Abstract
Cisplatin (Cis-DDP) is one of the most widely used anti-cancer drugs. It is applicable to many types of cancer, including lung, bladder, and breast cancer. However, its use is now limited because of drug resistance. p90 ribosomal S6 kinase (p90RSK) is one of the downstream effectors in the extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) pathway and high expression of p90RSK is observed in human breast cancer tissues. Therefore, we investigated the role of p90RSK in the Cis-DDP resistance-related signaling pathway and epithelial-mesenchymal transition (EMT) in breast cancer cells. First, we discovered that MDA-MB-231 cells exhibited more Cis-DDP resistance than other breast cancer cells, including MCF-7 and BT549 cells. Cis-DDP increased p90RSK activation, whereas the inactivation of p90RSK using a small interfering RNA (siRNA) or dominant-negative kinase mutant plasmid overexpression significantly reduced Cis-DDP-induced cell proliferation and migration via the inhibition of matrix metallopeptidase (MMP)2 and MMP9 in MDA-MB-231 cells. In addition, p90RSK activation was involved in EMT via the upregulation of mRNA expression, including that of Snail, Twist, ZEB1, N-cadherin, and vimentin. We also investigated NF-κB, the upstream regulator of EMT markers, and discovered that Cis-DDP treatment led to NF-κB translocation in the nucleus as well as its promoter activity. Our results suggest that targeting p90RSK would be a good strategy to increase Cis-DDP sensitivity in triple-negative breast cancers.
Keywords: Breast cancer cells, Cell proliferation, Cisplatin, Epithelial-mesenchymal transition, p90RSK
INTRODUCTION
Triple-negative breast cancer (TNBC), estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and human epidermal growth factor receptor (HER2)-negative is a subtype of cancer that exhibits the most aggressive behaviors among breast cancer subtypes (1). The treatment of TNBC is limited because TNBC is insensitive to most hormonal and therapeutic agents. Therefore, TNBC exhibits high recurrence and metastasis with poor prognosis (2). Although various clinical trials, with treatment with cisplatin (Cis-DDP) alone and in combination with other agents have been tested in TNBC, there is still a lack of information on the effect of breast cancer therapy due to drug resistance.
Cis-DDP is an anti-cancer drug that is classified as an alkylating agent. It is widely used in the treatment of ovarian and testicular cancers with a high curative effect and other cancers including small lung, head and neck, and bladder cancers. Cis-DDP exhibits anti-cancer effects via the formation of DNA adducts on inter- and intra-strand cross-links to induce the DNA damage response, cell cycle arrest, and mitochondria-mediated apoptosis. Chemotherapy with Cis-DDP improves the recurrence-free survival rate in women with locally advanced breast cancer (3). However, the development of resistance to Cis-DDP treatment leads to therapeutic failure and tumor recurrence. Therefore, it is important to discover molecular targets to increase the effectiveness of Cis-DDP without drug resistance.
Emerging reports have demonstrated that approximately 70% of TNBC is characterized as basal-like BC. Activated mitogen-activated protein kinase (MAPK) and Akt pathways are involved in TNBCs (4, 5). Extracellular signal-related kinase (ERK)1/2 is one of the major MAPK signaling pathways involved in cancer cell proliferation, differentiation, and migration, as well as epithelial-mesenchymal transition (EMT) (5). Various therapies that inhibit MEK-ERK1/2 signaling using a MEK inhibitor (trametinib) or a MEK nucleotide analog (gemcitabine) have been tested in clinical trials with patients who failed multiple therapies (6). However, patients that are treated with MEK inhibitors showed low efficacy with a number of side effects because MEK globally regulates many downstream substrates.
The activation of p90 ribosomal protein S6 kinase (p90RSK), which is a downstream substrate of ERK1/2 signaling, is associated with tumorigenesis and invasive cancer phenotypes (7). p90RSK contains two functional kinase domains, an N-terminal kinase domain (NTKD) and a C-terminal kinase domain (CTKD) connected by a linker domain (8). Once ERK1/2 is activated by various stimuli, such as carcinogens and growth factors, it can be docked to p90RSK to activate CTKD by phosphorylation of Thr577 (9). Sequential phosphorylation of p90RSK at Thr365 and Ser386 in the linker region induces 3-phosphoinositide-dependent protein kinase-1-mediated NTKD activation that is associated with the activation of p90RSK substrates, including transcription factors, c-Fos, and Myt1 (10). These transcription factors promote cell survival via the regulation of gene transcription, protein synthesis, and the cell cycle (11, 12). However, it has not been revealed whether p90RSK activation is associated with TNBC proliferation and migration via the regulation of transcription factors and the underlying signaling pathway.
In this study, we investigated the role of p90RSK, the downstream effector of the MAPK pathway in the proliferation and migration of Cis-DDP-treated breast cancer cells. We discovered that Cis-DDP treatment increased mRNA expression and protein activation of p90RSK. During the inhibition of p90RSK kinase activation using a p90RSK specific inhibitor, FMK, or a dominant-negative kinase defective mutant, DN-RSK1 transfection significantly decreased cell viability, cell migration, and EMT via the regulation of NF-κB transcriptional activity. Our study aimed to investigate whether the regulation of p90RSK activity is a critical therapeutic target for increasing Cis-DDP sensitivity in patients with TNBC.
RESULTS
Inhibition of p90RSK decreased cell proliferation in MDA-MB-231 cells
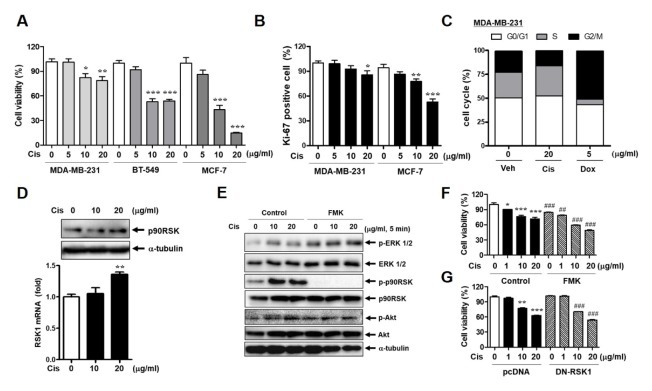
Human breast cancer cell lines MDA-MB-231, BT-549, and MCF-7 were treated with 0, 5, 10, and 20 μg/ml of Cis-DDP and cell viability was assessed by the MTT assay. The most significant Cis-DDP-mediated cytotoxicity was shown in MCF-7 cells, whereas MDA-MB-231 and BT-549 cells showed less cytotoxicity after Cis-DDP treatment than MCF-7 cells (Fig. 1A). Ki-positive cell data also showed the Cis-DDP resistance in MDA-MB 231 cytotoxicity compared with MCF-7 cells (Fig. 1B). As MDA-MB-231 cells presented the strongest Cis-DDP resistance, we determined the cell cycle changes by Cis-DDP in MDA-MB-231 compared with doxorubicin (Dox) treatment. Cis-DDP treatment did not significantly change the G1-S-G2/M cell cycle phase, whereas 5 μg/ml of Dox treatment induced G1 arrest compared with the control sample (Fig. 1C). Next, we evaluated p90RSK expression and activation in Cis-DDP-treated cells. The mRNA level of p90RSK was significantly increased by 20 μg/ml of Cis-DDP treatment (Fig. 1D). To identify the p90RSK-mediated signaling pathway associated with Cis-DDP resistance in MDA-MB-231 cells, cells were pretreated with a p90RSK specific inhibitor, FMK, for 1 h. FMK treatment only inhibited p90RSK activation, not the phosphorylation of ERK1/2, p38, and Akt (Fig. 1E), which showed the specific inhibitory role of FMK on p90RSK activation. It was also investigated whether p90RSK activation and expression in MCF-7 cells were affected by Cis-DDP treatment as in MDA-MB-231, but Cis-DDP treatment did not alter both p90RSK activation and expression in MCF-7 cells (Fig. S1A–C). When we examined the inhibitory effect of FMK on p90RSK isoforms, such as RSK1 and RSK2, only the mRNA expression of RSK1 was downregulated by FMK (Fig. S2A). Cis-DDP-induced p90RSK activation was completely blocked by FMK pretreatment (Fig. 1E). In addition, FMK treatment for 24 h resulted in down-regulation of phosphorylation and protein expression of p90RSK (Fig. S2B). FMK treatment and DN-RSK1 transfection-induced inhibition of p90RSK activation significantly sensitized cells to Cis-DDP-induced cell toxicity compared with the control, as shown in Fig. 1F and G.
Fig. 1.
Inhibition of p90RSK decreases cell proliferation in MDAMB-231 cells. (A) MDA-MB-231, BT549, and MCF-7 breast cancer cells were stimulated with Cis-DDP for 24 h and cell viability was determined by an MTT assay. (B) Cells were treated with the Cis-DDP for 36 h and cell proliferation was determined by FACS using a Ki-67 proliferation kit. (C) MDA-MB-231 cells were stimulated with 20 μg/ml of Cis-DDP or 5 μg/ml of Dox for 24 h and the cell cycle was investigated using FACS. (D) MDA-MB-231 cells were stimulated with Cis-DDP for 24 h and the protein and mRNA expression of p90RSK were determined by western blotting (upper panel) and qPCR (lower bar graph). (E) MDA-MB-231 cells were stimulated with Cis-DDP for 5 min and whole-cell lysates were subjected to western blot analysis against the indicated antibodies. (F, G) MDA-MB-231 cells were treated with 10 μM of FMK for 3 h (F) or transfected with pcDNA or DN-RSK1 for 18 h (G) followed by treatment with the indicated dose of Cis-DDP for 24 h and cell viability was determined by an MTT assay. The data are presented as means ± SEM (n = 3). *P < 0.05, **P < 0.01 or ***P < 0.001 compared with 0 sample; #P < 0.05, ##P < 0.01 or ###P < 0.001 compared with each control.
Effect of p90RSK activation on cell migration in Cis-DDP-induced MDA-MB-231 cells
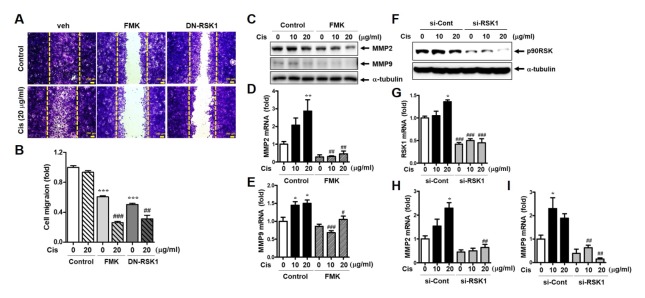
The inhibitory effect of p90RSK activation on MDA-MB-231 cell migration was demonstrated by a scratch wound-healing assay. FMK significantly decreased Cis-DDP-induced migration in MDA-MB-231 cells. Furthermore, the overexpression of dominant-negative p90RSK1 (DN-RSK1) decreased Cis-DDP induced cell migration (Fig. 2A and B). Next, we investigated the effect of protein and mRNA expression of matrix metallopeptidase (MMP)2 and MMP9 in migration behavior. Although migrated cells were less visible in cisplatin treatment for 36 hr compared to untreated sample in Fig. 2A, both protein and mRNA expression of MMP2 and MMP9 were significantly increased by Cis-DDP treatment for 12 hr (Fig. 2C–I). In addition, deactivation and depletion of p90RSK using a FMK treatment and a small interfering RNA (siRNA) completely inhibited Cis-DDP-induced mRNA levels of MMP2 and 9, respectively (Fig. 2D–I). Protein expression of p90RSK was depleted by 70% by siRNA of p90RSK compared to siRNA of control (Fig. 2F).
Fig. 2.
Effect of p90RSK activation on cell migration in Cis-DDP-induced MDA-MB-231 cells. (A) Cells were pretreated with 10 μM of FMK for 3 h or transfected with DN-RSK for 18 h followed by treatment with 20 μg/ml of Cis-DDP. After 36 h, cells were fixed, stained with crystal violet, and photographed using an Olympus microscope. The bar indicates 200 μm. (B) The migration area was measured using Image J software and indicated as fold change compared to the control (0) sample. (C–E) MDA-MB-231 cells were treated with the same conditions as above (Figure 2A) and whole protein lysates and RNA samples were subjected to western blotting (C) or qPCR (D, E) against MMP2 and MMP9. (F–I) MDA-MB-231 cells were transfected with siRNA from the control (si-Cont) or RSK1 (si-RSK1) for 48 h followed by treatment with 10 or 20 μg/ml of Cis-DDP for 24 h. (F) p90RSK protein expression was determined by western blotting. (G–I) Total RNA samples were subjected to qPCR against RSK1, MMP2, and MMP9 primers. The data are presented as means ± SEM (n = 3). *P < 0.05, **P < 0.01 or ***P < 0.001 compared with 0 sample; #P < 0.05, ##P < 0.01 or ###P < 0.001 compared with each control.
Involvement of p90RSK activation in Cis-DDP-induced EMT
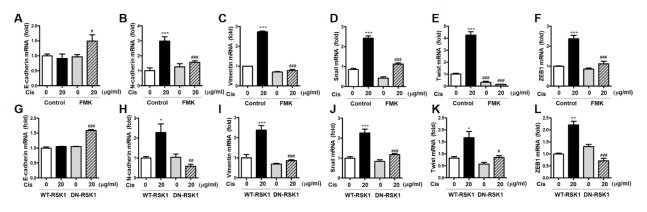
We investigated whether p90RSK regulated EMT on stimulation with Cis-DDP. As shown in Fig. 3A–F, mRNA expression of E-cadherin, a tumor repressor, was enhanced in cells treated with Cis-DDP and FMK, whereas mesenchymal markers (N-cadherin and vimentin) and EMT transcription factors (Snail, Twist, and ZEB1) were inhibited in the same condition. To demonstrate the effect of p90RSK activation on Cis-DDP-induced EMT, MDA-MB-231 cells were overexpressed with WT-RSK1 and DN-RSK1 and the mRNA levels of mesenchymal markers and EMT transcription factors were examined. Consistent with the FMK effects shown in Fig. 3A–F, DN-RSK1 overexpression, unlike WT-RSK1 overexpression, caused the suppression of EMT by the downregulation of N-cadherin, vimentin, Snail, Twist, and ZEB1 in Cis-DDP-induced cells (Fig. 3G–L).
Fig. 3.
Involvement of p90RSK activation in Cis-DDP-induced EMT. (A–F) MDA-MB-231 cells were pretreated with 10 μM of FMK for 1 h followed by treatment with 20 μg/ml of Cis-DDP for 6 h. (G–L) Cells were transfected with WT-RSK1 or DN-RSK1 plasmids for 18 h followed by treatment with 20 μg/ml of Cis-DDP for 6 h. Total RNA samples were subjected to qPCR against E-cadherin (A, G), N-cadherin (B, H), vimentin (C, I), Snail (D, J), Twist (E, K), and ZEB1 (F, L) primers. The data are presented as means ± SEM (n = 3). *P < 0.05, **P < 0.01 or ***P < 0.001 compared with 0 sample; #P < 0.05, ##P < 0.01 or ###P < 0.001 compared with each control.
Effect of p90RSK activation on NF-κB transcriptional activity
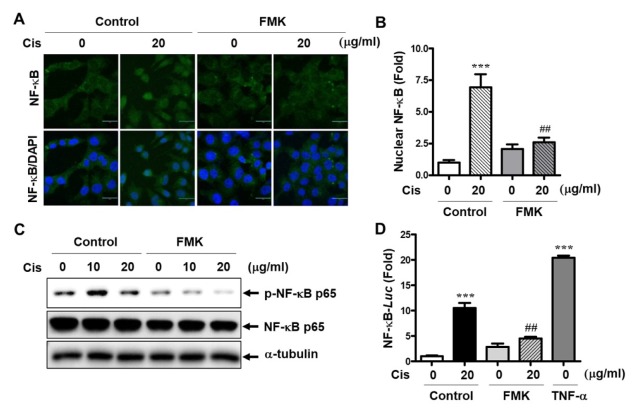
As we discovered that p90RSK activation is involved in mRNA expression of EMT genes in MDA-MB-231 cells, we examined NF-κB translocation and transcriptional activity (Fig. 4). As shown in Fig. 4A and B, Cis-DDP increased NF-κB translocation; however, FMK pretreatment significantly decreased Cis-DDP-induced NF-κB nuclear expression. In addition, Cis-DDP-induced phosphorylation of NF-κB was completely inhibited by FMK treatment (Fig. 4C). Next, we investigated the role of p90RSK activation on NF-κB promotor activity. NF-κB promotor activity was strongly increased by 20 μg/ml of Cis-DDP treatment, whereas FMK treatment blocked Cis-DDP-increased NF-κB promotor activity (Fig. 4D). TNF-α stimulation was used as a positive control.
Fig. 4.
Effect of p90RSK activation on NF-κB transcriptional activity. (A–C) MDA-MB-231 cells were pretreated with 10 μM of FMK for 1 h followed by treatment with 20 μg/ml of Cis-DDP for 1 h. (A) The p65 expression in cells was observed using a laser scanning confocal spectral microscope (Nanoscope systems). Bars indicate 30 μm. (B) Many nuclear p65 NF-κBs were indicated as fold change compared to the control (0) sample. (C) Total protein lysates were subjected to western blotting using anti-phospho or -total p65 antibodies. (D) MDA-MB-231 cells were co-transfected with the pNF-κB-luc and pRL-Renilla reporter construct. At 18 h after transfection, cells were treated with 10 μM of FMK for 1 h followed by treatment with Cis-DDP or TNF-α (positive control) for 12 h. The luciferase activities of the extracts were determined and normalized based on Renilla luciferase expression. The data are presented as means ± SEM (n = 3). ***P < 0.001 compared with 0 sample; ##P < 0.01 compared with each control.
DISCUSSION
The purpose of this study was to determine the role of p90RSK activation and its underlying signaling pathway on Cis-DDP drug resistance in breast cancer cells. We evaluated Cis-DDP resistance on cell viability in MDA-MB-231, BT-549, and MCF-7 cells and discovered that in TNBC, MDA-MB-231 exhibited the greatest resistance to cell viability after Cis-DDP treatment. Consistent with the cell viability results, the cell cycle analysis results revealed that 20 μg/ml of Cis-DDP treatment failed to arrest G0/G1, whereas 5 μg/ml of Dox, used as a positive control, strongly arrested G0/G1. We demonstrated the important role of p90RSK activation in Cis-DDP resistance in TNBC. The Cis-DDP treated sample showed both increased protein and mRNA expression of p90RSK in TNBC, MDA-MB-231 cells. This study is the first to show the involvement of p90RSK in Cis-DDP resistance in TNBC.
Recent studies have reported the role of p90RSK in cancer development and progression in various types of cancer (13, 14). p90RSK is a downstream effector of the ERK1/2 signaling pathway and leads to mammalian target of rapamycin complex 1 (mTORC1) activation that results in an increase in BRAF-mutated melanoma cell proliferation in vitro (15). p90RSK has also been proposed as an important mediator of cancer cell migration and EMT (16). Furthermore, a recent study showed a high protein expression level of p90RSK in human metastatic breast cancer tissue (7). The depletion of p90RSK induces the inhibition of CD44 (a tumor-initiating cell phenotype) expression at the cell surface (17). In agreement with previous reports, our data showed that p90RSK phosphorylation was involved in Cis-DDP resistance by inducing cell viability, migration, and EMT. Although a previous report has suggested that the phosphorylation of p90RSK is a potential predictive marker for chemotherapy resistance in ER-positive breast cancer via the Ras/Raf/ERK/p90RSK signaling pathway (18), our results showed that p90RSK expression was higher in TNBC (MDA-MB-231) cells than ER-positive BC (MCF-7) cells. In addition, we demonstrated that MDA-MB-231 cells presented more cis-DDP resistance than MCF-7 cells, with reduced levels of cell viability, proliferation, and G0/G1 arrest.
In Fig. 1E, we found that FMK treatment inhibited the phosphorylation of p90RSK at Ser380 within 5 min, but not the protein expression of p90RSK. Since we were able to see the changes in the mRNA level of RSK1 by FMK treatment for 24 h, we also tested whether FMK changed protein expression of p90RSK or not. As shown in Fig. S2B, Cis-DDP treatment led to an increase in both phosphorylation and protein expression of p90RSK. As the amount of protein expression of p90RSK increases, p90RSK phosphorylation can last for 24 hr. If ubiquitin (Ub) binds to p90RSK and FMK abolishes the Ub binding, FMK-mediated Ub modifications can be altered to p90RSK stability. Therefore, we would like to investigate whether ubiquitination could be involved in protein the stability of p90RSK in the next study.
EMT occurs due to the loss of E-cadherin via many signaling pathways, including the TGF-β signaling pathway and NF-κB signaling pathway (19). We found that p90RSK activation induced NF-κB nuclear translocation and transcriptional activity (Fig. 4). Ras-activated MAPK also promotes EMT via the Twist signaling pathway (20). An EMT transcription factor, Twist correlates with MAPK, which is one of the signaling pathways involved in the promotion of breast cancer cell invasion (21). Various transcription factors are related to EMT and cell invasion, and Slug, Snail, and Twist are transcription factors that have been reported to regulate the expression of tumor suppressor such as E-cadherin (22). Our results indicated that p90RSK activation was involved in the upregulation of mesenchymal markers, such as Snail, Twist, ZEB1, N-cadherin, and Vimentin in Cis-DDP-stimulated MDA-MB-231 cells. The overexpression of WT-RSK1 increased the number of mesenchymal markers induced by Cis-DDP, whereas the inhibition of p90RSK kinase activation reduced the mRNA level of mesenchymal markers and the increased mRNA level of E-cadherin. Since ERK1/2 increases p90RSK activation to stimulate tumorigenesis and invasive cancer phenotypes (5), ERK1/2-mediated p90RSK activation could be involve in NF-κB activation. Many EMT transcription factors including Snail, Twist, and ZEB-1 are activated when NF-κB translocates to the nucleus (23). Therefore, ERK1/2-p90RSK signaling pathway results in NF-κB transactivation-mediated target gene expression, such as Snail, Twist, and ZEB-1.
In conclusion, our study demonstrated, for the first time, that p90RSK kinase was involved in Cis-DDP-mediated cell viability, cell migration, and EMT. Cis-DDP-induced p90RSK activation regulated cell migration via MMP2 and MMP9 expression and EMT via the Snail/Twist/ZEB1 signaling pathway in MDA-MB-231 cells. The inhibition of Cis-DDP-induced p90RSK resulted in the inhibition of NF-κB nuclear translocation and suppressed NF-κB promotor activity. These discoveries reveal a new important mechanism in the research of Cis-DDP resistance in TNBC and that the regulation of p90RSK activity can be a critical therapeutic target for increasing Cis-DDP sensitivity in patients with TNBC.
MATERIALS AND METHODS
Cell culture
Human mammary carcinoma cell lines MDA-MB-231 (HTB-26TM) or MCF-7 (AHTB-22TM) and BT549 (HTB-122TM) were obtained from the American Type Culture Collection (Manassas, VA, USA).
Cell transfection
Rat RSK1 (NM031107) was mutated to K94A/K447A to create a kinase dead protein (DN-p90RSK1) with the QuickChange II site-directed mutagenesis kit (#200521, Agilent) as described previously (24).
Luciferase reporter assay
Cells were transiently co-transfected with pNF-κB-luc and p-TK-renilla reporter plasmid by the DEAE-dextran methods as described previously (25).
Real-Time Polymerase Chain Reaction assay
The quantitative RT-PCR (qRT-PCR) assay was used to analyze the mRNA expression of RSK1, RSK2, MMP2, MMP9, E-cadherin, N-cadherin, vimentin, Snail, Twist, and ZEB1 as described previously (24). The relative gene expression was calculated using a 2−Δct method, which was normalized by GAPDH. All primer sequences used in qRT-PCR experiments are listed in Supplementary Table 1.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (version 5.02, GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparison was performed. A P value < 0.05 was considered significant. All experiments were expressed as the mean ± SEM and were performed independently at least 3 times.
Supplementary Information
ACKNOWLEDGEMENTS
This research was supported by Basic Research Lab grant of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1A4A1015860).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Jung J, Jang K, Ju JM, et al. Novel cancer gene variants and gene fusions of triple-negative breast cancers (TNBCs) reveal their molecular diversity conserved in the patient-derived xenograft (PDX) model. Cancer Lett. 2018;428:127–138. doi: 10.1016/j.canlet.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Park J, Jang JH, Park GS, Chung Y, You HJ, Kim JH. BLT2, a leukotriene B4 receptor 2, as a novel prognostic biomarker of triple-negative breast cancer. BMB Rep. 2018;51:373–377. doi: 10.5483/BMBRep.2018.51.8.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma N, Muller AK, Kothari C, et al. Targeting of PYK2 Synergizes with EGFR Antagonists in Basal-like TNBC and Circumvents HER3-Associated Resistance via the NEDD4-NDRG1 Axis. Cancer Res. 2017;77:86–99. doi: 10.1158/0008-5472.CAN-16-1797. [DOI] [PubMed] [Google Scholar]
- 5.Qin H, Liu X, Li F, et al. PAD1 promotes epithelial-mesenchymal transition and metastasis in triple-negative breast cancer cells by regulating MEK1-ERK1/2-MMP2 signaling. Cancer Lett. 2017;409:30–41. doi: 10.1016/j.canlet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HB, Myung SJ. Clinical implications of the Hippo-YAP pathway in multiple cancer contexts. BMB Rep. 2018;51:119–125. doi: 10.5483/BMBRep.2018.51.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwik KA, Campbell JP, Li M, et al. Development of a RSK Inhibitor as a Novel Therapy for Triple-Negative Breast Cancer. Mol Cancer Ther. 2016;15:2598–2608. doi: 10.1158/1535-7163.MCT-16-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L, White SA, Hu K. Role of p90RSK in Kidney and Other Diseases. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20040972. pii: E972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Mackintosh C. Differential regulation of NHE1 phosphorylation and glucose uptake by inhibitors of the ERK pathway and p90RSK in 3T3-L1 adipocytes. Cell Signal. 2009;21:1984–1993. doi: 10.1016/j.cellsig.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Ikuta M, Kornienko M, Byrne N, et al. Crystal structures of the N-terminal kinase domain of human RSK1 bound to three different ligands: Implications for the design of RSK1 specific inhibitors. Protein Sci. 2007;16:2626–2635. doi: 10.1110/ps.073123707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tas I, Han J, Park SY, et al. Physciosporin suppresses the proliferation, motility and tumourigenesis of colorectal cancer cells. Phytomedicine. 2019;56:10–20. doi: 10.1016/j.phymed.2018.09.219. [DOI] [PubMed] [Google Scholar]
- 12.Melhuish TA, Kowalczyk I, Manukyan A, et al. Myt1 and Myt1l transcription factors limit proliferation in GBM cells by repressing YAP1 expression. Biochim Biophys Acta Gene Regul Mech. 20181861:983–995. doi: 10.1016/j.bbagrm.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Cassia Viu Carrara R, Fontes AM, Abraham KJ, et al. Expression differences of genes in the PI3K/AKT, WNT/b-catenin, SHH, NOTCH and MAPK signaling pathways in CD34+ hematopoietic cells obtained from chronic phase patients with chronic myeloid leukemia and from healthy controls. Clin Transl Oncol. 2018;20:542–549. doi: 10.1007/s12094-017-1751-x. [DOI] [PubMed] [Google Scholar]
- 14.Lim W, Yang C, Park S, Bazer FW, Song G. Inhibitory Effects of Quercetin on Progression of Human Choriocarcinoma Cells Are Mediated Through PI3K/AKT and MAPK Signal Transduction Cascades. J Cell Physiol. 2017;232:1428–1440. doi: 10.1002/jcp.25637. [DOI] [PubMed] [Google Scholar]
- 15.Zhao T, Li R, Tan X, et al. Simulated Microgravity Reduces Focal Adhesions and Alters Cytoskeleton and Nuclear Positioning Leading to Enhanced Apoptosis via Suppressing FAK/RhoA-Mediated mTORC1/NF-kappaB and ERK1/2 Pathways. Int J Mol Sci. 2018;19:1994. doi: 10.3390/ijms19071994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng W, Chen C, Dong M, et al. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017;8:e3147. doi: 10.1038/cddis.2017.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwano M, Shibata T, Watari K, Ono M. Oncogenic Y-box binding protein-1 as an effective therapeutic target in drug-resistant cancer. Cancer Sci. 2019;110:1536–1543. doi: 10.1111/cas.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon HG, Yi JK, Kim HS, et al. Phosphorylation of p90RSK is associated with increased response to neoadjuvant chemotherapy in ER-positive breast cancer. BMC Cancer. 2012;12:585. doi: 10.1186/1471-2407-12-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashaie MA, Chowdhury EH. Cadherins: The Superfamily Critically Involved in Breast Cancer. Curr Pharm Des. 2016;22:616–638. doi: 10.2174/138161282205160127095338. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhou BP. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin J Cancer. 2011;30:603–611. doi: 10.5732/cjc.011.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong J, Zhou J, Fu J, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71:3980–3990. doi: 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin S, Zhang C, Liu F, et al. Actinomycin V Inhibits Migration and Invasion via Suppressing Snail/Slug-Mediated Epithelial-Mesenchymal Transition Progression in Human Breast Cancer MDA-MB-231 Cells In Vitro. Mar Drugs. 2019;17:305. doi: 10.3390/md17050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harquail J, LeBlanc N, Landry C, Crapoulet N, Robichaud GA. Pax-5 Inhibits NF-kappaB Activity in Breast Cancer Cells Through IKKepsilon and miRNA-155 Effectors. J Mammary Gland Biol Neoplasia. 2018;23:177–187. doi: 10.1007/s10911-018-9404-4. [DOI] [PubMed] [Google Scholar]
- 24.Heo KS, Chang E, Takei Y, et al. Phosphorylation of protein inhibitor of activated STAT1 (PIAS1) by MAPK-activated protein kinase-2 inhibits endothelial inflammation via increasing both PIAS1 transrepression and SUMO E3 ligase activity. Arterioscler Thromb Vasc Biol. 2013;33:321–329. doi: 10.1161/ATVBAHA.112.300619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heo KS, Le NT, Cushman HJ, et al. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J Clin Invest. 2015;125:1299–1310. doi: 10.1172/JCI76453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.