Abstract
Background
Leptin is an adipokine related to overweight and cardiovascular diseases. However, the leptin expression level in epicardial adipose tissue (EAT) of humans and its association with coronary atherosclerosis has never been investigated.
Material/Methods
Patients receiving cardiac surgery were divided into a coronary artery disease group (CAD group) and a non-CAD group (NCAD group). Blood samples from coronary vein, biopsies of subcutaneous adipose tissue (SAT), and EAT were acquired during the surgery. Serum leptin level and leptin level in EAT and SAT were tested with ELISA, quantitative PCR, and immunohistochemistry and were compared between the CAD group and NCAD group, as well as between stenosis and non-stenosis subgroups. Logistic regression analysis was performed to explore the risk factors for coronary artery stenosis.
Results
No statistically significant differences were found in demographic and clinical data between groups (all P>0.05). Serum leptin concentration and leptin expression in EAT and SAT of the CAD group were much higher in than in the NCAD group (all P<0.05). In subgroup analysis, there was no difference in serum leptin and expression in SAT of stenosis and non-stenosis patients (All P>0.05). The leptin expression level in EAT of stenosis patients was significantly higher than in non-stenosis patients (P=0.0431). By multivariate logistic regression analysis, we demonstrated that leptin expression level in EAT was an independent risk factor for coronary artery stenosis [OR=1.09, 95%CI (1.01±1.18), P=0.031].
Conclusions
Leptin expression in EAT and SAT were both increased for CAD patients. Leptin expression in EAT was an independent risk factor for coronary atherosclerosis in the adjacent artery, while leptin in SAT was not associated.
MeSH Keywords: Adipose Tissue, Atherosclerosis, Coronary Artery Disease, Leptin, Risk Factors
Background
Coronary artery disease (CAD) has become the leading cause of death worldwide, in which the fundamental pathophysiological change is atherosclerosis [1]. Despite numerous studies performed over decades, the precise mechanism underlying coronary atherosclerosis remains elusive. Recent years have witnessed the emerging recognition of the promising role of epicardial adipose tissue (EAT) in the onset, development, and prognosis of CAD [2]. EAT, previously thought to be merely a structural fat protecting the heart, is considered as an active organ with crosstalk with the myocardium and coronary artery, which are connected directly without any fascia [3]. One of the studied relationships between EAT and CAD is the paracrine function in secreting cytokines or adipokines, including adiponectin [4], omentin-1 [5], chemerin [6], and others [7].
Produced in white adipose tissue, leptin is a plasmatic peptide hormone involved in cardiovascular homeostasis and atherosclerosis [8]. It was demonstrated that circulating leptin or serum leptin can independently predict acute cardiovascular events, restenosis after percutaneous coronary intervention, and cerebral stroke [9]. However, the expression of leptin in EAT and its relationship with CAD has seldom been investigated. In this study, we examined leptin expression in serum, subcutaneous adipose tissue (SAT), and EAT of CAD patients and control patients, then analyzed its relationship with coronary stenosis.
Material and Methods
Subjects
From March 2016 to October 2018 in the Department of Cardiovascular Diseases, Xi’an No. 1 Hospital, a total of 38 patients receiving coronary artery bypass grafts (CABG) and 40 patients receiving valve operation and ASD repair were recruited in this study. All patients enrolled met the following criteria. We included patients diagnosed with CAD and prepared to receive CABG and those diagnosed with valve disease or atrium septal defect (ASD). We excluded patients with CAD on coronary angiography and prepared to receive a valve operation or ASD repair, patients over 80 years old, and patients with severe organ dysfunction, metabolism disorder, infectious disease, or tumors. According to their primary disease, enrolled patients were assigned to the CAD group or the NCAD group. Within the CAD group, we divided patients into the stenosis group or the non-stenosis group depending on whether there was local coronary stenosis near the right coronary artery ostium.
We collected demographic data, laboratory results, and clinical data from the electronic record system of the hospital. The Committee on Biomedical Ethics of Xi’an No. 1 Hospital approved this study, which conformed to the Declaration of Helsinki. Signed informed consent was were also acquired from all patients enrolled in this study.
Definition
In the present study, CAD was defined as a stenosis diameter of ≥50% in the left main coronary artery (LMCA) and ≥70% in vessels other than the LMCA (10), while local coronary stenosis near the right coronary artery ostium was defined as diameter stenosis of ≥70% (5). Type 2 diabetes mellitus (T2DM) diagnosis was made based on the American Diabetes Association criteria (11).
Sample acquisition
We collected venous blood samples from all patients in sodium heparin Vacutainers (Becton-Dickinson) at admission for routine laboratory tests. Blood samples were centrifuged (15 min, 3000×g) to obtain serum, which was then stored at −80°C. The blood samples were acquired before breakfast after fasting for more than 12 h.
Different types of adipose tissue were acquired during the surgery at different time points. As soon as the skin was incised, the SAT sample (average 0.5 g each) was acquired at the chest incision. Before the cardiopulmonary bypass, EAT was acquired near the right coronary artery ostium. The 2 portions of each biopsy sample were handled differently – the part used for RNA isolation was stored at −80°C and the part used for immunohistochemistry was kept in neutralized formalin. After the heart was opened, the blood sample from the coronary vein was acquired and handled as described above, which was used for leptin examination.
Serum leptin test
Human leptin ELISA kits (ab179884, Abcam, USA) were used to examine the serum leptin level of all patients, following the manufacturer’s instructions. The intra-assay and inter-assay coefficients of variation were both <5%. We measured all samples twice.
RNA expression measurement
As previously described, we measured the RNA expression level with quantitative real-time PCR (5). The primer sequences were forward 5′-CCTGTGCGGATTCTTGTGG-3′, reverse 5′-GGTGACTTTCTGTTTGGAGGA-3′ We calculated the relative gene expression via the threshold cycle value (CT) and the formula 2-CT.
Histology
Immunohistochemistry for all adipose samples was conducted according to a previously described method [6]. Primary antibody of leptin was purchased from Abcam (ab3583, 1: 200 dilution, Abcam, USA). With Image J software, we calculated the integrated optical density (IOD) of each section from 8 different 400×magnified fields.
Statistical analysis
We used IBM SPSS Statistics, version 19.0 (SPSS, Inc, Armonk, NY) for statistical analysis. We conducted the normality distribution test of the variables at first to check the distribution condition. Normally distributed continuous variables are presented as mean±standard deviations, and continuous variables that were not normally distributed are expressed as median (lower quartile, upper quartile). Categorical variables are presented as proportions.
We used the independent samples t test for comparison of normally distributed continuous variables, while the Mann-Whitney test was used for the statistical analysis of continuous variables that were not normally distributed. The chi-square test was performed in different evaluations of categorical variables. Multivariate logistic regression was used to analyze the risk factors for local artery stenosis. Statistical significance was confirmed when the p-value was less than 0.05.
Results
Patient characteristics
Demographic data, laboratory results, and clinical data of the CAD group and NCAD group are shown in Table 1. For demographic data, there was no statistical difference between the 2 groups in age, sex, BMI, or smoking status (All P>0.05). Cardiac function (NYHA grade, LVEF) and comorbidities were similar between the 2 groups (all P>0.05). Among the laboratory test results, the BNP of the NCAD group was higher than in the CAD group, but the difference was not statistically significant (P=0.076). Other indicators were also similar between 2 groups, including FBG, HbAlc, hs-CRP, cTnI, serum creatinine, triglycerides, total cholesterol, HDL-C, and LDL-C (all P>0.05). We also compared medication use between the 2 groups, which showed that the CAD group had more use of aspirin and nitrates (both P<0.001), while there was no significant difference in usage of statins, ACEI/ARB, or β-blockers (all P>0.05).
Table 1.
Baseline characteristics and clinical data of CAD group and NCAD group.
| Variables | CAD group (n=38) | NCAD group (n=40) | χ2/t | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 63.5±5.8 | 61.1±7.7 | 1.531 | 0.130 |
| Sex (% male) | 24 (63.2%) | 20 (50.0%) | 1.372 | 0.241 |
| BMI (kg/m2) | 24.2±2.6 | 23.6±4.3 | 0.688 | 0.493 |
| Smoking | 0.535 | 0.765 | ||
| Non-smoker | 26 (68.4%) | 29 (72.5%) | ||
| Current smoker | 10 (26.3%) | 8 (20.0%) | ||
| Former smoker | 2 (5.3%) | 3 (7.5%) | ||
| NYHA functional class | 7.373 | 0.061 | ||
| I | 8 (21.1%) | 7 (17.5%) | ||
| II | 18 (47.4%) | 10 (25.0%) | ||
| III | 7 (18.4%) | 8 (20.0%) | ||
| IV | 5 (13.2%) | 15 (37.5%) | ||
| LVEF (%) | 54.3±6.5 | 52.3±6.2 | 1.354 | 0.180 |
| Comorbidities | ||||
| Hypertension | 7 (18.4%) | 5 (12.5%) | 0.525 | 0.541 |
| T2DM | 17 (44.7%) | 11 (27.5%) | 2.516 | 0.113 |
| Stroke | 2 (5.3%) | 4 (10.0%) | 0.616 | 0.433 |
| COPD | 4 (10.5%) | 4 (10.0%) | 0.006 | 0.939 |
| Dyslipidemia | 18 (47.4%) | 12 (30.0%) | 2.484 | 0.115 |
| Laboratory examination | ||||
| FBG (mmol/L) | 6.18±2.15 | 5.81±1.63 | 0.845 | 0.401 |
| HbAlc (%) | 5.55±1.32 | 5.68±1.23 | 0.425 | 0.672 |
| hs-CRP (mg/L) | 1.96±1.15 | 2.05±1.2 | 0.326 | 0.745 |
| BNP (pg/ml) | 177.24±168.46 | 254±205.36 | 1.800 | 0.076 |
| cTnI (μg/L) | 0.05±0.03 | 0.06±0.03 | 0.864 | 0.390 |
| Serum creatinine (μmol/L) | 75.89±25.67 | 72.95±25.82 | 0.505 | 0.615 |
| Triglycerides (mmol/L) | 1.55±0.24 | 1.44±0.36 | 1.579 | 0.118 |
| Total cholesterol (mmol/L) | 4.45±1.21 | 4.55±1.36 | 0.3424 | 0.733 |
| HDL-C (mmol/L) | 0.94±0.21 | 1.05±0.32 | 1.785 | 0.078 |
| LDL-C (mmol/L) | 3.19±1.04 | 3.01±1.21 | 0.703 | 0.484 |
| Medications | ||||
| Aspirin (%) | 20 (52.6%) | 1 (2.5%) | 24.892 | <0.001 |
| Statins (%) | 14 (36.8%) | 8 (20.0%) | 2.730 | 0.099 |
| Nitrates (%) | 35 (92.1%) | 4 (10.0%) | 52.547 | <0.001 |
| ACEI/ARB (%) | 7 (18.4%) | 5 (12.5%) | 0.525 | 0.469 |
| β-blockers (%) | 5 (13.2%) | 3 (7.5%) | 0.556 | 0.456 |
| Major heart disease | NA | NA | ||
| CAD | 38 | 0 | ||
| Valve disease | 0 | 21 | ||
| Congenital heart disease | 0 | 19 | ||
BMI – body mass index; NYHA – New York Heart Association; LVEF – left ventricular ejection fraction; T2DM – type 2 diabetes mellitus; COPD – chronic obstructive pulmonary disease; FBG – fasting blood glucose; HbAlc – glycated hemoglobin; hs-CRP – hypersensitive C-reactive protein; BNP – brain natriuretic peptide; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; ACEI/ARB – angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers; CAD – coronary artery disease.
Table 2 shows the same characteristics of subgroups of CAD patients, who were divided into a stenosis group and a non-stenosis group depending on the stenosis status of the coronary artery near the right coronary artery ostium, where EAT samples were collected. Comparisons of demographic data showed that stenosis patients were significantly older (P=0.003) and more likely to be obese (P=0.002), while there was no difference in sex or smoking status (all P>0.05). As in between-group analysis, there was also no significant difference between stenosis patients and non-stenosis patients in cardiac function, comorbidities, laboratory test results, or medications (all P>0.05).
Table 2.
Baseline characteristics and clinical data of stenosis and non-stenosis patients.
| Variables | Stenosis (n=20) | Non-stenosis (n=18) | χ2/t | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 66.1±4.4 | 60.7±6.0 | 3.191 | 0.003 |
| Sex (%male) | 12 (60.0%) | 12 (66.7%) | 0.181 | 0.671 |
| BMI (kg/m2) | 25.4±1.8 | 22.8±2.8 | 3.311 | 0.002 |
| Smoking | 8 (40.0%) | 4 (22.2%) | 1.386 | 0.239 |
| NYHA functional class | 1.631 | 0.652 | ||
| I | 3 (15.0%) | 5 (27.8%) | ||
| II | 11 (55.0%) | 7 (38.9%) | ||
| III | 4 (20.0%) | 3 (16.7%) | ||
| IV | 2 (10.0%) | 3 (16.7%) | ||
| LVEF (%) | 55.4±6.9 | 53.0±5.9 | 1.147 | 0.259 |
| Comorbidities | ||||
| Hypertension | 3 (15.0%) | 4 (22.2%) | 0.329 | 0.566 |
| T2DM | 11 (55.0%) | 6 (33.3%) | 1.799 | 0.180 |
| Stroke | 0 (0%) | 2 (11.1%) | 2.346 | 0.126 |
| COPD | 3 (15.0%) | 1 (5.6%) | 0.897 | 0.344 |
| Dyslipidemia | 10 (47.4%) | 8 (30.0%) | 0.117 | 0.732 |
| Laboratory examination | ||||
| FBG (mmol/L) | 6.63±2.08 | 5.68±2.17 | 1.374 | 0.178 |
| HbAlc (%) | 5.85±1.26 | 5.23±1.34 | 1.465 | 0.151 |
| hs-CRP (mg/L) | 1.92±1.10 | 2.01±1.24 | 0.248 | 0.806 |
| BNP (pg/ml) | 154.55±125.08 | 202.44±207.37 | 0.872 | 0.389 |
| cTnI (μg/L) | 0.05±0.03 | 0.05±0.03 | 0.722 | 0.475 |
| Serum creatinine (μmol/L) | 74.75±24.49 | 77.17±27.59 | 0.286 | 0.776 |
| Triglycerides (mmol/L) | 1.45±0.22 | 1.60±0.30 | 1.769 | 0.085 |
| Total cholesterol (mmol/L) | 4.58±1.29 | 4.31±1.04 | 0.705 | 0.485 |
| HDL-C (mmol/L) | 0.88±0.26 | 1.01±0.11 | 1.966 | 0.057 |
| LDL-C (mmol/L) | 3.25±1.15 | 3.02±0.87 | 0.689 | 0.495 |
| Medications | ||||
| Aspirin (%) | 10 (50.0%) | 10 (55.6%) | 0.117 | 0.732 |
| Statins (%) | 8 (40.0%) | 6 (33.3%) | 0.181 | 0.671 |
| Nitrates (%) | 20 (100%) | 15 (83.3%) | 3.619 | 0.057 |
| ACEI/ARB (%) | 3 (15.0%) | 4 (22.2%) | 0.329 | 0.566 |
| β-blockers (%) | 3 (15.0%) | 2 (11.1%) | 0.125 | 0.723 |
| Coronary atherosclerosis with stenosis >50% | 3.541 | 0.170 | ||
| One vessel | 4 (20.0%) | 4 (22.2%) | ||
| Two vessels | 14 (70.0%) | 8 (44.4%) | ||
| Three vessels | 2 (10.0%) | 6 (33.3%) | ||
BMI – body mass index; NYHA – New York Heart Association; LVEF – left ventricular ejection fraction; T2DM – type 2 diabetes mellitus; COPD – chronic obstructive pulmonary disease; FBG – fasting blood glucose; HbAlc – glycated hemoglobin; hs-CRP – hypersensitive C-reactive protein; BNP – brain natriuretic peptide; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; ACEI/ARB – angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers; CAD – coronary artery disease.
Serum leptin level
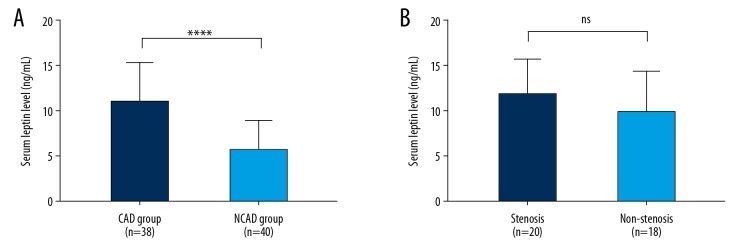
Serum leptin level was examined by ELISA, and the results are shown in Figure 1. The mean serum leptin level in the CAD group was 10.92±4.32 ng/mL, while the that of the NCAD group was 5.64±3.30 ng/mL, which was much lower than in the CAD group (P<0.001). In subgroup analysis, no statistically significant difference was found in serum leptin level between stenosis patients and non-stenosis patients [(11.93±3.84) vs. (9.80±4.64) ng/mL, P=0.136].
Figure 1.
Serum leptin level. (A) Serum leptin level of CAD and NCAD groups; (B) Serum leptin level of stenosis and non-stenosis patients. ** P<0.001; ns – not significant.
Leptin expression in adipose tissue
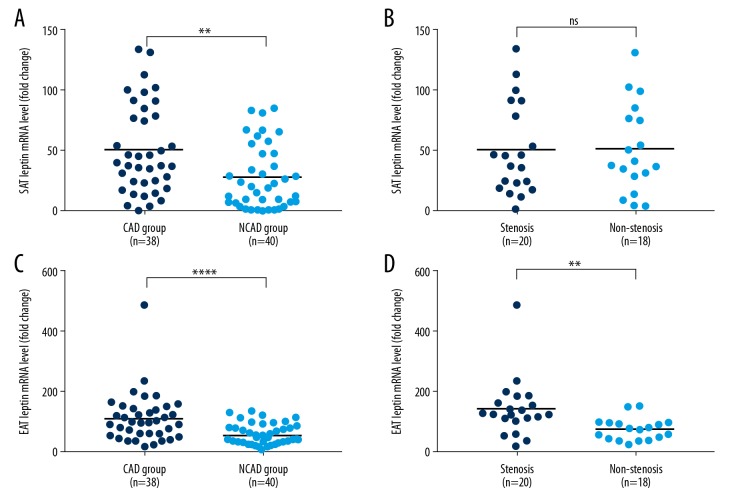
We used qPCR to test the mRNA expression level and used immunohistochemistry to examine the protein expression level of leptin in SAT and EAT, which is shown in Figures 2–4. SAT leptin mRNA level was significantly higher in the CAD group than in the NCAD group [38.8 (22.1, 80.2) vs. 19.5 (6.9, 48.1), P=0.002], as shown in Figure 2A. However, there was no statistically significant difference between stenosis patients and non-stenosis patients [41.3 (19.9, 87.9) vs. 38.7 (24.5, 78.6), P=0.983], as shown in Figure 2B. For EAT leptin mRNA level, there was a significant difference between the CAD group and the NCAD group [98.5 (57.1, 145.1) vs. 43.3 (32.4, 79.7), P<0.001] and between stenosis and non-stenosis patients [126.1 (103.6, 181.2) vs. 75.3 (43.9, 97.0), P=0.002], as shown in Figure 2C and 2D.
Figure 2.
Leptin mRNA expression level. (A) SAT mRNA expression level of CAD and NCAD groups; (B) SAT mRNA expression level of stenosis and non-stenosis patients; (C) EAT mRNA expression level of CAD and NCAD groups; (D) EAT mRNA expression level of stenosis and non-stenosis patients. **** P<0.001; ** P=0.002; ns – not significant.
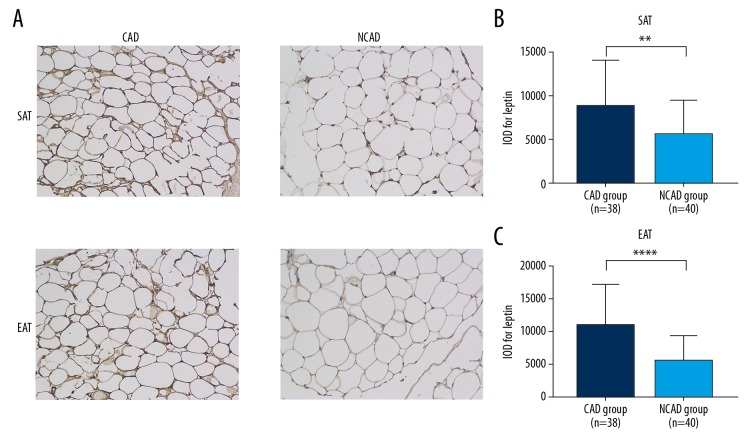
Figure 3.
Immunohistochemistry results of CAD and NCAD groups. (A) representative sections; (B) IOD value of leptin in SATs; (C) IOD value of leptin in EAT. **** P<0.001; ** P=0.002; ns – not significant.
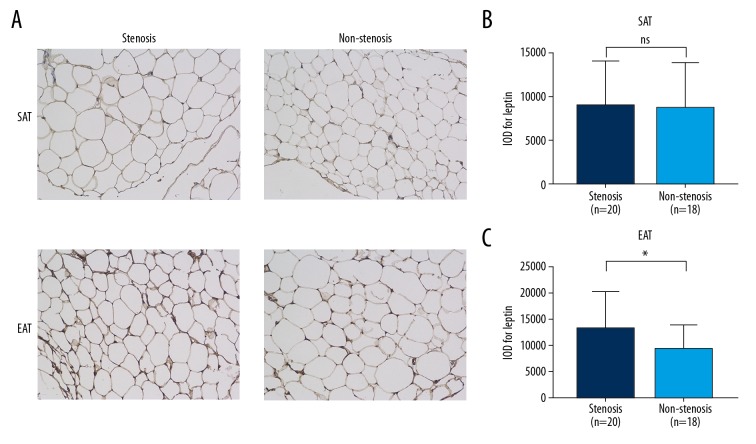
Figure 4.
Immunohistochemistry results of stenosis and non-stenosis patients. (A) representative sections; (B) IOD value of leptin in SAT; (C) IOD value of leptin in EAT. * P=0.043; ns – not significant.
Immunohistochemistry presented similar trends as qPCR results. Figure 3 shows representative sections of the SAT and EAT of CAD and NCAD groups, which demonstrates that the IOD values of the CAD group were significantly higher than in the NCAD group in both SAT [(8793±5158) vs. (5557±3803), P=0.002] and EAT [(11035±6136) vs. (5683±3737), P<0.001]. The SAT and EAT sections of stenosis and non-stenosis patients are shown in Figure 4. There was no difference between stenosis and non-stenosis patients in IOD value for leptin of SAT [(8984±5245) vs. (8621±5209), P=0.832]. However, the IOD value for leptin of EAT was significantly higher in stenosis patients than in non-stenosis patients [(13141±6990) vs. (9140±4649), P=0.043].
Multivariate logistic regression analysis
In Table 2, univariate linear analysis was conducted to find the potential risk factors for coronary stenosis, showing that only age and BMI were significant (P<0.05), with sex, LVED, serum leptin, SAT leptin mRNA expression, and EAT leptin mRNA expression included in the final regression model. As demonstrated in Table 3, we found that age [OR 1.88 (1.02±3.47), P=0.042] and EAT leptin mRNA expression [OR 1.09 (1.01±1.18), P=0.031] were independent risk factors for local coronary artery stenosis.
Table 3.
Multivariate logistic regression analysis of risk factors for coronary stenosis.
| Variables | B | S.E. | Wald | Exp(B) | P |
|---|---|---|---|---|---|
| Age | 0.633 | 0.311 | 4.134 | 1.88 (1.02±3.47) | 0.042 |
| Gender* | −5.299 | 2.758 | 3.691 | 0.005 (0±1.11) | 0.055 |
| BMI | 1.208 | 0.665 | 3.303 | 3.35 (0.91±12.32) | 0.069 |
| LVEF | −0.153 | 0.139 | 1.213 | 0.86 (0.65±1.13) | 0.271 |
| Serum leptin | 0.252 | 0.246 | 1.051 | 1.29 (0.79±2.08) | 0.305 |
| SAT leptin mRNA expression | −0.064 | 0.035 | 3.335 | 0.94 (0.88±1) | 0.068 |
| EAT leptin mRNA expression | 0.086 | 0.040 | 4.647 | 1.09 (1.01±1.18) | 0.031 |
| Constant | −65.205 | 33.317 | 3.830 | NA | NA |
Gender: male to female
Discussion
By examining the leptin expression in serum, SAT, and EAT of CAD patients, as well as controls, we found that: (1) Compared with controls, leptin expression levels were increased systematically in serum, SAT, and EAT; (2) CAD patients with stenosis near the right coronary artery ostium, where the EAT samples were collected, had even higher leptin expression levels; and (3) The leptin expression level in EAT was an independent risk factor for local coronary stenosis.
Leptin is a critical cytokine and biomarker for obesity, T2DM, chronic kidney disease, and cardiovascular disorders [12]. The IARS cohort results showed that high leptin level was associated with obesity-related metabolic disturbances and incident CAD in Asian Indians [13]. Khafaji et al. examined the links between serial serum leptin level and the degree of coronary atherosclerosis, coronary reperfusion, echocardiographic findings, and clinical outcome in patients with acute myocardial infarction (AMI) receiving thrombolysis [14]. They found that serum leptin levels significantly increased after AMI, and serum leptin level can indicate the left ventricular ejection fraction and the degree of atherosclerosis but not the degree of coronary reperfusion [14]. However, not all studies confirmed positive results. Although higher serum leptin levels have been associated with a modestly higher incidence of cardiovascular disease, a prospective cohort study in the USA found that leptin levels are not associated with incident cardiovascular events [15]. Previous studies focusing on the underlying mechanism of leptin indicates that it exerts various atherogenic effects such as increasing endothelial dysfunction; facilitation of inflammatory reaction; induction of oxidative stress; mitigation of paraoxonase activity; platelet aggregation, migration, and hypertrophy; and proliferation of vascular smooth muscle cells [12]. They also found that leptin-deficient and leptin receptor-deficient mice are protected from arterial thrombosis and neointimal hyperplasia in response to arterial wall injury [9]. In the present study, we also demonstrated that serum leptin level was elevated in CAD patients and the leptin expression levels in SAT and EAT were also clearly increased.
Leptin is produced by adipocytes and is usually considered to be a circulating hormone [16]. It is now believed that adipose tissue acts as an endocrine organ, which secrets many active cytokines and adipokines and participates in the pathogenesis of metabolism and cardiovascular disorders [17]. EAT, also known as pericardial adipose tissue, or epicardial fat, has received attention due to its significant relationship with several cardiovascular diseases, including atrial fibrillation [18], heart failure [19], and atherosclerosis [20]. We compared leptin expression levels in serum, SAT, and EAT in CAD patients with controls, showing that all 3 indicators were significantly higher in CAD patients, which also validated previous studies and further confirmed that EAT and is a reservoir for adipokines. Gruzdeva also explored the leptin expression level in EAT and found that it is much more highly expressed in CAD patients [21]. Considering the paracrine effects of EAT reported before [22], we decided to conduct a subgroup analysis between patients with stenosis and those without, which demonstrated that patients with stenosis had higher leptin expression levels. Further logistic regression analysis also proved that leptin expression level was an independent risk factor for local stenosis, but there was no significant difference in SAT or serum leptin. In AF patients, Qing et al. demonstrated that leptin was less expressed in EAT compared with paracardial adipose tissue [23], but they did not assess the leptin levels of CAD patients.
We must note several limitations of this study. First, all patients included in this study were complicated with heart disease, especially in the NCAD group, which might have led to systematic errors. However, these errors cannot be eliminated because of the ethical considerations in acquiring EAT samples. Second, all patients were enrolled non-randomly. Although no statistically significant difference was found, it still might have affected our conclusions. Last, the sample size and investigating methods also limit the findings of the study.
Conclusions
Leptin expression levels in EAT and SAT were both higher in CAD patients. Leptin expression in EAT, but not in SAT, was an independent risk factor for coronary atherosclerosis in the adjacent artery.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Boudoulas KD, Triposciadis F, Geleris P, Boudoulas H. Coronary atherosclerosis: Pathophysiologic basis for diagnosis and management. Prog Cardiovasc Dis. 2016;58:676–92. doi: 10.1016/j.pcad.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Mancio J, Oikonomou EK, Antoniades C. Perivascular adipose tissue and coronary atherosclerosis. Heart. 2018;104:1654–62. doi: 10.1136/heartjnl-2017-312324. [DOI] [PubMed] [Google Scholar]
- 3.Lazaros G, Antonopoulos A, Antoniades C, Tousoulis D. The role of epicardial fat in pericardial diseases. Curr Cardiol Rep. 2018;20:40. doi: 10.1007/s11886-018-0986-7. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima T, Yokota T. Impaired mitochondrial oxidative phosphorylation capacity in epicardial adipose tissue is associated with decreased concentration of adiponectin and severity of coronary atherosclerosis. Sci Rep. 2019;9:3535. doi: 10.1038/s41598-019-40419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Ji Q, Cai L, et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc Diabetol. 2016;15:90. doi: 10.1186/s12933-016-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Mi S, Zhang F, et al. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawicka M, Janowska J, Chudek J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int J Cardiol. 2016;222:581–89. doi: 10.1016/j.ijcard.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Jerez-Valero M, Meliveo-Garcia A, Jordan-Martinez L, et al. [Role of serum leptin in the severity of coronary artery disease in patients with stable angina]. Med Clin. 2016;147:7–12. doi: 10.1016/j.medcli.2016.03.040. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 9.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Verma B, Katyal D, Patel A, et al. Relation of systolic and diastolic epicardial adipose tissue thickness with presence and severity of coronary artery disease (The EAT CAD study) J Family Med Prim Care. 2019;8:1470–75. doi: 10.4103/jfmpc.jfmpc_194_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes. 2017;9:320–24. doi: 10.1111/1753-0407.12524. [DOI] [PubMed] [Google Scholar]
- 12.Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin. 2018;39:1176–88. doi: 10.1038/aps.2018.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanker J, Rao VS, Ravindran V, et al. Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost. 2012;108:769–80. doi: 10.1160/TH12-04-0263. [DOI] [PubMed] [Google Scholar]
- 14.Khafaji HA, Bener AB, Rizk NM, Al Suwaidi J. Elevated serum leptin levels in patients with acute myocardial infarction; Correlation with coronary angiographic and echocardiographic findings. BMC Res Notes. 2012;5:262. doi: 10.1186/1756-0500-5-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin SS, Blaha MJ, Muse ED, et al. Leptin and incident cardiovascular disease: The Multi-ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239:67–72. doi: 10.1016/j.atherosclerosis.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23:770–84. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawicka M, Janowska J, Chudek J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int J Cardiol. 2016;222:581–89. doi: 10.1016/j.ijcard.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 18.Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017;38:1294–302. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 19.Berg G, Miksztowicz V, Morales C, Barchuk M. Epicardial adipose tissue in cardiovascular disease. Adv Exp Med Biol. 2019;1127:131–43. doi: 10.1007/978-3-030-11488-6_9. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Sata M. Roles of perivascular adipose tissue in the pathogenesis of atherosclerosis. Front Physiol. 2018;9:3. doi: 10.3389/fphys.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruzdeva O, Uchasova E, Dyleva Y, et al. Relationship between epicardial and perivascular fatty tissue and adipokine-cytokine level in coronary artery disease patients. PLoS One. 2019;14:e0208156. doi: 10.1371/journal.pone.0208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Trasancos Á, Guerola-Segura R, Paradela-Dobarro B, et al. Glucose and Inflammatory Cells Decrease Adiponectin in Epicardial Adipose Tissue Cells: Paracrine Consequences on Vascular Endothelium. J Cell Physiol. 2016;231:1015–23. doi: 10.1002/jcp.25189. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Xi W, Yin L, et al. Human epicardial adipose tissue cTGF expression is an independent risk factor for atrial fibrillation and highly associated with atrial fibrosis. Sci Rep. 2018;8:3585. doi: 10.1038/s41598-018-21911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]