Abstract
Background
Use of smokeless tobacco (SLT) products has been linked to multiple adverse effects, especially precancer and cancer of oral cavity. However, the association of SLT use with risk of coronary heart disease (CHD) is shrouded with controversy due to conflicting results in the literature. The present meta-analysis aimed to evaluate the risk of CHD among adult ever-users of SLT products along with sub-group analysis.
Methods
The analysis included studies retrieved from a systematic literature search for published articles assessing risk of CHD with SLT use. Two authors independently extracted risk estimates and study characteristics of the included studies. Summary relative risks were estimated using the random-effect model.
Results
Twenty studies from four WHO regions were included in the analysis. The summary risk of CHD in SLT users was not significantly positive (1.05, 95% CI = 0.96 to 1.15) although a higher risk of fatal CHD was seen (1.10, 95% CI = 1.00 to 1.20). The risk was significant for users in European Region (1.30, 95% CI = 1.14 to 1.47). The results remained unchanged even after strict adjustment for smoking. Product-wise analysis revealed a significant positive association of fatal CHD with snus/snuff use (1.37, 95% CI = 1.14 to 1.61). The SLT-attributable fraction of fatal CHD was calculated to be 0.3%, highest being for European region (5%).
Conclusion
A significant positive association was detected between SLT use and risk of fatal CHD, especially for European users and those consuming snus/snuff. In view of the positive association even after strict adjustment for smoking, these results underscore the need for inclusion of cessation efforts for smokeless tobacco in addition to smoking for control of fatal cardiovascular diseases.
Implications
The present meta-analysis demonstrates a global perspective of association between coronary heart disease (CHD) and use of smokeless tobacco (SLT), especially for fatal cardiac events, even with strict adjustment for smoking. There appears to be some difference in this effect based on the type of SLT product used. These results highlight the independent deleterious effect of SLT products on the outcome of CHD and might help to resolve the long-standing controversy regarding the association of SLT with the risk of CHD. Hence, we propose that in addition to smoking, cessation efforts should be directed towards SLT products as well, for control of cardiovascular diseases.
Introduction
Among middle-aged adults, tobacco use has been projected to be the most important avoidable risk factor for premature death in males and second most common in women in 2010–2025.1 Tobacco consumption, as smoking or smokeless tobacco (SLT), is one of the major preventable causes of cardiovascular diseases.2 Cardiovascular effects of cigarette smoking, linked to the toxic constituents of tobacco smoke, have been amply documented in the literature.3,4 The association of cigarette smoking with ischemic heart disease (coronary heart disease, CHD) and heart failure has also been demonstrated in many studies.5
Over the last three decades, global age-standardized prevalence of smoking among men and women has seen a downward trend, especially in high income countries.1 More than 350 million people across the globe are users of SLT in one form or the other.6 In addition to addiction, SLT use has been linked to various health effects such as cancers of oral cavity, esophagus and pancreas as well as adverse reproductive outcomes.7,8 The published meta-analyses of studies evaluating SLT use with CHD have not found an overall significant higher risk of CHD in SLT users. However, a positive association has been demonstrated between SLT use and fatal CHD. The meta-analysis reported by Boffetta and Straif9 included only Swedish and American studies while that by Vidyasagaran et al.10 and Sinha et al.11 included Asian reports as well. None of the earlier meta- analyses undertaken on this topic have attempted to evaluate the results on the basis of type of SLT product consumed.
The SLT-attributable fraction of cardiovascular disease was calculated in the meta-analysis by Boffetta and Straif. In their analysis, 0.5% of fatal CHD in the United States and 5.6% of such events in Sweden could be attributed to SLT use.9 Sinha et al.11 estimated the global SLT-attributable CHD mortality as well as for European region but not for American or Southeast Asian regions. However, the recent meta-analysis by Vidyasagaran et al. did not comment on the SLT-attributable fraction.10
The present systematic review and meta-analysis, hence, aimed at conducting an updated and comprehensive global review of the risk of CHD with SLT use. We also attempted to include additional regions and perform sub-group analysis for smoking-adjusted studies and type of SLT product used. The SLT-attributable fraction of fatal CHD, both globally and region-wise was also calculated.
Materials and Methods
The review included articles retrieved through a systematic literature search performed in 2017. SLT use was considered as the main exposure variable and CHD as the outcome variable. Multiple databases including PubMed/ Medline, EMBASE, Web of Science and Cochrane Library were searched and reference lists of included studies checked for additional citations.
Study Definition
SLT exposure was defined as any use, current or past. Studies including smokers as well as SLT users were considered only if separate results for SLT users were reported.
Literature Search
The search terms used were: “smokeless tobacco,” “chewing tobacco,” “chewable tobacco,” “oral tobacco,” “spit tobacco,” “snuff,” “snus,” “nasvar,” “gul,” and “cardiovascular disease,” “myocardial infarction,” “ ischemic heart disease,” “coronary heart disease,” and “heart disease.” The strategy followed for literature search and evaluation of studies is depicted in Supplementary Figure 1.
A combined search for SLT and CHD yielded 226 results from PubMed. Of these, 35 irrelevant articles (pertaining neither to SLT nor to CHD) were excluded. References dealing in general with tobacco-related issues or related to smoking were excluded (n = 50). Studies focusing only on betel quid, betel nut or areca nut chewing without other SLT products were also excluded (n = 3). References pertaining to SLT-related issues other than health effects were also excluded (n = 33). Also, articles pertaining to issues related to cardiovascular diseases like prevalence estimation, risk factors and preventive strategies were excluded from the review (n = 16).
The remaining references (n = 89) were reviewed for relevance to the topic under study. Studies related to health effects other than cardiovascular disease (n = 25) or those evaluating risk factors for CHD rather than the outcome (n = 14) were excluded. References of letters and reviews on SLT and CHD (n = 24) were excluded. Twenty six (26) studies exploring the association between SLT use and CHD were left after these exclusions. Of these, six studies (two whose full-text could not be accessed, three studies including heart failure in the outcome and one study evaluating mortality risk after SLT cessation) were excluded. Hence, 20 studies were finally included in the meta-analysis.
Data Extraction and Statistical Analysis
The quality of the reporting of the included studies was evaluated using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.12
The Cochrane Q-test and I2 statistics were used to evaluate the statistical heterogeneity among studies under the assumption of random-effect model. A p value of <.05 for Q-test or I2 > 50% was considered statistically significant heterogeneity among the studies. Publication bias was assessed using the funnel plot, Egger’s regression, and Begg’s tests. In addition, p values for all the included studies were calculated using the formula suggested by Altman and Bland.13P curve was subsequently drawn using the online tool available at http://p-curve.com/. Analysis was performed using all available studies together and as per region. Further rigorous selection of studies was done to exclude the data where smoking was not adjusted as a confounding variable or study subjects were not exclusive SLT users. Five studies were excluded on this basis and hence, further sub-group analysis was performed on remaining 15 studies for global as well as regional estimates. Additional sub-group analysis was performed on the basis on type of SLT product. All data analysis was performed by Stata software STATA 12.1 (StataCorp, Lakeway Drive, Texas, USA).
The attributable fraction, as a measure of burden of CHD due to SLT use, was estimated using the relative risk and proportion of exposed population by the formula: proportion of exposed population × (relative risk − 1) divided by [proportion of exposed population × (relative risk − 1)] + 1, based on the previously published data of total number of adult SLT users.6
Results
The characteristics of selected 20 studies included in the analysis are depicted in Supplementary Table 1.
Qualitative Analysis
Of the 20 studies, six demonstrated significant positive association of SLT use with risk of CHD.13–19 Three studies found significant risk of CHD only in selected sub-group (CHD outcome or gender-specific).20–22 The remaining 11 studies did not find such an association.23–33
Among the 10 European studies, only two found higher risk of fatal CHD in SLT users,14,20 though one of these reported no significantly higher risk of CHD overall or of nonfatal outcome.20 The studies from region of Americas and Eastern Mediterranean region (EMR) differed in their results, with one study demonstrating significant association of SLT use and CHD15,18 and the other not finding similar results.31,32 Of the five studies from South East Asian region, two16,17 demonstrated significantly higher risk of nonfatal CHD with SLT use while two studies showed an association of CHD in only female SLT users.21,22 The fifth study did not find any significant association between SLT use and CHD.33
A large multicountry (52 countries) study by Teo et al. found a significantly higher risk of CHD in users of chewing tobacco, even with adjustment for smoking.19
Quantitative analysis
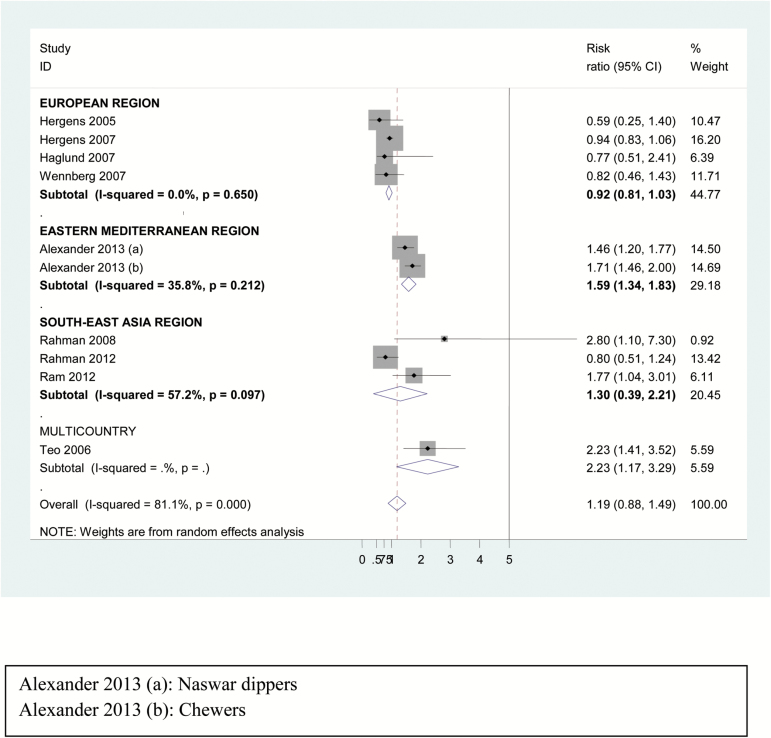
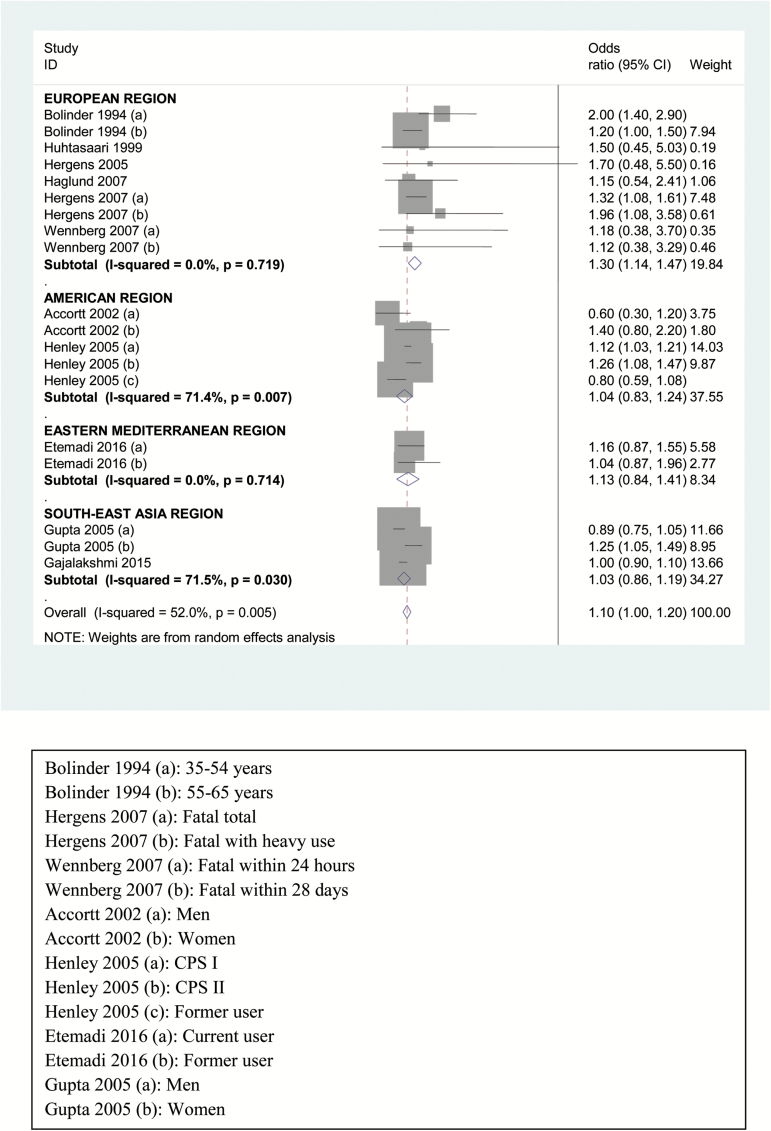
The summary relative risk for CHD was not significant in random-effect model (1.05, 95% CI = 0.96 to 1.15) though region-wise analysis showed higher risk for users in EMR (1.41, 95% CI = 1.13 to 1.69), as shown in Supplementary Figure 2. Based on the CHD outcome, risk for nonfatal CHD (Figure 1) was significantly positive only in EMR (1.59, 95% CI = 1.34 to 1.83) though overall risk estimate for nonfatal CHD was not significant (1.19, 95% CI = 0.88 to 1.49). In contrast, the risk for fatal CHD was higher in SLT users (1.10, 95% CI = 1.00 to 1.20) depicted in Table 1 and Figure 2), especially in European region (1.30, 95% CI = 1.14 to 1.47) whereas the same was not true for the other three regions included. On restriction of studies to those adjusting for smoking or including only nonsmokers, the overall results as well as those for fatal CHD did not alter much (1.10, 95% CI = 1.00 to 1.21), as shown in Table 1 and Supplementary Figure 3.
Figure 1.
Forest plot of risk estimates of nonfatal CHD among users of smokeless tobacco with region-wise analysis—random effect model.
Table 1.
Results of meta-analysis including all available studies and sub-group analysis
| Stratification group | No. of studies | Begg’s test (p value) | Random effects model OR (95% CI) |
|---|---|---|---|
| Total | 20 | .49 | 1.05 (0.96–1.15) |
| Region | |||
| European Region | 10 | .54 | 0.93 (0.81–1.06) |
| American Region | 2 | .47 | 1.04 (0.83–1.24) |
| Eastern Mediterranean region | 2 | .20 | 1.41 (1.13–1.69) |
| South-east Asian region | 5 | .03 | 1.02 (0.86–1.18) |
| Smoking-adjusted studies | |||
| Total | 15 | .56 | 1.05 (0.95–1.16) |
| Region | |||
| European Region | 6 | .67 | 0.95 (0.86–1.04) |
| Region of the Americas | 2 | .47 | 1.04 (0.83–1.24) |
| Eastern Mediterranean region | 2 | .20 | 1.41 (1.13–1.69) |
| South-east Asian region | 4 | .08 | 1.00 (0.85–1.15) |
| CHD outcome | |||
| Nonfatal | 8 | .85 | 1.19 (0.88–1.49) |
| Region | |||
| European | 3 | 0.92 (0.81–1.03) | |
| Eastern Mediterranean | 1 | 1.59 (1.34–1.83) | |
| South East Asian | 3 | 1.30 (0.39–2.21) | |
| Fatal | 11 | .38 | 1.10 (1.00–1.20) |
| Region | |||
| European | 6 | 1.30 (1.14–1.47) | |
| American | 2 | 1.04 (0.83–1.24) | |
| Eastern Mediterranean | 1 | 1.13 (0.84–1.41) | |
| South East Asian | 2 | 1.03 (0.86–1.19) | |
| Type of SLT Product used | |||
| Snuff | 7 | 0.96 (0.86–1.06) | |
| Fatal CHD with snuff | 4 | 1.37 (1.14–1.61) | |
| Chewing tobacco | 7 | 1.13 (0.92–1.33) | |
| Fatal CHD with chewing tobacco | 1.07 (0.91–1.23) | ||
| Nass/Nasvar | 2 | 1.30 (1.06–1.54) | |
Figure 2.
Forest plot of risk estimates of region-wise analysis of fatal CHD among users of smokeless tobacco products—random effect model.
Sub-group analysis
Sub-group analysis based on type of SLT product used revealed an overall significant positive association of CHD with use of nass/nasvar (1.30, 95% CI = 1.06 to 1.54). The risk of fatal CHD was found to be higher in users of snus/snuff (1.37, 95% CI = 1.14 to 1.61), whereas chewing tobacco did not yield a significant positive association (Supplementary Figure 4).
Publication bias
Egger’s regression test and Begg’s test revealed similar statistically non-significant result, indicating presence of publication bias among the included studies. Supplementary Figure 5 depicts the funnel plot for included studies. P–curve gave the power as 79% with 95% CI as 53–93%.
The proportion of fatal CHD attributable to SLT, using the global estimate relative risk was calculated to be 0.3%. The proportion of CHD deaths attributable to SLT use was calculated to be 5% for Sweden, 0.14% for United States, and 0.77% for Southeast Asia.
Discussion
This meta-analysis presents a comprehensive analysis of the available literature on association of SLT product use with risk of CHD, both nonfatal and fatal. We have classified the studies as per WHO-defined regions and included four regions—European, American, South-East Asian, and Eastern Mediterranean in our analysis. We would like to highlight the lack of reports of association of SLT use and risk of CHD from some of the regions, namely African and Western Pacific regions. The previous meta-analyses cited in literature were limited in the geographic regions included. Boffetta and Straif included only Swedish and American studies and excluded the Asian studies on association of SLT use and CHD.9 Vidyasagaran et al. included all available studies (Europe, America, and Asia) in their analysis;10 however, the authors categorized a study from Pakistan in Asian region. Sinha et al.11 also included studies available from three regions (European region, regions of the Americas, and South East Asian Region).
The overall risk estimate for CHD in the present analysis was not significant in the random effect model (1.05, 95% CI = 0.96 to 1.15). Our results are similar to results of earlier meta-analysis by Boffetta and Straif9 who gave an overall summary risk estimate for myocardial infarction as 0.99 (95% CI = 0.89 to 1.10) and that reported by Vidyasagaran et al.10 with risk estimate of 1.14 (95% CI = 0.92 to 1.42). Outcome-based analysis showed a significantly higher overall risk for fatal CHD (1.10, 95% CI = 1.00 to 1.20), which is in consonance with that reported by earlier meta-analyses.9–11 In the present meta-analysis, the risk of fatal CHD was highest (13%) in European SLT users. This also is in agreement with the earlier reports by Boffetta and Straif, Vidyasagaran et al., and Sinha et al.9–11
The previously reported meta-analyses have included few studies that did not adjust smoking as a confounder.23,24,34 Inclusion of former smokers in the study group is likely to lead to overestimation of relative risk of CHD and including such studies in the meta-analysis may lead to erroneous results. We performed a rigorous analysis of the available data after exclusion of such studies where smoking was not adjusted as a confounder. Our results demonstrate a significant positive association of SLT use and fatal CHD even after this analysis.
The present review analyzed, for the first time, the risk of CHD with different SLT products and found a significant positive association of fatal CHD with snus/snuff use (1.37, 95% CI = 1.14 to 1.61). This finding is supported by an earlier study that demonstrated reduction of post-MI mortality risk in snus quitters.35 Of importance is the observation of lack of heterogeneity among various studies included in the group of snus/snuff. Though snus/snuff is most commonly consumed in Europe and to some extent in the United States, the existence of internet marketing of SLT products is transgressing geographic boundaries and hence, these results are likely to assume global significance. On the other hand, we did not find a significant positive association of chewing tobacco with fatal CHD (1.07, 95% CI = 0.91 to 1.23). This may partly be attributed to the small number of studies in this group and significant heterogeneity between these studies. Though nass/nasvar showed a significant positive association with CHD (Supplementary Figure 4), there were only two studies in this group and hence, these results require further support by robust large studies. Further well-designed research studies are imperative on this topic for conclusive evidence. Studies are also required for detailed chemical analysis of various SLT products in order to understand the basis of cardiovascular effects and the observed difference between products.
The fraction of fatal CHD attributable to SLT use, in the present analysis, was calculated as 0.30% using the global data. Fraction of CHD deaths attributable to SLT use was seen to be higher in Sweden (5%) compared with United States (0.14%) and Southeast Asia (0.77%). Our results are in agreement with those of Boffetta and Straif who reported attributable fraction of myocardial infarction deaths as 0.5% in the United States and 5.6% in Sweden.9 The meta-analysis by Vidyasagaran et al. did not attempt the calculation of attributable fraction.10 These results suggest that probably SLT products used in Europe have a more deleterious effect on the outcome of CHD compared to those used in Americas or southeast Asia. The attributable fraction may come useful to policymakers for understanding of the disease burden of fatal CHD due to SLT use, thus making a case for an urgent need of implementing SLT control policies.
The main strengths of the present meta-analysis include the thoroughness of literature search and the strict inclusion criteria used. We performed the analysis including all studies initially followed by a rigorous exclusion of those studies where smoking was not adjusted as a confounder. This is of importance since smoking has been documented as a risk factor for cardiovascular diseases.4 With this view, the results of the present meta-analysis, including only smoking-adjusted studies, emphasize the positive association of SLT use with fatal CHD, especially for European SLT users. There results would, to a large extent, put to rest the raging controversy about association of SLT use with fatal CHD in so far as the European region is concerned. Since the inclusion criteria defined adjustment of smoking as confounder, the positive results of this meta-analysis signify the independent effect of SLT use on the risk of fatal CHD.
We, for the first time, performed analysis of product-based effect of SLT on risk of CHD and found a significant positive association between snus/snuff use and fatal CHD. However, further studies are required to corroborate this association.
Certain limitations in the present meta-analysis also need mention. The number of studies from American region was much less than those from Europe and, hence, the results could not be compared well. Since none of the studies included biochemical confirmation of SLT exposure, a possibility of misclassification of tobacco exposure based entirely on self-reporting cannot be ruled out. Another major limitation is the lack of information on some important confounders like alcohol, serum lipids in many studies included in our analysis. Presence of publication bias and heterogeneity among the included studies is another limitation that deserves mention. The same could be attributed to the limited number of studies conducted in only a few countries. Moreover, due to fewer number of studies, mixed effect model as well as level 2 and 3 models could not be applied.
Conclusion
Our meta-analysis reaffirms and emphasizes the increased risk of fatal CHD with SLT use. The risk was found to be higher for European users and those who consume snus/snuff. These results, in light of the strict adjustment for smoking, warrant that public health intervention for control of cardiovascular diseases should also lay emphasis on efforts for cessation of smokeless tobacco use in addition to those directed towards smoking.
Funding
AWB acknowledges past support from RC2DA028793, R21DA033813 and R43DA041211.
Declaration of Interests
The authors declare that they have no competing interests.
Supplementary Material
Acknowledgments
RG and SG contributed equally for this study.
References
- 1. Eriksen M, Mackay J, Schluger NW, Comeshtapeh FI, Drope J.. The Tobacco Atlas. Fifth edition. Atlanta: The American Cancer Society; 2015. http://3pk43x313ggr4cy0lh3tctjh.wpengine.netdna-cdn.com/wp-content/uploads/2015/03/TA5_2015_WEB.pdf. Accessed May 24, 2017. [Google Scholar]
- 2. Pechacek TF, Asma S, Blair N, Eriksen MP. Tobacco: global and community solutions. In: Yusuf S, Cairns JA, Camm AJ, Fallen EL, Gersh BJ, eds. Evidence-based cardiology. 2nd edn. London: BMJ Books; 2003:103–113. [Google Scholar]
- 3. Parish S, Collins R, Peto R et al. Cigarette smoking, tar yields, and non-fatal myocardial infarction: 14,000 cases and 32,000 controls in the United Kingdom. The International Studies of Infarct Survival (ISIS) Collaborators. BMJ. 1995;311(7003):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302(4):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ezzati M, Lopez AD. Measuring the accumulated hazards of smoking: global and regional estimates for 2000. Tob Control. 2003;12(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sinha DN, Agarwal N, Gupta PC. Prevalence of smokeless tobacco use and number of users in 121 countries. Br J Med Med Res. 2015;9:1–20. [Google Scholar]
- 7. Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9(7):667–675. [DOI] [PubMed] [Google Scholar]
- 8. Piano MR, Benowitz NL, Fitzgerald GA et al. ; American Heart Association Council on Cardiovascular Nursing. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122(15):1520–1544. [DOI] [PubMed] [Google Scholar]
- 9. Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ. 2009;339:b3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vidyasagaran AL, Siddiqi K, Kanaan M. Use of smokeless tobacco and risk of cardiovascular disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(18):1970–1981. [DOI] [PubMed] [Google Scholar]
- 11. Sinha DN, Suliankatchi RA, Gupta PC et al. Global burden of all-cause and cause-specific mortality due to smokeless tobacco use: systematic review and meta-analysis. Tob Control. 2018;27(1):35–42. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343:d2304. [DOI] [PubMed] [Google Scholar]
- 14. Bolinder G, Alfredsson L, Englund A, de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am J Public Health. 1994;84(3):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States). Cancer Causes Control. 2005;16(4):347–358. [DOI] [PubMed] [Google Scholar]
- 16. Rahman MA, Zaman MM. Smoking and smokeless tobacco consumption: possible risk factors for coronary heart disease among young patients attending a tertiary care cardiac hospital in Bangladesh. Public Health. 2008;122(12):1331–1338. [DOI] [PubMed] [Google Scholar]
- 17. Ram RV, Trivedi AV. Smoking, smokeless tobacco consumption and coronary artery disease – a case control study. Natl J Community Med. 2012;3:264–268. [Google Scholar]
- 18. Alexander M. Tobacco use and the risk of cardiovascular diseases in developed and developing countries. Cambridge: University of Cambridge; 2013. [Google Scholar]
- 19. Teo KK, Ounpuu S, Hawken S et al. ; INTERHEART Study Investigators. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–658. [DOI] [PubMed] [Google Scholar]
- 20. Hergens MP, Alfredsson L, Bolinder G, Lambe M, Pershagen G, Ye W. Long-term use of Swedish moist snuff and the risk of myocardial infarction amongst men. J Intern Med. 2007;262(3):351–359. [DOI] [PubMed] [Google Scholar]
- 21. Gupta PC, Pednekar MS, Parkin DM, Sankaranarayanan R. Tobacco associated mortality in Mumbai (Bombay) India. Results of the Bombay Cohort Study. Int J Epidemiol. 2005;34(6):1395–1402. [DOI] [PubMed] [Google Scholar]
- 22. Gajalakshmi V, Kanimozhi V. Tobacco chewing and adult mortality: a case-control analysis of 22,000 cases and 429,000 controls, never smoking tobacco and never drinking alcohol, in South India. Asian Pac J Cancer Prev. 2015;16(3):1201–1206. [DOI] [PubMed] [Google Scholar]
- 23. Huhtasaari F, Asplund K, Lundberg V, Stegmayr B, Wester PO. Tobacco and myocardial infarction: is snuff less dangerous than cigarettes?BMJ. 1992;305(6864):1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huhtasaari F, Lundberg V, Eliasson M, Janlert U, Asplund K. Smokeless tobacco as a possible risk factor for myocardial infarction: a population-based study in middle-aged men. J Am Coll Cardiol. 1999;34(6):1784–1790. [DOI] [PubMed] [Google Scholar]
- 25. Hergens MP, Ahlbom A, Andersson T, Pershagen G. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005;16(1):12–16. [DOI] [PubMed] [Google Scholar]
- 26. Johansson SE, Sundquist K, Qvist J, Sundquist J. Smokeless tobacco and coronary heart disease: a 12-year follow-up study. Eur J Cardiovasc Prev Rehabil. 2005;12(4):387–392. [DOI] [PubMed] [Google Scholar]
- 27. Wennberg P, Eliasson M, Hallmans G, Johansson L, Boman K, Jansson JH. The risk of myocardial infarction and sudden cardiac death amongst snuff users with or without a previous history of smoking. J Intern Med. 2007;262(3):360–367. [DOI] [PubMed] [Google Scholar]
- 28. Haglund B, Eliasson M, Stenbeck M, Rosén M. Is moist snuff use associated with excess risk of IHD or stroke? A longitudinal follow-up of snuff users in Sweden. Scand J Public Health. 2007;35(6):618–622. [DOI] [PubMed] [Google Scholar]
- 29. Hansson J, Pedersen NL, Galanti MR et al. Use of snus and risk for cardiovascular disease: results from the Swedish Twin Registry. J Intern Med. 2009;265(6):717–724. [DOI] [PubMed] [Google Scholar]
- 30. Janzon E, Hedblad B. Swedish snuff and incidence of cardiovascular disease: a population-based cohort study. Eur J Cardiovasc Prev Rehab. 2009;12:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Accortt NA, Waterbor JW, Beall C, Howard G. Chronic disease mortality in a cohort of smokeless tobacco users. Am J Epidemiol. 2002;156(8):730–737. [DOI] [PubMed] [Google Scholar]
- 32. Etemadi A, Khademi H, Kamangar F et al. ; Golestan Cohort Study Team. Hazards of cigarettes, smokeless tobacco and waterpipe in a Middle Eastern Population: a Cohort Study of 50 000 individuals from Iran. Tob Control. 2017;26(6):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rahman MA, Spurrier N, Mahmood MA, Rahman M, Choudhury SR, Leeder S. Is there any association between use of smokeless tobacco products and coronary heart disease in Bangladesh?PLoS One. 2012;7(1):e30584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bolinder GM, Ahlborg BO, Lindell JH. Use of smokeless tobacco: blood pressure elevation and other health hazards found in a large-scale population survey. J Intern Med. 1992;232(4):327–334. [DOI] [PubMed] [Google Scholar]
- 35. Arefalk G, Hambraeus K, Lind L, Michaëlsson K, Lindahl B, Sundström J. Discontinuation of smokeless tobacco and mortality risk after myocardial infarction. Circulation. 2014;130(4):325–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.