Abstract
Large B-cell lymphoma with IRF4 rearrangement is a provisional entity in the 2017 World Health Organization classification. In order to characterize these lymphomas in children from the United States, IRF4 fluorescence in situ hybridization and immunohistochemical stains were performed on 32 follicular (FL) and diffuse large B-cell lymphomas (DLBCL) from Children’s Oncology Group studies. Two DLBCLs (6%) had IRF4 rearrangements, one involving the ileocecal valve and another involving the tonsil and cerebrospinal fluid. Both cases had strong, diffuse IRF4/MUM1 immunohistochemical staining, which may be a pathologic clue to the diagnosis. Reclassification of these cases may have prognostic and therapeutic implications.
Keywords: diffuse large B cell lymphoma, follicular lymphoma, IRF4, lymphoma, pediatric
INTRODUCTION
Non-Hodgkin lymphoma (NHL) accounts for 5-7% of childhood cancers, with diffuse large B-cell lymphoma (DLBCL) representing 10-20% and follicular lymphoma (FL) 1-2% of pediatric NHL.1 FL in pediatric patients can be divided into “pediatric-type” and “usual/adult” variants based upon morphology, immunohistochemistry, genetics, and clinical information.2–4 In 2011, a German group identified translocations between IGH, IGL, and IGK with IRF4 in 20 of 427 (5%) cases of DLBCL and high grade FL.5 These lymphomas were more frequent in children under age 18 years, typically had limited stage in the head and neck region including the Waldeyer ring, and showed a better prognosis. The revised 2017 World Health Organization (WHO) classification of hematopoietic and lymphoid neoplasms introduced a new provisional entity of large B-cell lymphoma with IRF4 rearrangement, encompassing these higher grade FL (3B) and DLBCLs.6 Herein, pediatric cases of FL and DLBCL from Children’s Oncology Group (COG) studies were screened for this translocation and immunohistochemical properties in order to assess the incidence of these newly classified lymphomas in United States cohorts.
METHODS
FL from COG rare NHL registry ANHL04B17 and DLBCL (excluding mediastinal) from COG B-NHL trials ANHL1131 and CCG-59618 were identified from the central pathology review archives and approved for use in this study by COG (protocol #ANHL16B2-Q and #ANHL16B3-Q) and the Cancer Therapy Evaluation Program. Informed consent was obtained by the patient and/or parent/guardian. Cases with sufficient unstained formalin-fixed paraffin embedded slides were included, and hematoxylin and eosin-stained (H&E) slides were reviewed to confirm the submitted diagnoses. Fluorescence in situ hybridization (FISH) using the IRF4/DUSP22 break-apart probe (Kreatech probe KI-10613, Leica Biosystems, Buffalo Grove, IL) was performed, with tissues demonstrating ≥11% of cells with separation of 5’ and 3’ signals considered rearranged based on our clinical laboratory validation. Immunohistochemistry (IHC) staining for MUM1/IRF4, CD10, BCL6, and/or BCL2 (Supplemental Information Table S1) was performed if not previously reported at diagnosis or done during central review. Cases were considered positive if ≥30% tumor cells stained.9 A minimum of ten 200x fields of each section were examined in IHC analyses. Clinical characteristics including age, gender, biopsy site, disease site(s), cytogenetics, clinical stage, and outcome were obtained from COG files (Supplemental Information Table S2).
RESULTS
FISH for IRF4 rearrangements was successfully performed on 32 cases. H&E slide review confirmed 20 cases of DLBCL and 11 cases of pediatric-type FL; however, one FL case was reclassified as DLBCL in a background of follicular hyperplasia. The male to female ratios were 9:2 and 16:9 with median ages of 13.0 years (range 6.4-18.6) and 12.8 years (range 4.5-18.1) for the FL and DLBCL cases, respectively.
IRF4 rearrangements were identified in 2/32 cases. The first case was the reclassified DLBCL which occurred in a 5.9-year-old female with stage IV disease involving the tonsil and cerebrospinal fluid (CSF). Figure 1 demonstrates the histology of her tonsil, positive MUM1 staining in >95% of cells, and weak BCL6 staining; this lymphoma lacked expression of BCL2 and CD10. This child is alive without evidence of relapse 1379 days after registry enrollment, with therapy including cyclophosphamide, doxorubicin, etoposide, methotrexate, cytarabine, and vincristine with intrathecal cytarabine and methotrexate. The second IRF4-rearranged case was a DLBCL occurring in a 16.3-year-old male with isolated disease in his ileocecal valve, Murphy stage II. Figure 2 demonstrates the histology from his ileum and strongly positive MUM1 in >90% of the cells. This lymphoma lacked CD10 and BCL2 expression; staining for BCL6 failed, likely due to long-term storage of the unstained slide prior to testing. This patient is also alive without evidence of relapse 1446 days after enrollment and treatment on the CCG-5961 trial.8
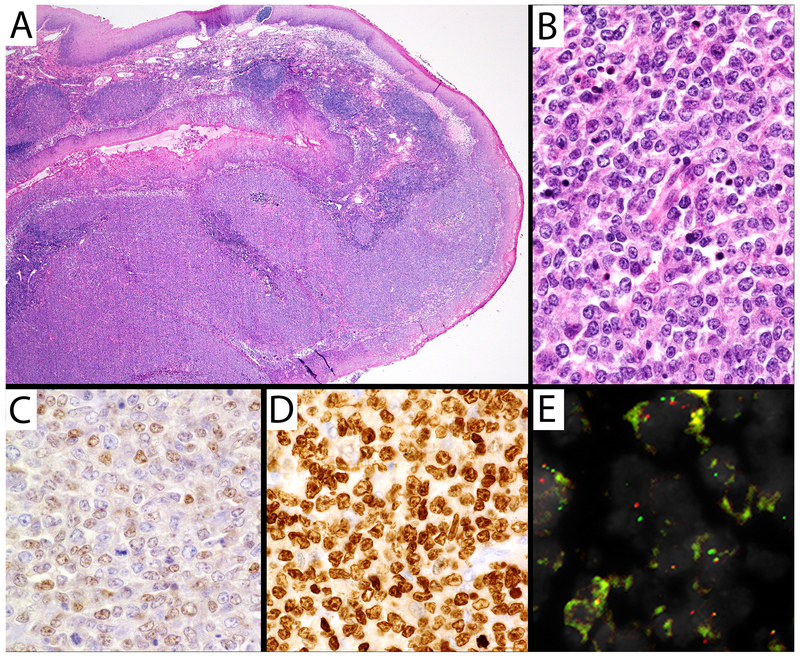
Figure 1. Large B-cell lymphoma with IRF4 rearrangement from the tonsil.
A) H&E 20X of tonsil demonstrating squamous mucosa overlying an atypical lymphoid proliferation. B) H&E 400X demonstrating atypical cells with round to irregular nuclear contours, vesicular chromatin, and prominent nucleoli. C, A subset of atypical lymphocytes expressed weak BCL6 (400X), while (D) >95% of atypical lymphocytes strongly expressed MUM1 (400X). The cells lacked BCL2 and CD10. E) FISH demonstrated separated red and green probes from the IRF4 gene break-apart probe.
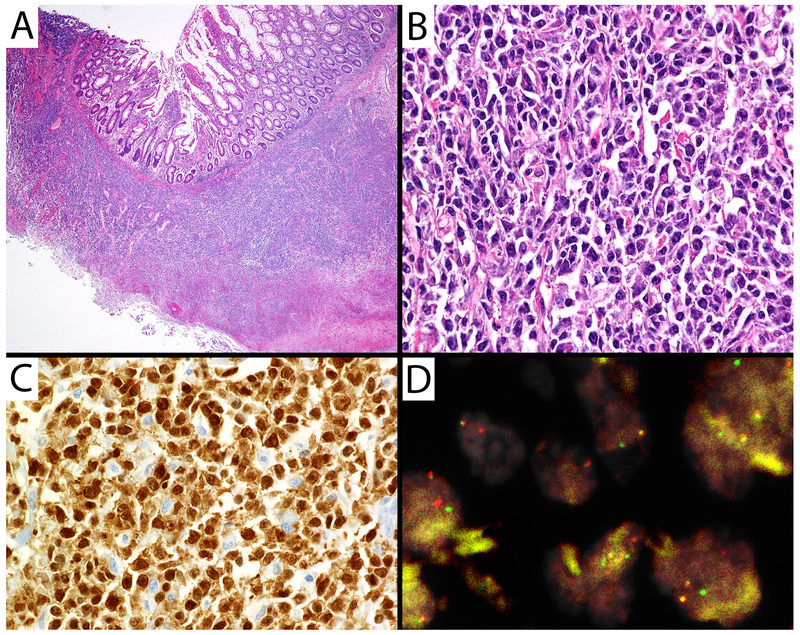
Figure 2. Large B-cell lymphoma with IRF4 rearrangement of the small intestine.
A) H&E 20X demonstrating small intestinal mucosa overlying an atypical lymphoid proliferation. B) H&E 400X demonstrating atypical cells with irregular nuclear contours, coarse chromatin, and variably conspicuous nucleoli. C) >90% of atypical lymphocytes strongly expressed MUM1 (400X). The cells lacked CD10 and BCL2 expression. BCL6 staining failed. D) FISH demonstrated separated red and green probes from the IRF4 gene break-apart probe.
MUM1 IHC staining was successfully performed in 29/32 cases: 3 cases had >90% positive cells, 4 had 60-90% positive cells, 3 had 30-60% positive cells, and 16 were negative (<30%); 3 additional cases had positive staining reported by the submitting institution but without quantitation. CD10 was positive in 20/31 cases, BCL6 in 18/23 cases, and BCL2 in 9/25 cases. Two of the 3 cases with >90% MUM1 positive cells demonstrated IRF4 gene rearrangements. The third case with strong, diffuse MUM1 staining was a DLBCL that also expressed BCL6 and CD10 in >80% of cells. This case had stage IV disease involving retroperitoneal and inguinal lymph nodes, the nasopharynx, spleen, lung, intestine, and parameninges with an abnormal karyotype of 46,XY,del(6)(q13q23),del(11)(p11.2p15)[21]/46,XY[1]. While not all IRF4 translocations can be demonstrated with current techniques6 and some reports have described pediatric Waldeyer ring lymphomas with IRF4/MUM1 overexpression lacking these gene rearrangements,10,11 the high stage, aggressive disease in this patient is not suggestive of a large B-cell lymphoma with IRF4 rearrangement.
DISCUSSION
Our series confirms that large B-cell lymphomas with IRF4 rearrangements occur at a low frequency, herein 6%, and 5% in the largest published series of 20 cases.5 The head and neck region including the Waldeyer ring is the most common site,3,5 as seen in the tonsil lymphoma in this series. The other case in this series occurred at the ileocecal valve, a less common but described location, found in 4 cases in the German cohort.5 The German series also found that 84% of cases had limited disease stage, consistent with the current ileocecal case, but not the tonsil case (which demonstrated CSF involvement). Yet, the reported better prognosis and overall survival5 is supported by both of the cases in our series showing no evidence of disease more than 3.5 years after diagnosis, though both were treated with intense chemotherapy.
Morphologically, large B-cell lymphomas with IRF4 rearrangements can appear as high grade FL, DLBCL, or FL with diffuse components. The identification of a diffuse component can be helpful in differentiating an IRF4-rearranged lymphoma from a pediatric-type FL, as demonstrated by the tonsil case which was originally submitted as a pediatric-type FL but reclassified as DLBCL. This case also co-expressed MUM1 and BCL6, an atypical finding in normal germinal centers12 and pediatric-type FL,2 as IRF4 usually suppresses BCL6 expression. In the German series, MUM1 was expressed in all 20 cases of large B-cell lymphoma with IRF4 rearrangement (having strong intensity in 17/20), BCL6 in 94% of cases, and CD10 and BCL2 in approximately 65% of cases each.5 MUM1 and BCL6 co-expression has been reported in other reports of these lymphomas.3,11,13 Of the 22 cases with MUM1 and BCL6 IHC staining in this series, only 9 were positive for both stains, including the IRF4-rearranged DLBCL of the tonsil (the ileal case failed BCL6 staining). Of note, the two cases with IRF4 gene rearrangements had strong intense staining of >90% cells, and this strong MUM1 staining has been reported in most of the IRF4-rearranged cases in the literature.3,5,11,13
In conclusion, IRF4 rearrangements occurred at low frequency in patients enrolled in these COG studies. These cases would be reclassified under WHO 2017 criteria as large B-cell lymphomas with IRF4 rearrangements, which may have important therapeutic implications due the associated good prognosis. Studies will need to be undertaken to determine if chemotherapy can be reduced or a watch and wait strategy can be utilized for those with localized disease after excision. Strong and diffuse MUM1 staining may be suggestive of this diagnosis, so this stain should be assessed routinely on pathologic examination, with follow-up FISH evaluation to confirm the diagnosis.
Supplementary Material
Supplemental Information Table S1: Immunohistochemical stain antibody information
Supplemental Information Table S2: Clinical and pathologic characteristics of 32 COG cases.
ACKNOWLEDGMENTS
Samples from Children’s Oncology Group rare NHL registry ANHL04B1 and COG B-NHL trials ANHL1131 and CCG-5961 were used in this study (COG protocol #ANHL16B2-Q and #ANHL16B3-Q) and we acknowledge the following COG grants associated with the research: NCTN Operations Center Grant U10 CA180886, NCTN Statistics and Data Center Grant U10 CA180899, and Human Specimen Banking in NCI-Sponsored Clinical Trials U24CA114766. This research is also supported by St. Baldrick’s Foundation. We thank the patients, families, physicians, and institutions who participated in these COG studies. We also thank the dedicated members of the Immunohistochemistry Laboratory at ARUP Laboratories for performing the immunohistochemical stains. This work was supported by the ARUP Institute for Clinical and Experimental Pathology, William L. Roberts Memorial Fund.
Abbreviation key:
- COG
Children’s Oncology Group
- CSF
Cerebrospinal fluid
- DLBCL
Diffuse large B cell lymphoma
- FISH
Fluorescence in situ hybridization
- FL
Follicular lymphoma
- H&E
Hematoxylin and eosin-stained
- IHC
Immunohistochemistry
- NHL
Non-Hodgkin lymphoma
- WHO
World Health Organization
Footnotes
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest
Previous publication: This paper was presented as “IRF4 Translocation status in pediatric follicular and diffuse large B cell lymphoma patients enrolled in Children’s Oncology Group Trials” at the 6th International Symposium on Childhood, Adolescent and Young Adult Non-Hodgkin Lymphoma, Rotterdam, Netherlands, September 26-29, 2018. Abstract published in British Journal of Haematology. 182(S1): 81, #131.
REFERENCES
- 1.Lorsbach RB, Shay-Seymore D, Moore J, et al. Clinicopathologic analysis of follicular lymphoma occurring in children. Blood. 2002;99:1959–1964. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL and Siebert R. Paediatric-type follicular lymphoma In: Swerdlow SH, Campo E, Harris NL, et al. , (eds.). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer, 2017, p. 278–279. [Google Scholar]
- 3.Liu Q, Salaverria I, Pittaluga S, et al. Follicular lymphomas in children and young adults: a comparison of the pediatric variant with usual follicular lymphoma. Am J Surg Pathol. 2013;37:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oschlies I, Salaverria I, Mahn F, et al. Pediatric follicular lymphoma--a clinico-pathological study of a population-based series of patients treated within the Non-Hodgkin’s Lymphoma--Berlin-Frankfurt-Munster (NHL-BFM) multicenter trials. Haematologica. 2010;95:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salaverria I, Philipp C, Oschlies I, et al. Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood. 2011;118:139–147. [DOI] [PubMed] [Google Scholar]
- 6.Pittaluga S, Harris NL, Siebert R, et al. Large B-cell lymphoma with IRF4 rearrangement In: Swerdlow SH, Campo E, Harris NL, et al. , (eds.). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer, 2017, p. 280–281. [Google Scholar]
- 7.O’Suoji C, Welch JJ, Perkins SL, et al. Rare Pediatric Non-Hodgkin Lymphomas: A Report From Children’s Oncology Group Study ANHL 04B1. Pediatr Blood Cancer. 2016;63:794–800. [DOI] [PubMed] [Google Scholar]
- 8.Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Al-Kzayer LF, Liu T, et al. IFR4/MUM1-positive lymphoma in Waldeyer ring with co-expression of CD5 and CD10. Pediatr Blood Cancer. 2017;64:311–314. [DOI] [PubMed] [Google Scholar]
- 11.Quintanilla-Martinez L, Sander B, Chan JK, et al. Indolent lymphomas in the pediatric population: follicular lymphoma, IRF4/MUM1+ lymphoma, nodal marginal zone lymphoma and chronic lymphocytic leukemia. Virchows Arch. 2016;468:141–157. [DOI] [PubMed] [Google Scholar]
- 12.Martinez A, Pittaluga S, Rudelius M, et al. Expression of the interferon regulatory factor 8/ICSBP-1 in human reactive lymphoid tissues and B-cell lymphomas: a novel germinal center marker. Am J Surg Pathol. 2008;32:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma A, Epari S, Gujral S, et al. An unusual presentation of large B-cell lymphoma with interferon regulatory factor 4 gene rearrangement. Indian J Pathol Microbiol. 2018;61:271–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information Table S1: Immunohistochemical stain antibody information
Supplemental Information Table S2: Clinical and pathologic characteristics of 32 COG cases.