Abstract
Almost 800 million people are chronically undernourished worldwide, of whom 98% are in low- and middle-income countries where tuberculosis is endemic. In many tuberculosis-endemic countries, undernutrition is a driver of tuberculosis incidence and associated with a high population attributable fraction of tuberculosis and poor treatment outcomes. Data suggest that undernutrition impairs innate and adaptive immune responses needed to control Mycobacterium tuberculosis infection and may affect responses to live vaccines, such as BCG. Given its impact on tuberculosis, addressing undernutrition will be a vital component of the World Health Organization End TB strategy. This narrative review describes the effect of undernutrition on the immune response, vaccine response, and tuberculosis incidence, severity, and treatment outcomes.
Keywords: Undernutrition, tuberculosis, nutrition disorders, body mass index, latent Mycobacterium tuberculosis infection
Undernutrition, which affects almost 800 million people worldwide, impairs innate and adaptive immune responses against Mycobacterium tuberculosis infection. This narrative review describes the effect of undernutrition on the immune response, vaccine response, and tuberculosis incidence, severity, and treatment outcomes.
Mycobacterium tuberculosis, the bacterium that causes tuberculosis, is regarded as the most lethal infectious organism in the world. In 2016, 10.4 million people developed tuberculosis and 1.3 million people died from the disease [1]. The 2017 report by the Food and Agriculture Organization estimated that 815 million individuals are undernourished, with the majority living in low- and middle-income counties, particularly in sub-Saharan Africa and Southeast Asia [2]. These regions also have high rates of tuberculosis [1]. This geographical overlap makes it important to understand the relationship between undernutrition and tuberculosis. In fact, the World Health Organization (WHO) estimates that undernutrition is responsible for twice the number of tuberculosis cases as human immunodeficiency virus (HIV) globally [1]. Therefore, the WHO End TB strategy, which aims to decrease tuberculosis incidence by 90% and tuberculosis mortality by 95% by 2035, is unlikely to succeed without targeting undernutrition [3].
There has been a recent rise in studies of tuberculosis and undernutrition. A PubMed search of “tuberculosis and malnutrition” found 651 articles published between 2008–2018; selected relevant articles on the topic were evaluated from the PubMed search, and references from key articles were reviewed. This narrative review aims to delineate what is known about the effect and mechanism of undernutrition on tuberculosis incidence, severity, and outcomes.
As the effect of micronutrient deficiency on tuberculosis has been well summarized elsewhere, this review focuses on undernutrition particularly related to protein and calories and its impact on tuberculosis [4]. Protein undernutrition can be studied as an isolated entity in animal models since nutrients can be selectively withdrawn from the diet of experimental animals. However, common anthropometric measures of nutrition used in human studies cannot single out one particular nutrient deficiency. Indeed, micronutrient deficiencies often coexist with protein undernutrition [5]. Hence, the term “undernutrition” is used while reviewing epidemiological studies.
MEASURES OF NUTRITION
A variety of methods exist to measure the complex condition of undernutrition. In adults, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) is used most commonly and is categorized as severely underweight (<16), underweight (16–18.4), normal weight (18.5–24.9), overweight (25–29.9), and obese (≥30) [6]. BMI has limitations as it does not differentiate adipose tissue, muscle, water, or bone content. Body composition in undernourished adults can be assessed using a variety of alternative techniques, such as mid-upper arm circumference (MUAC), triceps skin-fold thickness, bioelectrical impedance analysis, and dual-energy X-ray absorptiometry [6, 7]. However, these methods are not as commonly used as BMI and MUAC.
In children, measures of nutritional status are commonly standardized to global norms [6]. Acute malnutrition can be defined as deviation from median growth standards. The WHO categorizes severe acute malnutrition as having a weight-for-height z score 3 SDs below the median. MUAC can also be used to assess acute undernutrition in children during 6–59 months of age. Manifestations of acute undernutrition include marasmus (characterized by wasting) and kwashiorkor (characterized by periorbital and peripheral edema attributed to hypoalbuminemia). Edema invalidates metrics such as weight-for-height z score and signifies severe malnutrition. Chronic undernutrition in children can result in stunting (quantified by the height-for-age z score). Although acute malnutrition is responsive to refeeding, stunting is difficult to reverse. Differences in the measures used to define undernutrition make pooling studies and comparing results challenging and should be considered when interpreting published results.
Impact of Undernutrition on Host Immunity and Vaccine Response
Undernutrition and Host Immunity
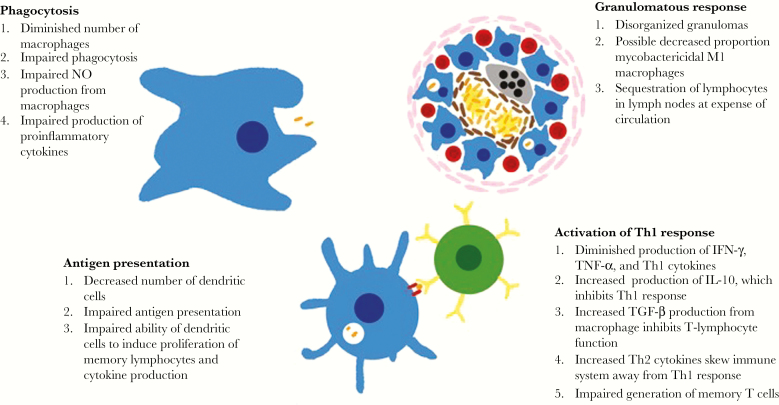
Undernutrition is thought to be the most common cause of secondary immunodeficiency worldwide (Figure 1) [8]. Animal models have yielded important insights about the impact of undernutrition in the immune response against M. tuberculosis [9]. Phagocytosis of M. tuberculosis by alveolar macrophages is the first step in the immune response. Peritoneal macrophages in mice fed a low-protein diet have been shown to have decreased mobility, chemotaxis, attachment, and ingestion of opsonized Candida albicans [10].
Figure 1.
Undernutrition blunts both adaptive and immune responses against Mycobacterium tuberculosis through multiple mechanisms. IFN-γ, interferon γ; IL-10, interleukin 10; NO, nitric oxide; Th1, T-helper type 1; Th2, T-helper type 2; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
Macrophages produce proinflammatory cytokines such as tumor necrosis factor α (TNF-α) to stimulate the inflammatory response to pathogens. TNF-α acts through tumor necrosis factor receptor 1 (TNFR1) to initiate a cascade of events that includes activation of nuclear factor κB (NF-κB), which promotes production of cytokines and chemokines while also inducing expression of adhesion molecules and inducible nitric oxide synthase (iNOS) [11]. Among mice fed an isocaloric diet containing 83% less protein than that fed to control animals, decreased expression of TNFR1 in peritoneal macrophages was observed after 28 days [11]. Protein-deficient animals also showed diminished NF-κB phosphorylation and decreased production of downstream cytokines (interleukin 1β [IL-1β] and interleukin 12 [IL-12]) after stimulation with TNF-α. Contrasting results have been reported, however, which may be explained by the macrophage populations used (bone marrow versus peritoneal), their differing ability to phagocytose, and the diets fed to the mice [12].
Cytokines play an important role in the regulation of adaptive immunity. Consistent with changes to the innate cytokine response, mice fed a protein-deficient diet had different clinical and immune responses after exposure to M. tuberculosis than mice fed an isocaloric protein-sufficient diet. While only 20% of the protein-sufficient mice died, 100% of the protein-deficient mice died of tuberculosis [13]. Deficits in innate immunity likely underpin this difference in outcome. Mice fed the protein-deficient diet that were exposed to M. tuberculosis had higher mycobacterial burdens and disorganized granulomas [13]. The undernourished mice were shown to have lower production of NOS2, IFN-γ, and TNF-α, cytokines important for antimycobacterial activity in the granuloma. These effects on the immune system could be reversed by feeding the protein-deficient mice a normal diet for 5–10 days. Protein-deficient guinea pigs exposed to M. tuberculosis also showed small, poorly defined granulomas but were capable of forming large and well-defined granulomas when they received intraperitoneal injection of T-cell–enriched lymphocytes from the bronchotracheal lymph nodes of well-nourished guinea pigs that had been exposed to M. tuberculosis [14]. Studies of the microenvironment of tuberculosis granulomas from mice suggest that M1 macrophages expressing NOS2 restrict growth of intracellular M. tuberculosis, while M2 macrophages expressing arginase are permissive to M. tuberculosis growth [15]. The impact of undernutrition on the distribution of M1 and M2 macrophages is unknown.
By presenting antigen to T cells and B cells, dendritic cells serve as emissaries between the innate and adaptive systems. Mice starved for 48 hours, compared with well-nourished controls, had significantly fewer liver and spleen dendritic cells [16]. Additionally, dendritic liver cells from starved mice produced lower levels of IL-12 in response to hepatitis B virus surface antigen (HBsAg) and were unable to induce interferon γ (IFN-γ) production from HBsAg-specific memory lymphocytes. Furthermore, liver dendritic cells from starved mice also did not induce proliferation of HBsAg-specific memory lymphocytes.
Once activated by dendritic cells, T cells are critical in the adaptive immune response. CD4+ T cells produce important cytokines such as interleukin 2 (IL-2) and IFN-γ and coordinate the T-helper type 1 (Th1) response, which leads to granuloma formation. CD8+ T cells also produce cytokines and have cytotoxic functions. Compared with guinea pigs fed a 30% ovalbumin diet, those fed a protein-deficient 10% ovalbumin diet had increased numbers of CD4+ and CD8+ T cells in their bronchotracheal lymph node but had marked reductions in CD4+ and CD8+ lymphocytes in the blood and the spleen [17]. Sequestration of lymphocytes in the lymph nodes at the expense of the peripheral circulation may explain the deficient immune response in undernourished animals. Furthermore, studies have shown that alveolar macrophages in protein-deficient guinea pigs produce higher quantities of transforming growth factor β, which suppresses T-lymphocyte function in infected animals [9].
In contrast to the wealth of literature on undernutrition and immunity in animals, there are relatively few human studies investigating the effects of undernutrition on immunity. Necropsies of severely malnourished children with kwashiorkor and marasmus who died of infectious diseases revealed thymolymphatic atrophy that resembled atrophy seen in animals that had undergone neonatal thymectomies, resulting in the notion of a “nutritional thymectomy” [18]. In malnourished children, thymic atrophy is reversible with nutritional repletion but takes longer to normalize than anthropometric markers, such as BMI [19].
Consistent with the idea that undernutrition impairs cytokine levels, human studies have shown that undernourished individuals had decreased concentrations of Th1 cytokines and increased concentrations of Th2 cytokines. Skewing the cytokine response away from Th1 differentiation could impair the defense against intracellular pathogens such as M. tuberculosis. In a small study that compared 5 undernourished children who were hospitalized with respiratory or gastrointestinal illness to 12 well-nourished children with similar illnesses and 11 well-nourished children without illnesses, expression of cytokines necessary for the Th1 response (IL-2 and IFN-γ) was found to be reduced in the lymphocytes of undernourished children. Diminished IL-2 production was attributed to diminished populations of IL-2–producing CD45RO+ memory cells. Conversely, interleukin 4 and IL-10, cytokines associated with the Th2 and T-regulatory cell responses were increased in the undernourished children [20]. Additionally, undernourished children in the study had diminished intensity of CD69 and CD25 in immunofluorescence studies, when compared to the well-nourished controls, which suggested impaired T-cell activation. Another small study compared 16 well-nourished children hospitalized with bacterial respiratory or gastrointestinal illnesses to 25 children with varying degrees of malnutrition and found decreased production of Th1 cytokines (IL-12, IL-18, and IL-21) in the undernourished children [21]. Greater undernourishment, as determined by anthropometric means, was associated with greater suppression in cytokines.
Little has been done to investigate the impact of undernutrition on the human immune response to M. tuberculosis in particular. In a study of 56 South Indian adults with latent M. tuberculosis infection as determined by a tuberculin skin test (TST) or IFN-γ–release assay, individuals with a BMI <18.5 had diminished circulating levels of IFN-γ and TNF-α as compared to individuals with a BMI >18.5. Additionally, induction of IFN-γ, TNF-α, interleukin 6, IL-1α, and IL-1β in response to M. tuberculosis antigens (ESAT-6, CFP-10, and TB 7.7) was diminished in the undernourished adults. By contrast, circulating levels of some type 2 cytokines (interleukin 5 and interleukin 13), transforming growth factor β, and IL-10 were increased [22].
Undernutrition and Vaccine Response
Millions of infants across the world receive BCG vaccine every year, and several novel tuberculosis vaccine trials are ongoing [23]. Therefore, it is important consider how undernutrition affects the vaccine response. Malnourished mice inoculated with BCG had increased mycobacterial dissemination to lymph nodes and the thymus than well-fed mice, which raises concern for the development of immune tolerance to M. tuberculosis and consequently a poor pathogen-specific T-cell response [24]. Protein deficiency during or after vaccination also blunted the efficacy of the H56-CAF01 subunit vaccine in mice, which could be reversed through protein supplementation [25]. Splenocytes derived from guinea pigs receiving a protein-deficient diet and vaccinated with M. tuberculosis H37Ra were capable of activating macrophages from well-nourished guinea pigs but did not activate autologous macrophages in vitro, which indicated that protein deficiency had impaired generation of antigen-specific lymphocyte responses [26].
Several studies have shown that undernutrition in humans is associated with a delay in reactivity to TST following BCG vaccination [27]. The delay in TST reactivity and diminished size of induration is suggestive of impaired cell-mediated immunity. Severity of malnutrition correlated with greater diminution of TST reactivity [28]. However, these studies were heterogeneous and conducted in different countries with different measures of nutritional status, and some studies showed no effect of undernutrition on TST reactivity after BCG vaccination [29].
The impact of undernutrition on the response to new tuberculosis vaccines will depend on vaccine formulation. Malnourished persons can generally mount effective responses to protein, subunit, killed, and polysaccharide vaccines, as recently reviewed [24]. Of note, the impact of undernutrition on vaccine response is confounded by coincident micronutrient deficiencies, malabsorption, and infections (eg, diarrhea) and is affected by route of delivery [30]. Therefore, the nutritional status of vaccine recipients must be a consideration in the ongoing efforts to develop an effective tuberculosis vaccine.
UNDERNUTRITION AND TUBERCULOSIS EPIDEMIOLOGY
Undernutrition and Tuberculosis Incidence
For >70 years, studies have suggested an association between undernutrition and the progression from M. tuberculosis infection to tuberculosis. In German prisoner camps during World War II, British soldiers consumed nutritional supplements from the Red Cross that yielded a daily caloric intake that was twice that for Soviet soldiers and included thirty extra grams of animal proteins in milk, meat, and fish. Soviet soldiers had an almost 16-fold higher incidence of tuberculosis (19.0% vs 1.2%) [31]. The British soldiers were more capable of producing granulomas. Soviet soldiers were more likely to develop large cavities and die of miliary tuberculosis within 6 months.
Annual chest radiography, TSTs, contact investigations, and hospital admission records of US Navy recruits during 1949–1955 and 1958–1967 first documented a dose-response relationship between body habits and tuberculosis incidence [32]. Similar findings were reported from a national study of Norwegians screened for tuberculosis with compulsory radiography between 1963–1975 [33]. Subsequent studies in other countries confirmed these findings and provided further evidence for an increased risk of tuberculosis for those with a low BMI, which is used in many studies as a proxy for malnutrition [9]. A study of National Health and Nutrition Examination Study data found that, compared with persons with a normal BMI, the population-estimated hazard ratio (HR) of developing tuberculosis for persons with a BMI <18.5 was 12.4 (95% confidence interval [CI], 5.7–26.9), after controlling for confounders [34]. In particular, protein intake less than 50% of normal was strongly associated with tuberculosis incidence.
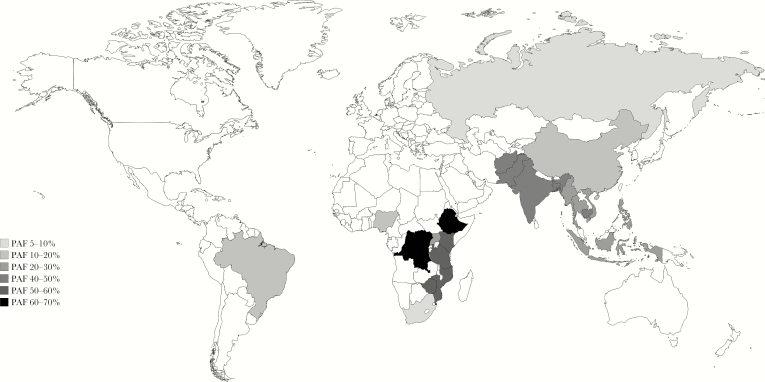
A systematic literature review and meta-analysis of 6 cohort studies in high-income countries found a 13.8% reduction (95% CI, 13.4%–14.2%) in tuberculosis incidence per unit increase in BMI [35]. Among people living with HIV, a BMI <18.5 was associated with a 4-fold increased tuberculosis risk within 6 months of initiating antiretroviral therapy, an effect comparable to having a CD4+ T-cell count < 50 cells/mm3 [36]. In 2009, data suggested that the population attributable fraction of tuberculosis due to undernutrition in 22 countries with a high tuberculosis burden was 26.9% (Figure 2) [37]. The population attributable fraction of tuberculosis due to malnutrition in India, a country with a quarter of the estimated global tuberculosis burden, is as high as 61.5% in women and 57.4% in men [1, 38].
Figure 2.
Population attributable fractions (PAFs) of tuberculosis due to undernutrition in 22 countries with a high tuberculosis burden. The map was created using data derived from Lönnroth et al [35].
Although the association between undernutrition and tuberculosis in humans has been repeatedly demonstrated, causality is difficult to prove [9]. Since tuberculosis can have an insidious onset, the duration of disease prior to diagnosis can be difficult to establish, making it challenging to know which came first—malnutrition or tuberculosis. Weight loss and undernutrition in patients with tuberculosis can be caused by decreased food intake or factors due to tuberculosis (ie, cachexia due to metabolic dysfunction, poor absorption, fever, and anorexia). The altered metabolism from tuberculosis may lead to a so-called anabolic block, whereby dietary protein is used more for energy production than anabolism [39]. Furthermore, anthropometric measures of malnutrition, such as BMI, MUAC, and weight-for-height z score, cannot differentiate between the cachexia due to tuberculosis and that due to undernutrition.
Although a causal relationship between undernutrition and tuberculosis is difficult to prove, longitudinal and circumstantial data, as well as results from animal studies, provide substantial support for such a relationship. Further, there is reason to believe that this may be an actionable relationship. The Papworth experiment was conducted in the preantibiotic era (ie, in 1918–1943) to study the effect of socioeconomic interventions and improved nutrition on tuberculosis transmission and incidence. Patients discharged from sanatoria were ensured a job, were provided adequate nutrition and lodging, and were monitored closely for tuberculosis. A reanalysis of data from this study showed that, although there was no reduction in tuberculosis transmission, children <5 years of age born within the Papworth settlement had a markedly lower tuberculosis incidence than those born outside the village (0 vs 1217 cases/100000 person-years) [40]. This lends credence to the notion that improved nutrition, in addition to socioeconomic interventions, can decrease the incidence of tuberculosis. A recent modeling study projected that modest reductions in undernutrition in the central-eastern states of India could reduce tuberculosis incidence by approximately 4.8 million (95% uncertainty range, 0.5 million–17.1 million) over 20 years [41].
Undernutrition and Tuberculosis Severity
Limited data suggest that tuberculosis severity may be greater among undernourished patients. A retrospective study of 995 adults with multidrug-resistant tuberculosis in Latvia found that a BMI <18.5 at diagnosis was associated with culture positivity (adjusted OR [aOR], 1.7; 95% CI, 1.0–2.9) and bilateral cavitation (aOR, 2.1; 95% CI, 1.3–3.5), controlling for age and reported weight loss; findings were similar for retreatment and new cases [42]. In Malawi, a BMI <19 was associated with far advanced lung disease, as defined by a radiography-based international classification system for tuberculosis severity (aOR, 8.83; 95% CI, 3.64–21.42); the model controlled for age, sex, HIV infection, and other confounders but not for duration of illness, which may predispose to weight loss and low BMI [43]. This is an important limitation because a decreased BMI may be a symptom of severe tuberculosis, rather than a cause of it. Additional studies are needed to assess the influence of undernutrition on tuberculosis severity and to address mycobacterial burden, controlling for duration of symptoms.
Undernutrition and Tuberculosis Treatment Outcomes
Nutritional status may also be an important tool for predicting treatment success, outcomes, and mortality. Several studies suggest that undernutrition is associated with worse treatment outcomes. A retrospective cohort study in Indonesia of 212 patients with multidrug-resistant tuberculosis found that a pretreatment BMI <16 (vs >18) was associated with a longer time to sputum conversion (adjusted HR, 0.55; 95% CI, .37–.84), controlling for sex, age, drug resistance, and sputum acid-fast bacilli grade [44]. Furthermore, insufficient weight gain, defined as ≤ 5% in the first 2 months of treatment, has been associated with higher rates of relapse [45].
Several studies suggest that undernutrition is associated with tuberculosis mortality. In Ethiopia, a retrospective cohort study of 810 patients with smear-positive tuberculosis found that a weight <35 kg at the time of treatment initiation had an aHR of 3.9 (95% CI, 1.6–9.3) for mortality during tuberculosis treatment [46]. Similarly, a 2016 South African study of extensively drug-resistant tuberculosis demonstrated that a weight <50 kg at the start of treatment was the strongest predictor of an unfavorable outcome [47]. An Indian study of pulmonary tuberculosis found that, after adjustment for age, sex, treatment category, sputum smear, and HIV status, an increased BMI lowered the odds of death during treatment (aOR, 0.78 [95% CI, .68–.90] for each unit increase in BMI) [48].
Macronutrient Supplementation and Treatment Outcomes
Research on therapeutic macronutrient supplementation for patients with active tuberculosis during therapy is sparse. A Cochrane systematic review identified only 6 studies of sufficient quality that studied the effect of macronutrient supplementation on treatment outcomes, weight gain, and quality of life [49]. Overall, the systematic review found no evidence for earlier sputum clearance (relative effect, 1.08; 95% CI, 0.86–1.37) and low-grade evidence for improvement in quality of life through macronutrient supplementation, although there was moderate evidence that supplementation led to weight gain. Tuberculosis mortality in the included trials was too low to draw conclusions about the efficacy of macronutrient supplementation on decreasing tuberculosis mortality. The studies included in the Cochrane review were small, underpowered, and heterogeneous in terms of the nutritional interventions and outcomes studied. Furthermore, there is a possibility that the nutritional supplementation was insufficient. Only 2 of 6 trials confirmed that their intervention actually improved caloric intake, and neither trial exceeded 2500 kcal/day, which is the average recommended requirement for uninfected individuals. Given the anabolic block described in tuberculosis and the hypercatabolic nature of this febrile illness, these nutritional interventions may have been underdosed. Larger studies with richer supplementation and closer monitoring of nutritional intake may be necessary.
NEED FOR FUTURE RESEARCH
Future studies are needed to address gaps in knowledge around undernutrition and tuberculosis, as outlined in the WHO guideline for the nutritional care and support of patients with tuberculosis (Table 1) [50]. Information is needed to understand better the impact of undernutrition on the time to sputum culture conversion, relapse, and mortality [8]. The consequences of undernutrition for close contacts of patients with tuberculosis and the opportunities to mitigate the risk of transmission through nutritional interventions should also be considered. Additional studies on the role of nutritional supplementation are necessary to understand the caloric requirements for patients with tuberculosis, as well as the metabolic and immunological consequences of malnutrition and refeeding. The precise mechanistic basis of undernutrition-mediated modifications to host resistance against tuberculosis needs to defined. Well-powered studies from diverse contexts are necessary to understand the potential of nutritional supplementation during active tuberculosis to improve treatment outcomes and adherence.
Table 1. Nutritional Supplementation Research Priorities Outlined by the World Health Organization [50]
| Nutritional Requirements | Nutritional Supplementation | Assessment & Counselling | Programs |
|---|---|---|---|
| Determining energy requirements for TB patients | Effect of micronutrient supplementation on TB treatment outcomes | Developing successful counseling strategies to enhance uptake of nutritional advice. | Effect of WHO nutrition and TB programs on nutritional recovery and treatment outcomes in TB patients. |
| Quantifying protein & fat requirements and utilization in patients with active TB | Effect of nutritional supplementation in pregnant women with TB on neonatal outcomes | Identifying good nutritional measures in pregnant women with TB | Determining the relative importance of supplementation on adherence |
| Characterizing risk of micronutrient deficiencies among active TB patients | Benefits of nutritional supplementation on growth and development in children with active TB | Determining optimal BMI for pregnant women with TB to ensure optimal maternal and neonatal outcomes | |
| Identifying causes of malnutrition in TB patients | Defining treatment outcomes and nutritional parameters for supplementation trials | ||
| Defining trajectory of weight change during the intensive phase of treatment |
Abbreviations: BMI, body mass index; WHO, World Health Organization.
CONCLUSION
Prospective and retrospective studies have found convincing evidence of an association between undernutrition and tuberculosis incidence, which is corroborated by animal studies. Furthermore, there is strong evidence for associations between undernutrition and poor tuberculosis treatment outcomes, likely mediated by effects on innate and adaptive immune responses. Further research is necessary that uses modern immunological methods to understand how undernutrition blunts the immune response. Such research will be a vital component of the End TB strategy.
Notes
Financial support. This work was supported by the US Civilian Research and Development Foundation (CRDF; award USB-31150-XX-13 to N. S. H., C. R. H., and P. S.); the National Science Foundation (cooperative agreement OISE-9531011 to N. S. H., C. R. H., and P. S.), with federal funds from the Government of India’s Department of Biotechnology, the Indian Council of Medical Research, the National Institutes of Health, the National Institute of Allergy and Infectious Diseases, and the Office of AIDS Research and distributed in part by CRDF Global; grant from the Warren Alpert Foundation and Boston University School of Medicine (N.S.H and P. S); the Clinical and Translational Sciences Institute (grant 1UL1TR001430 to N. S. H.); the Providence/Boston Center for AIDS Research (grant P30AI042853 to C. R. H.); and the Boston University/Rutgers Tuberculosis Research Unit (grant U19AI111276 to C. R. H.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization (WHO). Global tuberculosis report 2017. Geneva: WHO, 2017:1–3. [Google Scholar]
- 2. Food and Agriculture Organization of the United Nations (FAO), International Fund for Agricultural Development, United Nations Children’s Fund, World Food Programme, World Health Organization. The state of food security and nutrition in the world 2017. Building resilience for peace and food security. Rome: FAO, 2017:13. [Google Scholar]
- 3. Lönnroth K, Raviglione M. The WHO’s new End TB Strategy in the post-2015 era of the sustainable development goals. Trans R Soc Trop Med Hyg 2016; 110:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor CE, Camargo CA Jr. Impact of micronutrients on respiratory infections. Nutr Rev 2011; 69:259–69. [DOI] [PubMed] [Google Scholar]
- 5. Abubakar N, Atiku M, Alhassan A, Mohammed I, Garba R, Gwarzo G. An assessment of micronutrient deficiency: a comparative study of children with protein-energy malnutrition and apparently healthy controls in Kano, Northern Nigeria. Trop J Med Res 2017; 20:61–5. [Google Scholar]
- 6. Webb P, Bhatia R.. Manual: measuring and interpreting malnutrition and mortality. Rome: Nutrition Service WFP, 2005:15–21. [Google Scholar]
- 7. Hildreth HG, Johnson RK, Goran MI, Contompasis SH. Body composition in adults with cerebral palsy by dual- energy X-ray absorptiometry, bioelectrical impedance analysis, and skinfold anthropometry compared with the 18O isotope-dilution technique. Am J Clin Nutr 1997; 66:1436–42. [DOI] [PubMed] [Google Scholar]
- 8. Bhargava A. Undernutrition, nutritionally acquired immunodeficiency, and tuberculosis control. BMJ 2016; 355. [DOI] [PubMed] [Google Scholar]
- 9. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8:286–98. [PubMed] [Google Scholar]
- 10. De la Fuente M, Munoz M. Impairment of phagocytic process in macrophages from young and old mice by protein malnutrition. Ann Nutr Metab 1992; 36:41–7. [DOI] [PubMed] [Google Scholar]
- 11. de Oliveira DC, Hastreiter AA, Mello AS, et al. The effects of protein malnutrition on the TNF-RI and NF-κB expression via the TNF-α signaling pathway. Cytokine 2014; 69:218–25. [DOI] [PubMed] [Google Scholar]
- 12. Wang C, Yu X, Cao Q, et al. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol 2013; 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan J, Tian Y, Tanaka KE, et al. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci U S A 1996; 93:14857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mainali ES, McMurray DN. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect Immun 1998; 66:927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattila JT, Ojo OO, Kepka-Lenhart D, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol 2013; 191:773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abe M, Akbar F, Matsuura B, Horiike N, Onji M. Defective antigen-presenting capacity of murine dendritic cells during starvation. Nutrition 2003; 19:265–9. [DOI] [PubMed] [Google Scholar]
- 17. Mainali ES, McMurray DN. Adoptive transfer of resistance to pulmonary tuberculosis in guinea pigs is altered by protein deficiency. Nutr Res 1998; 18:309–17. [Google Scholar]
- 18. Purtilo DT, Connor DH. Fatal infections in protein-calorie malnourished children with thymolymphatic atrophy. Arch Dis Child 1975; 50:149–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chevalier P. Thymic ultrasonography in children, a non-invasive assessment of nutritional immune deficiency. Nutr Res 1997; 17:1271–6. [Google Scholar]
- 20. Rodríguez L, González C, Flores L, Jiménez-Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol 2005; 12:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. González-Torres C, González-Martínez H, Miliar A, et al. Effect of malnutrition on the expression of cytokines involved in Th1 cell differentiation. Nutrients 2013; 5:579–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anuradha R, Munisankar S, Bhootra Y, et al. Coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin Vaccine Immunol 2016; 23:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaufmann SH, Weiner J, von Reyn CF. Novel approaches to tuberculosis vaccine development. Int J Infect Dis 2017; 56:263–7. [DOI] [PubMed] [Google Scholar]
- 24. Prendergast AJ. Malnutrition and vaccination in developing countries. Phil Trans R Soc B 2015; 370:20140141. doi:10.1098/rstb.2014.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoang T, Agger EM, Cassidy JP, Christensen JP, Andersen P. Protein energy malnutrition during vaccination has limited influence on vaccine efficacy but abolishes immunity if administered during Mycobacterium tuberculosis infection. Infect Immun 2015; 83:2118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai G, McMurray DN. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect Immun 1998; 66:3562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition–a systematic review. PLoS One 2014; 9:e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMurray DN, Loomis SA, Casazza LJ, Rey H, Miranda R. Development of impaired cell-mediated immunity in mild and moderate malnutrition. Am J Clin Nutr 1981; 34:68–77. [DOI] [PubMed] [Google Scholar]
- 29. Greenwood BM, Bradley-Moore AM, Bradley AK, Kirkwood BR, Gilles HM. The immune response to vaccination in undernourished and well-nourished Nigerian children. Ann Trop Med Parasitol 1986; 80:537–44. [DOI] [PubMed] [Google Scholar]
- 30. Hoest C, Seidman JC, Pan W, et al. Evaluating associations between vaccine response and malnutrition, gut function, and enteric infections in the MAL-ED cohort study: methods and challenges. Clin Infect Dis 2014; 59(Suppl 4):S273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leyton GB. Effects of slow starvation. Lancet 1946; 2:73–9. [PubMed] [Google Scholar]
- 32. Palmer CE, Jablon S, Edwards PQ. Tuberculosis morbidity of young men in relation to tuberculin sensitivity and body build. Am Rev Tuberc 1957; 76:517–39. [DOI] [PubMed] [Google Scholar]
- 33. Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis 1986; 69:355–62. [PubMed] [Google Scholar]
- 34. Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971–1992. Am J Epidemiol 2012; 176:409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 36. Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr 2011; 56:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet 2010; 375:1814–29. [DOI] [PubMed] [Google Scholar]
- 38. Hochberg NS, Sarkar S, Horsburgh CR Jr, et al. Comorbidities in pulmonary tuberculosis cases in Puducherry and Tamil Nadu, India: opportunities for intervention. PLoS One 2017; 12:e0183195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Macallan DC, McNurlan MA, Kurpad AV, et al. Whole body protein metabolism in human pulmonary tuberculosis and undernutrition: evidence for anabolic block in tuberculosis. Clin Sci (Lond) 1998; 94:321–31. [DOI] [PubMed] [Google Scholar]
- 40. Bhargava A, Pai M, Bhargava M, Marais BJ, Menzies D. Can social interventions prevent tuberculosis? The Papworth experiment (1918–1943) revisited. Am J Respir Crit Care Med 2012; 186:442–9. [DOI] [PubMed] [Google Scholar]
- 41. Oxlade O, Huang CC, Murray M. Estimating the Impact of reducing under-nutrition on the tuberculosis epidemic in the Central Eastern States of India: a dynamic modeling study. PLoS One 2015; 10:e0128187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Podewils LJ, Holtz T, Riekstina V, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect 2011; 139:113–20. [DOI] [PubMed] [Google Scholar]
- 43. Van Lettow M, Kumwenda JJ, Harries AD, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis 2004; 8:211–7. [PubMed] [Google Scholar]
- 44. Putri FA, Burhan E, Nawas A, et al. Body mass index predictive of sputum culture conversion among MDR-TB patients in Indonesia. Int J Tuberc Lung Dis 2014; 18:564–70. [DOI] [PubMed] [Google Scholar]
- 45. Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med 2006; 174:344–8. [DOI] [PubMed] [Google Scholar]
- 46. Birlie A, Tesfaw G, Dejene T, Woldemichael K. Time to death and associated factors among tuberculosis patients in Dangila Woreda, Northwest Ethiopia. PLoS One 2015; 10:e0144244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kvasnovsky CL, Cegielski JP, van der Walt ML. Treatment outcomes for patients with extensively drug-resistant tuberculosis, KwaZulu-Natal and Eastern cape Provinces, South Africa. Emerg Infect Dis 2016; 22:1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhargava A, Chatterjee M, Jain Y, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One 2013; 8:e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grobler L, Nagpal S, Sudarsanam TD, Sinclair D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. World Health Organization (WHO). Guideline: nutritional care and support for patients with tuberculosis. Geneva: WHO, 2013:18–9. [PubMed] [Google Scholar]