Abstract
The inability to achieve adequate intracellular antiretroviral concentrations may contribute to HIV persistence within the brain and to neurocognitive deficits in opioid abusers. To investigate, intracellular antiretroviral concentrations were measured in primary human astrocytes, microglia, pericytes, and brain microvascular endothelial cells (BMECs), and in an immortalized brain endothelial cell line (hCMEC/D3). HIV-1 Tat and morphine effects on intracellular antiretroviral concentrations also were evaluated. After pretreatment for 24 h with vehicle, HIV-1 Tat, morphine, or combined Tat and morphine, cells were incubated for 1 h with equal concentrations of a mixture of tenofovir, emtricitabine, and dolutegravir at one of two concentrations (5 μM or 10 μM). Intracellular drug accumulation was measured using LC-MS/MS. Drug penetration differed depending on the drug, the extracellular concentration used for dosing, and cell type. Significant findings included: 1) Dolutegravir (at 5 μM or 10 μM) accumulated more in HBMECs than other cell types. 2) At 5 μM, intracellular emtricitabine levels were higher in microglia than other cell types; while at 10 μM, emtricitabine accumulation was greatest in HBMECs. 3) Tenofovir (5 or 10 μM extracellular dosing) displayed greater accumulation inside HBMECs than in other cell types. 4) After Tat and/or morphine pretreatment, the relative accumulation of antiretroviral drugs was greater in morphine-exposed HBMECs compared to other treatments. The opposite effect was observed in astrocytes in which morphine exposure decreased drug accumulation. In summary, the intracellular accumulation of antiretroviral drugs differed depending on the particular drug involved, the concentration of the applied antiretroviral dose, and cell type targeted. Moreover, morphine, and to a lesser extent Tat, exposure also had differential effects on antiretroviral accumulation. These data highlight the complexity of optimizing brain-targeted HIV therapeutics, especially in the setting of chronic opioid use or misuse.
Keywords: neuro-human immunodeficiency virus (neuroHIV), tenofovir, emtricitabine, dolutegravir, opioid, blood-brain barrier, mass spectrometry
Introduction
Despite the aggressive use of combination antiretroviral therapy (cART) and following years of suppressive therapy, HIV viral loads persist within the CNS [1–3]. The lack of maximally effective penetration of some antiretroviral drugs into the CNS likely contributes to low levels of ongoing HIV replication and production of viral proteins, causing chronic inflammation and leading to the development of neurocognitive impairment [4–6]. Opioid abuse can exacerbate HIV progression and increase the severity and incidence of neurocognitive impairment [7,8]. Several studies demonstrate that opioids can increase HIV and simian immunodeficiency virus (SIV) replication [9,10], alter host immune function [11], increase viral loads [10,12], and hasten the progression to AIDS [13–15].
The blood-brain barrier (BBB) is a selective barrier, which restricts the free movement of substances between the blood and the CNS. The principal cell type comprising the BBB is the brain microvascular endothelial cell (BMEC). Barrier properties and functions are also influenced by interactions with many other cell types such as astrocytes, pericytes, microglia and neurons, which collectively and in interaction with the endothelial cells, are termed the neurovascular unit [16,17]. The complex interactions within the neurovascular unit contribute to normal brain homeostasis and protect the brain from the extracellular environment [16,17].
Microglia, astrocytes, BMECs, and pericytes have been reported to harbor HIV infection. Microglia are a major site of productive infection within the CNS [18], while astrocytes, pericytes, and BMECs are typically thought to be sites of non-productive viral infection [19–22]. Astrocytes, BMECs, and microglia express μ-opioid receptors, while μ-opioid receptor expression has not been detected in pericytes despite their displaying functional responses to morphine [23–26].
Within weeks after infection, HIV crosses the BBB and enters the CNS [27]. The CNS is a major HIV reservoir site and triggers the neuropathologic complications of HIV infection [17]. There are multiple routes by which HIV is thought to cross the BBB to enter the CNS, including passive diffusion of free virus either transcellularly (through the cell) or paracellularly (between the cells). HIV can also enter the CNS through infected monocytes (the “trojan horse” mechanism) or can infect the BMECs or other neurovascular unit cells, which causes damage to the BBB and increases the access of HIV into the CNS.
Eradication of HIV from the CNS has remained a challenge, in part, because of poor penetration of antiretroviral drugs. Successful therapy requires effective therapeutic concentrations within each cell type that harbors HIV infection [3]. Except for entry and fusion inhibitors, all currently marketed antiretroviral drugs have intracellular targets. However, to date, little is known about intracellular antiretroviral concentrations in the primary cell types of the neurovascular unit. Nor is there much known about how opioid exposure may affect the antiretroviral concentrations. Our lab has previously demonstrated that morphine exposure to mice results in decreased brain concentrations of select antiretroviral drugs (in a region-specific manner) and also demonstrated that morphine exposure, and to a lesser extent HIV-1 Tat, resulted in damage to the BBB [28]. The prior study, however, focused on brain tissue concentrations rather than intracellular concentrations within specific cell types of the neurovascular unit of the BBB.
The purpose of this study was to analyze intracellular penetration of the cART regimen consisting of tenofovir, emtricitabine, and dolutegravir among different CNS cell types (primary HBMECs, astrocytes, microglia, pericytes, or an immortalized brain endothelial cell line, hCMEC/D3) to better understand if there are any cell type specific differences in intracellular accumulation. Additionally, we examined the effects of morphine and/or Tat exposure on the intracellular concentrations.
Methods
Chemicals and reagents
The following reagents were obtained through the National Institutes of Health (NIH) AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), tenofovir, emtricitabine, and dolutegravir. Tenofovir-d6 and emtricitabine-13C 15N2 were obtained from Toronto Research Chemical Inc. (Ontario, Canada). Dolutegravir-d5 was purchased from BDG Synthesis (Wellington, NZ). Human brain microvascular endothelial cells, hCMEC/D3, were generously provided by Dr. Babette Weksler of Weill Cornell Medical College, Cornell University, New York, NY. These cells were grown in EBM-2 medium (Lonza, Walkersville, MD) and supplemented as described previously [29] and all experiments were used between passage number 28 and 32. Primary human pericytes and astrocytes, as well as the appropriate culture medium were purchased from ScienCell, Inc. (Carlsbad, CA). Primary human microglia and medium were obtained from Celprogen, Inc. (Torrance, CA). Primary human brain endothelial cells and medium were purchased from Cell Systems, Inc. (Kirkland, WA). Recombinant HIV-1 Tat was obtained from ImmunoDX, Inc. (Woburn, MA). All other compounds were purchased from Fisher Scientific.
Treatment
All cells were grown at 5% CO2, 37°C and 95% relative humidity and when they reached 90% confluency, were treated with Tat (100 nM) and/or morphine (500 nM) for 24 h. The concentration of Tat and time interval for treatment used in this study is based on previously published reports and it reflects the levels observed clinically [29–33]. The concentration and time of morphine treatment were also based on previous studies of maximal stimulation of μ-opioid receptors by morphine [31,33–35]. Final concentrations of each antiretroviral drug within the cocktail was 5 μM or 10 μM and the final concentration of DMSO within the cell culture was less than 0.01% (v/v). The selected antiretroviral concentrations were based on the Cmax values for each drug in clinical trials [36–39]. Each cell type was treated with HIV-1 Tat, morphine, or combined Tat and morphine for 24 h and then treated for 1 h with a combination (cocktail) of three drugs: tenofovir, emtricitabine, and dolutegravir. Two different concentrations of the antiretroviral cocktail were used, one in which each drug of the three drug combination was at a final concentration of 5 μM and the other in which each drug of the three drug combination was at a final concentration of 10 μM. After 1 h, cells were rinsed, and the intracellular accumulation of each antiretroviral drug was measured.
Intracellular accumulation of antiretroviral drugs
At the end of treatments (described above), cells were washed thrice with 1 mL of ice cold HBSS. Ice-cold methanol was added on each well and the plates were placed on the ice for 15 min. After cells were collected by scraping of the culture well surface, the resultant lysate was collected and centrifuged at 7500 rpm. Supernatant (200 μL) from each sample was aliquoted into a 96-well plate, and 20 μL of 50 ng/mL internal standard for each analyte was added. The pellet containing cell debris was incubated with 50 μL lysis buffer (NP40 + complete protease inhibitor) for 5 min on ice, then sonicated and analyzed for protein content using the BCA Protein Assay. Lysate samples were vortex mixed for 5 min at 750 rpm and centrifuged for 15 min at 5700 rpm. 150 μL of supernatant was collected, filtered through a 0.45 μm pore-diameter multiscreen HTS filter plate (centrifuged for 5 min at 3500 rpm) and dried under a steady stream of nitrogen at 45 °C. The dried extracts were reconstituted with 150 μL of water, vortex mixed for 10 min at 1250 rpm and re-centrifuged again for 15 min at 5700 rpm. 50 μL of the final extract was injected for LC-MS/MS analysis, as described previously [40].
LC-MS/MS method
Details of the instrumentation and extraction procedure used were provided in our previously published paper [40]. Low- and high-quality control (QC) samples at concentrations of 2 ng/mL and 75 ng/mL were prepared in different brain cell samples to validate the applicability of the protocol on different brain cell types (data not shown). Precision and accuracy were within ±15% for all QC samples.
Statistical Analysis
Antiretroviral accumulation was assessed by calculating the drug concentration normalized to the protein content of cell extract (pmol/mg protein) and by the proportional change from baseline (% change from control) via separate 3-way repeated-measures ANOVAs. Between-subject’s factors included cell type (HBMECs, astrocytes, microglia, pericytes, or hCMEC/D3), antiretroviral condition (dolutegravir, emtricitabine, or tenofovir), and treatment condition (control, Tat, morphine, or combined Tat and morphine) with antiretroviral concentration as the within-subjects factor (5 μM or 10 μM). All interactions were delineated via simple main effects followed by main effect contrasts. All possible pairwise comparisons were assessed with alpha controlled for family-wise error. Analyses were considered significant when p < 0.05.
Results
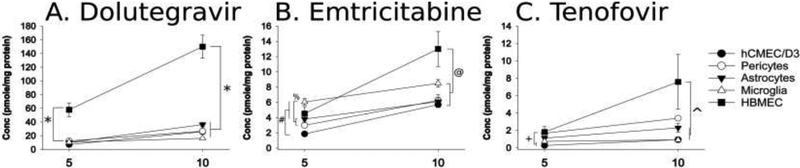
Intracellular accumulation of antiretroviral drugs significantly differed by the cell type examined and the type and concentration of antiretroviral used [F(8,300)=13.54, p<0.05]. Dolutegravir concentrations were significantly higher in HBMECs compared to any other cell type (p < 0.0001), irrespective of the extracellular concentration added (i.e., dosed) (5 or 10 μM; Fig. 1A). By contrast, the accumulation of other antiretroviral drugs was significantly influenced by antiretroviral dosing concentration and cell type.
Figure 1.
Intracellular content of each antiretroviral drugs across all five cell types. Each cell type was incubated in a medium containing a 5 or 10 μM cocktail of dolutegravir, emtricitabine, and tenofovir for 1 h, at which time the cells were harvested via methanol extraction and stored at −80 °C until LC-MS/MS analysis. (A) Dolutegravir intracellular concentrations were significantly higher in HBMECs than all other cell types examined (*). (B) Intracellular accumulation of emtricitabine was significantly higher in microglia as compared to astrocytes, pericytes and hCMEC/D3 cells exposed to 5 μM dosing concentration (%). Intracellular concentrations of emtricitabine were lowest with hCMEC/D3 cells, which was significantly lower as compared to HBMECs, pericytes, and microglia (#). In contrast, in cells exposed to 10 μM emtricitabine-containing medium, the achieved intracellular emtricitabine concentrations were highest in the HBMECs as compared to the other cell types examined (@). (C) Tenofovir, which had the lowest intracellular concentrations as compared to dolutegravir or emtricitabine, displayed the greatest accumulation in HBMECs. HBMECs accumulated significantly greater amounts than microglia or hCMEC/D3 cells (+) when they were incubated in 5 μM tenofovir. At 10 μM, HBMECs showed a significantly greater capacity to accumulate tenofovir intracellularly than microglia, pericytes and hCMEC/D3 cells where intracellular tenofovir concentrations remained lower (^). Data are expressed as mean ± SEM (picomoles/mg of protein) for n = 6 independent experiments (p < 0.05 for each comparison).
Unlike dolutegravir, which accumulated preferentially in HBMECs at either concentration, incubating cells with 5 μM emtricitabine resulted in significantly higher concentrations in microglia than in astrocytes (p < 0.0001) or pericytes (p = 0.005) and accumulated most poorly in hCMEC/D3 cells. Furthermore, intracellular emtricitabine levels in hCMEC/D3 cells were significantly lower than those observed in all other cell types (p < 0.0001–0.02; Fig. 1B), except in astrocytes. By contrast, incubating cells with 10 μM emtricitabine caused significantly greater accumulation in HBMECs than in other cell types (p < 0.0001–0.02; Fig. 1B).
Treating cells with 5 μM tenofovir showed significantly greater drug accumulation in HBMECs than microglia (p = 0.03) or hCMEC/D3 cells (where it accumulated least; p = 0.001). Astrocytes also demonstrated significantly greater accumulation of tenofovir than hCMEC/D3 cells (p = 0.003; Fig. 1C). Like other antiretrovirals examined, incubating in the higher concentration of tenofovir (10 μM) resulted in significantly greater accumulation of the drug in HBMECs than any other cell type (p = 0.003 – 0.02), except for astrocytes which did not differ from HBMECs.
To examine the effects of Tat and/or morphine on the relative changes in antiretroviral drug accumulation, intracellular accumulation of composite antiretroviral concentrations was converted to a percent change from its own control within the same cell type. Cell type, antiretroviral concentration, and morphine/Tat treatment significantly influenced the proportional change in antiretroviral drugs from their respective control groups [F(12,300)=2.636, p<0.05] (Table 2). Although Tat had few effects, relative changes in antiretroviral intracellular drug levels differed depending the extracellular antiretroviral concentration during incubation, cell type targeted and, in some instances, morphine exposure.
Table 2.
Morphine and HIV-1 Tat effects on the relative changes in antiretroviral intracellular accumulation among five cell types
| Astrocytes | Pericytes | Microglia | HBMEC | hCMEC/D3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 μM | 10 μM | 5 μM | 10 μM | 5 μM | 10 μM | 5 μM | 10 μM | 5 μM | 10 μM | |
| Control | 0.00 ± 13.79 | 0.00 ± 13.17 | 0.00 ± 16.25 | 0.00 ± 16.61 | 0.00 ± 19.78 | 0.00 ± 6.98 | 0.00 ± 14.04 | 0.00 ± 15.14 | 0.00 ± 8.24 | 0.00 ± 10.65 |
| Tat | 2.95 ± 16.31 | 14.46 ± 13.59 | −22.09 ± 11.05 | −16.63 ± 19.11 | 26.36 ± 15.83 | −20.46 ± 3.91 | 7.38 ± 18.06 | −7.46 ± 15.17 | −21.84 ± 5.14 | −10.88 ± 7.18 |
| Morphine | *−35.88 ± 6.94 | −4.15 ± 8.82 | 7.00 ± 16.15 | −30.00 ± 10.79 | 11.01± 18.17 | −11.04± 4.61 | #163.4 ± 81.15 | 67.57 ± 63.84 | −19.30 ± 5.96 | 17.59 ± 24.85 |
| Tat + Morphine | *−37.15 ± 6.78 | −7.20 ± 6.18 | 2.11 ± 13.43 | −32.80 ± 9.08 | 0.28 ± 10.25 | −13.20 ± 7.34 | 5.71 ± 15.62 | 193.63 ± 136.86 | −14.67 ± 8.87 | 2.88 ± 8.75 |
Each cell types was treated with HIV-1 Tat (100 nM), morphine (500 nM), or combined Tat and morphine for 24 h and then treated with combined antiretrovirals at 5 μM or 10 μM for 1 h prior to cell harvesting. Data are expressed as a percent of control of the intracellular accumulation of the antiretroviral drugs, as a composite value.
In astrocytes, morphine administered alone or with Tat had significantly lower intracellular accumulation than cells treated with Tat only for the 5 μM antiretroviral treatment concentrations. Although in astrocytes, morphine exposure also tended to reduce the relative amount of antiretroviral accumulation as compared to placebo controls, this effect was not statistically significant.
In HBMECs, morphine exposure resulted in a significantly greater relative amount of intracellular antiretroviral accumulation than placebo controls, Tat or Tat + morphine treated cells (at the 5 μM antiretroviral treatment concentration). No significant effects of Tat or morphine on antiretroviral intracellular concentrations in pericytes, microglia or hCMEC/D3 cells. All data are expressed as mean ± SEM (percent of control) for n=6 experiments (p<0.05).
Morphine exposure significantly reduced the proportion of intracellular antiretroviral (5 μM) accumulation in astrocytes when administered alone (p = 0.04) or in conjunction with Tat (p = 0.04) compared to Tat treatment alone (Table 2). Although morphine tended to reduce the relative amount of antiretroviral drug accumulation in astrocytes (when they were exposed to 5 μM antiretrovirals) compared vehicle-treated astrocytes, the effect was not significant (pmorphine = 0.068; pmorphine/Tat = 0.057). Neither Tat nor morphine exposure significantly affected the relative concentration of antiretroviral drugs in microglia, pericytes, or hCMEC/D3 cells (Table 2).
In contrast, in HBMECs dosed with the 5 μM antiretroviral drug cocktail, the proportion of accumulated drugs was significantly greater among cells exposed to morphine compared to the other treatment groups (p = 0.02 – 0.03; Table 2). This effect was not evident at the higher (10 μM) antiretroviral concentration.
Discussion
It is, perhaps, not surprising that there are cell-type specific differences in intracellular antiretroviral concentrations. There is experimental evidence of differential penetration and/or differential efficacy of antiretroviral drugs between cell types. Lower intracellular concentrations of select antiretroviral drugs have been reported in human microglia when compared to lymphocytes, resulting in significantly higher half maximal effective concentration (EC50) values in human microglia [41]. Similarly, it has also been reported that nucleoside reverse transcriptase inhibitor (NRTI) drugs have significantly lower efficacy in astrocytes than in monocyte-derived macrophages or peripheral mononuclear blood cells [42]. Less is known, however, about intracellular accumulation of antiretroviral drugs among each of the various cell types comprising the neurovascular unit.
In the cell types examined and conditions of our study, the rank order of intracellular accumulation (from highest to lowest concentration) was dolutegravir > emtricitabine > tenofovir, with dolutegravir being significantly higher than emtricitabine and tenofovir in each cell type examined. These data are consistent with what has been reported in the literature for CNS penetration [43,44]. Furthermore, our data demonstrate, for a given antiretroviral drug, differential accumulation between the five cell types (Fig. 1). Pharmacologic factors that influence a drug’s capacity to penetrate cells include the drug’s physicochemical properties, such as molecular size, charge and lipophilicity, substrate specificity for specific transport proteins (if applicable), as well as qualitative and quantitative differences in the cell-specific patterns of expression and function of individual uptake and efflux transport proteins. It is difficult to predict the role of particular transport proteins in mediating the observed dissimilarities in antiretroviral accumulation among cell types because of differences in the expression and/or activity of each of 15 or more major classes of transport proteins within each of the cell types examined have not been fully elucidated [45–50].
The biological implications for the differential intracellular antiretroviral content in the context of HIV are not fully understood. However, of the cell types examined, microglia are the most important site of active HIV replication [18]. Low antiretroviral concentrations within microglia could contribute to the maintenance of the microglia as an important reservoir for HIV and sub-optimal inhibition of viral replication could lead to the development of resistant strains. Astrocytes and pericytes also harbor HIV, albeit with only low level replication or as latent virus [21,51]. Sustaining high intracellular levels of antiretroviral drugs may be important even within latently infected cells to prevent the emergence from latency and HIV reactivation.
When cells were incubated with 10 μM drugs, HBMECs accumulated all three antiretroviral drugs at higher concentrations than other cell types (Fig. 1). BMECs are the primary physical barrier of the neurovascular unit, which protects the brain from toxins through controlling the flux of compounds into the brain [17]. Moreover, HBMECs can sequester drugs intracellularly within lysosomes [52] and perhaps other organellar/subcellular compartments, depending on the physicochemical properties of the drug. The observation of increased antiretroviral accumulation within the HBMECs may involve sequestration as a protective mechanism. Future studies would be necessary to verify this theory but are beyond the scope of the current study.
In our studies, we were also interested in learning whether HIV-1 Tat and/or morphine exposure would alter the intracellular accumulation of antiretroviral drugs. In most cell types examined, Tat exposure did not affect antiretroviral accumulation. In astrocytes, however, Tat and morphine interacted to reduce composite antiretroviral exposure was significantly less in Tat-treated astrocytes as compared to morphine or Tat and morphine co-exposed groups, but not control cells.
Morphine exposure significantly impacted the overall accumulation of antiretroviral drugs in astrocytes and HBMECs, and the directionality of the change depended on the cell type. Within astrocytes, morphine-exposure alone or in combination with Tat resulted in significantly lower antiretroviral accumulation (when incubated in 5 μM drugs) than astrocytes treated with Tat by itself. Astrocytes are well known targets of HIV-1, contribute to disease progression, and may harbor latent infection [51,53]. Astrocytes are also important cellular targets for opioid-HIV-1 interactions. They can express opioid receptors, and opioid drugs can disrupt intracellular calcium homeostasis and increase pro-inflammatory cytokine release—especially in the presence of HIV proteins [33,54]. Accordingly, morphine-mediated decreases in antiretroviral penetration within astrocytes, as observed in our studies, may decrease antiviral efficacy, thereby enhancing their vulnerability to HIV and limiting their ability to support neurons.
In contrast, within HBMEC cells, morphine exposure significantly increased antiretroviral accumulation in HBMECs compared to vehicle, Tat, or combined Tat and morphine-treated HBMEC cells. Morphine exposure can augment the expression of platelet derived growth factor within human brain microvascular endothelial cells and compromise barrier function in vitro [55]. Morphine exposure also can increase P-glycoprotein expression in primary human brain microvascular endothelial cells, perhaps through increased trafficking from nuclear stores to the plasma membrane [56] or through activation of the NMDA/cyclo-oxygenase-2 cascade [57], while decreasing the expression of ZO-1 and occludin junctional proteins [58]. The mechanisms by which morphine altered antiretroviral concentrations within these studies was not examined, however, future studies examining the extent and mechanism(s) by which morphine exposure differentially affects the expression and function of individual drug transporters are warranted.
We have previously demonstrated μ-opioid receptor (MOR-1) expression in human astrocytes and microglia [23,24]. In contrast, μ-opioid receptor expression was not detected in pericytes. Interestingly, HBMEC cells were found to express the novel μ-opioid receptor splice variant, MOR1-K but not the canonical MOR-1 [23]. MOR-1K is preferentially found in the brain, is expressed intracellularly and couples to G∝S and stimulates the release of nitric oxide and increases intracellular calcium. Thus, the differential responses to morphine exposure by each cell type observed in these studies may be a consequence of the variability in μ-opioid receptor expression and the associated function. Furthermore, even within a particular class of cell, the functional consequences of μ-opioid receptor activation are highly contextual and influenced by regional differences, the duration of exposure, and stage of development, and may also depend on other factors such as ongoing physiological or pathological processes [59,60].
Besides Tat negating morphine-dependent increases in 5 μM antiretroviral accumulation in HBMECs (Table 2), few interactions between Tat and morphine were observed in these studies. While several studies [33,58,61] have demonstrated that morphine can exacerbate the inflammatory effects of Tat, this is not consistently observed in isolated cell types and may differ among brain regions. For example, morphine-driven positive proinflammatory feedback between μ-opioid receptor-expressing astroglia and microglia results in spiraling inflammation that is not seen with either glial type alone [15]. Morphine can exacerbate Tat-dependent cytokine release from astrocyte-enriched cultures from striatum [32,33], but this is not observed in astrocyte-enriched cultures isolated from the cerebral cortex, cerebellum, or spinal cord [62].
It is perhaps not surprising that we found a significant discordance in the concentration of antiretroviral drugs in immortalized hCMEC/D3 cells and primary HBMECs. hCMEC/D3 cells are widely used, well characterized, and are convenient to model some aspects of human BBB structure and function. This cell line retains the expression of several transporters and receptors expressed in the human BBB in vivo [63], at least up to passage number 38 [64]. Nonetheless, some significant differences between hCMEC/D3 and primary cells have also been reported, which may contribute to discrepancies with primary endothelial cells. Although hCMEC/D3 cell monolayers express characteristic tight junction proteins, the levels of expression of claudin-5, junctional adhesion molecule (JAM)-2, glucose transporter (Glut)-1 and the insulin receptor are lower than in isolated primary microvessels [65]. Claudin-5 is important for junctional tightness and the lower expression levels may contribute to overall decreased cell-to-cell junctional adhesiveness that has been observed in the hCMEC/D3 cells [63]. Another critical difference between the two cell types is that the profile of expression of endocytosis-related genes differs markedly between primary brain microvascular endothelial cells and hCMEC/D3 cells [66]. Manipulation of culture conditions, including the activation of the Wnt/β-catenin pathway, activation of nuclear receptors, or using microfluidics to mimic the shear force associated with blood flow can tighten the barrier properties [63] and may also alter the experimental outcome. Accordingly, some caution is warranted when interpreting data generated using hCMEC/D3 cells as the model for the BBB.
Nucleoside reverse transcriptase inhibitors (NRTIs), such as tenofovir and emtricitabine, need to be converted to their di- and tri-phosphorylated forms, respectively, to be pharmacologically active [67,68]. The studies herein measured only intracellular concentrations of the parent drug, not their phosphorylated, active forms. Therefore, we may have overlooked potential effects of Tat and/or morphine on the phosphorylation, and thus activation, of these drugs. However, this is unlikely because it typically takes up to 18 h for antiretroviral drugs to become phosphorylated intracellularly [69], while our experiments only lasted 1 h. Regardless, future studies that include NRTI drugs should examine the effects of Tat and morphine treatment on total concentrations of the inactive, and di- and tri-phosphorylated forms of each drug.
Overall, our findings demonstrate that the accumulation of antiretroviral drugs in the CNS differ in cell-type specific manner, and that morphine, and to a lesser extent Tat, can uniquely influence antiretroviral levels in each cell type. Since reservoirs of both active and latent HIV infection can be differentially established within distinct CNS cell types early during the disease, it is important to determine the bioavailability of individual antiretrovirals in each cell type. Moreover, exposure to HIV-1 Tat or morphine further affected antiretroviral concentrations in a cell-type specific manner, which highlights the complexity and challenges in developing brain-targeted HIV-1 therapeutics, especially in individuals who abuse opioids. Our studies examined these phenomena within isolated cell types of the neurovascular unit. Future studies, beyond the scope of the present findings, are warranted to begin to understand the complexities of drug transport through the neurovascular unit, and how each individual cell type influences the kinetics of drug flux and the behavior of other cell types within the neurovascular unit.
Table 1.
Intracellular Concentrations of Antiretroviral Drugs under Control Conditions
| Astrocytes | Pericytes | Microglia | HBMEC | hCMEC/D3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 μM | 10 μM | 5 μM | 10 μM | 5 μM | 10 μM | 5 μM | 10 μM | 5 μM | 10 μM | |
| Tenofovir | 2.08 ± 0.59 | 3.36 ± 0.69 | 1.08 ± 0.30 | 2.83 ± 1.07 | 0.65 ± 0.23 | 1.03 ± 0.12 | 1.05 ± 0.41 | 2.83 ± 1.02 | 0.40 ± 0.09 | 0.82 ± 0.15 |
| Emtricitabine | 3.61 ± 0.68 | 6.29 ± 1.85 | 4.10 ± 1.16 | 7.21 ± 1.30 | 4.99 ± 0.89 | 9.45 ± 1.02 | 3.26 ± 0.44 | 10.52 ± 2.73 | 1.89 ± 0.13 | 5.68 ± 0.99 |
| Dolutegravir | 12.06 ± 3.35 | 35.55 ± 7.82 | 13.19 ± 4.39 | 35.46 ± 11.54 | 11.41 ± 5.60 | 18.36 ± 2.90 | 47.76 ± 7.98 | 151.22 ± 28.98 | 7.88 ± 0.92 | 27.70 ± 6.34 |
Intracellular content (picomole/mg protein) of each antiretroviral drug when given in the culture medium as a 5 or 10 μM cocktail. Data are represented at means ± SEM, n= 6.
Highlights.
Intracellular antiretroviral accumulation within cells of the neurovascular unit differs depending on drug and cell type
In response to morphine exposure, there is decreased intracellular exposure of antiretroviral drugs within astrocytes
In response to morphine exposure, there is increased intracellular exposure of antiretroviral drugs within HBMECs
Acknowledgements.
This work was supported by funds from NIH: R21 DA045630 (MPM), K02 DA027374 (KFH), R00 DA039791 (JJP), and VCU’s CTSA (UL1TR000058 from the National Center for Advancing Translational Sciences) and the CCTR Endowment Fund of Virginia Commonwealth University (MPM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW, Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation., AIDS. 28 (2014) 2251–8. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, Winston A, Van Sighem A, Miro JM, Podzamczer D, Olson A, Arribas JR, Moreno S, Meyer L, Del Romero J, Dabis F, Bucher HC, Wandeler G, Vourli G, Skoutelis A, Lanoy E, Gasnault J, Costagliola D, Hernán M. a., Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions, Neurology. 83 (2014) 134–141. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Calcagno A, Di Perri G, Bonora S, Pharmacokinetics and Pharmacodynamics of Antiretrovirals in the Central Nervous System, Clin. Pharmacokinet 53 (2014) 891–906. doi: 10.1007/s40262-014-0171-0. [DOI] [PubMed] [Google Scholar]
- [4].Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R, Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus, Virus Res. 111 (2005) 194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [5].Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A, Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein., Proc. Natl. Acad. Sci. U. S. A 110 (2013) 13588–93. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC, HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment, Nat. Rev. Neurol (2016). doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur JC, Grant I, Group C, Neurocognitive impact of substance use in HIV infection., J. Acquir. Immune Defic. Syndr 58 (2011) 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, Navia B, Cohen R. a., Neurocognitive Effects of HIV, Hepatitis C, and Substance Use History, J. Int. Neuropsychol. Soc 18 (2012) 68–78. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH, Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures., AIDS. 4 (1990) 869–73. [DOI] [PubMed] [Google Scholar]
- [10].Bokhari SM, Hegde R, Callen S, Yao H, Adany I, Li Q, Li Z, Pinson D, Yeh H-W, Cheney PD, Buch S, Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques., J. Neuroimmune Pharmacol 6 (2011) 626–39. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Roy S, Ninkovic J, Banerjee S, Charboneau R, Das S, Dutta R, Kirchner V, Koodle L, Ma J, Meng J, Opioid Drug Abuse and Modulation of Immune Function: Consequences in the Susceptibility to Opportunistic Infections, J. Neuroimmune Pharmacol 6 (2013) 442–465. doi: 10.1007/s11481-011-9292-5.Opioid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reddy PVB, Pilakka-Kanthikeel S, Saxena SK, Saiyed Z, Nair MPN, Interactive effects of morphine on HIV infection: Role in HIV-associated neurocognitive disorder, AIDS Res. Treat 2012 (2012). doi: 10.1155/2012/953678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dutta R, Roy S, Mechanism(s) Involved in Opioid Drug Abuse Modulation of HAND, Curr. HIV Res. 10 (2012) 469–477. doi: 10.2174/157016212802138805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A, Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques., Virology. 354 (2006) 192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- [15].Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE, Opiate drug use and the pathophysiology of neuroAIDS., Curr. HIV Res 10 (2012) 435–52. doi:22591368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Egleton RD, Abbruscato T, Drug abuse and the neurovascular unit., Adv. Pharmacol 71 (2014) 451–80. doi: 10.1016/bs.apha.2014.06.019. [DOI] [PubMed] [Google Scholar]
- [17].Shawahna R, Physical and metabolic integrity of the blood-brain barrier in HIV infection: a special focus on intercellular junctions, influx and efflux transporters and metabolizing enzymes, Curr. Drug Metab 16 (2015) 105–123. [DOI] [PubMed] [Google Scholar]
- [18].Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE, Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis., Am. J. Pathol 155 (1999) 1599–611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DFJ, a Thompson K, Gabuzda D, McArthur JC, a Pardo C, Wesselingh SL, Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues., J. Neurovirol 12 (2006) 146–52. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- [20].Nottet HS, Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood - brain barrier function., J. Neurovirol 5 (1999) 659–69. [DOI] [PubMed] [Google Scholar]
- [21].Nakagawa S, Castro V, Toborek M, Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier., J. Cell. Mol. Med 16 (2012) 2950–7. doi: 10.1111/j.1582-4934.2012.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ, Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry., AIDS. 10 (1996) 573–85. [DOI] [PubMed] [Google Scholar]
- [23].Dever SM, Costin BN, Xu R, El-Hage N, Balinang J, Samoshkin A, O’Brien MA, McRae M, Diatchenko L, Knapp PE, Hauser KF, Differential expression of the alternatively spliced OPRM1 isoform μ-opioid receptor-1K in HIV-infected individuals., AIDS. 28 (2014) 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stiene-Martin A, Zhou R, Hauser KF, Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes., Glia. 22 (1998) 249–59. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luk K, Boatman S, Johnson KN, Dudek OA, Ristau N, Vang D, Nguyen J, Gupta K, Influence of Morphine on Pericyte-Endothelial Interaction: Implications for Antiangiogenic Therapy, J. Oncol 2012 (2012) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hauser KF, Knapp PE, Interactions of HIV and drugs of abuse: the importance of glia, neural progenitors, and host genetic factors., 2014. doi: 10.1016/B978-0-12-801284-0.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jaureguiberry-Bravo M, Wilson R, Carvallo L, Berman JW, Opioids and Opioid Maintenance Therapies: Their Impact on Monocyte-Mediated HIV Neuropathogenesis., Curr. HIV Res 14 (2016) 417–430. doi: 10.2174/1570162X14666160324124132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim W-K, Knapp PE, Kashuba ADM, Hauser KF, McRae M, HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity., J. Neurovirol (2019). doi: 10.1007/s13365-019-00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Patel S, Leibrand CR, Palasuberniam P, Couraud P-O, Weksler B, Jahr FM, McClay JL, Hauser KF, McRae M, Effects of HIV-1 Tat and Methamphetamine on Blood-Brain Barrier Integrity and Function In Vitro., Antimicrob. Agents Chemother 61 (2017) in press. doi: 10.1128/AAC.01307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nath A, Conant K, Chen P, Scott C, Major EO, Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon., J. Biol. Chem 274 (1999) 17098–102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- [31].Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE, Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at muopioid receptor-expressing glia, Brain. 134 (2011) 3613–3628. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF, Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription., PLoS One. 3 (2008) e4093. doi: 10.1371/journal.pone.0004093.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF, Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat., Glia. 50 (2005) 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stiene-Martin A, Hauser KF, Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro., J. Neurosci. Res 29 (1991) 538–48. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC, mu-Opioid receptor-induced Ca2+ mobilization and astroglial development: morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca(2+)-dependent mechanism., Brain Res. 720 (1996) 191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Anderson PL, Zheng JH, King T, Bushman LR, Predhomme J, Meditz A, Gerber J, Fletcher CV, Concentrations of zidovudine- and lamivudine-triphosphate according to cell type in HIV-seronegative adults, Aids. 21 (2007) 1849–1854. doi: 10.1097/QAD.0b013e3282741feb. [DOI] [PubMed] [Google Scholar]
- [37].I. Gilead Sciences, Viread® [Package Insert], 2012.
- [38].I. Gilead Sciences, Emtriva® [Package Insert], 2012.
- [39].GlaxoSmithKline, TIVICAY ® [Package Insert], 2013.
- [40].Patel SH, Ismaiel OA, Mylott WR, Yuan M, Hauser KF, McRae M, Simultaneous determination of intracellular concentrations of tenofovir, emtricitabine, and dolutegravir in human brain microvascular endothelial cells using liquid chromatography-tandem mass spectrometry (LC-MS/MS), Anal. Chim. Acta 1056 (2019) 79–87. doi: 10.1016/j.aca.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Asahchop EL, Meziane O, Mamik MK, Chan WF, Branton WG, Resch L, Gill MJ, Haddad E, V Guimond J, Wainberg MA, Baker GB, Cohen EA, Power C, Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain., Retrovirology. 14 (2017) 47. doi: 10.1186/s12977-017-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gray LR, Tachedjian G, Ellett AM, Roche MJ, Cheng W-J, Guillemin GJ, Brew BJ, Turville SG, Wesselingh SL, Gorry PR, Churchill MJ, The NRTIs lamivudine, stavudine and zidovudine have reduced HIV-1 inhibitory activity in astrocytes., PLoS One. 8 (2013) e62196. doi: 10.1371/journal.pone.0062196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Letendre SL, Mills AM, Tashima KT, Thomas DA, Min SS, Chen S, Song IH, Piscitelli SC, ING116070: A study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects, Clin. Infect. Dis 59 (2014) 1032–1037. doi: 10.1093/cid/ciu477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, CHARTER Group, Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system., Arch. Neurol 65 (2008) 65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Koepsell H, Endou H, The SLC22 drug transporter family, Pflugers Arch. Eur. J. Physiol 447 (2004) 666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- [46].Taneva E, Crooker K, Park SH, Su JT, Ott A, Cheshenko N, Szleifer I, Kiser PF, Frank B, Mesquita PMM, Herold BC, Differential mechanisms of tenofovir and tenofovir disoproxil fumarate cellular transport and implications for topical preexposure prophylaxis, Antimicrob. Agents Chemother 60 (2016) 1667–1675. doi: 10.1128/AAC.02793-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR, Mechanism of active renal tubular efflux of tenofovir., Antimicrob. Agents Chemother 50 (2006) 3297–304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB, The expression and functional characterization of ABCG2 in brain endothelial cells and vessels., FASEB J. 17 (2003) 2085–7. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- [49].Ronaldson PT, Persidsky Y, Bendayan R, Regulation of ABC membrane transporters in glial cells: Relevance to the pharmacotherapy of brain HIV-1 infection, Glia. 56 (2008) 1711–1735. doi: 10.1002/glia.20725. [DOI] [PubMed] [Google Scholar]
- [50].Seithel A, Karlsson J, Hilgendorf C, Björquist A, Ungell AL, Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: Comparison between human segments and Caco-2 cells, Eur. J. Pharm. Sci 28 (2006) 291–299. doi: 10.1016/j.ejps.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [51].Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR, Extensive astrocyte infection is prominent in human immunodeficiency virus - associated dementia, Ann. Neurol 66 (2009) 253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- [52].Noack A, Gericke B, von Köckritz-Blickwede M, Menze A, Noack S, Gerhauser I, Osten F, Naim HY, Löscher W, Mechanism of drug extrusion by brain endothelial cells via lysosomal drug trapping and disposal by neutrophils, Proc. Natl. Acad. Sci 115 (2018) E9590–E9599. doi: 10.1073/pnas.1719642115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brack-Werner R, Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis., AIDS. 13 (1999) 1–22. [DOI] [PubMed] [Google Scholar]
- [54].Hauser KF, El-Hage N, Buch S, Nath A, Tyor WR, Bruce-Keller AJ, Knapp PE, Impact of opiate-HIV-1 interactions on neurotoxic signaling, J. Neuroimmune Pharmacol 1 (2006) 98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- [55].Wen H, Lu Y, Yao H, Buch S, Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PLoS One. 6 (2011) e21707. doi: 10.1371/journal.pone.0021707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schaefer CP, Arkwright NB, Jacobs LM, Jarvis CK, Hunn KC, Largent-Milnes TM, Tome ME, Davis TP, Chronic morphine exposure potentiates p-glycoprotein trafficking from nuclear reservoirs in cortical rat brain microvessels, PLoS One. 13 (2018) 1–16. doi: 10.1371/journal.pone.0192340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yousif S, Chaves C, Potin S, Margaill I, Scherrmann J-M, Declèves X, Induction of P-glycoprotein and Bcrp at the rat blood-brain barrier following a subchronic morphine treatment is mediated through NMDA/COX-2 activation., J. Neurochem 123 (2012) 491–503. [DOI] [PubMed] [Google Scholar]
- [58].Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, Chawda R, Shanahan TC, Schwartz SA, Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability., J. Clin. Immunol 28 (2008) 528–41. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- [59].Hauser KF, Mangoura D, Diversity of the endogenous opioid system in development. Novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation., Perspect. Dev. Neurobiol 5 (1998) 437–49. [PubMed] [Google Scholar]
- [60].Hauser KF, Knapp PE, Opiate Drugs with Abuse Liability Hijack the Endogenous Opioid System to Disrupt Neuronal and Glial Maturation in the Central Nervous System, Front. Pediatr 5 (2018) 1–23. doi: 10.3389/fped.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF, Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro., Neuroscience. 102 (2001) 555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE, Regional Heterogeneity and Diversity in Cytokine and Chemokine Production by Astroglia: Differential Responses to HIV-1 Tat, gp120, and Morphine Revealed by Multiplex Analysis, J. Proteome Res 9 (2010) 1795–1804. doi: 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Weksler B, Romero IA, Couraud P-O, The hCMEC/D3 cell line as a model of the human blood brain barrier., Fluids Barriers CNS. 10 (2013) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tai LM, Reddy PS, Lopez-Ramirez MA, Davies HA, Male ADK, Loughlin AJ, Romero IA, Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123, Brain Res. 1292 (2009) 14–24. [DOI] [PubMed] [Google Scholar]
- [65].Urich E, Lazic SE, Molnos J, Wells I, Freskgård P-O, Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models., PLoS One. 7 (2012) e38149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ilina P, Partti S, Niklander J, Ruponen M, Lou YR, Yliperttula M, Effect of differentiation on endocytic profiles of endothelial and epithelial cell culture models, Exp. Cell Res 332 (2015) 89–101. doi: 10.1016/j.yexcr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [67].Robbins BL, V Srinivas R, Kim C, Bischofberger N, Fridland A, Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA., Antimicrob. Agents Chemother. 42 (1998) 612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cihlar T, Ray AS, Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine., Antiviral Res. 85 (2010) 39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- [69].Shen L, Peterson S, Sedaghat AR, a McMahon M, Callender M, Zhang H, Zhou Y, Pitt E, Anderson KS, Acosta EP, Siliciano RF, Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs., Nat. Med 14 (2008) 762–766. doi: 10.1038/nm1777. [DOI] [PMC free article] [PubMed] [Google Scholar]