Abstract
Lactobacillus (Lab.) is a human probiotic beneficial for the prevention and improvement of disease, yet properties of different Lab. strains are diverse. To obtain a Lab. strain that possesses greater potential against gastrointestinal dysfunction, we isolated Lactobacillus plantarum TCI378 (TCI378) from naturally fermented Korean kimchi. TCI378 has shown potential as probiotic since it can survive at pH 3.0 and in the presence of 0.3% bile acid. The bile salt hydrolase activity of TCI378 was shown by formation of opaque granular white colonies on solid de Man Rogosa Sharpe (MRS) medium supplemented with taurodeoxycholic acid, and its cholesterol-lowering ability in MRS medium supplemented with cholesterol. The metabolites of TCI378 from liquid culture in MRS medium prevented emulsification of bile salts. Moreover, both the metabolites of TCI378 and the dead bacteria reduced oil droplet accumulation in 3T3-L1, as detected by Oil red O staining. The expressions of adipocyte-specific genes perilipin 1 and glucose transporter type 4 were suppressed by the metabolites of TCI378, indicating TCI378 may have anti-obesity effects in adipocytes. These in vitro data show the potential of the prophylactic applications of TCI378 and its metabolites for reducing fat and lowering cholesterol.
Keywords: Lactobacillus plantarum TCI378, bile salt hydrolase activity, cholesterol-lowering, anti-obesity
INTRODUCTION
The incidence of chronic diseases, such as metabolic syndrome, hypertension, dyslipidemia, diabetes, and obesity, is increasing worldwide and is attributed to long-term exposure to an unhealthy lifestyle. As a prophylactic agent against disease, supplementation food with probiotics has various benefits on human health. Clinical studies have shown the effectiveness of probiotics for immunomodulation, reducing serum cholesterol, and preventing diseases such as obesity, insulin resistance syndrome, type 2 diabetes, gastrointestinal diseases, allergic diseases, and colon cancer (Markowiak and Śliżewska, 2017; Sánchez et al., 2017).
Among the probiotics, Lactobacillus plantarum (Lab. plantarum) is supplemented in food, and shows beneficial effect on the intestinal balance and health of the host. Lab. plantarum can be isolated from human breast milk (Jiang et al., 2016), human feces (Ait Seddik et al., 2017; Costa et al., 2014), dairy products (Jabbari et al., 2017; Papadopoulou et al., 2018), and Asian fermented fruits and vegetables (Arasu and Al-Dhabi, 2017; Chen et al., 2010; Endo et al., 2008; Leisner et al., 2001; Patra et al., 2016; Swain et al., 2014; Tamang et al., 2005). Interestingly, the function and efficacy of probiotics depend on the strain, and may vary by the isolation sources. Kimchi is a popular and widely consumed Korean traditional fermented vegetable, which has been evaluated as one of the healthiest food in the world. Kimchi contains various bioactive components, including dietary fiber, vitamin C, and β-carotene. The intake of kimchi, which contains lactic acid bacteria, may have protective benefits on human organs, such as the gut, heart, and brain (Liu et al., 2018).
Lab. plantarum is one of the important lactic acid bacteria identified during the fermentation of kimchi (Jung et al., 2014; Patra et al., 2016; Swain et al., 2014). As intestinal microbes, some Lab. plantarum strains have potential bile salt hydrolase (BSH) activity (Bosch et al., 2014; Kumar et al., 2012; Lambert et al., 2008; Ren et al., 2011). Strains of probiotics with BSH activity are considered capable of reducing serum cholesterol levels (Kumar et al., 2012; Öner et al., 2014; Tsai et al., 2014). The main mechanism by which probiotics lower cholesterol is thought to lie in the biotransformation reaction of bile salt deconjugation, which is functional in bile detoxification (Ishimwe et al., 2015; Öner et al., 2014; Pavlović et al., 2012). Isolation and identification of probiotics that are present in natural foods can be an alternative method of prevention and a treatment strategy for hypercholesterolemia.
Another definable benefit of Lab. plantarum is its efficacy for regulating obesity. Obesity increases circulating cholesterol and triglyceride levels, and causes metabolic syndrome (Haslam and James, 2005). Peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT-enhancer-binding protein α (C/EBPα) regulate the early stage of adipocyte differentiation by activating adipogenic markers such as fatty acid synthase (FAS), cluster of differentiation 36 (CD36), leptin, glyceraldehyde-3-phosphate dehydrogenase (GPDH), and adipocyte fatty acid binding protein (aP2), which trigger accumulation of lipid in the cells. Lab. plantarum has been reported to inhibit the differentiation and lipid accumulation in adipocytes by downregulating expression of PPARγ, C/EBPα, FAS, aP2, leptin, GPDH, and CD36 (Ilavenil et al., 2015; Park et al., 2013b; Park et al., 2018). Administration of Lab. plantarum to diet-induced obese mice induced reductions in adipocyte size and white adipose tissue weight, and anti-obesity effects including decreases in serum total cholesterol and leptin concentrations (Lee et al., 2018; Park et al., 2013a; Park et al., 2015; Takemura et al., 2010).
Lab. plantarum has the generally recognized as safe (GRAS) status from the European Union and qualified presumption of safety (QPS) status from the U.S. Food and Drug Administration (Seddik et al., 2017). This study aimed to isolate a Lab. plantarum strain from naturally fermented kimchi that can be used as a dietary supplement for healthcare. We isolated the Lab. plantarum strain TCI378, and confirmed the potential abilities of TCI378 and its metabolites on gastrointestinal functions, fat metabolism and anti-obesity effects by in vitro examination.
MATERIALS AND METHODS
Isolation and preliminary identification of Lab. plantarum
Homogenates of naturally fermented kimchi were mixed with de Man Rogosa Sharpe (MRS) broth (Difco Laboratories Inc., Detroit, MI, USA) at a ratio (v/v) of 1:100. The mixture was cultured in anaerobic conditions (with an oxygen concentration <1%, AnaeroPack® System, Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan) at 37°C for 18 h. The overnight culture was streaked on a solid MRS agar plate and cultured overnight in anaerobic conditions at 37°C for single colony growth. Isolates were Gram-stained and characterized by carbohydrate utilization patterns using an API 50 CH system (bioMérieux, Inc., Durham, NC, USA). All isolates were subcultured three times prior to experimental analysis.
Candidate isolates were identified by phylogenetic analysis of the 16S rDNA sequence. Chromosomal DNA from each strain was extracted and 16S rRNA was amplified using universal primers. Polymerase chain reaction (PCR) primer sequences were as follows: forward primer, 5′-AGA GTT TGA TCC TGG CTC AG-3′; reverse primer, 5′-GGT TAC CTT TGT TAC GAC TT-3′ (Tri-I Biotech, Inc., Taipei, Taiwan). The thermal cycling parameters were: denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1 min, polymerization at 72°C for 40 s, and final polymerization at 72°C for 5 min. Amplified products were purified using a Gel Extraction Kit (GeneMark, Taichung, Taiwan), and sequenced. Sequence homologies of 16S rDNA were examined by blasting the online DNA database of National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Low pH and bile salts tolerance test
The ability of bacteria to grow at low pH was evaluated as described previously (Ehrmann et al., 2002). Bacteria were grown in MRS broth at 37°C overnight, then subcultured in fresh MRS broth and incubated for a further 24 h. Cultures were pelleted, washed twice and resuspended in sterile phosphate-buffered saline (PBS). Bacteria were diluted 1/100 in PBS at pH 3.0 and incubated at 37°C for 3 h. Bacteria incubated in PBS at pH 7.0 were used as controls. Bacterial cultures (100 μL) was transferred to MRS agar and broth, and incubated anaerobically at 37°C for 48 h. Counts of surviving cells were determined on MRS agar plates.
The tolerance of bacteria to bile salts was determined as described previously (Gilliland et al., 1984) with modifications. The bacteria were grown overnight in MRS broth. Freshly-prepared overnight cultures (2%) were inoculated in tubes containing MRS broth with 0.3% oxgall bile acids (Difco Laboratories Inc.); MRS broth without bile acids was used as the control. Tolerance to bile acid was evaluated by measuring cell viability after 3 h incubation at 42°C. Bacterial cultures (100 μL) were plated in duplicate onto MRS agar plates and bacterial numbers were counted after 48 h incubation.
BSH activity assay
The bile salt hydrolase activity was determined as previously described (Dashkevicz and Feighner, 1989). Lab. plantarum strains were cultured on MRS agar plates containing 0.5% taurodeoxycholic acid sodium (TDCA, Sigma, St. Louis, MO, USA). After incubation at 37°C for 2 days, colonies surrounded by a halo of precipitated-free bile acids and those forming opaque granular white colonies were regarded as having high BSH activity. The bile salt emulsification process was created as described (Fratter et al., 2014) with modifications. The cell-free supernatant of Lab. plantarum cultured in MRS broth containing 0.3% bile acids was collected and filtered to purify metabolites. Soy oil (1 mL) was mixed with 4 mL MRS broth containing bile salts or purified metabolites of bacterial culture, and the emulsification effect was observed.
Cholesterol-lowering test
Cholesterol-lowering activity was determined by using the cholesterol quantitation kit (#MAK043, Sigma) and by reference to a previously described method (Ziarno, 2008). Bacteria were cultured at 37°C in MRS broth supplemented with 0.2% oxgall bile acids (Difco Laboratories Inc.) and 0.1 g water soluble cholesterol. Following incubation, bacterial cells were harvested by centrifugation (10,000 rpm, 5 min). The supernatant and un-inoculated control MRS broth were assayed to determine the content of cholesterol through measuring absorbance at 570 nm using a spectrophotometer. The ability of each culture to lower cholesterol was calculated through the reduction of cholesterol concentration in broth supernatant at the end of the experiment.
Adipocyte culture and sample treatment
3T3-L1 cells were derived from the American Type Culture Collection (CL-173, Manassas, VA, USA). Culture of 3T3-L1 cells and adipogenesis induction were performed as previously described (Lee et al., 2015) with modifications. Cells were cultured in pre-adipocyte expansion medium based on Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco). Cells were seeded at a density of 8×104 cells/well in 24-well culture plates in triplicate (n=3). Confluent cells were cultured in adipocyte differentiation cocktail medium [0.5 mM methylisobutylxanthine (IBMX, Sigma), 1 μM dexamethasone (Sigma), and 1 μg/mL insulin (Sigma) in DMEM supplemented with 10% fetal bovine serum (FBS)] for 4 days, then in adipocyte maintenance medium (DMEM supplemented with 10% FBS and 1 μg/mL insulin) for 2 days, followed by DMEM supplemented with 10% FBS for a further 8 days. Cells were then cultured in a CO2 incubator at 37°C for adipocyte differentiation to complete. Differentiated adipocytes were treated with 1% MRS as a blank, or 1% culture supernatant of TCI378 without cells, and cultured in a CO2 incubator at 37°C for 2 days.
Oil red O staining of adipocytes
Intracellular lipid accumulation was measured using Oil red O staining. Oil red O staining of 3T3-L1 cells were performed as previously described (Lee et al., 2015) with modifications. 3T3-L1 cells were fixed with 10% formaldehyde/PBS and stained with Oil red O (Sigma). To assess lipid accumulation, the dye was extracted from stained cells with isopropanol, and was measured spectrophotometrically at 510 nm (Green and Kehinde, 1975). The percentage of Oil red O stained material from treated wells relative to that from control wells was calculated.
Quantification of adipocyte-specific gene expressions by real-time PCR
Treated adipocytes were harvested, and total RNA was isolated from cells using an RNA purification kit (Gene-Mark). DNA-free total RNA was reverse transcribed to cDNA using the SuperScriptTM Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA). Quantitative real-time PCR was conducted using ABI StepOnePlusTM Real-Time PCR System (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the SYBR Green Master Mix (KAPA Biosystems, Inc., Wilmington, MA, USA). Cycling conditions were as follows: one cycle at 95°C for 20 s, then 40 cycles of 95°C for 1 s, and 60°C for 20 s. The following primers were used for amplification: perilipin 1 (PLIN1, XM_011250776), forward (5′-GGG ACC TGT GAG TGC TTC C-3′) and reverse (5′-GTA TTG AAG AGC CGG GAT CTT TT-3′); glucose transporter type 4 (GLUT4, NM_001359114), forward (5′-GTG ACT GGA ACA CTG GTC CTA-3′) and reverse (5′-CCA GCC ACG TTG CAT TGT AG-3′). The β-actin gene (NM_007393) was used for normalization, amplified using the primers: forward (5′-GGC TGT ATT CCC CTC CAT CG-3′) and reverse (5′-CCA GTT GGT AAC AAT GCC ATG T-3′). Data were analyzed using the ABI StepOneTM Software v2.2.3 (Thermo Fisher Scientific, Inc.). All PCR assays were performed in duplicate three times.
Statistical asnalysis
All values are expressed as mean±standard deviation (SD). The statistical significance of the differences between two sample populations was determined by unpaired two-tailed Student’s t-tests. Statistical significance was considered at P<0.05.
RESULTS AND DISCUSSION
Lab. plantarum TCI378 was isolated from naturally fermented kimchi
A total of 32 bacterial colonies were isolated from naturally fermented kimchi, and were subjected to autosequencing for identity confirmation. Among these 32 colonies, 23 were identified as Lab. plantarum, 5 were identified as Leuconostoc mesenteroides and 4 were identified as Lactobacillus coryniformis by 16S rDNA sequence analysis. Strain of each species with the best growth rates were subjected to the Oil red O staining assay. Quantification results showed the Lab. plantarum strain reduced 15% of lipid droplets, the Leu. mesenteroides strain reduced 12%, and Lab. coryniformis strain reduced 9% (data not shown). In this study, we focused on the novel Lab. plantarum strain TCI378, which has 99% sequence identity homology at the 16S rRNA gene. Lab. plantarum TCI378 was deposited at the Food Industry Research and Development Institute (Hsinchu, Taiwan) under the accession number BCRC 910760 and at German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen) under the accession number DSM 32451. Although many Lab. plantarum strains have been isolated from fermented foods, the probiotic characteristics of each are considered to be source-specific (Haghshenas et al., 2016; Ramos et al., 2013; Yadav et al., 2016; Yadav et al., 2017). Lab. plantarum strains isolated from kimchi have been reported to have beneficial functions in immunoregulation, cancer treatment, skin moisturizing, and anti-obesity (Hong et al., 2014; Kim et al., 2015; Kwak et al., 2014; Park et al., 2017). The identification of Lab. plantarum strain TCI378 from indigenous kimchi enabled us to investigate its probiotic properties.
Lab. plantarum TCI378 is resistant to low pH and bile salt
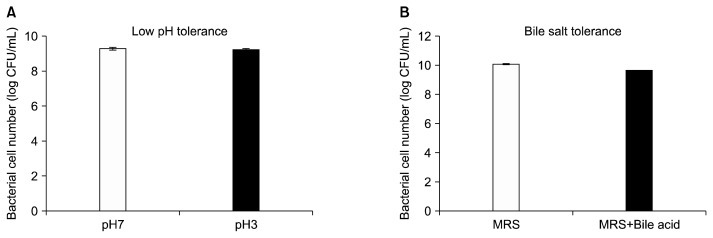
In the low pH tolerance test, the cell count of TCI378 in MRS medium of pH 7.0 was 1.97×109 colony-forming unit (CFU)/mL. In MRS medium of pH 3.0, the cell count of TCI378 was 1.76×109 CFU/mL. The survival rate of TCI378 in pH 3.0 was calculated from the base 10 logarithm, and showed a cell viability of 99.5% compared with the cell number for those cultured at pH 7.0 (Fig. 1A). In the bile salt tolerance test, the cell count of TCI378 in MRS medium lacking bile acids was 1.2×1010 CFU/mL, whereas the cell count of TCI378 cultured in MRS medium supplemented with 0.3% bile acids was 4.3×109 CFU/mL. The survival rate of TCI378 in 0.3% bile acids was 95.6% compared to the number of viable cells cultured in the control culture (Fig. 1B). These results show TCI378 is capable of survival at a low pH and in bile salts, indicating that TCI378 has potential as a probiotics to grow in gastrointestinal juice and function in the upper digestive tract (Papadimitriou et al., 2015).
Fig. 1.
Lactobacillus plantarum TCI378 (TCI378) is tolerant to low pH and bile acids. (A) The survival cell number of TCI378 cultured in MRS broth of pH 7.0 or pH 3.0 was calculated after taking the base 10 logarithm. (B) The survival cell number of TCI378 cultured in MRS broth only (MRS) or MRS broth supplement with 0.3% bile acids (MRS+bile acid) was calculated after taking the base 10 logarithm. The data are presented as mean±SD. CFU, colony-forming unit.
Lab. plantarum TCI378 possesses bile salt hydrolase and cholesterol-lowering activities
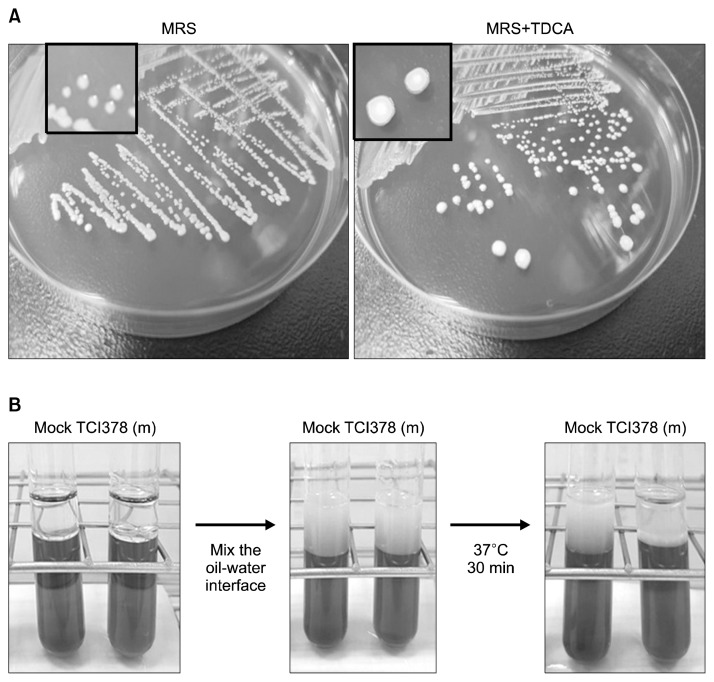
In MRS medium containing TDCA, taurine-conjugated bile acid is deconjugated and deoxycholic acid is produced by bile salt hydrolase-producing Lactobacilli (Dashkevicz and Feighner, 1989). Lab. plantarum TCI378 grown on MRS agar plates containing 0.5% TDCA formed opaque granular white colonies (Fig. 2A). Therefore, TCI378 was regarded as having bile salt hydrolase activity.
Fig. 2.
Lactobacillus plantarum TCI378 (TCI378) possessed the bile salt hydrolase activity and reduced the emulsification effect of bile salts. (A) TCI378 was streaked on MRS agar plates (MRS) or MRS agar plates containing 0.5% taurodeoxycholic acid (MRS+TDCA). Comparing to the regular TCI378 colonies (close view of left plate), TCI378 formed opaque granular white colonies on MRS+TDCA plates (close view of right plate). (B) The purified metabolite of TCI378 was added to the MRS medium containing bile salts and soy oil [TCI378(m)]. After mix the oil-water interface, the emulsification was observed. The emulsified layer was depleted in the mixture containing TCI378(m) after incubation at 37°C for 30 min, while the emulsification remained in the control (mock).
The biological function of bile salts in lipid digestion and absorption in the intestine involves high amounts of bile salt micelles in oil-in-water emulsions. The micelles promote fat absorption by facilitating transportation of a high concentration of lipids from the water layer to the mucosal cells (Torcello-Gómez et al., 2012). Mixing the oil with MRS broth containing bile salts produced an emulsified layer at the oil-water interface. Addition of TCI378 metabolites was able to deplete the emulsification and micelle formation of bile salts, indicating that TCI378 culture has bile salt hydrolase activity (Fig. 2B). Therefore, TCI378 may have an ability to reduce lipid absorption in the intestinal tract.
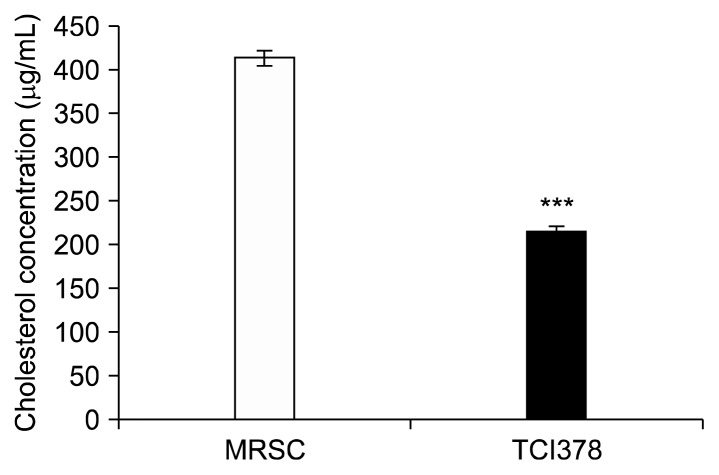
Bile salt hydrolase activity influences cholesterol metabolism. The ability of probiotic Lactobacilli to reduce serum cholesterol levels in vivo has often been correlated with their bile salt hydrolase activity (Patel et al., 2010). In general, strains with high BSH activities show high bile salt deconjugation abilities. Increases in biliary concentrations consequently decrease cholesterol removal and the viability of the bacteria (Öner et al., 2014). In vitro cholesterol-lowering tests showed TCI378 reduced the cholesterol concentration to 216 μg/mL, 48% lower than the control (413 μg/mL) (Fig. 3). Combined with its bile salt hydrolase activity, TCI378 may potentially be used for reducing serum cholesterol levels in prophylactical management of hypercholesterolemia.
Fig. 3.
Lactobacillus plantarum TCI378 (TCI378) cholesterollowering activity. TCI378 was cultured in MRS broth supplemented with 0.2% oxgall bile acids and 0.1 g water soluble cholesterol. The cholesterol concentration was determined in the supernatants of control (MRSC) and TCI378 culture (TCI378). Data are presented as mean±SD. ***P <0.001 (n=3).
Many potential mechanisms have been proposed for the cholesterol-lowering activities of probiotics with BSH activity, including regulations of bile acid binding to the farnesoid X receptor, and cholesterol export through the adenosine triphosphate binding cassette G5/G8 and Niemann-Pick C1-like 1 (Ishimwe et al., 2015; Jones et al., 2013). The main mechanism for the cholesterol-lowering effect of probiotics is thought to depend on the biotransformation reaction of bile salt deconjugation, which is functional in bile detoxification (Ishimwe et al., 2015; Öner et al., 2014). Hydrophobic deconjugated bile salts are reabsorbed less readily in the intestine, resulting in higher excretion in feces. Without probiotic bacteria, cholesterol is precipitated due to the reduced solubility of deconjugated bile salts. Therefore, coprecipitation of cholesterol with deconjugated bile salts at lower pH values is more required for cholesterol removal rather than assimilation or uptake of cholesterol by bacterial cells (Sridevi et al., 2009). Since TCI378 has shown potential to grow at a low pH and in bile salts, consumption may assist coprecipitation of cholesterol with deconjugated bile salts in gastrointestinal juice of the upper digestive tract.
Lab. plantarum TCI378 and its metabolites suppress adipogenesis and levels of lipogenesis-related factors
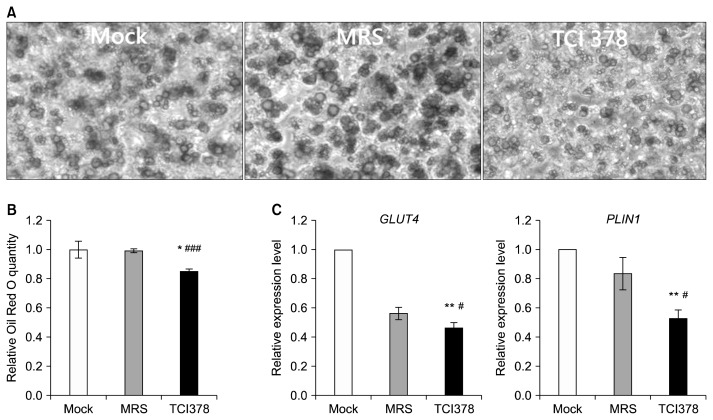
Differentiated adipocytes accumulate large amounts of oil droplets, demonstrated by control cells cultured in adipocyte maintenance medium, both with and without 1% MRS broth (Fig. 4A). However, the sizes and amounts of lipid droplets were reduced in 3T3-L1 cells treated with 1% TCI378 metabolites (Fig. 4A). Quantification of total lipid accumulation showed a 14% reduction in adipocytes treated with 1% TCI378 metabolites compared with controls (Fig. 4B). In addition, supplementation with dead TCI378 bacteria reduced lipid accumulation in 3T3-L1 by 9% compared to control cells (P<0.01) (unpublished data). We further examined expression of genes related to adipogenesis and lipolysis. Treatment of 3T3-L1 cells with 1% TCI378 metabolites decreased mRNA expression for GLUT4 and PLIN1 by 10% and 31%, respectively (Fig. 4C).
Fig. 4.
Lactobacillus plantarum TCI378 (TCI378) metabolites inhibit 3T3-L1 cell adipogenesis and lipolysis. Differentiated 3T3-L1 cells were left untreated (Mock), treated with 1% MRS (blank; MRS), or treated with 1% culture supernatant without TCI378 cells (TCI378), and cultured for 2 days. (A) Images of Oil red O-stained 3T3-L1 cells on plates. (B) Levels of oil accumulation were determined by quantifying Oil red O staining. (C) Real-time RT-PCR assays were performed for the adipogenic marker gene GLUT4 and lipolytic maker gene PLIN1. Data are presented as mean±SD. *P <0.05 and **P <0.01 compared with control, and #P <0.05 and ###P <0.001 compared with MRS (n=3).
In adipocytes, expression of GLUT4 is induced by PPARγ during the late stages of differentiation. Once cells are transformed into mature adipocytes, GLUT4 plays a major role in metabolic regulation by glucose transportation (Fernyhough et al., 2007). Therefore, a reduction in GLUT4 will decrease fat formation in adipocytes. Under basal conditions, PLIN1 is found on the surface of lipid droplets and is expressed specifically in adipocytes and steroidogenic cells. Overexpression of PLIN1 in 3T3-L1 preadipocytes suppresses lipolysis and limits access of lipases to lipid droplets (Brasaemle et al., 2000). Conversely, suppression of PLIN1 may promote fragmentation of larger lipid droplets into smaller droplets during lipolysis, which could be advantageous for providing a larger accessible surface area for lipases (MacPherson and Peters, 2015). To summarize, we suggest that Lab. plantarum TCI378 and its metabolites may inhibit lipid accumulation and reduce fat formation in adipocytes through suppression of adipogenic and lipolytic activities involving GLUT4 and PLIN1.
Many Lab. plantarum strains have been isolated from fermented kimchi. Probiotic characteristics of different strains are thought to be source-specific. Specifically, differing functions of Lab. plantarum strains isolated from kimchi have been reported, including: anti-obesity effects of Ln4 and HAC01 (Lee et al., 2018; Park et al., 2017), anti-pathogenic effects of KC21, DK211/303, YML007, and AF1 (Ahmad Rather et al., 2013; Baick and Kim, 2015; Lim and Im, 2009; Yang and Chang, 2010), and immunoregulatory effects of CJLP55/56/133/136, PM008, CLP-0611, and HY7712 (Jang et al., 2014; Jang et al., 2011; Jang et al., 2013; Won et al., 2011). In this study, we reported the functional specificity of Lab. plantarum strain TCI378. TCI378 shows probiotic BSH activity in cholesterol removal and anti-obesity; these results provide insight into the mechanisms of action of probiotic bacteria against obesity and hypercholesterolemia.
In conclusion, we used in vitro evaluation to show the indigenous Lab. plantarum strain TCI378 isolated from naturally fermented kimchi may promote gastrointestinal function, cholesterol metabolism, and anti-obesity effects. As candidate probiotics, TCI378 and its metabolites may be used as dietary supplements to improve human health.
ACKNOWLEDGEMENTS
This research was entirely funded by TCI Co., Ltd..
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Ahmad Rather I, Seo BJ, Rejish Kumar VJ, Choi UH, Choi KH, Lim JH, et al. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML007 and its application as a food preservative. Lett Appl Microbiol. 2013;57:69–76. doi: 10.1111/lam.12077. [DOI] [PubMed] [Google Scholar]
- Ait Seddik H, Bendali F, Cudennec B, Drider D. Anti-pathogenic and probiotic attributes of Lactobacillus salivarius and Lactobacillus plantarum strains isolated from feces of Algerian infants and adults. Res Microb. 2017;168:244–254. doi: 10.1016/j.resmic.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Arasu MV, Al-Dhabi NA. In vitro antifungal, probiotic, and antioxidant functional properties of a novel Lactobacillus paraplantarum isolated from fermented dates in Saudi Arabia. J Sci Food Agric. 2017;97:5287–5295. doi: 10.1002/jsfa.8413. [DOI] [PubMed] [Google Scholar]
- Baick SC, Kim CH. Assessment of characteristics and functional properties of Lactobacillus species isolated from kimchi for dairy use. Korean J Food Sci Anim Resour. 2015;35:339–349. doi: 10.5851/kosfa.2015.35.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Fuentes MC, Audivert S, Bonachera MA, Peiró S, Cuñé J. Lactobacillus plantarum CECT 7527, 7528 and 7529: probiotic candidates to reduce cholesterol levels. J Sci Food Agric. 2014;94:803–809. doi: 10.1002/jsfa.6467. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- Chen YS, Wu HC, Liu CH, Chen HC, Yanagida F. Isolation and characterization of lactic acid bacteria from jiang-sun (fermented bamboo shoots), a traditional fermented food in Taiwan. J Sci Food Agric. 2010;90:1977–1982. doi: 10.1002/jsfa.4034. [DOI] [PubMed] [Google Scholar]
- Costa GN, Marcelino-Guimarães FC, Vilas-Bôas GT, Matsuo T, Miglioranza LHS. Potential fate of ingested Lactobacillus plantarum and its occurrence in human feces. Appl Environ Microbiol. 2014;80:1013–1009. doi: 10.1128/AEM.02588-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashkevicz MP, Feighner SD. Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol. 1989;55:11–16. doi: 10.1128/AEM.55.1.11-16.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann MA, Kurzak P, Bauer J, Vogel RF. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J Appl Microbiol. 2002;92:966–975. doi: 10.1046/j.1365-2672.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- Endo A, Mizuno H, Okada S. Monitoring the bacterial community during fermentation of sunki, an unsalted, fermented vegetable traditional to the Kiso area of Japan. Lett Appl Microbiol. 2008;47:221–226. doi: 10.1111/j.1472-765X.2008.02404.x. [DOI] [PubMed] [Google Scholar]
- Fernyhough ME, Okine E, Hausman G, Vierck JL, Dodson MV. PPARγ and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007;33:367–378. doi: 10.1016/j.domaniend.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Fratter A, Frare C, Uras G, Bonini M, Casari Bariani E, Ragazzo B, et al. New chitosan salt in gastro-resistant oral formulation could interfere with enteric bile salts emulsification of diet fats: preliminary laboratory observations and physiologic rationale. J Med Food. 2014;17:723–729. doi: 10.1089/jmf.2013.0131. [DOI] [PubMed] [Google Scholar]
- Gilliland SE, Staley TE, Bush LJ. Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct. J Dairy Sci. 1984;67:3045–3051. doi: 10.3168/jds.S0022-0302(84)81670-7. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Haghshenas B, Haghshenas M, Nami Y, Yari Khosroushahi A, Abdullah N, Barzegari A, et al. Probiotic assessment of Lactobacillus plantarum 15HN and Enterococcus mundtii 50H isolated from traditional dairies microbiota. Adv Pharm Bull. 2016;6:37–47. doi: 10.15171/apb.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Hong YF, Kim H, Kim HR, Gim MG, Chung DK. Different immune regulatory potential of Lactobacillus plantarum and Lactobacillus sakei isolated from kimchi. J Microbiol Biotechnol. 2014;24:1629–1635. doi: 10.4014/jmb.1406.06062. [DOI] [PubMed] [Google Scholar]
- Ilavenil S, Kim DH, Valan Arasu M, Srigopalram S, Sivanesan R, Choi KC. Phenyllactic acid from Lactobacillus plantarum promotes adipogenic activity in 3T3-L1 adipocyte via up-regulation of PPAR-γ2. Molecules. 2015;20:15359–15373. doi: 10.3390/molecules200815359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimwe N, Daliri EB, Lee BH, Fang F, Du G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol Nutr Food Res. 2015;59:94–105. doi: 10.1002/mnfr.201400548. [DOI] [PubMed] [Google Scholar]
- Jabbari V, Khiabani MS, Mokarram RR, Hassanzadeh AM, Ahmadi E, Gharenaghadeh S, et al. Lactobacillus plantarum as a probiotic potential from Kouzeh cheese (traditional Iranian cheese) and its antimicrobial activity. Probiotics Antimicrob Proteins. 2017;9:189–193. doi: 10.1007/s12602-017-9255-0. [DOI] [PubMed] [Google Scholar]
- Jang SE, Han MJ, Kim SY, Kim DH. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. Int Immunopharmacol. 2014;21:186–192. doi: 10.1016/j.intimp.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Jang SE, Hyun YJ, Trinh HT, Han MJ, Kim DH. Anti-scratching behavioral effect of Lactobacillus plantarum PM008 isolated from kimchi in mice. Immunopharmacol Immunotoxicol. 2011;33:539–544. doi: 10.3109/08923973.2010.549136. [DOI] [PubMed] [Google Scholar]
- Jang SE, Joh EH, Lee HY, Ahn YT, Lee JH, Huh CS, et al. Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J Microbiol Biotechnol. 2013;23:414–421. doi: 10.4014/jmb.1210.10010. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang F, Wan C, Xiong Y, Shah NP, Wei H, et al. Evaluation of probiotic properties of Lactobacillus plantarum WLPL04 isolated from human breast milk. J Dairy Sci. 2016;99:1736–1746. doi: 10.3168/jds.2015-10434. [DOI] [PubMed] [Google Scholar]
- Jones ML, Tomaro-Duchesneau C, Martoni CJ, Prakash S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biol Ther. 2013;13:631–642. doi: 10.1517/14712598.2013.758706. [DOI] [PubMed] [Google Scholar]
- Jung JY, Lee SH, Jeon CO. Kimchi microflora: history, current status, and perspectives for industrial kimchi production. Appl Microbiol Biotechnol. 2014;98:2385–2393. doi: 10.1007/s00253-014-5513-1. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim HR, Jeong BJ, Lee SS, Kim TR, Jeong JH, et al. Effects of oral intake of kimchi-derived Lactobacillus plantarum K8 lysates on skin moisturizing. J Microbiol Biotechnol. 2015;25:74–80. doi: 10.4014/jmb.1407.07078. [DOI] [PubMed] [Google Scholar]
- Kumar R, Grover S, Batish VK. Bile salt hydrolase (Bsh) activity screening of lactobacilli: in vitro selection of indigenous Lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiotics Antimicrob Proteins. 2012;4:162–172. doi: 10.1007/s12602-012-9101-3. [DOI] [PubMed] [Google Scholar]
- Kwak SH, Cho YM, Noh GM, Om AS. Cancer preventive potential of kimchi lactic acid bacteria (Weissella cibaria, Lactobacillus plantarum) J Cancer Prev. 2014;19:253–258. doi: 10.15430/JCP.2014.19.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JM, Bongers RS, de Vos WM, Kleerebezem M. Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Appl Environ Microbiol. 2008;74:4719–4726. doi: 10.1128/AEM.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Jung SR, Lee SY, Lee NK, Paik HD, Lim SI. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients. 2018;10:E643. doi: 10.3390/nu10050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Song JL, Park ES, Ju J, Kim HY, Park KY. Anti-obesity effects of starter fermented kimchi on 3T3-L1 adipocytes. Prev Nutr Food Sci. 2015;20:298–302. doi: 10.3746/pnf.2015.20.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisner JJ, Vancanneyt M, Rusul G, Pot B, Lefebvre K, Fresi A, et al. Identification of lactic acid bacteria constituting the predominating microflora in an acid-fermented condiment (tempoyak) popular in Malaysia. Int J Food Microbiol. 2001;63:149–157. doi: 10.1016/S0168-1605(00)00476-1. [DOI] [PubMed] [Google Scholar]
- Lim SM, Im DS. Screening and characterization of probiotic lactic acid bacteria isolated from Korean fermented foods. J Microbiol Biotechnol. 2009;19:178–186. doi: 10.4014/jmb.0804.269. [DOI] [PubMed] [Google Scholar]
- Liu YW, Liong MT, Tsai YC. New perspectives of Lactobacillus plantarum as a probiotic: the gut-heart-brain axis. J Microbiol. 2018;56:601–613. doi: 10.1007/s12275-018-8079-2. [DOI] [PubMed] [Google Scholar]
- MacPherson RE, Peters SJ. Piecing together the puzzle of perilipin proteins and skeletal muscle lipolysis. Appl Physiol Nutr Metab. 2015;40:641–651. doi: 10.1139/apnm-2014-0485. [DOI] [PubMed] [Google Scholar]
- Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:E1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öner Ö, Aslim B, Aydaş SB. Mechanisms of cholesterol-lowering effects of lactobacilli and bifidobacteria strains as potential probiotics with their bsh gene analysis. J Mol Microbiol Biotechnol. 2014;24:12–18. doi: 10.1159/000354316. [DOI] [PubMed] [Google Scholar]
- Papadimitriou K, Zoumpopoulou G, Foligné B, Alexandraki V, Kazou M, Pot B, et al. Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol. 2015;6:58. doi: 10.3389/fmicb.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou OS, Argyri AA, Varzakis EE, Tassou CC, Chorianopoulos NG. Greek functional Feta cheese: enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiol. 2018;74:21–33. doi: 10.1016/j.fm.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, et al. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013a;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Oh SH, Cha YS. Lactobacillus plantarum LG42 isolated from gajami sik-hae inhibits adipogenesis in 3T3-L1 adipocyte. BioMed Res Int. 2013b;2013 doi: 10.1155/2013/460927. 460927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Ji Y, Jung HY, Park H, Kang J, Choi SH, et al. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl Microbiol Biotechnol. 2017;101:1605–1614. doi: 10.1007/s00253-016-7953-2. [DOI] [PubMed] [Google Scholar]
- Park SY, Cho SA, Lee MK, Lim SD. Effect of Lactobacillus plantarum FH185 on the reduction of adipocyte size and gut microbial changes in mice with diet-induced obesity. Korean J Food Sci Anim Resour. 2015;35:171–178. doi: 10.5851/kosfa.2015.35.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim S, Lim SD. The inhibitory effect of L. plantarum Q180 on adipocyte differentiation in 3T3-L1 and reduction of adipocyte size in mice fed high-fat diet. Korean J Food Sci Anim Resour. 2018;38:99–109. doi: 10.5851/kosfa.2018.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Singhania RR, Pandey A, Chincholkar SB. Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol. 2010;162:166–180. doi: 10.1007/s12010-009-8738-1. [DOI] [PubMed] [Google Scholar]
- Patra JK, Das G, Paramithiotis S, Shin HS. Kimchi and other widely consumed traditional fermented foods of Korea: a review. Front Microbiol. 2016;7:1493. doi: 10.3389/fmicb.2016.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlović N, Stankov K, Mikov M. Probiotics–interactions with bile acids and impact on cholesterol metabolism. Appl Biochem Biotechnol. 2012;168:1880–1895. doi: 10.1007/s12010-012-9904-4. [DOI] [PubMed] [Google Scholar]
- Ramos CL, Thorsen L, Schwan RF, Jespersen L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013;36:22–29. doi: 10.1016/j.fm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Ren J, Sun K, Wu Z, Yao J, Guo B. All 4 bile salt hydrolase proteins are responsible for the hydrolysis activity in Lactobacillus plantarum ST-III. J Food Sci. 2011;76:M622–M628. doi: 10.1111/j.1750-3841.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600240. doi: 10.1002/mnfr.201600240. 1600240. Available from: [DOI] [PubMed] [Google Scholar]
- Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins. 2017;9:111–122. doi: 10.1007/s12602-017-9264-z. [DOI] [PubMed] [Google Scholar]
- Sridevi N, Vishwe P, Prabhune A. Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Res Int. 2009;42:516–520. doi: 10.1016/j.foodres.2009.02.016. [DOI] [Google Scholar]
- Swain MR, Anandharaj M, Ray RC, Rani RP. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol Res Int. 2014;2014 doi: 10.1155/2014/250424. 250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp Biol Med. 2010;235:849–856. doi: 10.1258/ebm.2010.009377. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Tamang B, Schillinger U, Franz CMAP, Gores M, Holzapfel WH. Identification of predominant lactic acid bacteria isolated from traditionally fermented vegetable products of the Eastern Himalayas. Int J Food Microbiol. 2005;105:347–356. doi: 10.1016/j.ijfoodmicro.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Torcello-Gómez A, Jódar-Reyes AB, Maldonado-Valderrama J, Martín-Rodríguez A. Effect of emulsifier type against the action of bile salts at oil-water interfaces. Food Res Int. 2012;48:140–147. doi: 10.1016/j.foodres.2012.03.007. [DOI] [Google Scholar]
- Tsai CC, Lin PP, Hsieh YM, Zhang ZY, Wu HC, Huang CC. Cholesterol-lowering potentials of lactic acid bacteria based on bilesalt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci World J. 2014;2014 doi: 10.1155/2014/690752. 690752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won TJ, Kim B, Song DS, Lim YT, Oh ES, Lee DI, et al. Modulation of Th1/Th2 balance by Lactobacillus strains isolated from Kimchi via stimulation of macrophage cell line J774A.1 in vitro. J Food Sci. 2011;76:H55–H61. doi: 10.1111/j.1750-3841.2010.02031.x. [DOI] [PubMed] [Google Scholar]
- Yadav R, Puniya AK, Shukla P. Probiotic properties of Lactobacillus plantarum RYPR1 from an indigenous fermented beverage Raabadi. Front Microbiol. 2016;7:1683. doi: 10.3389/fmicb.2016.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Singh PK, Puniya AK, Shukla P. Catalytic interactions and molecular docking of bile salt hydrolase (BSH) from L. plantarum RYPR1 and its prebiotic utilization. Front Microbiol. 2017;7:2116. doi: 10.3389/fmicb.2016.02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Chang HC. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int J Food Microbiol. 2010;139:56–63. doi: 10.1016/j.ijfoodmicro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Ziarno M. In vitro cholesterol uptake by Lactobacillus acidophilus isolates. Acta Sci Pol Technol Aliment. 2008;7:65–74. [Google Scholar]