Abstract
Dual catalytic light-driven cross-coupling methodologies utilizing a Ni(II) salt with a photocatalyst (PC) have emerged as promising methodologies to forge aryl C–N bonds under mild conditions. The recent discovery that the PC can be omitted and the Ni(II) complex directly photoexcited suggests that the PC may perform energy transfer (EnT) to the Ni(II) complex, a mechanistic possibility that has recently been proposed in other systems across dual Ni photocatalysis. Here, we report the first studies in this field capable of distinguishing EnT from electron transfer (ET), and the results are consistent with Förster-type EnT from the excited state [Ru(bpy)3]Cl2 PC to Ni-amine complexes. The structure and speciation of Ni-amine complexes that are the proposed EnT acceptors were elucidated by crystallography and spectroscopic binding studies. With the acceptors known, quantitative Förster theory was utilized to predict the ratio of quenching rate constants upon changing the PC, enabling selection of an organic phenoxazine PC that proved to be more effective in catalyzing C–N cross-coupling reactions with a diverse selection of amines and aryl halides.
Graphical Abstract

INTRODUCTION
Dual catalytic, light-driven C–N,1,2 C–O,3 C–C,4 C–P,5 and C–S6 cross-coupling methodologies utilizing a Ni(II) catalyst along with a photocatalyst (PC) have recently emerged as promising systems for synthetic chemistry with advantages over traditional Pd catalysis in terms of sustainability, cost, and mildness of reaction conditions.7 As such, mechanistic understanding of these reactions is essential in order to rationally design effective catalysts and unlock new reactivity.
In 2016, two dual catalytic, C–N cross-coupling systems were independently developed. In both cases, a wide range of amines and aryl halides were coupled under blue light irradiation employing the same Ni(II) precatalyst (i.e., NiBr2·glyme) and Ir(III) PC (Figure 1A).1,2 The Ir(III) PC used was [Ir(dF(CF3)ppy)2(dtbbpy)]PF6, which is a strong photo-oxidant. In contrast, our group recently found that organic PCs (e.g., dihydrophenazines8 and phenoxazines9) that are strong photoreductants also functioned efficiently for dual catalytic C–N cross-coupling under similar conditions (Figure 1B).10 These results suggest two potential scenarios. In one case, the Ni(II) precatalyst is sufficiently robust that C–N cross-coupling can occur through both reductive and oxidative electron transfer (ET) cycles utilizing a photo-oxidant and a photoreductant, respectively. Alternatively, the Ni(II) precatalyst might be activated via energy transfer (EnT) from the PC; notably, the possibility of an EnT cycle has seldom been considered to date in C–N cross-coupling, with only one reported example describing the coupling of sulfonamides with aryl halides.11
Figure 1.
Reported C–N cross-coupling systems utilizing (A) a photo-oxidant, (B) photoreductants, or (C) no added PC. This work (D) on C–N bond formation promoted by energy transfer from a PC to the Ni-amine complex. In (A), the Ir PC used was [Ir(dF(CF3)ppy)2(dtbbpy)]PF6, where dF(CF3)ppy = 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine and dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyr-idine. The organic PCs used in (B) were 3,7-di([1,1′-biphenyl]-4-yl)-10-(naphthalen-1-yl)-10H-phenoxazine and 5,10-di(naphthalen-2-yl)-5,10-dihydrophenazine. PC = photocatalyst; DMAc = N,N-dimethylacetamide. ArBr = aryl bromide.
Furthermore, we recently discovered that a PC can be omitted from the dual catalytic C–N cross-coupling system. Specifically, upon amine addition to a solution containing Ni(II) and aryl halide, we observed in situ formation of Ni-amine complexes that can be directly photoexcited with 365 nm LEDs for the formation of the desired aryl C–N product (Figure 1C).12 The existence of a direct Ni(II) irradiation route supports the possibility of an EnT quenching mechanism as elucidated herein (Figure 1D). In the absence of an added PC, the Ni excited state is accessed directly through photoexcitation. Similarly, in a dual catalytic system, an analogous (but possibly distinct) Ni excited state can be accessed through EnT from a PC.
This discovery is complemented by the finding that a PC can be omitted for dual catalytic C–O13 or C–C14 systems, as the Ni complex can similarly be directly photoexcited, suggesting the possibility of an EnT pathway. More broadly, methods across photocatalysis that involve the direct excitation of transition metal complexes15–19 suggest systems that may be conducive to EnT upon addition of a PC. Importantly, EnT pathways have been proposed to be operative with Ni(II) or Cu(I) complexes serving as the acceptor in several systems across light-driven dual catalysis.20–23 However, to date, no study has utilized time-resolved techniques capable of distinguishing between electron and energy transfer to support that the excited state PC does indeed react via energy transfer in these systems.
Notably, obtaining spectroscopic evidence of an EnT pathway can unlock pivotal practical advances for a methodology as illustrated by the C–S cross-coupling of alkenes/alkynes with disulfides in which replacement of the precious metal Ir(III) PC with an organic PC of higher triplet energy both accelerated the rate of product formation and alleviated sustainability concerns.24 Furthermore, dual catalysis enables mild visible light irradiation, while 365 nm light was required previously to directly excite the Ni complex. Thus, addition of a PC can enable use of UV-sensitive substrates.
Herein, we provide spectroscopic evidence in support of EnT from an excited state PC to Ni-amine complexes under conditions relevant for dual catalytic C–N cross-coupling driven by visible light (Figure 1D). In particular, using nanosecond transient absorption (TA) spectroscopy, we observed the excited state of [Ru(bpy)3]Cl2 (bpy = 2,2′-bipyridine) reacting with Ni-amine complexes formed in situ in C–N cross-coupling reaction mixtures. The spectral data are consistent with an EnT pathway proceeding primarily through a Förster-type mechanism, a result that is notably distinct from the Dexter-type pathway typically invoked in the literature in catalytic cycles involving EnT that results in substrate sensitization.25 Next, speciation studies elucidated the Ni-amine complexes that serve as EnT acceptors (or as light absorbers in the direct excitation method).12 Finally, these mechanistic insights were utilized in conjunction with quantitative Förster theory to select an organic phenoxazine PC (the same shown in Figure 1B) that proved to be more effective than [Ru(bpy)3]Cl2 in the C–N coupling of 13 substrate pairs.
RESULTS AND DISCUSSION
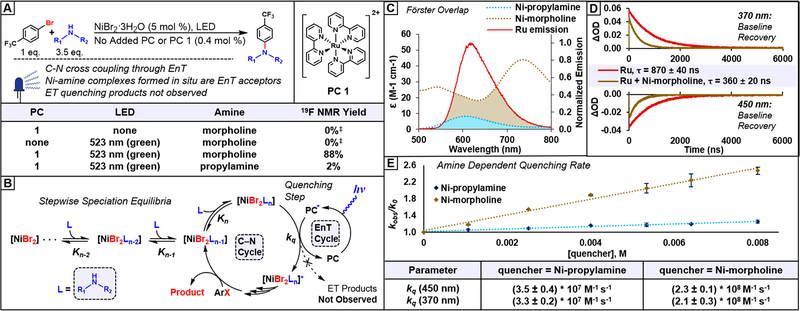
[Ru(bpy)3]Cl2 was chosen as the PC for spectroscopic studies due to the extensive body of photophysical literature describing its spectral changes upon EnT or ET26,27 and the precedence for its use as a PC in related dual catalytic C–N cross-coupling systems.1,28 Here, we initially confirmed that [Ru(bpy)3]Cl2 (pC 1) is an effective PC for C–N cross-coupling involving 4-bromobenzotrifluoride and morpholine, achieving 88% conversion as measured by 19F NMR after 22 h of irradiation with a green (i.e., λmax = 523 nm) LED (Figure 2A). Importantly, no product was observed either in the absence of light or PC under these conditions, indicating that the direct excitation of the Ni complex was not a significant pathway, and thus the observed reactivity can be completely ascribed to the role of PC 1.
Figure 2.
(A) Dual catalytic C–N cross-coupling control reactions. Reaction details: 0.4 mmol in aryl bromide in DMAc, 22 h reaction time. (B) Simplified catalytic cycle highlighting the focus of this work: the PC quenching step probed by transient absorption (TA) experiments and the speciation of the Ni catalyst. For the full proposed cycle, see Figure S71. (C) Förster EnT efficiency approximated by overlap between PC 1 emission and Ni-amine complex absorption. (D) TA single wavelength kinetic traces acquired with λpump = 532 nm and λprobe = 450 or 370 nm for PC 1 and a mixture (80:1 molar ratio of Ni:PC) containing PC 1 (0.1 mM) with Ni-morpholine complexes. (E) Stern–Volmer plot and extracted quenching rate constants. Quenchers are mixtures of Ni-amine complexes formed in situ. The species formed are determined later in this work. ‡We note that debromination was not observed (Figure S72); only unreacted aryl bromide was detected by 19F NMR. EnT = energy transfer. ET = electron transfer. kq = quenching rate constant. k0 = decay rate constant of PC 1. kobs = observed decay rate constant in the presence of quencher. Kn = equilibrium binding constant. See SI, Section 2, for TA experimental details and spectra.
The bimolecular quenching step (Figure 2B) between the excited state of PC 1 and the Ni-amine complex was further elucidated via nanosecond TA experiments. We note that the speciation of the Ni-amine complexes formed in situ is detailed later in this work. Laser irradiation at λpump = 532 nm in N,N-dimethylacetamide (DMAc) solvent containing PC 1 produced the long-lived (i.e., 870 ± 40 ns in DMAc, Figure S4) metal-to-ligand charge-transfer (MLCT) triplet excited state characterized by an excited state absorption (ESA) feature at λ = 370 nm and a prominent ground state bleach (GSB) at λ = 450 nm (see SI, Figure S3). The MLCT excited state lifetime measured here is consistent with previous reported values ranging from 800 to 1000 ns in polar, aprotic solvents.29
We note that the MLCT state can be approximated as a formal reduction of one bpy ligand to the radical anion along with formal oxidation of the Ru center to Ru(III). As such, the ESA at λ = 370 nm has been previously assigned to a transition involving the radical anion of one bpy ligand on the basis of comparison to spectroelectrochemical measurement of the radical anion of free bpy and is thus diagnostic of MLCT state formation.26 In addition, the GSB at λ = 450 nm was attributed to the presence of Ru(III) in the MLCT state, which lacks a transition in this region. As such, quenching through ET is characterized by persistence of the ESA at λ = 370 nm in the case of reductive quenching since [Ru(bpy)3]Cl2 is formally reduced, while oxidative quenching leads to persistence of the GSB at λ = 450 nm.27 However, in the case of EnT, both signals fully return to baseline since the ground state is recovered, and a decrease in the excited state lifetime is observed.27
In a C–N cross-coupling reaction mixture consisting of the same molar ratio of components detailed in Figure 2A, we monitored excited state quenching of PC 1 using λpump = 532 nm, consistent with the green LED used in cross-coupling reactions. We note that under these conditions Ni-morpholine complexes are formed in situ (vide infra) and are active in quenching PC 1’s excited state. Importantly, consistent with an EnT pathway, signals corresponding to PC 1’s excited state return fully to the baseline at all wavelengths from λ = 300–800 nm (Figure S11), indicative of recovery of the ground state of PC 1. In particular, kinetic traces of PC 1 at both λprobe = 370 nm and λprobe = 450 nm returned fully to baseline (Figure 2D), and thus neither oxidized nor reduced PC 1 indicative of an ET mechanism was observed.
We further note that cyclic voltammetry experiments suggest that an ET quenching mechanism (either oxidative or reductive) is unlikely to occur (Section 4). Specifically, E0* ([Ru(bpy)3]*2+/[Ru(bpy)3]3+) = −1.19 V vs Fc/Fc+ (−0.74 V vs SCE) as measured in this work in DMAc, while the first reduction of a Ni-morpholine complex occurs at Ep = −1.71 V vs Fc/Fc+ (−1.26 V vs SCE); thus, an oxidative quenching pathway is thermodynamically unfavorable. Similarly, reductive quenching is unlikely as E0*([Ru(bpy)3]*2+/[Ru(bpy)3]+) = 0.27 V vs Fc/Fc+ (0.72 V vs SCE), and the first oxidation of a Ni-morpholine complex occurs at Ep/2 ≈ 0.48 V vs Fc/Fc+ (0.93 V vs SCE).
Furthermore, the presence of Ni-morpholine complexes led to significant reduction in the excited state lifetime of PC 1 from 870 ± 40 ns to 360 ± 20 ns, while free morpholine did not lead to quenching (Figures S5 and S6). Further control experiments showed that electron transfer products were not observed even under high laser power (Figures S9 and S10). We note that subtraction of spectra did not yield any signals that could be assigned to an excited state Ni complex, likely due to the excited state lifetime being too short to measure (Figure S12); for example, a square planar Ni(II) aryl halide complex was observed to have a 4.2 ns lifetime,13 too short to be detectable with our setup. Overall, these results suggest that C–N cross-coupling reactivity is derived from excited Ni-amine complexes, which can be accessed through either EnT from a PC as in this work or through direct photoexcitation as in our previous work.12 As such, we propose that mechanistic steps following the EnT step will mirror those we proposed previously (Figure S71, for the catalytic cycle).
Next, we observed that when using different types of amines and the Ni:amine ratio is held at 1:70, the same molar ratio used in C–N reactions, PC 1 was quenched to different degrees. To explore this relationship, a Stern–Volmer quenching study was performed with ratios of Ni:PC 1 increasing from 10:1 to 80:1. Notably, use of morpholine gives kq = (2.3 ± 0.1) × 108 M−1 s−1 at λprobe = 450 nm, while propylamine quenched with a significantly reduced rate constant of kq = (3.5 ± 0.4) × 107 M−1 s−1 (Figure 2E), consistent with the lower cross-coupling performance of primary amines relative to secondary amines observed herein and previously under direct excitation with 365 nm irradiation.12 Since the same PC was used throughout and none of the other reaction components were suitable ET or EnT quenchers (Figures S5–S8), this variation in kq must arise from changes in electronic structure of the Ni-amine complex, and thus the speciation of the complexes formed must be determined.
Unlike typical organic substrates for which the contribution of Förster-type EnT can be deduced to be minimal a priori on account of lacking significant spectral overlap with the excited PC,27 Ni-amine complexes absorb significantly in the wavelength range of PC 1 phosphorescence (Figure 2C). Further, the overlap area for absorption of Ni-morpholine and Ni-propylamine mixtures appears correlated with the rate of quenching, supporting the hypothesis of Förster-type EnT. However, the components of the quenching mixture must be elucidated in order to determine the identity and molar absorptivity of the EnT acceptor(s) to utilize Förster theory quantitatively in examining this hypothesis. Turning to classic reports30–35 describing monodentate primary and secondary amine ligands binding to Ni(II), the available information is insufficient due to the lack of both crystallographic characterization and speciation studies in DMAc solution.
To address this challenge, we first grew single crystals from concentrated Ni-amine mixtures, and analysis by single crystal X-ray diffraction (XRD) revealed for the first time that propylamine, morpholine, and quinuclidine form 6-, 5-, and 4-coordinate Ni(II) bromide complexes, respectively (Figure 3A). Importantly, these complexes are likely the EnT acceptors, but it must be confirmed that the structures obtained in solid state can serve as reasonable approximations of the geometry of complexes formed in situ in DMAc solution. To confirm that this assumption is reasonable, single crystals were examined through solid-state UV–visible absorption spectroscopy (Figure 3B; see SI, Section 8, for details). Qualitatively, similar absorption features are observed in solution to those found in the single crystals for all complexes, suggesting that structures obtained from XRD are not changed significantly upon DMAc solvation. Further, a control UV–vis experiment supports that the same complexes also exist in the full C–N coupling reaction mixtures (i.e., with aryl halide and PC added; Figure S58). As such, some bands in the UV–vis spectra of the quenching mixtures used in TA experiments can now be assigned to specific complexes, namely, [NiBr2(morpholine)3] (i.e., λmax,1 = 427 nm, λmax,2 = ~740 nm) and [NiB2(propylamine)4] (λmax,1 = 379 nm, λmax,2 = ~620 nm).
Figure 3.
(A) Crystal structures of [NiBr2(propylamine)4], [NiBr2(morpholine)3], and [NiBr2(quinuclidine)2] shown at 50% thermal ellipsoids with hydrogens omitted for clarity. (B) Left axis: molar absorptivity of Ni-amine complexes in DMAc solution. Right axis: solid-state UV–visible absorption spectra of single crystals of complexes shown in (A). Inset: photographs of crystals of each complex at 40× magnification. (C) Selected bond distances and angles. See SI, Section 5, for further experimental details.
Notably, the structure obtained for [NiBr2(morpholine)3] closely matches the density functional theory (DFT)-optimized ground state geometry in DMAc solution described in our previous work,12 supporting this assignment as well as the accuracy of our reported DFT calculations. Further, the formation of [NiBr2(propylamine)4] suggests the general trend that primary amines form six-coordinate NiBr2 complexes as supported by the isolation of [NiBr2(aniline)4] and [NiBr2(cyclohexylamine)4] (Figures S48 and S49). Ni-amine complexes of the type characterized here are proposed to form across Ni catalysis in systems lacking exogenous ligand and employing amines in conjunction with NiBr2, regardless of the NiBr2 source used (e.g., NiBr2·glyme, NiBr2·3H2O, or anhydrous NiBr2; Figure S57). The same complexes also form in the presence of all PCs used in C–N coupling reactions (Ir(III), Ru(II), and phenoxazine; Figures S54–S56), suggesting they may more broadly serve as mechanistically relevant species in many dual catalytic Ni(II) cross-coupling systems.
Reported magnetic moment measurements of identical34 or related36–38 Ni(II) complexes, as well as our previous DFT calculations, are consistent with [NiBr2(propylamine)4] and [NiBr2(morpholine)3] possessing triplet ground states in DMAc solution, suggesting that the d–d absorptions in the region of Förster overlap are of triplet-to-triplet nature. As such, Förster EnT is allowed based on the conservation of spin angular momenta39 from the triplet excited state of PC 1 as the donor to form a triplet excited state Ni-amine complex. In order to employ Förster theory in modeling this excited state reactivity, the molar absorptivity of each acceptor complex overlapping with PC 1 emission must be known. However, UV–vis absorption peaks are observed in the Ni-amine solutions which remain to be assigned (e.g., the feature at ~550 nm in the Ni-morpholine DMAc solution, Figure 2C) that might belong to viable EnT acceptors. We hypothesized that these features must originate from other Ni-amine complexes with fewer amine ligands, since a stepwise series of amine additions is required in the formation of the observed complexes from the NiBr2·3H2O precatalyst. These binding equilibria were directly observed through UV–vis isothermal titrations in which mixtures were analyzed with increasing ratios of the amine ligand:Ni.
UV–vis of these mixtures shows the evolution and demise of species with increasing numbers of amine ligands in the Ni-morpholine mixture (Figure 4A). Initially, we note that the DMAc solvent forms the salt [Ni(DMAc)6][NiBr4] prior to amine addition (Figure S51) and that multiple pathways exist for the first two amine additions (Figures S69 and S70). However, all pathways converge to form the tetrahedral [NiBr2(morpholine)2] as the product of K2, which can be assigned to the signal with λmax,1 = ~550 nm and λmax,2 = ~850 nm. To precisely determine the ratio of these species in the Ni-morpholine quenching mixture, the titration data were fitted to four variants (flavors)40 of a 1:3 (metal:ligand or host:guest) binding model. This analysis was performed using a Matlab code based on the analytical solution to the system of equations for the 1:3 equilibria, similar to that described in a NMR study on a 3:1 complexation of a bis-antimony receptor with halide anions.41 In our work we performed a global analysis42 using the UV–vis binding isotherms from λ = 395 to 1200 nm (see SI, Section 7, for details). Comparing how the various flavors of the 1:3 binding model fitted the data40,43 clearly showed that the “full” 1:3 model, which assumes (i) cooperativity and (ii) that the 1:1, 1:2, and 1:3 stepwise complexes have distinct spectra, gave a significantly better fit to the data than the other binding models (flavors) considered.
Figure 4.
(A) Selected UV–vis traces from one replicate titration experiment showing equilibria between Ni-morpholine complexes. Arrows indicate features that rise or fall in the forward direction of each equilibrium. A 70 equiv amount of amine ligand added (relative to Ni) corresponds to the exact conditions used in C–N cross-coupling reactions. (B) Calculated average molar absorptivity (n = 3 replicates) of Ni-morpholine complexes. (C) Scheme defining stepwise series of equilibria upon addition of amine ligands. Equilibrium constants and molar absorptivities were extracted from titration data via a global analysis fitting procedure (Figures S62–S65 for details). aWe note that the NiBr2 precatalyst forms a tetrabromonickelate salt in DMAc solution from which multiple amine addition pathways are possible for K1 and K2. bValues in kcal mol−1. See SI for details.
The full model allowed us to extract not only the stepwise equilibrium binding constants but also the molar absorptivity (Figure 4B) of each species, giving K1 = (6 ± 3) × 103 M−1, K2 = 130 ± 30 M−1, and K3 = 2.6 ± 0.5 M−1 with the corresponding free energies (after correcting for statistical factors) ΔG1 = −2.0 ± 0.3 kcal mol−1, ΔG2 = −1.3 ± 0.1 kcal mol−1, and ΔG3 = −0.5 ± 0.1 kcal mol−1, respectively (Figure 4C). The increasing ΔG values indicate negative binding cooperativity or less favorable addition of morpholine to Ni with each successive association. Notably, ΔG3 = −0.5 kcal mol−1 is reasonably close to the value of ΔG3 = −1.1 kcal mol−1 calculated by DFT in our previous work.12
Furthermore, we calculate that under C–N coupling conditions (i.e., 70:1 amine:Ni molar ratio), the Ni-morpho-line quenching mixture consists of 73% [NiBr2(morpholine)3], 27% [NiBr2(morpholine)2], and <0.3% other complexes. As shown in Figure 2C, both [NiBr2(morpholine)2] and [NiBr2(morpholine)3] demonstrate absorptions that overlap significantly with PC 1’s emission. While [NiBr2(morpholine)2] shows the largest overlap, it is present in lower concentration. Interestingly, [NiBr2(morpholine)3] contains a morpholine bound in the apical position (Figure 3A) with a significantly shortened Ni–N bond of 2.050(4) Å compared to the other morpholines (i.e., 2.099(4) and 2.101(4) Å); thus, this morpholine is the strongest donor and may facilitate the subsequent proposed mechanistic step, intramolecular ET to generate a Ni(I) center and a morpholino radical cation (Figure S71, for our proposed mechanism). As such, both [NiBr2(morpholine)2] and [NiBr2(morpholine)3] show positive features for EnT catalysis, and we conclude that both are the EnT acceptors.
With the acceptors and their respective molar absorptivities known, we turned to classical Förster theory, in which the theoretical energy transfer rate constant, kEnT, can be calculated as follows:27
| (1) |
where
| (2) |
and
| (3) |
In these equations, R is the donor–acceptor distance, R0 is the critical Förster distance defined in eq 2, kr,D is the radiative decay constant of the donor in the absence of acceptor, κ is the dipolar orientation factor, η is the refractive index of the solvent, and J is the spectral overlap integral defined in eq 3, which involves FD, the area-normalized emission spectrum of the donor, and εA, the molar absorptivity of the acceptor as a function of frequency. In order to apply this equation to the reaction between excited state PC 1 and a Ni-amine complex, we needed to eliminate variables R and κ, which are difficult to measure in solution with freely tumbling donors and acceptors.
Utilizing an approach similar to that first demonstrated in the study of a system involving intramolecular EnT from a Re donor to a transition metal acceptor,44,45 an expression was derived (see SI, Section 3, for details) to evaluate the ratio of quenching rate constants (kEnT,1/kEnT,2) as the donor, PC 1, was held constant but the acceptor was changed, from the Ni-morpholine quenching mixture to the Ni-propylamine quenching mixture, according to the following equation:
| (4) |
Using eq 4, the ratio for PC 1 was calculated as kEnT,A1/kEnT,A2 = 4.6, which compares favorably with the experimental value of 6.5 obtained from the TA experiments described above (Table S5, for J integrals used in the calculation). This level of agreement compares very well with that obtained for similar calculations in the literature,44 supporting our assignment of the EnT quenching step as a Förster-type EnT process. Thus, based on eqs 1–3, it can be seen that selection of a PC with higher radiative rate constant kr,D and higher overlap integral J will result in a higher EnT rate.
As such, we hypothesized that changing the donor PC from PC 1 to the organic phenoxazine PC 2 would impart improved performance in C–N cross-coupling reactions given that PC 2 covers an increased spectral range in its emission compared to PC 1 (Figure S33) and has a radiative rate constant reported46 to be (4 ± 1) × 106 s−1, which is significantly higher than that of PC 1 (i.e., 7 × 104 s−1).29 First, we confirmed that PC 2 also reacts via EnT as opposed to ET through spectroscopic (Figures S24–S32) and electrochemical (see SI, Section 4) control experiments. To theoretically probe the hypothesis that PC 2 will increase the rate of EnT, a second equation was derived (see SI, Section 3) to predict the ratio of quenching rate constants as the PC is changed but the Ni-morpholine mixture is kept constant as the acceptor:
| (5) |
We note that the photophysics of PC 2 are more complicated than those of PC 1 in that both singlet and triplet excited states are populated. The triplet state is unlikely to react at a kinetically significant rate via a Förster-type pathway given the extremely low phosphorescence radiative decay rates of organic PCs in solution, typically on the order of 100−103 s−1.47 Thus, the fluorescence spectrum and fluorescence radiative decay constant were used in conjunction with eq 5 to calculate the ratio of kEnT,PC2:kEnT,PC1 = 20.4, which is within a factor of 3 of the value of 12.7 determined experimentally from quenching studies (see above for PC 1 and SI, Section 3, for PC 2). Agreement between these values suggests that the observed EnT occurs through primarily a Förster-type pathway in the case of both PCs and with a much higher rate constant for PC 2 of (2.9 ± 0.2) × 109 M−1 s−1 (Figure S36) as compared to that for PC 1 of (2.3 ± 0.1) × 108 M−1 s−1.
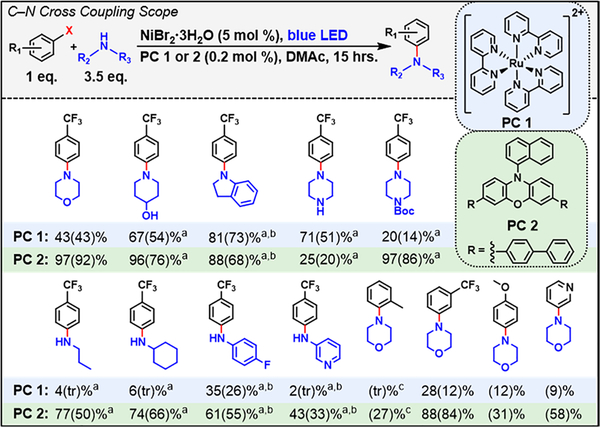
With these results in hand, we compared the performance of PCs 1 and 2 in C–N cross-coupling reactions (Figure 5; see SI, Section 9, for experimental details and product characterization). In order to directly compare the PCs, we chose to use the exact same conditions for all reactions. In particular, blue 457 nm light irradiation was chosen since PC 2 cannot absorb green light. Further, an irradiation time of 15 h was chosen to allow for direct comparison with our previous work.12 Control reactions confirmed that no product is formed in the absence of PC under blue irradiation (Table S9), and we further note that for PC 1 blue LED irradiation accesses the same absorption band as green irradiation, forming the same lowest MLCT excited state that we propose reacts via EnT.
Figure 5.
Scope of C–N coupling reactions using PC 1 and PC 2; PC 1 = [Ru(bpy)3]Cl2 and PC 2 = 3,7-di([1,1′-biphenyl]-4-yl)-10-(naphthalen-1-yl)-10H-phenoxazine. Yields were determined using 19F NMR for products containing fluorine. Isolated yields are reported in parentheses. X = Br unless otherwise indicated. aOne equiv of KBr was used as an additive; b1.5 equiv of the amine was used with 1.5 equiv of added quinuclidine. cAn aryl iodide was used. tr = trace product isolated. Reactions were performed with 0.4 mmol of aryl halide at room temperature. See SI, Section 9, for details and characterizations. The blue LED emission λmax = 457 nm.
Under these conditions, the performance with PC 1 and morpholine is significantly worsened (e.g., 43% conversion vs 88% in Figure 2A), and this can be attributed to a combination of factors, namely, the lower catalyst loading, which leads to a loss of 23% conversion (Table S9), a lower luminous flux of the blue LED as compared with the green LED (1:4.4, blue:green; Table S1), and the inner-filter effect resulting from increased unproductive absorption of blue vs green excitation light by the Ni-morpholine complexes (3:1, blue:green; Figure S33). Despite these worsened conditions, PC 1 is effective for coupling of secondary aliphatic amines with 4-bromobenzotrifluoride, highlighted by coupling of unprotected piperazine (51%) and indoline (73%), nitrogen heterocycles that are among those most frequently used in medicinal chemistry.48
On the other hand, PC 2 is more broadly effective, achieving higher yields than PC 1 with almost all substrates (Figure 5). Notably, PC 2 is effective in coupling difficult aliphatic (e.g., propylamine, 50%) and aromatic primary amines such as 4-fluoroaniline (55%) and 3-aminopyridine (33%) with 4-bromobenzotrifluoride, while PC 1 is ineffective, achieving only trace product formation with primary aliphatic amines. PC 2’s emission extends ~100 nm further into the blue than PC 1, overlapping an absorption band of [NiBr2(propylamine)4] that PC 1 cannot access (Figure S33). Since the efficiency of EnT has been shown to depend on specific electronic transitions,45 we hypothesize that this blue-shifted band may facilitate EnT and thus might explain PC 2’s increased performance.
With aromatic amines (e.g., 4-fluoroaniline), quinuclidine was employed as a base additive based on a beneficial effect on yield observed in our previous work.12 The effect of quinuclidine is likely due at least in part to its role in controlling the speciation of the Ni catalyst; investigation by UV–vis revealed that quinuclidine binds more strongly than aniline to NiBr2 (Figure S59), forming [NiBr2(quinuclidine)2], which has good overlap between its absorption and PC 2’s emission (Figure S33). In addition, the use of 1 equiv of KBr as an additive improved the performance of some amines such as propylamine and unprotected piperazine (14% and 26% increase, respectively; Table S10, and following supplemental discussion). With propylamine, we hypothesize that KBr improves reactivity by inhibiting the speciation equilibria as observed by UV–vis (Figures S60 and S61); this inhibition increases the concentration of [NiBr2(propylamine)3], which has better overlap with PC 2 than [NiBr2(propylamine)4.
With regard to the aryl halide coupling partner, PC 2 successfully promotes coupling of morpholine with aryl halides containing electron-withdrawing groups (EWGs, e.g., 4-bromobenzotrifluoride, 92%) as well as electron-donating groups (EDGs, e.g., 4-bromoanisole, 31%). Notably, a heterocyclic aryl bromide, 3-bromopyridine, could be coupled in good yield (58%). In addition, the difficult ortho-substituted aryl halide 2-iodotoluene could be coupled with morpholine in moderate yield (27%), constituting the first example of C–N bond formation with this substrate pair in light-driven Ni catalysis.
Trends in reactivity for both PCs mirror those observed in our previous work with secondary > primary > primary aromatic amines in terms of yield. Similarly, aryl halides containing EWGs gave greater yields than those containing EDGs. Overall, the similarity in these trends across both PCs to trends obtained in our previous work12 supports that a similar Ni-amine excited state intermediate forms in both cases that can be accessed either via EnT from a PC under visible light or via direct excitation under 365 nm irradiation.
CONCLUSION
In sum, spectroscopic evidence supports an EnT quenching mechanism in dual catalytic C–N cross-coupling that proceeds via a Förster-type pathway. In addition, the EnT acceptors have been identified via single-crystal XRD and spectroscopic binding studies as a series of [Ni(II)Br2(amine)x] complexes formed in situ in C–N cross-coupling reaction mixtures. These complexes are proposed to form more broadly across catalytic systems utilizing NiBr2 and amines and thus constitute mechanistically significant intermediates across Ni catalysis. Elucidating the speciation enabled the use of quantitative Förster theory to calculate EnT rate constant ratios that agreed with values determined experimentally from spectroscopic studies. Employment of this mechanistic knowledge through selection of phenoxazine PC 2 led to increased performance relative to [Ru(bpy)3]Cl2 PC 1 in catalyzing C–N bond formation between diverse amines and aryl halides under mild conditions. Ultimately, future work utilizing quantitative Förster theory has the potential to both predict and discover new reactivity in energy transfer systems across light-driven Ni catalysis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Colorado State University, the NIH (R35GM119702), and the Alfred P. Sloan Foundation. P.T. would like to thank the ARC for support (CE140100036 and DP190101892). M.K. would like to thank Dr. Brian Newell for aid and instruction in X-ray diffraction structure analysis and Prof. Richard G. Finke for many insightful discussions regarding this work. We would also like to thank the reviewers of this manuscript for their insightful comments.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b11049.
Transient absorption spectroscopic data, cyclic voltammetry data, derivations of equations using Förster theory, emission spectroscopy, single-crystal X-ray crystallography procedures, UV–visible spectroscopy and data analysis, a proposed catalytic cycle, and details regarding C–N cross-coupling reaction procedures and product characterization (PDF)
X-ray crystallographic data (CIF)
X-ray crystallographic data (CIF)
X-ray crystallographic data (CIF)
X-ray crystallographic data (CIF)
X-ray crystallographic data (CIF)
X-ray crystallographic data (CIF)
Additional information (ZIP)
Additional information (ZIP)
Additional information (ZIP)
The authors declare no competing financial interest.
REFERENCES
- (1).Corcoran EB; Pirnot MT; Lin S; Dreher SD; DiRocco DA; Davies IW; Buchwald SL; MacMillan DWC Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353 (6296), 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Oderinde MS; Jones NH; Juneau A; Frenette M; Aquila B; Tentarelli S; Robbins DW; Johannes JW Highly Chemoselective Iridium Photoredox and Nickel Catalysis for the Cross-Coupling of Primary Aryl Amines with Aryl Halides. Angew. Chem., Int. Ed 2016, 55 (42), 13219–13223. [DOI] [PubMed] [Google Scholar]
- (3).Terrett JA; Cuthbertson JD; Shurtleff VW; MacMillan DWC Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 2015, 524 (7565), 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zuo Z; Ahneman DT; Chu L; Terrett JA; Doyle AG; MacMillan DWC Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345 (6195), 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Xuan J; Zeng T-T; Chen J-R; Lu L-Q; Xiao W-J Room Temperature C–P Bond Formation Enabled by Merging Nickel Catalysis and Visible-Light-Induced Photoredox Catalysis. Chem. -Eur. J 2015, 21 (13), 4962–4965. [DOI] [PubMed] [Google Scholar]
- (6).Oderinde MS; Frenette M; Robbins DW; Aquila B; Johannes JW Photoredox Mediated Nickel Catalyzed Cross-Coupling of Thiols With Aryl and Heteroaryl Iodides via Thiyl Radicals. J. Am. Chem. Soc 2016, 138 (6), 1760–1763. [DOI] [PubMed] [Google Scholar]
- (7).Butters M; Catterick D; Craig A; Curzons A; Dale D; Gillmore A; Green SP; Marziano I; Sherlock J-P; White W Critical Assessment of Pharmaceutical Processes: A Rationale for Changing the Synthetic Route. Chem. Rev 2006, 106 (7), 3002–3027. [DOI] [PubMed] [Google Scholar]
- (8).Lim C-H; Ryan MD; McCarthy BG; Theriot JC; Sartor SM; Damrauer NH; Musgrave CB; Miyake GM Intramolecular Charge Transfer and Ion Pairing in N, N-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization. J. Am. Chem. Soc 2017, 139 (1), 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pearson RM; Lim C-H; McCarthy BG; Musgrave CB; Miyake GM Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts. J. Am. Chem. Soc 2016, 138 (35), 11399–11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Du Y; Pearson RM; Lim C-H; Sartor SM; Ryan MD; Yang H; Damrauer NH; Miyake GM Strongly Reducing, Visible-Light Organic Photoredox Catalysts as Sustainable Alternatives to Precious Metals. Chem. - Eur. J 2017, 23 (46), 10962–10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kim T; McCarver SJ; Lee C; MacMillan DWC Sulfonamidation of Aryl and Heteroaryl Halides through Photosensitized Nickel Catalysis. Angew. Chem., Int. Ed 2018, 57 (13), 3488–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lim C-H; Kudisch M; Liu B; Miyake GM C–N Cross-Coupling via Photoexcitation of Nickel–Amine Complexes. J. Am. Chem. Soc 2018, 140 (24), 7667–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shields BJ; Kudisch B; Scholes GD; Doyle AG Long-Lived Charge-Transfer States of Nickel(II) Aryl Halide Complexes Facilitate Bimolecular Photoinduced Electron Transfer. J. Am. Chem. Soc 2018, 140 (8), 3035–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ishida N; Masuda Y; Ishikawa N; Murakami M Cooperation of a Nickel–Bipyridine Complex with Light for Benzylic C-H Arylation of Toluene Derivatives. Asian J. Org. Chem 2017, 6 (6), 669–672. [Google Scholar]
- (15).Creutz SE; Lotito KJ; Fu GC; Peters JC Photoinduced Ullmann C–N Coupling: Demonstrating the Viability of a Radical Pathway. Science 2012, 338 (6107), 647. [DOI] [PubMed] [Google Scholar]
- (16).Ziegler DT; Choi J; Muñ oz-Molina JM; Bissember AC; Peters JC; Fu GC A Versatile Approach to Ullmann C–N Couplings at Room Temperature: New Families of Nucleophiles and Electrophiles for Photoinduced, Copper-Catalyzed Processes. J. Am. Chem. Soc 2013, 135 (35), 13107–13112. [DOI] [PubMed] [Google Scholar]
- (17).Shields BJ; Doyle AG Direct C(sp3)−H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals. J. Am. Chem. Soc 2016, 138 (39), 12719–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hwang SJ; Powers DC; Maher AG; Anderson BL; Hadt RG; Zheng S-L; Chen Y-S; Nocera DG Trap-Free Halogen Photoelimination from Mononuclear Ni(III) Complexes. J. Am. Chem. Soc 2015, 137 (20), 6472–6475. [DOI] [PubMed] [Google Scholar]
- (19).Kainz QM; Matier CD; Bartoszewicz A; Zultanski SL; Peters JC; Fu GC Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 2016, 351 (6274), 681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Heitz DR; Tellis JC; Molander GA Photochemical Nickel-Catalyzed C–H Arylation: Synthetic Scope and Mechanistic Investigations. J. Am. Chem. Soc 2016, 138 (39), 12715–12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dewanji A; Krach PE; Rueping M The Dual Role of Benzophenone in Visible-Light/Nickel Photoredox-Catalyzed C-H Arylations: Hydrogen-Atom Transfer and Energy Transfer. Angew. Chem., Int. Ed 2019, 58 (11), 3566–3570. [DOI] [PubMed] [Google Scholar]
- (22).Yoo W-J; Tsukamoto T; Kobayashi S Visible Light-Mediated Ullmann-Type C–N Coupling Reactions of Carbazole Derivatives and Aryl Iodides. Org. Lett 2015, 17 (14), 3640–3642. [DOI] [PubMed] [Google Scholar]
- (23).Welin ER; Le C; Arias-Rotondo DM; McCusker JK; MacMillan DWC Photosensitized, energy transfer-mediated organometallic catalysis through electronically excited nickel(II). Science 2017, 355 (6323), 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Teders M; Henkel C; Anhäuser L; Strieth-Kalthoff F; Gómez-Suárez A; Kleinmans R; Kahnt A; Rentmeister A; Guldi D; Glorius F The energy-transfer-enabled biocompatible disulfide–ene reaction. Nat. Chem 2018, 10 (9), 981–988. [DOI] [PubMed] [Google Scholar]
- (25).Strieth-Kalthoff F; James MJ; Teders M; Pitzer L; Glorius F Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev 2018, 47 (19), 7190–7202. [DOI] [PubMed] [Google Scholar]
- (26).Brown AM; McCusker CE; McCusker JK Spectroelectrochemical identification of charge-transfer excited states in transition metal-based polypyridyl complexes. Dalton Trans 2014, 43 (47), 17635–17646. [DOI] [PubMed] [Google Scholar]
- (27).Arias-Rotondo DM; McCusker JK The photophysics of photoredox catalysis: a roadmap for catalyst design. Chem. Soc. Rev 2016, 45 (21), 5803–5820. [DOI] [PubMed] [Google Scholar]
- (28).Le CC; Wismer MK; Shi Z-C; Zhang R; Conway DV; Li G; Vachal P; Davies IW; MacMillan DWCA General Small-Scale Reactor To Enable Standardization and Acceleration of Photocatalytic Reactions. ACS Cent. Sci 2017, 3 (6), 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Caspar JV; Meyer TJ Photochemistry of tris(2,2′-bipyridine)ruthenium(2+) ion (Ru(bpy)32+). Solvent effects. J. Am. Chem. Soc 1983, 105 (17), 5583–5590. [Google Scholar]
- (30).Shukla P; Narain G An Spectroscopic Investigation of Complex Formation in nickel sulphate-aliphatic amine system. J. Prakt. Chem 1968, 38 (5–6), 289–294. [Google Scholar]
- (31).Pavkovic SF; Rapp B Nickel complexes with long-chain primary amines. Inorg. Chem 1970, 9 (12), 2800–2801. [Google Scholar]
- (32).Lee-Thorp JA; Rüede JE; Thornton DA The infrared spectra (3500—150 cm-1) of aniline complexes of cobalt(II), nickel(II), copper(II) and zinc(II) halides. J. Mol. Struct 1978, 50 (1), 65–71. [Google Scholar]
- (33).Jansky MT; Yoke JT Hydrates and tertiary amine complexes of cobalt(II) and nickel(II) trifluoromethanesulfonates. J. Inorg. Nucl. Chem 1979, 41 (12), 1707–1709. [Google Scholar]
- (34).Palazón J; Gálvez J; García G; Lopez G Some complexes of nickel (II) with morpholine. Polyhedron 1983, 2 (12), 1353–1356. [Google Scholar]
- (35).Kenessey G; Carson BR; Allan JR; Wadsten T; Liptay G Primary aliphatic amine complexes of transition-metal halides. J. Therm. Anal 1997, 50 (1), 167–173. [Google Scholar]
- (36).Drago RS; Meek DW; Longhi R; Joesten MD Spectrochemical Studies of the Primary Alkylamine Complexes of Nickel(II) and an Evaluation of the Donor Properties of Amines. Inorg. Chem 1963, 2 (5), 1056–1060. [Google Scholar]
- (37).Cotton FA; Goodgame DML New Tetrahedral Complexes of Nickel(II). J. Am. Chem. Soc 1960, 82 (22), 5771–5774. [Google Scholar]
- (38).Goodgame DML; Goodgame M; Cotton FA Electronic Spectra of Some Tetrahedral Nickel(II) Complexes. J. Am. Chem. Soc 1961, 83 (20), 4161–4167. [Google Scholar]
- (39).Guo D; Knight TE; McCusker JK Angular Momentum Conservation in Dipolar Energy Transfer. Science 2011, 334 (6063), 1684. [DOI] [PubMed] [Google Scholar]
- (40).Howe ENW; Bhadbhade M; Thordarson P Cooperativity and Complexity in the Binding of Anions and Cations to a Tetratopic Ion-Pair Host. J. Am. Chem. Soc 2014, 136 (20), 7505–7516. [DOI] [PubMed] [Google Scholar]
- (41).Qiu J; Song B; Li X; Cozzolino AF Solution and gas phase evidence of anion binding through the secondary bonding interactions of a bidentate bis-antimony(iii) anion receptor. Phys. Chem. Chem. Phys 2018, 20 (1), 46–50. [DOI] [PubMed] [Google Scholar]
- (42).Thordarson P Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev 2011, 40 (3), 1305–1323. [DOI] [PubMed] [Google Scholar]
- (43).Brynn Hibbert D; Thordarson P The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun 2016, 52 (87), 12792–12805. [DOI] [PubMed] [Google Scholar]
- (44).Knight TE; Guo D; Claude JP; McCusker JK Energy Transfer Dynamics in ReI-Based Polynuclear Assemblies: A Quantitative Application of Förster Theory. Inorg. Chem 2008, 47 (16), 7249–7261. [DOI] [PubMed] [Google Scholar]
- (45).Knight TE; McCusker JK Orbital-Specific Energy Transfer. J. Am. Chem. Soc 2010, 132 (7), 2208–2221. [DOI] [PubMed] [Google Scholar]
- (46).Sartor SM; McCarthy BG; Pearson RM; Miyake GM; Damrauer NH Exploiting Charge-Transfer States for Maximizing Intersystem Crossing Yields in Organic Photoredox Catalysts. J. Am. Chem. Soc 2018, 140 (14), 4778–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy; Lakowicz JR, Ed; Springer US: Boston, MA, 2006; pp 1–26. [Google Scholar]
- (48).Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57 (24), 10257–10274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.