Abstract
Benign prostatic hyperplasia (BPH) is an age-related disease characterized by prostatic enlargement and is the most common urologic symptoms in elderly men 60 years of age and older. Previously, we documented that 70% ethanol (EtOH) seed extract of Quisqualis indica (QI) attenuates pathological condition of testosterone propionate (TP)-induced BPH rat model via modulation of proliferation and apoptosis of prostate cells. Based on this potential of QI, we produced standardized seed extract of QI (HU-033) in order to prove further mechanisms. In this study, we aimed to suggest further mechanisms underlying the relationship between BPH and HU-033. Through not only cellular and nuclear receptor functional assays, but TP-mediated BPH rat model treated with HU-033, we demonstrated that HU-033 exerted antagonist effect on α1A- and α1D-adrenergic receptors in vitro and inhibitory effect on protein expression of androgen receptor and estrogen receptor alpha in vivo. Taken together, these results suggest that HU-033 is a novel candidate for the management of BPH.
Keywords: HU-033, benign prostate hyperplasia, α1-adrenergic receptor, estrogen receptor α, androgen receptor
INTRODUCTION
The rapid increase in the age of the general population has been a pivotal factor in the increased prevalence of senescence-related disorders, including cancer, cardiovascular disease, and neurodegenerative symptoms; subsequently, these have emerged as a significant public health issue (Brunet et al., 2014). Benign prostatic hyperplasia (BPH), a representative gender-dependent disease, is the most common pathological condition in elderly men 60 years of age and older (Lepor, 2005; Woodard et al., 2016).
BPH, the result of gradual overgrowth of the prostate gland that lies at the base of the bladder and encircles the urethra, is generally associated with impairment in urinary tract function, including urinary intermittency, frequency, weak stream, and nocturia (Shabbir et al., 2004). Drug therapy using α1-adrenergic receptor antagonist (α1-blockers), 5α-reductase inhibitors, muscarinic receptor antagonists, and phosphodiesterase 5 inhibitors is common for the treatment of BPH. However, these drugs may cause drug-related adverse effects such as erectile dysfunction, sexual desire disorder, ectopic disorder, and cardiovascular effects such as orthostatic hypotension (Lepor, 2011; Huri et al., 2014). Because natural materials derived from plants are generally observed to lead to fewer side effect, many patients with BPH are interested in the use of complementary and alternative medicine.
Previously, we disclosed that seed extract of Quisqualis indica (QI) protected against testosterone propionate (TP)-mediated BPH in rats by decreasing expression level of dihydrotestosterone (DHT) and modulating proliferation and apoptosis of prostate cancer cells (Ub Wijerathne et al., 2017). As the standardized seed extract of QI, we turned out HU-033 on a mass production basis by adding dextrin as an exipient under the process of material standardization. By utilizing this substance, we examined further molecular mechanisms underlying the anti-BPH activity of HU-033.
MATERIALS AND METHODS
Preparation of HU-033
The seeds of QI were obtained from a local herbal market (Ansan, Korea), authenticated by Dr. Yeon, and deposited in the herbarium of the HUONS Research Center (Voucher no. HU033/SKJA150427, Ansan, Korea). The dried seeds of QI were homogenized to a fine powder (50 kg) and extracted by reflux with 500 L of 70% ethanol at 80°C for 6 h. The solution was concentrated under vacuum until elimination of the organic solvent was complete. Maltodextrin was then mixed with QI seed extract (total soluble solids content) in a 1:1 mass ratio. The final product (HU-033) obtained after mixing with maltodextrin and spray-drying (ODA-25, SeoGang Engineering, Cheonan, Korea) was collected and stored at room temperature.
High-performance liquid chromatography (HPLC) assay
For the identification of quisqualic acid from other amino acids, all types of amino acids were subjected to pre-column derivatization with o-phthaldialdehyde and ethanethiol, as described previously (Fernstrom et al., 1981). Free amino acids were measured by HPLC performed using an Agilent 1200 series system (Agilent Technologies, Santa Clara, CA, USA). The separation of HU-033 was performed at 30°C with a ZORBAX Eclipse Plus C18 column (4.6×250 mm, 5 μm particle size; Agilent Technologies). Sodium acetate buffer (solvent A; 0.01 M, pH 4.6) with methanol (solvent B) was used as the mobile phase, at a flow rate of 1 mL/min, with a programmed gradient of: 0~5 min, 20% B; 5~14 min, 30% B; 30 min, 50% B; and 35 min, 100% B; this was followed by equilibration to 20% B for 5 min. The absorbance at 338 nm was measured. The administered HU-033 was standardized to a quisqualic acid content of 1% by using a validated HPLC assay, as described by the Hong Kong Chinese Materia Medica Standards office.
Experimental animals
Eight-week-old male Sprague-Dawley (SD) rats were purchased from Orient Bio Inc. (Seongnam, Korea). Animals were maintained in an environment of 20~24°C and 45~55% humidity under a 12-h light/dark cycle and allowed ad libitum access to a standard rodent chow and sterilized tap water. All experiments were conducted according to the guidelines of the Animal Experimental Ethics Committee of Chungnam National University (Daejeon, Korea; Approval number: CNU-00961).
Establishment of TP-induced BPH rat model
The rats were acclimatized for 1 week prior to randomized division into 6 groups (6 rats/group): the normal control (NC) group, which received an oral administration of PBS and a subcutaneous injection of corn oil; the BPH group, which received an oral administration of PBS and a subcutaneous injection of 3 mg/kg TP; the finasteride (Fina) group, used as a positive control, which received an oral administration of 10 mg/kg Fina and a subcutaneous injection of 3 mg/kg TP; and three HU-033 groups, which received an oral administration of 50, 150, and 300 mg/kg HU-033, respectively, and a subcutaneous injection of 3 mg/kg TP. All rats received the indicated treatments daily for 4 weeks and body weight was measured once per week. At the end of the experiment, the rats were fasted overnight, anesthetized by the intraperitoneal injection of pentobarbital (100 mg/kg), and dissected to obtain blood samples from the caudal vena cava. The whole blood samples were centrifuged at 1,000 g for 10 min to obtain serum, which was stored at −80°C until further analysis. The prostate of each rat was also carefully recovered and weighed. The prostatic index and percentage inhibition were calculated from the following equation:
(Patil et al., 2016). The ventral prostate of each rat was fixed overnight in 10% neutral buffered formalin (NBF) and the rest of the prostate was snap-frozen in liquid nitrogen for protein assays.
Cell counting kit-8 (CCK-8) assay
The cytotoxic effect of HU-033 on LNCaP cells was evaluated by using the 2-(2-methoxy-4-nitrophenyl)-3-(4-ni trophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8) assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The cells were plated at 1×104 cells/well in 96-well plates in 100 μL Roswell Park Memorial Institute-1640 medium, to which HU-033 (25~3,200 μg/mL) was added. After incubation at 37°C for 72 h, the medium was replaced with 10 μL WST-8 reagent and incubated under the same conditions for a further 2 h. The absorbance at 450 nm was determined by using a microscope reader, from which the cell viability was calculated from the following equation:
Evaluation of antagonist effect against human α1-adrenergic receptors
The antagonist effect of HU-033 on α1A-, α1B-, and α1D-adrenergic receptors was determined using Eurofins Cerep (Celle-Lévescault, France). Briefly, Chinese hamster ovary (CHO) cells expressing the α1A and α1B receptors, and rat basophil leukemia cells expressing the α1D receptor were used to measure the effect on α1A agonist-induced cytosolic Ca2+ ion mobilization, α1B agonist-induced cyclic adenosine monophosphate (cAMP) production, and α1D agonist-induced cytosolic Ca2+ ion mobilization, respectively.
Western blotting
Tissues were homogenized in radioimmunoprecipitation assay lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) supplemented with protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific, Waltham, MA, USA). For western blotting, 30~40 μg of protein was separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membranes. For experiments using prostate tissue, the membranes were probed with anti-androgen receptor (AR; Invitrogen, Carlsbad, CA, USA), antiestrogen receptor alpha (ERα; Abcam, Cambridge, MA, USA), or anti-β-actin (housekeeping gene; Sigma-Aldrich Co., St. Louis, MO, USA) antibodies diluted in Tris-buffered saline [0.05% Tween-20 (TBS-T) and 5% skim milk]. After washing in TBS-T, the membranes were developed by using an enhanced chemiluminescence kit (Thermo Fisher Scientific).
Immunohistochemical (IHC) analysis
IHC staining was performed by using a Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, CA, USA) in accordance with the manufacturer’s instructions. After antigen retrieval, the sections were blocked in normal serum and then incubated with anti-AR (Invitrogen) or ERα (Abcam) antibodies at 4°C. After incubation overnight, the sections were then incubated in biotinylated secondary antibody and developed by using a diaminobenzidine peroxidase substrate kit (Vector Laboratories Inc.).
Statistical analysis
Experiments were conducted at least three times and the data were expressed as the mean±standard deviation (SD) or standard error of the mean (SEM). All statistical analyses were computed by using Statistical Package for the Social Sciences 16.0 software (IBM Corp., New York, NY, USA). Student’s t-test was used to compare the parameters between two groups, whereas analysis of variance followed by Tukey’s post hoc test was used to compare the parameters between three groups; in all analyses, P<0.05 was considered to indicate statistical significance.
RESULTS AND DISCUSSION
HPLC analysis of HU-033
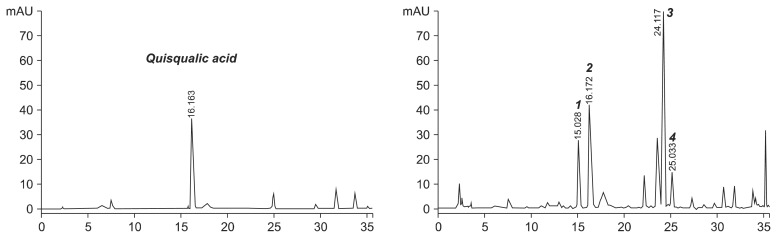
We verified the test substance, HU-033 through validated HPLC analysis. As shown in Fig. 1, HU-033 was standardized by the content of quisqualic acid (left) and several amino acids contained in HU-033 (1, asparagine; 2, quisqualic acid; 3, arginine; 4, glutamic acid) were determined (right).
Fig. 1.
High-performance liquid chromatography (HPLC) analysis of standardized seed extract of Quisqualis indica (HU-033). Chromatogram of quisqualic acid standard (left) and HPLC profile of amino acids in standardized HU-033 (right). 1, asparagine; 2, quisqualic acid; 3, arginine; 4, glutamic acid.
Antagonistic effect of HU-033 on α1-adrenergic receptors
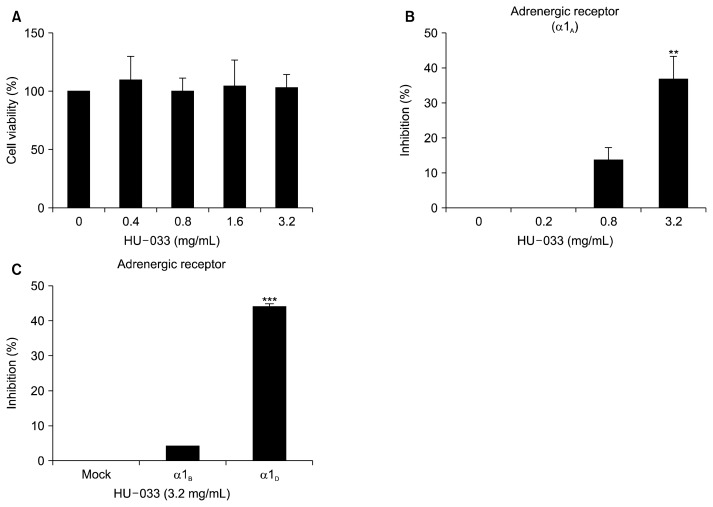
Prior to beginning in vitro studies, we evaluated the cytotoxicity of HU-033 in different concentrations (from 0.4 mg/mL up to 3.2 mg/mL). As a result, we confirmed that HU-033 did not affect cell viability at all concentrations (Fig. 2A). And then, we disclosed the interaction between HU-033 and subtypes of α1-adrenergic receptors including α1A, α1B, and α1D. As shown in Fig. 2B and 2C, we found that expression of α1A and α1D-adrenergic receptors were selectively inhibited by HU-033 in vitro. It is well established that 5α-reductase is an essential enzyme in the formation of DHT, which is a key factor in BPH. An increase in the level of this principle prostatic androgen triggers the occurrence of BPH (Bartsch et al., 2002; Carson and Rittmaster, 2003). Hence, 5α-reductase inhibitors, such as finasteride and dutasteride, are widely used for the treatment of BPH and act through a reduction in prostate volume and the consequent improvement of lower urinary tract symptoms (Kim et al., 2018). In addition to the androgen-signaling axis, alpha adrenergic receptors including doxazosin, tamsulosin, and alfuzosin, induce the relaxation of the smooth muscle in the prostate and around the neck of the bladder, effectively relieving BPH symptoms (Janknegt et al., 1993; Chon et al., 1999; Shelbaia et al., 2013; Chapple, 2005). Among the subfamilies of alpha adrenergic receptors, α1A receptors are linked with relaxation of prostate smooth muscle and α1D receptors are linked with weak urine flow and acute retention (Forray et al., 1994). Thus, these results suggest the potential of HU-033 as the inhibitor of BPH pathogenesis-associated α1A and α1D except for α1B.
Fig. 2.
Effect of seed extract of Quisqualis indica (HU-033) on α1-adrenergic receptor in vitro. (A) The toxicity of HU-033 (0.4, 0.8, 1.6, and 3.2 mg/mL) to LNCaP cells was analyzed by using a cell counting kit-8 assay. The antagonist activity of HU-033 on the α1A, α1B, and α1D-adrenergic receptors was evaluated in CHO cells treated with 0.2, 0.8, and 3.2 mg/mL (B) or 3.2 mg/mL (C) HU-033. **P <0.01 and ***P <0.001 vs. the control group.
Therapeutic effect of HU-033 administration on prostate weight of rats
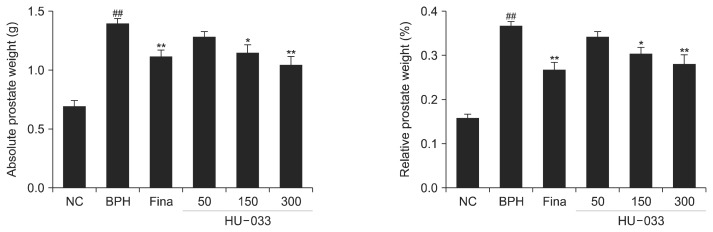
At the end of the TP-induced BPH rat model study, all rats were sacrificed and we measured the prostate weight isolated from them treated with finasteride or HU-033 (50, 150, and 300 mg/kg). As shown in Fig. 3, treatment of finasteride that is a selective competitive inhibitor of 5α-reductase type 2 reduced weight of enlarged prostate caused by TP injection. In addition, administration of HU-033 decreased prostate weight with concentration of 150 and 300 mg/kg in comparison with BPH group (negative control).
Fig. 3.
Effect of seed extract of Quisqualis indica (HU-033) on the weight of prostate tissues isolated from testosterone propionate (TP)-induced benign prostatic hyperplasia (BPH) rats. The absolute and relative prostate weights were calculated. NC, normal control, corn oil injection and PBS treatment; BPH, TP (3 mg/kg) injection and PBS treatment; Fina, TP (3 mg/kg) injection and finasteride (10 mg/kg) treatment; HU-033-50, 150, and 300, TP (3 mg/kg) injection and HU-033 (50, 150, and 300 mg/kg) treatment, respectively. ##P <0.01 vs. the NC group and *P <0.05 and **P <0.01 vs. the BPH group.
Inhibitory effect of HU-033 administration on AR and ERα expression in prostate tissues
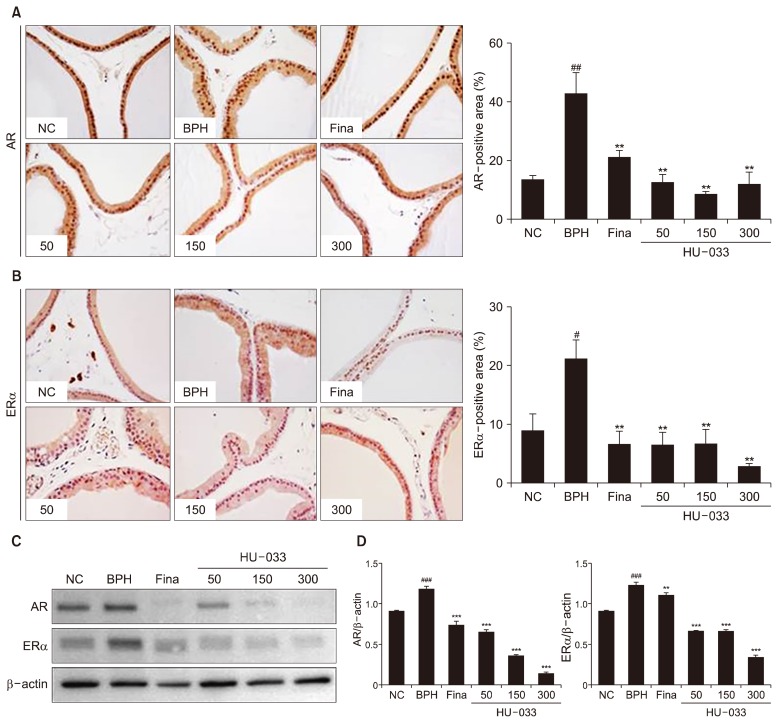
Our previous study revealed that 70% EtOH seed extract of QI suppressed protein expression of AR in vitro (Ub Wijerathne et al., 2017). But, in vivo effect of QI seed extract on AR and ERα expression has been unknown. Hence, molecular mechanisms underlying HU-033-mediated BPH treatment were investigated through IHC analysis with AR and ERα antibody. HU-033 significantly down-regulated the degree of elevated AR-positive region in prostate tissues from BPH group (Fig. 4A). HU-033 also negatively regulated expression of ERα in prostate tissues (Fig. 4B). This observation was reaffirmed by western blotting using protein lysates of prostate tissues from experimental rat groups (Fig. 4C and 4D). As the key molecular mechanisms for BPH modulation, androgen/ AR signaling induces the development of BPH development via control of the interaction between epithelial and stromal cells, and their subsequent proliferation. The selective knockout of AR in stromal smooth-muscle cells negatively regulated BPH development in vitro and blockade of this signaling reduced BPH volume in vivo (Izumi et al., 2013). ERα also contributed to bladder impairment in the pathogenesis of BPH. It was proven that ERα conditional-knockout mice did not suffer from BPH despite the co-treatment of TP and 17β-estradiol, which suggested a stimulatory role for ERα in BPH (Nicholson et al., 2015). In line with in vitro study, administration of HU-033 on TP-induced BPH rat model blocked pathological condition of prostate tissues by regulating the expression of AR and ERα in vivo.
Fig. 4.
Effect of seed extract of Quisqualis indica (HU-033) on the expression of androgen receptor (AR) and estrogen receptor (ER) α in vivo. (A) Representative photomicrographs of prostate tissues (magnification × 400, left). The percentage of AR-positive area in the prostate tissue (right). (B) Representative photomicrographs of prostate tissues (magnification ×400, left). The percentage of ERα-positive area in the prostate tissue (right). (C) The protein expression of AR, ERα, and β-actin in rat prostate tissues was analyzed by using western blotting. (D) The relative ratio of band intensity was calculated for AR/β-actin and ERα/β-actin. #P <0.05, ##P <0.01, and ###P <0.001 vs. the normal control (NC) group and **P <0.01 ***P <0.001 vs. the benign prostatic hyperplasia (BPH) group.
Previously, we reported the first documented case of seed extract of QI in BPH. In BPH rat models, serum concentration of DHT, and mRNA level of 5α-reductase type 2 were significantly inhibited following the treatment with QI. Also, QI exerted anti-proliferative and pro-apoptotic activities of prostate cells through regulating proliferating cell nuclear antigen, cyclin D1, caspase-3, and -9 (Ub Wijerathne et al., 2017). Based on this research, we tried to disclose further mechanisms by using standardized material of QI, HU-033. In this study, we demonstrated additional preclinical data against BPH through usage of HU-033 as the standardized material. We confirmed not only antagonistic effect on α1-adrenergic receptors in vitro but also inhibitory effect on AR and ERα in vivo. This preclinical research disclosed that HU-033 is a dietary supplement for overcoming prostate disease.
ACKNOWLEDGEMENTS
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the Agri-Bio Industry Technology Development Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (2016190262).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the role of 5 alpha-reductase inhibitors in benign prostatic hyperplasia. Urologe A. 2002;41:412–424. doi: 10.1007/s00120-002-0230-2. [DOI] [PubMed] [Google Scholar]
- Brunet A, Berger SL. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014;69:S17–S20. doi: 10.1093/gerona/glu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson C, 3rd, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61:2–7. doi: 10.1016/S0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Chapple CR. A comparison of varying alpha-blockers and other pharmacotherapy options for lower urinary tract symptoms. Rev Urol. 2005;4:S22–S30. [PMC free article] [PubMed] [Google Scholar]
- Chon JK, Borkowski A, Partin AW, Isaacs JT, Jacobs SC, Kyprianou N. Alpha 1-adrenoceptor antagonists terazosin and doxazosin induce prostate apoptosis without affecting cell proliferation in patients with benign prostatic hyperplasia. J Urol. 1999;161:2002–2008. doi: 10.1016/S0022-5347(05)68873-8. [DOI] [PubMed] [Google Scholar]
- Fernstrom MH, Fernstrom JD. Rapid measurement of free amino acids in serum and CSF using high-performance liquid chromatography. Life Sci. 1981;29:2119–2130. doi: 10.1016/0024-3205(81)90669-X. [DOI] [PubMed] [Google Scholar]
- Forray C, Bard JA, Wetzel JM, Chiu G, Shapiro E, Tang R, et al. The alpha 1-adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacological properties of the cloned human alpha 1c subtype. Mol Pharmacol. 1994;45:703–708. [PubMed] [Google Scholar]
- Huri HZ, Xin CH, Sulaiman CZ. Drug-related problems in patients with benign prostatic hyperplasia: a cross sectional retrospective study. PLoS One. 2014;9:e86215. doi: 10.1371/journal.pone.0086215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182:1942–1949. doi: 10.1016/j.ajpath.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknegt RA, Chapple CR. Efficacy and safety of the alpha-1 blocker doxazosin in the treatment of benign prostatic hyperplasia. Analysis of 5 studies. Doxazosin Study Groups. Eur Urol. 1993;24:319–326. doi: 10.1159/000474321. [DOI] [PubMed] [Google Scholar]
- Kim EH, Brockman JA, Andriole GL. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian J Urol. 2018;5:28–32. doi: 10.1016/j.ajur.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepor H. Medical treatment of benign prostatic hyperplasia. Rev Urol. 2011;13:20–33. [PMC free article] [PubMed] [Google Scholar]
- Lepor H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev Urol. 2005;7:S3–S12. [PMC free article] [PubMed] [Google Scholar]
- Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, et al. Estrogen receptor-α is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol. 2015;193:722–729. doi: 10.1016/j.juro.2014.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AA, Veeresh B, Yadav A. Combination of lauric acid and myristic acid prevents benign prostatic hyperplasia (BPH) symptoms in animal model. Afr J Pharm Pharmacol. 2016;10:101–106. [Google Scholar]
- Shabbir M, Mumtaz FH. Benign prostatic hyperplasia. J R Soc Promot Health. 2004;124:222–227. doi: 10.1177/146642400412400519. [DOI] [PubMed] [Google Scholar]
- Shelbaia A, Elsaied WM, Elghamrawy H, Abdullah A, Salaheldin M. Effect of selective alpha-blocker tamsulosin on erectile function in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Urology. 2013;82:130–135. doi: 10.1016/j.urology.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Ub Wijerathne C, Park HS, Jeong HY, Song JW, Moon OS, Seo YW, et al. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol Pharm Bull. 2017;40:2125–2133. doi: 10.1248/bpb.b17-00468. [DOI] [PubMed] [Google Scholar]
- Woodard TJ, Manigault KR, McBurrows NN, Wray TL, Woodard LM. Management of benign prostatic hyperplasia in older adults. Consult Pharm. 2016;31:412–424. doi: 10.4140/TCP.n.2016.412. [DOI] [PubMed] [Google Scholar]