in individuals with CF (4, 5). It has been shown that CF airway infections are polymicrobial (6, 7), and the composition and interspecies interactions within the polymicrobial communities can have profound and diverse consequences on bacterial growth (8–10), as well as on disease progression and therapeutic outcomes (6, 11).
An example of such microbial interactions includes the CF-associated streptococcal species, the presence of which may influence the growth and/or virulence of other CF pathogens, including those of the important pathogen Pseudomonas aeruginosa (12, 13). In turn, P. aeruginosa can impact the growth and/or persistence of streptococci (8–10, 14), with the net impact of these interactions resulting in exacerbation in some cases (4, 15–17) or in less severe loss of lung function under other circumstances (11, 16, 18–21). Thus, the impact of such polymicrobial interactions on host outcomes is complex in the clinical setting. Therefore, understanding how these pathogens interact with each other and their multicellular host to impact disease progression, as well as how these interactions are modified by the CF airway environment, is of high significance.
Zinc is an essential micronutrient for all organisms, serves as a structural or catalytic cofactor in 5 to 6% of proteins in the bacterial proteome (22–24), and can play an important role in the expression of key virulence factors (25–32). However, high concentrations of zinc are toxic, possibly due to competition with other relevant metal ions (24, 33), inhibition of key enzymes and essential metabolic reactions in the cell (32, 34, 35), and/or induction of membrane stress (36). For most pathogens, there is intense competition for zinc in the face of the host’s nutritional immunity defense mechanisms (37) and, conversely, elevating zinc levels is also a strategy used by macrophages to kill pathogens encapsulated in their phagosomes (38, 39).
To maintain zinc homeostasis, bacterial species have evolved multiple systems to import and export zinc in environments of zinc limitation or excess, respectively (40, 41). In P. aeruginosa, the two best-studied zinc acquisition systems are the high-affinity ZnuABC system (42–44) and the pseudopaline system (45, 46). ZnuABC is a member of the ATP-binding cassette (ABC) transporters and consists of a zinc-specific binding periplasmic protein (ZnuA), an inner membrane permease (ZnuB), and an ATPase (ZnuC) (42, 44, 47). Loss of ZnuA in P. aeruginosa PAO1 results in an ∼60% reduction in cellular zinc accumulation (42). The pseudopaline system is primarily involved in zinc uptake in zinc-poor environments, and zinc transport into the cell is achieved via the action of a four-gene operon (cntOLMI, abbreviated cntO-I and also termed zrmABCD) (45, 46). This operon includes the genes for a TonB-dependent outer membrane pseudopaline receptor (CntO), two biosynthetic enzymes (CntL and CntM) responsible for synthesizing pseudopaline, and an inner membrane transporter (CntI) involved in pseudopaline secretion (43, 45, 46). Both the ZnuABC system and the pseudopaline operon are negatively regulated by zinc level through the Fur-like zinc uptake regulator Zur (42, 44, 46), which can sense and respond to femtomolar changes of cytosolic zinc concentrations (48). When zinc is bound, Zur represses transcription of the zinc uptake systems, and thus loss of Zur results in higher cytoplasmic zinc levels in P. aeruginosa (43, 44).
Similarly, several zinc transporters contribute to zinc homeostasis and virulence in pathogenic streptococci (30, 31). In most streptococci, zinc acquisition involves a high-affinity zinc ABC transporter, which is comprised of an integral membrane component (AdcB), an ATPase (AdcC), and one or several zinc-binding proteins (AdcA, AdcAII, Lbp, and/or Lmb) (30, 49–51). The streptococcal AdcR repressor, a MarR family regulator, is involved in regulation of this transporter system (52).
Recent studies have suggested that total zinc levels are much higher in sputum from CF patients than in that from healthy controls (53), but zinc availability may be limited in the lung mucosa, leading to zinc starvation for P. aeruginosa during CF lung infection (54). Moreover, the host also employs zinc starvation or intoxication to retard streptococcal growth during colonization and infection (30, 31, 55). Nevertheless, the potential significance of zinc in the interplay between P. aeruginosa and Streptococcus, especially in the context of a polymicrobial infection in the CF airway, remains to be explored.
We recently showed that P. aeruginosa can enhance the growth of multiple oral Streptococcus spp. in coculture conditions (8, 10), but the molecular mechanism underlying such interactions is still poorly understood. In the present work, we used a comprehensive Streptococcus sanguinis SK36 mutant library to determine the genetic requirements for S. sanguinis SK36 to benefit from interaction with P. aeruginosa. Our results show that efficient zinc acquisition by S. sanguinis SK36 plays a critical role in P. aeruginosa-induced growth enhancement. During cocultivation, we observed increased transcription of zinc uptake genes by S. sanguinis SK36 and demonstrated that P. aeruginosa and S. sanguinis SK36 compete for zinc. Furthermore, by coupling analysis of microbial communities and zinc content within CF sputum samples, we discovered a relationship between the relative abundance of Streptococcus and Pseudomonas bacteria with the concentrations of total zinc in CF sputum. These results suggest that zinc availability may play a role in Pseudomonas-Streptococcus interactions in vivo, and, furthermore, that changes in zinc levels may also be related to the development of the respiratory microflora in patients with CF.
RESULTS
P. aeruginosa and its conditioned medium stimulate S. sanguinis growth on both plastic and CF-derived airway cells.
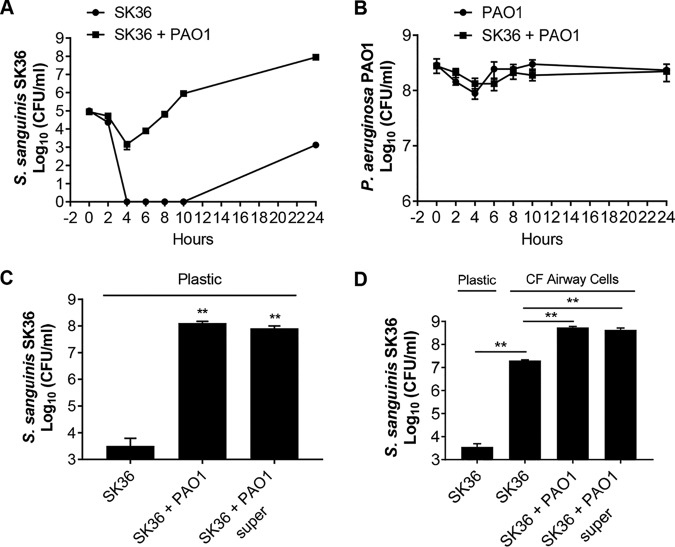
We have previously shown that P. aeruginosa can enhance S. sanguinis SK36 growth as either planktonic or biofilm cells in coculture compared to that of S. sanguinis SK36 in monoculture, whereas the P. aeruginosa population was not significantly impacted by the presence of S. sanguinis (8, 10), a finding confirmed here (see Fig. S1A in the supplemental material). Furthermore, an assay monitoring biofilm formation over time shows that the higher viable count of S. sanguinis SK36 cells in coculture with P. aeruginosa is due to enhanced growth of S. sanguinis SK36 after a modest reduction in viability (Fig. 1A). Interestingly, S. sanguinis SK36 did show enhanced growth in monoculture at later time points, indicating that P. aeruginosa may stimulate transition out of the lag phase as one possible mechanism of the observed increased viable count of S. sanguinis SK36 cells observed in the coculture assay system. The viable counts of P. aeruginosa cells were similar in monoculture and coculture at all time points tested (Fig. 1B).
FIG 1.
P. aeruginosa can stimulate the growth of S. sanguinis when grown in cocultures. (A and B) Growth kinetics of S. sanguinis SK36 (labeled SK36) and P. aeruginosa PAO1 (labeled PAO1) in a 96-well deep-well plate either in coculture or as a monoculture. (C and D) P. aeruginosa cells or a 1:2 dilution of P. aeruginosa supernatant (super) enhances S. sanguinis growth in coculture on a plastic substratum (C) or on CFBE monolayers (D). Error bars indicate standard deviation of the means from a representative triplicate assay. **, P < 0.01 (Student’s t test).
In an attempt to understand the basis of this observed growth enhancement of S. sanguinis SK36, we tested whether a cell-free supernatant of P. aeruginosa PAO1 could stimulate the growth of S. sanguinis SK36. We found that P. aeruginosa cell-free supernatant was indeed capable of promoting S. sanguinis biofilm growth on plastic (Fig. 1C). We noted a significant increase in the number of S. sanguinis cells grown in the presence of a one-half or one-quarter dilution of the original P. aeruginosa PAO1 supernatant (Fig. S1B), with a dose-dependent decrease in growth enhancement with increasing dilution of the supernatant. Undiluted P. aeruginosa PAO1 supernatant showed a less robust effect on S. sanguinis SK36 growth than the one-half or one-quarter dilution, perhaps due to the known antimicrobial factors in P. aeruginosa supernatant, including siderophores, phenazines, cyanide, and elastase (56–58). Taken together, these data suggest that one or more secreted factors in the P. aeruginosa PAO1 supernatant can enhance growth of S. sanguinis SK36.
To determine whether the interactions between Streptococcus and P. aeruginosa also occur when these microbes are grown in the presence of airway cells derived from patients with CF, we extended our observation to CF-derived bronchial epithelial cells homozygous for the ΔF508 allele of CFTR (referred to here as cystic fibrosis bronchial epithelial [CFBE] monolayers) (59). We evaluated S. sanguinis SK36 growth on CFBE monolayers in the absence or presence of P. aeruginosa cells or supernatants. As a control, S. sanguinis growth on tissue culture plates without host cells was also included (Fig. 1D, leftmost column). Interestingly, S. sanguinis growth enhancement was observed with monoculture biofilms on host cells, and P. aeruginosa cells and supernatants further enhanced the growth of S. sanguinis SK36 by an additional ∼2 logs (Fig. 1D). Collectively, our results demonstrate the ability of P. aeruginosa PAO1 cells and supernatant to promote the growth of S. sanguinis SK36 under a variety of conditions.
Identification of S. sanguinis mutants defective in P. aeruginosa-mediated growth enhancement.
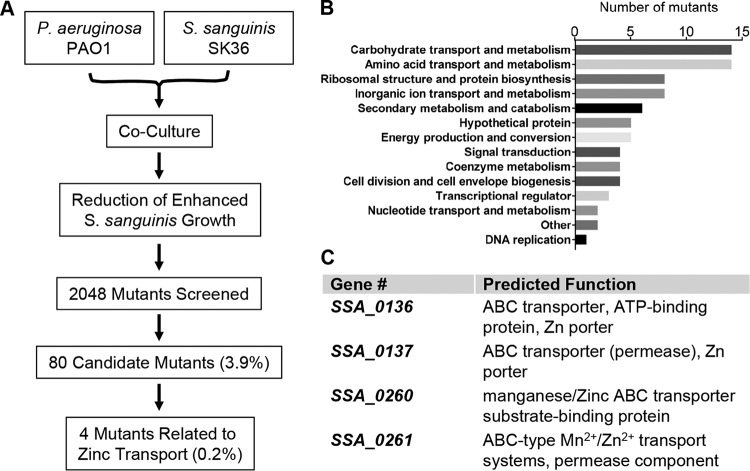
To identify S. sanguinis genes that are potentially required for the increase in S. sanguinis SK36 growth in the P. aeruginosa PAO1-S. sanguinis SK36 coculture, we performed a genome-wide screen of an available comprehensive S. sanguinis SK36 mutant library (60) that covers ∼90% of the predicted 2,270 protein coding genes in the S. sanguinis SK36 genome. In this screen, we sought to identify mutants with reduced enhancement phenotypes in the presence of P. aeruginosa PAO1 (Fig. 2A). Briefly, a 96-pin replicator was used to transfer inocula from the frozen library plates (each well of the plate contained a single S. sanguinis SK36 mutant strain) to a 96-well plate containing 150 μl of Bacto Todd-Hewitt (TH) broth supplemented with 0.5% (wt/vol) yeast extract (THY) broth per well. The freshly inoculated plate was then incubated statically for 24 h at 37°C with 5% CO2. In parallel, a P. aeruginosa PAO1 culture was grown overnight in lysogeny broth (LB) and adjusted to an optical density of 600 nm (OD600) of 0.05 in minimal essential medium (MEM) supplemented with 2 mM l-glutamine (MEM+l-Gln), and 400 μl of this adjusted inoculum suspension was added to each well of a 96-well deep-well plate. The 96-pin replicator was then used to transfer 2 to 3 μl of the 24-h culture from the mutant library plate into the 96-well deep-well plate containing P. aeruginosa PAO1. Unattached bacteria were removed after 2 h, 400 μl of fresh MEM+l-Gln was added to each well, and the plates were grown for an additional 20 h at 37°C with 5% CO2. The 96-pin replicator was again used to disrupt the biofilms into the planktonic fraction, large petri dish plates containing either Pseudomonas isolation agar (PIA) or blood agar supplemented with 10 μg/ml neomycin and 10 μg/ml polymyxin B (SBA) medium were spot inoculated with each culture, and the growth of P. aeruginosa or S. sanguinis, respectively, was assessed. Candidate mutants that showed low or undetectable growth compared to that of the wild-type S. sanguinis SK36 (which formed small lawns when inoculated onto an agar plate by the 96-pin replicator) were stored at −80°C in 30% glycerol in a sterile 96-well plate. To confirm the phenotype, a second and third round of mutant screening were performed as described above. Of 2,048 mutants screened, a total of 80 mutants showed a measurable and repeatable reduction in the enhancement of S. sanguinis growth (Table S1).
FIG 2.
Identification of S. sanguinis SK36 mutants that exhibit reduced P. aeruginosa-mediated growth enhancement. (A) Schematic diagram of the genome-wide screen of the S. sanguinis SK36 mutant library. (B) Functional classification of screen hits using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology (KO) database, shown here as the number of candidate mutants identified in each of the indicated pathways. (C) Genes required for zinc uptake identified in the screen and corresponding functions (https://www.genome.jp/dbget-bin/get_linkdb?-t+genes+gn:T00473).
Among those 80 candidates, mutations were found in genes belonging to a wide variety of functional classes (Fig. 2B), including (i) carbohydrate transport and metabolism (SSA_0222, SSA_0773, SSA_1261, SSA_1521, SSA_1749, etc.), (ii) amino acid transport and metabolism (SSA_0564, SSA_1043, SSA_1044, SSA_1341-1343, etc.), (iii) cell division and cell envelope biogenesis (SSA_0015, SSA_0655, etc.), (iv) coenzyme metabolism (SSA_1201, SSA_1202, SSA_1536, etc.), (v) DNA replication (SSA_1626), (vi) nucleotide transport and metabolism (SSA_0568, SSA_1163, etc.), and (vii) translation, ribosomal structure and protein biosynthesis (SSA_0820, SSA_1272, SSA_1611, SSA_1613, SSA_1895, SSA_1896, SSA_2033, SSA_2058, etc.). We also found genes predicted to be involved in metal ion (zinc and manganese) transport (SSA_0136, SSA_0137, SSA_0260, SSA_0261, and SSA_2365-2367) and FeS cluster assembly (SSA_1955 and SSA_1956). A complete list of mutants and the documented or proposed functions of the encoded gene products of the mutated loci is provided in Table S1.
Zinc is required for the promotion of S. sanguinis growth by P. aeruginosa.
To further probe the basis for the enhanced S. sanguinis growth mediated by P. aeruginosa, we focused on the role of zinc importers identified in our screen (SSA_0136, SSA_0137, SSA_0260, and SSA_0261) (Fig. 2C). In S. sanguinis SK36, the SSA_0136 and SSA_0137 genes encode components of an Adc zinc ATP-binding cassette (ABC) transporter, which is involved in zinc uptake in several pathogenic streptococci. The SSA_0136 and SSA_0137 genes are called adcC and adcB, respectively (30, 50). The SSA_0260 gene, also termed ssaB (S. sanguinis adhesin B) (61), encodes an LraI family lipoprotein and serves as the substrate-binding protein for a Mn/Zn ABC import system (62). The SSA_0261 gene, also named ssaC, is located upstream of SSA_0260 and encodes a putative Mn/Zn ABC transporter permease (UniProt identifier A3CKL5). The SSA_0260 and SSA_0261 genes are predicted to be cotranscribed.
To validate our screen results, the four zinc transporter mutants were further tested in the coculture assay with P. aeruginosa PAO1. We found that the SSA_0136 and SSA _0137 mutants showed an ∼2-log reduction in growth enhancement relative to that of the wild-type S. sanguinis SK36, while the SSA_0260 and SSA_0261 mutants displayed a 1-log reduction in growth enhancement compared to that of the wild-type S. sanguinis SK36 (Fig. 3A). In addition, the four mutants were also tested in monoculture to determine their growth behavior; the results revealed that these mutants grew like the S. sanguinis wild type (Fig. S2), suggesting that the growth enhancement deficiency observed for these mutants was not due to a general growth defect.
FIG 3.
Zinc is required for the P. aeruginosa-induced enhancement of S. sanguinis growth. (A) Growth of wild-type S. sanguinis and zinc transporter mutants with P. aeruginosa in media with or without addition of zinc at the indicated concentration. Statistical significance was assessed by one-way analysis of variance (ANOVA) with a Tukey’s multiple-comparison test, and different letters indicates statistically significant differences (P < 0.05). Identical letters indicate no significant difference. In this and all subsequent panels, the SSA designation indicates the wild-type gene, while the Ssx designation indicates a mutation in that gene, using the convention reported in the original description of these mutant strains (60). (B) Complementation assays with the zinc transporter mutant strains. Significant differences in growth compared to the uncomplemented vector control are indicated. *, P < 0.05; **, P < 0.01 (Student's t test). (C) Growth of indicated S. sanguinis strains with P. aeruginosa on CF airway cells in the presence or absence of 1 μM zinc. Statistical significance was determined by one-way ANOVA with a Tukey’s multiple-comparison test. Different letters indicate statistically significant differences (P < 0.05). Identical letters indicate no significant difference. (D) Growth of indicated S. sanguinis strains in monoculture on CF airway cells in the presence or absence of 1 μM zinc. Data are representative of three experiments performed in triplicate, and none of the differences are significant.
To confirm that the observed growth promotion defect was indeed caused by inactivation of the individual genes, we complemented each of the mutants with its corresponding gene under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter, as previously reported (63). As shown in Fig. 3B, the complemented mutants showed growth enhancement phenotypes similar to that of wild-type S. sanguinis SK36, indicating that the mutation in these genes is solely responsible for the observed growth enhancement deficiency. Together, our data indicate that the functions of these genes are required for S. sanguinis SK36 to show enhanced growth in coculture with P. aeruginosa.
As zinc ABC transporters are required for zinc acquisition (30), we reasoned that the defects in growth enhancement of these mutants during coculture might be due to the lack of zinc uptake. Consequently, the effect of supplementing additional zinc to the coculture medium was tested. We first measured the zinc levels of the medium used in our coculture conditions (MEM+l-Gln) and found that the concentration of zinc was 0.024 ± 0.007 μM. Supplementation of the medium with 0.1 μM, 1 μM, and 10 μM zinc chloride restored the growth enhancement phenotype of each of the mutants to wild-type levels (Fig. 3A), and growth of wild-type S. sanguinis SK36 was slightly promoted upon addition of 0.1 μM and 1 μM zinc (Fig. 3A). There was no significant difference in P. aeruginosa growth in monoculture or coculture in the zinc-amended and unamended media (Fig. S3). These results indicate that zinc is required for efficient growth enhancement of S. sanguinis during coculture with P. aeruginosa. Notably, zinc added to 10 μM slightly inhibited the growth of wild-type S. sanguinis SK36 and the SSA_0136 and SSA _0137 mutants but not that of the SSA_0260 and SSA_0261 mutants (Fig. 3A). Taken together, our data suggest that high concentrations of zinc are toxic to S. sanguinis, and that these zinc transporters play an important role in zinc acquisition and may have distinct affinities for zinc.
As mentioned above, P. aeruginosa can also promote S. sanguinis SK36 growth on airway cells (Fig. 1D). To further investigate the role of zinc in P. aeruginosa-induced growth enhancement, we extended our observation to cocultures on CFBE monolayers. The wild-type S. sanguinis SK36 strain and zinc transporter mutants were cocultured with P. aeruginosa with 1 μM zinc chloride added to the medium. Similarly to the observations in our coculture model on plastic, the zinc transporter mutants exhibited reduced growth enhancement, and supplementing the medium with 1 μM zinc rescued the growth enhancement defect of these mutants (Fig. 3C). It is worth noting that there were no significant differences in growth of wild-type S. sanguinis SK36 and the zinc transporter mutants in monoculture on CFBE monolayers with or without addition of zinc (Fig. 3D). The growth of S. sanguinis was enhanced when cultured with CFBE cells in the presence or absence of added zinc (Fig. 1D), indicating that CFBE monolayers produce a factor(s) other than zinc that can enhance the growth of S. sanguinis. Taken together, these data demonstrate that enhanced S. sanguinis growth mediated by P. aeruginosa requires the zinc importers of S. sanguinis SK36.
Coculture with P. aeruginosa upregulates zinc transporter gene expression in S. sanguinis.
We next examined the expression patterns of zinc transporter genes in both S. sanguinis and P. aeruginosa using reverse transcription-quantitative PCR (qRT-PCR). As shown in Fig. 1A, in the first 2 h of coculture with P. aeruginosa PAO1, S. sanguinis colonies did not show a significant enhancement in growth. The mixed cultures showed enhanced S. sanguinis growth at 6 h. We saw no differences between the pure and the mixed cultures when we examined the growth kinetics of P. aeruginosa PAO1 over the course of 24 h. As a consequence of these findings, qRT-PCR studies were performed on samples taken at 2 h and 6 h for both monocultures and cocultures.
At 2 h, S. sanguinis zinc transporter genes SSA_0136 and SSA_0137 were upregulated 2.2- and 2.3-fold, respectively, in coculture compared to in S. sanguinis monoculture (Fig. 4A), while the expression of SSA_0260 and SSA_0261 genes were not significantly changed at this time point (Fig. 4A). At 6 h, S. sanguinis expression of all of the four zinc transporter genes was upregulated (4.4-fold for SSA_0136, 4.7-fold for SSA_0137, 8.2-fold for SSA_0260, and 4.3-fold for SSA_0261) when cocultured with P. aeruginosa (Fig. 4A). In contrast, the addition of exogenous zinc (1 μM) to the coculture medium reduced the expression of S. sanguinis zinc transporter genes to the same level as that in monocultures with zinc supplementation (Fig. 4B).
FIG 4.
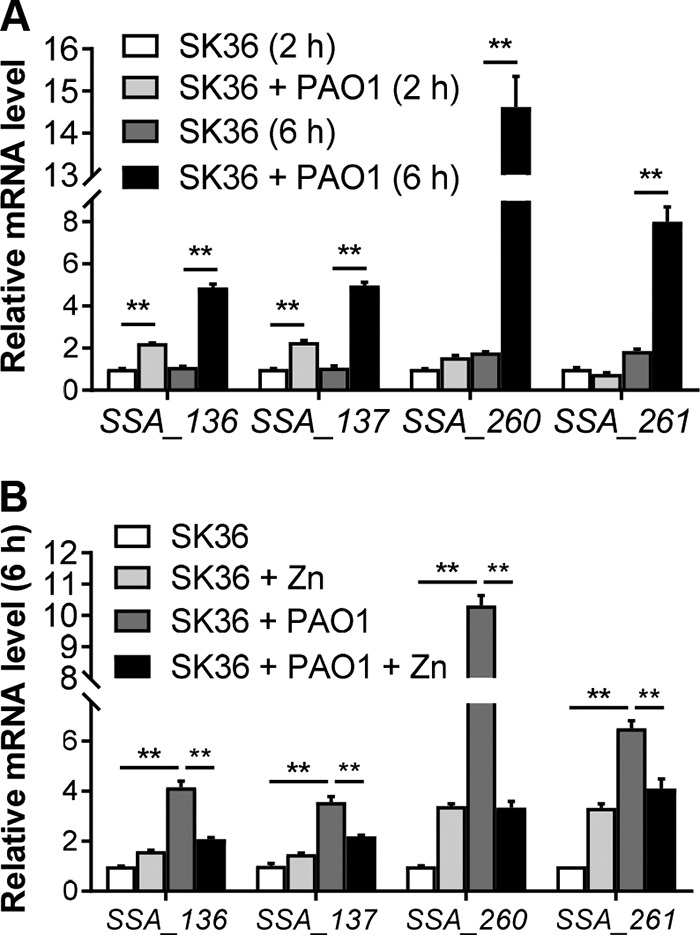
S. sanguinis zinc transporter genes are upregulated in the presence of P. aeruginosa. (A) Relative mRNA expression of S. sanguinis zinc transporter genes in monoculture and coculture with P. aeruginosa at 2 h and 6 h. The relative mRNA expression was measured using qRT-PCR, normalized to the expression of the gyrA control, and calculated using the threshold cycle (2−ΔΔCT) method, setting the value of SK36 (2 h) as 1. (B) Relative expression of S. sanguinis zinc transporter genes at 6 h in monoculture and coculture with P. aeruginosa with or without zinc (1 μM) supplementation. Error bars represent deviations of the means. ANOVA with a Tukey’s multiple-comparison test was used for statistical analysis. **, P < 0.01.
The expression of P. aeruginosa PAO1 zinc uptake and regulator genes (zur, znuA, cntI, and cntO) was minimally impacted by the presence of S. sanguinis compared to that of P. aeruginosa PAO1 alone at either 2 h or 6 h (Fig. S4), although the zinc transporters of P. aeruginosa PAO1 were significantly upregulated in monoculture and coculture at 6 h compared to 2 h (Fig. S4). Notably, the robust increase in zinc transporter gene expression by S. sanguinis at 6 h corresponds to the induction of P. aeruginosa uptake systems at this same time point.
Taken together, our studies are consistent with a model that S. sanguinis zinc transporter genes are induced by zinc deficiency when S. sanguinis is grown in coculture with P. aeruginosa, presumably due to zinc competition between these organisms.
Mutations in zinc-related genes of P. aeruginosa promote modest growth of S. sanguinis in coculture.
Our data suggest that in coculture, P. aeruginosa and Streptococcus bacteria compete for zinc. As shown above, S. sanguinis SK36 mutants defective in zinc uptake show less robust viability when grown in coculture with P. aeruginosa. Thus, we predict that mutations in zinc uptake in P. aeruginosa would result in S. sanguinis SK36 more effectively competing for zinc, likely reflected by an increase in viable counts of S. sanguinis.
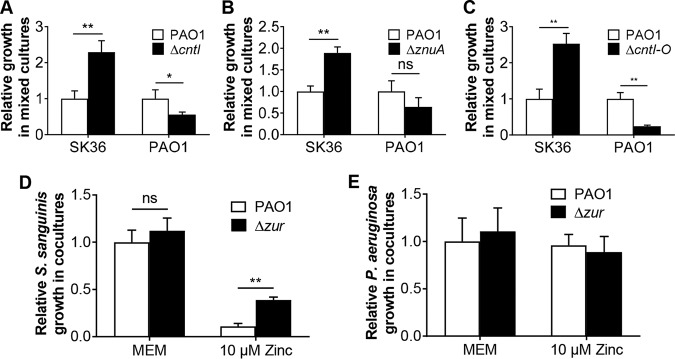
We first assessed the impact of mutating zinc transport systems of P. aeruginosa PAO1 by testing strains with mutations in the cntI, znuA, and cntI-O genes (Fig. 5A to C). In all cases, coculture with the P. aeruginosa zinc transport mutants resulted in a modest enhancement (∼2- to 3-fold) in growth of S. sanguinis SK36 on top of the typical ∼4-log growth promotion by P. aeruginosa compared to that in coculture with the wild-type P. aeruginosa PAO1. That is, eliminating zinc competition only has a small positive effect on S. sanguinis SK36 viable counts, which cannot account for the robust increase in growth we observe in the typical coculture experiment, for example, as shown in Fig. 1. One confounding factor in the interpretation of these results is that all of the P. aeruginosa zinc transport mutants show a small but consistent ∼50% reduction in growth in coculture, which may be due to the lack of effective zinc transport or to other anticipated defects, as previously reported (42, 44).
FIG 5.
Analysis of zinc transporter mutants. (A to C) Growth of S. sanguinis SK36 with P. aeruginosa PAO1 wild-type and indicated mutant strains in coculture. The strains tested carry deletion mutations in the cntI (A), znuA (B) and cntI-O (C) genes of P. aeruginosa PAO1. (D and E) Growth of S. sanguinis SK36 (D) and P. aeruginosa wild-type and ΔzurA mutant strains (E) in coculture assays in MEM (no added zinc) or with 10 μM added zinc (growth-inhibitory level of zinc). ANOVA with a Tukey’s multiple-comparison test was used for statistical analysis (**, P < 0.01) in panel D. There were no significant differences in panel E. SK36, S. sanguinis SK36; PAO1, P. aeruginosa PAO1.
In P. aeruginosa, the expression of zinc importer genes is controlled by the Zur protein, a zinc responsive repressor (43), and loss of Zur results in a higher intracellular zinc concentration in P. aeruginosa (44). We generated a P. aeruginosa PAO1 Δzur mutant and cocultured this mutant with S. sanguinis SK36 with or without zinc addition; coculture of wild-type P. aeruginosa PAO1 with S. sanguinis served as the control. In the absence of added zinc, there was no difference in the growth of S. sanguinis SK36 in the presence of the P. aeruginosa PAO1 wild type versus that of the Δzur mutant (Fig. 5D and E). As described above, in mixed cultures of S. sanguinis and wild-type P. aeruginosa PAO1, we found that excessive zinc concentration (10 μM) led to a 9-fold decline in S. sanguinis growth compared to that in cells grown without zinc supplementation (Fig. 3A and Fig. 5D). In contrast, mixed cultures of S. sanguinis and the P. aeruginosa PAO1 Δzur mutant in the presence of 10 μM zinc resulted in a 3.5-fold increase in S. sanguinis growth compared to that observed for coculture with wild-type P. aeruginosa PAO1 (Fig. 5D). There was no difference in the growth of P. aeruginosa PAO1 wild type versus that of the Δzur mutant in coculture (Fig. 5E). Given the protective action of the zinc hyper-accumulating P. aeruginosa PAO1 Δzur mutant toward S. sanguinis SK36, these results further support the idea that P. aeruginosa and S. sanguinis compete for a shared pool of zinc when grown in coculture.
The relationship between sputum zinc concentration and the relative abundances of Streptococcus and Pseudomonas spp.
In previous studies, we collected sputum samples from patients with CF and assessed the relative abundances of Streptococcus and Pseudomonas spp. in these samples (64). These same samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS), as previously reported (64, 65), to determine the concentration of zinc in the sputum. We observed that the concentration of zinc in these samples ranged from 4.8 to 145 μM, with a median of 36.4 μM (n = 118 sputum samples) (see Fig. S5).
We examined the relationship between zinc concentration and the relative abundances of Streptococcus and Pseudomonas spp. in these samples. We observed that Streptococcus generally had greater relative abundance in sputum samples with lower zinc concentrations compared to that in sputum samples in which the zinc concentration was relatively high (Fig. 6, orange dots), although there are some exceptions to this pattern. The relative abundance of Pseudomonas varied across these samples (Fig. 6, blue dots). Interestingly, the most frequent examples of low relative abundance of Pseudomonas spp. (<10%) seemed to occur at the lowest zinc concentrations. Although measures like Pearson’s or Spearman’s correlations have been used to quantify this type of relationship and attach statistical significance, the compositional nature of bacterial community abundance suggests that these statistical approaches are inappropriate (66, 67). Therefore, we interpret in vivo associations observed between Streptococcus abundance, Pseudomonas abundance, and zinc concentration as broadly consistent with the hypothesis that they are related without attaching a specific measure of statistical significance.
FIG 6.
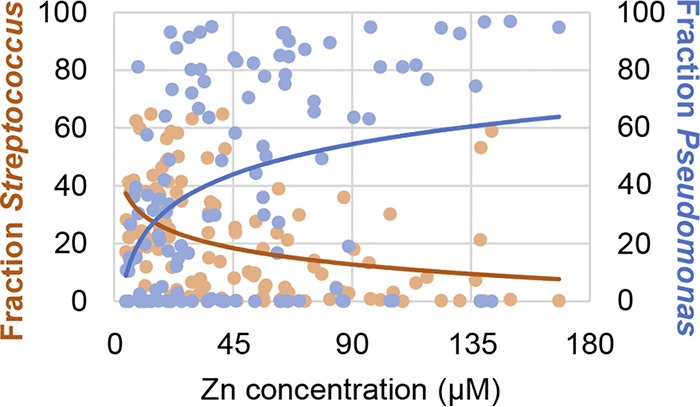
The relationship between sputum zinc concentration and the relative abundances of Streptococcus and Pseudomonas spp. The relative abundance of Streptococcus (orange dots) and Pseudomonas (blue dots) in each sputum sample (indicated as a fraction of 100% of the total microbial community) is plotted versus the concentration of zinc in the corresponding sample. For each sputum sample, there are two dots, one for Streptococcus and one for Pseudomonas. Total zinc was measured by ICP-MS analysis of nitric acid-dissolved samples and normalized to the volume of the sample.
Physiologically relevant levels of zinc impact the competition between Streptococcus and Pseudomonas.
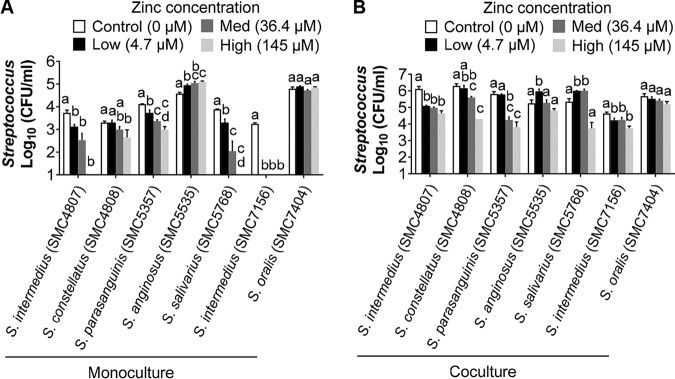
Given the observations described above, we decided to assess how various, but physiologically relevant, zinc concentrations (see Fig. S5) might impact the competition between various Streptococcus species found in the CF airway and P. aeruginosa (Fig. 7). For clinical isolates of Streptococcus intermedius (two isolates), Streptococcus constellatus, Streptococcus parasanguinis, and Streptococcus salivarius, all of which have been found in the CF airway (11), increasing zinc across a range of concentrations measured in sputum resulted in progressively lower viability of many of these streptococci when grown in monoculture (Fig. 7A) or in coculture with P. aeruginosa (Fig. 7B). Thus, many of these strains appear to be sensitive to clinically relevant concentrations of zinc. Interestingly, two isolates (Streptococcus anginosus and Streptococcus oralis) showed robust growth even at the highest level of zinc tested, indicating that these strains have increased tolerance to zinc for reasons we do not understand. Interestingly, the data in Fig. 6 show that some patients with high levels of zinc in their sputum also have high relative abundance of Streptococcus spp.; perhaps these patients harbor zinc-resistant streptococci.
FIG 7.
Impact of zinc level on viability of Streptococcus in monoculture and coculture with Pseudomonas. Growth of the indicated streptococci when grown in monoculture (A) or coculture with P. aeruginosa PAO1 (B) at the indicated concentration of supplemented zinc chloride. The 0 addition has only the zinc present in the medium (0.024 ± 0.007 μM). The other zinc additions were based on the data shown in Fig. S5 and indicate the lowest (4.7 μM), median (36.4 μM), and highest (145 μM) concentrations of zinc found in the 118 sputum samples analyzed. Statistical significance was determined by one-way ANOVA with a Tukey’s multiple-comparison test. Different letters indicate statistically significant differences (P < 0.05). Identical letters indicate no significant difference.
DISCUSSION
In this study, we characterized the interaction between P. aeruginosa and S. sanguinis SK36 in a dual-species-coculture model system. We demonstrated that zinc uptake by S. sanguinis SK36 was necessary for the P. aeruginosa-mediated promotion of S. sanguinis SK36 growth, and our findings are consistent with the model that P. aeruginosa competes with S. sanguinis SK36 for zinc during cocultivation. Additionally, we described a potential association between zinc levels and the abundance of Pseudomonas and Streptococcus spp. in CF sputum, thus highlighting the potential role of zinc in the interaction between these CF pathogens as well as a potential role for this metal in shaping microbiome dynamics in the context of polymicrobial CF airway infections.
We report that cocultivation with P. aeruginosa results in enhanced growth of S. sanguinis SK36 on either plastic or CF-derived airway cells, while P. aeruginosa growth is relatively unaffected during coculture. These data are consistent with previous reports from our group and others that coculture of streptococci with P. aeruginosa promotes the growth of streptococci, but with no obvious benefit to P. aeruginosa growth (8–10). Interestingly, in a study examining the interactions between P. aeruginosa and oral streptococci, including S. sanguinis (12, 68), P. aeruginosa growth was inhibited when streptococci were grown as a pioneer colonizer; streptococci can produce hydrogen peroxide (H2O2) to react with excess nitrite in the medium to generate reactive nitrogenous intermediates (RNI) for the inhibition of P. aeruginosa growth (12, 13, 68). We note that P. aeruginosa was inoculated simultaneously with S. sanguinis in our study under conditions that differ from these previous reports (including no added nitrate in our experiments), and we did not observe streptococcus-mediated inhibition of P. aeruginosa. These observations suggest that the relationships between P. aeruginosa and streptococci are complex and are likely influenced by metabolic/environmental factors and colonization sequence. Understanding how various in vitro models reflect dynamic in vivo environments will contribute to efficiently synthesizing and better understanding the data obtained from various laboratories.
By screening a genome-wide nonessential gene mutant library of S. sanguinis SK36, we discovered 80 mutants with attenuation in P. aeruginosa-mediated growth enhancement (summarized in Table S1 in the supplemental material). The large number of genes (3.5% of the S. sanguinis SK36 genome) found to be involved in this interspecies interaction and the variety of functions performed by these gene products provide new insights into the mechanism(s) of interaction between these two pathogens. Among the candidate genes we identified in our screen, we focused on a set of genes involved in the import of zinc. We demonstrate that the ability to obtain zinc as one factor contributing to the P. aeruginosa-induced enhancement of S. sanguinis SK36 growth, both in the presence and absence of human airway cells. Future studies will focus on the other genes identified in our screen.
Our observations here raise the question of whether zinc is a factor that drives growth enhancement of streptococci in the presence of P. aeruginosa, or whether efficient uptake of zinc is required to allow the growth-promoting factors produced by P. aeruginosa to exert their effect. We favor the latter model, in large part because we show that adding additional zinc to monocultures of S. sanguinis SK36 or other streptococci has, at best, a very modest effect on growth of streptococci. Similarly, as shown in Fig. 5, mutating the zinc uptake system of P. aeruginosa PAO1 results in only a modest 2- to 3-fold increase in S. sanguinis SK36 growth on top of the typical ∼4-log growth promotion by P. aeruginosa. That is, eliminating zinc competition only has a small effect on S. sanguinis SK36 viable counts, which cannot account for the robust increase in growth we observe in our typical coculture experiment, for example, as shown in Fig. 1. Thus, it does not appear that the streptococci are obtaining zinc from P. aeruginosa to enhance streptococcal growth by the ∼4 logs we typically observe. Instead, we observed that zinc starvation might be triggered by competition for this metal between P. aeruginosa and S. sanguinis SK36. Indeed, the elevated expression of the zinc transporters of wild-type S. sanguinis SK36 in mixed cultures, and the lack of such induction with zinc supplementation in the coculture medium, indicates that S. sanguinis SK36 becomes zinc starved during its interaction with P. aeruginosa. In contrast, there was no significant change in the expression of P. aeruginosa zinc transporters under coculture conditions, indicating that P. aeruginosa is not lacking this metal under these conditions; thus, it is unlikely that S. sanguinis is “stealing” the zinc in the culture from P. aeruginosa. Taking these results together, we argue that zinc provided by P. aeruginosa is not a key factor enhancing the robust (∼4-log) streptococcal growth in coculture. Given these findings, we conclude that other yet-to-be-identified factors (besides zinc) are provided to S. sanguinis by P. aeruginosa to stimulate the robust growth enhancement of S. sanguinis observed in coculture (Fig. 1).
An intriguing observation here is the relationship between the sputum zinc levels and the relative fraction of Pseudomonas and Streptococcus in the CF sputum. In the human body, the zinc concentration varies among different tissues, and total zinc concentration in induced sputum from control patients is around 1 μM (50 μg/liter) (30, 69), while higher levels of total sputum zinc have been reported in patients with CF (53), a finding consistent with our sputum measurements here. Furthermore, the zinc-sequestering protein calprotectin is present in CF sputum in high concentrations (70, 71), potentially resulting in limited bioavailability of zinc for CF pathogens like P. aeruginosa (54). Thus, at present, it is difficult to conclude how much of the increased zinc measured in CF sputum is actually available to the microbes; knowing the answer to this question may be key to understanding disease progression. For example, based on in vitro studies, efficient zinc uptake is critical for P. aeruginosa to express several virulence traits associated with lung colonization, including swarming, swimming motility, and the ability to form biofilms (54). It has been shown that high concentrations of total zinc are correlated with airway inflammation (53), indicating that perhaps P. aeruginosa can access some of the large pool of zinc in some circumstances. Furthermore, sputum zinc levels were found to decrease following antibiotic treatment of CF exacerbation (53, 54), which is again suggestive that loss of access to zinc reduces virulence. Interestingly, we found a possible relationship between the concentrations of zinc and relative abundance of Pseudomonas and Streptococcus spp.; however, because of the relative abundance data available for this analysis, determining a statistical correlation is difficult. Nevertheless, we do note that at higher measured zinc concentrations, the relative abundance of Streptococcus spp. appears to be lower than that at low concentrations of this metal, which may be related to the toxicity observed when Streptococcus is grown under high levels of zinc, which results in impaired growth or death (72). These data do suggest that increased total zinc may be associated with increased bioavailable zinc. A more detailed analysis of the physiology of zinc metabolism in CF sputum and measurements of the bioavailability of this metal will be required to definitively address the questions raised here, and these questions should be addressed in future studies.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table S2 in the supplemental material. S. sanguinis SK36 and other clinical streptococcal isolates were grown statically in Bacto Todd-Hewitt (TH) broth supplemented with 0.5% (wt/vol) yeast extract (THY), or on Trypticase soy agar plates supplemented with 5% (vol/vol) defibrinated sheep blood (blood agar) at 37°C with 5% CO2. P. aeruginosa and Escherichia coli strains were grown in lysogeny broth (LB) medium (73) with shaking or on LB agar at 37°C unless otherwise noted. As indicated, the following antibiotics and concentrations were used: 500 μg/ml kanamycin and 200 μg/ml spectinomycin for S. sanguinis; 50 μg/ml gentamicin and 150 μg/ml carbenicillin for P. aeruginosa; and 10 μg/ml gentamicin, 50 μg/ml carbenicillin and 100 μg/ml spectinomycin for E. coli. For IPTG-inducible plasmids, IPTG was added to cultures to a 100 μM final concentration.
Coculture assays.
Coculture assays were performed as previously described with minor modifications (8). Briefly, overnight cultures of P. aeruginosa and Streptococcus spp. were centrifuged at 13,000 × g for 3 min, washed twice with phosphate-buffered saline (PBS), and resuspended in minimal essential medium (MEM) supplemented with 2 mM l-glutamine (MEM+l-Gln). For coculture samples, P. aeruginosa inoculum was prepared in MEM+l-Gln to an OD600 of 0.05, and Streptococcus spp. inoculum was prepared to an OD600 of 0.001. For monoculture controls, the inocula for P. aeruginosa or Streptococcus spp. were prepared to the same OD600 as for the coculture samples. Three wells of a 96-well deep-well plate were inoculated with 400 μl per well per monoculture and coculture condition. Culture plates were then incubated statically at 37°C with 5% CO2 for 2 h, at which point the unattached planktonic cells were removed by aspiration, and 400 μl of fresh MEM+l-Gln was once again added to each well. The cultures were then incubated for an additional 20 h, and both planktonic and biofilm cells were harvested together using a 96-pin replicator. Bacterial growth was determined by 10-fold serial dilutions in PBS, and cultures were plated in 3-μl aliquots on Pseudomonas isolation agar (PIA) or blood agar supplemented with 10 μg/ml neomycin and 10 μg/ml polymyxin B (SBA) for P. aeruginosa and Streptococcus spp. selective growth, respectively. After overnight incubation, bacterial colonies were counted and the CFU per milliliter of culture was determined.
Kinetic growth assay.
The relative ability of S. sanguinis SK36 and P. aeruginosa PAO1 to grow in the coculture model system was determined by kinetic growth assays. Briefly, S. sanguinis SK36 was grown in coculture with P. aeruginosa PAO1 as described above in a 96-well deep-well plate. The cultures were grown at 37°C with 5% CO2 for 24 h and were assessed for viable cell counts (CFU/milliliter) at the following 7 time points: 0, 2, 4, 6, 8, 10, and 24 h. The 0-h time point corresponds to the initial inoculum. Cells were collected at the 2-h time point prior to the 2-h medium replacement. At each time point, a combination of the planktonic and biofilm cells from triplicate wells were serially diluted and plated on PIA and SBA agar media to select for P. aeruginosa and Streptococcus, respectively, and CFU counts were determined after overnight incubation as described above.
P. aeruginosa supernatant assays.
To prepare P. aeruginosa conditioned medium, bacteria were grown in 0.5 ml per well of MEM+l-Gln in 24-well plates with medium changes as described above. At the 22-h time point, the planktonic fractions were collected and centrifuged, and supernatants were filter-sterilized through a 0.22-μm syringe filter. The effect of the sterile P. aeruginosa supernatants on the growth of S. sanguinis was tested on both plastic and CFBE monolayers. For experiments on plastic, supernatant at different dilutions in fresh MEM+l-Gln were added to S. sanguinis monocultures when the medium was replaced at the 2-h time point. For assays on CF airway cells, a one-half dilution of P. aeruginosa supernatant in MEM+l-Gln was supplemented with 0.4% l-arginine and gently added to each well of CF airway cells that had been cocultured with S. sanguinis for 1 h or 5.5 h when the medium was exchanged. S. sanguinis growth was evaluated after 22 h of incubation at 37°C with 5% CO2 as described above.
Tissue culture cells and coculture on CFBE monolayers.
The cystic fibrosis bronchial epithelial (CFBE) monolayers used in the coculture model (59, 74) are immortalized cells that overexpress ΔF508 cystic fibrosis transmembrane conductance regulator (75). CFBE monolayers were grown as previously described (10, 74). In brief, the CFBE monolayers were seeded at a concentration of 100,000 cells/well in a 24-well tissue culture plate and fed every other day with MEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 μg/ml puromycin, and 5 μg/ml plasmocin. Cells were grown at 37°C with 5% CO2 for 5 to 7 days to form a confluent monolayer and tight junctions before inoculation with bacteria. For coculture assays with mono- and dual-bacterial species, liquid cultures of P. aeruginosa and Streptococcus spp. were prepared as described above, and 500 μl of bacterial inocula were gently added to triplicate wells of CFBE monolayers that had been washed twice with MEM. The cocultures were incubated at 37°C with 5% CO2 for 1 h, at which point unattached bacteria were removed by aspiration and 500 μl of MEM+l-Gln + 0.4% l-arginine was added to each well and incubated for an additional 4.5 h. At this point, planktonic cells were removed by aspiration, and 500 μl of fresh MEM+l-Gln + 0.4% l-arginine was once again added into each well. The established coculture was incubated for an additional 16.5 h. At 22 h postinoculation, both planktonic and biofilm-grown bacteria were collected together by scraping with a pipette tip, and bacteria were serially diluted and plated on PIA and SBA plates, as described above, to identify P. aeruginosa and Streptococcus spp., respectively. Following overnight incubation, the resulting colonies were counted, and the CFU/ml of the culture was determined.
Zinc supplementation.
For zinc supplementation assays, wild-type Streptococcus sanguinis SK36 and individual mutants with growth enhancement defects, wild-type P. aeruginosa PAO1, and P. aeruginosa PAO1 zinc homeostasis-associated mutants were grown as described above for monoculture and coculture assays. After 2 h, unattached bacteria were removed by aspiration, MEM+l-Gln with or without additional zinc (at the indicated concentrations, diluted from 1 mM zinc chloride stock solution in MEM+l-Gln) was added to each well, and cocultures were then treated as described above. Nutritional complementation on CF airway cells were performed as described for biofilms on plastic, except that MEM+l-Gln supplemented with 0.4% arginine (to enhance biofilm formation), with or without additional zinc chloride at the indicated concentration, was used for medium exchange when indicated.
Construction of mutants and complementation.
In-frame deletions of P. aeruginosa genes were constructed by allelic exchange employing the sucrose counterselection system with the gene replacement vector pEX18Ap (76). Mutant strains were confirmed by PCR analysis of genomic DNA. S. sanguinis mutants were derived from a defined mutant library described previously (60). For complementation of each targeted S. sanguinis gene, a suicide vector, pJFP126, was used to allow for the insertion of complementing genes into an ectopic chromosomal site (SSA_0169) via homologous recombination and expression of each gene is under the control of an IPTG-inducible promoter, hyper-spank (63). Transformation was performed essentially as described previously (77) with a competence-stimulating peptide (CSP; sequence DLRGVPNPWGWIFGR) custom synthesized by GenScript, Inc. (Piscataway, NJ). Primers used for PCR amplification of selected genes are listed in Table S3.
Expression studies.
For qRT-PCR studies, the overnight culture used as the inoculum was prepared as described above; from this inoculum, three replicate cocultures each of P. aeruginosa and S. sanguinis or the S. sanguinis or the P. aeruginosa monoculture were prepared in 100 ml of warm MEM+l-Gln in a 250-ml flask. The inocula of P. aeruginosa- and S. sanguinis were prepared and brought to an OD600 of 0.05 and 0.02, respectively, as described above. Cultures were incubated at 37°C with 5% CO2. After 2 h and 6 h of incubation, samples were pelleted, and bacterial cells were mechanically lysed with 10 30-s cycles of bead beating, with each cycle followed by 30 s on ice, with a 1:1 mixture of 0.1 mm and 0.5 mm glass beads. Total RNA was isolated using TRIzol and the Direct-zol RNA miniprep kit (catalog no. R2053; Zymo Research) with two rounds of in-column DNase I treatment according to the manufacturer’s instructions. RNA purity and concentration were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific). For each sample, 1 μg of RNA was converted to cDNA using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) and then diluted 1:50. qRT-PCR were carried out in triplicate in a StepOnePlus real-time PCR system (Applied Biosystems) using iTaq Universal SYBR green supermix (Bio-Rad). The qRT-PCR primers are listed in Table S3. Relative gene expression was calculated using the threshold cycle (2−ΔΔCT) method with DNA gyrase subunit gene gyrA as a normalization control for S. sanguinis (78, 79) and PA2875 as the reference gene for P. aeruginosa (80).
Measurement of zinc in sputum samples.
Sputum samples for zinc analysis were stored at −80°C until processed. Sputum zinc was quantified by inductively coupled plasma-mass spectrometry (ICP-MS) following nitric acid digestion of organic material according to the method of Heck et al. and is expressed as micromolar zinc (65).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 7, and results were expressed as the mean values plus or minus standard deviations. Unless otherwise noted, one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test or Student’s t test analysis was performed to determine statistical significance of the data. See the figure legends or Results for other specific statistical tests used.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Cystic Fibrosis Foundation (grant OTOOLE16GO) and NIH (grant R37 AI83256-06) to G.A.O. and by a China Scholarship Council (CSC) grant (no. 201708330005) to K.L. T.H.H. is supported by the DartCF CF-BBC (P30-DK117469). We acknowledge support from the CF-Research Development Program (STANTO19R0) for the CFBE cells. The ICP-MS studies were performed by the Dartmouth Trace Element Analysis Core, funded by grant P42ES007373 from the National Institutes of Health.
We thank Brian Jackson for performing the ICP-MS studies. We also thank P. Xu for sharing the S. sanguinis mutant library.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Elborn J. 2016. Cystic fibrosis. Lancet 388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 2.Cutting GR. 2015. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe SM, Miller S, Sorscher EJ. 2005. Cystic fibrosis. N Engl J Med 352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 4.Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, Surette MG. 2008. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A 105:15070–15075. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherrard LJ, Tunney MM, Elborn JS. 2014. Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. Lancet 384:703–713. doi: 10.1016/S0140-6736(14)61137-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A 109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filkins LM, O’Toole GA. 2015. Cystic fibrosis lung infections: polymicrobial, complex, and hard to treat. PLoS Pathog 11:e1005258. doi: 10.1371/journal.ppat.1005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott J, Li K, Filkins L, Zhu B, Kuchma S, Schwartzman J, O’Toole G. 2019. Pseudomonas aeruginosa can inhibit growth of streptococcal species via siderophore production. J Bacteriol 201:e00014-19. doi: 10.1128/JB.00014-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoffield JA, Duan D, Zhu F, Wu H. 2017. A commensal Streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS Pathog 13:e1006300. doi: 10.1371/journal.ppat.1006300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price KE, Naimie AA, Griffin EF, Bay C, O’Toole GA. 2016. Tobramycin-treated Pseudomonas aeruginosa PA14 enhances Streptococcus constellatus 7155 biofilm formation in a cystic fibrosis model system. J Bacteriol 198:237–247. doi: 10.1128/JB.00705-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O’Toole GA. 2012. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol 194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scoffield JA, Wu H. 2015. Oral streptococci and nitrite-mediated interference of Pseudomonas aeruginosa. Infect Immun 83:101–107. doi: 10.1128/IAI.02396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scoffield JA, Wu H. 2016. Nitrite reductase is critical for Pseudomonas aeruginosa survival during co-infection with the oral commensal Streptococcus parasanguinis. Microbiology 162:376–383. doi: 10.1099/mic.0.000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastaert F, Kheir S, Saint-Criq V, Villeret B, Dang PM, El-Benna J, Sirard JC, Voulhoux R, Sallenave JM. 2018. Pseudomonas aeruginosa LasB subverts alveolar macrophage activity by interfering with bacterial killing through downregulation of innate immune defense, reactive oxygen species generation, and complement activation. Front Immunol 9:1675. doi: 10.3389/fimmu.2018.01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sibley CD, Grinwis ME, Field TR, Parkins MD, Norgaard JC, Gregson DB, Rabin HR, Surette MG. 2010. McKay agar enables routine quantification of the ‘Streptococcus milleri’ group in cystic fibrosis patients. J Med Microbiol 59:534–540. doi: 10.1099/jmm.0.016592-0. [DOI] [PubMed] [Google Scholar]
- 16.Scott JE, O’Toole GA. 2019. The yin and yang of Streptococcus lung infections in cystic fibrosis: a model for studying polymicrobial interactions. J Bacteriol 201:e00115-19. doi: 10.1128/JB.00115-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis EA, Coats MT, Griffin S, Pang B, Briles DE, Crain MJ, Swords WE. 2018. Hyperencapsulated mucoid pneumococcal isolates from patients with cystic fibrosis have increased biofilm density and persistence in vivo. Pathog Dis 76:fty073. doi: 10.1093/femspd/fty073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta N, Whelan FJ, Somayaji R, Poonja A, Surette MG, Rabin HR, Parkins MD. 2017. The evolving cystic fibrosis microbiome: a comparative cohort study spanning 16 years. Ann Am Thorac Soc 14:1288–1297. doi: 10.1513/AnnalsATS.201609-668OC. [DOI] [PubMed] [Google Scholar]
- 19.Flight WG, Smith A, Paisey C, Marchesi JR, Bull MJ, Norville PJ, Mutton KJ, Webb AK, Bright-Thomas RJ, Jones AM, Mahenthiralingam E. 2015. Rapid detection of emerging pathogens and loss of microbial diversity associated with severe lung disease in cystic fibrosis. J Clin Microbiol 53:2022–2029. doi: 10.1128/JCM.00432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta N, Heirali A, Somayaji R, Surette MG, Workentine ML, Sibley CD, Rabin HR, Parkins MD. 2018. Sputum microbiota is predictive of long-term clinical outcomes in young adults with cystic fibrosis. Thorax 73:1016–1025. doi: 10.1136/thoraxjnl-2018-211510. [DOI] [PubMed] [Google Scholar]
- 21.Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Zhang Y, Surendra A, Gong Y, Elizabeth Tullis D, Yau YC, Waters VJ, Hwang DM, Guttman DS. 2015. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreini C, Banci L, Bertini I, Rosato A. 2006. Zinc through the three domains of life. J Proteome Res 5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 23.Finney LA, O’Halloran TV. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 24.Mikhaylina A, Ksibe AZ, Scanlan DJ, Blindauer CA. 2018. Bacterial zinc uptake regulator proteins and their regulons. Biochem Soc Trans 46:983–1001. doi: 10.1042/BST20170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, Prentice BM, Gilston BA, Doster RS, Gaddy JA, Chazin WJ, Caprioli RM, Skaar EP. 2016. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat Commun 7:11951. doi: 10.1038/ncomms11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeShazer D. 2019. A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex. Microbiol Res 226:48–54. doi: 10.1016/j.micres.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Zavala B, Manz H, Englert F, Rogers PD, Morschhauser J. 2018. A hyperactive form of the zinc cluster transcription factor Stb5 causes Yor1 overexpression and beauvericin resistance in Candida albicans. Antimicrob Agents Chemother 62:e01655-18. doi: 10.1128/AAC.01655-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider RO, Diehl C, Dos Santos FM, Piffer AC, Garcia AWA, Kulmann MIR, Schrank A, Kmetzsch L, Vainstein MH, Staats CC. 2015. Effects of zinc transporters on Cryptococcus gattii virulence. Sci Rep 5:10104. doi: 10.1038/srep10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg SL. 2019. The role of metal ions in the virulence and viability of bacterial pathogens. Biochem Soc Trans 47:77–87. doi: 10.1042/BST20180275. [DOI] [PubMed] [Google Scholar]
- 30.Shafeeq S, Kuipers OP, Kloosterman TG. 2013. The role of zinc in the interplay between pathogenic streptococci and their hosts. Mol Microbiol 88:1047–1057. doi: 10.1111/mmi.12256. [DOI] [PubMed] [Google Scholar]
- 31.Makthal N, Kumaraswami M. 2017. Zinc’ing it out: zinc homeostasis mechanisms and their impact on the pathogenesis of human pathogen group A streptococcus. Metallomics 9:1693–1702. doi: 10.1039/c7mt00240h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcheron G, Garenaux A, Proulx J, Sabri M, Dozois CM. 2013. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:e00090. doi: 10.3389/fcimb.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG, Paton JC. 2011. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog 7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K, Ishihama A. 2005. Transcriptional response of Escherichia coli to external zinc. J Bacteriol 187:6333–6340. doi: 10.1128/JB.187.18.6333-6340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hantke K. 2005. Bacterial zinc uptake and regulators. Curr Opin Microbiol 8:196–202. doi: 10.1016/j.mib.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Poole K, Hay T, Gilmour C, Fruci M. 2019. The aminoglycoside resistance-promoting AmgRS envelope stress-responsive two-component system in Pseudomonas aeruginosa is zinc-activated and protects cells from zinc-promoted membrane damage. Microbiology 165:563–571. doi: 10.1099/mic.0.000787. [DOI] [PubMed] [Google Scholar]
- 37.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong CL, Gillen CM, Barnett TC, Walker MJ, McEwan AG. 2014. An antimicrobial role for zinc in innate immune defense against group A Streptococcus. J Infect Dis 209:1500–1508. doi: 10.1093/infdis/jiu053. [DOI] [PubMed] [Google Scholar]
- 39.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. 2011. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blencowe DK, Morby AP. 2003. Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev 27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]
- 41.Capdevila DA, Wang J, Giedroc DP. 2016. Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J Biol Chem 291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pederick VG, Eijkelkamp BA, Begg SL, Ween MP, McAllister LJ, Paton JC, McDevitt CA. 2015. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci Rep 5:13139. doi: 10.1038/srep13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez MR, Ducret V, Leoni S, Perron K. 2019. Pseudomonas aeruginosa zinc homeostasis: key issues for an opportunistic pathogen. Biochim Biophys Acta Gene Regul Mech 1862:722–733. doi: 10.1016/j.bbagrm.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Ellison ML, Farrow JM IIII, Parrish W, Danell AS, Pesci EC. 2013. The transcriptional regulator Np20 is the zinc uptake regulator in Pseudomonas aeruginosa. PLoS One 8:e75389. doi: 10.1371/journal.pone.0075389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lhospice S, Gomez NO, Ouerdane L, Brutesco C, Ghssein G, Hajjar C, Liratni A, Wang SL, Richaud P, Bleves S, Ball G, Borezee-Durant E, Lobinski R, Pignol D, Arnoux P, Voulhoux R. 2017. Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline. Sci Rep 7:17132. doi: 10.1038/s41598-017-16765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastropasqua MC, D’Orazio M, Cerasi M, Pacello F, Gismondi A, Canini A, Canuti L, Consalvo A, Ciavardelli D, Chirullo B, Pasquali P, Battistoni A. 2017. Growth of Pseudomonas aeruginosa in zinc poor environments is promoted by a nicotianamine-related metallophore. Mol Microbiol 106:543–561. doi: 10.1111/mmi.13834. [DOI] [PubMed] [Google Scholar]
- 47.Patzer SI, Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol 28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 48.Outten CE, O’Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 49.Plumptre CD, Eijkelkamp BA, Morey JR, Behr F, Couñago RM, Ogunniyi AD, Kobe B, O’Mara ML, Paton JC, McDevitt CA. 2014. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol Microbiol 91:834–851. doi: 10.1111/mmi.12504. [DOI] [PubMed] [Google Scholar]
- 50.Moulin P, Patron K, Cano C, Zorgani MA, Camiade E, Borezee-Durant E, Rosenau A, Mereghetti L, Hiron A. 2016. The Adc/Lmb system mediates zinc acquisition in Streptococcus agalactiae and contributes to bacterial growth and survival. J Bacteriol 198:3265–3277. doi: 10.1128/JB.00614-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linke C, Caradoc-Davies TT, Young PG, Proft T, Baker EN. 2009. The laminin-binding protein Lbp from Streptococcus pyogenes is a zinc receptor. J Bacteriol 191:5814–5823. doi: 10.1128/JB.00485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panina EM, Mironov AA, Gelfand MS. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A 100:9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray RD, Duncan A, Noble D, Imrie M, O’Reilly DSJ, Innes JA, Porteous DJ, Greening AP, Boyd AC. 2010. Sputum trace metals are biomarkers of inflammatory and suppurative lung disease. Chest 137:635–641. doi: 10.1378/chest.09-1047. [DOI] [PubMed] [Google Scholar]
- 54.Mastropasqua MC, Lamont I, Martin LW, Reid DW, D’Orazio M, Battistoni A. 2018. Efficient zinc uptake is critical for the ability of Pseudomonas aeruginosa to express virulence traits and colonize the human lung. J Trace Elem Med Biol 48:74–80. doi: 10.1016/j.jtemb.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Makthal N, Nguyen K, Do H, Gavagan M, Chandrangsu P, Helmann JD, Olsen RJ, Kumaraswami M. 2017. A critical role of zinc importer AdcABC in Group A Streptococcus-host interactions during infection and its implications for vaccine development. Ebiomedicine 21:131–141. doi: 10.1016/j.ebiom.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Britigan BE, Railsback MA, Cox CD. 1999. The Pseudomonas aeruginosa secretory product pyocyanin inactivates alpha1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease. Infect Immun 67:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyczak JB, Cannon CL, Pier GB. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2:1051–1060. doi: 10.1016/S1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 58.Gallagher LA, Manoil C. 2001. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol 183:6207–6214. doi: 10.1128/JB.183.21.6207-6214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson GG, Moreau-Marquis S, Stanton BA, O’Toole GA. 2008. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun 76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu P, Ge X, Chen L, Wang X, Dou Y, Xu JZ, Patel JR, Stone V, Trinh M, Evans K, Kitten T, Bonchev D, Buck GA. 2011. Genome-wide essential gene identification in Streptococcus sanguinis. Sci Rep 1:125. doi: 10.1038/srep00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das S, Kanamoto T, Ge X, Xu P, Unoki T, Munro CL, Kitten T. 2009. Contribution of lipoproteins and lipoprotein processing to endocarditis virulence in Streptococcus sanguinis. J Bacteriol 191:4166–4179. doi: 10.1128/JB.01739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crump KE, Bainbridge B, Brusko S, Turner LS, Ge X, Stone V, Xu P, Kitten T. 2014. The relationship of the lipoprotein SsaB, manganese and superoxide dismutase in Streptococcus sanguinis virulence for endocarditis. Mol Microbiol 92:1243–1259. doi: 10.1111/mmi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhodes DV, Crump KE, Makhlynets O, Snyder M, Ge X, Xu P, Stubbe J, Kitten T. 2014. Genetic characterization and role in virulence of the ribonucleotide reductases of Streptococcus sanguinis. J Biol Chem 289:6273–6287. doi: 10.1074/jbc.M113.533620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gifford AH, Alexandru DM, Li Z, Dorman DB, Moulton LA, Price KE, Hampton TH, Sogin ML, Zuckerman JB, Parker HW, Stanton BA, O’Toole GA. 2014. Iron supplementation does not worsen respiratory health or alter the sputum microbiome in cystic fibrosis. J Cyst Fibros 13:311–318. doi: 10.1016/j.jcf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, Duell EJ. 2009. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect 117:1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carr A, Diener C, Baliga NS, Gibbons SM. 2019. Use and abuse of correlation analyses in microbial ecology. ISME J 13:2647. doi: 10.1038/s41396-019-0459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall LI, McDonald D, Melnik AV, Morton JT, Navas J, Quinn RA, Sanders JG, Swafford AD, Thompson LR, Tripathi A, Xu ZZ, Zaneveld JR, Zhu Q, Caporaso JG, Dorrestein PC. 2018. Best practices for analysing microbiomes. Nat Rev Microbiol 16:410–422. doi: 10.1038/s41579-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 68.Whiley RA, Fleming EV, Makhija R, Waite RD. 2015. Environment and colonisation sequence are key parameters driving cooperation and competition between Pseudomonas aeruginosa cystic fibrosis strains and oral commensal streptococci. PLoS One 10:e0115513. doi: 10.1371/journal.pone.0115513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayaram L, Chunilal S, Pickering S, Ruffin RE, Zalewski PD. 2011. Sputum zinc concentration and clinical outcome in older asthmatics. Respirology 16:459–466. doi: 10.1111/j.1440-1843.2011.01932.x. [DOI] [PubMed] [Google Scholar]
- 70.Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. 2010. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros 9:193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Gray RD, MacGregor G, Noble D, Imrie M, Dewar M, Boyd AC, Innes JA, Porteous DJ, Greening AP. 2008. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med 178:444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong CL, Walker MJ, McEwan AG. 2015. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci Rep 5:10799. doi: 10.1038/srep10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bertani G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186:595–600. doi: 10.1128/jb.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreau-Marquis S, Redelman CV, Stanton BA, Anderson GG. 2010. Co-culture models of Pseudomonas aeruginosa biofilms grown on live human airway cells. J Vis Exp (44)::2186. doi: 10.3791/2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hentchel-Franks K, Lozano D, Eubanks-Tarn V, Cobb B, Fan L, Oster R, Sorscher E, Clancy JP. 2004. Activation of airway Cl− secretion in human subjects by adenosine. Am J Respir Cell Mol Biol 31:140–146. doi: 10.1165/rcmb.2004-0012OC. [DOI] [PubMed] [Google Scholar]
- 76.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 77.Chen L, Ge X, Xu P. 2015. Identifying essential Streptococcus sanguinis genes using genome-wide deletion mutation. Methods Mol Biol 1279:15–23. doi: 10.1007/978-1-4939-2398-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu B, Ge X, Stone V, Kong X, El-Rami F, Liu Y, Kitten T, Xu P. 2017. ciaR impacts biofilm formation by regulating an arginine biosynthesis pathway in Streptococcus sanguinis SK36. Sci Rep 7:17183. doi: 10.1038/s41598-017-17383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu L, Zhang Y, Fan J, Herzberg MC, Kreth J. 2011. Characterization of competence and biofilm development of a Streptococcus sanguinis endocarditis isolate. Mol Oral Microbiol 26:117–126. doi: 10.1111/j.2041-1014.2010.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costaglioli P, Barthe C, Fayon M, Christoflour N, Bui S, Derlich L, Domblides P, Crouzet M, Vilain S, Garbay B. 2014. Selection of Pseudomonas aeruginosa reference genes for RT-qPCR analysis from sputum of cystic fibrosis patients. Mol Cell Probes 28:10–12. doi: 10.1016/j.mcp.2013.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.