Abstract
The concept that the positive feedback effect of ovarian estradiol (E2) results in GnRH and gonadotropin surges is a well-established principle. However, a series of studies investigating the rapid action of E2 in female rhesus monkeys has led to a new concept that neuroestradiol, synthesized and released in the hypothalamus, also contributes to regulation of the preovulatory GnRH surge. This unexpected finding started from our surprising observation that E2 induces rapid stimulatory action in GnRH neurons in vitro. Subsequently, we confirmed that a similar rapid stimulatory action of E2 occurs in vivo. Unlike subcutaneous injection of E2 benzoate (EB), a brief (10–20 min), direct infusion of EB into the median eminence in ovariectomized (OVX) female monkeys rapidly stimulates release of GnRH and E2 in a pulsatile manner, and the EB-induced GnRH and E2 release is blocked by simultaneous infusion of the aromatase inhibitor, letrozole. This suggests that stimulated release of E2 is of hypothalamic origin. To further determine the role of neuroestradiol we examined the effects of letrozole on EB-induced GnRH and LH surges in OVX females. Results indicate that letrozole treatment greatly attenuated the EB-induced GnRH and LH surges. Collectively, neuroestradiol released from the hypothalamus appears to be necessary for the positive feedback effect of E2 on the GnRH/LH surge.
Keywords: neuroestradiol, GnRH surge, kisspeptin surge, hypothalamus, monkeys
Introduction:
In addition to the gonads, the brain is one of the major organs that synthesizes estradiol (E2). For more than 4 decades, a role of E2 in the regulation of neuronal function has been predicted. As early as 1971, Naftolin and colleagues reported that aromatase, the enzyme that converts androgens to E2, is present in the hypothalamus: Incubation of diencephalon obtained from male human fetuses with radiolabeled androstenedione yielded E2 and estrone (Naftolin et al., 1971). Subsequently, several pioneers in the field reported the presence of aromatase in the brain (Selmanoff et al., 1977; Steiner and Huchison, 1980; Ellinwood et al., 1984; MacLusky et al., 1986; Roselli et al., 1987; Roselli et al., 2001) and have speculated about possible roles of neuroestrogens in brain functions as a neurotransmitter or neuromodulator (see Balthazart and Ball, 2006; Saldanha et al., 2011). This speculation is based on the facts that 1) E2 induces rapid changes in neuronal activity and behaviors (Yagi et al., 1973; Kelly et al., 1976; Hojo et al., 2008; Cross & Roselli, 1999; Cornil et al, 2005 & 2006; Kow & Pfaff, 2004; Remage-Healey et al., 2011), and 2) aromatase is present in axon terminals and the presynaptic boutons of the brain in various animals including monkeys and humans (Naftolin et al., 1996; Hojo et al., 2004; Peterson et al., 2005). In 2008, Remage-Healey and colleagues (2008) reported the most convincing evidence for neuroestradiol release in the songbird auditory cortex by direct in vivo measurements showing that local E2 concentration was increased when the male bird is exposed to another male’s songs.
Ovarian E2 is indispensable for female reproductive functions. E2 is also known to facilitate learning/memory and protect from neuronal cell death throughout life (Morrison et al., 2006; Spencer et al., 2008; Brinton, 2009). Although a possible role of neuroestradiol in sex behavior, learning/memory, and neuroprotective function has been conceptually accepted, it has been completely ignored in reproductive neuroendocrinology. Perhaps, this is due to the well-established concept that reproductive cycles are regulated by the hypothalamo-pituitary-gonadal feedback circuit. However, while we were studying the rapid action of E2 on GnRH neurons, we almost accidentally found that neuroestradiol is significantly important for pulsatile release of GnRH and the preovulatory GnRH surge. In this review, I summarize a recent series of the findings in my laboratory supporting the concept that neuroestradiol participates in the control of GnRH secretion.
Rapid action of estradiol on GnRH neurons:
Using GnRH primary cultures derived from the monkey embryonic olfactory placode, we found that E2 induces rapid excitatory action on GnRH neurons. First, we observed that E2 at a 1 nM concentration stimulates firing activity of GnRH neurons within 60–120 sec, lasting over 25 minutes (Abe and Terasawa, 2005). Second, exposure of GnRH neurons to E2 at 1 nM stimulates the frequency of intracellular calcium ([Ca2+]i) oscillations, the number of cells exhibiting [Ca2+]i oscillations, and the synchronization frequency of [Ca2+]i oscillations within 10 minutes (Abe et al., 2008, Noel et al., 2008). Third, exposure of GnRH neurons to E2 (1 nM) also stimulates GnRH release within 10 minutes (Noel et al., 2008). This rapid E2 action on GnRH neurons appears to be direct, as our primary cultures of GnRH neurons usually do not contain other types of neurons or glia, although numerous fibroblasts, epithelial cells and unidentified non-neuronal cells are present (Terasawa et al., 1993; Richter et al., 2002). Together, we concluded that E2 induces a direct rapid excitatory action on primate GnRH neurons.
Rapid action of E2 on GnRH neurons has been known for some time. E2 hyperpolarizes guinea pig GnRH neurons, alters cAMP production in GT1-cells and stimulates or inhibits [Ca2+]i oscillations within 15 minutes (Lagrange et al., 1995; Navarro et al., 2003; Temple et al., 2004; Romano et al., 2008). Importantly, all these studies including ours were conducted in in vitro systems, such as cultured GnRH neurons or sliced brains. Thus, the question arises as to whether “rapid E2 action” seen in in vitro studies also occurs in vivo?
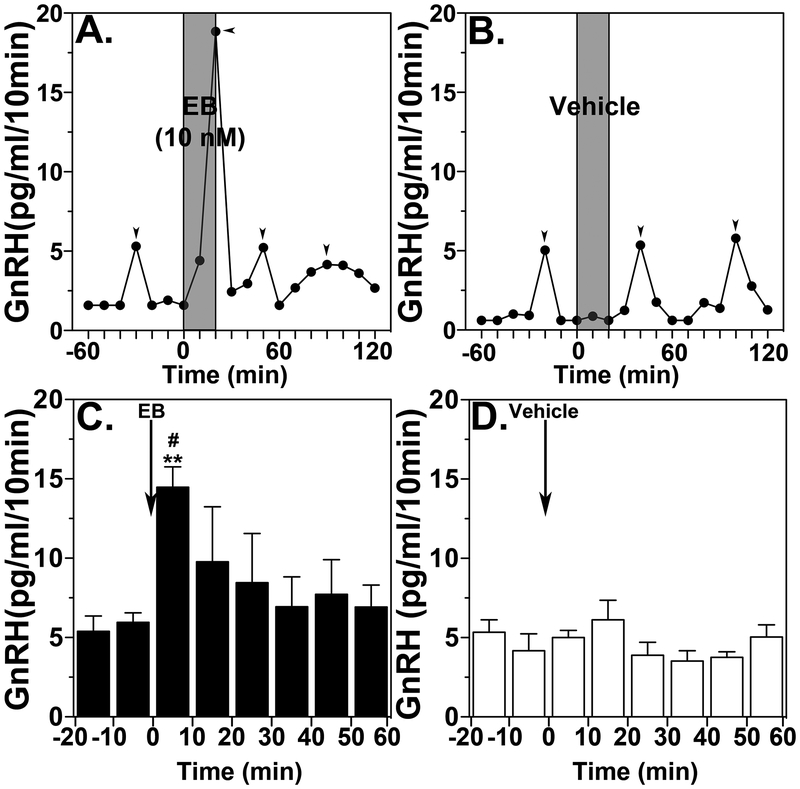
The negative and positive feedback effects of E2 on GnRH/LH release are well established concepts. For example, systemic administration of E2 benzoate (EB) in OVX monkeys results in suppression of GnRH/LH release with a latency of ~2 hours (Mizuno and Terasawa, 2005), whereas stimulatory action occurs with a latency of ~24 hours (Yamaji et al., 1971; Karsch et al., 1973, Terasawa et al., 1982). However, there was so far no report showing that rapid action of E2 on GnRH release occurs with a latency shorter than 20 minutes in vivo in any mammalian species. Therefore, we examined the effects of a brief EB infusion into the median eminence of the hypothalamus on GnRH release using a microdialysis approach. In the monkey dense GnRH neuroterminals and some GnRH cell bodies are distributed in the median eminence (Goldsmith et al., 1993). Continuous infusion of EB stimulated, did not suppress, GnRH release in ovariectomized (OVX) monkeys in vivo within 10–20 minutes, similar to what had been seen in vitro (Fig. 1, Kenealy et al, 2013). Furthermore, a similar brief infusion of EB also stimulated kisspeptin release (see Kenealy et al, 2015). Kisspeptin neuroterminals and cell bodies are also seen in the median eminence (Ramaswamy et al., 2009).
Figure 1:
Brief direct infusion of EB into the median eminence induces GnRH release. Representative case and group data (means ± SEM) from EB infusion (A and C) or vehicle infusion (B and D) are shown. Time zero designates the beginning of a 20-min EB or vehicle infusion (indicated by grey bars). Two-way ANOVA, indicates that EB significantly stimulated GnRH release (C) over vehicle control (D, p=0.0018). Post-hoc analysis indicates that GnRH levels during the first 10-min of EB infusion were higher than those during the control period (C, **: p<0.01) as well as the corresponding time period of vehicle infusion (D, #: p<0.05). Arrowheads indicate pulses identified by PULSAR. From Kenealy et al., 2013. Permission pending.
These findings led to another question: What is the underlying mechanism differentiating the E2 action resulting from the 2 modes of EB administration (systemic injection vs. local infusion to the median eminence)? There are at least 3 possible explanations. First, the difference may be due to the exposure period of the median eminence to E2. While the exposure period in the study of Kenealy et al (2013) was 10–20 minutes, systemic EB injection would sustain elevated circulating levels for at least 18 hours. Second, the difference may be due to the E2 concentration at the median eminence. While the EB exposure dose in the study by Kenealy et al (2013) was 1 or 10 nM, in systemic injection we used 50μg/kg and the local concentration produced in the median eminence is unknown. Third, the difference may be attributable to the site of action within the hypothalamus.
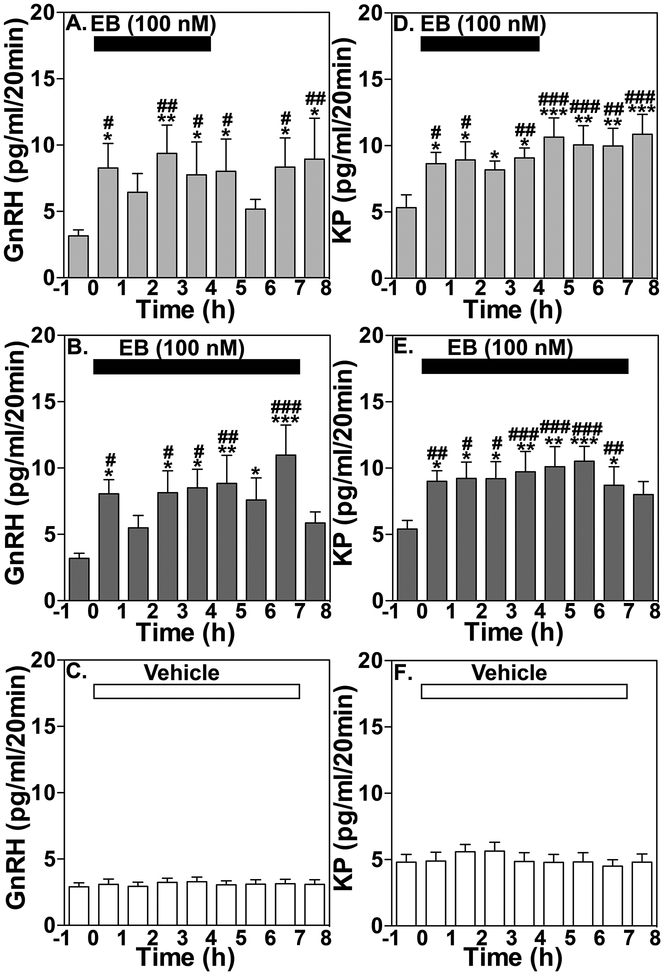
The results from a subsequent study indicate that the exposure period does not account for the difference in the 2 modes of EB administration: EB infusion into the median eminence for a prolonged period (4 or 7 hours) keeps stimulating release of GnRH and kisspeptin over several hours (Kenealy et al., 2015), similar to the release observed with a brief (10 or 20 minutes) infusion (Kenealy et al, 2013, Fig. 2). Likewise, the EB concentration at the median eminence does not likely differentiate the 2 modes of EB administration. Continuous or brief infusion of EB at doses ranging from 1 nM to 1 μM, which translates into one tenth of the concentration (0.1 to 100 nM) in the tissue at the median eminence due to the permeability of microdialysis membrane (Frost et al., 2008), stimulated release of GnRH and kisspeptin, whereas subcutaneous injection of EB at 50 μg/kg, which induces concentrations of 300 to 500 pg/ml or 1–1.5 nM in the circulation (Mizuno and Terasawa, 2005) suppressed both GnRH and kisspeptin release (Kenealy et al., 2015). In contrast, the site of EB exposure is critical for stimulating the release of GnRH and kisspeptin by EB. In the EB infusion experiment, the region exposed to EB is restricted within the median eminence, as the tips of microdialysis probes (determined by x-ray ventriculography) are all located within the median eminence (Kenealy et al., 2015), whereas systemically administered EB reaches a large portion of the brain as well as the pituitary gland, as shown by autoradiography (Pfaff et al., 1976). It is speculated that systemically administered EB binds to estrogen receptor alpha (ERα, ESR1) located in infundibular (arcuate) kisspeptin and neurokinin-B neurons (Rance, 2009) resulting in inhibitory action, whereas direct infusion of EB into the GnRH and kisspeptin neuroterminals induces rapid actions mediated through a membrane-initiated mechanism.
Figure 2:
Effects of prolonged EB infusion for 4 or 7 hours on release of GnRH and kisspeptin. Group data (Means ± SEM) showing the effect of 4 h EB infusion on GnRH (A) and kisspeptin (KP, D) release, or 7 h EB infusion on GnRH (B) and KP (E) release, and vehicle infusion on GnRH (C) and KP (F) release. Mean GnRH release in either the 4 h (p<0.02, n=7) or 7 h (p<0.0001, n=8) treatment groups was significantly higher than the 2 h respective control period before treatment as well as vehicle control (p<0.0001 for both, n=8). Similarly, the EB-induced increase in kisspeptin levels in both the 4 h and 7 h EB infusion treatment groups were significantly higher than the 2 h control period before (p<0.0001 for both) and vehicle control (p<0.0001 for both). Bonferroni’s post hoc analysis indicates that in 4 h and 7 h EB infusion experiments mean GnRH and KP release are significantly higher than the 2 h period before EB infusion as indicated (*: p < 0.05; **: p<0.01; ***: p<0.001 vs. before) and also significantly elevated compared to vehicle control at the corresponding time period as indicated (#: p< 0.05; ##: p<0.01; ###: p<0.001 vs. vehicle). From Kenealy et al., 2015. Permission pending.
Previously, we have shown that rapid action of E2 in vitro is mediated through two E2 membrane receptors, GPR30 (GPER1) and the STX-sensitive membrane receptors (Noel et al., 2009; Kenealy et al., 2011a, Kenealy et al., 2011b). This is significantly different from receptors implicated in the positive and negative feedback effects of E2. The feedback regulation of E2 requires estrogen receptor ERα (ESR1) or ERβ (ESR2), through which E2 activates nuclear transcription with a longer latency (see Herbison 2015). As discussed in the following sections, regulation of GnRH neurosecretory activity by neuroestradiol occurs as a rapid E2 action.
Role of neuroestradiol in pulsatile GnRH release:
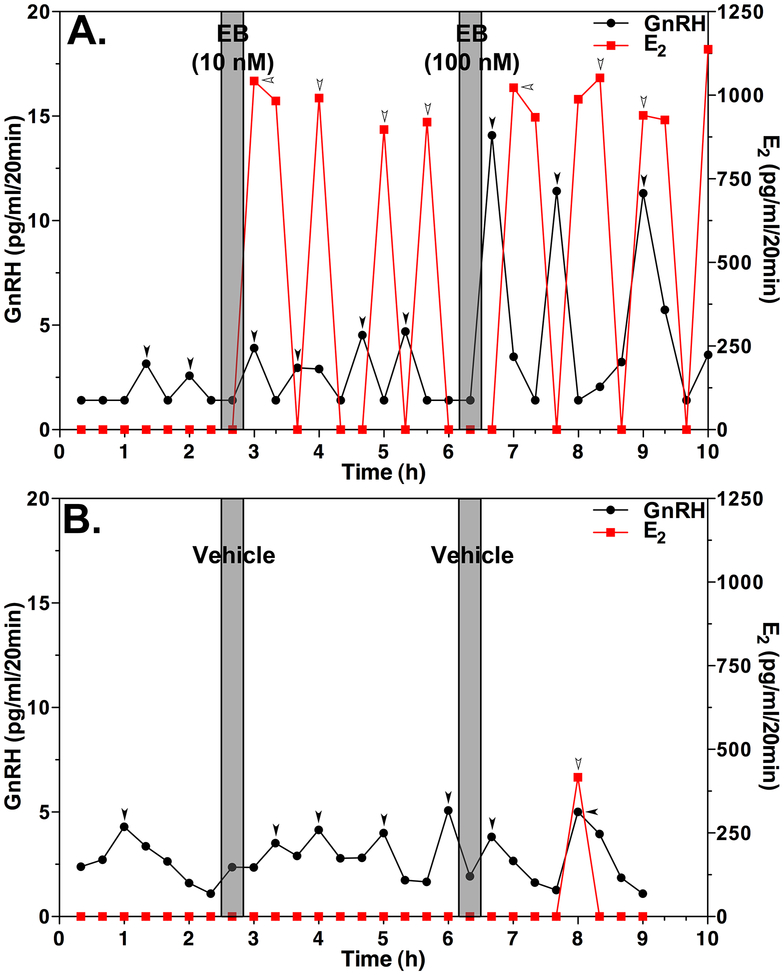
During the course of the experiments examining the rapid action of E2 on GnRH release in vivo, we assessed the pattern of local E2 levels as a consequence of EB injection. Results indicate that E2 release in response to EB challenge, measured by liquid chromatography followed by tandem mass-spectrometry (LC/MS/MS), is pulsatile (Fig. 3). The pulsatile release pattern of E2 was rather surprising to us, as we were used to seeing an asymmetric bell shape pattern in circulating E2 after systemic injection of EB, i.e., E2 rapidly increases reaching a plateau, and then slowly returns to the pre-injection level, because the E2 radioimmunoassay is cross-reacting with EB. We soon realized that LC/MS/MS distinguishes E2 from EB because of its different mass-to-charge ratios, such that infusion of EB into the median eminence must have induced local release of E2. If the hypothalamus releases E2 in response to an EB infusion, we should also be able to observe the release without EB treatment. Indeed, we found that electrical stimulation of the medial basal hypothalamus (MBH) of OVX monkeys initially suppresses and then stimulates E2 release (Kenealy et al., 2013).
Figure 3:
Brief (20 min), direct infusion of EB into the median eminence induces release of GnRH (black line with filled circle) and E2 (red line with filled square) in OVX female monkeys in vivo. Representative cases from EB infused (A) or vehicle infused (B) animals are shown. Gray bars designate the 20-min EB (10 or 100 nM) or vehicle infusion. Note that EB induced increases in GnRH release and pulsatile E2 release (A). While the amplitude of GnRH responses appears to be EB dose-dependent, the amplitude of E2 did not vary. In contrast, vehicle infusion does not cause any significant effects (B). In this case, a single spontaneous E2 peak is seen at 8 h. Arrowheads indicate pulses identified by PULSAR. From Kenealy et al., 2013. Permission pending.
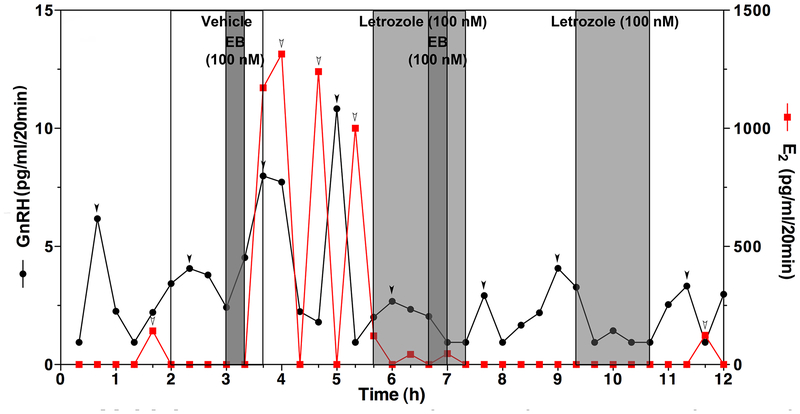
In addition, if the EB-induced E2 release is of hypothalamic origin, we should observe that inhibition of local E2 synthesis blocks stimulatory EB action. Therefore, we examined the effects of the aromatase inhibitor letrozole on the EB-induced GnRH and E2 release. In this experiment, we infused letrozole (or its vehicle for control) into the median eminence for 100 minutes, during which EB was also infused for 20 min. The results indicated that in the presence of letrozole EB did not induce E2 release nor GnRH release, whereas EB induced both GnRH and E2 increases in the absence of letrozole (Fig. 4, Kenealy et al., 2013). Importantly, letrozole also suppressed the EB-induced GnRH release as well as spontaneous GnRH pulses (Fig. 4). At this point we could have concluded that neuroestradiol plays an important role in regulation of pulsatile GnRH release. However, during the course of the study we found that spontaneous neuroestradiol release in the median eminence of OVX monkeys is rare and that when spontaneous release occurs, the amplitude of peaks is much smaller than for peaks evoked by EB or electrical stimulation (Kenealy et al., 2013). Therefore, we concluded that the role of neuroestradiol on regulation of pulsatile GnRH release might be limited to certain circumstances and there must be another role of hypothalamic neuroestradiol in GnRH regulation. We have addressed this issue with the next series of experiments.
Figure 4:
An example showing that infusion of letrozole (100nM, light grey bars) into the median eminence inhibits the EB-induced as well as spontaneous release of GnRH (black line with filled circle) and E2 (red line with filled square). EB challenge (100 nM, dark grey bars) was first infused for 20 min with vehicle, subsequently letrozole (100 nM, light grey bars) was continuously infused for 100 min, during which EB (100 nM) infusion was added for 20 min (60–80 min after the initiation of letrozole infusion), and finally letrozole (100 nM) alone was infused for 80 min. Note that control EB infusion stimulates release of GnRH and E2, whereas EB infusion in the presence of letrozole fails stimulating release of GnRH or E2. Letrozole infusion alone also blocks spontaneous GnRH pulses (A). From Kenealy et al., 2013. Permission pending.
Role of neuroestradiol in GnRH surge:
A rise in ovarian E2 from the primary follicles is the signal triggering the preovulatory GnRH/LH surge. Using an experimental model, it has been shown that systemic injections of EB to OVX monkeys induce GnRH and LH surges, i.e., sustained GnRH and LH elevations for over 36 hours with a latency of ~24 hours (Levine et al, 1985; Xia et al., 1992; Yamaji et al., 1973; Terasawa, 1985, Schultz and Terasawa, 1988). Because our observations show that prolonged infusion of EB into the median eminence keeps GnRH release elevated for several hours (Kenealy et al., 2015) and that the sustained elevation of GnRH release is reminiscent of the preovulatory GnRH surge, we hypothesized that neuroestradiol release is necessary for the estrogen-induced LH/GnRH surge.
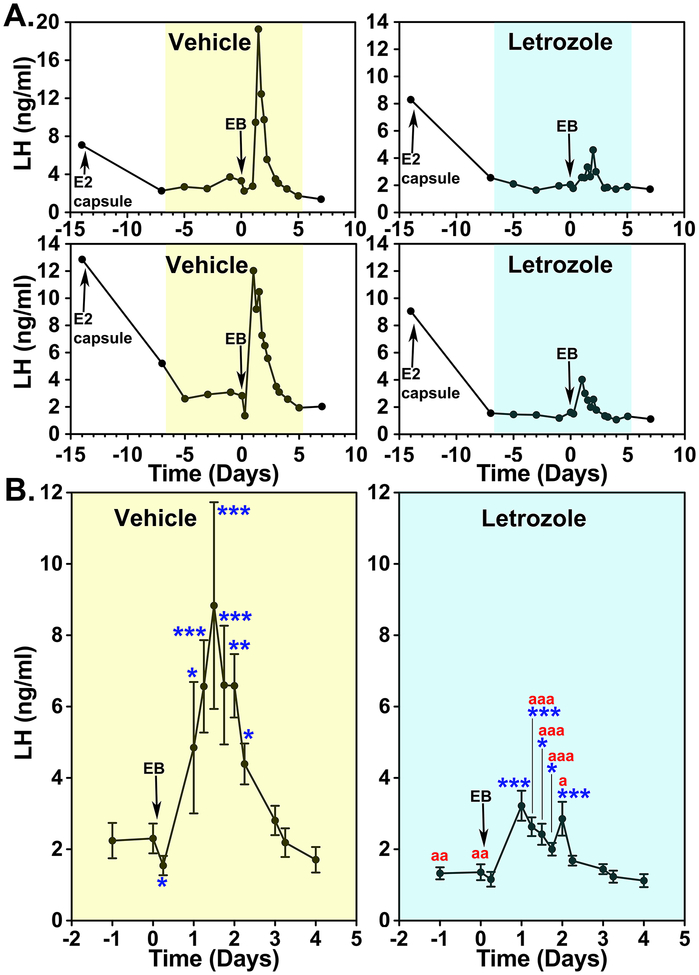
We tested this hypothesis with two experiments. In the first experiment OVX monkeys implanted with an E2 capsule (this results in LH levels similar to the level during the follicular phase) were treated with daily injections of letrozole for 1 week and then EB was injected systemically mimicking the preovulatory E2 increase. Results indicated that while EB injection induced full LH surges in controls, the letrozole treatment greatly attenuated EB-induced LH surges (Fig. 5, Kenealy et al., 2017). Importantly, the latency to the LH surge after EB was not affected by letrozole (Kenealy et al., 2017). These observations suggest that 1) an increase in circulating E2 triggers the LH surge, but 2) an elevated level of circulating E2 is insufficient for sustaining LH surges and E2 synthesized in the hypothalamus and/or pituitary gland is critically important for the EB-induced LH surge.
Figure 5:
Letrozole attenuates the EB-induced LH surge in OVX female monkeys. (A) Changes in LH release from two examples in each (vehicle and letrozole treated groups shown in yellow and blue, respectively) during the entire course of the experiment and (B) group data (mean±sem) highlighting the EB-induced LH surge are shown. All animals were implanted with capsules containing estradiol (E2) on Day −14 and received EB injection on Day 0. In both groups, E2 capsule lowered LH levels, similar to those during the early follicular phase. Analysis indicated that EB induced significant LH elevations over baseline LH levels before EB injection in both vehicle (n=5)- and letrozole (n=5)-treated animals (for both p<0.001) and that the EB-induced LH surge with letrozole was significantly (p<0.001) smaller than that with vehicle. Post hoc analysis indicated that the LH levels in the vehicle group measured at Day 0.25 were significantly (*: p<0.05) lower than before EB and LH levels at Day 1, 1.25, 1.5, 1.75, 2, and 2.25 were (*: p<0.05, **: p<0.01, and ***: p<0.001) significantly higher than before EB treatment. Similarly, LH levels in the letrozole treated group at Day 1, 1.25, 1.5, 1.75, and 2 were (*: p<0.05 and ***: p<0.001) higher than before treatment. Importantly, LH levels on Day −3, −1, 0, 1.25, 1.5, 1.75, and 2 in the letrozole treatment were significantly (a: p<0.5, aa: p<0.01, and aaa: p<0.001) lower than those in the vehicle group. From Kenealy et al., 2017. Permission pending.
There are abundant aromatase-expressing cells in the hypothalamus and anterior pituitary gland (MacLusky et al., 1986; Roselli et al., 2001; Galmiche et al., 2006; Kadioglu et al., 2008). Thus, the activity of aromatase synthesizing cells might be influenced by circulating E2. This view is consistent with a report in female baboons showing that aromatase activity in the amygdala and preopotic area assessed by PET scan with 11C-vorozole uptake fluctuates during the menstrual cycle (Pareto et al., 2013).
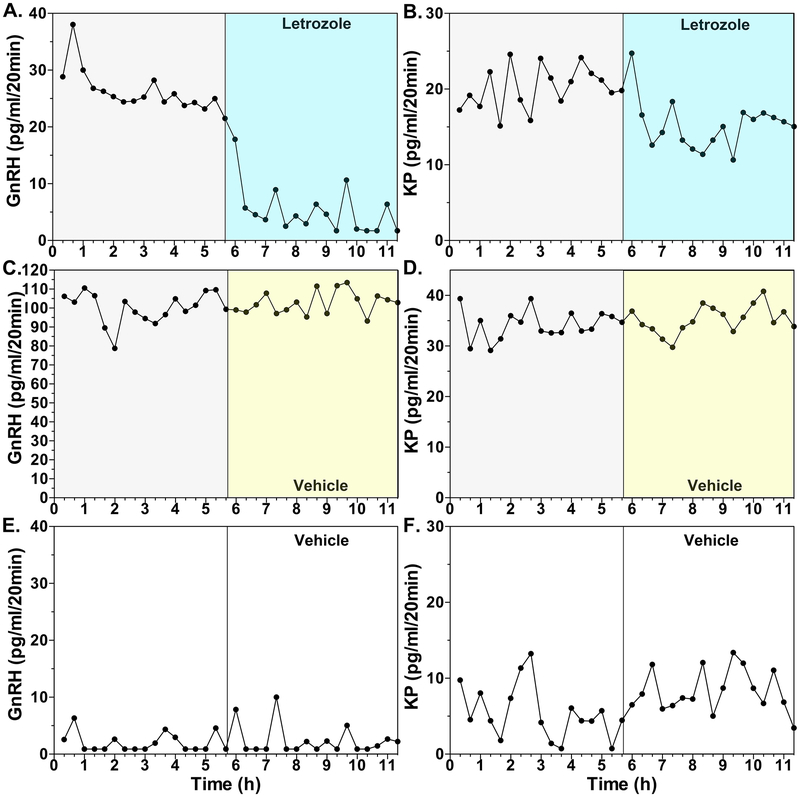
In the second experiment, we examined whether blocking locally synthesized E2 in the hypothalamus by letrozole infusion into the median eminence reduces the EB-induced GnRH surge. Although in the previous study (Kenealy et al., 2013) we showed that neuroestradiol in the hypothalamus is involved in regulation of pulsatile GnRH release in OVX females, we had not examined the involvement of neuroestradiol when there is elevated circulating E2. In this experiment, we used a single high EB injection model in OVX monkeys for the induction of GnRH surges. Results indicate that EB-induced GnRH surges are attenuated within 60 minutes of the letrozole infusion into the median eminence (Figs. 6A vs. 6C, Kenealy et al., 2017). Similarly, EB-induced kisspeptin surges are also reduced in the presence of letrozole, when compared to those in vehicle control (Figs. 6B vs. 6D, Kenealy et al., 2017). We speculate that letrozole infusion reduces the EB-induced E2 release, as shown in the previous study (Kenealy et al., 2013). We now plan to measure E2 release in the median eminence in future experiments.
Figure 6:
The effects of letrozole (A and B) or vehicle (C and D) infusion into the median eminence on EB-induced GnRH (left column) and kisspeptin (right column) surges, examined using microdialysis in OVX female monkeys. While in panels A, B, C, and D, EB was injected 36 h before sample collection, in panels E and F only oil (vehicle for EB) was injected 36 h before the start of dialysate collection. Both GnRH and kisspeptin levels during the first 5.7 h in EB injected animals (A, B, C, and D) were higher than those in oil controls (E and F) indicating EB induced GnRH and kisspeptin surges. In EB-treated animals letrozole infusion (A and B) suppressed both GnRH and kisspeptin release within 20 min, whereas vehicle infusion (C and D) did not change release of GnRH and kisspeptin. Note that there was considerable individual variation in elevated GnRH and kisspeptin levels after EB injection. From Kenealy et al., 2017. Permission pending.
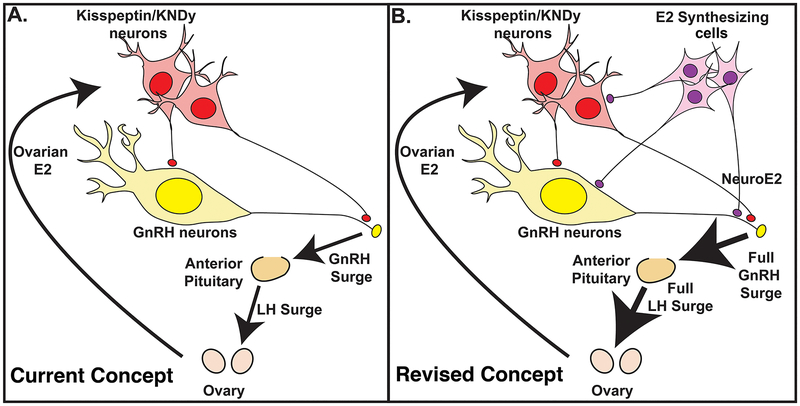
The results from these two experiments provide a new insight into the well-established positive feedback effects of E2. An increase in circulating E2 feeds back to interneurons, such as kisspeptin neurons, that stimulate GnRH neurons resulting in the GnRH surge (Dungan et al, 2007, Clarkson et al., 2008). At the same time elevated circulating E2 stimulates E2 synthesizing cells in the hypothalamus and locally synthesized neuroestradiol further augments the GnRH surge directly or indirectly through interneurons (Fig. 7B). This view is quite different from the current view in which an increase in circulating E2 solely feeds back to kisspeptin neurons, through which GnRH surges are generated (Fig. 7A).
Figure 7:
Schematic illustration of the positive feedback effects of E2. The current concept shown in (A) is that an increase in E2 from ovarian follicles during the late follicular phase gives a signal to interneurons expressing estrogen receptor (ER) α, such as kisspeptin neurons, in the hypothalamus, which stimulates perikarya and neuroterminals of GnRH neurons resulting in GnRH surge and subsequent LH surge from the pituitary. However, the results of a recent study indicate that the current concept is insufficient, as the removal of locally synthesized E2 (neuroestradiol) in the median eminence by the aromatase inhibitor, letrozole, reduces the amount of GnRH and LH. Therefore, we will need to revise the concept (B): An increase in E2 from the ovary stimulates not only the interneurons such as kisspeptin neurons, but also cells that locally synthesize E2 (neuroestradiol), which stimulates GnRH neurons directly or indirectly resulting in a full GnRH and LH surge (B). From Kenealy et al., 2017. Permission pending.
A small increase in circulating progesterone during the late follicular phase in humans or a progesterone increase after E2 priming in OVX monkeys defines the timing of the GnRH/LH surge (Clifton et al., 1975; Terasawa et al., 1982; Hoff et al., 1983). A question arises as to whether GnRH/LH surges augmented by progesterone in the presence of E2, are also accompanied by the release of neuroestradiol. Although additional experiments with direct measurements of E2 in the median eminence are necessary, it is unlikely that progesterone action during the preovulatory phase is affected by letrozole, as letrozole does not influence the latency of the EB-induced LH surge.
The stimulatory role of neuroprogesterone, locally synthesized by hypothalamic astrocytes, in the preovulatory LH surge in female rats has been reported in detail (see Micevych and Sinchak, 2011). So far, we have not examined the role of neuroprogesterone in the E2-induced GnRH surge. Nevertheless, it can be expected that the role of neuroprogesterone in the preovulatory GnRH surge in primates would significantly differ from that reported in rodents. This view is based on the difference in the role of progesterone in the preovulatory surge between rodents and primates. For example, whereas in E2-treated OVX female rats progesterone augments the timing and amplitude of the LH surge (DePaolo and Barraclough, 1979), in primates in E2-treated OVX female monkeys progesterone only modifies the timing, but not the overall amplitude, of the LH surge (Terasawa et al., 1979). As a consequence, unlike in rodents, the progesterone induced LH surge in OVX female monkeys treated with a low dose of E2 (Terasawa et al., 1980) does not quite resemble a spontaneous preovulatory LH surge or the LH surge induced by a high dose of E2 in OVX female monkeys (Yamaji et al. 1971; Karsch et al., 1973).
Extending the issue of the species differences, the role of neuroestradiol in the preovulatory GnRH/LH surge might be specific to primates or species with a luteal phase. For example, animals with a true luteal phase, such as monkeys and sheep, exhibit a long preovulatory GnRH surge, lasting well after the end of the LH surge (Levine et al., 1985; Xia et al., 1992; Moenter et al., 1990). By contrast in rats, which lack the luteal phase, the preovulatory GnRH surge terminates at the same time as the LH surge (Sarkar et al., 1976). Moreover, in OVX monkeys letrozole greatly attenuates the E2-induced GnRH and LH surges (Kenealy et al., 2017), whereas in OVX aromatase-deficient mice E2 or E2 plus progesterone readily induces LH surges (Szymanski and Bakker, 2012). It is speculated that the prolonged GnRH surge is a result of neuroestradiol and that it might be required for the establishment of the corpus luteum.
Conclusions and perspectives:
In this review article, I summarized our findings during the past ~15 years showing that E2 rapidly and directly stimulates GnRH neuronal activity and this rapid action of E2 has an important implication in the release of GnRH as well as in the local synthesis of E2 (neuroestradiol) in the hypothalamus. It turns out that neuroestradiol is released in the median eminence and this neuroestradiol release plays an important role in the regulation of pulsatile GnRH release as well as during the estrogen-induced GnRH surge (i.e., preovulatory GnRH surge). Because the preovulatory GnRH surge is the key event for successful pregnancy and contraception, the findings on the role of neuroestradiol in the preovulatory surge potentially open up a new avenue for treatments of fertility and sterility. In fact, brain specific exon 1 of the human aromatase gene has been reported (Honda et al., 1994).
The investigation of neuroestradiol in the regulation of reproductive function is just beginning and many questions remain to be answered. Does the release of neuroestradiol in the hypothalamus switch estrogen’s action from the negative feedback effect to the positive feedback effect? Is the role of neuroestradiol in the regulation of GnRH release limited to females, given that the male brain generally expresses a higher level of aromatase than the female brain (Roselli and Resko, 1997)? We previously postulated that neuroestradiol may be a substrate for the “Prepubertal Central Inhibition”, as release of neuroestradiol in the median eminence in prepubertal female monkeys is higher than that in early pubertal female monkeys (Kenealy et al., 2016), but how does neuroestradiol play a role in the suppression of GnRH release? Does neuroestradiol play any role in Alzheimer’s disease, as aromatase expression in the hypothalamus in Alzheimer patients is lower than in the age-matched control hypothalamus (Ishunina et al., 2005). Is neuroestradiol synthesized in neurons, glia, or both? If synthesized in neurons, what is their cell type? We have no data regarding receptors mediating neuroestradiol action in vivo. Unlike genomic estrogen actions mediated through ESR1, rapid actions of E2 occur through membrane associated estrogen receptors, such as GPER1 (GPR30) (see Terasawa and Kenealy, 2012), but these studies were conducted in vitro. Finally, why did such a complicated system evolve in primates including actions of E2 not only from the ovary but also from the brain?
As stated in the Introduction, the role of neuroestadiol has been described for learning and memory, stroke, and in behavioral contexts, such as sex behaviors. Together with current findings on the role of neuroestradiol in the regulation of GnRH release, we are still in the early stages of understanding the role of neuroestradiol in the brain.
Highlights:
Estradiol induces rapid changes in GnRH neuronal activity
Neuroestradiol is synthesized and released in the hypothalamus
Neuroestradiol contributes to the regulation of pulsatile GnRH release
Hypothalamic neuroestradiol is obligatory for the full GnRH surge
Acknowledgement:
The author expresses her sincere appreciation to Brian P. Kenealy, Ph.D. for his contributions to the series of studies described in this article that were conducted during his tenure in the lab, as a graduate student first and then a postdoctoral research fellow. She also thanks Senior Research Specialist, Kim L. Keen for all aspects of this project, colleagues, Toni Ziegler, Ph.D. and Amita Kapoor, Ph.D. for measurement of estradiol with LC/MS/MS, and the former postdoctoral research fellow, Hideki Abe, Ph.D., former and current graduate students, Sekoni D. Noel, and James P. Garcia, respectively for early or currently on-going projects. This work is supported by R01HD015433 and R21HD07747 from the Eunice K Shriver NICHD to ET. The work was made possible by support from the NIH Office of the Director for the Wisconsin National Primate Research Center (OD011106).
Funding: Supported by NIH grants: R21HD077447 and R01HD015433 to ET. The work was made possible by support (OD011106) for the Wisconsin National Primate Research Center.
Footnotes
Conflict of Interest: The author declares no competing financial interests.
References:
- Abe H, Keen KL, Terasawa E, 2008. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 149, 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E, 2005. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 146, 4312–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF, 2006. Is brain estradiol a hormone or a neurotransmitter? Trends in Neurosci. 29, 241–249. [DOI] [PubMed] [Google Scholar]
- Brinton RD, 2009. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol. Sci 30, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE, 2008. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci 235, 8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton DK, Steiner RA, Resko JA, Spies HG, 1975. Estrogen-induced gonadotropin release in ovariectomized rhesus monkeys and its advancement by progesterone. Biol. Reprod 13,190–194. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J, 2005. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior, Endocrinology. 146, 3809–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J, 2006. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm. Behav 49, 45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E, Roselli CE, 1999. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats, Am. J. Physiol 276, R1346–R1350. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA, 2007. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci 27, 12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA, 1984. Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys. J. Clin. Endocrinol. Metab 59, 1088–1096. [DOI] [PubMed] [Google Scholar]
- Frost SI, Keen KL, Levine JE, Terasawa E, 2008. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the Rhesus monkey. J. Neurosci. Methods 168, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche G, Richard N, Corvaisier S, Kottler ML, 2006. The expression of aromatase in gonadotropes is regulated by estradiol and gonadotropin-releasing hormone in a manner that differs from the regulation of luteinizing hormone. Endocrinology. 147, 4234–4244. [DOI] [PubMed] [Google Scholar]
- Goldsmith PC, Lamberts R, Brezina LR, 1993. Gonadotropin-releasing hormone neurons and pathways in the primate hypothalamus and forebrain, in Norman RL (Ed.), Neuroendocrine Aspect of Reproduction. Academic Press, New York, pp. 7–45. [Google Scholar]
- Herbison AE, 2015. Physiology of the adult gonadotropin-releasing hormone neuronal network, in Plant TM and Zeleznik AJ (Ed.), Physiology of Reproduction. Academic Press, New York, pp. 399–467. [Google Scholar]
- Hoff JD, Quigley ME, Yen SS, 1983. Hormonal dynamics at midcycle: a reevaluation. J. Clin. Endocrinol. Metab 57, 792–796. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii H, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Nat. Acad. Sci. U.S.A 101, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S, 2008. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol. Cell Endocrinol 290, 31–43. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Takagi Y 1994. Novel exon 1 of the aromatase gene specific for aromatase transcripts in human brain. Biochem. Biophys. Res. Commun 198,1153–1160. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, van Beurden D, van der Meulen G, Unmehopa UA, Hol EM, Huitinga I, Swaab DF, 2005. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer’s disease. Neurobiol. Aging 26, 173–194. [DOI] [PubMed] [Google Scholar]
- Kadioglu P, Oral G, Sayitoglu M, Erensoy N, Senel B, Gazioglu N, Sav A, Cetin G, Ozbek U, 2008. Aromatase cytochrome P450 enzyme expression in human pituitary. Pituitary. 11, 29–35. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dierschke DK, Weick RF, Yamaji T, Hotchkiss J, Knobil E, 1973. Positive and negative feedback control by estrogen of luteinizing hormone secretion in the rhesus monkey. Endocrinology. 92, 799–804. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA, 1976. Differential sensitivity of the preopticseptal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 114, 152–157. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E, 2013. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J. Neurosci 33, 19071–19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Rønnekleiv OK, and Terasawa E, 2011a. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 152, 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Garcia JP, Kohlenberg LK, Terasawa E, 2017. Obligatory role of neuroestradiol in the hypothalamus during the LH surge in female ovariectomized rhesus monkeys. Proc. Natl. Acad. Sci. U.S.A, 114, 13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Garcia JP, Richter DJ, Terasawa E, 2015. Prolonged infusion of estradiol benzoate into the stalk median eminence stimulates release of GnRH and kisspeptin in ovariectomized female rhesus macaques. Endocrinology. 156, 1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Kapoor A, Terasawa E, 2016. Neuroestradiol in the stalk median eminence of female rhesus macaques decreases in association with puberty onset. Endocrinology. 157, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Terasawa E, 2011b.. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor alpha and estrogen receptor beta. Steroids. 76, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW, 2004. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc. Natl. Acad. Sci. U.S.A. 101, 12354–12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ, 1995. Estradiol-17 and –opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 136, 2341–2344. [DOI] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG, 1985. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 117, 711–721. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Goldman-Rakic PS, 1986. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey, Proc. Natl. Acad. Sci. U.S.A. 83, 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, 2011. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol (Lausanne). 2:90 doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Terasawa E, 2005. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta). J. Neuroendocrinol 17, 238–245. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ, 1990. The estradiol-induced surge of gonadotropin releasing hormone in the ewe. Endocrinology. 127, 1375–1384. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC, 2006. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J. Neurosci 26, 10332–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J, 1996. Aromatase immunoreactivity in axon terminals of the vertebrate brain: an immunocytochemical study on quail, rat, monkey, and human tissues, Neuroendocrinology. 63, 149–155. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z, 1971. Aromatization of androstenedione by the diencephalons, J. Clin. Endocrinol. Metab 33, 368–370. [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ, 2003. Regulation of cyclic adenosine 3’, 5’-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol. Endocrinol 17, 1792–1804. [DOI] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E, 2008. Involvement of G-protein coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol. Endocrinol 23, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareto D, Biegon A, Alexoff D, Carter P, Shea C, Muench L, Xu Y, Fowler JS, Kim SW, Logan J, 2013. In vivo imaging of brain aromatase in female baboons: [11C]vorozole kinetics and effect of the menstrual cycle. Mol. Imaging 12, 518–524. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ, 2005. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain, Proc. Bio. Sci 272, 2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA, 1976. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J. Comp. Neurol 170, 279–293. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM 2008. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 149, 4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, 2009. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 30, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA, 2011. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J. Neurosci 31, 10034–10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA, 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci 11, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Keen KL, Terasawa E, 2002. Synchronization of Ca(2+) oscillations among primate LHRH neurons and nonneuronal cells in vitro. J. Neurophysiol 88, 1559–1567. [DOI] [PubMed] [Google Scholar]
- Romanò N, Lee K, Abrahám IM, Jasoni CL, Herbison AE, 2008. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 149, 5335–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, 1997. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J. Steroid Biochem. Mol. Biol 61, 365–374. [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA, 1987. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol. Reprod 37, 628–633. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman S, Resko JA, 2001. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J. Comp. Neurol 439, 208–223. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA, 2011. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr. Rev 32, 532–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz NJ, Terasawa E, 1988. Posterior hypothalamic lesions advance the time of the pubertal changes in luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology. 123, 445–455. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK, 1977. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology. 101, 841–848. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS, 2008. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocrinol 29, 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T, Hutchison JB, 1980. Aromatization of testosterone within a discrete hypothalamic area associated with the behavioral action of androgen in the male dove. Brain Res. 192, 586–591. [DOI] [PubMed] [Google Scholar]
- Szymanski L, Bakker J, 2012. Aromatase knockout mice show normal steroid-induced activation of gonadotrophin-releasing hormone neurones and luteinising hormone surges with a reduced population of kisspeptin neurones in the rostral hypothalamus. J. Neuroendocrinol 24, 1222–1233. [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S, 2004. Direct action of estradiol on gonadotropin- releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J. Neurosci 24, 6326–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, 1985. Developmental changes in the positive feedback effect of estrogen on luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology. 117, 2490–2497. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Noonan J, Bridson WE, 1982. Anaesthesia with pentobarbitone blocks the progesterone-induced luteinizing hormone surge in the ovariectomized rhesus monkey. J. Endocrinol 92, 327–339. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P, 1993. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 133, 2379–2390. [DOI] [PubMed] [Google Scholar]
- Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M, 1992. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology. 131, 2812–2820. [DOI] [PubMed] [Google Scholar]
- Yagi K, 1973. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Res. 53, 343–352. [DOI] [PubMed] [Google Scholar]
- Yamaji T, Dierschke DJ, Hotchkiss J, Bhattacharya AN, Surve AH, Knobil E, 1971. Estrogen induction of LH release in the rhesus monkey. Endocrinology. 89, 1034–1041. [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Barraclough CA, 1979. Interactions of estradiol and progesterone on pituitary gonadotropin secretion: possible sites and mechanisms of action. Biol. Reprod 20, 1173–1185. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Rodriguez-Sierra JF, Dierschke DJ, Bridson WE, Goy RW, 1980. Positive feedback effect of progesterone on luteinizing hormone (LH) release in cyclic female rhesus monkeys: LH response occurs in two phases. J. Clin. Endocrinol. Metab 51, 1245–1250. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Yeoman RR, Schultz NJ, 1984. Factors influencing the progesterone-induced luteinizing hormone surge in rhesus monkeys: diurnal influence and time interval after estrogen. Biol. Reprod 31, 732–741. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM, 1976. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264, 461–463. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Kenealy BP, 2012. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front. Neuroendocrinol 33, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]