Abstract
Non-coding RNAs have gained increasing attention, as their physiological and pathological functions are being gradually uncovered. MicroRNAs are the most well-studied ncRNAs, which play essential roles in translational repression and mRNA degradation. In contrast, long non-coding RNAs are distinguished from other small/short non-coding RNAs by length and regulate chromatin remodeling, gene transcription and posttranscriptional modifications. Recently, circular RNAs have emerged as endogenous, abundant, conserved and stable in mammalian cells. It has been demonstrated that circular RNAs can function as miRNA sponges. Other possible biological functions of circular RNAs are still under investigation. In this review, the biogenesis and biological functions of the three major types of ncRNAs, including miRNAs, lncRNAs and circRNAs, are overviewed. In addition, the role of ncRNAs in human diseases and potential clinical applications of ncRNAs are discussed.
Keywords: non-coding RNA, microRNA, long-non coding RNA, circular RNA, biological functions
SUMMARY
Introduction
-
Biogenesis and functions of non-coding RNAs
2.1 MicroRNA
2.2 Long non-coding RNA
2.3 Circular RNA
-
Interaction between different non-coding RNAs
3.1 CircRNAs function as miRNA sponges
3.2 The interaction of pseudogene and non-coding transcripts with miRNAs
-
Non-coding RNA and diseases
4.1 MicroRNAs and diseases
4.2 Long non-coding RNAs and diseases
4.3 Circular RNAs and diseases
Future perspectives
1. Introduction
In the human genome, only 1.5%-2% genes are protein-coding genes1,2. The vast majorities are known as non-protein coding genes, and have attracted increasing attention as their importance in physiological and pathological functions are being reported. Among the initial functional non-coding genes reported was a class of small non-coding RNA (ncRNA) called microRNAs (miRNA)3. Thereafter, other non-coding RNAs, such as PIWI-interacting RNAs (pi-RNAs), small nucleolar RNAs (snoRNAs), long non-coding RNAs (lncRNAs), and pseudogene were discovered and demonstrated to play roles in various development and disease processes4-10. Recently, the important functions of another class of ncRNAs, circular RNAs (circRNAs) has been illuminated, nearly 40 years following the initial characterization of circRNAs11,12.
Previously, ncRNAs were categorized according to their length. Although there is no clear borderline between classes, ncRNAs are broadly divided into short/small, mid-size, and long ncRNA13. The short ncRNAs includes miRNAs, piRNAs and tiRNAs. The snoRNAs belongs to mid-size RNAs. The large intergenic non-coding RNAs (lincRNAs) and the transcribed ultraconserved regions (T-UCR) with the size over 200bp are regarded as long ncRNAs13. Due to the special structure, circRNAs are classified either as an independent type of ncRNAs or as a class of long ncRNAs14.
In this review, we will give an overview of the biological functions of the three major types of ncRNAs, including miRNAs, lncRNAs and circRNAs. The biogenesis of these ncRNAs, especially circRNAs will also be briefly summarized. In addition, we will highlight the role of ncRNAs in the development of human diseases. Finally, we will bring to a discussion the existing and potential clinical application of ncRNAs.
2. Biogenesis and functions of non-coding RNAs
2.1 MicroRNA
MicroRNAs (miRNAs) are among the most well-studied ncRNAs. These are small, single-stranded RNAs of 18-24 nucleotides in length, that repress the transcription of their target genes3,15,16. It has been estimated that miRNAs regulate the translation of over 60% of protein-coding RNAs16,17. By controlling the majority of protein coding genes, miRNA have now been acknowledged for their essential roles in a range of physiological and pathological processes18,19.
2.1.1 Biogenesis of miRNA
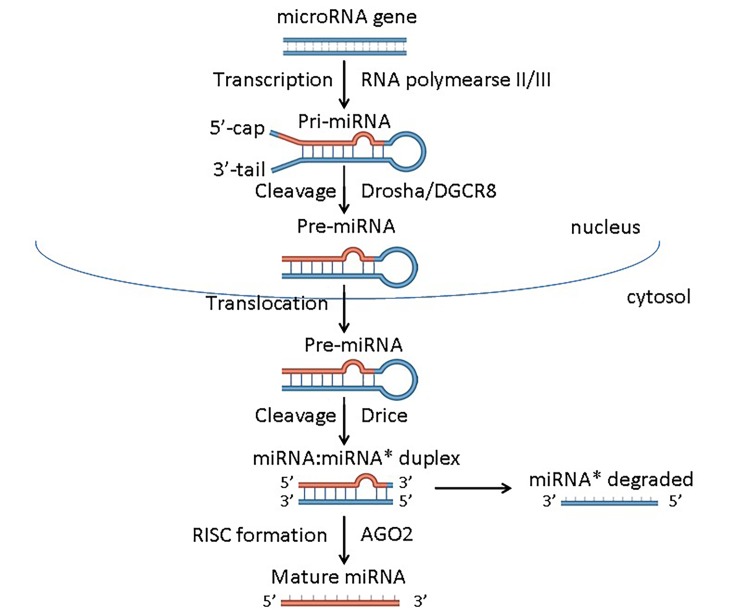
Primary miRNAs (pri-miRNAs) are mostly transcribed by RNA Polymerase II (RNA Pol II), and RNA Pol III has been implicated in miRNA transcription in some viruses20,21. Following transcription, pri-miRNAs undergo nuclear processing by the Drosha/DGCR8 microprocessor complex, followed by export into the cytoplasm as pre-miRNAs complexed with exportin5 and RAN· GTP22-25. In the cytoplasm, the pre-miRNAs are further processed by Dicer, releasing a small RNA duplex26-28. Following Dicer cleavage, the small RNA duplex is subsequently bound by an Argonaute (AGO) protein, and forms a complex known as the RNA induced silencing complex (RISC)29-31. After assembly into RISC, the miRNAs are regarded as mature miRNAs which target the 3’-untranslated regions (3’UTR) of mRNAs. This leads to post-transcriptional repression via destabilizing mRNA and inhibiting translation initiation17,32. The process of miRNA biogenesis is summarized in Figure 1. In addition to the canonical pathway, a minor portion of miRNAs are generated by alternative pathways. The biogenesis of miR-451, for example, bypasses the microprocessor or Dicer33-36. The maturation of miR-451 is independent of Dicer because the precursor of miR-451 (pre-miR-451) which is cleaved by Dosha/DGCR8 is too short (~18 bp) to be a Dicer substrate. Alternatively, the pre-miR-451 is directly incorporated into AGO2 followed by spliced on the 3’ hairpin arm and further resected to generate mature miR-45135,36. In addition, the biogenesis of miRNAs can be regulated by transcription factors, such as p53, MYC, ZEB1 and ZEB2, and by epigenetic modification (e.g. methylation, adenylation)37-39.
Figure 1. Figure 1. The biogenesis of microRNA.
The miRNA is initially transcribed by RNA Polymerase II (RNA Pol II) or RNA Pol III as pri-miRNA, then processed into pre-miRNA by Drosha/DGCR8 microprocessor complex. After transported from the nucleus to the cytoplasm, pre-miRNA is further processed by Dicer, releasing a small RNA duplex which is bound by an AGO protein and forms a complex with RISC. Then, the miRNA is regarded as mature miRNA. Abbreviations: pri-miRNA, primary miRNA; pre-miRNA, precursor miRNA; DGCR8, DiGeorge syndrome critical region 8; AGO, Argonaute; RISC, RNA-induced silencing complex.
2.1.2 Functions of miRNA
As mentioned above, miRNAs form base pairs with complementary sequences on the 3’UTRs of the target mRNAs resulting in RNA silencing at post-transcriptional levels40. miRNAs perform their gene silencing functions via repressing translation, deadenylation and destabilizing mRNAs.
Translational repression
Translational repression may occur through four different mechanisms: inhibition of translation initiation, suppression of translation elongation, degradation of co-translational protein and acceleration of translation termination.
Pillai et al. first reported that miRNAs could inhibit the initiation of translation41. In this study as well as two other studies, it was demonstrated that miRNAs mediated repression of mRNA translation, and that this required the 5' cap structure and/or the 3' poly(A) tail41-43. A later in vitro study demonstrated that endogenous let-7 miRNAs inhibited the translation initiation by targeting the m7G-cap–recognition process44. Furthermore, another study demonstrated that Ago proteins could compete with eIF4E for cap binding, leading to the repression of translation initiation45. These studies all suggested that miRNAs targeted an early step of translation initiation.
Before these function of miRNAs on the inhibition of translation initiation were characterized, miRNAs were thought to suppress translation at the post-initiation stage. The evidence was found in both the C. elegans and mammalian cells46-48. In C. elegans, lin-4 miRNA repressed the translation of the lin‑14 and lin‑28 mRNAs. The lin‑14 and lin‑28 mRNAs could be detected in polysomes after the protein translation was repressed47,48. Subsequent studies in mammalian cells supported these findings in C. elegans, by providing evidence that miRNAs caused ribosome drop-off and silenced translation independent of the cap structure, indicating that miRNAs induced a premature termination of translation48. Nottrott et al using nascent polypeptide coimmunoprecipitation experiments demonstrated that human let-7a miRNA and mRNA associated in polysomes resulting in a degradation of the newly synthesized polypeptide49. These studies suggested that the repression of translation by miRNA could occur after translation initiation.
mRNA deadenylation and degradation
Other than directly regulating translation, miRNAs also can induce target mRNA degradation in both C. elegans and metazoans32,50. Studies showed that if specific miRNAs were transfected into cultured cells, the abundance of mRNAs which contained the complementary binding sites was decreased32,51,52. Vice versa, depletion of a miRNA or the essential components of the miRNA processing pathway, such as Dicer or AGO proteins resulted in a corresponding increase of the target mRNAs51-53. In miRNA directed mRNA degradation, deadenylation of the mRNAs, a widespread consequence of miRNA regulation, occurs before they undergo 5’-3’ mRNA decay pathway54,55.
2.2 Long non-coding RNA
Long non-coding RNAs (lncRNAs) are primary spliced non-protein-coding RNA transcripts that are longer than 200 nucleotides in length, and do not fit into known classes of non-coding RNAs9,56. The number of lncRNAs ranges from 10,000 to greater than 200,000, which indicates that lncRNA may make up the largest portion of the mammalian non-coding transcriptome9,57. Increasing evidence indicates that lncRNAs are crucial regulators of numerous biological pathways8,55.
2.2.1 Biogenesis of lncRNA
LncRNAs can be transcribed from intergenic regions (lincRNAs), promoter regions (promoter-associated lncRNAs), introns of annotated genes (long intronic ncRNAs), the opposite strand of mRNAs (antisense lncRNAs), or pseudogenes58-61. Like coding genes, lncRNAs are transcribed by RNA polymerase II and then undergo post-transcriptional processing, including 5’capping, alternative splicing, RNA editing, and polyadenylation . However, unlike coding RNAs, cytoplasmic lncRNAs generally do not encode proteins62.
2.2.2 Functions of lncRNA
Epigenetic regulation and chromatin remodeling
Long ncRNAs can mediate epigenetic changes as recruiters, tethers or scaffolds for chromatin remodeling. For example, Hox transcript antisense RNA (HOTAIR) represses the transcription of the HOXD in trans by inducing a repressive chromatin state through interaction with the Polycomb chromatin remodeling complex (PRC2)63. Similarly, a 17kb lncRNA, Xist and a related transcript called RepA also recruit PRC264. Other than PRC2, HOTAIR can also bind LSD1/CoREST/REST complex that demethylates histone H3K4 to serve as a scaffold65. In addition, the imprinted lncRNA, named Air, also interacts with H3K9 methyltransferase G9a to epigenetically silence transcription66. A very recent study has identified another lncRNA, named myosin heavy-chain-associated RNA transcripts (Mhrt), which can bind to the helicase domain of Brg1 and sequester Brg1 from its genomic DNA targets to prevent chromatin remodeling67.
Transcriptional regulation
Long ncRNAs regulate gene transcription through a diverse set of mechanisms. Long ncRNAs can directly regulate gene transcription by acting as a decoy for transcription factors, transcriptional coregulators, or Pol II inhibitors. Some lncRNAs bind to certain RNA-binding proteins to silence gene expression. The ncRNA cyclin D1 (CCND1) can negatively regulate the parent CCND1 transcription by binding with and modulating the activities of the RNA-binding protein TLS, that subsequently suppress the histone acetyltransferase activities of CREB binding protein and p30068. A new identified lncRNA lnc-DC, which is expressed in human conventional dendritic cells, can bind directly to transcription factor STAT3 in the cytoplasm and promote STAT3 phosphorylation, via preventing STAT3 dephosphorylation by SHP169. In addition to acting through RNA-binding proteins, lncRNAs can directly inhibit Pol II activity. Two lncRNAs in mammalian cells, mouse B2 RNA and human Alu RNA, both prevent Pol II from establishing interactions with the promoter during closed complex formation, leading to a disruption of these interactions associated with transcriptional repression70,71.
Post-transcriptional regulation
Long ncRNAs may also play a role in the regulation of mRNA degradation, protein stability and translation. The long ncRNA HOTAIR enhances Plk1-dependent ubiquitination of SUZ12 and ZNF198, two transcription repression factors, and significantly reduce SUZ12 and ZNF198 stability72. Another lncRNA, FAL1, has been demonstrated to be associated with the epigenetic repressor Polycomb complex protein, BMI1 and regulates its stability in order to modulate the transcription of a large set of genes that are related with cell cycle and apoptosis73. A long intergenic noncoding RNA (lincRNA) homologous to the mouse plasmacytoma variant translocation gene (PVT1) is reported to control levels of MYC through regulation of the MYC protein stability and consequently cooperate to promote cancer cell proliferation74,75. In addition to controlling mRNA and protein stability, lncRNAs also exert their effects at the level of translational regulation. Our previous study has demonstrated that tumour suppressor candidate-2 pseudogenes (TUSC2P) interact with several miRNAs resulting in increased translation of TUSC24. Moreover, it has been reported that lincRNA-p21 associates with JunB, CTNNB1 and b-catenin mRNAs, selectively altering their translation via the translation repressor Rck76.
2.3. Circular RNA
Circular RNAs (circRNA) were first characterized in viruses in the 1970s77. However, due to their unique structure and limitations of detection techniques, the roles of circRNA have been underestimated over the past several decades. Last year, large amounts of endogenous circRNA were revealed in mammalian cells with the improvement of computational techniques and higher throughput sequencing methods12,78-80. Unlike linear RNA, circRNAs form a covalently closed continuous loop81 and its expression levels are not correlated with those of their linear isoforms79. Recently, circRNA have been identified as a type of non-coding RNA. CircRNAs now have been categorized into three types: exonic, circular intronic and retained-intron circRNAs, based on their origins and sequences12,14,78-82.
2.3.1 Biogenesis and properties of circRNA
The biogenesis of circRNAs is still under investigation. Most circRNA molecules in eukaryotic cells are produced via backsplicing by the spliceosomal machinery which joins a splice donor to an upstream splice acceptor81,83. Originally, the majority of circRNAs were regarded as the by-products of mis-splicing. However, a series of recent studies indicated that the formation of circRNAs is fully regulated. Over 250,000 circRNAs were identified from 14.4% of actively transcribed genes in human fibroblasts and these circRNAs were demonstrated to be stable and conserved78. Generally, the biogenesis of circRNA is as shown inFigure 2. A recent report demonstrated that exon circularization required flanking intronic complementary sequences. In addition, alternative circularization, resulting from the competition between the flanking introns or individual introns, may lead to a production of multiple circRNA transcripts from a single gene81. In accordance with this study, Ashwal-Fluss, et al. also elucidated that the production rate of circRNAs is mainly determined by flanking introns and precursor mRNA splicing, competing with circularization of exons83. In addition, short intronic repeats in the flanking introns were demonstrated to be critical for circularization84. These results all suggest that a close proximity of exon-intron junctions resulting from complementary base pairing of inverted repeats in the flanking introns facilitate the biogenesis of circRNAs. On the other hand, circRNAs have also been suggested to be formed by exon skipping75,85. Exon skipping leads to an exon-containing lariat, which could be further internally spliced to an exonic circular RNA molecule78,85.
Figure 2. Figure 2. The biogenesis of circRNA.
A. The biogenesis of extronic circRNA. The extronic circRNA can be formed via either backsplicing or extron skipping. Backsplicing is a downstream splicer donor pairs with unspliced upstream splice acceptor and the invervening RNA is circularized. Extron skipping leads to an exon-containing lariat, which could be further internally spliced to an exonic circular RNA molecule.
B. The biogenesis of circular intronic RNA and extron-intro circRNA. The biogenesis of circular intronic RNAs (ciRNAs) depends on a consensus motif containing a GU-rich element near the 5’ splice site and a C-rich element close to the branchpoint site. These motifs lead to a failure of debranching, which escape the lariat introns from branching and facilitate the formation of ciRNAs. The exon-intron circRNAs are generated from circularized exons with introns retained between exons.
In contrast to the circRNAs from exon back-splicing, the circular intronic RNAs (ciRNAs) are synthesized from the Group I or Group II introns which are excised from precursor RNAs. The previous studies have demonstrated that the full-length ciRNAs from Group I introns are generated via autocatalytic ribozyme action in Tetrahymena.
In brief, the circularization of RNAs is initiated by sequential site-specific hydrolysis followed by a transesterification action requiring guanosine as cofactor86. Another recent study has found that Group I intron could perform 3’, 5’ ligation to form circRNA and the exogenous guanosine cofactor is added during the steps of self-splicing87. The formation of ciRNAs which are from Group II introns depend on a consensus motif containing a 7 nt GU-rich element near the 5’ splice site and an 11 nt C-rich element close to the branchpoint site14. These motifs lead to a failure of debranching, which escape the lariat introns from branching and facilitate the formation of ciRNAs14. Recently, another subclass of circRNAs, named exon-intron circRNAs, have been highlighted. Exon-intron circRNAs are generated from circularized exons with introns retained between exons88. Both the ciRNAs and exon-intron circRNAs have been suggested to be involved in Pol II transcription14,88. The role of these circRNAs on the regulation of transcription will be discussed in the following section.
The biogenesis of circRNAs can be regulated by some RNA-binding proteins. The second exon of the splicing factor muscleblind (MBL/MBNL1) has been reported to promote the circularization of its own exons during circMbl biogenesis, by strongly and specifically binding to the conserved MBL binding sites in circMbl and its flanking introns83. Another RNA binding protein, Quaking (QKI), has been demonstrated to be a major regulator of circRNA biogenesis in epithelial-mesenchymal transition (EMT). Unlike MBL, QKI does not regulate the formation of its own circRNA. Instead, knockdown of QKI reduces the abundance of 105 out of 300 circRNAs by more than 2-fold in mesHMLE cells89. In accordance, a very recent study reported that a conserved RNA-editing enzyme, ADAR, may also be involved in circRNA biogenesis90.
2.3.2 Functions of circRNA
A large amount of circRNAs have been identified using genome-wide analysis78-80. Identification of the essential physiological roles of circRNAs has become a hotspot of ncRNA research. It may be possible that circRNAs function as miRNA sponges, thereby regulating gene expression at the transcriptional and translational levels.
Regulation of transcription
Among the first identified function of circRNA was that of a miRNA sponge, through which circRNAs indirectly regulated the transcription of target mRNA. In this review, the interaction of circRNAs and miRNAs will be summarized in the later section. Other than serving as a miRNA sponge however, circRNAs can also form base-pairs with RNA or sequester RNA-binding proteins12,91. The ciRNAs contain very few miRNA binding sites and act mainly in the nucleus. Some ciRNAs accumulate to the site of transcription bind with RNA Pol II and act as a positive regulator of RNA Pol II transcription, by which they control the expression of their parent genes14. Interestingly, besides these transcription sites, ciRNAs may also play a trans regulatory effect for the other target genes14. Exon-intron circRNAs have been demonstrated to be predominantly localized in the nucleus and exert cis regulatory effect on mRNA via interaction with Pol II, U1 snRNP and gene promoters88.
Regulation of translation
It is possible that an endogenous circRNA with an internal ribosome entry site (IRES) and a start codon could undergo translation. However, there remains no evidence that ATG-containing exonic circRNAs undergo translation in eukaryotes. The only natural circRNA which can encode a protein is circular hepatitis d in the hepatitis B virus92.
3. Interaction between different non-coding RNAs
3.1 CircRNAs function as miRNA sponges
In 2013, two groups published their findings regarding circular CDR1 antisense (CDR1as) or circular transcript ciRS-7, and the ability to suppress miR-7 activity11,12. There are more than 70 conventional seed–target regions for miR-7 located on the CDR1as/ciRS-7. In addition, CDR1as/ciRS-7 is densely associated with AGO proteins in a miR-7-dependent manner11,12. Knockdown of CDR1as/ciRS-7 leads to a decreased expression of miR-7 target genes. Overexpression of CDR1as/ciRS-7 results in the phenotypes that are similar to miRNA inhibition12. Besides CDR1as, circular sry (sex-determining region Y) can also serve as miRNA sponges for miR-138, indicating that the functions of circRNA as miRNA sponges may be observed in multiple biological contexts11. In a recent study, the function of circRNA as a miRNA sponge was further confirmed. During the development of esophageal squamous cell carcinoma (ESCC), circITCH serves as a sponge for miR-7, miR-17 and miR-214, thereby increasing levels of the ITCH mRNA, inhibiting the Wnt/β-catenin pathway93. Interestingly, another study has recently argued that most circRNAs are low-abundance and have less miRNA binding site than CDR1as. It remains to be seen whether other circRNAs function as miRNA sponges, as effectively as CDR1as94. Whether more circRNAs act as miRNA sponges warrants further investigation.
3.2 The interaction of pseudogene and non-coding transcripts with miRNAs
Pseudogenes are a subclass of lncRNAs. Our previous studies have demonstrated that the pseudogene TUSC2P can interact with endogenous miRNAs including miR-17, miR-93, miR-299-3p, miR-520a, miR-608 and miR-661, resulting in increased translation of TUSC2, TIMP2 and TIMP34. Another lncRNA PTEN pseudogene, PTENP1, serves as a tumour suppressive, by acting as a decoy for PTEN-targeting miR-17, miR-21, miR-214, miR-19 and miR-26 families10. In addition, the non-coding fragments of mRNAs can also function as sponge to regulate miRNA activities. For example, HMGA2 can function as a competing endogenous RNA to regulate miRNA activity95. The 3’-untranslated regions of versican96-98, CD4499,100, and nephronetin101,102 can bind to a number of endogenous miRNAs and function as sponges to modulate miRNA activities.
4. Non-coding RNAs and diseases
4.1 MicroRNAs and diseases
Following over a decade of research, miRNAs are now widely acknowledged to play crucial roles in carcinogenesis, as either tumor suppressors or oncogenes. Many studies are devoted to investigate have elucidated the diverse roles of miRNAs in the initiation and development of cancer. For example, our previous studies have demonstrated that miR-17 exerts dual effects on tumorigenesis. In hepatocarcinoma and prostate cancers, miR-17 promotes tumor growth and invasion by inhibiting the expression of tumor suppressors, such as PTEN or TIMP3103,104. In breast cancer, miR-24, miR-378, miR-93 and miR-199 enhances cell growth, survival, invasion, angiogenesis and metastasis by targeting different tumor suppressors105-108. In melanoma cells, miR-17 represses growth by targeting STAT3 and stimulating downstream host immune responses109. In addition, miR-17 also reduces cell adhesion, migration and proliferation in different normal or cancer cells110. Even in the same tumor cells, miR-17 shows dual roles in cell growth: it reduces the tumor proliferation but prolongs tumor cell survival and induces angiogenesis111. In accordance, other studies have also identified the dual roles of miR-17 or other miRNAs in other cancer types112,113.
Other than their roles in cancer, miRNAs are also involved in a variety of human diseases and biological processes. Our recent work shows that miRNAs, including miR-17 and miR-378 are important to wound healing, senescence as well as lipid metabolism114-117. We’ve also found that miR-17 controls the organ development. Transgenic mice overexpressing miR-17 showed growth retardation and smaller organs110. In the older mice, spontaneous hepatocellular carcinoma developed103. Further examination of the mice showed that larger spleens were observed in miR-17 transgenic mice compared to the wildtype mice (Figure 3). This suggests inflammation occurred in the miR-17 transgenic mice. The molecular mechanism awaits further investigation.
Figure 3. Figure 3. Role of miR-17 in organ size.

The spleen size of miR-17 transgenic mice (miR-17) is significantly increased compared to wide type mice (WT).
In addition, a number of large scale studies have identified associations between miRNAs and a range of diseases118. Several databases have been created to help the researches to understand the association between miRNAs and human diseases, such as human miRNA-associated disease database (http://cmbi.bjmu.edu.cn/hmdd), miR2Disease (http://www.mir2disease.org/) and PhenomiR (http://mips.helmholtz-muenchen.de/ phenomir)119-121. The investigation of the relationship between miRNA and human disease will help to identify more biomarkers and potential therapeutic targets.
4.2 Long non-coding RNAs and diseases
There is an increasing interest in researching the potential involvement of lncRNAs in diseases, due to their diverse functions in differentiation and developmental processes. lncRNAs appear to be new players in cancer and their potential roles have been demonstrated to involve in both oncogenic and tumor suppressive pathways122-124. It has been demonstrated that the aberrant expression of lncRNAs are associated with a variety of human tumors, such as breast, ovarian, prostate, and hepatocellular carcinoma10,72-74,125. Our previous study found that the pseudogene TUSC2P could inhibit cell proliferation, survival, migration, invasion and colony formation, and increase tumour cell death in breast cancer cells, via regulating TUSC2P, TIMP2 and TIMP34. Other than in cancer, lncRNAs may also play important roles in the development of neurodegenerative diseases (e.g. Alzheimer’s disease) and autoimmune disease (e.g. systemic lupus erythematosus)126,127. Similar to miRNAs, there are also useful database resources providing the putative or validated association of lncRNAs and diseases128-130, such as LncRNADisease database (http://cmbi.bjmu.edu.cn/lncrnadisease),lncRNAdb (http://www.lncrnadb.org/), and lncRNome (http://genome.igib.res.in/lncRNome), and other ones. Although all of the databases have certain limitations, these lncRNA databases help to delineate the relationships between lncRNA transcripts and their functions.
4.3 Circular RNAs and diseases
Due to its special structure and functions, circRNAs are considered to play critical roles in physiological and pathological processes and, therefore, may be involved in the initiation and development of certain diseases. To check the enrichment of genes associated with particular biological processes, a set of protein coding genes in the miRNA-circRNA interactome of diseases were analyzed using Gene Ontology (GO) enrichment analysis131. A database of disease-circRNA association was compiled in Circ2Traits, which was the first database of potential correlations between circular RNAs with human diseases131. In this study, it has been demonstrated that circRNAs are related to a variety of diseases, including gastric, esophageal, and prostate cancers, as well as neurodegenerative diseases like Parkinson's disease, Alzheimer's disease, Multiple sclerosis, and schizophrenia131.
circRNA and neurological disorders
circRNAs are highly enriched in the mammalian brain and Drosophila neural tissues1,12,132,133. On one hand, circRNAs, such as CDR1as/ciRS-7, could possibly be involved in diseases associated with miRNA owing to its function as a miRNA sponge12. For example, CiRS-7 is significantly decreased in the Alzheimer’s disease (AD) hippocampal CA1 samples compared to those in the age-matched controls134. Deficiency of ciRS-7 may result in an increase of miR-7 levels in AD brain cells, which is expected to downregulate the expression of miR-7 target genes relevant to AD134. On the other hand, circRNAs have been demonstrated to be upregulated during neuronal differentiation and development, and highly enriched in synapses in mouse and human brains132. In Drosophila, circRNAs also accumulate in the brain and increase with aging, which indicates the circRNAs may play a role in neural aging and have the potential to be an aging marker in neural system133.
circRNA and cancer
Based on a gene ontology (GO) enrichment analysis, Ghosal et al. established a global view of the potential association of circRNAs with cancer131. In this study, more than 60 of the circRNA which interacted with mRNAs were associated with breast and gastric cancers, and almost 200 of such circRNA-interacted mRNAs were correlated to cervical cancer. Further to this, some studies have begun to examine the role of circRNAs in specific cancers. Li P et al. identified that the circRNA hsa_circ_002059 was significantly downregulated in gastric cancer tissues compared with paired adjacent non-tumorous tissues, indicating this circRNA may hold promise as a potential biomarker for the diagnosis of gastric carcinoma135. In addition, another study has indicated that the circRNA abundance is low in colorectal cancer cell lines and cancer compared to normal tissues after detecting over 1,800 circular RNAs, suggesting a negative correlation of circular RNA abundance and proliferation136. In accordance with these studies, Li F, et al. also delineated that circITCH expression was low in ESCC compared to the peritumoral tissue93. In contrast, emerging data from our group has indicated that overexpression of some circRNAs could significantly promote tumorigenesis. Therefore, whether circRNAs are regarded as oncogenes or tumor suppressors will likely depend on the downstream target genes or proteins that are regulated by individual circRNAs.
5. Future perspectives
With the development of emerging computational, high throughput sequencing and biological techniques, we are able to further investigate and understand ncRNAs generated from different mechanisms. The RNAs previously thought as ‘junk’ RNA are now considered to be the treasures of the modern RNA research world. Only approximately 1.4% of the human genome is translated into proteins, whereas about 25% of mammalian genomes are predicted to be transcribed but not translated1,2,56,137, giving rise to a vast array of ncRNA which may play critical biological functions. Progressively, over the last four decades, researchers have found the structures of ncRNAs to range from short to long, and from linear to circular. Long ncRNAs and circular RNAs have been demonstrated to interact with miRNAs and exert a diverse range of biological functions. There remain large gaps in our understanding of ncRNAs, including the proportion and the range of ncRNA that are functional, and a mechanistic basis of these functions. The biogenesis pathways of these ncRNAs still warrants further study and is not clearly understood. As for miRNAs, which are the most studied ncRNAs, a cancer-targeted miRNA drug, MRX34 (a liposome-based miR-34 mimic), was recently launched into Phase I clinical trials in patients with advanced hepatocellular carcinoma in April 2013138,139. In addition, an LNA anti-miR against miR-122, was recently evaluated in Phase I and Phase IIa clinical trials for the treatment of hepatitis C virus140,141. The positive results from these clinical trials are encouraging, giving scientists, physicians and patients reason to further investigate the therapeutic potential of ncRNAs. Since lncRNAs and cirRNAs are more stable and specific for the target genes than miRNAs are, they may provide a new set of opportunities for clinical translation, following detailed preclinical examination of these ncRNAs.
Although non-coding RNAs do not encode proteins, they are essential in controlling the biological process associated with transcription, translation and/or post-transcriptional and post-translational regulation.
LncRNAs and circRNAs interact with miRNAs and exert a diverse range of biological functions.
Non-coding RNAs play critical roles in the initiation and progression of a variety of diseases.
Recent clinical trials for the application of miRNA-based therapeutics in certain diseases are encouraging, which indicates the great potential for the bench-to-bedside translation of lncRNAs and circRNAs in the future.
Acknowledgments
Our studies were supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC; 227937-2012) to BBY who is the recipient of a Career Investigator Award (CI 7418) from the Heart and Stroke Foundation of Ontario.
Footnotes
Conflict of interests: The authors declare no conflict of interest.
non-coding RNA (ncRNA); microRNAs (miRNA); PIWI-interacting RNA (pi-RNA); small nucleolar RNA (snoRNA); long non-coding RNA (lncRNA); circular RNA (circRNA); large intergenic non-coding RNAs (lincRNA); transcribed ultraconserved regions (T-UCR); RNA Polymerase II (RNA Pol II); RNA induced silencing complex (RISC); Argonaute protein (AGO protein); Hox transcript antisense RNA (HOTAIR); Polycomb chromatin remodeling complex (PRC2); plasmacytoma variant translocation gene (PVT1); tumour suppressor candidate-2 pseudogenes (TUSC2P); Circular RNAs (circRNA); circular intronic RNAs (ciRNA); epithelial-mesenchymal transition (EMT); internal ribosome entry site (IRES).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Initial sequencing and analysis of the human genome. Lander E S, Linton L M, Birren B, Nusbaum C, Zody M C, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov J P, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin J C, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston R H, Wilson R K, Hillier L W, McPherson J D, Marra M A, Mardis E R, Fulton L A, Chinwalla A T, Pepin K H, Gish W R, Chissoe S L, Wendl M C, Delehaunty K D, Miner T L, Delehaunty A, Kramer J B, Cook L L, Fulton R S, Johnson D L, Minx P J, Clifton S W, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng J F, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs R A, Muzny D M, Scherer S E, Bouck J B, Sodergren E J, Worley K C, Rives C M, Gorrell J H, Metzker M L, Naylor S L, Kucherlapati R S, Nelson D L, Weinstock G M, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith D R, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee H M, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis R W, Federspiel N A, Abola A P, Proctor M J, Myers R M, Schmutz J, Dickson M, Grimwood J, Cox D R, Olson M V, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans G A, Athanasiou M, Schultz R, Roe B A, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie W R, de la Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey J A, Bateman A, Batzoglou S, Birney E, Bork P, Brown D G, Burge C B, Cerutti L, Chen H C, Church D, Clamp M, Copley R R, Doerks T, Eddy S R, Eichler E E, Furey T S, Galagan J, Gilbert J G, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson L S, Jones T A, Kasif S, Kaspryzk A, Kennedy S, Kent W J, Kitts P, Koonin E V, Korf I, Kulp D, Lancet D, Lowe T M, McLysaght A, Mikkelsen T, Moran J V, Mulder N, Pollara V J, Ponting C P, Schuler G, Schultz J, Slater G, Smit A F, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf Y I, Wolfe K H, Yang S P, Yeh R F, Collins F, Guyer M S, Peterson J, Felsenfeld A, Wetterstrand K A, Patrinos A, Morgan M J, de Jong P, Catanese J J, Osoegawa K, Shizuya H, Choi S, Chen Y J, Szustakowki J. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 2.The sequence of the human genome. Venter J C, Adams M D, Myers E W, Li P W, Mural R J, Sutton G G, Smith H O, Yandell M, Evans C A, Holt R A, Gocayne J D, Amanatides P, Ballew R M, Huson D H, Wortman J R, Zhang Q, Kodira C D, Zheng X H, Chen L, Skupski M, Subramanian G, Thomas P D, Zhang J, Gabor Miklos G L, Nelson C, Broder S, Clark A G, Nadeau J, McKusick V A, Zinder N, Levine A J, Roberts R J, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian A E, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman T J, Higgins M E, Ji R R, Ke Z, Ketchum K A, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov G V, Milshina N, Moore H M, Naik A K, Narayan V A, Neelam B, Nusskern D, Rusch D B, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng M L, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers Y H, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint N N, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril J F, Guigó R, Campbell M J, Sjolander K V, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang Y H, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. Science (New York, N.Y.) 2001;291(5507):1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Wightman B, Ha I, Ruvkun G. Cell. 1993;75(5):855–62. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 4.The pseudogene TUSC2P promotes TUSC2 function by binding multiple microRNAs. Rutnam Zina Jeyapalan, Du William W, Yang Weining, Yang Xiangling, Yang Burton B. Nature communications. 2014;5:2914. doi: 10.1038/ncomms3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Developmentally regulated piRNA clusters implicate MILI in transposon control. Aravin Alexei A, Sachidanandam Ravi, Girard Angelique, Fejes-Toth Katalin, Hannon Gregory J. Science (New York, N.Y.) 2007;316(5825):744–7. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 6.Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Watanabe Toshiaki, Tomizawa Shin-ichi, Mitsuya Kohzoh, Totoki Yasushi, Yamamoto Yasuhiro, Kuramochi-Miyagawa Satomi, Iida Naoko, Hoki Yuko, Murphy Patrick J, Toyoda Atsushi, Gotoh Kengo, Hiura Hitoshi, Arima Takahiro, Fujiyama Asao, Sado Takashi, Shibata Tatsuhiro, Nakano Toru, Lin Haifan, Ichiyanagi Kenji, Soloway Paul D, Sasaki Hiroyuki. Science (New York, N.Y.) 2011;332(6031):848–52. doi: 10.1126/science.1203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Ni J, Tien A L, Fournier M J. Cell. 1997;89(4):565–73. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 8.A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Huarte Maite, Guttman Mitchell, Feldser David, Garber Manuel, Koziol Magdalena J, Kenzelmann-Broz Daniela, Khalil Ahmad M, Zuk Or, Amit Ido, Rabani Michal, Attardi Laura D, Regev Aviv, Lander Eric S, Jacks Tyler, Rinn John L. Cell. 2010;142(3):409–19. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long non-coding RNAs: insights into functions. Mercer Tim R., Dinger Marcel E., Mattick John S. Nature Reviews Genetics. 2009;10(3):155-159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 10.A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Poliseno Laura, Salmena Leonardo, Zhang Jiangwen, Carver Brett, Haveman William J, Pandolfi Pier Paolo. Nature. 2010;465(7301):1033–8. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natural RNA circles function as efficient microRNA sponges. Hansen Thomas B, Jensen Trine I, Clausen Bettina H, Bramsen Jesper B, Finsen Bente, Damgaard Christian K, Kjems Jørgen. Nature. 2013;495(7441):384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 12.Circular RNAs are a large class of animal RNAs with regulatory potency. Memczak Sebastian, Jens Marvin, Elefsinioti Antigoni, Torti Francesca, Krueger Janna, Rybak Agnieszka, Maier Luisa, Mackowiak Sebastian D, Gregersen Lea H, Munschauer Mathias, Loewer Alexander, Ziebold Ulrike, Landthaler Markus, Kocks Christine, le Noble Ferdinand, Rajewsky Nikolaus. Nature. 2013;495(7441):333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 13.Non-coding RNAs in human disease. Esteller Manel. Nature Reviews Genetics. 2011;12(12):861-874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 14.Circular intronic long noncoding RNAs. Zhang Yang, Zhang Xiao-Ou, Chen Tian, Xiang Jian-Feng, Yin Qing-Fei, Xing Yu-Hang, Zhu Shanshan, Yang Li, Chen Ling-Ling. Molecular cell. 2013;51(6):792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 15.MicroRNAs: target recognition and regulatory functions. Bartel David P. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Most mammalian mRNAs are conserved targets of microRNAs. Friedman Robin C, Farh Kyle Kai-How, Burge Christopher B, Bartel David P. Genome research. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MicroRNAs: small RNAs with a big role in gene regulation. He Lin, Hannon Gregory J. Nature reviews. Genetics. 2004;5(7):522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 18.Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Wang Yangming, Baskerville Scott, Shenoy Archana, Babiarz Joshua E, Baehner Lauren, Blelloch Robert. Nature genetics. 2008;40(12):1478–83. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germline deletion of the miR-17∼92 cluster causes skeletal and growth defects in humans. de Pontual Loïc, Yao Evelyn, Callier Patrick, Faivre Laurence, Drouin Valérie, Cariou Sandra, Van Haeringen Arie, Geneviève David, Goldenberg Alice, Oufadem Myriam, Manouvrier Sylvie, Munnich Arnold, Vidigal Joana Alves, Vekemans Michel, Lyonnet Stanislas, Henrion-Caude Alexandra, Ventura Andrea, Amiel Jeanne. Nature genetics. 2011;43(10):1026–30. doi: 10.1038/ng.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MicroRNA genes are transcribed by RNA polymerase II. Lee Yoontae, Kim Minju, Han Jinju, Yeom Kyu-Hyun, Lee Sanghyuk, Baek Sung Hee, Kim V Narry. The EMBO journal. 2004;23(20):4051–60. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Identification of microRNAs of the herpesvirus family. Pfeffer Sébastien, Sewer Alain, Lagos-Quintana Mariana, Sheridan Robert, Sander Chris, Grässer Friedrich A, van Dyk Linda F, Ho C Kiong, Shuman Stewart, Chien Minchen, Russo James J, Ju Jingyue, Randall Glenn, Lindenbach Brett D, Rice Charles M, Simon Viviana, Ho David D, Zavolan Mihaela, Tuschl Thomas. Nature methods. 2005;2(4):269–76. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 22.Processing of primary microRNAs by the Microprocessor complex. Denli Ahmet M, Tops Bastiaan B J, Plasterk Ronald H A, Ketting René F, Hannon Gregory J. Nature. 2004;432(7014):231–5. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 23.The Drosha-DGCR8 complex in primary microRNA processing. Han Jinju, Lee Yoontae, Yeom Kyu-Hyun, Kim Young-Kook, Jin Hua, Kim V Narry. Genes & development. 2004;18(24):3016–27. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuclear export of microRNA precursors. Lund Elsebet, Güttinger Stephan, Calado Angelo, Dahlberg James E, Kutay Ulrike. Science (New York, N.Y.) 2004;303(5654):95–8. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 25.Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. Bohnsack Markus T, Czaplinski Kevin, Gorlich Dirk. RNA (New York, N.Y.) 2004;10(2):185–91. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Hutvágner G, McLachlan J, Pasquinelli A E, Bálint E, Tuschl T, Zamore P D. Science (New York, N.Y.) 2001;293(5531):834–8. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 27.Signaling-mediated regulation of MicroRNA processing. Shen Jia, Hung Mien-Chie. Cancer research. 2015;75(5):783–91. doi: 10.1158/0008-5472.CAN-14-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DICER1: mutations, microRNAs and mechanisms. Foulkes William D, Priest John R, Duchaine Thomas F. Nature reviews. Cancer. 2014;14(10):662–72. doi: 10.1038/nrc3802. [DOI] [PubMed] [Google Scholar]
- 29.TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Chendrimada Thimmaiah P, Gregory Richard I, Kumaraswamy Easwari, Norman Jessica, Cooch Neil, Nishikura Kazuko, Shiekhattar Ramin. Nature. 2005;436(7051):740–4. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The structure of human argonaute-2 in complex with miR-20a. Elkayam Elad, Kuhn Claus-D, Tocilj Ante, Haase Astrid D, Greene Emily M, Hannon Gregory J, Joshua-Tor Leemor. Cell. 2012;150(1):100–10. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Making RISC. Kawamata Tomoko, Tomari Yukihide. Trends in biochemical sciences. 2010;35(7):368–76. doi: 10.1016/j.tibs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Mammalian microRNAs predominantly act to decrease target mRNA levels. Guo Huili, Ingolia Nicholas T, Weissman Jonathan S, Bartel David P. Nature. 2010;466(7308):835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canonical and alternate functions of the microRNA biogenesis machinery. Chong Mark M W, Zhang Guoan, Cheloufi Sihem, Neubert Thomas A, Hannon Gregory J, Littman Dan R. Genes & development. 2010;24(17):1951–60. doi: 10.1101/gad.1953310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Yang Jr-Shiuan, Lai Eric C. Molecular cell. 2011;43(6):892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Cheloufi Sihem, Dos Santos Camila O, Chong Mark M W, Hannon Gregory J. Nature. 2010;465(7298):584–9. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Yang Jr-Shiuan, Maurin Thomas, Robine Nicolas, Rasmussen Kasper D, Jeffrey Kate L, Chandwani Rohit, Papapetrou Eirini P, Sadelain Michel, O'Carroll Dónal, Lai Eric C. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(34):15163–8. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The widespread regulation of microRNA biogenesis, function and decay. Krol Jacek, Loedige Inga, Filipowicz Witold. Nature reviews. Genetics. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 38.Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Heo Inha, Ha Minju, Lim Jaechul, Yoon Mi-Jeong, Park Jong-Eun, Kwon S Chul, Chang Hyeshik, Kim V Narry. Cell. 2012;151(3):521–32. doi: 10.1016/j.cell.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 39.Human RNA methyltransferase BCDIN3D regulates microRNA processing. Xhemalce Blerta, Robson Samuel C, Kouzarides Tony. Cell. 2012;151(2):278–88. doi: 10.1016/j.cell.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Lim Lee P, Lau Nelson C, Garrett-Engele Philip, Grimson Andrew, Schelter Janell M, Castle John, Bartel David P, Linsley Peter S, Johnson Jason M. Nature. 2005;433(7027):769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 41.Inhibition of translational initiation by Let-7 MicroRNA in human cells. Pillai Ramesh S, Bhattacharyya Suvendra N, Artus Caroline G, Zoller Tabea, Cougot Nicolas, Basyuk Eugenia, Bertrand Edouard, Filipowicz Witold. Science (New York, N.Y.) 2005;309(5740):1573–6. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 42.MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Humphreys David T, Westman Belinda J, Martin David I K, Preiss Thomas. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(47):16961–6. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Recapitulation of short RNA-directed translational gene silencing in vitro. Wang Bingbing, Love Tara M, Call Matthew E, Doench John G, Novina Carl D. Molecular cell. 2006;22(4):553–60. doi: 10.1016/j.molcel.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 44.MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Mathonnet Géraldine, Fabian Marc R, Svitkin Yuri V, Parsyan Armen, Huck Laurent, Murata Takayuki, Biffo Stefano, Merrick William C, Darzynkiewicz Edward, Pillai Ramesh S, Filipowicz Witold, Duchaine Thomas F, Sonenberg Nahum. Science (New York, N.Y.) 2007;317(5845):1764–7. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 45.An mRNA m7G cap binding-like motif within human Ago2 represses translation. Kiriakidou Marianthi, Tan Grace S, Lamprinaki Styliani, De Planell-Saguer Mariangels, Nelson Peter T, Mourelatos Zissimos. Cell. 2007;129(6):1141–51. doi: 10.1016/j.cell.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 46.The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Olsen P H, Ambros V. Developmental biology. 1999;216(2):671–80. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 47.Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Seggerson Kathy, Tang Lingjuan, Moss Eric G. Developmental biology. 2002;243(2):215–25. doi: 10.1006/dbio.2001.0563. [DOI] [PubMed] [Google Scholar]
- 48.Short RNAs repress translation after initiation in mammalian cells. Petersen Christian P, Bordeleau Marie-Eve, Pelletier Jerry, Sharp Phillip A. Molecular cell. 2006;21(4):533–42. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 49.Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nottrott Stephanie, Simard Martin J, Richter Joel D. Nature structural & molecular biology. 2006;13(12):1108–14. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 50.Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Bagga Shveta, Bracht John, Hunter Shaun, Massirer Katlin, Holtz Janette, Eachus Rachel, Pasquinelli Amy E. Cell. 2005;122(4):553–63. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 51.Widespread changes in protein synthesis induced by microRNAs. Selbach Matthias, Schwanhäusser Björn, Thierfelder Nadine, Fang Zhuo, Khanin Raya, Rajewsky Nikolaus. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 52.The impact of microRNAs on protein output. Baek Daehyun, Villén Judit, Shin Chanseok, Camargo Fernando D, Gygi Steven P, Bartel David P. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Effects of Dicer and Argonaute down-regulation on mRNA levels in human HEK293 cells. Schmitter Daniela, Filkowski Jody, Sewer Alain, Pillai Ramesh S, Oakeley Edward J, Zavolan Mihaela, Svoboda Petr, Filipowicz Witold. Nucleic acids research. 2006;34(17):4801–15. doi: 10.1093/nar/gkl646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Behm-Ansmant Isabelle, Rehwinkel Jan, Doerks Tobias, Stark Alexander, Bork Peer, Izaurralde Elisa. Genes & development. 2006;20(14):1885–98. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deadenylation is a widespread effect of miRNA regulation. Eulalio Ana, Huntzinger Eric, Nishihara Tadashi, Rehwinkel Jan, Fauser Maria, Izaurralde Elisa. RNA (New York, N.Y.) 2009;15(1):21–32. doi: 10.1261/rna.1399509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schönbach C, Gojobori T, Baldarelli R, Hill D P, Bult C, Hume D A, Quackenbush J, Schriml L M, Kanapin A, Matsuda H, Batalov S, Beisel K W, Blake J A, Bradt D, Brusic V, Chothia C, Corbani L E, Cousins S, Dalla E, Dragani T A, Fletcher C F, Forrest A, Frazer K S, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson I J, Jarvis E D, Kanai A, Kawaji H, Kawasawa Y, Kedzierski R M, King B L, Konagaya A, Kurochkin I V, Lee Y, Lenhard B, Lyons P A, Maglott D R, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan W J, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius J U, Qi D, Ramachandran S, Ravasi T, Reed J C, Reed D J, Reid J, Ring B Z, Ringwald M, Sandelin A, Schneider C, Semple C A M, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor M S, Teasdale R D, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming L G, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander E S, Rogers J, Birney E, Hayashizaki Y. Nature. 2002;420(6915):563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 57.Epigenetic regulation by long noncoding RNAs. Lee Jeannie T. Science (New York, N.Y.) 2012;338(6113):1435–9. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 58.Poly A- transcripts expressed in HeLa cells. Wu Qingfa, Kim Yeong C, Lu Jian, Xuan Zhenyu, Chen Jun, Zheng Yonglan, Zhou Tom, Zhang Michael Q, Wu Chung-I, Wang San Ming. PloS one. 2008;3(7):e2803. doi: 10.1371/journal.pone.0002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.RNA maps reveal new RNA classes and a possible function for pervasive transcription. Kapranov Philipp, Cheng Jill, Dike Sujit, Nix David A, Duttagupta Radharani, Willingham Aarron T, Stadler Peter F, Hertel Jana, Hackermüller Jörg, Hofacker Ivo L, Bell Ian, Cheung Evelyn, Drenkow Jorg, Dumais Erica, Patel Sandeep, Helt Gregg, Ganesh Madhavan, Ghosh Srinka, Piccolboni Antonio, Sementchenko Victor, Tammana Hari, Gingeras Thomas R. Science (New York, N.Y.) 2007;316(5830):1484–8. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 60.lincRNAs: genomics, evolution, and mechanisms. Ulitsky Igor, Bartel David P. Cell. 2013;154(1):26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pseudogenes: pseudo-functional or key regulators in health and disease? Pink Ryan Charles, Wicks Kate, Caley Daniel Paul, Punch Emma Kathleen, Jacobs Laura, Carter David Raul Francisco. RNA (New York, N.Y.) 2011;17(5):792–8. doi: 10.1261/rna.2658311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribosome Profiling Provides Evidence that Large Noncoding RNAs Do Not Encode Proteins. Guttman Mitchell, Russell Pamela, Ingolia Nicholas T., Weissman Jonathan S., Lander Eric S. Cell. 2013;154(1):240-251. doi: 10.1016/j.cell.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Rinn John L, Kertesz Michael, Wang Jordon K, Squazzo Sharon L, Xu Xiao, Brugmann Samantha A, Goodnough L Henry, Helms Jill A, Farnham Peggy J, Segal Eran, Chang Howard Y. Cell. 2007;129(7):1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Zhao Jing, Sun Bryan K, Erwin Jennifer A, Song Ji-Joon, Lee Jeannie T. Science (New York, N.Y.) 2008;322(5902):750–6. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Long noncoding RNA as modular scaffold of histone modification complexes. Tsai Miao-Chih, Manor Ohad, Wan Yue, Mosammaparast Nima, Wang Jordon K, Lan Fei, Shi Yang, Segal Eran, Chang Howard Y. Science (New York, N.Y.) 2010;329(5992):689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Nagano Takashi, Mitchell Jennifer A, Sanz Lionel A, Pauler Florian M, Ferguson-Smith Anne C, Feil Robert, Fraser Peter. Science (New York, N.Y.) 2008;322(5908):1717–20. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- 67.A long noncoding RNA protects the heart from pathological hypertrophy. Han Pei, Li Wei, Lin Chiou-Hong, Yang Jin, Shang Ching, Nuernberg Sylvia T, Jin Kevin Kai, Xu Weihong, Lin Chieh-Yu, Lin Chien-Jung, Xiong Yiqin, Chien Huanchieh, Zhou Bin, Ashley Euan, Bernstein Daniel, Chen Peng-Sheng, Chen Huei-Sheng Vincent, Quertermous Thomas, Chang Ching-Pin. Nature. 2014;514(7520):102–106. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Wang Xiangting, Arai Shigeki, Song Xiaoyuan, Reichart Donna, Du Kun, Pascual Gabriel, Tempst Paul, Rosenfeld Michael G, Glass Christopher K, Kurokawa Riki. Nature. 2008;454(7200):126–30. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Wang Pin, Xue Yiquan, Han Yanmei, Lin Li, Wu Cong, Xu Sheng, Jiang Zhengping, Xu Junfang, Liu Qiuyan, Cao Xuetao. Science (New York, N.Y.) 2014;344(6181):310–3. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 70.B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Yakovchuk Petro, Goodrich James A, Kugel Jennifer F. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5569–74. doi: 10.1073/pnas.0810738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mariner Peter D, Walters Ryan D, Espinoza Celso A, Drullinger Linda F, Wagner Stacey D, Kugel Jennifer F, Goodrich James A. Molecular cell. 2008;29(4):499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 72.PLK1 and HOTAIR Accelerate Proteasomal Degradation of SUZ12 and ZNF198 during Hepatitis B Virus-Induced Liver Carcinogenesis. Zhang Hao, Diab Ahmed, Fan Huitao, Mani Saravana Kumar Kailasam, Hullinger Ronald, Merle Philippe, Andrisani Ourania. Cancer research. 2015;75(11):2363–74. doi: 10.1158/0008-5472.CAN-14-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Hu Xiaowen, Feng Yi, Zhang Dongmei, Zhao Sihai D, Hu Zhongyi, Greshock Joel, Zhang Youyou, Yang Lu, Zhong Xiaomin, Wang Li-Ping, Jean Stephanie, Li Chunsheng, Huang Qihong, Katsaros Dionyssios, Montone Kathleen T, Tanyi Janos L, Lu Yiling, Boyd Jeff, Nathanson Katherine L, Li Hongzhe, Mills Gordon B, Zhang Lin. Cancer cell. 2014;26(3):344–357. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Guan Yinghui, Kuo Wen-Lin, Stilwell Jackie L, Takano Hirokuni, Lapuk Anna V, Fridlyand Jane, Mao Jian-Hua, Yu Mamie, Miller Melinda A, Santos Jennifer L, Kalloger Steve E, Carlson Joseph W, Ginzinger David G, Celniker Susan E, Mills Gordon B, Huntsman David G, Gray Joe W. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(19):5745–55. doi: 10.1158/1078-0432.CCR-06-2882. [DOI] [PubMed] [Google Scholar]
- 75.PVT1 dependence in cancer with MYC copy-number increase. Tseng Yuen-Yi, Moriarity Branden S, Gong Wuming, Akiyama Ryutaro, Tiwari Ashutosh, Kawakami Hiroko, Ronning Peter, Reuland Brian, Guenther Kacey, Beadnell Thomas C, Essig Jaclyn, Otto George M, O'Sullivan M Gerard, Largaespada David A, Schwertfeger Kathryn L, Marahrens York, Kawakami Yasuhiko, Bagchi Anindya. Nature. 2014;512(7512):82–6. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LincRNA-p21 suppresses target mRNA translation. Yoon Je-Hyun, Abdelmohsen Kotb, Srikantan Subramanya, Yang Xiaoling, Martindale Jennifer L, De Supriyo, Huarte Maite, Zhan Ming, Becker Kevin G, Gorospe Myriam. Molecular cell. 2012;47(4):648–55. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Sanger H L, Klotz G, Riesner D, Gross H J, Kleinschmidt A K. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(11):3852–6. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Circular RNAs are abundant, conserved, and associated with ALU repeats. Jeck William R, Sorrentino Jessica A, Wang Kai, Slevin Michael K, Burd Christin E, Liu Jinze, Marzluff William F, Sharpless Norman E. RNA (New York, N.Y.) 2013;19(2):141–57. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cell-type specific features of circular RNA expression. Salzman Julia, Chen Raymond E, Olsen Mari N, Wang Peter L, Brown Patrick O. PLoS genetics. 2013;9(9):e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. Salzman Julia, Gawad Charles, Wang Peter Lincoln, Lacayo Norman, Brown Patrick O. PloS one. 2012;7(2):e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Complementary sequence-mediated exon circularization. Zhang Xiao-Ou, Wang Hai-Bin, Zhang Yang, Lu Xuhua, Chen Ling-Ling, Yang Li. Cell. 2014;159(1):134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Transcriptome-wide discovery of circular RNAs in Archaea. Danan Miri, Schwartz Schraga, Edelheit Sarit, Sorek Rotem. Nucleic acids research. 2012;40(7):3131–42. doi: 10.1093/nar/gkr1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.circRNA biogenesis competes with pre-mRNA splicing. Ashwal-Fluss Reut, Meyer Markus, Pamudurti Nagarjuna Reddy, Ivanov Andranik, Bartok Osnat, Hanan Mor, Evantal Naveh, Memczak Sebastian, Rajewsky Nikolaus, Kadener Sebastian. Molecular cell. 2014;56(1):55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Short intronic repeat sequences facilitate circular RNA production. Liang Dongming, Wilusz Jeremy E. Genes & development. 2014;28(20):2233–47. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Zaphiropoulos P G. Molecular and cellular biology. 1997;17(6):2985–93. doi: 10.1128/mcb.17.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.The ability to form full-length intron RNA circles is a general property of nuclear group I introns. Nielsen Henrik, Fiskaa Tonje, Birgisdottir Asa Birna, Haugen Peik, Einvik Christer, Johansen Steinar. RNA (New York, N.Y.) 2003;9(12):1464–75. doi: 10.1261/rna.5290903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.A natural ribozyme with 3',5' RNA ligase activity. Vicens Quentin, Cech Thomas R. Nature chemical biology. 2009;5(2):97–9. doi: 10.1038/nchembio.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Exon-intron circular RNAs regulate transcription in the nucleus. Li Zhaoyong, Huang Chuan, Bao Chun, Chen Liang, Lin Mei, Wang Xiaolin, Zhong Guolin, Yu Bin, Hu Wanchen, Dai Limin, Zhu Pengfei, Chang Zhaoxia, Wu Qingfa, Zhao Yi, Jia Ya, Xu Ping, Liu Huijie, Shan Ge. Nature structural & molecular biology. 2015;22(3):256–64. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 89.The RNA binding protein quaking regulates formation of circRNAs. Conn Simon J, Pillman Katherine A, Toubia John, Conn Vanessa M, Salmanidis Marika, Phillips Caroline A, Roslan Suraya, Schreiber Andreas W, Gregory Philip A, Goodall Gregory J. Cell. 2015;160(6):1125–34. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Ivanov Andranik, Memczak Sebastian, Wyler Emanuel, Torti Francesca, Porath Hagit T, Orejuela Marta R, Piechotta Michael, Levanon Erez Y, Landthaler Markus, Dieterich Christoph, Rajewsky Nikolaus. Cell reports. 2015;10(2):170–7. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 91.Circular RNAs: splicing's enigma variations. Hentze Matthias W, Preiss Thomas. The EMBO journal. 2013;32(7):923–5. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.The hepatitis delta (delta) virus possesses a circular RNA. Kos A, Dijkema R, Arnberg A C, van der Meide P H, Schellekens H. Nature. 1986;323(6088):558–60. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- 93.Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Li Fang, Zhang Liyuan, Li Wei, Deng Jieqiong, Zheng Jian, An Mingxing, Lu Jiachun, Zhou Yifeng. Oncotarget. 2015;6(8):6001–13. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Expanded identification and characterization of mammalian circular RNAs. Guo Junjie U, Agarwal Vikram, Guo Huili, Bartel David P. Genome biology. 2014;15(7):409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Kumar Madhu S, Armenteros-Monterroso Elena, East Philip, Chakravorty Probir, Matthews Nik, Winslow Monte M, Downward Julian. Nature. 2014;505(7482):212–7. doi: 10.1038/nature12785. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Versican 3'-untranslated region (3'-UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. Fang Ling, Du William W, Yang Xiangling, Chen Kui, Ghanekar Anand, Levy Gary, Yang Weining, Yee Albert J, Lu Wei-Yang, Xuan Jim W, Gao Zhongli, Xie Feng, He Chengyan, Deng Zhaoqun, Yang Burton B. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(3):907–19. doi: 10.1096/fj.12-220905. [DOI] [PubMed] [Google Scholar]
- 97.A 3'-untranslated region (3'UTR) induces organ adhesion by regulating miR-199a* functions. Lee Daniel Y, Shatseva Tatiana, Jeyapalan Zina, Du William W, Deng Zhaoqun, Yang Burton B. PloS one. 2009;4(2):e4527. doi: 10.1371/journal.pone.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Expression of versican 3'-untranslated region modulates endogenous microRNA functions. Lee Daniel Y, Jeyapalan Zina, Fang Ling, Yang Jennifer, Zhang Yaou, Yee Albert Y, Li Minhui, Du William W, Shatseva Tatiana, Yang Burton B. PloS one. 2010;5(10):e13599. doi: 10.1371/journal.pone.0013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.The non-coding 3' UTR of CD44 induces metastasis by regulating extracellular matrix functions. Rutnam Zina Jeyapalan, Yang Burton B. Journal of cell science. 2012;125(Pt 8):2075–85. doi: 10.1242/jcs100818. [DOI] [PubMed] [Google Scholar]
- 100.Expression of CD44 3'-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Jeyapalan Zina, Deng Zhaoqun, Shatseva Tatiana, Fang Ling, He Chengyan, Yang Burton B. Nucleic acids research. 2011;39(8):3026–41. doi: 10.1093/nar/gkq1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.A non-coding transcript of nephronectin promotes osteoblast differentiation by modulating microRNA functions. Lee Shao-Chen, Fang Ling, Wang Chia-Hui, Kahai Shireen, Deng Zhaoqun, Yang Burton B. FEBS letters. 2011;585(16):2610–6. doi: 10.1016/j.febslet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 102.MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. Kahai Shireen, Lee Shao-Chen, Lee Daniel Y, Yang Jennifer, Li Minhui, Wang Chia-Hui, Jiang Zide, Zhang Yaou, Peng Chun, Yang Burton B. PloS one. 2009;4(10):e7535. doi: 10.1371/journal.pone.0007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. Shan S. W., Fang L., Shatseva T., Rutnam Z. J., Yang X., Du W., Lu W.-Y., Xuan J. W., Deng Z., Yang B. B. Journal of Cell Science. 2013;126(6):1517-1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 104.Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Yang Xiangling, Du William W., Li Haoran, Liu Fengqiong, Khorshidi Anna, Rutnam Zina Jeyapalan, Yang Burton B. Nucleic Acids Research. 2013;41(21):9688-9704. doi: 10.1093/nar/gkt680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.MicroRNA miR-24 enhances tumor invasion and metastasis by targeting PTPN9 and PTPRF to promote EGF signaling. Du W. W., Fang L., Li M., Yang X., Liang Y., Peng C., Qian W., O'Malley Y. Q., Askeland R. W., Sugg S. L., Qian J., Lin J., Jiang Z., Yee A. J., Sefton M., Deng Z., Shan S. W., Wang C.-H., Yang B. B. Journal of Cell Science. 2013;126(6):1440-1453. doi: 10.1242/jcs.118299. [DOI] [PubMed] [Google Scholar]
- 106.The intermediate filament vimentin mediates microRNA miR-378 function in cellular self-renewal by regulating the expression of the Sox2 transcription factor. Deng Zhaoqun, Du William W., Fang Ling, Shan Sze Wan, Qian Jun, Lin Jiang, Qian Wei, Ma Jichun, Rutnam Zina Jeyapalan, Yang Burton B. Journal of Biological Chemistry. 2014;289(16):11567-11567. doi: 10.1074/jbc.M112.418830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Fang Ling, Du William W., Yang Weining, Rutnam Zina Jeyapalan, Peng Chun, Li Haoran, O'Malley Yunxia Q., Askeland Ryan W., Sugg Sonia, Liu Mingyao, Mehta Tanvi, Deng Zhaoqun, Yang Burton B. Cell Cycle. 2012;11(23):4352-4365. doi: 10.4161/cc.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. Shatseva T., Lee D. Y., Deng Z., Yang B. B. Journal of Cell Science. 2011;124(16):2826-2836. doi: 10.1242/jcs.077529. [DOI] [PubMed] [Google Scholar]
- 109.MicroRNA-17 inhibits tumor growth by stimulating T-cell mediated host immune response. Li Haoran, Gupta Shaan, Du William W., Yang Burton B. Oncoscience. 2014;1:531. doi: 10.18632/oncoscience.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Shan Sze Wan, Lee Daniel Y., Deng Zhaoqun, Shatseva Tatiana, Jeyapalan Zina, Du William W., Zhang Yaou, Xuan Jim W., Yee Siu-Pok, Siragam Vinayakumar, Yang Burton B. Nature Cell Biology. 2009;11(8):1031-1038. doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- 111.Stress Response of Glioblastoma Cells Mediated by miR-17-5p Targeting PTEN and the Passenger Strand miR-17-3p Targeting MDM2. Li Haoran, Yang Burton B. Oncotarget. 2012;3(12) doi: 10.18632/oncotarget.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.MicroRNA: Biogenesis, Function and Role in Cancer. MacFarlane Leigh-Ann, R. Murphy Paul. Current Genomics. 2010;11(7):537-561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.MicroRNA-375 plays a dual role in prostate carcinogenesis. Costa-Pinheiro Pedro, Ramalho-Carvalho João, Vieira Filipa Quintela, Torres-Ferreira Jorge, Oliveira Jorge, Gonçalves Céline S, Costa Bruno M, Henrique Rui, Jerónimo Carmen. Clinical Epigenetics. 2015;7(1) doi: 10.1186/s13148-015-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anti-microRNA-378a Enhances Wound Healing Process by Upregulating Integrin Beta-3 and Vimentin. Li Haoran, Chang Leslie, Du William W, Gupta Shaan, Khorshidi Azam, Sefton Michael, Yang Burton B. Molecular Therapy. 2014;22(10):1839-1850. doi: 10.1038/mt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.miR-17 extends mouse lifespan by inhibiting senescence signaling mediated by MKP7. Du W W, Yang W, Fang L, Xuan J, Li H, Khorshidi A, Gupta S, Li X, Yang B B. Cell Death & Disease. 2014;5(7):e1355-e1355. doi: 10.1038/cddis.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.The microRNA miR-17-3p inhibits mouse cardiac fibroblast senescence by targeting Par4. Du W. W., Li X., Li T., Li H., Khorshidi A., Liu F., Yang B. B. Journal of Cell Science. 2014;128(2):293-304. doi: 10.1242/jcs.158360. [DOI] [PubMed] [Google Scholar]
- 117.Inhibition of Dexamethasone-induced Fatty Liver Development by Reducing miR-17-5p Levels. Du William W, Liu Fengqiong, Shan Sze Wan, Ma Xindi Cindy, Gupta Shaan, Jin Tianru, Spaner David, Krylov Sergey N, Zhang Yaou, Ling Wenhua, Yang Burton B. Molecular Therapy. 2015;23(7):1222-1233. doi: 10.1038/mt.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MicroRNAs in development and disease. Sayed Danish, Abdellatif Maha. Physiological reviews. 2011;91(3):827–87. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 119.An analysis of human microRNA and disease associations. Lu Ming, Zhang Qipeng, Deng Min, Miao Jing, Guo Yanhong, Gao Wei, Cui Qinghua. PloS one. 2008;3(10):e3420. doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.miR2Disease: a manually curated database for microRNA deregulation in human disease. Jiang Qinghua, Wang Yadong, Hao Yangyang, Juan Liran, Teng Mingxiang, Zhang Xinjun, Li Meimei, Wang Guohua, Liu Yunlong. Nucleic acids research. 2009;37(Database issue):D98–104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.PhenomiR: a knowledgebase for microRNA expression in diseases and biological processes. Ruepp Andreas, Kowarsch Andreas, Schmidl Daniel, Buggenthin Felix, Brauner Barbara, Dunger Irmtraud, Fobo Gisela, Frishman Goar, Montrone Corinna, Theis Fabian J. Genome biology. 2010;11(1):R6. doi: 10.1186/gb-2010-11-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Long, abundantly expressed non-coding transcripts are altered in cancer. Perez Damon S, Hoage Tiffany R, Pritchett Jay R, Ducharme-Smith Allison L, Halling Meredith L, Ganapathiraju Sree C, Streng Paul S, Smith David I. Human molecular genetics. 2008;17(5):642–55. doi: 10.1093/hmg/ddm336. [DOI] [PubMed] [Google Scholar]
- 123.Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Calin George A, Liu Chang-gong, Ferracin Manuela, Hyslop Terry, Spizzo Riccardo, Sevignani Cinzia, Fabbri Muller, Cimmino Amelia, Lee Eun Joo, Wojcik Sylwia E, Shimizu Masayoshi, Tili Esmerina, Rossi Simona, Taccioli Cristian, Pichiorri Flavia, Liu Xiuping, Zupo Simona, Herlea Vlad, Gramantieri Laura, Lanza Giovanni, Alder Hansjuerg, Rassenti Laura, Volinia Stefano, Schmittgen Thomas D, Kipps Thomas J, Negrini Massimo, Croce Carlo M. Cancer cell. 2007;12(3):215–29. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 124.Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Hindorff Lucia A, Sethupathy Praveen, Junkins Heather A, Ramos Erin M, Mehta Jayashri P, Collins Francis S, Manolio Teri A. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Yu Wenqiang, Gius David, Onyango Patrick, Muldoon-Jacobs Kristi, Karp Judith, Feinberg Andrew P, Cui Hengmi. Nature. 2008;451(7175):202–6. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Faghihi Mohammad Ali, Modarresi Farzaneh, Khalil Ahmad M, Wood Douglas E, Sahagan Barbara G, Morgan Todd E, Finch Caleb E, St Laurent Georges, Kenny Paul J, Wahlestedt Claes. Nature medicine. 2008;14(7):723–30. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Kino Tomoshige, Hurt Darrell E, Ichijo Takamasa, Nader Nancy, Chrousos George P. Science signaling. 2010;3(107):ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.LncRNADisease: a database for long-non-coding RNA-associated diseases. Chen Geng, Wang Ziyun, Wang Dongqing, Qiu Chengxiang, Liu Mingxi, Chen Xing, Zhang Qipeng, Yan Guiying, Cui Qinghua. Nucleic acids research. 2013;41(Database issue):D983–6. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.lncRNAdb v2.0: expanding the reference database for functional long noncoding RNAs. Quek Xiu Cheng, Thomson Daniel W, Maag Jesper L V, Bartonicek Nenad, Signal Bethany, Clark Michael B, Gloss Brian S, Dinger Marcel E. Nucleic acids research. 2015;43(Database issue):D168–73. doi: 10.1093/nar/gku988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.lncRNome: a comprehensive knowledgebase of human long noncoding RNAs. Bhartiya Deeksha, Pal Koustav, Ghosh Sourav, Kapoor Shruti, Jalali Saakshi, Panwar Bharat, Jain Sakshi, Sati Satish, Sengupta Shantanu, Sachidanandan Chetana, Raghava Gajendra Pal Singh, Sivasubbu Sridhar, Scaria Vinod. Database : the journal of biological databases and curation. 2013;2013:bat034. doi: 10.1093/database/bat034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Ghosal Suman, Das Shaoli, Sen Rituparno, Basak Piyali, Chakrabarti Jayprokas. Frontiers in genetics. 2013;4:283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Rybak-Wolf Agnieszka, Stottmeister Christin, Glažar Petar, Jens Marvin, Pino Natalia, Giusti Sebastian, Hanan Mor, Behm Mikaela, Bartok Osnat, Ashwal-Fluss Reut, Herzog Margareta, Schreyer Luisa, Papavasileiou Panagiotis, Ivanov Andranik, Öhman Marie, Refojo Damian, Kadener Sebastian, Rajewsky Nikolaus. Molecular cell. 2015;58(5):870–85. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 133.Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Westholm Jakub O, Miura Pedro, Olson Sara, Shenker Sol, Joseph Brian, Sanfilippo Piero, Celniker Susan E, Graveley Brenton R, Lai Eric C. Cell reports. 2014;9(5):1966–80. doi: 10.1016/j.celrep.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Circular RNA (circRNA) in Alzheimer's disease (AD). Lukiw Walter J. Frontiers in genetics. 2013;4:307. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Li Peifei, Chen Shengcan, Chen Huilin, Mo Xiaoyan, Li Tianwen, Shao Yongfu, Xiao Bingxiu, Guo Junming. Clinica chimica acta; international journal of clinical chemistry. 2015;444:132–6. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 136.Correlation of circular RNA abundance with proliferation--exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Bachmayr-Heyda Anna, Reiner Agnes T, Auer Katharina, Sukhbaatar Nyamdelger, Aust Stefanie, Bachleitner-Hofmann Thomas, Mesteri Ildiko, Grunt Thomas W, Zeillinger Robert, Pils Dietmar. Scientific reports. 2015;5:8057. doi: 10.1038/srep08057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Mattick John S. BioEssays : news and reviews in molecular, cellular and developmental biology. 2003;25(10):930–9. doi: 10.1002/bies.10332. [DOI] [PubMed] [Google Scholar]
- 138.miR-34 - a microRNA replacement therapy is headed to the clinic. Bader Andreas G. Frontiers in genetics. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Ling Hui, Fabbri Muller, Calin George A. Nature reviews. Drug discovery. 2013;12(11):847–65. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Treatment of HCV infection by targeting microRNA. Janssen Harry L A, Reesink Hendrik W, Lawitz Eric J, Zeuzem Stefan, Rodriguez-Torres Maribel, Patel Keyur, van der Meer Adriaan J, Patick Amy K, Chen Alice, Zhou Yi, Persson Robert, King Barney D, Kauppinen Sakari, Levin Arthur A, Hodges Michael R. The New England journal of medicine. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 141.Micromanaging hepatitis C virus. Lieberman Judy, Sarnow Peter. The New England journal of medicine. 2013;368(18):1741–3. doi: 10.1056/NEJMe1301348. [DOI] [PubMed] [Google Scholar]