Abstract
Aged humans display a chronic and low-grade inflammation, termed “inflammaging”, which has been potentially linked to the subsequent development of some aging-associated systemic disorders, including type 2 diabetes, atherosclerotic cardiovascular disease, Alzheimer’s disease and obesity. Though the origin of aging-associated systemic inflammation is uncertain, epidemiological studies show that inflammatory dermatoses (psoriasis and eczema) are risk factors for some aging-associated systemic disorders, such as type 2 diabetes and atherosclerotic cardiovascular disease. Moreover, recent studies demonstrate that epidermal dysfunction in aged skin not only causes cutaneous inflammation, but also a subsequent increase in circulating levels of proinflammatory cytokines, suggesting that the skin could be a major contributor to inflammaging. This hypothesis is further supported by reductions in circulating levels of proinflammatory cytokines in both aged humans and murine, following improvements in epidermal function with topical emollients. Therefore, correction of epidermal dysfunction could be a novel approach for the prevention and mitigation of certain inflammation-associated chronic disorders in aged humans.
Keywords: aging, inflammation, inflammaging, epidermis, systemic disorders
As humans age, they almost inevitably suffer from one or more disorders associated with chronological aging, including type 2 diabetes, atherosclerotic cardiovascular disease, Alzheimer’s disease, obesity, osteoporosis, and sarcopenia. While the pathogenesis of these chronic aging-associated disorders is still poorly understood, increasing evidence points to a provocative role of sustained, sub-clinical inflammation, often termed “inflammaging,” in the development of these chronic disorders.1–7 In support of this notion, chronologically aged humans (≥50 years) display elevated circulating levels of pro-inflammatory cytokines, particularly IL-6, IL-1β and TNFα. Moreover, subjects with chronic cutaneous inflammatory diseases, such as psoriasis and eczematous dermatitis, also display an increased prevalence of aging-associated disorders, including atherosclerotic cardiovascular disease, obesity and type 2 diabetes.8 Though anti-inflammatory regimens, such as inhibitors of IL-1βα and TNFα, as well as methotrexate, have been deployed in the management of these aging-associated disorders, the outcomes of treatments with these agents have been inconclusive.9 It is possible that the effective, pathogenesis-based preventive regimens remain unavailable, in large part, because of the uncertainty of the pathogenesis of “inflammaging”.
While many chronologically aged humans merely display marked evidence of inflammation, they nonetheless display elevated circulating levels of cytokines, suggesting that one or more, as yet identified organs, could account for the aging-associated increase in circulating cytokines. It seems reasonable to postulate that the responsible organ(s) must be large enough to sustain such an increase in circulating cytokines, even without noticeable inflammation. Although the musculoskeletal system is the largest organ in humans, most chronologically aged humans display no evidence of musculoskeletal inflammation. Other relatively large organs to be considered include the skin, intestines, lungs and liver. The skin, with a surface area of ≈1.5–2.0 m2, weighs about 20 lbs (with an additional, variable contribution from subcutaneous adipose tissues), while the weights of the intestines (surface area≈250 m2), lungs (surface≈50–75m2) and liver represent ≈7.5, 5.0 and 3.3 lbs, respectively. Because of their relatively lesser size, inflammation of the lungs, intestines and liver likely would not only need to be apparent, but also sustained if any of these organs could account for the increase in circulating levels of cytokines. Yet again, the majority of otherwise normal aged humans display few clinical signs or symptoms of inflammation in these organs. Hence, it seems unlikely that they could contribute substantially to “inflammaging” unless multiple organs simultaneously exhibit mild inflammation. Notably, the aged skin commonly exhibits signs and symptoms of inflammation, such as pruritus and senile xerosis.10–12
Because of its relatively large size, we hypothesized that the skin could be an important contributor to the elevated levels of circulating cytokines in chronologically aged humans, despite the fact that it typically displays little evidence of inflammation. Not only its size, but also its unique anatomic site, serving as the interface between the body and external environment, supports our hypothesis. In this site, it is continuously exposed to external physical and chemical stressors, which themselves can provoke inflammation, even as other less-exposed organs remain quiescent. In addition, chronologically aged humans display alterations in several key epidermal functions, each of which can provoke low-grade, chronic inflammation in the skin. For example, otherwise normal, chronologically aged humans display lower levels of stratum corneum hydration, as early as age ≈45 years, resulting in an increase in cutaneous cytokine production.10,13,14 Moreover, skin surface pH increases (from pH 4.5–5.0 to 5.5–6.0) in chronologically aged humans, beginning at ≈50 years of age.10,15 An elevated pH alone can not only compromise epidermal permeability barrier homeostasis (the primary life-enabling function of the skin in a desiccating terrestrial environment),16 but also increases the activity of kallikreins, which directly destroy epidermal structure, while also activating the protease-activated receptor 2, leading to inflammation and pruritus.17 Chronologically aged humans also often suffer from intractable pruritus, particularly in low humidity environments, which can provoke scratching, further disrupting the epidermal permeability barrier, in turn, exacerbating downstream inflammation (Figure 1). Prior studies have shown that the defective epidermal permeability barrier function in chronologically aged humans and mice stimulates the continuous production and release of epidermal cytokines, ultimately resulting in elevations in circulating cytokines.18,19
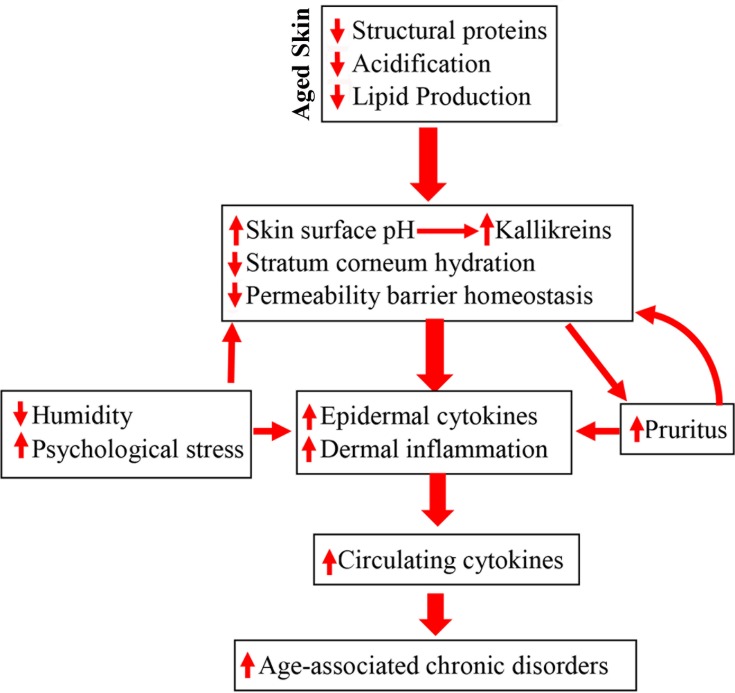
Figure 1.
Schematic diagram: pathogenic role of epidermal dysfunction in inflammaging and its associated disorders in the elderly. Due to reductions in epidermal lipid production, filaggrin and NHE1/sPLA2 expression, aged humans display multiple functional abnormalities in the epidermis, including delayed permeability barrier recovery, reduced stratum corneum hydration and elevated stratum corneum pH, which all can provoke cutaneous inflammation. In turn, cutaneous inflammation can also induce pruritus, leading to further barrier disruption because of scratching. Because of skin’s vast size, persistent cutaneous inflammation can induce sustained elevation in cytokine levels in the circulation, consequently leading to the development of inflammaging-associated disorders.
In considering chronologically aged versus young skin, cytokine production in the latter helps to restore permeability barrier homeostasis.20,21 But because normal barrier function can never be achieved in chronologically aged human skin due to defective acidification and reduced lipid production,15,22,23 a prolonged release of epidermal cytokines continues unabated in a futile attempt to restore normal barrier function. We hypothesized first, that when sustained over time, such elevations in cutaneous cytokines could eventually leak into circulation, either contributing or accounting for the systemic inflammation. In support of the hypothesis that chronic aging-associated alterations in epidermal function can account for elevations in circulating levels of inflammatory cytokines, i.e. “inflammaging”, we recently showed that otherwise normal, chronologically aged mice displayed elevations in both cutaneous and circulating levels of cytokines that parallel chronic aging-associated abnormalities in epidermal function.18 Moreover, in aged mice, the increase in serum cytokine levels occurred likely independent of T cell involvement, because disruption of the epidermal permeability barrier in athymic mice induced a comparable increase in circulating levels of inflammatory cytokines to that in normal mice.18 Conversely, correction of epidermal function in chronologically aged mice reduced both cutaneous and circulating levels of cytokines.18 Consistent with these findings in chronologically aged mice, a pilot study in aged humans (58 to 95 years old without skin diseases) also demonstrated that correction of epidermal function by repeated topical applications of an emollient, previously shown to enhance epidermal function in normal humans, reduced circulating levels of IL-1β, IL-6 and TNFα.19 Together, this evidence suggests a pathogenic role for epidermal keratinocytes, and possibly dermal fibroblasts and/or subcutaneous tissues, in chronic aging-associated systemic inflammation. Moreover, the fact that improving epidermal function alone lowered circulating cytokine levels unlikely supports the primary role of other organs or tissues, including adipose tissue, in the pathogenesis of “inflammaging”. Finally, because these cytokines have been linked to the development of aging-associated disorders,1–6 we speculate further that exposure of skin to additional exogenous stressors, such as a reduced ambient humidity or to excessive psychological stress, both of which can provoke and exacerbate epidermal permeability abnormality and inflammation, could accelerate the development of inflammaging-associated disorders (Figure 1).
Taken together, this evidence suggests that strategies which enhance epidermal function in aged skin could reduce circulating levels of inflammatory cytokines, suggesting the potential utility of such corrective approaches as a strategy to prevent and/or mitigate chronic aging-associated disorders. Yet, further studies are needed to confirm that skin-derived “inflammaging” provoke the downstream development of these disorders.
Acknowledgment
This work was supported, in part, by utilization of resources provided by the Veterans Affairs Medical Center, San Francisco, CA.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Patrick L, Uzick M. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern Med Rev. 2001;6(3):248–271. [PubMed] [Google Scholar]
- 2.Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130(10):837–844. doi: 10.1161/CIRCULATIONAHA.114.009990 [DOI] [PubMed] [Google Scholar]
- 3.Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(S3):12–29. doi: 10.1111/jdv.2013.27.issue-s3 [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135(3):721–8.e6. doi: 10.1016/j.jaci.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 5.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi: 10.1038/nrd4275 [DOI] [PubMed] [Google Scholar]
- 6.Marques-Vidal P, Bastardot F, von Känel R, et al. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study). Clin Endocrinol (Oxf). 2013;78(2):232–241. doi: 10.1111/cen.2012.78.issue-2 [DOI] [PubMed] [Google Scholar]
- 7.Lucas R, Parikh SJ, Sridhar S, et al. Cytokine profiling of young overweight and obese female African American adults with prediabetes. Cytokine. 2013;64(1):310–315. doi: 10.1016/j.cyto.2013.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanadi EC, Dimitrakakis G, Antoniou CK, et al. Metabolic syndrome and the skin: a more than superficial association. Reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9. doi: 10.1186/s13098-018-0311-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22(4):190–199. doi: 10.1159/000231524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Augustin M, Kirsten N, Körber A, et al. Prevalence, predictors and comorbidity of dry skin in the general population. J Eur Acad Dermatol Venereol. 2019;33(1):147–150. doi: 10.1111/jdv.2019.33.issue-1 [DOI] [PubMed] [Google Scholar]
- 12.Valdes-Rodriguez R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95(4):417–421. doi: 10.2340/00015555-1968 [DOI] [PubMed] [Google Scholar]
- 13.Ashida Y, Denda M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br J Dermatol. 2003;149(2):240–247. doi: 10.1046/j.1365-2133.2003.05408.x [DOI] [PubMed] [Google Scholar]
- 14.Denda M, Sato J, Tsuchiya T, Elias PM, Feingold KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111(5):873–878. doi: 10.1046/j.1523-1747.1998.00364.x [DOI] [PubMed] [Google Scholar]
- 15.Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127(12):2847–2856. doi: 10.1038/sj.jid.5700913 [DOI] [PubMed] [Google Scholar]
- 16.Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290(4):215–222. doi: 10.1007/s004030050293 [DOI] [PubMed] [Google Scholar]
- 17.Jairaman A, Yamashita M, Schleimer RP, Prakriya M. Store-operated Ca2+ release-activated Ca2+ channels regulate PAR2-activated Ca2+ signaling and cytokine production in airway epithelial cells. J Immunol. 2015;195(5):2122–2133. doi: 10.4049/jimmunol.1500396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Mauro TM, Dang E, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. 2017;137(6):1277–1285. doi: 10.1016/j.jid.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye L, Mauro TM, Dang E, et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: a pilot clinical study. J Eur Acad Dermatol Venereol. 2019;33(11):2197–2201. doi: 10.1111/jdv.v33.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye J, Garg A, Calhoun C, Feingold KR, Elias PM, Ghadially R. Alterations in cytokine regulation in aged epidermis: implications for permeability barrier homeostasis and inflammation. I. IL-1 gene family. Exp Dermatol. 2002;11(3):209–216. doi: 10.1034/j.1600-0625.2002.110303.x [DOI] [PubMed] [Google Scholar]
- 21.Barland CO, Zettersten E, Brown BS, Ye J, Elias PM, Ghadially R. Imiquimod-induced interleukin-1 alpha stimulation improves barrier homeostasis in aged murine epidermis. J Invest Dermatol. 2004;122(2):330–336. doi: 10.1046/j.0022-202X.2004.22203.x [DOI] [PubMed] [Google Scholar]
- 22.Ghadially R, Brown BE, Hanley K, Reed JT, Feingold KR, Elias PM. Decreased epidermal lipid synthesis accounts for altered barrier function in aged mice. J Invest Dermatol. 1996;106(5):1064–1069. doi: 10.1111/1523-1747.ep12338692 [DOI] [PubMed] [Google Scholar]
- 23.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest. 1995;95(5):2281–2290. doi: 10.1172/JCI117919 [DOI] [PMC free article] [PubMed] [Google Scholar]