Abstract
Objective:
In Persian medicine (PM), wet-cupping therapy (WCT) is the most utilized approach. WCT is mostly done between the shoulders, which is referred to as “hejamt-e-aam” in the Persian language. CD4+T cells also refer to T helper lymphocytes play a critical role in the immune system. Naïve CD4+ T cells differentiate into at least four subsets, T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17), and T regulatory (Treg) cells. The master regulator controlling each subset have been defined as follows, Tbet (Th1), Gata3 (Th2), RORγt (Th17), FoxP3 (Treg). The purpose of this study was to compare the effect of WCT and dry-cupping therapy (DCT) on the ratios of Th1/Th2 and Treg /Th17 in healthy individuals.
Materials and Methods:
Participants were divided randomly into two groups of 41 men in the WCT group and 40 men in the DCT group. Blood was taken, before, one and four weeks after the intervention. RNA was extracted from the peripheral blood mononuclear cells and the expression of T-bet, GATA-3, RORγt, and Foxp3 genes were determined by using SYBR green RT-PCR technique.
Results:
The results showed that WCT increased the expression ofGATA-3, RORγt, and Foxp3 transcription factor genes (p=0.009, p=0.001, and p=0.021, respectively). Although in the WCT group, the ratio of Foxp3/RORγt increased (p=0.048), but the ratio of T-bet/GATA-3 (Th1/Th2) decreased (p=0.971).
Conclusion:
Our findings indicated that WCT may regulate the T subsets of lymphocyte and reduce inflammation.
Key Words: Wet Cupping Therapy, Subsets of T lymphocyte, Persian medicine
Introduction
There is an increasing appeal for traditional, complementary, and alternative medicine throughout the world (Mahdavi et al., 2012 ▶). In Persian medicine (PM), there are five methods of withdrawing blood: phlebotomy (“Fasd”), wet cupping (El Sayed et al., 2014 ▶; Kordafshari et al., 2015 ▶; Montazer and Namavary, 2016 ▶), leeching, ear stinging, and blood rising from the nose. Among all these approaches, "hejamat" (in Persian) or "hijama" (in Arabic), which means "sucking", is popular in different cultures in Europe and Eastern Asia with some differences in each region (El Sayed et al., 2014 ▶).
Wet-cupping therapy (WCT), which is call “hejamt-e-aam”, is the most utilized approach of cupping in PM and it is mostly done between the shoulders butit can be also done on the head, legs, and waist. WCT is done by placing a plastic cup, which acts as a sucking tool, on the selected body part, and then the area is lacerated. Finally, the cup is placed on the lacerated area to drain the blood and interstitial fluids. The procedure should be repeated three to five times to drain the blood and fluids completely(El Sayed et al., 2014 ▶). Another cupping method is dry cupping therapy (DCT) where bloodletting is not involved.
Previous findings indicated that the interscapular site has special features due to its adjacent anatomical organs as well as the histological properties of the skin in that area. WCT has been used in PM for the treatment and prevention of various diseases (Kordafshari et al., 2015 ▶). Different characteristics of the cupping region of “hejamat-e aam” may contribute to its beneficial results. These features include the availability of brown adipose tissue or brown fat on the upper back of the chest and neck toward the shoulders( Yao et al., 2011 ▶), the proximity of the cupping region to the main vessel divisions carrying blood from the heart to the brain, the proximity to the sympathetic ganglia (stellate ganglion), and the thoracic duct. Based on Chinese medicine, the passage of five important channels of acupuncture, which can be stimulated by WCT, can release trapped energy (Wang and Wang, 2008 ▶) in the channels and help it flow correctly (Kim et al., 2011 ▶). Several studies showed that WCT may have positive effects on blood high-density lipoproteins (HDL), low-density lipoprotein (LDL), and cholesterol. The researchers showed that WCT can reduce LDL and cholesterol even a month after treatment (Arslan et al., 2014 ▶; Zarei et al., 2012 ▶). The most-frequently reported positive results of cupping therapy is pain relief (Cao et al., 2015 ▶), as well as improving acne (Wang and Wang, 2008 ▶), herpetic lesions (Cao et al., 2010 ▶), coughing, asthma (Goodwin and McIvor, 2011 ▶), eczema (Yao and Li, 2007 ▶), migraines (Tabatabaee et al., 2014 ▶) and back pain (AlBedah et al., 2015 ▶). Another beneficial effect of WCT is the improvement of the quality of life. According to Kordafshari et al., WCT can increase the quality of life one month after treatment (Kordafshari et al., 2017 ▶).
The immune system consists of two innate and adaptive components. Adaptive immunity is mediated by cells called lymphocytes, divided into two subsets of cellular and humoral (Abbas et al., 2015 ▶; Askari et al., 2016a ▶, 2016b ▶, 2018a,2018b; Askari and Shafiee-Nick , 2019a ▶,2019b ▶). T helper 1(Th1), T helper 2(Th2), T helper 17(Th17), and T regulatory (Treg) cells are all developed through differentiation of CD4+ T cells. The main function of Th1 cells is to activate macrophages. The most important cytokine produced by Th1 is interferon gamma (IFN-γ) which, along with interleukin 12 (IL-12), stimulates the differentiation of Th1 cells by activating transcription factors, T-bet, STAT4, and SATA1(Askari et al., 2016a ▶,2016b, 2018a,2018b; Askari and Shafiee-Nick, 2019a ▶, 2019b ▶; Kidd, 2003 ▶; Rahimi et al., 2017 ▶, 2018). Th2 cells, by secreting IgE, stimulate mast cells and eosinophils, which can eradicate parasitic infections. The cytokines produced by Th2 cells are interleukin 4 (IL-4), interleukin 5 (IL-5), and interleukin 13 (IL-13). IL-4 induces GATA-3 gene expression. GATA-3 is a transcription factor that acts as the most important trigger for the differentiation of Th2 (Boskabadi et al., 2018 ▶; Kidd, 2003 ▶; Rahimi et al., 2017 ▶,2018). Th17 cells recruit the leukocytes, especially neutrophils, into the infection site and IL-17is the most important cytokine produced by these cells. Th17 cell development depends on RORγt and STAT3 transcription factors. Th17 cells are an important component of the pathogenesis of many inflammatory diseases such as psoriasis, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis (Abbas et al., 2015 ▶; Grant et al., 2015 ▶). Regulatory T lymphocytes (Treg) are subtypes of CD4+ T cells that suppress immune responses and maintain self-tolerance (Grant et al., 2015 ▶). Transforming growth factor-beta (TGF-β) and interleukin 10 (IL-10) are two important cytokines secreted by Treg cells. The activation of Foxp3 transcription factor may result in the development and activation of these cells (Abbas et al., 2015 ▶; Askari et al., 2016a ▶,2016b,2018a,2018b; Askari and Shafiee-Nick, 2019a ▶, 2019b, Bacchetta et al., 2018 ▶).Several studies done using herbal medicine,evaluated changes in the ratios of lymphocyte cells subtypes and other cells involved in immunity; their finding suggested that cupping has a regulatory effect on T lymphocyte subsets (Askari et al.,2016 ▶, 2018a,2018b; Boskabady et al., 2016 ▶; Hashemzehi et al., 2016 ▶; Rahimi et al., 2018 ▶). The purpose of this study was to compare the effect of WCT and DCT on the ratios of Th1/Th2 and Treg /Th17 in healthy individuals.
Materials and Methods
Trial design and participants
This pre-post observational study investigated the effect of wet cupping on T cells subsets. The study subjects were selected from male patients who referred to the Persian Medicine clinic of the Mashhad University of Medical Sciences, Mashhad, Iran. The total number of subjects who participated in the study was 120. Participants were randomly divided into two groups using the random number table generated by computer. It was explained to each participant that he will be allocated in one of two different treatment groups, but they were not aware about the main intervention. The wet and dry cupping therapy groups consisted of 60 males. Information was taken from the records of the patients who attended the clinic in September 2017. From all subjects, 81 subjects who matched the inclusion and exclusion criteria, including 41 males in the WCT and 40 in the DCT groups, completed the study. The study process is shown in the consort flow diagram (Figure 1). The study was registered online at the Thai Clinical Trials Registry (registration No. TCTR20160609004) and Iranian Registry of Clinical Trials (registration No. IRCT20170806035515N2).
Figure 1.
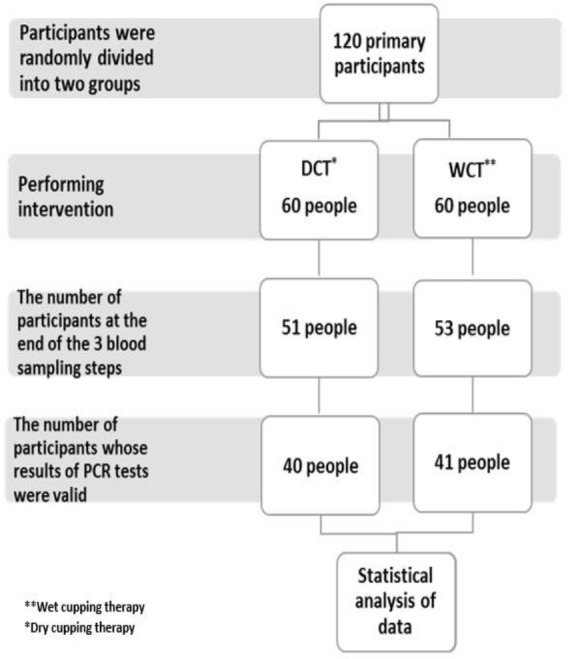
Consort flow diagram of the trial
Selection criteria
Inclusion criteria
Inclusion criteria consisted of healthy men aged between 25 and40 years old, weighed above 50 kg and had a body mass index (BMI) of 20-30. All participants had a normal body temperature (37±0.5˚C) and blood pressure (130/85±10 mmHg for systolic and diastolic, respectively). All individuals confirmed that they did not have a history of chronic diseases such as diabetes, coronary and pulmonary disease, anemia, coagulation disorders, neurology and psychiatry disorder, severe infection, or allergy and they were not taking any anti-allergic medication. Applicants who were scored over 61 by the General Health Questionnaire-28(GHQ-28) and were overweight (BMI >30), were excluded from the study.
Exclusion criteria
Exclusion criteria consisted of missing blood samples or becoming ill by any type of disease during the study.
Intervention
A verbal explanation was given to the eligible subjects, and then, their consent was obtained. All participants were examined by PM specialists. In the present study, the Persian subtitle of the GHQ-28, which was presented by Goldberg and Hiller, was employed(Goldberg and Hillier, 1979 ▶). The GHQ-28 was previously translated and approved in Iran by many studies (EBRAHIMI et al., 2007 ▶).
Before the intervention, 3 ml of venous blood was taken from each volunteer and placed in a tube (BD Co., England) with K3-EDTA anticoagulant in order to measure gene expression levels by real-time PCR method. For the WCT group, the area between the shoulders was disinfected by alcohol, and treated by oil, and slider cupping. Fixed cupping was performed on the "Aam" area and around it until the skin became expanded and reddened. Then, cups were exchanged with a 100- or 120-mlcup for 1 to 2 minutes with negative pressure cupping between the T2 and T4 on the backbones. Next, the cup was removed and seven scrapes in three rows (0.5 mm in depth) were applied by a scalpel (No. 22). Bloodletting was done about three times by applying a new disposable clean cup. In the end, the area of the procedure was cleaned using distilled water and dressed with honey. For the control DCT group, a similar procedure was used without skin scarification and bloodletting. The second blood sample (3 ml) was taken one week after the procedure. The same method was repeated during the fourth week after the procedure. Gene expression levels were measured using the SYBR green real-time PCR was done on both groups. For this purpose, the venous blood was used to extract the RNA and the cDNA was synthesized; then, transcription factor genes for T lymphocyte subsets, including T-bet, GATA-3, Foxp3, and RORyt using quantitative real-time PCR before the intervention and after one and four weeks.
RT-PCR method
Quantitative real-time PCR (qPCR) was conducted to examine the expression levels of the mRNA of the target genes in the Ficoll® isolated peripheral blood mononuclear cells (PBMCs) of the WCT group and DCT group according to the previous studies (Askari et al., 2018 ▶; Kianmehr et al., 2017 ▶), by the Roche LC96 real-time PCR system (Roche Biotechnology, Switzerland). The gene-specific primer for the qPCRs was designed based on the cDNA sequences of T-bet, GATA-3, Foxp3, and RORγt genes. The GAPDH gene was used as the internal control. The primer sequences used in the real-time PCR are shown in Table 1. The PCR SYBR® Premix Ex Taq (TliRNaseH Plus, Takara, Japan) was used according to the manufacturer's protocol. Total RNA was extracted from the PBMC using the Pars Tous Total RNA Extraction Kit (Pars Tous Biotechnology, Iran) according to the manufacturer’s protocol. The purity and concentration of total RNA were determined using a Nanodrop ND-2000 spectrophotometer (Thermo Electron Corporation, USA). The integrity of total RNA was checked by 1% agarose gel electrophoresis. cDNA was synthesized from 50µl of total RNA using the Yekta Tajhiz cDNA synthesis kit (YektaTajhizAzma, Iran) following the manufacturer’s protocol. The cDNA products were stored at -70°C to compare the gene expressions at a later time. No cell proliferation or stimulation was performed. The 2-∆∆Ct method was chosen to calculate the relative mRNA expression levels of the target genes. Normalization was performed against GAPDH expression level in each group. After the mathematical calculations, the standard curve was plotted for each gene, and after ensuring the efficiency, the Ct value results were used in the 2-∆∆Ct method for each sample. All data from the qPCRs were expressed as mean±standard error of the mean (mean±SEM).
Table 1.
The primer sequences used in the real-time PCR
| Gene | Primer | Sequence |
|---|---|---|
| GAPDH | Forward | 5'-CACTAGGCGCTCACTGTTCTC-3' |
| Reverse | 5'-CCAATACGACCAAATCCGTTGAC-3' | |
| T-bet | Forward | 5'-ATTGCCGTGACTGCCTACCAGA-3' |
| Reverse | 5'-GGAATTGACAGTTGGGTCCAGG-3' | |
| GATA-3 | Forward | 5'-ACCACAACCACACTCTGGAGGA-3' |
| Reverse | 5'-TCGGTTTCTGGTCTGGATGCCT-3' | |
| Foxp3 | Forward | 5'-GGCACAATGTCTCCTCCAGAGA-3' |
| Reverse | 5'-CAGATGAAGCCTTGGTCAGTGC-3' | |
| RORγt | Forward | 5'-CCCTGACAGAGATAGAGCACC-3' |
| Reverse | 5'-TTCCCACATCTCCCACATGG-3' |
Statistical analysis
The calculated sample size was 40 for each group based on previous studies and the Cochran formula with 5% error. Baseline data were presented as the mean±standard error of the mean. All statistical parameters were analyzed using SPSS software (version 19.0). The level of statistical significance was set at p=0.05 for all analyses. The generalized estimating equations (GEE) method was used to analyze the differences in genes expressed in each group before the intervention and after one and four weeks.
Results
All participants' of this study were male with an average age of 31.86±6.28 years old in the WCT group and 33.61±6.4 years old in the DCT group. The minimum acceptable level for hemoglobin (HB) was 12.5 g/dl and for hematocrit (HCT) was 38%. Subjects with HB and HCT below the minimum level were excluded from the study. Table 2 shows the clinical characteristics of the participants. The mean values of these variables did not show a significant difference between the two groups. Table 3 shows the descriptive statistics of hemoglobin levels at three different stages. The statistical details related to the GEE method are shown in Table 4.
Table 3.
The hemoglobin level in 3 different stages (mean±SD)
|
Hemoglobin
(g/dl) |
Before intervention
(mean±SD * ) |
One week after intervention
(mean±SD * ) |
Four weeks after intervention
(mean±SD * ) |
|||
|---|---|---|---|---|---|---|
| WCT | DCT | WCT | DCT | WCT | DCT | |
| 15.5±0.9 | 15.5±1 | 15.3±1.1 | 15.4±1 | 14.94±1 | 15.6±1 | |
*Standard deviation
Table 4.
The statistical details related to the GEE method
| Gene expression | Parameter | B | Std. Error |
Wald
Chi-Square |
df | p value ** |
|---|---|---|---|---|---|---|
| T-bet | WCT | - 0.703 | 0.4737 | 2.206 | 1 | 0.137 |
| Time | 0.003 | 0.0184 | 0.027 | 1 | 0.869 | |
| WCT*Time | 0.026 | 0.0257 | 1.058 | 1 | 0.304 | |
| GATA-3 | WCT | - 0.975 | 0.5995 | 2.645 | 1 | 0.104 |
| Time | ˉ 0.038 | 0.173 | 4.843 | 1 | 0.028* | |
| WCT*Time | 0.068 | 0.0259 | 6.899 | 1 | 0.009* | |
| RORγt | WCT | -1.103 | 0.4534 | 5.916 | 1 | 0.015* |
| Time | -0.037 | 0.0136 | 7.529 | 1 | 0.006* | |
| WCT*Time | 0.082 | 0.0196 | 17.680 | 1 | 0.00* | |
| Foxp3 | WCT | -1.350 | 0.4480 | 9.084 | 1 | 0.003* |
| Time | -0.012 | 0.0203 | 0.357 | 1 | 0.550 | |
| WCT*Time | 0.061 | 0.0263 | 5.353 | 1 | 0.021* | |
| T-bet/ GATA-3 | WCT | 0.219 | 3.3144 | 0.004 | 1 | 0.947 |
| Time | -0.010 | 1162 | 0.007 | 1 | 0.933 | |
| WCT*Time | 0.006 | 1678 | 0.001 | 1 | 0.971 | |
| Foxp3/ RORγt | WCT | -55.365 | 31.8656 | 3.019 | 1 | 0.082 |
| Time | -1.595 | 1.0378 | 2.363 | 1 | 0.124 | |
| WCT*Time | 3.341 | 1.6925 | 3.897 | 1 | 0.048* |
*p values that have been underlined and in bold show significant changes.
**Association of the analyzed parameter for each group for four weeks.
Table 2.
Clinical characteristics of each group
| WCT (mean±SD * ) | DCT (mean±SD * ) | |
|---|---|---|
| Age | 32.90±6.28 | 33.80±6.4 |
| Height | 175.19±5.61 | 177.05±6.19 |
| Weight | 79.24±10.92 | 81.17±12.04 |
| BMI | 25.82±3.4 | 25.84±3.22 |
*Standard deviation
The expression pattern of T-bet, GATA-3, RORγt, and FoxP-3 are shown in Figures 2, 3, 4 and 5, respectively. No significant changes showed by T-bet gene expression in both groups (Table 4). The expression levels of GATA-3 gene significantly varied over time (p<0.05) (Table 4). As in the first week, increased expression was observed in both groups as compared to the baseline, but at the end of the fourth week the expression of GATA-3 increased in the WCT group and decreased in the DCT group. RORγt expression changed significantly over time in both groups (p<0.01) (Table 4). Expression of RORγt increased in the WCT but decreased in DCT groups.
Figure 2.
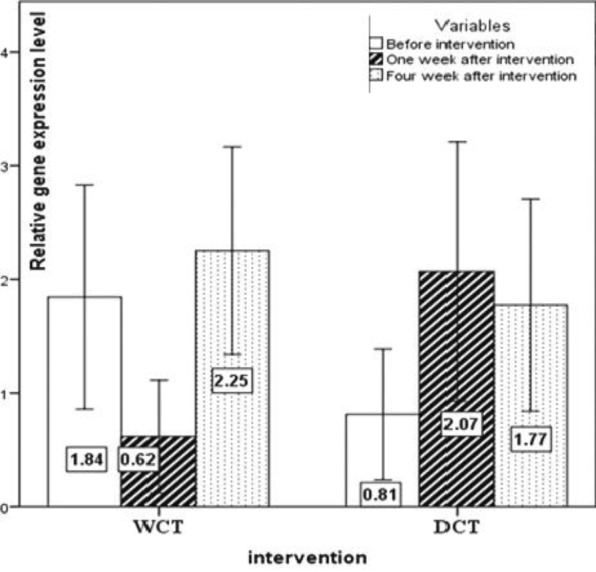
The expression pattern of the T-bet (Th1) transcription factor gene (mean±SEM)
Figure 3.
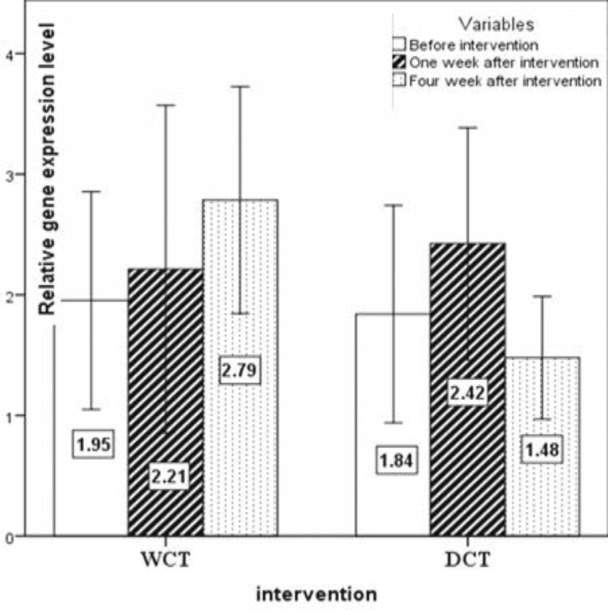
The expression pattern of the GATA-3 (Th2) transcription factor gene (mean±SEM). p<0.05 WCT*Time
Figure 4.
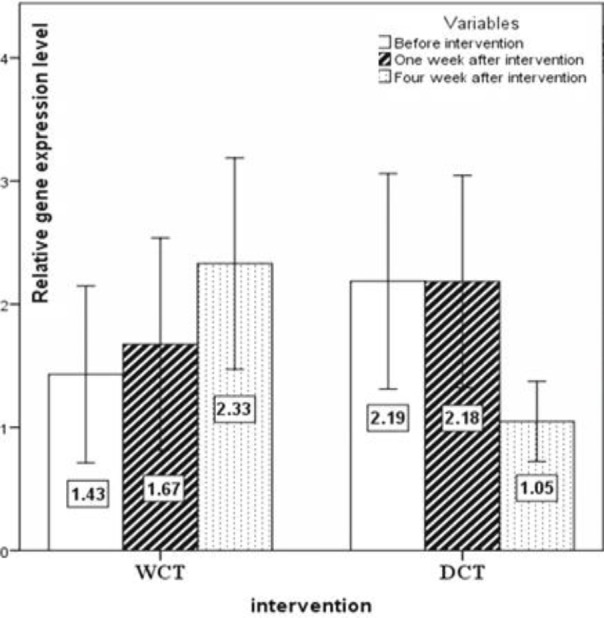
The expression pattern of the RORγt (Th17) transcription factor gene (mean±SEM). p<0.01 WCT*Time
Figure 5.
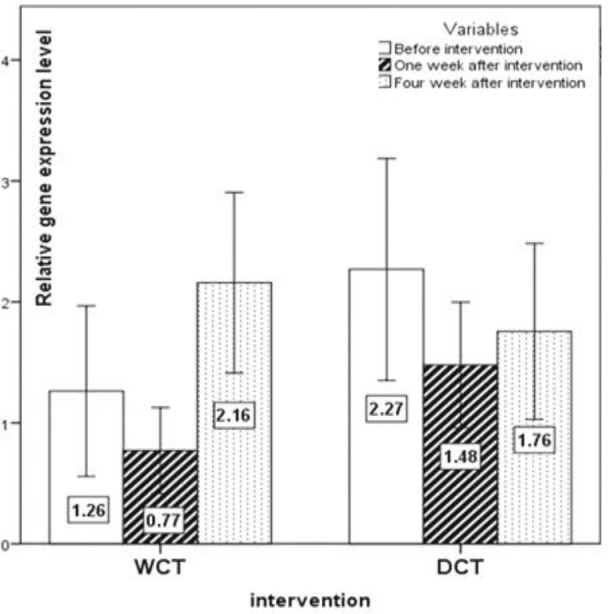
The expression pattern of the Foxp-3 (Treg) transcription factor gene (mean±SEM). p<0.05 WCT*Time
The changes in Foxp3 expression level were not significant over time (p<0.05) (Table 4). There were significant differences between the two groups over time, as during the four weeks the mean gene expression for GATA-3, RORγt, and Foxp3 in the WCT group were 0.068, 0.082, and 0.061 units more than the DCT group, respectively (p<0.001, p<0.001) (Table 4).
T-bet/GATA-3 and Foxp-3/RORγt gene expression ratio are shown in Figures 6 and 7, respectively. Though non-significantly, the T-bet/GATA-3 ratio decreased in the WCT group but increased in the DCT group four weeks after the intervention. The ratio of Foxp-3/RORγtwere not significantly change over time (p<0.05) (Table 4). However, there was a significant difference between the two groups over time (p=0.048) in terms of Foxp-3/RORγtratio, as after the four-week process, Foxp-3/RORγt ratio in the WCT group was 3.341 units higher than that of the DCT group.
Figure 6.
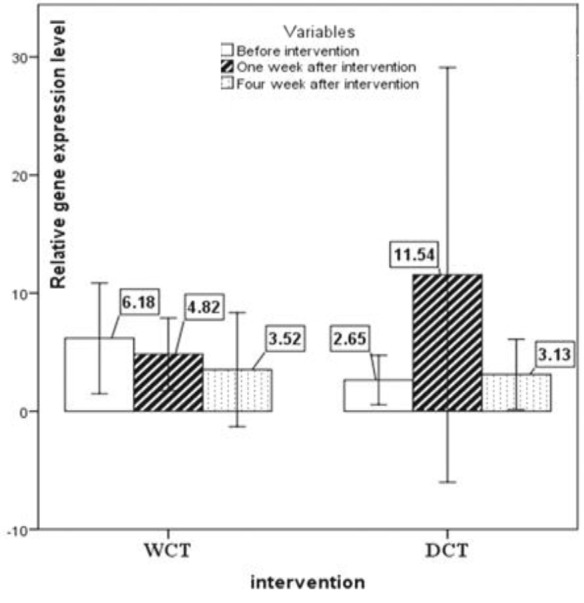
The gene expression ratio forT-bet/GATA-3 (Th1/Th2) (mean±SEM)
Figure 7.
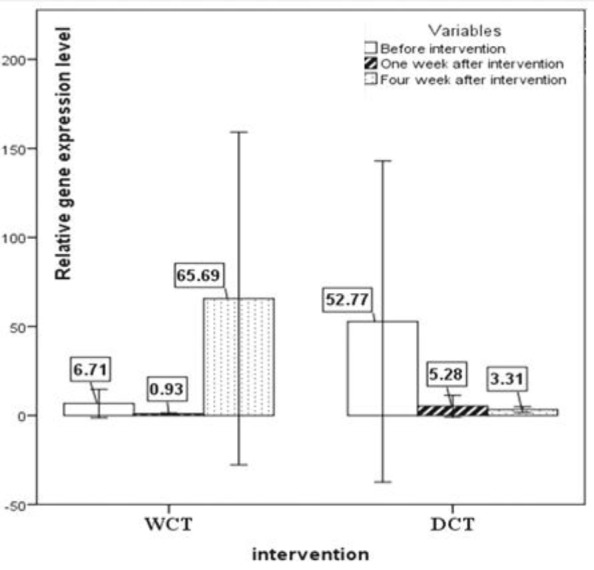
The gene expression ratio for the Foxp-3/RORγt (Treg/Th17) (mean±SEM). p<0.05 WCT*Time
The expression of the T-bet gene correlated with the expression of the GATA-3 in both groups before the intervention (p<0.05 and p<0.05); but, after the intervention, this correlation disappeared. In the WCT group, the expression of the Foxp3 gene had a positive relationship with RORγt gene expression in three time points of the study (p<0.001, R1=0.507, p<0.01, R2=0.680, p3<0.01, R3=0.327, respectively). In the DCT group, these two genes were positively correlated only in the pre-intervention stage (p<0.01, R=0.434).
Discussion
As a commonly used procedure in PM,WCT is employed for treatment as well as prevention of different diseases (Refaat et al., 2014 ▶). Based on clinical findings, it is hypothesized that WCT boosts the immune system. Many studies reported the positive effects of WCT on different types of pain and some diseases. WCT is practiced based on ancient medicine, and recently, this traditional procedure is becoming more popular around the world especially in the Middle East. Although a number of researches have shown the efficacy of WCT in some diseases, more studies are needed to better understand its efficacy. Another beneficial factor making WCT popular is that the procedure is a nonchemical and non-pharmacological approach, and there are no reports of antagonistic effects of WCT when co-administered with drugs or pharmacological treatments (Mahmoud et al., 2013 ▶).
Kordafshari et al. study revealed that WCT can improve general health(Kordafshari et al., 2017 ▶). In a study done by Ahmed et al., topical cupping improved the clinical conditions of rheumatoid arthritis patients (Ahmed et al., 2005 ▶). El Sayedet al. study demonstrated that WCT may treat iron overload conditions in thalassemia (El Sayed et al., 2014 ▶). Another study done by Dons’koi et al., showed that DCT decreases the number and activity of the natural killer cells (NKc) (Dons' koi, et al. 2016 ▶). A recent review about cupping therapy mentioned that cupping may affect the immune system by inducing local inflammation, activating the complement system, and increasing the level of immune products such as interferons (Al-Bedah et al., 2018 ▶). In the first phase of this study, evaluation of the effect of WCT on the health level of patients and hematological factors showed improved health levels and decreased blood hematocrit and hemoglobin levels.
The main aim of our study was to investigate the effect of WCT on the unique transcription factors of T-lymphocyte subsets. The most important limitation of this study was lack of blindness and lack of control over external factors that may affect the immune system such as nutrition, physical activity, and mental status. The major findings of this study were increases in the RORγt, Foxp3, GATA-3 gene expression, andFoxp-3/RORγtgene expression ratio in the WCT group compared to the DCT group over time. RORγt is the transcription factor of Th17 cells; hence, it can be assumed that WCT may increase the number of Th17 cells leading to increased levels of cytokines such as IL-17 and IL-22. Foxp3 is the transcription factor of Treg cells; therefore, it can be assumed that WCT can increase the number of Treg cells and may have an effect on the production of Treg cytokines such as TGF-β and IL-10. Differentiation of CD4+ T cells to Th17 or Treg is mediated by TGF-β. In the presence of IL-6 or IL-21, CD4+ T cells differentiate into Th17 cells, and in the absence of IL-6, TGF-β drives differentiation into Treg cells (Lee, 2018 ▶). The Th17 cells, which are activated by specific inflammatory cytokine, may lead to tissue damages (Lubberts, 2010 ▶). The Treg/Th17 ratio is a major issue in the immunopathology of cancer and autoimmune diseases (Fasching et al., 2017 ▶). Increased Treg/Th17 ratio indicates that the immune system is more tolerant towards its own antigens and is less likely to develop autoimmune diseases. After developing cancer, an increase in Treg/Th17 ratio can lead to cancer metastasis(Maruyama et al., 2010 ▶). In this study, Foxp-3/RORγtgene expression ratio (Treg/Th17) in the WCT group was increased compared to the DCT group over time, which means WCT may have a positive regulatory effect on the immune system.
Although the T-bet changes were not significant, in the second week after the intervention the mean gene expression of T-bet was decreased in the WCT group. T-bet is the transcription factor of Th1 cells. Th1 produces inflammatory cytokine, meaning that WCT may decrease inflammation in the body. Moreover, GATA-3 gene expression in the WCT group was higher than that of the DCT group following 4 weeks after the intervention. GATA-3 is the transcription factor of Th2 cells. Therefore, it can be assumed that WCT may increase the number of Th2 cells and consequently, the production of Th2 cytokines such as IL-4, IL-5, and IL-13. The Th2 response is dominant in some diseases like asthma and allergy, but this is not true for many others. In WCT, the Th2 response is dominant, and it is correlated with the suppression of inflammation and down-regulation of the Th1 response. In many inflammatory diseases, Th1/Th2 response is dominant. The Th1/Th2 ratio is considered an indicator of inflammation in the body. Reducing this ratio indicates the suppression of inflammation in the body and health improvement. In this study, T-bet/GATA-3gene expression ratio (Th1/Th2) decreased in the WCT group, which means that WCT can be effective in the suppression of inflammation in some inflammatory diseases although this change was not significant.
Although some of these results were not statistically significant, the clinical findings of this study indicated that WCT can reduce itchiness, warmness, pain, and inflammation in patients. In PM, WCT is a method to purify and cleanse the body. This technique is especially used in treating diseases that are characterized by heat and inflammation. Warm inflammation is usually accompanied by redness, itching, burning, and warmness. Some autoimmune diseases like psoriasis, rheumatoid arthritis, and lupus erythematosus have some of these symptoms. Base on the findings of this study, WCT may be useful in treating or reducing symptoms of these diseases by increasing Th2 and Treg cells and decreasing Th1 and Th17.
Acknowledgment
This study was part of a Ph.D. student thesis supported by a grant from the Vice Chancellor for Research of the Mashhad University of Medical Sciences.
Conflicts of interest
The authors have no conflict of interest to declare.
References
- Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Philadelphia: Elsevier/Saunders; 2015. pp. 317–323. [Google Scholar]
- Ahmed SM, Madbouly NH, Maklad SS, Abu-Shady EA. Immunomodulatory effects of bloodletting cupping therapy in patients with rheumatoid arthritis. Egypt Immunol. 2005;12:39–51. [PubMed] [Google Scholar]
- Al-Bedah AM, Elsubai IS, Qureshi NA, Aboushanab TS, Elgi , El-Olemy AT, Khalil AA, Khalil MK, Alqaed MS. The medical perspective of cupping therapy: Effects and mechanisms of action. J Trad Comp Med. 2019;9:90–97. doi: 10.1016/j.jtcme.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlBedah AM, Khalil A, Elolemy AA, Hussein M, AlQaed A, Mudaiheem AL, Abutalib RA, Bazaid FM, Bafail AS, Essa A. The use of wet cupping for persistent nonspecific low back pain: randomized controlled clinical trial. J Alter Comp Med. 2015;21:504–508. doi: 10.1089/acm.2015.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan M, Yeşilçam N, Aydin D, Yüksel R, Dane S. Wet cupping therapy restores sympathovagal imbalances in cardiac rhythm. J Alter Comp Med. 2014;20:318–321. doi: 10.1089/acm.2013.0291. [DOI] [PubMed] [Google Scholar]
- Askari VR, Alavinezhad A, Boskabady MH. The impact of “Ramadan fasting period” on total and differential white blood cells, haematological indices, inflammatory biomarker, respiratory symptoms and pulmonary function tests of healthy and asthmatic patients. Allergo et Immunopath. 2016;44:359–367. doi: 10.1016/j.aller.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Askari VR, BaradaranRahimi V, Rezaee SA, Boskabady MH. Auraptene regulates Th1/Th2/TReg balances, NF-κB nuclear localization and nitric oxide production in normal and Th2 provoked situations in human isolated lymphocytes. Phytomedicine. 2018;43:1–10. doi: 10.1016/j.phymed.2018.03.049. [DOI] [PubMed] [Google Scholar]
- Askari VR, Fereydouni N, BaradaranRahimi V, Askari N, Sahebkar AH, Rahmanian-Devin P, Samzadeh-Kermani A. Beta-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-gamma and regulates Mphi1/Mphi2 balances. Biomed Pharm Ther. 2018;101:438–446. doi: 10.1016/j.biopha.2018.02.098. [DOI] [PubMed] [Google Scholar]
- Askari VR, Fereydouni N, Rahimi VB N, Askari A H, Sahebkar AH, Rahmanian-Devin P, Samzadeh-Kermani A. β-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ 1/Mφ 2 balances. Biomed Pharm Ther. 2018;101:438–446. doi: 10.1016/j.biopha.2018.02.098. [DOI] [PubMed] [Google Scholar]
- Askari VR, Rezaee SA, Abnous K, Iranshahi M, Boskabady MH. The influence of hydro-ethanolic extract of Portulacaoleracea L on Th1/Th2 balance in isolated human lymphocytes. J Ethnopharm. 2016;194:1112–1121. doi: 10.1016/j.jep.2016.10.082. [DOI] [PubMed] [Google Scholar]
- Askari VR, Shafiee-Nick R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: A mechanistic study. Biochem Pharm. 2019;159:154–171. doi: 10.1016/j.bcp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Askari VR, Shafiee-Nick R. The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: A mechanistic evaluation. Life Sci. 2019;219:40–73. doi: 10.1016/j.lfs.2018.12.059. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Barzaghi F, Roncarolo MG. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci. 2018;14171:5–22. doi: 10.1111/nyas.13011. [DOI] [PubMed] [Google Scholar]
- Boskabadi J, Askari VR, Hosseini M, Boskabady MH. Immunomodulatory properties of captopril, an ACE inhibitor, on LPS-induced lung inflammation and fibrosis as well as oxidative stress. Inflammopharmacology. 2018;27:639–647. doi: 10.1007/s10787-018-0535-4. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Hashemzehi M, Khazdair MR, Askari VR. Hydro-ethanolic extract of Portulaca oleracea affects beta-adrenoceptors of guinea pig tracheal smooth muscle. IJPR. 2016;15:867–874. [PMC free article] [PubMed] [Google Scholar]
- Cao H, Han M, Zhu X, Liu J. An overview of systematic reviews of clinical evidence for cupping therapy. J Trad Chinese Med Sci. 2015;21:3–10. [Google Scholar]
- Cao H, Zhu C, Liu J. Wet cupping therapy for treatment of herpes zoster: a systematic review of randomized controlled trials. Alter Ther Health Med. 2010;16:48–54. [PMC free article] [PubMed] [Google Scholar]
- Dons' koi BV, Chernyshov VP, Osypchuk DV, Baksheev SM. Repeated cupping manipulation temporary decreases natural killer lymphocyte frequency, activity and cytotoxicity. J Integ Med. 2016;14:197–202. doi: 10.1016/S2095-4964(16)60250-9. [DOI] [PubMed] [Google Scholar]
- Ebrahimi AE H, Moulavi SG, Mousavi , BornaManesh A, Yaghoubi M. Psychometric properties and factor structure of General Health Questionnaire 28 (GHQ-28) in Iranian psychiatric patients. Horizon Med Sci. 2007;12:26–33. [Google Scholar]
- El Sayed SM, Al-quliti AS. Hijamah: in light of modern medicine and prophetic medicine. A J Med Bio Res. 2013:46–71. [Google Scholar]
- El Sayed SM, Baghdadi H, Abou-Taleb A H S, Mahmoud AH, Maria RA, Ahmed NS, Nabo MH. Al-hijamah and oral honey for treating thalassemia, conditions of iron overload, and hyperferremia: toward improving the therapeutic outcomes. J Blood Med. 2014;5:219–237. doi: 10.2147/JBM.S65042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017;22:134–170. doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychologicamedicine. 1979;9:139–145. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- Goodwin J, McIvor RA. Alternative therapy: cupping for asthma. Chest. 2011;139:475–476. doi: 10.1378/chest.10-2220. [DOI] [PubMed] [Google Scholar]
- Grant CR, Liberal R, Mieli-Vergani G, Vergani D, Longhi MS. Regulatory T-cells in autoimmune diseases: challenges, controversies and yet unanswered questions. Autoimmun Rev. 2015;14:105–116. doi: 10.1016/j.autrev.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Hashemzehi M, Khazdair M, Kiyanmehr M, Askari V, Boskabady MH. Portulacaolerace Affects muscarinic receptors of guinea pig tracheal smooth muscle. Indian J Pharm Sci. 2016;78:388–394. [Google Scholar]
- Kianmehr M, Rezaei A, Hosseini M, Khazdair MR, Rezaee R, Askari VR, Boskabady MH. Immunomodulatory effect of characterized extract of Zataria multiflora on Th1, Th2 and Th17 in normal and Th2 polarization state. Food Chem Toxicol. 2017;99:119–127. doi: 10.1016/j.fct.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Alter med review. 2003;8:223–246. [PubMed] [Google Scholar]
- Kim JI, Lee MS, Lee DH, Boddy K, Ernst E. Cupping for treating pain: a systematic review. Evi-Bas Comp Alter Med. 2011;2011:467014. doi: 10.1093/ecam/nep035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordafshari G, Ardakani MRS, Keshavarz M, Esfahani I, Nazem M, Moghimi A, Zargaran A, Kenari HM. Cupping therapy can improve the quality of life of healthy people in Tehran. J Trad Chin Med. 2017;37:558–562. [PubMed] [Google Scholar]
- Kordafshari G, Kenari HM, Esfahani MM, Ardakani MRS, Keshavarz M, Nazem E, Moghimi M, Zargaran A. Nutritional aspects to prevent heart diseases in traditional Persian medicine. J Evid-Base Comp Alter Med. 2015;20:57–64. doi: 10.1177/2156587214553939. [DOI] [PubMed] [Google Scholar]
- Lee GR. The Balance of Th17 versus Treg Cells in Autoimmunity. Inter J Mol Sci. 2018;19:730.–744. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi MRV, Ghazanfari T, Aghajani M, Danyali F, Naseri M. Evaluation of the effects of traditional cupping on the biochemical, hematological and immunological factors of human venous blood. A compendium of essays on alternative therapy. Croatia. 2012 In Tech 6. [Google Scholar]
- Mahmoud HS, Abou-El-Naga M, Omar NAA, El-Ghazzawy HA, Fathy YM, Nabo MMH, El Sayed SM. Anatomical sites for practicing wet cupping therapy (Al-Hijamah): in light of modern medicine and prophetic medicine. Alter Integr Med. 2013:138. [Google Scholar]
- Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Izawa S, Fujii H. Distribution of Th17 cells and FoxP3 (+) regulatory T cells in tumor‐infiltrating lymphocytes, tumor‐draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010;101:1947–1954. doi: 10.1111/j.1349-7006.2010.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazer R, Namavary D. Comparison biochemistries of obtained blood products between the hijama and phlebotomy techniques of traditional islamic remedy; healthy young adults at fasting state. J Hosp Med Manag. 2016;2:1–6. [Google Scholar]
- Rahimi VB, Askari VR, Shirazinia R, Soheili-Far S, Askari N, Rahmanian-Devin P, Sanei-Far Z, Mousavi SH, Ghodsi R. Protective effects of hydro-ethanolic extract of Terminalia chebula on primary microglia cells and their polarization (M1/M2 balance) MS Relat Disord. 2018;25:5–13. doi: 10.1016/j.msard.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Rahimi VB, Shirazinia R, Fereydouni N, Zamani P, Darroudi S, Sahebkar AH, Askari VR. Comparison of honey and dextrose solution on post-operative peritoneal adhesion in rat model. Biomed Pharm Therp. 2017;92:849–855. doi: 10.1016/j.biopha.2017.05.114. [DOI] [PubMed] [Google Scholar]
- Refaat B, El-Shemi AG, Ebid AA, Ashshi A, andBaSalamah MA. Islamic wet cupping and risk factors of cardiovascular diseases: effects on blood pressure, metabolic profile and serum electrolytes in healthy young adult men. Altern Integr Med. 2014;3:151–158. [Google Scholar]
- Tabatabaee A, Zarei M, Javadi SA, Mohammadpour A, Bidaki AA. "The effects of Wet-Cupping on intensity of headache in Migraine sufferers. JJCDC. 2014;3:1–12. [Google Scholar]
- Wang Qf, Wang GY. Therapeutic effect observation on treatment of acne with acupuncture plus moving cupping and blood-letting. J Acup Tuina Sci. 2008;6:212–214. [Google Scholar]
- Yao J, Li N. Clinical observation on pricking and blood-letting and cupping with a three-edge needle for treatment of acute eczema. Zhongguo zhen Jiu=Chinese Acup Moxibus. 2007;27:424–426. [PubMed] [Google Scholar]
- Yao X, Shan S, Zhang Y, Ying H. Recent progress in the study of brown adipose tissue. Cell Biosci. 2011;1:35–44. doi: 10.1186/2045-3701-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Hejazi S, Javadi SA, Farahani H. The efficacy of wet cupping in the treatment of hypertension. ARYA Atheroscler. 2012;8:S145–S148. [Google Scholar]


