Abstract
Copper (Cu) plays an essential role in the development and function of the brain. In humans, genetic disorders of Cu metabolism may cause either severe Cu deficiency (Menkes disease) or excessive Cu accumulation (Wilson disease) in the brain tissue. In either case, the loss of Cu homeostasis results in catecholamine misbalance, abnormal myelination of neurons, loss of normal brain architecture, and a spectrum of neurologic and/or psychiatric manifestations. Several metabolic processes have been identified as particularly sensitive to Cu dishomeostasis. This review focuses on the role of Cu in noradrenergic neurons and summarizes the current knowledge of mechanisms that maintain Cu homeostasis in these cells. The impact of Cu misbalance on catecholamine metabolism and functioning of noradrenergic system is discussed.
Keywords: Copper, catecholamines, dopamine-β-hydroxylase, locus coeruleus
The utilization and function of copper (Cu) in the brain
Copper (Cu) is required for the development, maturation, and function of human brain - as a cofactor of key metabolic enzymes and a signaling and regulatory molecule. In enzymes, Cu is most often used for electron transfer as well as binging and activation of oxygen. Detailed information on coordination environments and the types of Cu binding sites in biomolecules can be found in a recent review [1]. In mitochondria, cytochrome c oxidase uses its two Cu centers to facilitate transfer of electrons to oxygen during respiratory cycle. A binuclear, mixed valence (CuII/CuI) CuA center site is exposed into the inter-membrane space of mitochondria and serves as an acceptor of electrons from the reduced cytochrome. The bimetallic hemea3-Cu (CuB) center site is located within the transmembrane domain and is involved in O2 reduction [2]. To populate these centers, mitochondria employ a complex protein machinery for Cu delivery and insertion [3, 4]. Genetic defects in this machinery cause fatal disorders with a major impact on a heart, brain, and liver function [4].
In the cytosol, Cu enables activity of Cu/Zn-dependent superoxide dismutase 1 (SOD1). This ubiquitous and finely regulated enzyme detoxifies superoxide radicals by converting them into hydrogen peroxide [5]. SOD1 is a dimer, and each monomer has a Cu/Zn binuclear site. Cu plays the major role in the catalysis by transitioning from Cu(II) to Cu(I) state [6]. Loss of SOD1 activity is associated with a spectrum of severe neuropathologies, including progressive loss of motor function and cerebral atrophy [7]. Mutations that cause misfolding and aggregation of SOD1 have been linked to amyotrophic lateral sclerosis, a progressive and fatal neurodegenerative disease [8]. Other Cu-dependent enzymes in the brain have more specialized functions compared to cytochrome c oxidase and SOD1, but these functions are equally important. Peptidyl-α–monooxygenase (PAM) employs Cu to catalyze the first (hydroxylation) step in C-terminal amidation of neuropeptides [9]; this modification increases affinity of neuropeptides for their receptors. PAM has two Cu atoms in distinct sites (CuH and CuM) located 11Å apart; the peptide substrate bridges these sites and serves as a conduit for electron transfer [10]. PAM is especially abundant in the hippocampus and in olfactory cortex.
In noradrenergic neurons, Cu is needed for activity of dopamine-β-hydroxylase (DBH), which converts dopamine to norepinephrine and is required for catecholamine balance [11]. The catalytic core of DBH is structurally similar to PAM. DBH is a tetrameric protein and each subunit has two Cu atoms bound at distinct sites. In the CuH site, Cu is coordinated by three histidines; in the CuM site – by two histidines and one methionine. Binding of Cu to both sites is required for DBH catalysis [11]. Inactivation of DBH causes embryonic lethality in mice. Several other Cu-dependent enzymes, such as ceruloplasmin and hephaestin (involved in iron homeostasis), tyrosinase (pigmentation), lysyl oxidase (collagen cross-linking), SOD3 (wound healing), amino-oxidase 3 (regulation of metabolic fluxes and inflammation) are also expressed in the brain [12], but their specific roles in the CNS have not been characterized in detail.
In addition to its well-known role as an enzyme cofactor, Cu is involved in a wide range of cellular processes, in which it has regulatory and signaling roles. Cu is required for myelination of neurons [13], and it influences synaptic transmission by modulating functions of GABAA and NMDA receptors, as well as voltage-gated Ca2+ channels [14–17]. Changes in Cu levels alter glycolytic flux in astrocytes and affect lipid balance [18–21]. Dietary or genetic Cu deficiency causes significant metabolic abnormalities, hypomyelination, abnormal arborization of neurons, seizures, and - in Menkes disease (MNKD) – death [22]. Cu excess is also detrimental for the normal brain function. In Wilson disease (WD), Cu accumulates in the brain and other tissues. The increase in Cu levels causes a broad spectrum of neurologic and psychiatric pathologies, including depression, psychotic episodes, dystonia, tremor, and sleep abnormalities [23]. In both MNKD and WD, catecholamine homeostasis is disturbed [24, 25]. MNKD patients have increased levels of dopamine (DA) and lower levels on norepinephrine (NE), both in brain and in the serum. In WD, changes in catecholamine levels are region-dependent and variable [25], but the deficits in a dopaminergic system are frequently reported [26–28]. How Cu induces changes in the cellular machinery involved in dopamine signaling and uptake remains poorly understood. Cu misbalance in conjunction with a negative impact on noradrenergic neurons has also been reported for Parkinson’s disease, Alzheimer’s disease, and could be a contributing factor in etiology of other neurodegenerative disorders and aging [29].
Like many metabolic processes, the Cu-requiring biochemical reactions in the central nervous system (CNS) depend on a timely delivery to the brain of sufficient amounts of their key “metabolite”, namely Cu. Studies in human subjects determined that the concentration of Cu in the brain increases early in child development, and triples upon transition from a prenatal to an infant stage [30]. These age-related increases in the brain Cu are also seen in rodents [31, 32]. Both humans and rodents show gender-specific differences in the serum Cu levels, which are higher in females [33]. Whether gender specific differences exist in the uptake and processing of Cu by the CNS is unclear. Available data suggest that the adult females (humans and rats) have a larger number of noradrenergic neurons and more prolonged neurogenesis. Therefore, females would likely to require more Cu for the functional maturation of their noradrenergic system [34, 35]. Coincidentally, gender-dependent differences were reported for WD patients with neuropsychiatric symptoms. Not only neuropsychiatric WD is more frequently seen in men compared to women, male patients also have an earlier age of onset and a shorter time of disease latency compared to female patients [33, 36].
Copper entry into the brain
The mechanisms of Cu transport in and out of the brain are still being investigated. The two proposed entry routes are the brain capillary endothelial cells that form the blood-brain barrier (BBB) and the choroid plexus that forms the blood-cerebrospinal fluid barrier (BCB). A much higher content of Cu in the blood compared to the cerebrospinal fluid (0.3–0.5 μM or 22.3 ± 2.2 μg/L in a CSF versus 1129 ± 124 μg/L in a serum [37]) suggests that BBB and BCB limit entry of Cu into the brain. Experiments on perfusion of mice with radioactive Cu found a negligible increase of Cu in the CSF; whereas Cu uptake into the brain parenchyma was higher. These experiments led to the suggestion that BBB represents the primary route of Cu entry into the brain parenchyma, whereas a fine-tuned regulation of Cu entry into the brain occurs in BCB [38].
The Cu transporter ATP7A (defective in MNKD) appears to play the major role in exporting Cu from both brain barriers. ATP7A is highly abundant in the endothelial cells of choroid plexus [38], a BCB site, and in cerebrovascular endothelial cells that form BBB [39]. Studies of a recently identified patient with a somatic mosaicism for an ATP7A P1001L mutation provided evidence for significance of ATP7A in choroid plexus. In this patient, cells originating from the ectoderm (such as choroid plexus epithelia) had ATP7A mutation more frequently than cells originating from mesoderm (such as endothelial cells of BBB [40]). The patients had clinical manifestation typical for classic MNKD (a complete loss of ATP7A function), indicating that a partial activity of ATP7A in BBB is insufficient for normal Cu entry and brain development. Similarly to patients with MNKD, inactivation of ATP7A in brindle mice (an animal model of MNKD) results in Cu accumulation in brain capillaries and deficiency of Cu in the brain [41]. Overexpression of the recombinant ATP7A protein in choroid plexus along with Cu injections corrects ATP7A deficiency in brindle mice [42].
In WD (caused by ATP7B inactivation), Cu import into the brain is not impaired, but regulation of Cu balance is altered, resulting in a gradual accumulation of Cu in the brain tissue [23]. ATP7B, which is homologous to ATP7A, is also expressed in endothelial cells of choroid plexus but has different cellular behavior in response to Cu elevation. In these cells, Cu triggers ATP7A to traffic from intracellular compartments towards the apical membrane facing cerebrospinal fluid, a behavior typically associated with an increased Cu efflux from cells. By contrast, ATP7B moves towards the basolateral membrane (facing the blood), presumably to facilitate return of Cu to the circulation [39]. ATP7B may also buffer Cu levels in BCB and modulate ATP7A function. It should be noted that the pattern of polarized trafficking of the two Cu transporters in choroid plexus is opposite to other epithelial systems, where ATP7A was repeatedly shown to traffic to the basolateral membrane and ATP7B – to the apical membrane.
Cu is enriched in specific brain areas regions
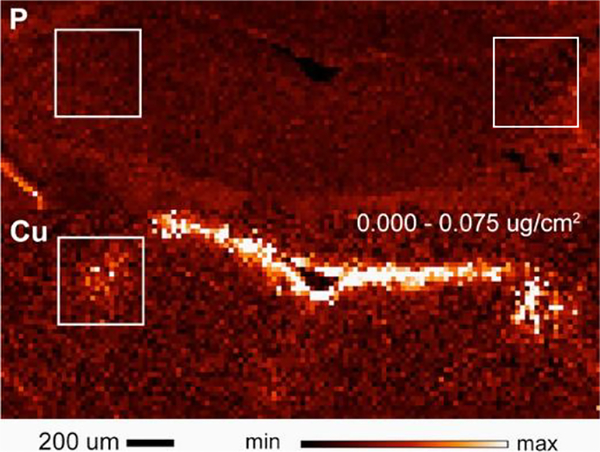
The distribution of Cu across the brain parenchyma is fairly even, with the exception of several regions, where Cu is notably high. Upon entry into the brain, Cu accumulates in the subventricular zone of brain ventricles (Fig.1), more specifically in the subset of astrocytes, which are thought to act as “storage depots” for Cu. Rats and mice show high Cu deposits in the vicinity of the 4th ventricle and in the regions of habenula and hypothalamus adjacent to the third ventricle. In a healthy brain, the concentration of Cu in cells located in these areas is in a millimollar range ([43] and our data), which is remarkably high. The ability of the brain to safely maintain Cu at such high concentration points to the existence of cellular mechanisms that effectively neutralize and stabilize the otherwise highly reactive Cu. The endogenous metal-chelating proteins metallothioneins could be part of this storage/neutralizing mechanism [44]. Another Cu-rich areas is locus coeruleus (LC) [45–47] (Fig.1 in boxes), which is a hub of noradrenergic neurons and will be discussed in more detail below.
Fig. 1. X-ray fluorescence imaging of the coronal section of mouse brain that includes ventricle and locus coeruleus (boxed).
Top: phosphate map, bottom: Cu map. Lighter color indicates a higher concentration of an element. The two boxed areas show locus coeruleus
The functional significance of Cu-rich cells in the subventricular zone is unclear: these cells may serve as a protective layer to efficiently “filter and store” excess Cu. Copper deposits in this brain region increase with age [48]. This change may reflect a diminished metabolic demand for Cu in an aging brain or, instead, a weakened ability to deliver Cu to the brain as a sign of aging. It is also thought that Cu storage may be necessary to accommodate neurogenesis, especially in injury, because the Cu-rich cells are located in the vicinity of neuronal stem cells. Existence of similar Cu-rich granules in the noradrenergic neurons of LC (see below) suggests that there could be additional, cell specific, functions for Cu deposits. More experimental data for the molecular identity and the roles of these Cu-depots are needed. The increasing resolution of a synchrotron X-ray fluorescence imaging promises to uncover many new details of subcellular Cu distribution. For example, recent studies of hippocampal neurons using X-ray fluorescence revealed enriched Cu in dendritic spines, especially within the spine neck [49]. This location led to suggestions of interactions between Cu and the components of cytoskeleton and, indeed, Cu chelation was shown to decrease the abundance of F-actin in dendrites and reduces the number of F-actin protrusions [49].
Proteins involved in Cu homeostasis
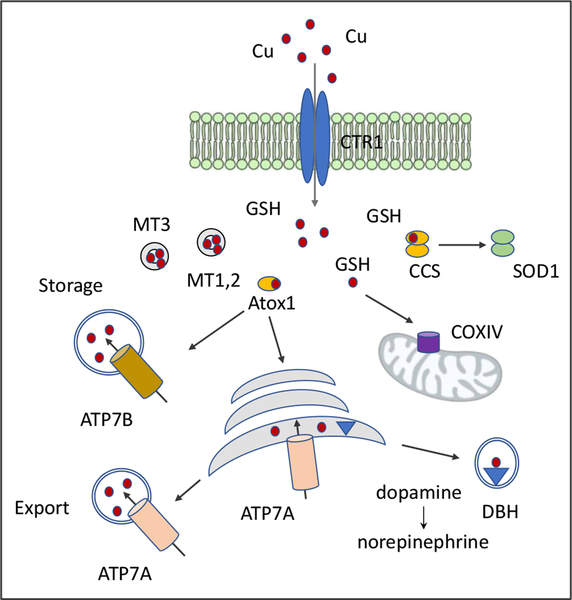
Cells of the CNS import Cu from the interstitial fluid via the high affinity Cu transporter CTR1 (SLC31A1). The uptake of Cu is facilitated by a tripeptide glutathione, GSH [50] (Fig.2). GSH also plays an important role in regulating Cu distribution between the intracellular compartments during neuronal differentiation [51]. Upon entry into the cell Cu binds to small cytosolic Cu-shuttle proteins or Cu-chaperones (Cox11, CCS, Atox1, and possibly others), and is transported to mitochondria (Cox11 and possibly others), cytosolic superoxide dismutase SOD1 (CCS), and the secretory pathway (Atox1). In the secretory pathway (the trans-Golgi network and endocytic vesicles) the Cu transporter ATP7A and a homologous ATP7B transfer Cu into the lumen of the pathway for its incorporation into the Cu-dependent enzymes (DBH, PAM, ceruloplasmin, tyrosinase, etc.) or to export excess Cu out of the cells (Fig.2).
Fig. 2. Cu distribution pathways in a generalized cell.
GSH, glutathione; MT1,2 and MT3; metallothioneins, CCS, copper chaperone for SOD1; Atox1, Cu chaperone for ATP7A and ATP7B.; COXIV cytochrome c reductase. ATP7A delivers Cu to Cu-dependent enzymes within the secretory pathway (DBH is shown), which are then trafficked to specialized vesicles or to the plasma membrane. Excess Cu is either stored in vesicles or exported via the plasma membrane.
Current data suggests that ATP7A is chiefly responsible for incorporation of Cu into PAM and DBH, and possibly other Cu-requiring enzymes in the CNS. The role of ATP7B in the CNS is less clear, and could be regulatory. Currently, the mechanistic understanding of Cu homeostasis in the brain is still in its infancy. In cultured hippocampal neurons, Cu elevation was shown to induce redistribution of ATP7A and ATP7B from their intracellular location in the trans-Golgi network (TGN) towards the plasma membrane to facilitate Cu export. ATP7A traffics along the axon and dendrites in response to high Cu or NMDA receptor signaling [52]. In the same neurons, trafficking of ATP7B in response to Cu elevation is restricted to the plasma membranes of a somato-dendritic domain [53]. Why some neurons express mostly ATP7A, while others have equally abundant ATP7A and ATP7B [54] remains a mystery.
Studies of motor neurons in vivo and a noradrenergic cell line (SH-SY5Y cells) in vitro found that changes in the oxidation state of glutathione that affect the cellular GSH:GSSG ratio also influence the oxidation state of Atox1. Atox1 binds Cu(I) in a two-coordination environment using cysteine residues of its Cys-XX-Cys motif. The redox potential of these residues are such that they become reversibly oxidized upon normal (within physiologic range) fluctuations in GSH:GSSG ratio [55]. Change in the oxidation state of Cu-coordinating cysteines, in turn, modulates the ability of Atox1 to bind Cu and transfer Cu to ATP7A and ATP7B [51]. Normally, such redox regulation of Atox1-mediated Cu transfer occurs upon neuronal differentiation; and it is used to determine the distribution of Cu between the secretory pathway and other cellular compartments [51].
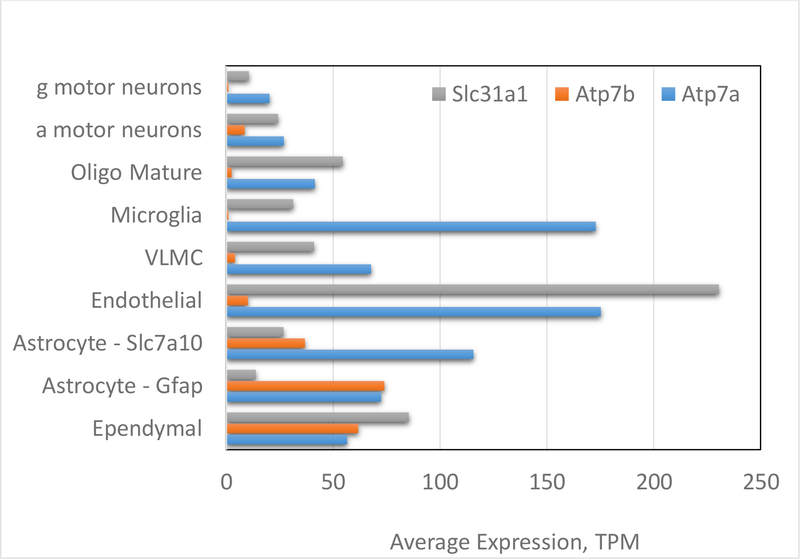
The precise composition of Cu handling machinery differs between various cell types in the brain. Recent single cells RNA sequencing revealed that all identified cell types (excitatory and inhibitory neurons, oligodendrocytes, astrocytes, ependymal cells, and microglia) express major Cu transporting proteins and chaperones. However, the abundance of ATP7A, ATP7B, CTR1 (SLC31a1) and Cu chaperones differs significantly between the cell types (Fig. 3). Endothelial cells have the highest expression levels of the transporters for Cu-uptake (CTR1) and Cu-efflux (ATP7A) as well as the Cu chaperone Atox1 (not shown), which is consistent with the proposed central role of these cells in Cu entry into the brain (see above). High levels of ATP7A, ATP7B, and CTR1 are also seen in ependymal cells that line ventricles and may be involved in regulation of Cu levels in the cerebral spinal fluid and parenchyma. Interestingly, different types of the inhibitory and excitatory neurons show significant difference in the composition of their Cu handling machinery (see examples in Fig.3) illustrating that regulation of Cu balance in the CNS is likely to be a complex and cell-specific process.
Fig. 3. Expression of major Cu transporters in different cell types in the CNS.
The graph has been generated using primary data from Rosenberg et al [54]. VLMC -vascular and leptomeningeal cells; g and a motor neurons are gamma and alpha motor neurons, respectively Further details and additional examples can be found in [54]
Cu in locus coeruleus.
Locus coeruleus (LC) represents a cluster of approximately 1500 neurons located in the pons (pontine tegmentum) region near the 4th ventricle (Fig.4a) [56]. This area of the brain is essential for catecholamine metabolism and is enriched in Cu (Fig.1). Catecholamines (dopamine and norepinephrine) are important neurotransmitters that regulate numerous activities of central and peripheral neurons.
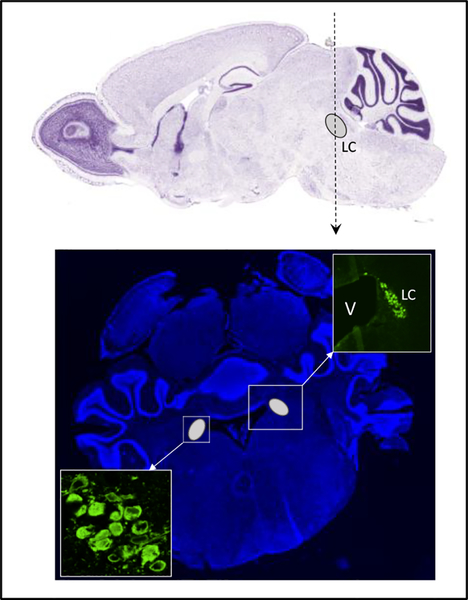
Figure 4. Localization of locus coeruleus in the mouse brain.
(Upper panel) Sagittal section, the location of LC is indicated by the grey oval and letters. The vertical dotted line indicates the position of the coronal section. (Lower) Coronal section. The LC is indicated by the grey ovals located on the left and right edge of the ventricle; the upper right insert shows a magnified view of the area that includes one LC and an edge of the ventricle (V); the lower insert shows a higher magnification image of neuronal cell bodies within the LC. The LC is identified by immunostaining for dopamine-β-hydroxylase (DBH, in green) as described in [60].
It is estimated that over 10 million neurons are catecholaminergic (dopaminergic and noradrenergic). Dopaminergic neurons are especially abundant in the substantia nigra and the ventral tegmental area of midbrain. These neurons control posture, initiation of movement, reward-associated behavior, attention, and memory. The noradrenergic neurons are concentrated in the LC. Although LC is small, it sends projections throughout the brain and represents the major source of norepinephrine in the CNS. The LC neurons are responsible for modulation of arousal, attention, and vigilance; they accomplish this function through regulation of target regions in a forebrain [57–59]. In the coronal sections of the brain (Fig.4b), LC has a characteristic “horn-like” appearance at the edges of the ventricle and can be easily identified by immunostaining for the Cu-dependent enzyme dopamine-β-hydroxylase, DBH, or tyrosine hydroxylase, which is an established marker of noradrenergic neurons (Fig.4b).
The morphology of LC is gender-dependent, at least in some species. In rats, the LC of adult females contains more noradrenergic neurons and is larger than LC in males [61–63]. This property has been attributed to a longer period of neurogenesis in females [63], and it may impact activities of target regions. It is not very clear how gender differences are established, but the effects of hormones throughout the development may play a role [61, 63]
Dopaminergic neurons and noradrenergic neurons express cell-type specific transporters, transcription factors, enzymes and signaling molecules [56]. The transcripts specifically enriched in the LC encode proteins that are involved in catecholamine metabolism (DBH, monoamine oxidase A, and cytochrome b561, which provides reducing equivalents for the DBH reaction) and retinoic acid-induced transcription factors AP-2β and AP-2α, which regulate expression of DBH. The high-affinity Cu transporter CTR1 (SLC31A1) is also abundantly expressed and specifically enriched in the LC. This high expression is likely to contribute to high Cu accumulation, which is apparent from the analysis of Cu distribution in mouse brain using X-ray fluorescence ([45] and Fig.1 (box)). High LC Cu was also reported for zebrafish, rats, and humans [46, 47, 64], pointing to an evolutionary conserved requirement for Cu in this neuronal cluster. In rat LC, Cu appeared to be higher in neuropil compared to neuronal cell bodies; the electron microscopy data also suggested enrichment of Cu at the synaptic membranes [64].
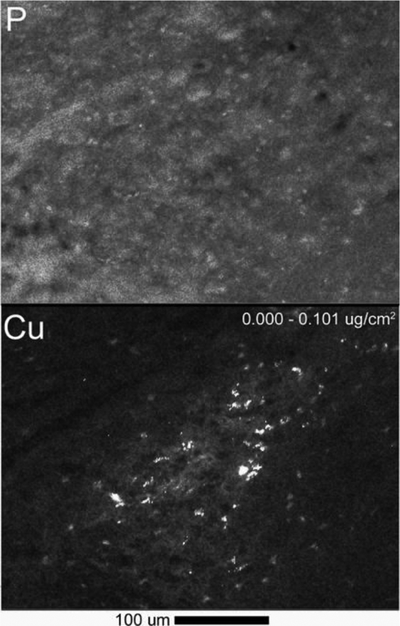
In either humans or mice, the Cu levels in noradrenergic neurons are uneven: some cells have modest Cu contents, whereas others show dense Cu clusters. Our recent XFM analysis of Cu distribution within the LC of adult mice revealed presence in this region of cells extremely rich in Cu (Fig.5).
Figure 5. Cu is elevated in LC (a) and is concentrated in “granules” in a subset of cells.
Phosphorus (P) shows location of cell nuclei. Lighter color indicates higher metal content.
The functional significance of these deposits as well as the identity of cells and intracellular structures in which Cu is located are currently unknown. The number of DBH-positive neurons present in a tissue section exceeds the number of Cu-rich cells (see the nuclei signal in the phosphorus map). This observation argues against the idea that the Cu-rich “depots” represent a Cu-bound DBH within secretory granules. Further studies are needed to determine whether the LC contains specialized Cu-storage cells or the Cu concentration in different subsets of the DBH expressing neurons is markedly different
It is worth noting that in Parkinson’s disease the levels of Cu and the Cu-transporter CTR1 in the LC are decreased along with the loss of neurons [65]. Similarly, the loss of neurons in the LC is observed in Alzheimer’s disease [66]. The cause-effect relationship between changes in Cu homeostasis and neuronal loss in the LC in either Parkinson’ disease or other neurodegenerative disorders has not yet been established and further studies are needed.
Copper and catecholamine balance.
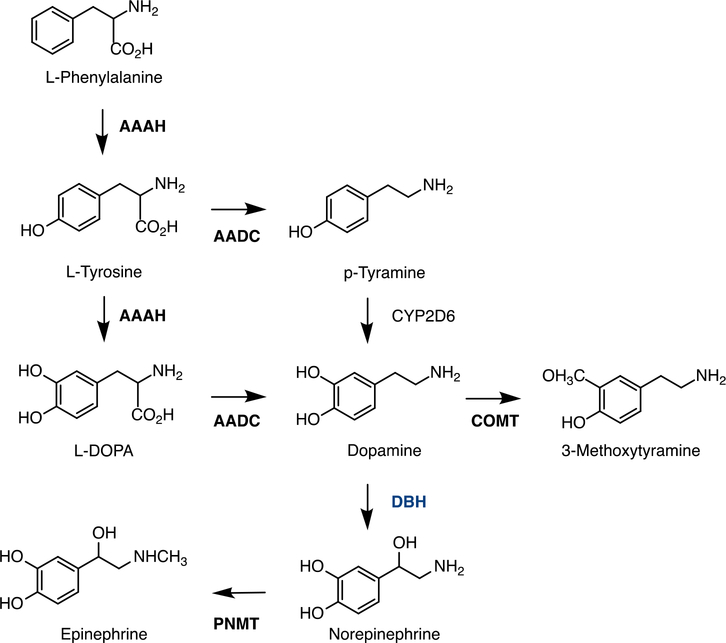
Catecholamines (dopamine, norepinephrine/noradrenaline, and epinephrine/adrenalin) are small molecules that have two adjacent hydroxyl groups on a benzene ring and one side-chain amine (Fig.6). The amino acids L-phenylalanine or L-tyrosine are precursors of catecholamines in a biosynthetic pathway that involves several enzymatic reactions (Fig.6). In this pathway, dopamine-β–hydroxylase (DBH) converts dopamine to norepinephrine; it is a rate-limiting and the only Cu-requiring enzyme.
Fig.6. Cartoon illustrating the biosynthesis of catecholamines and the step mediated by DBH.
AAAH- aromatic amino acid hydroxylase or tyrosine hydroxylase; AADC - aromatic L-amino acid decarboxylase or DOPA decarboxylase; COMT - catechol-O-methyltransferase; DBH - dopamine-β-hydroxylase; PNMT - phenylethanolamine N-methyltransferase
The catecholamine balance, especially levels of dopamine and norepinephrine, is altered in disorders of Cu metabolism. Infants with MNKD, which is caused by ATP7A inactivation, show marked Cu deficiency in the brain. The impaired Cu delivery to the CNS in these patients is associated with higher dopamine and lower norepinephrine levels in the brain and in a plasma, which is used in clinical diagnosis of the disease [67, 68]. The change in catecholamine levels is thought to be caused by the loss of Cu incorporation into DBH and hence its decreased enzymatic activity. Restoration of ATP7A expression in the mouse model of MNKD using an adenovirus-mediated targeted gene expression corrects the dopamine-to-norepinephrine ratio, especially if it is accompanied by extra Cu injections [24]
Similar to MNKD, the dietary Cu deficiency also alters catecholamine metabolism in the brain. Changes in the dopamine and norepinephrine levels were reported for the offspring of rats on a Cu deficient diet [69]. In one study, the norepinephrine levels were decreased in the cerebrum, mid-brain, corpus striatum, cerebellum, and medulla-pons but not in hypothalamus. Dopamine levels were elevated in the cerebellum, medulla, hypothalamus, and midbrain but unchanged in the cerebrum and striatum [69, 70]. In other study, Cu deficiency was associated with low dopamine in some but not all animals [70]. Norepinephrine concentrations were lower in the total brain, but higher in the hypothalamus. The reasons for this variability remain unclear.
The effect of high Cu on catecholamine levels also depends on the region of the brain. In rats, Cu treatment was found to increase catecholamines levels in the cerebellum, cortex, and midbrain [71]. Experiments on Cu supplementation in the diet of developing pigs also found increases in dopamine and norepinephrine levels in the midbrain and hypothalamic brain region along with an increased DBH activity in the midbrain[72]. However, the activity of DBH in hypothalamus was unaffected by Cu supplementation. The mechanisms behind these region-specific effects of Cu on DBH activity and catecholamine levels have not been explored, and further studies are needed to determine both the local and long-range effects of Cu imbalance in the CNS.
Inactivation of ATP7B is associated with Cu accumulation in the brain, which also impacts the catecholamine balance throughout the organism. In mice with a genetically inactivated ATP7B (Atp7b−/− mice), adrenal glands have reduced levels of norepinephrine and epinephrine even though ATP7B is not expressed in this tissue [73]. In another animal model of WD, toxic milk mice, ATP7B expression is not disrupted but protein is mutated. These animals with age demonstrate increase of Cu levels in the brain, motor deficits, and slight decrease in dopamine concentration in the striatum[74]. These changes do not fully reflect those reported for humans. In patients with WD, inactivation of ATP7B causes gradual Cu elevation in most regions of the brain, although specific effect of ATP7B inactivation on Cu levels in the LC remains to be determined. The significantly decreased levels of dopamine and increased norepinephrine were reported for the basal ganglia, whereas opposite was observed in the hypothalamus[75]. Long-Evans Cinnamon rats (another animal model of WD) show increased levels of dopamine and decreased norepinephrine levels in their cortex [76]. More systematic studies may help to understand these variations in catecholamine balance caused by loss of ATP7B.
Regulation of Cu balance in noradrenergic neurons
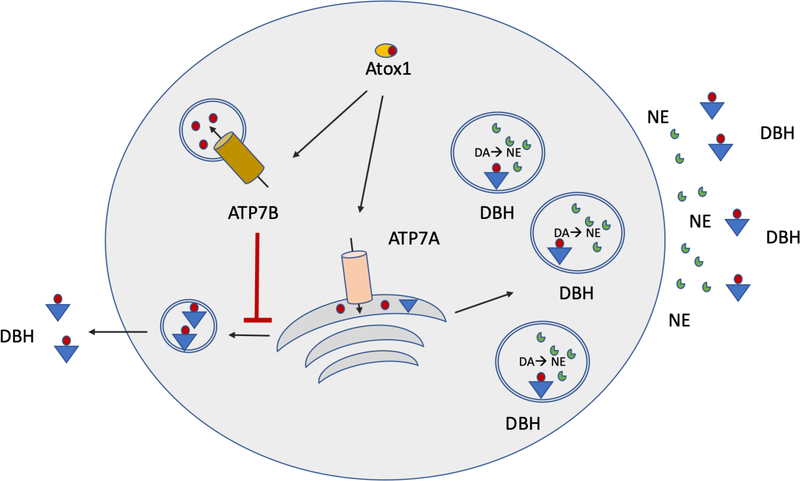
Noradrenergic neurons employ the same key components of the Cu balancing machinery as do other cells (Fig. 2). Recent studies directly demonstrated the presence in these cells of the Cu uptake transporter CTR1 along with the two Cu-transporting ATPases, ATP7A and ATP7B [45, 46]. ATP7A is thought to transfer Cu to DBH in the TGN, whereas ATP7B is located in vesicles and maintains Cu balance in the cytosol. It was proposed that the ATP7B activity modulates Cu availability to ATP7A and to downstream DBH [45]. The DBH-dependent conversion of dopamine into norepinephrine takes place in secretory granules, to which DBH traffics from the TGN (Fig.7). In response to electric or other stimuli, secretory granules fuse with the plasma membrane causing release of norepinephrine. Experiments with DBH-rich chromaffin cells of the adrenal medulla demonstrated that the membrane-bound components of secretory granules, including the membrane-bound DBH (see below), are subsequently and incrementally retrieved from the plasma membrane upon clathrin-mediated endocytosis.
Fig. 7. Signal dependent and constitutive secretion of DBH.
Cu levels in the cytosol of DBH expressing cells are regulated through a coordinated action of two Cu-transporters ATP7A and ATP7B. Both are thought to get their Cu from Cu chaperone Atox1. DBH receives Cu and is activated in the TGN, where it also undergoes partial proteolytic processing and sorting. The major fraction of DBH is sorted towards the secretory granules. The minor fraction is targeted for constitutive export
Synthesis and release of norepinephrine are regulated at several levels. In the rat primary sympathetic neurons, angiotensin II (Ang II) enhances norepinephrine synthesis by stimulating trafficking of DBH mRNAs to distal axons and also facilitates norepinephrine release while inhibiting its reuptake [77]. Noradrenergic neurons belong to the network of wake promoting neurons and norepinephrine release is required for sleep regulation. Recent studies in zebrafish provided direct evidence for the Cu-dependent involvement of released norepinephrine in the sleep / wake cycle [46]. In humans, an abnormal function of noradrenergic neurons may contribute to sleep abnormalities commonly observed in Wilson disease patients [78].
The molecular properties of DBH protein and its cellular behavior in noradrenergic neurons are also regulated. DBH is a tetrameric protein, which exists in at least three forms. These forms are generated through proteolysis and posttranslational modification, most likely during DBH functional maturation in the TGN. The membrane-bound form of DBH is a dimer of two noncovalently associated heterodimers. Each heterodimer consists of a membrane-bound and a soluble DBH subunit, generated from the membrane bound subunit by proteolysis and covalently linked to it via a disulfide bond. In addition to the membrane-bound tetramer, secretory granules contain a fully proteolized soluble form of DBH, which retains its tetrameric structure in solution. Upon neuronal signaling, the soluble DBH is released from cells along with norepinephrine.
In addition to a signal-dependent secretion of DBH, noradrenergic neurons in culture (and potentially in vivo) export enzymatically-active soluble tetrameric DBH via constitutive secretion (Fig. 7). The constitutive secretion of DBH is dependent on Cu availability [45]. Cu deficiency inhibits export of DBH from the neurons via this consecutive pathway. Down-regulation of ATP7B, which increases Cu availability in the cytosol, upregulates DBH export from cells even in the absence of neuronal signaling [45]. Thus, the catecholamine ratio (inside and outside on neuronal cells) under conditions of Cu misbalance would depend not only on the activity of DBH within the secretory granules but could also be influenced by the amount of active DBH exported into the extracellular milieu.
In conclusion, current experimental evidence from the in vitro and in vivo studies along with the clinical data highlights the essential role of Cu homeostasis for normal functioning of the central nervous system. The molecular mechanism maintaining Cu balance in various cell types in the brain remain to be fully explored. Accumulating data have identified locus coeruleus (a major site of norepinephrine production) as a Cu-enriched region, where Cu is utilized for (i) enzymatic activity of DBH, (ii) export of DBH into an extracellular milieu and (iii) possibly, for Cu storage. How these processes are coupled normally and how they are altered in human disease are fascinating topics of further studies.
Acknowledgements
This work was supported by the National Institute of Health grant R01 GM101502 to SL and R01 GM101502-S1 to CWH. The authors would like to thank O. Antipova for support and assistance with X-ray fluorescence microscopy data collection at the Advanced Photon Source part of the Argonne National laboratory supported by the Department of Energy, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement
The authors declare no conflicts
References
- 1.Solomon EI, et al. , Copper active sites in biology. Chem Rev, 2014. 114(7): p. 3659–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas MF, Rousseau DL, and Guallar V, Electron transfer pathways in cytochrome c oxidase. Biochim Biophys Acta, 2011. 1807(10): p. 1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timon-Gomez A, et al. , Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin Cell Dev Biol, 2018. 76: p. 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jett KA and Leary SC, Building the CuA site of cytochrome c oxidase: A complicated, redox-dependent process driven by a surprisingly large complement of accessory proteins. J Biol Chem, 2018. 293(13): p. 4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks CJ and Andersen JL, Mechanisms of SOD1 regulation by post-translational modifications. Redox Biol, 2019. 26: p. 101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirangelo I and Iannuzzi C, The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase. Molecules, 2017. 22(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, et al. , SOD1 deficiency: a novel syndrome distinct from amyotrophic lateral sclerosis. Brain, 2019. 142(8): p. 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huai J and Zhang Z, Structural Properties and Interaction Partners of Familial ALS-Associated SOD1 Mutants. Front Neurol, 2019. 10: p. 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousquet-Moore D, Mains RE, and Eipper BA, Peptidylgycine alpha-amidating monooxygenase and copper: a gene-nutrient interaction critical to nervous system function. J Neurosci Res, 2010. 88(12): p. 2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maheshwari S, et al. , Effects of copper occupancy on the conformational landscape of peptidylglycine alpha-hydroxylating monooxygenase. Commun Biol, 2018. 1: p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vendelboe TV, et al. , The crystal structure of human dopamine beta-hydroxylase at 2.9 A resolution. Sci Adv, 2016. 2(4): p. e1500980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. , An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci, 2014. 34(36): p. 11929–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takikita S, et al. , Increased apoptosis and hypomyelination in cerebral white matter of macular mutant mouse brain. Mol Genet Metab Rep, 2015. 4: p. 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGee TP, Houston CM, and Brickley SG, Copper block of extrasynaptic GABAA receptors in the mature cerebellum and striatum. J Neurosci, 2013. 33(33): p. 13431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasperini L, et al. , Prion protein and copper cooperatively protect neurons by modulating NMDA receptor through S-nitrosylation. Antioxid Redox Signal, 2015. 22(9): p. 772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetti C, Baranowska-Bosiacka I, and Gavazzo P, Multiple effects of copper on NMDA receptor currents. Brain Res, 2014. 1542: p. 20–31. [DOI] [PubMed] [Google Scholar]
- 17.Salazar-Weber NL and Smith JP, Copper Inhibits NMDA Receptor-Independent LTP and Modulates the Paired-Pulse Ratio after LTP in Mouse Hippocampal Slices. Int J Alzheimers Dis, 2011. 2011: p. 864753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nargund S, Qiu J, and Goudar CT, Elucidating the role of copper in CHO cell energy metabolism using (13)C metabolic flux analysis. Biotechnol Prog, 2015. 31(5): p. 1179–86. [DOI] [PubMed] [Google Scholar]
- 19.Scheiber IF and Dringen R, Copper accelerates glycolytic flux in cultured astrocytes. Neurochem Res, 2011. 36(5): p. 894–903. [DOI] [PubMed] [Google Scholar]
- 20.Morrell A, et al. , The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life, 2017. 69(4): p. 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn JF, et al. , Gender effects on plasma and brain copper. Int J Alzheimers Dis, 2011. 2011: p. 150916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafri SK, et al. , Menkes disease: A rare disorder. J Pak Med Assoc, 2017. 67(10): p. 1609–1611. [PubMed] [Google Scholar]
- 23.Czlonkowska A, et al. , Wilson disease. Nat Rev Dis Primers, 2018. 4(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaler SG and Holmes CS, Catecholamine metabolites affected by the copper-dependent enzyme dopamine-beta-hydroxylase provide sensitive biomarkers for early diagnosis of menkes disease and viral-mediated ATP7A gene therapy. Adv Pharmacol, 2013. 68: p. 223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barkhatova VP, et al. , [Pathological status of catecholamines in the corpus striatum in hepatocerebral dystrophy (Wilson-Konovalov disease)]. Zh Nevropatol Psikhiatr Im S S Korsakova, 1992. 92(4): p. 8–10. [PubMed] [Google Scholar]
- 26.Snow BJ, et al. , The nigrostriatal dopaminergic pathway in Wilson’s disease studied with positron emission tomography. J Neurol Neurosurg Psychiatry, 1991. 54(1): p. 12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westermark K, et al. , Neurological Wilson’s disease studied with magnetic resonance imaging and with positron emission tomography using dopaminergic markers. Mov Disord, 1995. 10(5): p. 596–603. [DOI] [PubMed] [Google Scholar]
- 28.Jeon B, et al. , Dopamine transporter imaging with [123I]-beta-CIT demonstrates presynaptic nigrostriatal dopaminergic damage in Wilson’s disease. J Neurol Neurosurg Psychiatry, 1998. 65(1): p. 60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandmann O, Weiss KH, and Kaler SG, Wilson’s disease and other neurological copper disorders. Lancet Neurol, 2015. 14(1): p. 103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahter M, et al. , Concentrations of copper, zinc and selenium in brain and kidney of second trimester fetuses and infants. J Trace Elem Med Biol, 1997. 11(4): p. 215–22. [DOI] [PubMed] [Google Scholar]
- 31.Wang LM, et al. , Bioimaging of copper alterations in the aging mouse brain by autoradiography, laser ablation inductively coupled plasma mass spectrometry and immunohistochemistry. Metallomics, 2010. 2(5): p. 348–53. [DOI] [PubMed] [Google Scholar]
- 32.Palm R, Wahlstrom G, and Hallmans G, Age related changes in weight and the concentrations of zinc and copper in the brain of the adult rat. Lab Anim, 1990. 24(3): p. 240–5. [DOI] [PubMed] [Google Scholar]
- 33.Litwin T, Gromadzka G, and Czlonkowska A, Gender differences in Wilson’s disease. J Neurol Sci, 2012. 312(1–2): p. 31–5. [DOI] [PubMed] [Google Scholar]
- 34.Busch C, Bohl J, and Ohm TG, Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging, 1997. 18(4): p. 401–6. [DOI] [PubMed] [Google Scholar]
- 35.Ohm TG, Busch C, and Bohl J, Unbiased estimation of neuronal numbers in the human nucleus coeruleus during aging. Neurobiol Aging, 1997. 18(4): p. 393–9. [DOI] [PubMed] [Google Scholar]
- 36.Li X, et al. , Sex Differences in Clinical Characteristics and Brain MRI Change in Patients With Wilson’s Disease in a Chinese Population. Front Physiol, 2018. 9: p. 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng W and Monnot AD, Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol Ther, 2012. 133(2): p. 177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi BS and Zheng W, Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res, 2009. 1248: p. 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu X, et al. , Regulation of copper transport crossing brain barrier systems by Cu-ATPases: effect of manganese exposure. Toxicol Sci, 2014. 139(2): p. 432–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donsante A, et al. , Somatic mosaicism in Menkes disease suggests choroid plexus-mediated copper transport to the developing brain. Am J Med Genet A, 2010. 152A(10): p. 2529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaler SG, ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol, 2011. 7(1): p. 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddad MR, et al. , Cerebrospinal Fluid-Directed rAAV9-rsATP7A Plus Subcutaneous Copper Histidinate Advance Survival and Outcomes in a Menkes Disease Mouse Model. Mol Ther Methods Clin Dev, 2018. 10: p. 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan B, et al. , Copper accumulation in rodent brain astrocytes: A species difference. J Trace Elem Med Biol, 2017. 39: p. 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan B, et al. , On the nature of the Cu-rich aggregates in brain astrocytes. Redox Biol, 2017. 11: p. 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt K, et al. , ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-beta-hydroxylase. J Biol Chem, 2018. 293(52): p. 20085–20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao T, et al. , Copper regulates rest-activity cycles through the locus coeruleusnorepinephrine system. Nat Chem Biol, 2018. 14(7): p. 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zecca L, et al. , The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci U S A, 2004. 101(26): p. 9843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pushkar Y, et al. , Aging results in copper accumulations in glial fibrillary acidic protein-positive cells in the subventricular zone. Aging Cell, 2013. 12(5): p. 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrin L, et al. , Zinc and Copper Effects on Stability of Tubulin and Actin Networks in Dendrites and Spines of Hippocampal Neurons. ACS Chem Neurosci, 2017. 8(7): p. 1490–1499. [DOI] [PubMed] [Google Scholar]
- 50.Maryon EB, Molloy SA, and Kaplan JH, Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am J Physiol Cell Physiol, 2013. 304(8): p. C768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatori Y, et al. , Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat Commun, 2016. 7: p. 10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlief ML, Craig AM, and Gitlin JD, NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci, 2005. 25(1): p. 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain S, Farias GG, and Bonifacino JS, Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1. Mol Biol Cell, 2015. 26(2): p. 218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenberg AB, et al. , Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science, 2018. 360(6385): p. 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatori Y, et al. , Functional partnership of the copper export machinery and glutathione balance in human cells. J Biol Chem, 2012. 287(32): p. 26678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grimm J, et al. , Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci U S A, 2004. 101(38): p. 13891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chamberlain SR and Robbins TW, Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol, 2013. 27(8): p. 694–718. [DOI] [PubMed] [Google Scholar]
- 58.Sara SJ, The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci, 2009. 10(3): p. 211–23. [DOI] [PubMed] [Google Scholar]
- 59.Szabadi E, Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol, 2013. 27(8): p. 659–93. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt K, et al. , Localization of the Locus Coeruleus in the Mouse Brain. J Vis Exp, 2019(145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guillamon A, de Blas MR, and Segovia S, Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res, 1988. 468(2): p. 306–10. [DOI] [PubMed] [Google Scholar]
- 62.Luque JM, et al. , Sexual dimorphism of the dopamine-beta-hydroxylaseimmunoreactive neurons in the rat locus ceruleus. Brain Res Dev Brain Res, 1992. 67(2): p. 211–5. [DOI] [PubMed] [Google Scholar]
- 63.Pinos H, et al. , The development of sex differences in the locus coeruleus of the rat. Brain Res Bull, 2001. 56(1): p. 73–8. [DOI] [PubMed] [Google Scholar]
- 64.Sato M, et al. , Localization of copper to afferent terminals in rat locus ceruleus, in contrast to mitochondrial copper in cerebellum. J Histochem Cytochem, 1994. 42(12): p. 1585–91. [DOI] [PubMed] [Google Scholar]
- 65.Davies KM, et al. , Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging, 2014. 35(4): p. 858–66. [DOI] [PubMed] [Google Scholar]
- 66.Pamphlett R and Kum Jew S, Different Populations of Human Locus Ceruleus Neurons Contain Heavy Metals or Hyperphosphorylated Tau: Implications for Amyloid-beta and Tau Pathology in Alzheimer’s Disease. J Alzheimers Dis, 2015. 45(2): p. 437–47. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein DS, Holmes CS, and Kaler SG, Relative efficiencies of plasma catechol levels and ratios for neonatal diagnosis of menkes disease. Neurochem Res, 2009. 34(8): p. 1464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoeldtke RD, et al. , Catecholamine metabolism in kinky hair disease. Pediatr Neurol, 1988. 4(1): p. 23–6. [DOI] [PubMed] [Google Scholar]
- 69.Prohaska JR and Bailey WR, Regional specificity in alterations of rat brain copper and catecholamines following perinatal copper deficiency. J Neurochem, 1994. 63(4): p. 1551–7. [DOI] [PubMed] [Google Scholar]
- 70.Miller DS and O’Dell BL, Milk and casein-based diets for the study of brain catecholamines in copper-deficient rats. J Nutr, 1987. 117(11): p. 1890–7. [DOI] [PubMed] [Google Scholar]
- 71.Moshtaghie AA, et al. , Protective effects of copper against aluminum toxicity on acetylcholinesterase and catecholamine contents of different regions of rat’s brain. Neurol Sci, 2013. 34(9): p. 1639–50. [DOI] [PubMed] [Google Scholar]
- 72.Yang W, et al. , High Dietary Copper Increases Catecholamine Concentrations in the Hypothalami and Midbrains of Growing Pigs. Biol Trace Elem Res, 2016. 170(1): p. 115–8. [DOI] [PubMed] [Google Scholar]
- 73.Gerbasi V, Lutsenko S, and Lewis EJ, A mutation in the ATP7B copper transporter causes reduced dopamine beta-hydroxylase and norepinephrine in mouse adrenal. Neurochem Res, 2003. 28(6): p. 867–73. [DOI] [PubMed] [Google Scholar]
- 74.Przybylkowski A, et al. , Neurochemical and behavioral characteristics of toxic milk mice: an animal model of Wilson’s disease. Neurochem Res, 2013. 38(10): p. 2037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nyberg P, et al. , Advanced catecholaminergic disturbances in the brain in a case of Wilson’s disease. Acta Neurol Scand, 1982. 65(1): p. 71–5. [DOI] [PubMed] [Google Scholar]
- 76.Saito T, et al. , Neurochemical and histochemical evidence for an abnormal catecholamine metabolism in the cerebral cortex of the Long-Evans Cinnamon rat before excessive copper accumulation in the brain. Neurosci Lett, 1996. 216(3): p. 195–8. [DOI] [PubMed] [Google Scholar]
- 77.Aschrafi A, et al. , Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine beta-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons. J Neurochem, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nevsimalova S, et al. , Sleep disorders in Wilson’s disease. Eur J Neurol, 2011. 18(1): p. 184–90. [DOI] [PubMed] [Google Scholar]