In pregnant women assessed for parasitemia every 28 days, the risk of placental malaria increased in a dose-response relationship with both increasing frequency and density of parasitemia; however, even women with only submicroscopic parasitemia were at risk for placental malaria.
Keywords: placental malaria, submicroscopic parasitemia, microscopic parasitemia, asymptomatic malaria infection, intermittent preventive treatment during pregnancy, dihydroartemisinin-piperaquine
Abstract
Background
Placental malaria is a major cause of adverse birth outcomes. However, data are limited on the relationships between longitudinal measures of parasitemia during pregnancy and placental malaria.
Methods
Data came from 637 women enrolled in a randomized controlled trial of intermittent preventive treatment of malaria in pregnancy (IPTp) from Uganda. Plasmodium falciparum parasitemia was assessed using microscopy and ultrasensitive quantitative PCR at intervals of 28 days from 12 to 20 weeks gestation through delivery. Multivariate analysis was used to measure associations between characteristics of parasitemia during pregnancy and the risk of placental malaria based on histopathology.
Results
Overall risk of placental malaria was 44.6%. None of the 34 women without parasitemia detected during pregnancy had evidence of placental malaria. Increasing proportion of interval assessments with parasitemia and higher parasite densities were independently associated with an increased risk of placental malaria. Higher gravidity and more effective IPTp were associated with a decreased risk of placental malaria. Women with parasitemia only detected before the third trimester still had an increased risk of placental malaria.
Conclusions
The frequency, density, and timing of parasitemia are all important risk factors for placental malaria. Interventions should target the prevention of all levels of parasitemia throughout pregnancy.
In Africa, approximately 30 million pregnant women are at risk of Plasmodium falciparum infection every year [1]. In this setting, a high proportion of infected women are asymptomatic, with low-level parasitemia that can nonetheless lead to placental sequestration. Placental malaria is common, with 25% to 40% of women in sub-Saharan Africa having evidence of placental malaria at time of delivery, and is associated with poor birth outcomes [2, 3]. Babies born to mothers with placental malaria have twice the risk of low birth weight (LBW) [4] and stillbirth [5] compared to those born to mothers without placental malaria. Malaria in pregnancy is estimated to cause 900 000 LBW deliveries and 100 000 infant deaths each year [6, 7]. In high-transmission areas, primigravidae are at greater risk of infection, and slowly acquire immunity to placental malaria through consecutive pregnancies [2]. Because of both parity-specific and age-related immunity, P. falciparum infection can be difficult to diagnose during pregnancy, as peripheral infections are often submicroscopic and asymptomatic, especially in multigravida women [8, 9]. Because of this, the World Health Organization (WHO) recommends the use of intermittent preventative treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP), which is intended to clear current infections and provide a period of prophylaxis to prevent future infections [2].
While associations between microscopic parasitemia during pregnancy and placental malaria have been reported in several studies, the impact of submicroscopic infection has been less well studied [2, 9]. Advances in molecular diagnostics, including the development of ultrasensitive nucleic-acid based assays such as the var gene acidic terminal sequence quantitative polymerase chain reaction (qPCR), now allow for detection of parasite densities as low as 0.06–0.15 parasites per microliter (p/µL) [10]. Most prior studies of parasitemia during pregnancy have been cross-sectional, and those that were longitudinal did not utilize ultrasensitive techniques or had limited interval assessments for parasitemia [11–13], meaning that some infections, particularly low-density ones, were likely to have been missed. Frequent assessments for parasitemia using ultrasensitive qPCR should allow for a more accurate estimation of the burden of parasitemia during pregnancy and improve our ability to investigate the impact of submicroscopic parasitemia on the development of placental malaria.
Recently, given widespread resistance to SP, there has been concern for diminishing efficacy of IPTp [14, 15]. Several studies have focused on the arteminisin-based combination therapy dihydroartemisinin-piperaquine (DP) as an alternative to SP. Three recent studies from East Africa showed that IPTp with DP was more effective than SP at reducing the risk of malaria during pregnancy and the risk of placental malaria at delivery [16–18]. In this study, we utilized ultrasensitive qPCR data obtained every 28 days from pregnant women enrolled in a randomized controlled trial comparing monthly IPTp with SP versus DP, in Uganda, to conduct a detailed longitudinal study of the impact of P. falciparum infection during pregnancy. A better understanding of how the frequency, density, and timing of parasitemia during pregnancy affects the risk of placental malaria is crucial for developing effective strategies to prevent malaria in pregnancy.
METHODS
Study Setting and Participants
Data came from a previously published double-blind, randomized controlled trial comparing monthly SP and monthly DP for IPTp conducted from September 2016 to December 2017 in Busia District, an area in southeastern Uganda where malaria transmission is perennial and intense [18]. In Busia District, the only vector control interventions conducted prior to or during this trial were 2 district-wide long-acting insecticide treated net (LLIN) distribution campaigns in May 2013 and April 2017. Briefly, eligible participants were HIV-uninfected women at least 16 years of age with a viable pregnancy between 12 and 20 weeks gestation confirmed by ultrasound. IPTp was administered every 4 weeks starting at 16 or 20 weeks gestational age. For the present study, all women with a singleton delivery at ≥28 weeks gestational age with placental histopathology results were included (Figure 1).
Figure 1.
Flowchart of study participants. Abbreviations: DP, dihydroartemisinin-piperaquine; HIV, human immunodeficiency virus; SP, sulfadoxine-pyrimethamine.
Study Procedures
Routine visits were conducted every 4 weeks, including collection of blood for the detection of malaria parasites by microscopy and qPCR. Women were not treated for a positive blood smear at a routine visit unless they were febrile, in accordance with the standard of care in Uganda. Women were encouraged to come to the clinic when ill. Those who presented with a tympanic temperature ≥38.0°C or history of fever in the previous 24 hours had blood collected for a thick blood smear. If the smear was positive, the patient was diagnosed with malaria and treated with artemether-lumefantrine. qPCR was not performed at unscheduled sick visits in accordance with study protocol. Placental tissue, placental blood, and maternal blood were collected at the time of delivery.
Laboratory Procedures
Blood smears were stained with 2% Giemsa and read by experienced microscopists. A blood smear was considered negative when the examination of 100 high-power fields did not reveal asexual parasites. All slides were read by a second microscopist and a third reviewer settled any discrepant readings. We collected 200 µL of blood at enrollment and at the time of each routine visit to test for the presence of parasitemia using an ultrasensitive qPCR assay targeting the multicopy conserved var gene acidic terminal sequence [10]. Placental blood was tested for the presence of malaria parasites by microscopy and loop-mediated isothermal amplification (LAMP) kit (Eiken Chemical, Japan), as previously described [19]. Placental tissue was evaluated for histopathological evidence of active or past infection with malaria parasites based on the detection of malaria parasites and/or hemozoin pigment in intervillous fibrin and macrophages, using standardized criteria, as previously described [20].
Variables of Interest
The primary outcome was the prevalence of placental malaria, defined as the presence of any parasites or hemozoin pigment detected by histopathology. To evaluate the magnitude of placental malaria, we also assessed the proportion of high-powered fields in which any hemozoin pigment was detected as a secondary outcome. Symptomatic malaria was defined as fever with a positive blood smear. Interval assessments for parasitemia were performed at the enrollment visit, at routine visits that occurred approximately every 4 weeks through delivery, and at unscheduled visits in febrile patients. For each enrollment and routine visit, we assessed for the presence or absence of (1) microscopic parasitemia and (2) any parasitemia (by microscopy or qPCR); at unscheduled visits, only microscopic parasitemia was assessed. The frequency of detection of parasitemia was summarized as the proportion of interval assessments with parasitemia (number of interval assessments with microscopic or any parasitemia divided by the total number of interval assessments). Proportion of interval assessments with parasitemia was then categorized into 5 strata: 0%, >0%–25%, >25%–50%, >50%–75%, and >75%–100%, to best demonstrate relationships with outcomes of interest. Geometric mean parasite density for each participant was calculated by adding 1 to each qPCR parasite density, taking the geometric mean of all parasite densities (microscopic and qPCR), and then subtracting 1. Geometric mean parasite density was then categorized into 3 strata: ≤1p/µL, >1–10 p/µL, and >10 p/µL, as this best reflected relationships with the outcome of interest. When parasite densities were available by both microscopy and qPCR, the qPCR value was used.
Statistical Analysis
Statistical analyses were performed using Stata software, version 14 (StataCorp) and Rstudio, version 1.0.143. Comparisons of simple proportions were made using the χ2 test or Fisher exact test. One-way analysis of variance (ANOVA) was used for normally distributed continuous variables. Associations between the proportion of interval assessments with parasitemia and density of parasitemia during pregnancy with the risk of placental malaria were made using univariate and multivariate generalized linear Poisson regression models with robust standard errors. All models were adjusted for gravidity and IPTp regimen. We assumed a 2-sided significance level of 0.05.
RESULTS
Among 782 women enrolled, 95 (12.1%) were excluded before delivery and 50 (6.4%) after delivery resulting in 637 women included in the analyses (Figure 1). At enrollment, mean maternal age, mean gestational age, and gravidity were similar between the 2 IPTp arms (Table 1). LLIN use was similar between the 2 groups with women in the SP arm reporting sleeping under a LLIN the previous night on 84.4% of clinic visits versus 85.7% in the DP arm. Participants underwent a median of 8 interval assessments (range 4–9) for parasitemia during pregnancy. There were no significant differences in the incidence of malaria (incidence rate ratio = 0.71; 95% confidence interval [CI], 0.33–1.55; P = .39) or measures of parasitemia before initiation of IPTp between the 2 treatment arms. After initiation of IPTp, women randomized to DP experienced a lower incidence of malaria (0.02 versus 0.54 episodes per person-years; P value <.001), and fewer interval assessments with microscopic parasitemia (0.5% versus 30.6%; P < .001) or any parasitemia (20.2% versus 64.2%; P < .001). IPTp with DP was also associated with a significantly lower prevalence of parasites detected in placental blood by microscopy (0.3% versus 8.8%; P < .001) or LAMP (2.2% versus 22.3%; P < .001), and any evidence of placenta malaria by histopathology (28.2% vs. 61.7%; P < .001). Notably, among those with any evidence of placental malaria by histopathology, parasites were detected in only 9.5%; therefore, we chose to use the presence of any parasites or pigment as the outcome for placental malaria by histopathology. Because histopathology provided the most sensitive measure of placental malaria, as has been shown in other studies [21], this was chosen as the primary outcome for the remainder of the analyses.
Table 1.
Characteristics of Study Participants
| Study Period | Characteristic | Treatment Arm | ||
|---|---|---|---|---|
| Monthly SP | Monthly DP | |||
| Enrollment | Number of participants | 311 | 326 | |
| Age in years, mean (SD) | 24.0 (6.0) | 23.9 (5.7) | ||
| Gestational age, weeks, mean (SD) | 15.7 (2.4) | 15.4 (2.3) | ||
| Gravidity, n (%) | 1 | 81 (26.1) | 72 (22.1) | |
| 2 | 65 (20.9) | 85 (26.1) | ||
| ≥3 | 165 (53.1) | 169 (51.8) | ||
| Before administration of first dose of IPTp | Episodes of malaria, n (incidencea) | 15 (1.13) | 11 (0.81) | |
| Interval assessments,b n | 591 | 621 | ||
| Interval assessments with microscopic parasitemia, n (%) | 301 (50.9) | 338 (54.4) | ||
| Interval assessments with any parasitemia, n (%) | 494 (83.6) | 504 (81.1) | ||
| After administration of first dose of IPTp | Episodes of malaria, n (incidencea) | 70 (0.54) | 3 (0.02) | |
| Interval assessments, n | 1516 | 1616 | ||
| Interval assessments with microscopic parasitemia, n (%) | 45 (30.6) | 9 (0.5) | ||
| Interval assessments with any parasitemia, n (%) | 973 (64.2) | 326 (20.2) | ||
| Placental outcomes | Parasites detected in placental blood by microscopy, n/N (%) | 27/307 (8.8) | 1/326 (0.3) | |
| Parasites detected in placental blood by LAMP, n/N (%) | 67/301 (22.3) | 7/323 (2.2) | ||
| Parasites or pigment detected by histopathology, n/N (%) | 192/311 (61.7) | 92/326 (28.2) | ||
Abbreviations: DP, dihydroartemisinin-piperaquine; IPTp, intermittent preventative treatment in pregnancy; LAMP, loop-mediated isothermal amplification; SP, sulfadoxine-pyrimethamine.
aEpisodes per person-years.
bIncludes enrollment visit.
Associations Between Proportion of Interval Assessments With Parasitemia and Placental Malaria
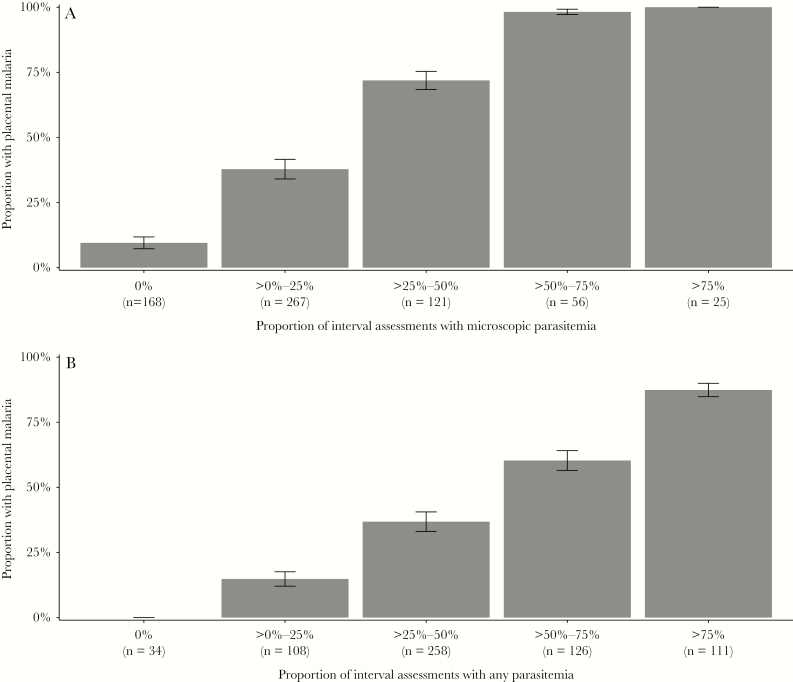
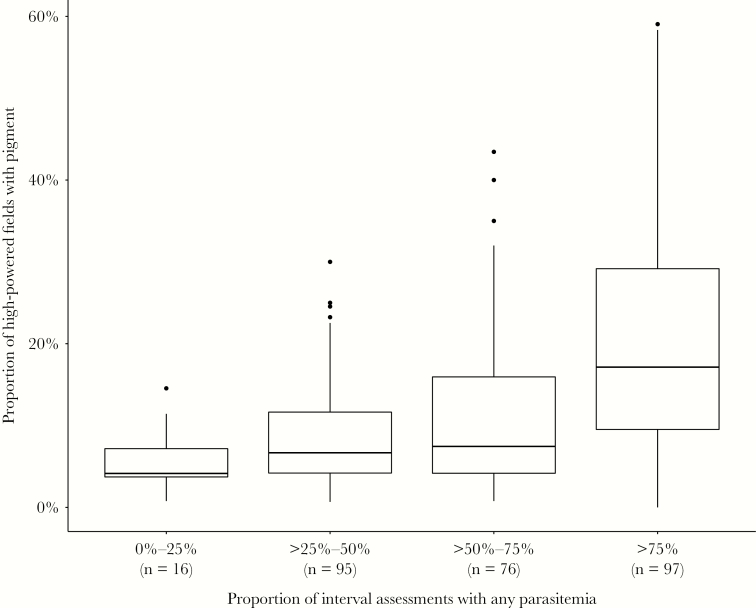
There was a strong “dose response” relationship between the proportion of interval assessments with microscopic or any parasitemia and the risk of placental malaria (P < .001 using χ2 test for trend for both comparisons; Figure 2). Among 34 women in which parasitemia was never detected by microscopy or qPCR during pregnancy, none had evidence of placental malaria. Notably, there were 16 mothers who developed placental malaria in whom only submicroscopic parasitemia was detected. In contrast, among 111 women with parasitemia detected in over 75% of interval assessments, the risk of placental malaria was 87.4%. When limiting the analysis to only parasitemia detected by microscopy, among 81 women with parasitemia detected in over 50% of interval assessments, all but 1 (98.8%) had evidence of placental malaria. Primigravida women were more likely to develop placental malaria than multigravidae, but the dose-response relationship between the proportion of interval assessments with microscopic or any parasitemia and the risk of placental malaria held within each category of gravidity (Supplementary Figure 1). Additionally, among the 284 women with placental malaria, an increasing proportion of interval assessments with any parasitemia was also associated with increasing proportion of high-powered fields with hemozoin pigment (P < .001; Figure 3). Thus, increasing frequency of detection of parasitemia during pregnancy was associated not only with an increased risk of placental malaria, but also with an increased amount of affected tissue in the placenta.
Figure 2.
Proportion of women with placental malaria by (A) proportion of interval assessments with microscopic parasitemia and (B) proportion of interval assessments with any parasitemia. Numbers at the bottom of each bar represent the total number of patients in the category (n). Error bars represent 95% confidence intervals of the proportions.
Figure 3.
Proportion of high-powered microscopy fields with pigment by proportion of interval assessments with any parasitemia among those with placental malaria. Numbers at the bottom of each bar represent the total number of patients in the category (n). Boxes represent interquartile range (IQR), with median represented as a horizontal band. Whiskers extend to Q3 + 1.5IQR and Q1 − 1.5IQR.
Associations Between Geometric Mean Parasite Density and Placental Malaria
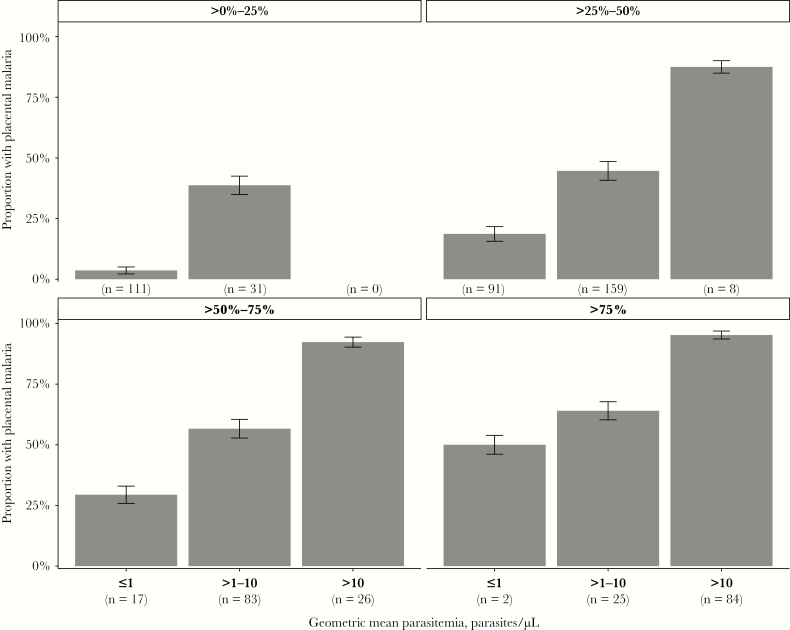
In addition to the presence or absence of parasitemia, we assessed whether the degree of parasitemia, as measured by the geometric mean parasite density, was associated with placental malaria. We found that there was a strong dose-response relationship between higher geometric mean parasite density and an increased risk of placental malaria; this relationship was preserved within each category of gravidity (Supplementary Figure 2). When stratified by proportion of interval assessments with any parasitemia, higher geometric mean parasite densities remained associated with a higher risk of placental malaria (P < .001; Figure 4); among 110 women with any parasitemia detected in over 50% of interval assessments and a geometric mean parasite density of >10 p/µL, the risk of placental malaria was 94.5%. Notably, the risk for placental malaria remained >50% in women with lower geometric mean parasite densities (between 1 and 10 p/µL) if they had greater than 50% of interval assessments with parasitemia. In the 182 women who never had a parasite density of >100 p/µL on any visit, that is likely missed by standard diagnostic tests, higher geometric mean parasite density was still associated with increased risk of placental malaria; in this group, only 6% of those with a geometric mean parasite density of ≤1 p/µL developed placental malaria, while 13.9% of those who had a geometric mean parasite density of >1 p/µL developed placental malaria (P = .07).
Figure 4.
Proportion of women with placental malaria by geometric mean parasite density, stratified by proportion of interval assessments with any parasitemia. Numbers at the bottom of each bar represent the total number of patients in the category (n). Error bars represent 95% confidence intervals of the proportions.
Adjusted Model Suggests Independent Associations of Measures of Parasitemia, IPTp, and Gravidity With Placental Malaria
In univariate analysis, an increase in the frequency and density of parasitemia were strongly associated with an increasing risk of placental malaria, as discussed above and presented in Table 2. Increasing gravidity and IPTp with DP were associated with a decreased risk of placental malaria, relationships that have been previously reported. Interestingly, in adjusted analysis, although all measures of associations decreased in magnitude (suggesting some degree of confounding), all of the risk factors assessed remained independently associated with placental malaria. These data provide further evidence that the frequency and density of parasitemia during pregnancy, although correlated, contribute independently towards the risk of developing placental malaria. Increasing gravidity and IPTp with DP also remained significantly associated with a lower risk of placental malaria in multivariate analysis, suggesting that immunity and antimalarial drugs may modulate the risk of developing placental malaria independent of the frequency and density of parasitemia in the peripheral blood.
Table 2.
Factors Associated with Placental Malaria
| Variable | Categories | n | Placental Malaria, n (%) | Unadjusted Analysis | Adjusted Analysis | ||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | ||||
| Proportion of interval assessments with any parasitemia | 0%–25% | 142 | 16 (11.3) | Reference group | Reference group | ||
| >25%–50% | 258 | 95 (36.8) | 3.27 (2.00–5.33) | <.001 | 1.95 (1.19–3.19) | .008 | |
| >50%–75% | 126 | 76 (60.3) | 5.35 (3.30–8.68) | <.001 | 2.31 (1.38–3.85) | .001 | |
| >75% | 111 | 97 (87.4) | 7.76 (4.86–12.4) | <.001 | 2.39 (1.41–4.06) | .001 | |
| Geometric mean parasite density | ≤1 p/μL | 221 | 27 (12.2) | Reference group | Reference group | ||
| >1–10 p/μL | 298 | 146 (49.0) | 4.01 (2.76–5.82) | <.001 | 2.61 (1.73–3.94) | <.001 | |
| >10 p/μL | 118 | 111 (94.1) | 7.7 (5.39–11.0) | <.001 | 3.03 (1.94–4.74) | <.001 | |
| Gravidity | 1 | 153 | 123 (80.4) | Reference group | Reference group | ||
| 2 | 150 | 70 (46.7) | .59 (.49–.71) | <.001 | .75 (.64–.88) | .001 | |
| ≥3 | 334 | 91 (27.3) | .34 (.28–.41) | <.001 | .48 (.40–.58) | <.001 | |
| IPTp regimen | SP | 311 | 192 (61.7) | Reference group | Reference group | ||
| DP | 326 | 92 (28.2) | .46 (.38–.56) | <.001 | .74 (.60–.92) | .007 | |
Abbreviations: CI, confidence interval; DP, dihydroartemisinin-piperaquine; IPTp, intermittent preventative treatment in pregnancy; p, parasite; RR, relative risk; SP, sulfadoxine-pyrimethamine.
Timing of Parasitemia
To explore the effect of the timing of parasitemia during gestation on the risk of placental malaria, we stratified the women into categories of those who never had parasitemia detected (n = 34), those with any parasitemia detected only prior to the third trimester (n = 204), those with any parasitemia detected only during the third trimester (n = 21), and those with any parasitemia both before and during the third trimester (n = 378). Compared to women with no parasitemia detected (none of whom developed placental malaria), the risk of placental malaria was similarly higher among those with parasitemia detected only prior to the third trimester (30.4%; P < .001) or only during the third trimester (23.8%; P = .003) and highest in those with parasitemia both before and during the third trimester (57.4%; P < .001; Table 3). A similar pattern was seen when limited to women who received IPTp with SP. In contrast, among women who received IPTp with DP, none of the 10 women with parasitemia detected only in the third trimester developed placental malaria, while the risk of placental malaria was similar among those with parasitemia only detected prior to the third trimester (31.1%) and those with parasitemia detected before and during the third trimester (32.5%). These data suggest that regardless of the IPTp regimen, parasitemia limited to before the third trimester is still associated with an increased risk of placental malaria.
Table 3.
Association Between the Timing of Parasitemia and Placental Malaria
| Timing of Parasitemia | Placental Malaria, n/N (%) | ||
|---|---|---|---|
| Total | Monthly SP | Monthly DP | |
| No parasitemia detected | 0/34 (0) | 0/8 (0) | 0/26 (0) |
| Only before the 3rd trimester | 62/204 (30.4) | 11/40 (27.5) | 51 /164 (31.1) |
| Only during the 3rd trimester | 5/21 (23.8) | 5/11 (45.5) | 0/10 (0) |
| Before and during the 3rd trimester | 217/378 (57.4) | 176/252 (69.8) | 41/126 (32.5) |
Abbreviations: DP, dihydroartemisinin-piperaquine; SP, sulfadoxine-pyrimethamine.
Discussion
We investigated associations between the frequency, density, and timing of parasitemia during pregnancy with the development of placental malaria in an intensely followed cohort of Ugandan women enrolled in a clinical trial of IPTp. The key novel aspects of this study were the frequent use of a highly sensitive qPCR assay in addition to traditional microscopy for the assessment of parasitemia during pregnancy and the use of histopathology to assess for placental malaria. When assessing for parasitemia every 4 weeks starting at 12–20 weeks of gestational age, there was a strong positive relationship between the proportion of interval assessments positive for parasitemia and the risk of placental malaria. Higher parasite densities were also independently associated with an increased risk of placental malaria; however, even women with only submicroscopic parasitemia were at risk for placental malaria. Notably, among women with no parasitemia detected during the study, none had evidence of placental malaria. Finally, regardless of the IPTp regimen, parasitemia limited to the period before the third trimester was still associated with an increased risk of placental malaria. DP did not appear to clear early infections already established in the placenta but was highly effective in preventing placental malaria when infections occurred only later in the course of pregnancy.
Most studies of parasitemia during pregnancy have been cross-sectional, with parasitemia only assessed at enrollment or delivery. One longitudinal study in Uganda screened mothers weekly with rapid diagnostic tests and found that multiple episodes of parasitemia and higher levels of parasitemia were both associated with adverse birth outcomes; however, this study did not assess mothers for submicroscopic parasitemia and placental histopathology was only available in a subset [22]. A study in Malawi found a dose-response relationship between the number of malaria infections diagnosed by microscopy and risk of LBW but also did not measure submicroscopic parasitemia or placental outcomes [23]. A longitudinal study that used a less sensitive PCR assay to detect submicroscopic parasitemia found that after controlling for microscopic parasitemia, each additional episode of submicroscopic parasitemia increased the risk of placental malaria [13]. Our study confirms the finding that increasing frequency of detection of any parasitemia during pregnancy increases the risk of placental malaria.
The longitudinal study, discussed above, using a less-sensitive PCR assay also demonstrated that women with only submicroscopic infections were more likely to have placental malaria than women without any documented infection during pregnancy [13]. Notably, in that study, 35% of women with evidence of placental malaria did not have any peripheral infections detected during pregnancy or at delivery [13]. However, in our cohort, women without documented parasitemia during pregnancy did not develop placental malaria. This implies that either the early gestational age at which most participants were enrolled, the frequency of screening, the use of ultrasensitive qPCR, or the combination of all three allowed us to more accurately assess the true burden of parasitemia in these women. Furthermore, it provides reassurance that ultrasensitive qPCR is sensitive enough to detect any level of parasitemia that might lead to sequestration of parasites in the placenta.
An alternative approach to IPTp is intermittent screening and treatment (ISTp), in which women are screened for parasitemia intermittently during pregnancy and only given antimalarial therapy if they test positive for malaria parasites. Two recent trials, using rapid diagnostic tests to assess women at regular intervals for parasitemia, evaluated ISTp with DP versus standard-of-care IPTp with SP. Both studies showed that ISTp with DP was inferior for preventing placental malaria compared to IPTp with SP [16, 24]. This was likely because the rapid diagnostic tests used for screening were not sensitive enough to detect low-density parasitemia, which can lead to placental malaria. Given the high burden of submicroscopic infections, and the fact that even women with only submicroscopic parasitemia had an increased risk of placental malaria, our study provides additional evidence that an ISTp strategy to prevent placental malaria has serious limitations given the level of sensitivity of currently available point-of-care diagnostic tests.
Prospective studies assessing the relationship between the timing of parasitemia during pregnancy and the risk of placental malaria have reached conflicting conclusions [25–31], and most have not included assessments for submicroscopic parasitemia or placental histopathology. Our data provide direct evidence that infections before the third trimester increase the risk for placental malaria even in those without any microscopic or submicroscopic parasitemia in the third trimester and provides support for the WHO recommendation to start dosing IPTp in the second trimester and to give at least 3 doses during pregnancy, no matter which antimalarial regimen is chosen.
This study had several limitations. Of the women enrolled in the primary trial, 18.5% were excluded from this analysis. The primary reason for exclusion was missing histopathology data. Reassuringly, neither gravidity nor prevalence of microscopic or submicroscopic parasitemia at enrollment varied significantly between those who were excluded and included. Furthermore, episodes of parasitemia were not genotyped; therefore, we cannot say whether repeated infections in women were new or persistent. However, because women were assessed every 28 days, we do have an accurate measurement of the proportion of time during pregnancy that they were actively infected. Another limitation of this analysis is that we did not investigate associations between parasitemia during pregnancy and birth outcomes. While one study in Benin showed an association between submicroscopic parasitemia and poor birth outcomes, two other studies have not [12, 13, 32]. Given that adverse birth outcomes are multifactorial and relatively uncommon events, we feel that this question is best addressed in a planned separate analysis. Additionally, because we enrolled women near the end of the first trimester and during the early second trimester, we cannot comment on the impact of parasitemia during the first trimester on the development of placental malaria. Finally, this study was conducted in a region with perennially high malaria transmission and the results may not be generalizable to areas that have a more seasonal transmission pattern or lower transmission intensity.
In conclusion, our data suggest that in a region with high malaria transmission intensity, the risk of placental malaria increases in a dose-response relationship with both increasing frequency and density of parasitemia. Importantly, however, even women with only submicroscopic infection have an increased risk of placental malaria compared to women without any parasitemia. Women with parasitemia limited to the period before the third trimester remain at risk for placental malaria. These results provide additional evidence for an IPTp strategy over ISTp to prevent placental malaria in women living in malaria endemic areas; furthermore, antimalarials used for IPTp should be effective at promptly clearing parasitemia and be dosed early and often.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the women who participated in the study, the dedicated study staff, and members of the Infectious Diseases Research Collaboration in Uganda.
Financial support. This work was supported by the National Institutes of Health /National Institute of Allergy and Infectious Diseases (grant number U19A1089674).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 2010; 7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 3. Chico RM, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA 2012; 307:2079–86. [DOI] [PubMed] [Google Scholar]
- 4. Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev 2004; 17:760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Geertruyden JP, Thomas F, Erhart A, D’Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg 2004; 71:35–40. [PubMed] [Google Scholar]
- 6. Guyatt HL, Snow RW. Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans R Soc Trop Med Hyg 2001; 95:569–76. [DOI] [PubMed] [Google Scholar]
- 7. Walker PG, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2014; 2:e460–7. [DOI] [PubMed] [Google Scholar]
- 8. Fried M, Duffy PE. Malaria during pregnancy. Cold Spring Harb Perspect Med 2017;7:a025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carmona-Fonseca J, Arango EM. Asymptomatic plasmodial infection in pregnant women: a global scenario. J Vector Borne Dis 2017; 54:201–6. [DOI] [PubMed] [Google Scholar]
- 10. Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 2015; 12:e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kapisi J, Kakuru A, Jagannathan P, et al. Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar J 2017; 16:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cottrell G, Moussiliou A, Luty AJ, et al. Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis 2015; 60:1481–8. [DOI] [PubMed] [Google Scholar]
- 13. Cohee LM, Kalilani-Phiri L, Boudova S, et al. Submicroscopic malaria infection during pregnancy and the impact of intermittent preventive treatment. Malar J 2014; 13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gutman J, Kalilani L, Taylor S, et al. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthetase reduces the effectiveness of sulfadoxine-pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis 2015; 211:1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranson H, N’guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 2011; 27:91–8. [DOI] [PubMed] [Google Scholar]
- 16. Desai M, Gutman J, L’lanziva A, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet 2015; 386:2507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kakuru A, Jagannathan P, Muhindo MK, et al. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 2016; 374:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kajubi R, Ochieng T, Kakuru A, et al. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a randomized controlled trial [published online ahead of print 22 March 2019]. Lancet doi: 10.1016/S0140-6736(18)32224-4. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins H, González IJ, Polley SD, et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 2013; 208:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Natureeba P, Ades V, Luwedde F, et al. Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 2014; 210:1938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fried M, Muehlenbachs A, Duffy PE. Diagnosing malaria in pregnancy: an update. Expert Rev Anti Infect Ther 2012; 10:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Beaudrap P, Turyakira E, White LJ, et al. Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospective cohort with intensive malaria screening and prompt treatment. Malar J 2013; 12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg 2010; 104:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madanitsa M, Kalilani L, Mwapasa V, et al. Scheduled intermittent screening with rapid diagnostic tests and treatment with dihydroartemisinin-piperaquine versus intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy in Malawi: an open-label randomized controlled trial. PLoS Med 2016; 13:e1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am J Trop Med Hyg 2007; 76:849–54. [PubMed] [Google Scholar]
- 26. Huynh BT, Fievet N, Gbaguidi G, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg 2011; 85:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valea I, Tinto H, Drabo MK, et al. ; FSP/MISAME Study Group An analysis of timing and frequency of malaria infection during pregnancy in relation to the risk of low birth weight, anaemia and perinatal mortality in Burkina Faso. Malar J 2012; 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Beaudrap P, Turyakira E, Nabasumba C, et al. Timing of malaria in pregnancy and impact on infant growth and morbidity: a cohort study in Uganda. Malar J 2016; 15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan AD, Nyirenda T, Cullinan T, Taylor T, Lau A, Meshnick SR. Placental haemozoin and malaria in pregnancy. Placenta 2000; 21:417–21. [DOI] [PubMed] [Google Scholar]
- 30. Kalilani-Phiri L, Thesing PC, Nyirenda OM, et al. Timing of malaria infection during pregnancy has characteristic maternal, infant and placental outcomes. PLoS One 2013; 8:e74643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore KA, Simpson JA, Wiladphaingern J, et al. Influence of the number and timing of malaria episodes during pregnancy on prematurity and small-for-gestational-age in an area of low transmission. BMC Med 2017; 15:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor SM, Madanitsa M, Thwai KL, et al. Minimal impact by antenatal subpatent Plasmodium falciparum infections on delivery outcomes in Malawian women: a cohort study. J Infect Dis 2017; 216:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.