Abstract
Cytokine-induced apoptosis inhibitor 1 (CIAPIN1), known as an anti-apoptotic and signal-transduction protein, plays a pivotal role in a variety of biological processes. However, the role of CIAPIN1 in inflammation is unclear. We investigated the protective effects of CIAPIN1 in lipopolysaccharide (LPS)-exposed Raw 264.7 cells and against inflammatory damage induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in a mouse model using cell-permeable Tat-CIAPIN1. Transduced Tat-CIAPIN1 significantly reduced ROS production and DNA fragmentation in LPS-exposed Raw 264.7 cells. Also, Tat-CIAPIN1 inhibited MAPKs and NF-κB activation, reduced the expression of Bax, and cleaved caspase-3, COX-2, iNOS, IL-6, and TNF-α in LPS-exposed cells. In a TPA-induced animal model, transduced Tat-CIAPIN1 drastically decreased inflammation damage and inhibited COX-2, iNOS, IL-6, and TNF-α expression. Therefore, these findings suggest that Tat-CIAPIN1 might lead to a new strategy for the treatment of inflammatory skin disorders.
Keywords: Cytokine, Inflammation, MAPK, Protein therapy, Tat-CIAPIN1
INTRODUCTION
Inflammation is a natural defense response to infection or injury, and it may lead to various human diseases, including cancer (1). Under the inflammatory responses, macrophages are activated and secrete pro-inflammatory mediator proteins, such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS) as well as pro-inflammatory cytokines, including interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) (2–5). Several studies have demonstrated that the nuclear factor-kappa B (NF-κB) and the mitogen-activated protein kinases (MAPKs) signaling pathways play a pivotal role in inflammatory responses, suggesting that modulation of NF-κB and MAPKs is a key point for therapeutic approaches to inflammatory diseases (6–10).
Cytokine-induced apoptosis inhibitor 1 (CIAPIN1) is known as an anti-apoptotic and signal-transduction protein, and many studies have revealed that CIAPIN1 may suppress apoptosis and regulate tumorigenesis (11–14). Park et al. (2011) reported that the CIAPIN1 protein protects neuronal MN9D cells against oxidative stress-induced cell death by increasing the expression of anti-apoptotic proteins (15). Wang et al. (2015) have shown that transfected CIAPIN1 genes in human multiple myeloma significantly inhibited the growth and proliferation of tumor cells, suggesting that CIAPIN1 is a potential tumor suppressor (16) and several studies have reported that chronic inflammation can lead to cancer by increasing pro-inflammatory mediators, ROS, intracellular signaling-pathway mediators, and transcription factors (17–19). Although the CIAPIN1 protein may be associated with the suppression of cancer and inflammation, there is no evidence about its exact roles in inflammation until now. Therefore, we investigated the effects of Tat-CIAPIN1 on inflammation with lipopolysaccharide (LPS)-exposed Raw 264.7 cells and a 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse edema model.
RESULTS AND DISCUSSION
Transduction and effects of Tat-CIAPIN1 against LPS-induced cytotoxicity in Raw 264.7 cells
Since it is known that protein transduction domains (PTDs) can deliver proteins into cells, many studies have suggested that PTDs can be used for application of therapeutic proteins to treat various diseases (20–30). Purified Tat-CIAPIN1 protein was identified (Supplementary Fig. S1). We showed that Tat-CIAPIN1 transduced into the Raw 264.7 cells concentration-and time-dependently as well as transduced Tat-CIAPIN1 levels persisted in the cells for 12 h (Supplementary Fig. S2A–S2C).
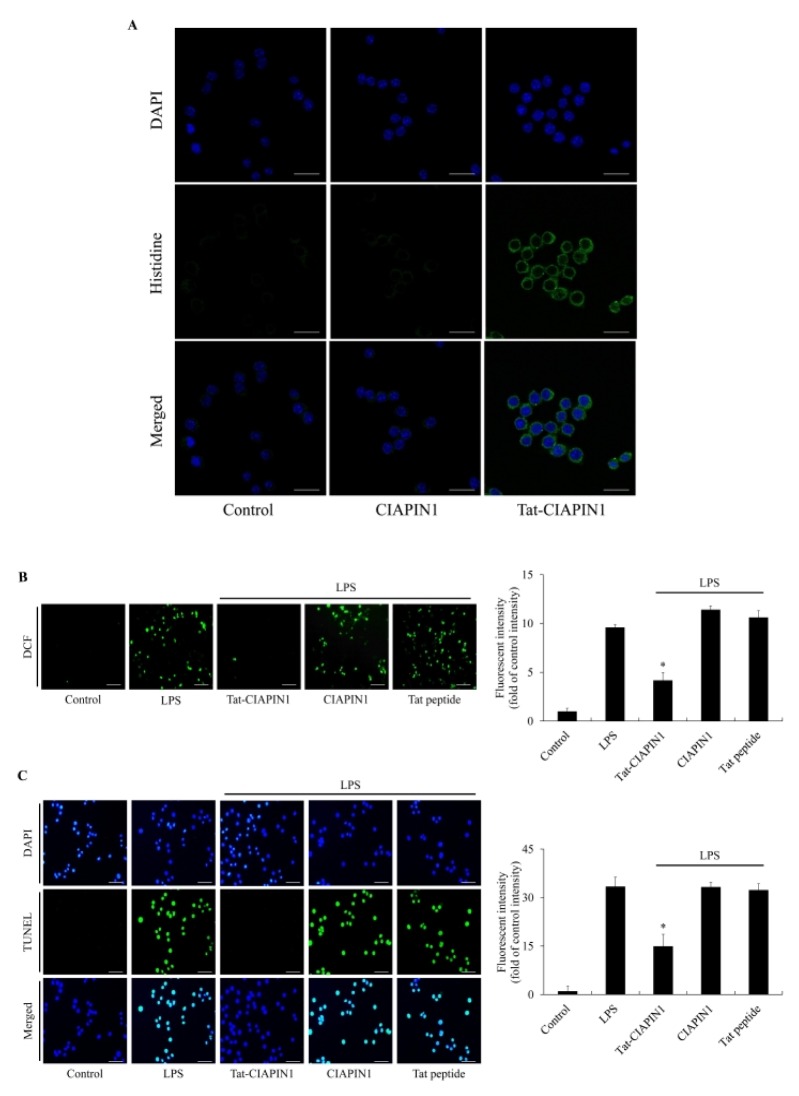
We also assessed the distribution of Tat-CIAPIN1 in Raw 264.7 cells using immunostaining with Alexa Fluor 488 and DAPI. Tat-CIAPIN1 transduced into both the cytosol and the nuclei of Raw 264.7 cells. However, CIAPIN1 did not transduce into the cells (Fig. 1A). Other studies have reported that LPS induces ROS production and DNA damage in various cells, including Raw 264.7 cells, finally leading to cell damage (2–4, 31). In agreement with these reports, we showed that ROS generation and DNA fragmentation levels were significantly increased in the cells exposed only to LPS, control CIAPIN1, and Tat peptide. However, transduced Tat-CIAPIN1 markedly inhibited ROS generation and DNA fragmentation in LPS-exposed cells (Fig. 1B and 1C).
Fig. 1.
Effects of transduced Tat-CIAPIN1 protein on LPS-induced ROS production and DNA fragmentation. The localization of transduced Tat-CIAPIN1 protein was examined by confocal fluorescence microscopy (A). Scale bar = 20 μm. Cells were treated with Tat-CIAPIN1 (3 μM) or CIAPIN1 protein for 1 h before treatment with 1 μg/ml of LPS for 3 h or 14 h. Then, intracellular ROS levels (B) and DNA fragmentation (C) were measured by DCF-DA staining and TUNEL staining. Fluorescence intensity was quantified using an ELISA plate reader. Scale bar = 50 μm. *P < 0.05, compared with LPS-treated cells.
Effects of Tat-CIAPIN1 on LPS-induced inflammatory responses in Raw 264. 7 cells
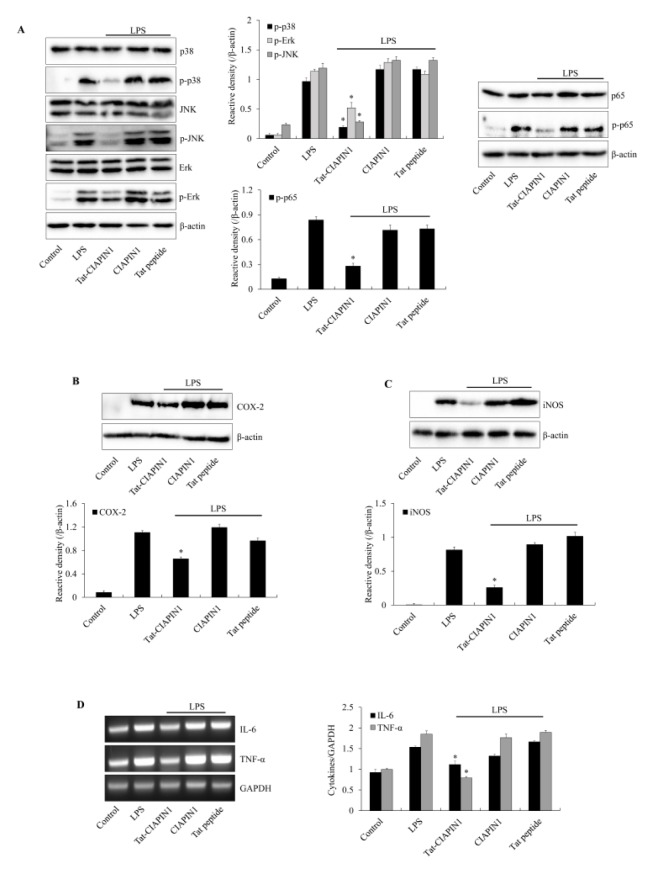
Other studies have reported that regulation of NF-κB and MAPKs signaling pathways are important to protect against LPS-induced inflammatory responses in Raw 264.7 cells (32, 33). To examine the effects of Tat-CIAPIN1 on LPS-induced signaling pathways (MAPKs and NF-κB), the cells were exposed to LPS (1 μg/ml). In the LPS-exposed cells, phosphorylated MAPKs and p65 expression were higher than in the control cells. CIAPIN1 and Tat peptide-exposed cells showed similar patterns. In contrast, Tat-CIAPIN1 significantly reduced the phosphorylated MAPKs and p65 expression (Fig. 2A). Many studies have described that NF-κB and MAPKs signaling pathways are crucial mediators in various cellular biological processes and play a key role in the process of inflammatory response by promoting the release of the pro-inflammatory cytokines (34–36).
Fig. 2.
Effects of Tat-CIAPIN1 protein on LPS-induced inflammatory responses in Raw 264.7 cells. The cells were treated with Tat-CIAPIN1 (3 μM) or CIAPIN1 protein for 1 h before being exposed to LPS (1 μg/ml). MAPK and NF-κB activation (A) and the expression levels of COX-2 (B) and iNOS (C) protein were analyzed by Western blotting. Total RNA was extracted from the cells. We analyzed cytokines (IL-6 and TNF-α) and GAPDH mRNA by RT-PCR using specific indicated primers (D). The band intensity was measured by densitometer. *P < 0.05, compared with LPS-treated cells.
We investigated the effect of Tat-CIAPIN1 against LPS-induced Bax, Bcl-2, and Caspase-3 expression levels. Other studies have shown that LPS induced apoptosis via a caspase-dependent mitochondrial death signaling pathway (37, 38). Supplementary Fig. S3A–S3C shows that Bcl-2 and Caspase-3 expression were reduced in the LPS-exposed Raw 264.7 cells. However, Tat-CIAPIN1 significantly increased Bcl-2 and Caspase-3 expression more than did those treated with LPS alone. In contrast, Bax and cleaved Caspase-3 expression showed an effect opposite to that of Bcl-2 and Caspase-3. There was no changes in CIAPIN1 and Tat peptide-treated cells. Consistent with our results, other studies have shown that overexpression of CIAPIN1 reduced cleaved Caspase-3 expression, whereas Caspase-3 expression was increased in CIAPIN1-depleted K562 cells, suggesting that CIAPIN1 has an anti-apoptotic function (39, 40). In addition, CIAPIN1 protected against neuronal cell death caused by increased Bcl-XL under oxidative stress conditions, suggesting that CIAPIN1 plays an important role in protecting neuronal cells against cell death induced by oxidative stress (15).
We also investigated whether Tat-CIAPIN1 suppresses the inflammatory response in LPS-induced Raw 264.7 cells. LPS markedly increased COX-2, iNOS, TNF-α, and IL-6 expression in Raw 264.7 cells. There were no changes in CIAPIN1 and Tat peptide-treated cells. However, Tat-CIAPIN1 drastically reduced COX-2, iNOS, TNF-α, and IL-6 expression in LPS-exposed Raw 264.7 cells (Fig. 2B–2D). Several studies have reported that LPS activated macrophages via the activation of NF-κB, MAPKs, pro-inflammatory proteins, and cytokines and led to cell death (32, 33, 41–43). These data indicate that Tat-CIAPIN plays an important role against LPS-induced Raw 264.7 cell injury. However, the precise mechanism involved in the target site of Tat-CIAPIN1 in inflammatory molecular signal pathways remains to be elucidated.
Effects of Tat-CIAPIN1 on TPA-induced ear edema model
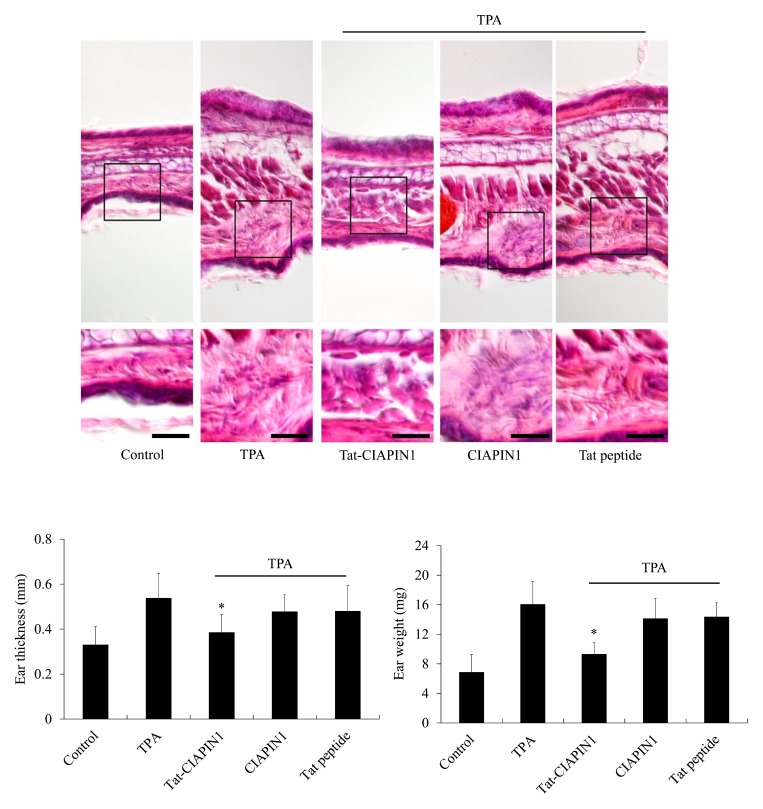
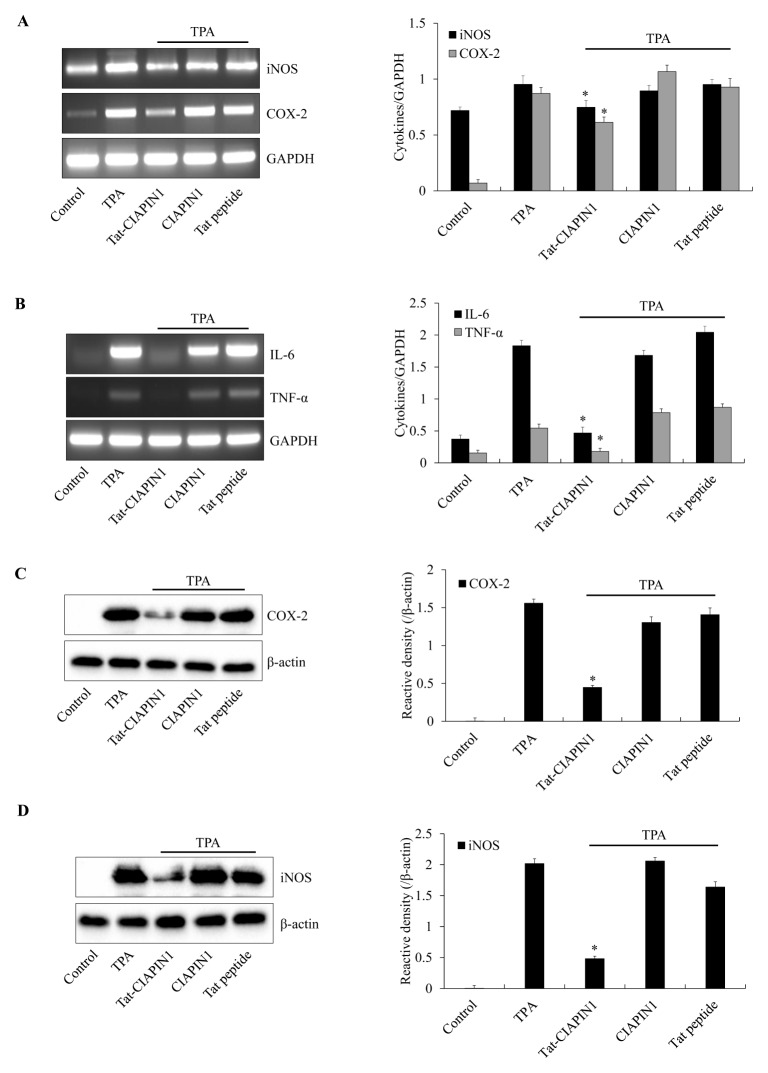
It has been reported that multiple applications of TPA to the ears of mice induce skin inflammation (44). TPA-induced skin tumors are highly related to the inflammatory response, including production of cytokines, iNOS, and COX-2 proteins (45–48). Therefore, we investigated whether transduced Tat-CIAPIN1 protects against inflammation in a TPA-induced ear edema animal model (Supplementary Fig. S4). As shown in Fig. 3, TPA significantly increased ear thickness and weight of mice as compared with the control-, CIAPIN1- and Tat peptide-treated groups. This increase in ear thickness and weight was markedly inhibited by treatment with Tat-CIAPIN1. We also examined whether Tat-CIAPIN1 could inhibit expression of pro-inflammatory cytokines and proteins. TPA markedly increased the COX-2, iNOS, TNF-α, and IL-6 expression in mice ears. CIAPIN1 and Tat peptide did not show the protective effects. However, Tat-CIAPIN1 drastically reduced the COX-2, iNOS, TNF-α, and IL-6 expression in a TPA-induced mice model of edema (Fig. 4). Our results coincide with those of Chung et al. (2007), who reported that pro-inflammatory protein and cytokines expression markedly increased in TPA-induced skin inflammation models (49).
Fig. 3.
Effects of Tat-CIAPIN1 protein on TPA-induced mice ear edema. Ears of mice were treated with TPA (1 μg/ear) once a day for 3 days. Tat-CIAPIN1 protein (10 μg) was topically applied to the mouse ears 1 h prior to TPA exposure over 3 days. Protective effects of Tat-CIAPIN1 protein were confirmed by hematoxylin and eosin staining, changes in ear weight, and ear thickness in a TPA-induced mice ear edema model. Scale bar = 50 μm. *P < 0.05, compared with TPA-treated mice.
Fig. 4.
Effects of Tat-CIAPIN1 protein on TPA-induced pro-inflammatory mediator protein (iNOS and COX-2) and cytokine (IL-6 and TNF-α) expression in mice ears. Mice were stimulated with TPA (1 μg/ear), after which Tat-CIAPIN1 protein (10 μg) was topically applied to the mouse ears for 3 days. Mouse ear extracts were prepared. After total RNA was extracted from ear biopsies, pro-inflammatory mediator proteins (iNOS and COX-2) and cytokine (IL-6 and TNF-α) expression levels were measured by RT-PCR using specific primers (A, B). The expression levels of pro-inflammatory mediator protein (iNOS and COX-2) were confirmed by Western blotting (C, D). The band intensity was measured by densitometer. *P < 0.05, compared with TPA-treated mice.
In summary, our study showed that Tat-CIAPIN1 inhibits LPS-induced inflammation damage by suppression of pro-inflammatory proteins and cytokines expression and of the NF-κB and MAPK signaling pathways. These finding imply that Tat-CIAPIN1 exerts a protective role in the inflammatory response. Although further studies are still needed to confirm the precise roles of Tat-CIAPIN1 in inflammation, these finding suggest that Tat-CIAPIN1 may be a potential therapeutic agent for skin inflammatory diseases.
MATERIALS AND METHODS
See supplementary information for this section.
Supplementary Information
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program (2017R1D1A3B04032007 & 2019R1A6A1A11036849) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Fan G, Jiang X, Wu X, et al. Anti-inflammatory activity of Tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-kappaB pathway. Inflammation. 2016;39:375–384. doi: 10.1007/s10753-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 2.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci. 2003;104:27–38. doi: 10.1042/cs1040027. [DOI] [PubMed] [Google Scholar]
- 3.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Chung HY, Cesari M, Anton S, et al. Molecular inflammation as an underlying mechanism of aging: the anti-inflammatory action of calorie restriction. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 6.Grilli M, Memo M. Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Death Differ. 1999;6:22–27. doi: 10.1038/sj.cdd.4400463. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 8.Riehemann K, Behnke B, Schulze-Osthoff K. Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-kB. FEBS Lett. 1999;442:89–94. doi: 10.1016/S0014-5793(98)01622-6. [DOI] [PubMed] [Google Scholar]
- 9.Ivashkiv LB. Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states. Eur J Immunol. 2011;41:2477–2481. doi: 10.1002/eji.201141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibayama H, Takai E, Matsumura I, et al. Identification of a cytokine-induced antiapoptotic molecule anamorsin essential for definitive hematopoiesis. J Exp Med. 2004;199:581–592. doi: 10.1084/jem.20031858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Wu K, Fan D. CIAPIN1 as a therapeutic target in cancer. Expert Opin Ther Targets. 2010;14:603–610. doi: 10.1517/14728221003774127. [DOI] [PubMed] [Google Scholar]
- 13.Hao Z, Li X, Qiao T, Du R, Hong L, Fan D. CIAPIN1 confers multidrug resistance by upregulating the expression of MDR-1 and MRP-1 in gastric cancer cells. Cancer Biol Ther. 2006;5:261–266. doi: 10.4161/cbt.5.3.2381. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Fan R, Zou X, et al. Reversal of multidrug resistance of gastric cancer cells by down-regulation of CIAPIN1 with CIAPIN1 siRNA. Mol Biol. 2008;42:102–109. doi: 10.1134/S0026893308010135. [DOI] [PubMed] [Google Scholar]
- 15.Park KA, Yun N, Shin DI, et al. Nuclear translocation of anamorsin during drug-induced dopaminergic neurodegeneration in culture and in rat brain. J Neural Transm. 2011;118:433–444. doi: 10.1007/s00702-010-0490-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Pan J, Li J. Cytokine-induced apoptosis inhibitor 1 inhibits the growth and proliferation of multiple myeloma. Mol Med Res. 2015;12:2056–2062. doi: 10.3892/mmr.2015.3656. [DOI] [PubMed] [Google Scholar]
- 17.Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1–15. doi: 10.1042/BSR20100136. [DOI] [PubMed] [Google Scholar]
- 18.Sethi G, Tergaonkar V. Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol Sci. 2009;30:313–321. doi: 10.1016/j.tips.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr Pharm Des. 2005;11:3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- 21.Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–56. doi: 10.1016/S0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;54:78–86. doi: 10.1016/j.pharmthera.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Li Y, Cheng Y, et al. Tat PTD-endostatin: A novel anti-angiogenesis protein with ocular barrier permeability via eye-drops. Biochim Biophys Acta. 20151850:1140–1149. doi: 10.1016/j.bbagen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Sakurazawa M, Katsura K, Saito M, Asoh S, Ohta S, Katayama Y. Mild hypothermia enhanced the protective effect of protein therapy with transductive anti-death FNK protein using a rat focal transient cerebral ischemia model. Brain Res. 2012;1430:86–92. doi: 10.1016/j.brainres.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Kim MJ, Park M, Kim DW, et al. Transduced PEP-1-PON1 proteins regulate microglial activation and dopaminergic neuronal death in a Parkinson’s disease model. Biomaterials. 2015;64:45–56. doi: 10.1016/j.biomaterials.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, Kim DW, Park JH, et al. PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radic Biol Med. 2013;63:432–445. doi: 10.1016/j.freeradbiomed.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Shin MJ, Kim DW, Lee YP, et al. Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic Biol Med. 2014;67:195–210. doi: 10.1016/j.freeradbiomed.2013.10.815. [DOI] [PubMed] [Google Scholar]
- 28.Yeo HJ, Shin MJ, Yeo EJ, et al. Tat-CIAPIN1 inhibits hippocampal neuronal cell damage through the MAPK and apoptotic signaling pathways. Free Radic Biol Med. 2019;135:68–78. doi: 10.1016/j.freeradbiomed.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Moon JI, Han MJ, Yu SH, et al. Enhanced delivery of protein fused to cell penetrating peptides to mammalian cells. BMB Rep. 2019;52:324–329. doi: 10.5483/BMBRep.2019.52.5.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo HJ, Yeo EJ, Shin MJ, et al. Protective effects of Tat-DJ-1 protein against streptozotocin-induced diabetes in a mice model. BMB Rep. 2018;51:362–367. doi: 10.5483/BMBRep.2018.51.7.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaiswal YK, Jaiswal MK, Agrawal V, Chaturvedi MM. Bacterial endotoxin (LPS)-induced DNA damage in preimplanting embryonic and uterine cells inhibits implantation. Fertil Steril. 2009;91:2095–2103. doi: 10.1016/j.fertnstert.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 32.Uto T, Suangkaew N, Morinaga O, Kariyazono H, Oiso S, Shoyama Y. Eriobotryae folium extract suppresses LPS-induced iNOS and COX-2 expression by inhibition of NF-kappaB and MAPK activation in murine macrophages. Am J Chin Med. 2010;38:985–994. doi: 10.1142/S0192415X10008408. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Jiang W, Dong C, et al. Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling pathways. Toxicol In Vitro. 2012;26:1–6. doi: 10.1016/j.tiv.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Cao F, Liu T, Xu Y, Xu D, Feng S. Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-κB and JNK signaling pathways. Int J Clin Exp Pathol. 2015;8:6037–6045. [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KN, Heo SJ, Yoon WJ, et al. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 2010;649:369–375. doi: 10.1016/j.ejphar.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 36.Ki YW, Park JH, Lee JE, Shin IC, Koh HC. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol Lett. 2013;218:235–245. doi: 10.1016/j.toxlet.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 38.Langford MP, McGee DJ, Ta KH, Redens TB, Texada DE. Multiple caspases mediate acute renal cell apoptosis induced by bacterial cell wall components. Ren Fail. 2011;33:192–206. doi: 10.3109/0886022X.2011.553304. [DOI] [PubMed] [Google Scholar]
- 39.Yun N, Lee YM, Kim C, et al. Anamorsin, a novel caspase-3 substrate in neurodegeneration. J Biol Chem. 2014;289:22183–22195. doi: 10.1074/jbc.M114.552679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Li Q, Wang C, et al. Knock-down of CIAPIN1 sensitizes K562 chronic myeloid leukemia cells to Imatinib by regulation of cell cycle and apoptosis-associated members via NF-kB and ERK5 signaling pathways. Biochem Pharmacol. 2016;99:132–145. doi: 10.1016/j.bcp.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Khan S, Shin EM, Choi RJ, et al. Suppression of LPS-induced inflammatory and NF-κB responses by anomalin in RAW 264.7 macrophages. J Cell Biochem. 2011;112:2179–2188. doi: 10.1002/jcb.23137. [DOI] [PubMed] [Google Scholar]
- 43.Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–1172. doi: 10.1016/j.intimp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Stanley PL, Steiner S, Havens M, Tramposch KM. Mouse skin inflammation induced by multiple topical applications of 12-O-tetradecanoylphorbol-13-acetate. Skin Pharmacol. 1991;4:262–271. doi: 10.1159/000210960. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann A, Baltimore D. Circuity of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 46.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discovery. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem Pharmacol. 2006;72:1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Murakami A, Nakamura Y, Torikai K, et al. Inhibitory effect of citrus nobiletin on phorbol esterinduced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60:5059–5066. [PubMed] [Google Scholar]
- 49.Chung WY, Park JH, Kim MJ, et al. Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skincarcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-kappa B. Carcinogen. 2007;28:1224–1231. doi: 10.1093/carcin/bgm005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.