Abstract
In this study the chemical composition, morphology, crystallinity, thermal, and pasting properties of Anchote (Coccinia abyssinica) starch were compared with commercial potato and wheat starches. Anchote starch showed lower total starch content than that of potato starch. Their morphological properties were investigated using scanning electron microscopy. The mean granule width of potato starch was four times greater than anchote starch and two times greater than that of wheat starch. The x-ray powder diffraction analysis revealed that anchote starch had a B-type crystallinity pattern. Differential scanning calorimetric (DSC) results showed the significant differences between the gelatinization temperature of anchote, wheat, and potato starches. The onset, peak, and conclusion temperature of anchote starch were 66.58 °C, 70.18 °C, and 73.98 °C, respectively. The gelatinization temperature of potato and wheat starches were 56.53 °C and 55.56 °C for onset, 61.46 °C and 61.14 °C for peak, 68.47 °C and 67.06 °C for conclusion, respectively. These properties of anchote starch make it an attractive candidate for industrial use.
Keywords: Anchote, Coccinia abyssinica, starch
1. Introduction
Due to its wide applicability special attention should be given to discover new crops and plant parts with high starch content and yield [1]. This investigation was performed to study the physicochemical, thermal, and pasting properties of Anchote (Coccinia abyssinica) and to compare its properties with commercially available potato and wheat starches. This investigation was performed to explore the feasibility of Anchote as a potential raw material for thermoplastic starch production.
Anchote (Coccinia abyssinica) is an indigenous tuber crop to Ethiopia; Anchote is a local name for Coccinia abyssinica. This tuber crop belongs to the order Cucurbitales and family Cucurbitaceae [2]. There are eight species of Coccinia in Ethiopia and most of them are wild, except Coccinia abyssinica [3]. Western, southern, and southwestern areas of Ethiopia are where Anchote is cultivated and utilized [4]. In addition to its nutritional value the society uses it for its medicinal properties [5].
The crop grows at wide range of altitudes, from 1300 to 2800 meters above sea level, and where the annual rainfall is 762–1016 mm [6]. Remarkably, anchote can give reasonable yields in conditions of acidic soils, drought, low soil fertility, and in intercropping with cereals [7]. In normal conditions it has the total yield of 150–180 quintals per hectare [2]. The proximate analysis and nutritional values have been performed by different groups revealing that the major chemical composition of anchote is starch [2, 7, 8].
Starch is polysaccharide molecule which is renewable, inexpensive, and the second most abundant organic polymer [9]. It is produced by green plants for energy storage over long periods, which is a storage polysaccharide [10]. Starch is found in the seeds and roots of plants in the form of granules [11]. Starch granules have a variety of shapes such as oval, angular, round, spherical or irregular and range in size from approximately 0.1 μm to more than 200μm[10].
The composition of starch can be categorized into two major groups. The first group contains the major components amylose and amylopectin. Amylose is 15 to 35% of the starch granules in most plants. It is primarily a linear polysaccharide with α-(1–4)-linked D-glucose units. Amylopectin is a highly branched molecule, with α-(1–4)-linked D-glucose backbones and exhibits about 5% of α-(1–6)-linked branches [10, 12, 13]. The second group contains the minor components of starch such as proteins, lipids, and minerals. In general, starch is classified as regular starches which have around 70–80% amylopectin, waxy starches which contain less than 10% amylose, or high amylose starches which have more than 40% amylose[11].
Starch is a natural polymer but doesn’t possess the physicochemical, thermal, or rheological properties that polymers which are used as thermoplastic materials exhibit. Native starch properties can be modified in the presence of plasticizers (such as glycerol, water, sorbitol, etc.) or with thermal or mechanical energy. This modified starch is called thermoplastic starch (TPS), which is an amorphous or semi-crystalline material developed from gelatinized or destructurized starch containing one or more plasticizers. TPS can be molded or shaped using heat and shear forces. Thermoplastic starch can be processed by conventional methods used in the plastic industry[14].
Native or modified starch finds uses in food and it can be an ingredient for different food stuffs such as, coffee whitener, snack foods, soups, sugar syrups, meat products, and others. Its non-edible applications include manufacturing of paper, bioplastics, adhesives, textiles, sanitary pads, building materials, seed coatings, pharmaceuticals, and for drilling purpose [15].
Starches have attractive characteristics such as natural accessibility, biodegradability and easy chemical and physical modifications that permit new applications [16]. Variations in physicochemical properties such as morphology, crystallinity, and composition, function properties which include thermal properties, rheological properties, swelling power and solubility of starch granules indicate their botanical origin and growth conditions. These properties could have significant influence on the final properties of the end product [17]. Therefore, it is necessary to understand the physicochemical and functional properties of starch from different sources before selecting it for some specific application.
2. Materials and Methods
2.1. Materials
Anchote (Coccinia abyssinica) tuber was obtained from the local market at Nekemet, Oromia Region, Ethiopia. Commercial wheat and potato starches were purchased fromSigma-Aldrich.
2.2. Starch Extraction
The starch of Anchote tuber was extracted using a modified literature procedure [18]. The raw Anchote tuber was washed to remove debris from the surface and peeled. The peeled tuber was cut into approximately 1 cm cubes. The size was further reduced using a mechanical blender for four minutes in order to get the crushed anchote tuber with a uniform size. A 10 % (w/v) crushed tuber and tap water suspension was prepared. The suspension was filtered with 250 μm sieve and the filtrate was kept for 12 h. The supernatant is decanted and the sediment was washed until it became pure white. For further purification, the suspension of distilled water and sediment was centrifuged (5810R, eppendorf, Germany)at 3000 × g for 10 minutes. The sediment was dried at 40 °C for 48 h in an oven. The starch power was made from dried sediment using a mortar and pestle.
2.3. Physicochemical Properties
2.3.1. Proximate composition
The proximate composition was carried out by using the methods in Association of Official Analytical Chemists (AOAC) standard methods. Total starch content was determined by the AOAC (2007) official method 996.11 using Total Starch Assay Kit (Megazyme International Ireland Ltd. Co.,Wicklow, Ireland), Moisture content was executed using the AOAC (2006) official method 950.46 moisture removal process, AOAC (2006) official method 992.15 was used to determine the crude protein. Ethylene diamine tetra acetic acid (EDTA—9.75% nitrogen) was used as a standard reference for calibration purposes as well as blanks and the crude protein content was calculated by multiplying with a nitrogen factor 6.25, the fat content was determined by the AOAC (2006) official method 989.05 ether extraction and percent of ash was determined using the ash oven method described in the AOAC (2006) official method 920.153.
2.3.2. Morphology
The morphology and particle size of the starch granules was investigated using scanning electron microscopy. Powder starch samples were evenly sprinkled on double sided adhesive tape and were attached to a circular specimen aluminum stub. The samples were coated with a 10 nm thick coating of gold using gold sputter coater (Desk II, Denton Vacuum). Photomicrographs were taken by Field Emission Scanning Electron Microscope (JSM-6500F, JEOL Ltd., Japan) using 5.0 kV of accelerated voltage and a 10 mm of working distance. The size of starch granules were estimated using Image J, image analyzer, software. Randomly, thirty granules were selected and their length and width were measured from the micrographs.
2.3.3. Crystallinity
The crystalline structure of the starch samples were investigated by X-ray powder diffraction/XRD ( Bruker D8 Davinci) set with Cu radiation at a wavelength of 1.5406 Å. The XRD was operated at 40 kV of target voltage and 40 mA current at room temperature. The scanning angle (2θ) was in the range from 5° to 50°.
2.4. Thermal Properties
Differential Scanning Calorimetery, DSC, (DSC Q20, TA instruments, USA) was used to determine the thermal or gelatinization properties of starches; the method developed by Zhou, Zhong and Chen [19] was followed with minor modification. The starch samples (3 g), mixed with distilled water with a ration of 1:3, starch to distilled water. The mixtures were agitated by magnetic stirrer at room temperature for one hour. The sample was quantitatively transferred into DSC pans. Then they were hermetically sealed. The samples were heated from 30 °C to 100 °C at a rate of 10 °C/minute. The gelatinization parameters such as onset (To), peak (Tp), conclusion (Tc) temperatures and gelatinization enthalpy were analyzed and calculated by DSC software (TA instruments, USA), The enthalpy of gelatinization was reported per gram of dry starchThe thermal stability and decomposition of starch samples were measured by TGA instrument (PerkinElmer Ltd., Waltham, USA). 7 to 10mg of starch samples were heated from 25 to 700 °C with a heating rate of 10 °C /min and under nitrogen gas flow rate of 20 mL/min.
2.5. Pasting Properties
The pasting characteristics were determined with a Rapid Visco-Analyzer (RVA series 4, Newport Scientific, NSW, Australia). A sample of 3 grams of dry starch was added to 25 mL of deionized water to make a 12 % (w/v) starch slurry. The starch slurry was heated from 50 °C to 95 °C for 4 min, held at 95 °C for 2 min, cooled from 95 °C to 50 °C in 4 min and held at 50 °C for 2 min. From RVA pasting curve pasting temperature, peak viscosity, pasting time, hold viscosity, breakdown viscosity, final viscosity and set back viscosity were obtained.
2.6. Statistical Analysis
Experiments were conducted in triplicate for each sample and results were presented as the mean ± standard deviation (SD). The software SPSS 20 was used for the determination of statically significance between samplecomparisons. Differences were considered at significant level of 95% and (p-value of < 0.05).
3. Results and Discussion
3.1. Proximate Composition
Moisture, crude protein, total starch, fat, and ash contents of starch samples are presented in Table 1. Crude protein content of starches extracted from anchote, potato, and wheat were 0.34%, 0.20% and 0.38%, respectively. The crude protein content of wheat and potato starches were less than previously reported values [20, 21]. The fat content in anchote starch was 0.20% which was relatively lower than the fat content of both potato and wheat starches. Anchote starch had ash content which was greater than wheat starch and less than potato starch ash content. The ash content, which is the residue after the combustion process into carbon-free product, comes from minerals and inorganic salts in the fresh tuber, fertilizer use, and can also come from the soil and air contamination during processing [22].
Table 1.
Proximate Composition of Anchote, Potato and Wheat Starches (w/w % in dry basis)
| Samples | Total Starch (%) | Crude Protein (%) | Fat (%) | Moisture Content (%) | Ash (%) |
|---|---|---|---|---|---|
| Anchote | 78.71 ± 0.07a | 0.34 ± 0.05a | 0.20 ± 0.00a | 9.06 ± 0.11a | 0.30 ± 0.00a |
| Potato | 89.30 ± 0.43b | 0.20 ± 0.05ab | 0.23 ± 0.06a | 4.03 ± 0.21b | 0.33 ± 0.15a |
| Wheat | 87.28 ± 1.02c | 0.39 ± 0.07ac | 0.27 ± 0.06a | 7.50 ± 0.20c | 0.19 ± 0.09a |
Data (mean ± SD) in the same column with different superscript letters , a, b, c, were significantly different (P ≤ 0.05)
Anchote starch had the lowest total starch content at around 78% while potato starch had the highest value. This value indicates all hydrolysable carbohydrates. Moisture contents were 9.06%, 4.03% and 7.50% in anchote, potato, and wheat starches, respectively. Anchote starch had the highest moisture content and is related with the method used to extract the starch. In this work, anchote starch was extracted using water alone, with the aim of make the process more green. The obtained results for protein and fat contents testify the efficiency of extraction process.
3.2. Morphology
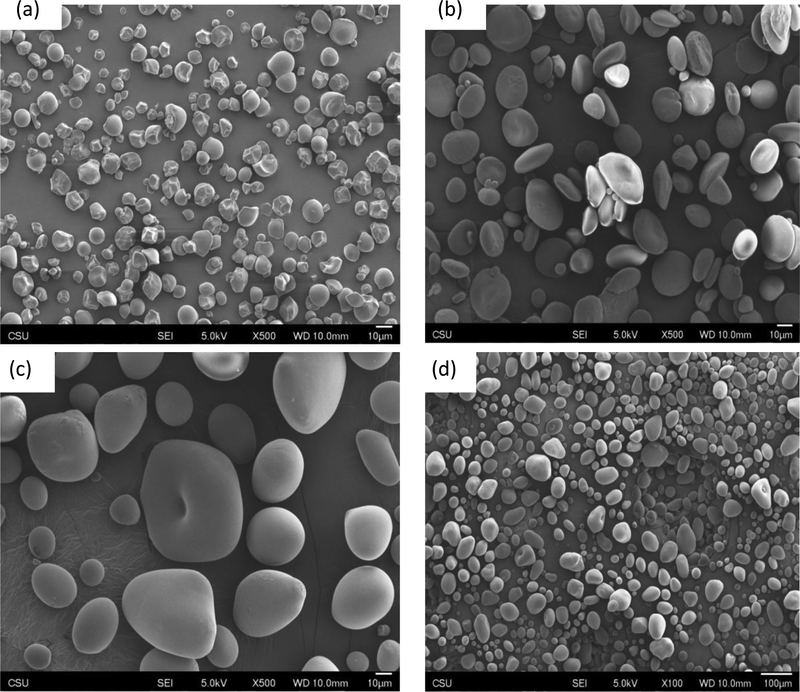
The morphological features of starch granules were evaluated using SEM. The SEM images in Fig. 1 depict that anchote starch granules had different morphological characteristics from that of wheat or potato starches. The observed microscopic appearance of potato and wheat starch granules shape is in agreement with the literature. Wheat and potato starches have granule shape which depends on their size. Wheat starch granules were composed of two different categories based on their size. The larger granules had mainly a disk shape, while the smaller granules exhibited mainly spherical or ellipsoidal shape [23–25]. In the case of potato starches the classification becomes broader. This range is due to the potato granules possessing broad particle size distributions as indicated in Table 2. For potato starch, large granules were mainly ellipsoidal in shape, the small granules were mainly spherical, whereas medium granules constituted of both ellipsoidal and spherical particles [26]. On the other hand, anchote starch granules were dome shaped with polygonal or irregular geometries on the other face. The remaining anchote granules had irregular shapes. It has been reported that the morphology of the starch granules are affected by physiology of the plant, biological origin, and the biochemistry of the chloroplast [27].
Figure 1.
SEM images of a) Anchote starch (500X), b) Wheat starch (500X), c) Potato starch (500X), and d) Potato starch (100X)
Table 2.
Anchote, Potato, and Wheat Starch Granule Size.
| Anchote | Potato | Wheat | |
|---|---|---|---|
| Mean granule width (μm) | 8.85 | 34.44 | 15.41 |
| Width range (μm) | 1.31 – 17.26 | 1.25 – 71.42 | 3.75 – 32.04 |
| Mean granule length (μm) | 11.50 | 51.42 | 22.49 |
| Length range (μm) | 3.35 – 18.09 | 5.13 – 87.89 | 5.01– 34.38 |
Starch granules size range, average width, and length of anchote, potato and wheat starches are summarized in Table 2. Approximately, the mean granule width of potato starch is four times greater than anchote starch and two times greater than that of wheat starch. The starches taken from root and tuber crop had granule sizes ranging from 1 μmto 110 μm, depending on the starch sources. By SEM, all granules appeared smooth [28]. The obtained results for anchote and potato starches strongly agree with previous work regarding the relationship between granules morphology and size.
3.3. Crystalline structure
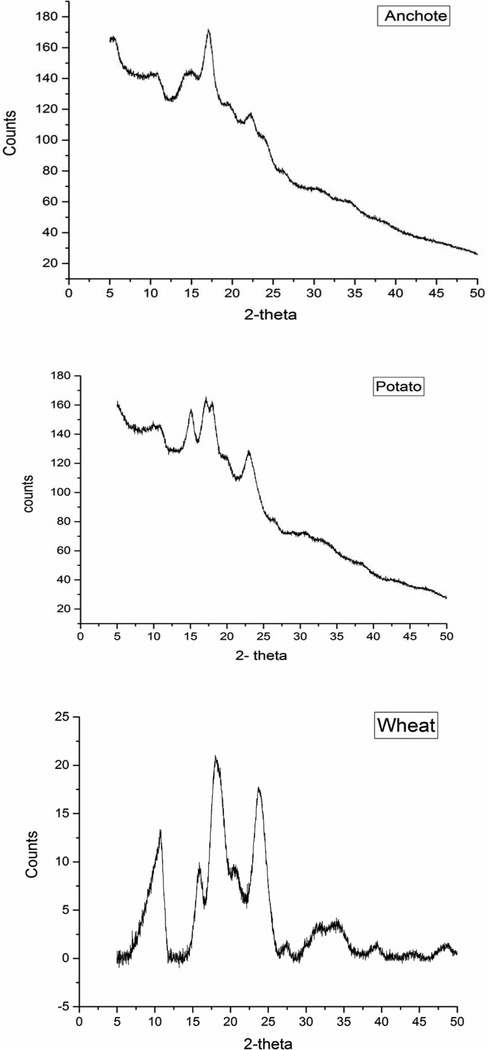
Fig.2 shows the x-ray diffraction pattern of anchote, potato, and wheat starches. The diffraction patterns provide information on the arrangement of amylopectin chain in double helices [29]. The x-ray pattern of starch has been classified as A-type, B-type, or C-type. A-type starches have a doublet at around 17° and 18° of 2θ and present strong diffraction peaks about 15° and 23° of 2θ. B-type patterns show a strong peak at 17° of 2θ and a characteristic peak about 5.6° of 2θ. In addition to these peaks, it has small peaks around 15°, 22°, and 24° of 2θ. C-type patterns are a combination of A- and B-type [30–32]. Potato starch demonstrated peaks at 5°, 15°, 17° and 22.96° of 2θ. This result supports that potato starch had type-B pattern, as previously documented [26, 28]. Wheat starch had diffraction peaks at 10.68°, 15.87°, 18° and 23.73° of 2θ. This result supports that wheat starch has a type-A pattern consistent with the previous findings [33]. Anchote starch showed strong diffraction peaks at 5° and 17° of 2θ with small peak at 22.25° of 2θ, in accord with a B-type diffraction pattern.
Figure 2.
X-ray powder diffraction patterns of anchote, potato, and wheat starches.
3.3. Thermal Properties
Thermal properties of starch granules are characterized by endothermic gelatinization enthalpy (ΔH) and gelatinization temperatures. These temperatures are further classified as onset (To), peak (Tp), and conclusion (Tc) gelatinization temperatures. Gelatinization is a process in which starch granules change their semi crystalline structure into an amorphous state. This transformation is attained by heating starch in the presence of water or different plasticizers such as glycerol, glucose, sorbitol, ethylene glycol, and amides [34]. Differential scanning calorimetry (DSC) is commonly used for starch thermal analysis.
The gelatinization temperatures, gelatinization temperature ranges, and gelatinization enthalpy (ΔH) of anchote, potato, and wheat starches are presented in Table 3. The onset temperature is the temperature when samples begin to gelatinize, whereas the maximum intensity of gelatinization occurs at peak temperature and conclusion temperature is the temperature where gelatinization concludes [35]. The onset, peak and conclusion temperatures of anchote starch were 66.58 °C, 70.18 °C and 73.98 °C, respectively. The gelatinization temperatures of potato and wheat starches were 56.53 °C and 55.56 °C for onset, 61.46 °C and 61.14 °C for peak, 68.47 °C and 67.06 °C for conclusion, respectively. Analogous results were reported for wheat starch [35] while the gelatinization temperature values obtained for potato starch were less than previously reported values [36]. These temperatures directly relate to the degree of arrangement of the molecules in starch granules [37]. Based on these observations, it can be concluded that anchote starch has higher crystallinity than both potato and wheat starch because of the observed higher gelatinization temperature values.
Table 3.
Thermal Characteristics of Anchote, Potato, and Wheat Starches.
| Sample | To (°C ) | Tp (°C ) | Tc (°C ) | Tc-To(°C ) | ΔH ( J/g) |
|---|---|---|---|---|---|
| Anchote | 66.58± 0.26a | 70.18± 0.04a | 73.98± 0.29a | 7.40± 0.45a | 11.25± 0.75a |
| Potato | 56.53± 0.34b | 61.46± 0.36b | 68.47± 1.47b | 11.93± 1.63b | 17.05± 1.09b |
| Wheat | 55.56± 0.12c | 61.14± 0.06b | 67.06± 0.39b | 11.50± 0.27b | 9.46± 0.10a |
Data (mean ± SD) in the same column with different superscript letters , a, b, c, were significantly different (P ≤ 0.05)
Gelatinization temperature ranges of anchote, potato, and wheat starches were 7.40 °C, 11.93 °C and 11.50 °C, respectively. Anchote starch had a narrow gelatinization range. The narrow gelatinization range comes from the presence of thermally least stable crystallites within the starch granules [38]. This result indicates that anchote starch has less stable crystallites than potato and wheat starches. The other important thermal properties analyzing parameter is gelatinization enthalpy (ΔH). The gelatinization enthalpy is energy needed for disrupting the ordered helical structure in starch granules [39]. The enthalpies of anchote, potato, and wheat starches were 11.25, 17.05, and 9.46 J/g, respectively. Comparatively, potato starch needed the highest energy to disorder granules semi crystalline structure.
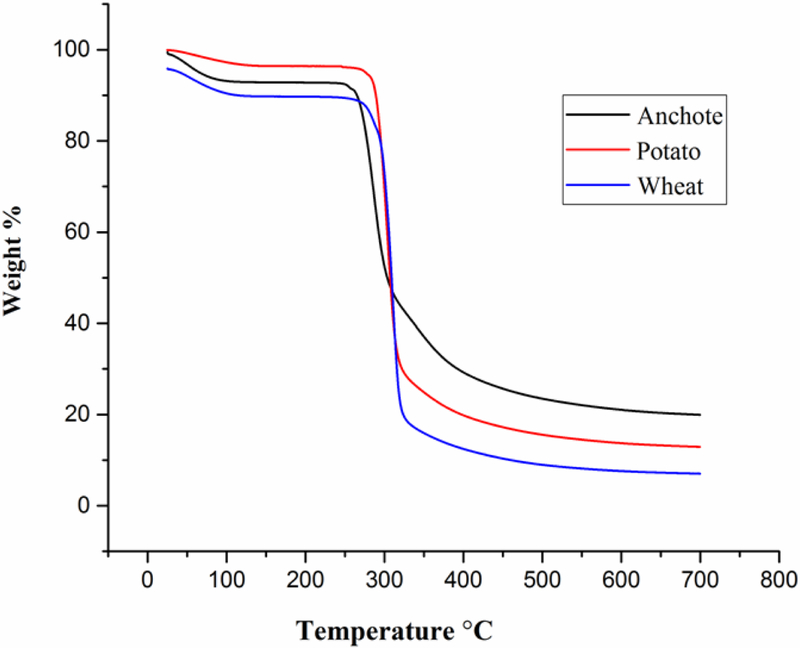
The thermal stability of the different sources of starch was evaluated using thermogravimetric analysis (TGA). TGA results of anchote, potato, and wheat starches are presented in Fig.3. Generally, weight loss occurred in three sections. In the first section, from 25 °C to 240 °C, the weight loss was 8%, 4%, and 11% for anchote, potato, and wheat, respectively. In this section, potato starch is thermally more stable than anchote and wheat starches. From the temperature range between 240 °C to 316 °C, the weight loss was 55%, 67% and 71% for anchote, potato, and wheat, respectively.
Figure 3.
TGA plot for anchote, potato, and wheat starches.
3.4. Pasting Properties
Table 4 shows Rapid-visco analyzer pasting parameters, such as pasting temperature, peak viscosity, peak time, trough viscosity, and final viscosity, for anchote, potato, and wheat starches. Pasting temperature is a temperature at which viscosity increases. Peak viscosity is the maximum viscosity during heating to or holding at specific temperature. Trough viscosity is the minimum viscosity after peak viscosity and final viscosity is the viscosity at the end of the run [34]. Wheat starch had the highest pasting temperature (76.95 °C) and anchote starch had a pasting temperature greater than potato starch, as presented in Table 4. Amylose content and particle size distribution are the main factors which affect pasting temperature [40]. Previously reported pasting temperatures were greater for potato and wheat starches [41, 42]. The peak viscosities of anchote, potato, and wheat starches were 5335 cP, 12429 cP, and 3428 cP, respectively. The highest peak viscosity recorded for potato starch granules. The peak viscosity value is directly proportional to the size of starch granules [43]. The value obtained for potato starch granules is in agreement with the literature.
Table 4.
Pasting properties of anchote, potato and wheat starches
| Parameters | Anchote | Potato | Wheat |
|---|---|---|---|
| Pasting Temperature (°C ) | 72.4 | 64.1 | 76.95 |
| Peak Viscosity (cP) | 5335 | 12429 | 3428 |
| Trough Viscosity (cP) | 4737 | 1462 | 2786 |
| Breakdown Viscosity (cP) | 598 | 10867 | 642 |
| Final Viscosity (cP) | 6996 | 3694 | 4124 |
| Setback Viscosity (cP) | 2259 | 2132 | 1338 |
| Peak Time (minute) | 5.8 | 3 | 6.33 |
Trough viscosity was found to be the highest for anchote starch (4737 cP) and the lowest for potato starch (1462 cP). The break down viscosity, which was obtained by subtracting the trough viscosity from the peak, is another important pasting parameter. This parameter provides insight into the thermal and shearing properties of starch granules. For instance, the lower breakdown values indicated the higher resistance of the starch to heating and mixing [44]. It can be concluded that anchote had the best resistance for heating and shearing. The capacity of the starch to form a viscous paste is expressed by the final viscosity [45]. From the results presented in Table 4, anchote starch had highest tendency to form a viscous paste. The last important parameter is setback viscosity. This parameter indicates the retrogradation of the starch paste induced by the amylose leached from the starch in the cooling process [44]. Regarding setback values wheat starch had the lowest and both potato and anchote starches had proportional values.
4. Conclusion
Anchote starch had 78% total starch content. Most of its granules were dome shaped with polygonal or irregular shape on geometry. The granules size varied from 1.31 to 18.09 μm. it gave B-type pattern when analyzed by x-ray powder diffraction. Its higher gelatinization temperature implies that the higher crystallinity is there in anchote starch granules. Anchote paste had good resistance for heating and shearing. The thermal stability of anchote starch makes it a potential candidate for thermoplastic starch products.
Acknowledgements
This study was funded by Addis Ababa University, Ethiopia, Colorado State University, and the National Institute of General Medical Sciences of the National Institutes of Health (R35GM119702). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nwokocha LM and Williams PA, “New starches : Physicochemical properties of sweetsop ( Annona squamosa ) and soursop ( Anonna muricata ) starches,” Carbohydr. Polym, vol. 78, pp. 462–468, 2009. [Google Scholar]
- [2].Gemede HF, “Nutritional composition , antinutritional factors and effect of boiling on nutritional composition of Anchote ( Coccinia abyssinica ) tubers,” J. Sci. Innov. Res, vol. 3, pp. 177–188, 2014. [Google Scholar]
- [3].Bekele A, Feyissa T, and Tesfaye K, “Genetic diversity of anchote ( Coccinia abyssinica ( Lam .) Cogn .) from Ethiopia as revealed by ISSR markers,” Genet Resour Crop Evol, vol. 61, pp. 707–719, 2014. [Google Scholar]
- [4].Mekbib Y and Deressa T, “Exploration and collection of root and tuber crops in East Wollega and Ilu Ababora zones : Rescuing declining genetic resources,” Indian J. Tradit. Knowl, vol. 15, pp. 86–92, 2016. [Google Scholar]
- [5].Parmar A, AgzaGebre B, Legesse A, Demelash Y, Fladung K, and Hensel O, “Nutritional Comparison of White and Red Coccinia Abyssinica (Lam.) Cong. Accessions: An Under-Utilised Edible Tuber of the Ethiopian Highlands,” Foods, vol. 6, pp. 1–8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mengesha D, Belew D, Gebreselassie W, and Sori W, “Growth and Yield Performance of Anchote [Coccinia abyssinica(Lam.) Cogn.] in Response to Contrasting Environment,” Asian J. Plant Sci, vol. 11, pp. 172–181, 2012. [Google Scholar]
- [7].Jibat GA and Buko DH, “Yield and nutrient concentration of Anchote [ Coccinia abyssinica ( Lam . ) Cogn .] affected by harvesting dates and in-situ storage,” African J. Crop Sci, vol. 3, pp. 156–161, 2015. [Google Scholar]
- [8].Desalegn BB and T Tadesse F, “Physical and Proximate Characterization of Anchote ( Coccinia abyssinica ) Accessions Grown under Hawassa and Wondo Genet,” Food Sci. Qual. Manag, vol. 42, pp. 62–75, 2015. [Google Scholar]
- [9].Ashogbon AO and Akintayo ET, “Recent trend in the physical and chemical modi fi cation of starches from different botanical sources : A review,” Starch/Stärke, vol. 66, pp. 41–57, 2014. [Google Scholar]
- [10].Perez S and Bertoft E, “The molecular structures of starch components and their contribution to the architecture of starch granules : A comprehensive review,” Starch/Stärke, vol. 62, pp. 389–420, 2010. [Google Scholar]
- [11].Schirmer M, Höchstötter A, Jekle M, Arendt E, and Becker T, “Physicochemical and morphological characterization of different starches with variable amylose / amylopectin ratio,” Food Hydrocoll, vol. 32, pp. 52–63, 2013. [Google Scholar]
- [12].Luchese CL, Spada JC, and Tessaro IC, “Industrial Crops & Products Starch content a ff ects physicochemical properties of corn and cassava starch-based fi lms,” Ind. Crop. Prod, vol. 109, no. May, pp. 619–626, 2017. [Google Scholar]
- [13].Goldstein A, Annor G, Putaux J, Hebelstrup KH, Blennow A, and Bertoft E, “Impact of full range of amylose contents on the architecture of starch granules,” Int. J. Biol. Macromol, vol. 89, pp. 305–318, 2016. [DOI] [PubMed] [Google Scholar]
- [14].Ebnesajjad S, HANDBOOK OF BIOPOLYMERS AND BIODEGRADABLE PLASTICS PROPERTIES , PROCESSING , AND. 2013. [Google Scholar]
- [15].Copeland L, Blazek J, Salman H, and Tang MC, “Form and functionality of starch,” Food Hydrocoll, vol. 23, pp. 1527–1534, 2009. [Google Scholar]
- [16].Bouza R, Rodríguez-llamazares S, Carrasco C, and Vinicius RVB, “Processing and characterization of starch-based materials from pehuen seeds ( Araucaria araucana ( Mol ) K . Koch ),” Carbohydr. Polym, vol. 88, pp. 299–307, 2012. [Google Scholar]
- [17].Li D, Yang N, Jin Y, Zhou Y, Xie Z, and Jin Z, “Changes in crystal structure and physicochemical properties of potato starch treated by induced electric field,” Carbohydr. Polym, vol. 153, pp. 535–541, 2016. [DOI] [PubMed] [Google Scholar]
- [18].Sit N, Misra S, and Deka SC, “Physicochemical , functional , textural and colour characteristics of starches isolated from four taro cultivars of North-East India,” Starch/Stärke, vol. 65, pp. 1011–1021, 2013. [Google Scholar]
- [19].Zhou D, Zhang B, Chen B, and Chen H, “Effects of oligosaccharides on pasting , thermal and rheological properties of sweet potato starch,” Food Chem, vol. 230, pp. 516–523, 2017. [DOI] [PubMed] [Google Scholar]
- [20].Alfauomy GA, Ibrahim OS, and Ali MMA, “Physico-chemical Characteristics of Starches from Different Cereal Grains,” Am. J. Food Sci. Technol, vol. 5, pp. 125–134, 2017. [Google Scholar]
- [21].Alvani K, Qi X, Tester RF, and Snape CE, “Physico-chemical properties of potato starches,” Food Chem, vol. 125, no. 3, pp. 958–965, 2011. [Google Scholar]
- [22].Cecep R, Andriansyah E, Rahman T, and Herminiati A, “Characteristics of Chemical and Functional Properties of Modified Cassava Flour ( Manihot esculenta ) by Autoclaving,” in Earth and Environmental Science 101, 2017. [Google Scholar]
- [23].Benavent-gil Y and Rosell CM, “Morphological and physicochemical characterization of porous starches obtained from different botanical sources and amylolytic enzymes,” Int. J. Biol. Macromol, vol. 103, pp. 587–595, 2017. [DOI] [PubMed] [Google Scholar]
- [24].Li C, Li C, Zhang R, Liang W, Kang X, and Jia Y, “Effects of drought on the morphological and physicochemical characteristics of starch granules in different elite wheat varieties,” J. Cereal Sci, vol. 66, pp. 66–73, 2015. [Google Scholar]
- [25].Menzel C, Seisenbaeva G, Agback P, Gällstedt M, Boldizar A, and Koch K, “Wheat starch carbamate : Production , molecular characterization , and film forming properties,” Carbohydr. Polym, vol. 172, pp. 365–373, 2017. [DOI] [PubMed] [Google Scholar]
- [26].Zhang L, Zhao Y, Hu W, Qian J, Ding X, and Guan C, “Multi-scale structures of cassava and potato starch fractions varying in granule size,” Carbohydr. Polym, vol. 200, no. August, pp. 400–407, 2018. [DOI] [PubMed] [Google Scholar]
- [27].Wang C, Tang C, Fu X, Huang Q, and Zhang B, “Granular size of potato starch affects structural properties, octenylsuccinic anhydride modification and flowability,” Food Chem, vol. 212, pp. 453–459, 2016. [DOI] [PubMed] [Google Scholar]
- [28].Hoover R, “Composition , molecular structure , and physicochemical properties of tuber and root starches : a review,” Carbohydr. Polym, vol. 45, pp. 253–267, 2001. [Google Scholar]
- [29].Jamir K and Seshagirirao K, “Isolation, characterization and comparative study of starches from selected Zingiberaceae species , a non-conventional source,” Food Hydrocoll, vol. 72, pp. 247–253, 2017. [Google Scholar]
- [30].Sun Q, Nan C, Dai L, and Xiong L, “Effect of heat-moisture treatment with maltitol on physicochemical properties of wheat starch,” LWT - Food Sci. Technol, vol. 62, no. 1, pp. 319–324, 2015. [Google Scholar]
- [31].Li M, Xie Y, Chen H, and Zhang B, “Effects of heat-moisture treatment after citric acid esteri fi cation on structural properties and digestibility of wheat starch , A- and B-type starch granules,” Food Chem, vol. 272, pp. 523–529, 2019. [DOI] [PubMed] [Google Scholar]
- [32].Guo Z, Jia X, Zhao B, Zeng S, and Xiao J, “C-type starches and their derivatives : structure and function,” Ann. N. Y. Acad. Sci, vol. 1398, pp. 1–15, 2017. [DOI] [PubMed] [Google Scholar]
- [33].Kong X, Zhou X, Sui Z, and Bao J, “Effects of gamma irradiation on physicochemical properties of native and acetylated wheat starches,” Int. J. Biol. Macromol, vol. 91, pp. 1141–1150, 2016. [DOI] [PubMed] [Google Scholar]
- [34].Linton A et al. , “Functional properties of arrowroot starch in cassava and sweet potato composite starches,” Food Hydrocoll, vol. 53, pp. 187–191, 2016. [Google Scholar]
- [35].Atrous H et al. , “Effect of γ-radiation on free radicals formation, structural changes and functional properties of wheat starch,” Int. J. Biol. Macromol, vol. 80, pp. 64–76, 2015. [DOI] [PubMed] [Google Scholar]
- [36].Hu X et al. , “Modification of potato starch by using superheated steam,” Carbohydr. Polym, vol. 198, pp. 375–384, 2018. [DOI] [PubMed] [Google Scholar]
- [37].Jane J et al. , “Effects of Amylopectin Branch Chain Length and Amylose Content on the Gelatinization and Pasting Properties of Starch 1,” Am. Assoc. Cereal Chem, vol. 76, pp. 629–637, 1999. [Google Scholar]
- [38].E. ID and H. DR, “The Effect of Solutes on the Gelatinization Temperature Range of Potato Starch,” Starch/Stärke, vol. 34, pp. 224–231, 1982. [Google Scholar]
- [39].Kim JH, Kim HR, Choi SJ, Park C, and Moon TW, “Production of an In Vitro Low-digestible Starch via Hydrothermal Treatment of Amylosucrase-modified Normal and Waxy Rice Starches and Its Structural Properties Production of an In Vitro Low-digestible Starch via Hydrothermal Treatment of Amylosucrase-modi,” J. Agric. Food Chem, vol. 64 (24), pp. 5045–5052, 2016. [DOI] [PubMed] [Google Scholar]
- [40].Zabot GL et al. , “Physicochemical, morphological, thermal and pasting properties of a novel native starch obtained from annatto seeds,” Food Hydrocoll, vol. 89, pp. 321–329, 2019. [Google Scholar]
- [41].Zhou F, Liu Q, Zhang H, Chen Q, and Kong B, “Potato starch oxidation induced by sodium hypochlorite and its effect on functional properties and digestibility,” Int. J. Biol. Macromol, vol. 84, pp. 410–417, 2016. [DOI] [PubMed] [Google Scholar]
- [42].Wang W, Guan L, Seib PA, and Shi Y, “Settling volume and morphology changes in cross-linked and unmodified starches from wheat , waxy wheat , and waxy maize in relation to their pasting properties,” Carbohydr. Polym, vol. 196, pp. 18–26, 2018. [DOI] [PubMed] [Google Scholar]
- [43].Arachchige H, Wickramasinghe M, Takigawa S, Matsuura-endo C, Yamauchi H, and Noda T, “Comparative analysis of starch properties of different root and tuber crops of Sri Lanka,” Food Chem, vol. 112, pp. 98–103, 2009. [Google Scholar]
- [44].Zhang W et al. , “Effects of potassium fertilization on potato starch physicochemical properties,” Int. J. Biol. Macromol, vol. 117, pp. 467–472, 2018. [DOI] [PubMed] [Google Scholar]
- [45].Sandhu KS and Singh N, “Some properties of corn starches II : Physicochemical , gelatinization , retrogradation , pasting and gel textural properties,” Food Chem, vol. 101, pp. 1499–1507, 2007. [Google Scholar]