Abstract
Background
Oral Epstein-Barr virus (EBV) status reflects host EBV activity and potentially links to EBV-associated diseases, however, factors influencing oral EBV loads or reactivation, such as environmental exposures or host factors, are not fully understood.
Methods
A 2-stage, multicenter, cross-sectional study of 6558 subjects from 21 administrative cities of southern China and 3 populations from representative geographical areas in China (referred to as the south, north, and northeastern populations) was performed. The relationships between demographical factors and environmental exposures to EBV loads were analyzed by logistic regression models.
Results
Current smoking, with a dose-response effect, was found to be strongly associated with higher oral EBV loads in the pooled data, with an odds ratio of 1.58 (95% confidence interval, 1.39–1.79), as well as in each of the separate populations. The odds ratio increased to 3.06 when current smokers in southern China were compared to never smokers in northern China. Additionally, higher oral EBV loads tended to be detected in older participants, male participants, and participants in southern China.
Conclusions
This study provided evidence linking the effect of host-environmental factors, particularly smoking, to oral EBV activity. It could strengthen our understanding of the possible causal roles of EBV-related diseases, which may help to prevent or mitigate EBV-associated diseases.
Keywords: Oral EBV DNA loads, cigarette smoking, cross-sectional study, China
Our multicenter cross-sectional study in China provided evidence linking the effect of host-environmental factors to oral Epstein-Barr virus (EBV) activity. Higher oral EBV loads tended to be detected in smokers, older participants, male participants, and participants in southern China.
More than 95% of adults worldwide have been infected with Epstein-Barr virus (EBV), and in most the virus persists inside resting memory B cells asymptomatically [1, 2]. It has been suggested that Waldeyer’s ring is the most common and important site for EBV replication and persistence, which is characterized by low-level viral shedding in saliva. EBV can be reactivated irregularly during one’s lifetime through yet unclear mechanisms, exhibiting a switch from latent infection to lytic replication and resulting in large quantities of EBV particles releasing into saliva [3]. Saliva exchange is the main mode of EBV transmission from person to person [4].
According to the latest report, 2.2 million new cancer cases (15.4%) were attributable to carcinogenic pathogens worldwide, and EBV, as the fifth most common infectious cause of cancer, caused about 120000 new cases in 2012, including lymphoid and epithelial malignancies [5]. A prospective study found that children with higher EBV antibody levels against lytic EBV have a 30-fold higher risk of Burkitt lymphoma than those with low antibody levels [6]. Another cohort study showed that elevated anti-EBV antibody levels could be observed in patients with Hodgkin disease before diagnosis [7, 8]. Several long-term prospective studies of nasopharyngeal carcinoma (NPC) also concluded similarly that people who carried elevated levels of immunoglobulin A (IgA) to viral capsid antigen, IgA to early antigen, or DNase may have a 20–30-fold increased risk of NPC in EBV-endemic areas in southern China and Taiwan, and the serological window could be detected several years before NPC diagnosis [9, 10]. All of these findings consistently suggest that individuals with high lytic EBV antibody levels have greatly increased malignancy risks, revealing that EBV reactivation could be present prior to clinical onset. Given that EBV reactivation appears to play an important role in the pathogenesis of EBV-associated disease [11–17], determining the inducing factors would be helpful in the primary prevention of EBV reactivation, even to the EBV-associated diseases. Several agents, such as tetradecanoylphorbol acetate, phorbol ester, calcium ionophores, and some extracts of food containing mycotoxin aflatoxin B1, Cantonese-style salted fish, Chinese herbs have been reported to contribute to EBV reactivation [18–25]. However, most of these synthetic or natural inducers of EBV reactivation are reported on the basis of in vitro or in vivo experiments and lack support by epidemiological data.
The aberrant elevations in global serum anti-EBV antibody levels have served as indirect markers of EBV activity. To date, however, characteristics of oral EBV infection, which directly reflect EBV activity, and oral EBV activation–associated host-environment factors have not been systematically studied, partly because of the variability in oral EBV loads or limited sample sizes [26–28].
Here, we performed a large epidemiological study to systematically evaluate the associations between oral EBV loads and both demographical factors and environmental exposures among healthy carriers in China. A 2-stage, cross-sectional study including 4 independent cohorts was conducted, and oral EBV loads were analyzed separately and jointly. The epidemiological study of oral EBV loads could improve understanding of oral EBV infection status; person-to-person EBV transmission that results in primary infection in infants or young children, reinfection in adults, and coinfection with different EBV subtypes in adults; and possibly the etiology of some EBV-related diseases, particularly NPC, which is prevalent in southern China.
METHODS
Study Populations
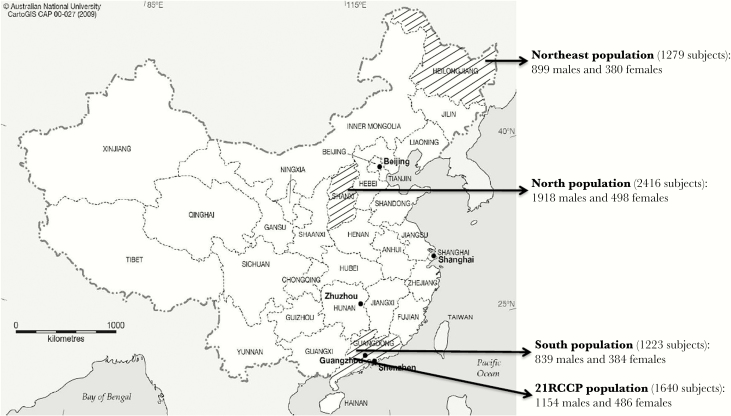
This project of the Chinese Environment, EBV, and Cancer Study (CEEC) was reviewed and approved by the Human Ethics Committee of Sun Yat-sen University Cancer Center. Written informed consent was obtained from each subject before the interview. Briefly, we conducted a 2-stage, multicenter study with 4 independent populations. One population, enrolled during the initial stage, was from 21 cities in Guangdong Province in southern China (defined as the 21RCCP population). The remaining 3 populations, enrolled during the validation stage, were from Sihui City in Guangdong Province (defined as the south population), Yangquan City in Shanxi Province (defined as the north population), and Mishan City in Heilongjiang Province (defined as the northeast population). The geographical distribution of the study populations is presented in Figure 1. All male and female individuals were eligible if they (1) were aged 20–80 years; (2) were free from any history of cancer, immunological diseases, or acute diseases; (3) were local residents who had lived in their ancestral home >10 years; and (4) could answer the questionnaire independently.
Figure 1.
The geographical distribution of the 4 independent populations in this study.
The 21RCCP population was taken from the healthy subjects reported in our previous study. Briefly, 2275 healthy controls were recruited from physical examination centers in 21 administrative cities in Guangdong Province between 1 October 2005 and 1 October 2007. This study included 1640 subjects with mouthwash samples; the sex of 1154 was male, the sex of 486 was female, and the mean age (±SD) was 46.40 ± 11.39.
To validate the findings in the 21RCCP population, we extended our study to 3 additional independent healthy populations. The south population was composed of healthy residents from several administrative villages in Sihui City who were enrolled between 1 October 2015 and 1 August 2016. Initially, 1234 healthy participants were recruited, but 11 participants were excluded because of a lack of samples. Of the 1223 enrolled subjects, the sex of 839 was male, the sex of 384 was female, and the mean age (±SD) was 46.89 ± 11.47 years. The north population was composed of healthy subjects who underwent physical examination in the First General Hospital of Yangquan City between 1 May and 1 October 2014. Initially, 2506 healthy volunteers were consecutively enrolled, but 90 subjects were excluded because they did not have saliva samples. Of the 2416 enrolled subjects, the sex of 1918 was male, the sex of 498 was female, and the mean age (±SD) was 46.74 ± 11.16 years. For the northeast population, healthy participants were enrolled from 2 locations. The first location was the General Hospital of Mishan City, where people underwent health check-ups, and the second location comprised several administrative villages in Mishan City. Healthy residents were recruited from both locations between 1 May and 1 September 2015. Initially, 1287 healthy participants were recruited, but 9 subjects were excluded because of a lack of samples. Of the 1279 enrolled subjects, the sex of 899 was male, the sex of 380 was female, and the mean age (±SD) was 46.17 ± 11.48 years.
Demographic and Behavioral Data Collection
All CEEC participants were asked to complete a comprehensive face-to-face interview conducted by well-trained investigators, using structured questionnaires. Demographic data (ie, age, sex, and education level) and history of cancer in first-degree relatives were collected. Data on environmental exposures, such as smoking, alcohol consumption, and consumption of preserved vegetables, were recorded. For the female participants in the north population, information about age, sex, education level, and smoking status were collected. Additionally, data on the consumption of the Cantonese dietary components of slow-cooked soup, tea, herbal tea, and salted fish were also collected specifically for the south population (Supplementary Materials).
Collection of Oral Samples, Extraction of EBV DNA, and Quantification of EBV DNA Loads
Saliva and mouthwash were recognized as 2 types of samples frequently used for oral EBV detection. For the 21RCCP population, 10-mL mouthwash samples were collected, and for the other 3 populations, 2–3-mL pure saliva samples were collected (Supplementary Materials). All samples were collected and processed by standard procedures. Oral EBV loads were quantified by real-time quantitative polymerase chain reaction (PCR) as the number of EBV DNA copies per milliliter in mouthwash or saliva specimens (Supplementary Materials) [29–31]. The sensitivity of real-time quantitative PCR (5 copies per reaction) was calculated by a standard serial dilution of DNA (Supplementary Figure 1). All samples were tested in duplicate by the same experimenters.
Statistical Analyses
Since the distribution of data on EBV loads in mouthwash or saliva samples were highly skewed, oral EBV loads were log10 transformed before analysis. In the comparison of EBV DNA loads among geographical areas, data from the 21RCCP population was not included because the quantity of virus loads in mouthwash specimens from this group was not comparable with that in saliva specimens from the 3 other populations. However, in the association analysis of oral EBV loads and risk factors, all data were included and treated as categorical data according to their corresponding median values. Univariate logistic regression and multivariable analysis were conducted to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for potential risk factors associated with higher oral EBV levels. Ordered logistic regression was used to assess the risk of higher salivary EBV loads among different populations by dividing the EBV loads from the 4 populations into quartiles. Additive interaction was calculated to analyze the synergistic effect between different geographical populations and smoking status on the risk of higher oral EBV loads. Considering the dramatic different NPC rates among different geographical areas, we combined the 21RCCP and south populations (defined as the southern China population), where the NPC incidence was 10–20 cases/100000, and we combined the north and northeast populations (defined as the northern China population), where the NPC incidence was <2 cases/100000, in the subgroup analysis. Analyses were performed in Stata 10.0 (Stata Corp, College Station, TX). The raw data in this article have been successfully uploaded and locked in the Research Data Deposit (number RDDA2018000552).
RESULTS
Geographical Difference of EBV DNA Loads
In total, 6558 subjects (1640 in the initial cross-sectional study and 4918 in the extended study) were included in this study. Oral EBV was detected in 78.88% of subjects (5173 of 6558). To find possible differences in salivary EBV levels among different geographical areas in China, median EBV DNA loads from populations in different areas were analyzed. We found that salivary EBV loads were higher in subjects in the south population, which has the highest risk of NPC among populations in this study, than in those in the north and northeast populations. The median log10-transformed EBV load was 5.58 copies/mL (interquartile range [IQR], 3.92–6.55 copies/mL) in the south population, 4.57 copies/mL (IQR, 2.89–6.08 copies/mL) in the northeast population, and 3.75 copies/mL (IQR, 0.00–5.40 copies/mL) in the north population. Ratios of the median EBV loads showed a 68-fold difference between the south population (3.83 × 105 copies/mL) and the north population (5.62 × 103 copies/mL) and a 10-fold difference between the south population and the northeast populations (3.71 × 104 copies/mL; Table 1).
Table 1.
Comparison of Salivary Epstein-Barr Virus (EBV) DNA Loads Among Healthy Subjects in Different Geographical Areas of China
| Salivary EBV Load | North Population (n = 2416) |
Northeast Population (n = 1279) |
South Population (n = 1223) |
|---|---|---|---|
| Overall, EBV NDA copies/mL, median (IQR)a | 3.75 (0.00–5.40) | 4.57 (2.89–6.08) | 5.58 (3.92–6.55) |
| <P 25,bsubjects, no. (%) | 809 (33.49) | 263 (20.56) | 156 (12.76) |
| P 25 to <P 50,bsubjects, no. (%) | 640 (26.49) | 356 (27.83) | 232 (18.97) |
| P 50 to <P 75,bsubjects, no. (%) | 581 (24.05) | 310 (24.24) | 345 (28.21) |
| ≥P 75,bsubjects, no. (%) | 386 (15.98) | 350 (27.37) | 490 (40.07) |
| OR (95% CI)c | 1.00 (reference) | 2.03 (1.79–2.31) | 3.80 (3.33–4.34) |
aMedians and interquartile ranges (IQRs) were used to describe the distribution of EBV loads among different populations, and log10-transformed numbers of EBV copies/mL of saliva was used since the original values were highly skewed.
bP25, P50, and P75 refer to the first quartile of 102.55, the median of 104.47, and third quartile of 105.95 EBV copies/mL of saliva, respectively, in the total population of 4918 subjects.
cOrdered logistic regression analyses were used to assess odds ratios (ORs) and 95% confidence intervals (CIs) by adjusting for age (continuous variable), sex (male or female), and education level (high school and less or university and greater).
Oral EBV DNA Loads and Demographic Factors
Several demographic factors, including age, sex, and education level, were associated with oral EBV levels in each of the 4 populations. Overall, log10-transformed median oral EBV loads in male subjects were higher than those in female subjects (4.78 copies/mL [IQR, 3.10–6.00 copies/mL] vs 3.60 copies/mL [IQR, 0–5.18 copies/mL]; adjusted OR, 2.05; 95% CI, 1.83–2.30). Oral EBV loads increased with age (P < .001). Subjects with higher education levels, especially those with bachelor’s degrees or higher, had lower oral EBV levels than those with low education levels of high school or less (adjusted OR, 0.88; 95% CI, .78–.98). No association was detected between family history of cancer and oral EBV load in this study (Table 2).
Table 2.
Associations Between Potential Risk Factors and Oral Epstein-Barr Virus (EBV) Levels Among Healthy Subjects in China
| Variablea | Subjects With Low Oral EBV Loads,b No. (%) |
Subjects With High Oral EBV Loads,b No. (%) |
OR (95% CI) | Adjusted OR (95% CI)c |
|---|---|---|---|---|
| Age, y | ||||
| ≤30 | 270 (57.20) | 202 (42.80) | 1.00 (reference) | 1.00 (reference) |
| 31–40 | 865 (52.78) | 774 (47.22) | 1.20 (.97–1.47) | 1.14 (.92–1.40) |
| 41–50 | 1106 (51.78) | 1030 (48.22) | 1.24 (1.02–1.52) | 1.15 (.93–1.41) |
| 51–60 | 715 (45.22) | 866 (54.78) | 1.62 (1.32–1.99) | 1.48 (1.19–1.83) |
| >60 | 321 (44.03) | 408 (55.97) | 1.70 (1.34–2.15) | 1.60 (1.26–2.04) |
| P trend d | <.001 | <.001 | ||
| Sex | ||||
| Female | 1102 (63.04) | 646 (36.96) | 1.00 (reference) | 1.00 (reference) |
| Male | 2175 (45.22) | 2635 (54.78) | 2.07 (1.85–2.31) | 2.05 (1.83–2.30) |
| Education level | ||||
| High school or less | 2284 (48.31) | 2444 (51.69) | 1.00 (reference) | 1.00 (reference) |
| University or greater | 968 (54.05) | 823 (45.95) | 0.79 (.71–.89) | 0.88 (.78–.98) |
| Family history of tumor | ||||
| No | 2713 (50.30) | 2681 (49.70) | 1.00 (reference) | 1.00 (reference) |
| Yes | 529 (48.27) | 567 (51.73) | 1.08 (.95–1.24) | 1.06 (.92–1.21) |
| Family history of NPC | ||||
| No | 3186 (49.97) | 3190 (50.03) | 1.00 (reference) | 1.00 (reference) |
| Yes | 56 (49.12) | 58 (50.88) | 1.03 (.71–1.50) | 1.11 (.76–1.61) |
| Cigarette smoking | ||||
| Never smoker | 1930 (57.46) | 1429 (42.54) | 1.00 (reference) | 1.00 (reference) |
| Former smoker | 314 (50.48) | 308 (49.52) | 1.32 (1.11–1.57) | 0.97 (.80–1.17) |
| Current smoker | 1026 (40.05) | 1536 (59.95) | 2.02 (1.82–2.24) | 1.58 (1.39–1.79) |
| P trend d | <.001 | <.001 | ||
| Alcohol consumption | ||||
| Nondrinker | 1493 (45.98) | 1754 (54.02) | 1.00 (reference) | 1.00 (reference) |
| ≤1 drink/day | 688 (51.08) | 659 (48.92) | 0.82 (.72–.93) | 0.74 (.65–.85) |
| >1 drink/day | 694 (47.86) | 756 (52.14) | 0.93 (.82–1.05) | 0.80 (.70–.91) |
| P trend d | .083 | <.001 | ||
| Preserved vegetable consumption | ||||
| Less than monthly | 2276 (47.52) | 2514 (52.48) | 1.00 (reference) | 1.00 (reference) |
| Monthly | 399 (45.39) | 480 (54.61) | 1.09 (.94–1.26) | 1.14 (.98–1.32) |
| Weekly or more | 190 (51.77) | 177 (48.23) | 0.84 (.68–1.04) | 0.83 (.67–1.03) |
| P trend d | .540 | .696 | ||
| Tea intake | ||||
| Less than monthly | 390 (41.01) | 561 (58.99) | 1.00 (reference) | 1.00 (reference) |
| Monthly | 309 (45.71) | 367 (54.29) | 0.83 (.68–1.01) | 0.81 (.66–1.00) |
| Weekly or more | 508 (41.20) | 725 (58.80) | 0.99 (.84–1.18) | 0.77 (.64–.93) |
| P trend d | .965 | .008 | ||
| Herbal tea intake | ||||
| Less than monthly | 262 (45.09) | 319 (54.91) | 1.00 (reference) | 1.00 (reference) |
| Monthly | 562 (39.63) | 856 (60.37) | 1.25 (1.03–1.52) | 1.26 (1.03–1.55) |
| Weekly or more | 376 (44.44) | 470 (55.56) | 1.03 (.83–1.27) | 1.00 (.81–1.25) |
| P trend d | .904 | .695 | ||
| Cantonese soup consumption | ||||
| Less than monthly | 50 (45.05) | 61 (54.95) | 1.00 (reference) | 1.00 (reference) |
| Monthly | 192 (36.02) | 341 (63.98) | 1.46 (.96–2.20) | 1.56 (1.02–2.38) |
| Weekly or more | 958 (43.57) | 1241 (56.43) | 1.06 (.72–1.56) | 1.15 (.78–1.70) |
| P trend d | .053 | .119 | ||
| Salted fish consumption | ||||
| Less than monthly | 1122 (42.27) | 1519 (57.73) | 1.00 (reference) | 1.00 (reference) |
| Monthly | 44 (35.48) | 80 (64.52) | 1.33 (.91–1.94) | 1.30 (.89–1.90) |
| Weekly or more | 45 (47.37) | 50 (52.63) | 0.81 (.54–1.23) | 0.80 (.52–1.22) |
| P trend d | .875 | .779 | ||
aData on Cantonese tea, Chinese herbal tea, slow-cooked soup, and salted fish were available only for the 21RCCP and south populations.
bOral EBV levels were divided into low and high levels according to the median number of EBV copies in saliva or mouthwash per milliliter. For mouthwash EBV loads in the 21RCCP population, a low EBV level refers to <104.55 copies/mL of mouthwash and a high EBV level refers to ≥104.55 copies/mL of mouthwash; for salivary EBV loads in the south, north, and northeast populations, a low EBV level refers to <104.47 copies/mL of saliva, and a high EBV level refers to ≥104.47 copies/mL of saliva.
cMultivariable logistic regression was used by adjusting for age (continuous variable), sex (male or female), and education level (high school and less or university and greater).
dLinear trends tests were performed by treating ordered categorical variables as continuous variables.
Current Smoking Enhances Oral EBV DNA Loads
Among the environmental factors we investigated, cigarette smoking was consistently associated with higher oral EBV levels, not only in the pooled data (adjusted OR, 1.58; 95% CI, 1.39–1.79) but also in each of the 4 populations. The relationship between smoking and higher oral EBV loads was only observed in current smokers and not in former smokers (Table 2 and Supplementary Table 1). To confirm the relationship between current smoking and higher oral EBV levels, we performed a subgroup analysis according to smoking status and found consistent and solid dose-response relationships between current smoking and higher oral EBV loads. Smokers with a longer smoking history, a greater number of cigarette packs smoked per day, a greater cumulative number of pack-years of smoking, and inhalation of smoke often had higher oral EBV levels both in the pooled data (Table 3) and in each of the 4 populations (Supplementary Table 2).
Table 3.
Dose-Response Between Cigarette Smoking and Oral Epstein-Barr Virus (EBV) Levels Among Healthy Subjects in China
| Variable | Current Smoker | Former Smoker | ||||
|---|---|---|---|---|---|---|
| Low/High EBV Loadsa | Adjusted OR (95% CI)b | P | Low/High EBV Loadsa | Adjusted OR (95% CI)b | P | |
| Age at smoking initiation, y | ||||||
| Never | 1930/1429 | 1.00 (reference) | 1930/1429 | 1.00 (reference) | ||
| ≥20 | 548/847 | 1.71 (1.48–1.98) | <.001 | 168/165 | 1.02 (.80–1.31) | .834 |
| <20 | 472/682 | 1.68 (1.43–1.97) | <.001 | 145/140 | 1.04 (.80–1.34) | .763 |
| P trend c | <.001 | .736 | ||||
| Daily smoking amount, packs | ||||||
| Never | 1930/1429 | 1.00 (reference) | 1930/1429 | 1.00 (reference) | ||
| <1 | 447/573 | 1.46 (1.25–1.72) | <.001 | 141/109 | 0.84 (.64–1.10) | .203 |
| ≥1 | 569/957 | 1.91 (1.64–2.21) | <.001 | 137/169 | 1.28 (1.00–1.65) | .054 |
| P trend c | <.001 | .169 | ||||
| Smoking duration, y | ||||||
| Never | 1930/1429 | 1.00 (reference) | 1930/1429 | 1.00 (reference) | ||
| ≤25 | 532/682 | 1.61 (1.37–1.88) | <.001 | 174/171 | 1.10 (.87–1.40) | .414 |
| >25 | 486/851 | 1.80 (1.54–2.11) | <.001 | 105/108 | 0.98 (.73–1.32) | .886 |
| P trend c | <.001 | .811 | ||||
| Cumulative amount smoked, pack-years | ||||||
| Never | 1930/1429 | 1.00 (reference) | 1930/1429 | 1.00 (reference) | ||
| <20 | 538/685 | 1.53 (1.32–1.79) | <.001 | 166/153 | 1.02 (.80–1.30) | .888 |
| ≥20 | 479/845 | 1.89 (1.62–2.21) | <.001 | 112/125 | 1.11 (.84–1.48) | .455 |
| P trend c | <.001 | .486 | ||||
| Smoking type, inhaled or not inhaled | ||||||
| Never | 1930/1429 | 1.00 (reference) | 1930/1429 | 1.00 (reference) | ||
| Did not inhale | 405/533 | 1.49 (1.26–1.75) | <.001 | 136/110 | 0.89 (.67–1.17) | .397 |
| Inhaled | 597/973 | 1.85 (1.60–2.14) | <.001 | 167/179 | 1.11 (.88–1.42) | .378 |
| P trend c | <.001 | 0.554 | .554 | |||
aOral EBV levels were divided into low and high levels according to the median number of EBV copies in saliva or mouthwash, per milliliter. For mouthwash EBV loads in the 21RCCP population, a low EBV level refers to <104.55 copies/mL of mouthwash and a high EBV level refers to ≥104.55 copies/mL of mouthwash; for salivary EBV loads in the south, north, and northeast populations, a low EBV level refers to <104.47 copies/mL of saliva, and a high EBV level refers to ≥104.47 copies/mL of saliva.
bLogistic regression analyses were used to assess odds ratios (ORs) and 95% confidence intervals (CIs) by adjusting for age (continuous variable), sex (male or female), education level (high school and less or university and greater), and alcohol consumption (nondrinker, ≤1 drink/day, or >1 drink/day).
cLinear trends tests were performed by treating ordered categorical variables as continuous variables.
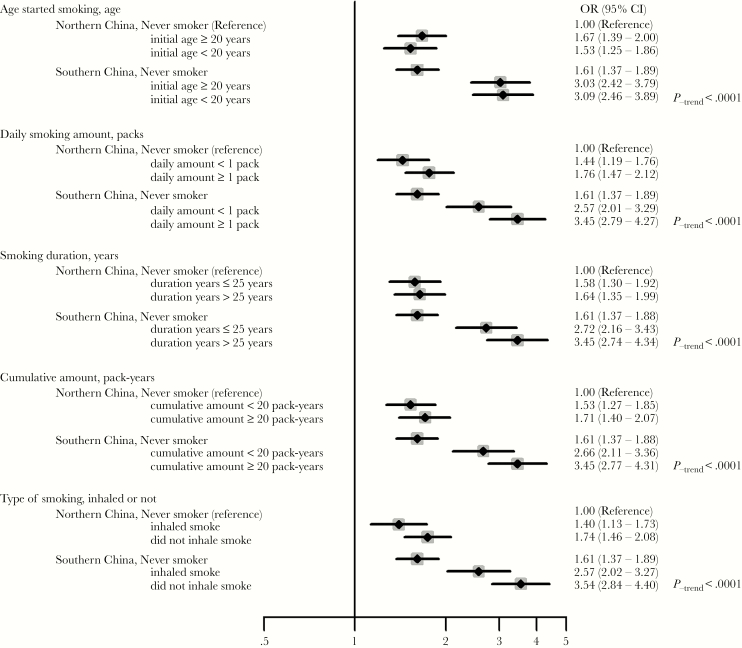
Moreover, we found a synergistic interaction between different geographical areas and smoking status on higher oral loads in an additive model (attributable proportion, 0.28 [95% CI, .14–.41]; synergism index, 1.69 [95% CI, 1.24–2.31]), although we did not find a similar interaction in the multiplicative model (Table 4). The risk of higher oral EBV loads for current smokers in southern China (ie, subjects in the 21RCCP and the south populations) were markedly increased, reaching levels 3-fold greater than the risk for never smokers in northern China (ie, subjects in the north and northeast populations; OR, 3.06; 95% CI, 2.55–3.67). More-detailed subgroup analyses between smoking status and higher oral EBV load risk among different geographical populations are presented in Figure 2.
Table 4.
Interaction Analysis of Smoking Status and Oral Epstein-Barr Virus (EBV) Levels Among Healthy Subjects in Different Geographical Areas of China
| Smoking Status |
Subjects in Northern Chinaa | Subjects in Southern Chinaa | ||||
|---|---|---|---|---|---|---|
| Low/High EBV Loads b | Adjusted OR (95%CI) c |
P | Low/High EBV Loads b | Adjusted OR (95%CI) c |
P | |
| Never | 1181/672 | 1.00 (reference) | / | 749/757 | 1.61 (1.37–1.89) | <.001 |
| Current | 711/815 | 1.61 (1.37–1.88) | <.001 | 315/721 | 3.06 (2.55–3.67) | <.001 |
| AP d | … | 0.28 (.14–.41) | … | … | ||
| SI d | … | 1.69 (1.24–2.31) | … | … | ||
aSubjects in northern China are from the north and northeast populations, where the annual incidence of nasopharyngeal carcinoma (NPC) was <1 case/100000 persons; subjects in southern China are from the 21RCCP and south populations, where the NPC incidence was >20 cases/100000 persons.
bOral EBV levels were divided into low and high levels according to the median number of EBV copies in saliva or mouthwash, per milliliter. For mouthwash EBV loads in the 21RCCP population, a low EBV level refers to <104.55 copies/mL of mouthwash and a high EBV level refers to ≥104.55 copies/mL of mouthwash; for salivary EBV loads in the south, north, and northeast populations, a low EBV level refers to <104.47 copies/mL of saliva, and a high EBV level refers to ≥104.47 copies/mL of saliva.
cLogistic regression analyses were used to assess odds ratios (ORs) and 95% confidence intervals (CIs) by adjusting for age (continuous variable), sex (male or female), education level (high school and less or university and greater), and alcohol consumption (nondrinker, ≤1 drink/day, or >1 drink/day).
dThe attributable proportion (AP) and synergism index (SI) due to additive interaction were calculated.
Figure 2.
The relationship between cigarette smoking and oral Epstein-Barr virus (EBV) DNA loads in populations from areas of differing risks of nasopharyngeal carcinoma (NPC), by smoking status. The southern China population includes the 21RCCP and south populations, where the NPC annual incidence was >20 cases/100000 persons, and the northern China population includes the north and northeast populations, where the NPC incidence was very rare (ie, <1 case/100000 persons). We stratified smoking categories by median values as the cutoffs for subgroup analysis (former smokers were excluded): age started smoking (≥20 vs <20 years), smoking amount per day (<1 vs ≥1 pack), smoking duration (≤25 vs >25 years), cumulative amount (<20 vs ≥20 pack-years), and smoking type (did not inhale vs inhaled). Unconditional multivariable logistic regression for a higher oral EBV load risk among different populations was calculated by adjusting age (continuous variables), sex (male and female), education level (high school and less vs university and greater), and alcohol consumption (nondrinker, ≤1 drink/day, and >1 drink/day), with never smokers in low-risk areas as the reference. All tests were 2-sided. Squares denote study-specific odds ratios (ORs), horizontal lines denote study-specific 95% confidence intervals (CIs), and the solid vertical line denotes an OR of 1.0.
Additionally, we found other factors associated with oral EBV loads in the pooled data. The consumption of herbal tea and Cantonese soup were positively associated with higher oral EBV loads, and the consumption of alcohol and tea were negatively associated with higher oral EBV loads, although we did not observe similar associations in each population separately (Table 2 and Supplementary Table 1).
DISCUSSION
Our study was a large-scale, multicenter epidemiological study to investigate the oral EBV loads among subjects in multiple independent healthy populations in China. To ensure the reliability and accuracy of the data, several steps have been taken in our study design. First, uniformly structured questionnaires and consecutive enrollment of participants were used throughout our cross-sectional studies. This may reduce possible bias due to nonrandom selection of subjects, recall bias, and reporting bias. This was evidenced by the nearly matched prevalence of current smoking between our study population (52.9% for male subjects and 1.2 % for female subjects) and findings from the Global Adult Tobacco Survey in China from the latest WHO report (52.9% and 2.4%, respectively). Second, the validity of the questionnaires was tested in 906 subjects from the 21RCCP population by comparison of self-reported smoking status with serum cotinine levels, an objective indicator of smoking. Self-reported smoking status was highly concordant with median serum cotinine levels, with values of 0.99 ng/mL (IQR, 0.60–1.53 ng/mL), 1.44 ng/mL (IQR, 0.88–2.75 ng/mL), and 143.92 ng/mL (IQR, 73.01–228.33 ng/mL) for never smokers, former smokers, and current smokers. Oral EBV loads were correlated with serum cotinine levels (P = .0008; data not shown). In addition, we established a reliable method to quantify the oral EBV loads by using a quantitative PCR method. Until now, it was difficult to compare EBV loads across different studies owing to the lack of a uniform reference standard for oral EBV quantification. In this study, we designed efficient primers and a probe targeting the EBV BamHI-W genomic region and successfully improved the EBV DNA detection sensitivity to 5 copies/reaction. Since EBV can be reactivated periodically and virus shedding is relatively stable over short periods but fluctuates through 3.5–5.5 logs over longer periods, it is difficult to describe the characteristics of oral EBV without an epidemiological study design with an adequate sample size. A multicenter cross-sectional study with a large sample size is a feasible strategy to describe the factors associated with oral EBV loads. There is no doubt that performance of a large-scale prospective study with a dynamic analysis of the oral EBV loads at different time points during long periods would be very meaningful in the future [26–28, 32].
With reliable data, the risk factors of environmental exposures that could affect oral EBV loads or reactivation were determined. The strong epidemiological evidence of geographical and smoking history and its correlation with high oral EBV loads provides important information for clarifying the pathogenesis of EBV-associated diseases such as NPC in southern China, Southeast Asia, and North Africa. Previous studies have reported that smoking was associated with many EBV-associated diseases. The established mechanisms of cigarette smoking in cancer development are the exposure to carcinogens, the formation of covalent bonds between the carcinogens and DNA adducts, and the resulting permanent mutations in critical genes of somatic cells and so on [33]. It has been reported that PI3K and MAPK pathways play an important role in recurrent chemical reactivations of EBV, which promotes genome instability and enhances tumor progression [17]. Given that EBV reactivation appears to play an important role in the pathogenesis of EBV-associated diseases [15, 16], we think smoking-induced EBV reactivation could contribution to the pathogenesis of EBV-associated diseases.
The large variation in oral EBV loads among individuals, from undetectable levels (<5 copies in this study) to several billion copies/mL, was consistent with previous findings of virus shedding dynamics in healthy carriers [32]. However, oral EBV DNA loads have been seldom reported from other populations, especially those with a large sample size. Nevertheless, there are still several interesting studies. In a study from the United States [32], 2 subjects were asked to rinse repeatedly with 5 mL of fluid, and EBV copies in 8 repeated rinses were detected. The average virus per rinse was 4.5 × 105 copies/5 mL for subject 1 and 8.5 × 107 copies/5 mL for subject 2. The EBV DNA loads ranged from 104 to 107 copies/mL, which was quite comparable with our results. Another study in Japan did not detect oral EBV loads, but they reported a prevalence of salivary oral EBV of 90% in 48 adults aged 21–57 years, compared with 78.88% in our study [34]. We observed a difference in both salivary EBV detection rates (69.54%, 82.49%, and 90.76%) and median salivary EBV loads (3.83 × 105, 3.71 × 104, and 5.62 × 103 copies/mL) among participants from the north, northeast, and south populations. The salivary EBV loads in the south population, where NPC is endemic, were higher than those in the northeast and north populations, where NPC is nonendemic. Moreover, higher oral EBV loads were found in male subjects, older individuals, and those with lower education levels, which may be related to chronic interpersonal stress, relatively unhealthy environments, or weakened host immune systems in these populations [35]. It is noteworthy that all of the above results suggest that EBV tends to be reactivated in the host oral cavity in subgroups of populations that are at high risk for NPC. We therefore speculate that the high oral EBV loads in these populations may contribute to the incidence of NPC, although elsewhere it was suggested that establishment of a latent and transforming infection in epithelial cells is potentially an important causative factor for the development of NPC [36]. Nevertheless, our study provides strong epidemiological evidence for differences in oral EBV loads between populations in NPC-endemic and nonendemic regions and supports the need for further investigations into the possible role of oral EBV biology in NPC.
EBV reactivation can be triggered by many factors, including various chemical agents or biological stimuli in vitro or in vivo [18–25, 37]. Interestingly, in this epidemiological study, we found a dose-dependent relationship between current smoking and higher oral EBV DNA loads in all populations (Ptrend < .001). EBV in the host oral cavity tended to be reactivated in the current-smoker subgroup, which was consistent with the reported subpopulations with a high incidence of EBV-associated diseases [38–40]. Moreover, the EBV loads were lower in those who quit smoking, suggesting that smoking cessation might create unfavorable conditions for EBV reactivation. In our previous study, we described the relationship between smoking and elevated levels of serum IgA to viral capsid antigen, the indirect marker of host immunity to EBV lytic replication, and the cell biology assays in that study provided direct evidence that cigarette smoke extract could induce EBV reactivation in vitro [41]. Recently, a prospective study conducted in Hong Kong reported similar results that cigarette smoking was associated with plasma EBV DNA loads [42]. Whether smoking may lead to higher levels of EBV reactivation systemically needs to be further investigated. Here, we provided population-based evidence that environmental inducers for oral EBV reactivation, such as smoking, indeed exist. Although the underlying mechanisms of smoking-related virus reactivation are not fully understood, some earlier studies suggested that impaired immunity of smokers could interfere with the ability of the host to clear virus infection [43, 44]. However, our data indicate that smoking might directly trigger EBV reactivation and increase the chance of EBV infection of nasopharyngeal epithelial cells.
In addition to current smoking, other risk factors, such as consumption of herbal tea and Cantonese soup with herbs, were also found to be associated with higher oral EBV loads in our study. A previous in vitro study found that some Chinese herbs could induce EBV expression in B cells [24]. Herbal tea includes complex components, and some components had been reported to participate in EBV reactivation. For example, phorbol ester (tetradecanoylphorbol acetate), which exists in many plants of the family Euphorbiaceae and can be used as a traditional Chinese herbal medicine [45], has been widely reported to induce EBV reactivation via NF-kB and AP-1 as regulated by PKC and MAPK [46]. Interestingly, the consumption of tea was associated with lower oral EBV loads in the 21RCCP and south populations, and a previous study found that epigallocatechin-3-gallate, the most abundant catechin in tea, inhibited EBV lytic replication in vitro [13]. Additionally, we found that consumption of alcohol was associated with lower oral EBV loads. There are other factors, such as exercise, stress, and occasional infections, that could induce EBV reactivation [47–49]. However, because this information was not collected in this study, we could not adjust for them as covariates in the analysis.
Together, these data strongly support further investigation of the roles of environmental factors in EBV reactivation. We recommended behavioral and dietary changes, even chemoprevention, to reduce the risk of EBV reactivation.
Supplementary Material
Notes
Acknowledgments. We thank Hui-Jun Li (First General Hospital of Yangquan city), Qi-Hong Huang (Sihui Institute for Cancer Prevention and Control), Hong-Shen (General Hospital of Mishan city), and Xiu-Hua Yu (General Hospital of Mishan city) for their contributions in the recruitment of the study population.
Financial support. This work was supported by the National Key Research and Development Program of China (grant 2016YFC1302704); the National Key Research and Development Program of China (grant 2016YFC0902001); the Sino-Sweden Joint Research Program (grant 81861138006); the Science and Technology Planning Project of Guangzhou, China (grant 201804020094); the Special Support Program for High-level Professionals on Scientific and Technological Innovation of Guangdong Province, China (grant 2014TX01R201); the National Science Fund for Distinguished Young Scholars of China (grant 81325018); the Key Project for International Cooperation and Exchange of the National Natural Science Foundation of China (grant 81220108022); and the Science and Technology project of Guangdong Province (grant 2014B050504004
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity 1998; 9:395–404. [DOI] [PubMed] [Google Scholar]
- 2. Henle G, Henle W, Clifford P, et al. . Antibodies to Epstein-Barr virus in Burkitt’s lymphoma and control groups. J Natl Cancer Inst 1969; 43:1147–57. [PubMed] [Google Scholar]
- 3. Niederman JC, Miller G, Pearson HA, Pagano JS, Dowaliby JM. Infectious mononucleosis. Epstein-Barr-virus shedding in saliva and the oropharynx. N Engl J Med 1976; 294:1355–9. [DOI] [PubMed] [Google Scholar]
- 4. Morgan DG, Niederman JC, Miller G, Smith HW, Dowaliby JM. Site of Epstein-Barr virus replication in the oropharynx. Lancet 1979; 2:1154–7. [DOI] [PubMed] [Google Scholar]
- 5. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Global Health 2016; 4:e609–16. [DOI] [PubMed] [Google Scholar]
- 6. de-Thé G, Geser A, Day NE, et al. . Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature 1978; 274:756–61. [DOI] [PubMed] [Google Scholar]
- 7. Goldman JM, Aisenberg AC. Incidence of antibody to EB virus, herpes simplex, and cytomegalovirus in Hodgkin’s disease. Cancer 1970; 26:327–31. [DOI] [PubMed] [Google Scholar]
- 8. Mueller N, Evans A, Harris NL, et al. . Hodgkin’s disease and Epstein-Barr virus. Altered antibody pattern before diagnosis. N Engl J Med 1989; 320:689–95. [DOI] [PubMed] [Google Scholar]
- 9. Cao SM, Liu Z, Jia WH, et al. . Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One 2011; 6:e19100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chien YC, Chen JY, Liu MY, et al. . Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 2001; 345:1877–82. [DOI] [PubMed] [Google Scholar]
- 11. Fagin U, Nerbas L, Vogl B, Jabs WJ. Analysis of BZLF1 mRNA detection in saliva as a marker for active replication of Epstein-Barr virus. J Virol Methods 2017; 244:11–6. [DOI] [PubMed] [Google Scholar]
- 12. Ma SD, Hegde S, Young KH, et al. . A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol 2011; 85:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu S, Li H, Chen L, et al. . (-)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis 2013; 34:627–37. [DOI] [PubMed] [Google Scholar]
- 14. Arvey A, Tempera I, Tsai K, et al. . An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe 2012; 12:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu CC, Fang CY, Huang SY, Chiu SH, Lee CH, Chen JY. Perspective: contribution of Epstein-Barr virus (EBV) reactivation to the carcinogenicity of nasopharyngeal cancer cells. Cancers 2018; 10: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li H, Liu S, Hu J, et al. . Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis. Int J Biol Sci 2016; 12:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang CY, Lee CH, Wu CC, et al. . Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int J Cancer 2009; 124:2016–25. [DOI] [PubMed] [Google Scholar]
- 18. Accardi R, Gruffat H, Sirand C, et al. . The mycotoxin aflatoxin B1 stimulates Epstein-Barr virus-induced B-cell transformation in in vitro and in vivo experimental models. Carcinogenesis 2015; 36:1440–51. [DOI] [PubMed] [Google Scholar]
- 19. Ben-Sasson SA, Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer 1981; 28:131–5. [DOI] [PubMed] [Google Scholar]
- 20. Eliasson L, Kallin B, Patarroyo M, Klein G, Fujiki H, Sugimura T. Activation of the EBV-cycle and aggregation of human blood lymphocytes by the tumor promoters teleocidin, lyngbyatoxin A, aplysiatoxin and debromoaplysiatoxin. Int J Cancer 1983; 31:7–11. [DOI] [PubMed] [Google Scholar]
- 21. Faggioni A, Zompetta C, Grimaldi S, Barile G, Frati L, Lazdins J. Calcium modulation activates Epstein-Barr virus genome in latently infected cells. Science 1986; 232:1554–6. [DOI] [PubMed] [Google Scholar]
- 22. Jiang JH, Wang N, Li A, et al. . Hypoxia can contribute to the induction of the Epstein-Barr virus (EBV) lytic cycle. J Clin Virol 2006; 37:98–103. [DOI] [PubMed] [Google Scholar]
- 23. Luka J, Kallin B, Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 1979; 94:228–31. [DOI] [PubMed] [Google Scholar]
- 24. Zeng Y, Zhong JM, Ye SQ, et al. . Screening of Epstein-Barr virus early antigen expression inducers from Chinese medicinal herbs and plants. Biomed Environ Sci 1994; 7:50–5. [PubMed] [Google Scholar]
- 25. Shao YM, Poirier S, Ohshima H, et al. . Epstein-Barr virus activation in Raji cells by extracts of preserved food from high risk areas for nasopharyngeal carcinoma. Carcinogenesis 1988; 9:1455–7. [DOI] [PubMed] [Google Scholar]
- 26. Yao QY, Rickinson AB, Epstein MA. A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int J Cancer 1985; 35:35–42. [DOI] [PubMed] [Google Scholar]
- 27. Haque T, Crawford DH. PCR amplification is more sensitive than tissue culture methods for Epstein-Barr virus detection in clinical material. J Gen Virol 1997; 78 (Pt 12):3357–60. [DOI] [PubMed] [Google Scholar]
- 28. Ling PD, Lednicky JA, Keitel WA, et al. . The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: a 14-month longitudinal study. J Infect Dis 2003; 187:1571–80. [DOI] [PubMed] [Google Scholar]
- 29. Lo YM, Chan LY, Lo KW, et al. . Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 1999; 59:1188–91. [PubMed] [Google Scholar]
- 30. Zheng XH, Lu LX, Li XZ, Jia WH. Quantification of Epstein-Barr virus DNA load in nasopharyngeal brushing samples in the diagnosis of nasopharyngeal carcinoma in southern China. Cancer Sci 2015; 106:1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xue WQ, He YQ, Liao XY, et al. . Decreased oral Epstein-Barr virus DNA loads in patients with nasopharyngeal carcinoma in Southern China: A case-control and a family-based study. Cancer Med 2018. doi: 10.1002/cam4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog 2009; 5:e1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg 2006; 391:603–13. [DOI] [PubMed] [Google Scholar]
- 34. Ikuta K, Satoh Y, Hoshikawa Y, Sairenji T. Detection of Epstein-Barr virus in salivas and throat washings in healthy adults and children. Microbes Infect 2000; 2:115–20. [DOI] [PubMed] [Google Scholar]
- 35. Fagundes CP, Jaremka LM, Glaser R, et al. . Attachment anxiety is related to Epstein-Barr virus latency. Brain Behav Immun 2014; 41:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol 2015; 235:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer 1984; 33:27–32. [DOI] [PubMed] [Google Scholar]
- 38. Lin JH, Jiang CQ, Ho SY, et al. . Smoking and nasopharyngeal carcinoma mortality: a cohort study of 101,823 adults in Guangzhou, China. BMC Cancer 2015; 15:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamper-Jørgensen M, Rostgaard K, Glaser SL, et al. . Cigarette smoking and risk of Hodgkin lymphoma and its subtypes: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph). Ann Oncol 2013; 24:2245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hernán MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol 2001; 154:69–74. [DOI] [PubMed] [Google Scholar]
- 41. Xu FH, Xiong D, Xu YF, et al. . An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J Natl Cancer Inst 2012; 104:1396–410. [DOI] [PubMed] [Google Scholar]
- 42. Chan KCA, Chu SWI, Lo YMD. Ambient temperature and screening for nasopharyngeal cancer. N Engl J Med 2018; 378:962–3. [DOI] [PubMed] [Google Scholar]
- 43. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 2012; 91:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barton SE, Maddox PH, Jenkins D, Edwards R, Cuzick J, Singer A. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change?Lancet 1988; 2:652–4. [DOI] [PubMed] [Google Scholar]
- 45. Tomei LD, Noyes I, Blocker D, Holliday J, Glaser R. Phorbol ester and Epstein-Barr virus dependent transformation of normal primary human skin epithelial cells. Nature 1987; 329:73–5. [DOI] [PubMed] [Google Scholar]
- 46. Gao X, Ikuta K, Tajima M, Sairenji T. 12-O-tetradecanoylphorbol-13-acetate induces Epstein-Barr virus reactivation via NF-kappaB and AP-1 as regulated by protein kinase C and mitogen-activated protein kinase. Virology 2001; 286:91–9. [DOI] [PubMed] [Google Scholar]
- 47. Glaser R, Pearson GR, Jones JF, et al. . Stress-related activation of Epstein-Barr virus. Brain Behav Immun 1991; 5:219–32. [DOI] [PubMed] [Google Scholar]
- 48. Stowe RP, Pierson DL, Barrett AD. Elevated stress hormone levels relate to Epstein-Barr virus reactivation in astronauts. Psychosom Med 2001; 63:891–5. [DOI] [PubMed] [Google Scholar]
- 49. Bennett JM, Glaser R, Malarkey WB, Beversdorf DQ, Peng J, Kiecolt-Glaser JK. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav Immun 2012; 26:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.