Abstract
Purpose
This report presents a procedure for performing power Doppler ultrasound-guided sialography using the phenomenon of increased blood flow and illustrates its application to practical patient cases.
Materials and Methods
The salivary gland was scanned using ultrasound equipment (GE LOGIQ5 Expert® device; GE Medical Systems, Milwaukee, WI, USA) to identify pathological findings related to the patient's chief complaint. To identify the orifice of the main duct, it should be cannulated using a lacrimal dilator. After inserting the catheter into the cannulated main duct, the position of the catheter within the duct was confirmed by ultrasound. A contrast agent was injected until the patient felt fullness, and ultrasound (B-mode) was used to confirm whether the contrast agent filled the main canal and secondary and tertiary ducts. Then, power Doppler ultrasound was performed to determine whether the salivary gland had increased blood flow.
Results
In 2 cases in this report, a power Doppler ultrasound scan showed a significant increase in blood flow after contrast medium injection, which was not observed on a preoperative scan.
Conclusion
Power Doppler ultrasound was found to be a simple, safe, and effective tool for real-time sialography monitoring.
Keywords: Sialography, Ultrasonography, Vasodilation
Introduction
Sialography is a traditional technique used to diagnose pathological conditions of the salivary glands. Although marked improvements and changes in major salivary non-neoplastic diseases can be seen on computed tomography (CT) and magnetic resonance imaging (MRI),1 sialography is the most accurate technique for describing anatomical structures and pathological changes within the ductal system. Additionally, it is advantageous compared to CT because of a smaller radiation dose, and it is more accessible than MRI.2 Sialography is mainly used to diagnose obstructive diseases such as sialolithiasis, Sjögren syndrome, and duct stenosis, which are accompanied by changes in the ductal system due to inflammation.3 These diseases show similar clinical symptoms, but each has characteristic imaging findings on sialography, making this technique very useful for differential diagnosis.
Sialography is an invasive procedure in which a radiopaque contrast material is injected retrogradely into the gland's ductal system via the intraoral orifice of the Wharton or Stensen duct. It involves a number of sophisticated steps prior to conventional radiography. Technical errors such as extravasation during these complex steps can lead to complications or low-quality sialography.4 Our previous study introduced a new sialography technique that utilized ultrasound (US) to reduce the risk of complications such as ductal perforation or contrast medium leakage and demonstrated that this technique could be successfully used as a diagnostic aid in patients with symptomatic salivary glands.5 In particular, by observing the sialography procedure directly or indirectly through US images, it was possible to overcome the limitations of conventional sialography, which relies only on a fluoroscopic instrument.
Conventional Doppler US of vessels measures the speed and direction of moving blood cells, based on the principle that the faster their speed, the greater the Doppler shift, measured in hertz. It is difficult to express the speed, direction, and intensity of the Doppler signal using color alone, so only speed and direction are presented. However, power Doppler US displays only the intensity of the Doppler signal in color, not the other parameters of speed and direction. All moving blood cells produce color signals, regardless of the direction or speed of movement. The more cells in motion there are, the stronger the color signal, expressed in white.6
When fluid is injected into the catheter during US-guided sialography, vasodilation and increased blood flow in the salivary gland can be detected on power Doppler US images. This ability to indirectly visualize the filling of fluid into the parenchyma of the salivary gland with power Doppler US, based on the phenomenon of increased blood flow during sialography, enables the presence of the contrast medium within the salivary gland to be confirmed. This report describes our method of performing power Doppler US-guided sialography using the phenomenon of increased blood flow and discusses the diagnostic efficacy and usefulness of this technique.
Materials and Methods
Power Doppler US-guided sialography was performed according to the following sequence of steps. Before the procedure, the salivary gland associated with the patient's chief complaint was scanned using a GE LOGIQ5 Expert® device (GE Medical Systems, Milwaukee, WI, USA) with a 12-MHz linear probe. The area was examined in the vertical and horizontal directions in B-mode and power Doppler mode to evaluate the status of the duct and parenchyma.
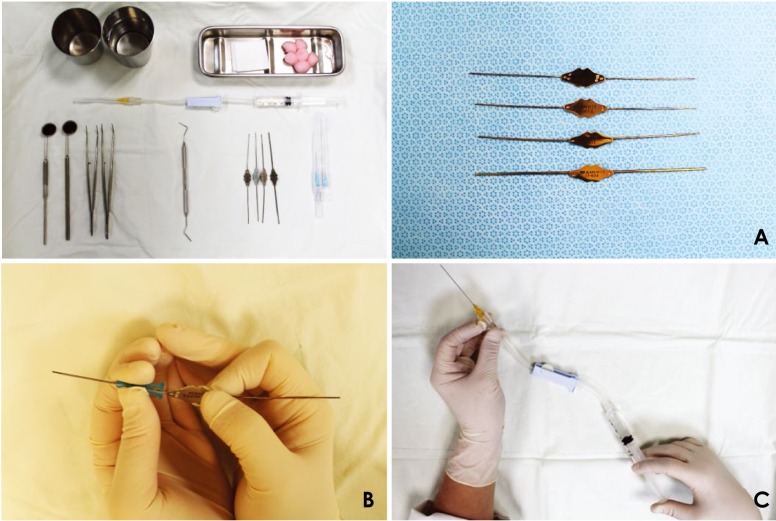
The first step in the sialography process was to check the orifice of the parotid or submandibular gland. After the mucous membranes were dried with gauze, the parotid or submandibular gland was milked using a secretagogue such as lemon drops, and the orifice of the Stensen or Wharton duct was checked with a periodontal probe. Next, the main duct was cannulated with a lacrimal dilator (Bowman lacrimal probe®, #0.000–#0.0; Karl Storz GmbH & Co. KG, Tuttlingen, Germany) (Fig. 1A). The plastic section of an intravenous catheter (20 G, Sewoon Medical Co., LTD., Cheonan, Korea) was inserted into the main duct that had been cannulated with a lacrimal dilator (Fig. 1B). Proper catheter insertion into the main duct was confirmed with US imaging through the presence of metallic posterior acoustic shadows. Non-ionic, water-soluble contrast medium (Iopamiro 370®, 0.5–2 mL; Ilsung Co., Seoul, Korea) was injected via a universal cannula (25 G, 50 mm; Nanum Company, Daegu, Korea) connected to a 5-mL syringe until patients experienced a feeling of fullness in the salivary gland (Fig. 1C).
Fig. 1. Instruments used for power Doppler ultrasound-guided sialography. A. Lacrimal dilators (Bowman lacrimal probe®, #0.000 – #0.0). B. The plastic section of an intravenous catheter (20 G) with a lacrimal dilator. C. Universal cannula connected to a 5-mL syringe and intravenous infusion set.
A series of US images (B-mode) was obtained in real time to confirm pooling of the contrast agent in the primary, secondary, and tertiary ducts. Furthermore, the salivary glands were scanned in the power Doppler mode of US to confirm vasodilation and increased blood flow in the salivary glands after contrast medium filling.
Results
Patient 1
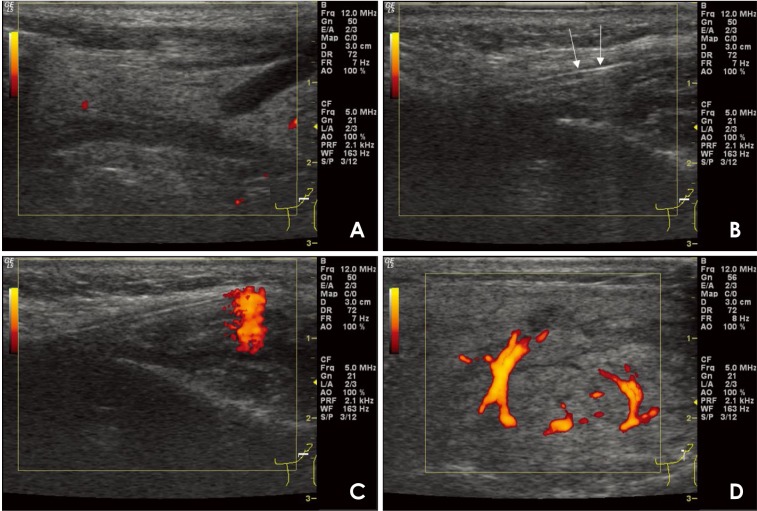
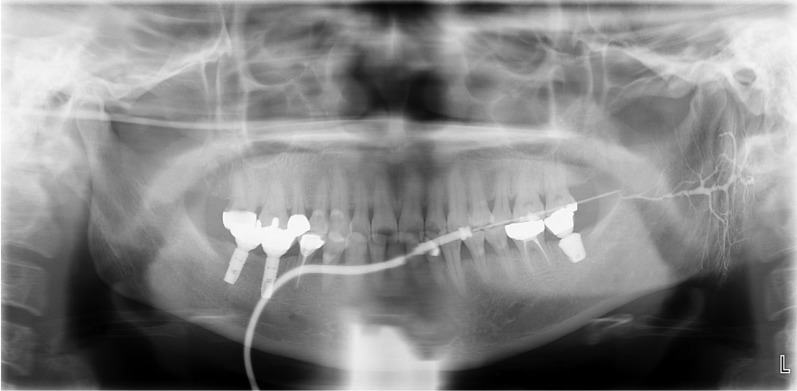
A 51-year-old female patient presented to the department of oral medicine at a dental hospital with the complaint of irregular and intermittent dull pain in the left parotid gland area that had lasted for 6 months. She had no systemic disease other than hypertension. No swelling, redness, or tenderness on palpation at the left parotid gland was noted on an oral examination. Power Doppler US-guided sialography was performed on the left parotid gland. On preoperative US images, no pathological signs such as ductal enlargement or sialectasis in the parotid gland were observed (Fig. 2A). During sialography, the position of the catheter in the main duct was confirmed by a linear hyperechoic signal with posterior enhancement on the US image (Fig. 2B). Increased blood flow was observed at the tip of the universal cannula during contrast medium injection when performing power Doppler US (Fig. 2C), followed by ductal dilatation due to filling of the contrast medium into the salivary duct. Furthermore, vasodilation and increased blood flow were observed around the duct, which were absent on preoperative US images (Fig. 2D). Sialography showed slight dilatation of the main and intraglandular ducts, but normal ramification of the ductal system in the parenchymal portion of the salivary gland and no evidence of sialectasis or extravasation. The patient was diagnosed with sialodochitis (Fig. 3).
Fig. 2. Power Doppler ultrasound (US)-guided sialography (51/F) for diagnosing sialodochitis of the left parotid gland. A. On preoperative US images, there are no observable pathologic changes in the parotid gland. B. The position of the catheter in the main duct is confirmed by a linear hyperechoic signal with posterior enhancement (arrow). C. During fluid injection, an increase in blood flow is observed at the tip of the universal cannula. D. As the contrast agent entered, parotid gland duct dilatation, periductal vasodilation, and increased blood flow are observed.
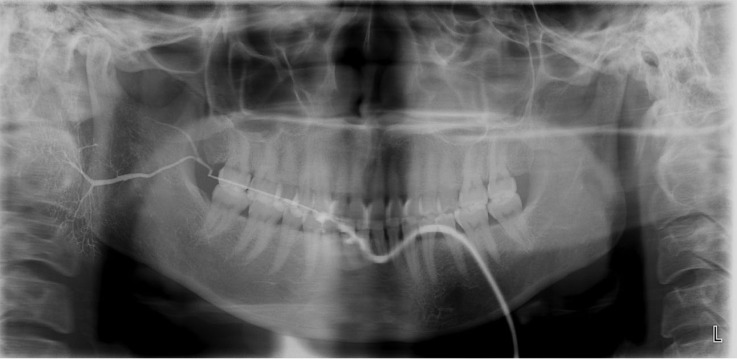
Fig. 3. Sialography of the left parotid gland in case 1 shows sialodochitis without sialadenitis. The Stensen duct exhibited dilatation with local strictures, and the intraglandular ductules showed irregular dilatation with constriction.
Patient 2
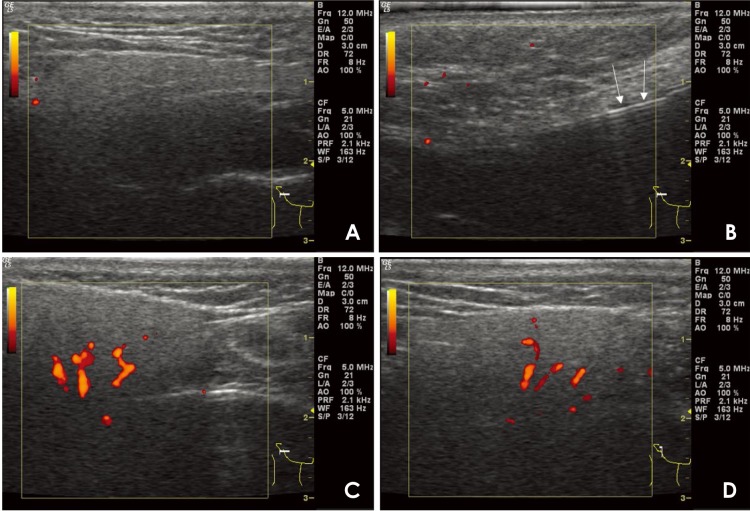
A 30-year-old female patient presented to the department of oral medicine at a dental hospital with the complaint of a 6-month history of dry, burning mouth sensations. The symptoms were aggravated by brushing and spicy foods. However, an examination revealed no clinical signs of oral mucosal lesions such as mucosal damage, ulcers, or oral candidiasis. On preoperative power Doppler US images, no abnormal findings were observed (Fig. 4A). During sialography, the position of the catheter in the main duct was confirmed by a linear hyperechoic signal with posterior enhancement (Fig. 4B). After the contrast medium injection, the power Doppler US images revealed an increased blood flow in the salivary parenchyma (Figs. 4C and D). The patient was diagnosed with atrophic changes in the parotid ductal system on sialography (Fig. 5).
Fig. 4. Power Doppler ultrasound (US)-guided sialography (30/F) for diagnosing xerostomia. A. On preoperative US images, there are no observable pathologic changes in the ductal system or the parenchymal portion of the right parotid gland. B. The position of the catheter in the main duct is confirmed by a linear hyperechoic signal with posterior enhancement (arrow). C and D. As the contrast agent enters, parotid gland duct dilatation, periductal vasodilatation, and increased blood flow are observed.
Fig. 5. Sialography shows atrophic changes in the ductal system of the right parotid gland, with narrowing of the secondary and tertiary ductules, but without sialectasis.
Discussion
In this study, power Doppler US images showed vasodilation and increased blood flow in the salivary gland when contrast medium was injected into the duct during sialography. However, the mechanism of this increase in blood flow has not yet been fully elucidated and could be due to a wide variety of factors. In a previous study, saline and various contrast agents were injected into a Doppler phantom made of thin tubes at a rate of 0.001–0.1 mL/s to analyze the relationship between Doppler signal intensity and the fluid type, concentration, and velocity. The contrast agent used in the present study did not show any Doppler signal on sialography, implying that the Doppler effect on sialography was not caused by the simple flow of fluid.7 In mongrel adult dogs, the blood flow in the submandibular gland increased by 1.5 to 2 times when a saline solution was injected through the main salivary duct.8 Thus, blood flow was accelerated by the reaction of backward pressure on the salivary gland. Experiments conducted to evaluate whether these results were due to humoral factors such as bradykinin or neural reflexes have shown that nerve fibers play an important role in accelerating blood flow. The results imply that baroreceptors distributed in the submandibular glands of the dog play some important physiological roles in regulating the gland's blood supply. Another study demonstrated that increased interstitial fluid due to the reaction of backward pressure on the salivary gland can change capillary flow. It has been suggested that pressure in the interstitial fluid may be an active force that contributes to fluid flow through the capillary, as in acute inflammatory reactions and traumatic conditions.9,10
Power Doppler US, which is a technique that displays Doppler signal strength, rather than flow velocity and direction information, has the advantage of being highly sensitive to flow and is relatively independent of the angle of the US beam to the blood vessel, which results in a better delineation of tortuous vessels. In particular, by encoding the moving blood flow signal with different hues, the signal can be easily differentiated from noise, thereby enabling the identification of blood vessels that were not found previously.11 In this study, the use of power Doppler US enabled the detection of minute changes in blood flow caused by the injection of contrast agents and real-time monitoring of the sialography process. Particularly in our first case, a dramatic increase in blood flow was observed on power Doppler US in response to the contrast injection. We believe this may help to promote the healing response to inflammation, resulting in rapid recovery and a fairly good prognosis in patients with significant blood flow response.
The main limitations of this study are the small number of patients observed and the lack of objective indicators for assessing the increased blood flow. In future studies, it may be necessary to evaluate the clinical efficacy of this technique with objective evaluation indices and a larger sample size. Additionally, it must be investigated whether this technique can be used to diagnose patients and to predict their prognosis through a comparative analysis of the pattern of increased blood flow between normal volunteers and patients with salivary gland disease. Moreover, this technique could be applied in conservative treatment techniques such as salivary gland irrigation using saline instead of a contrast agent.
Footnotes
Conflicts of Interest: None
References
- 1.Abdel Razek AA, Mukherji SK. State-of-the-art imaging of salivary gland tumors. Neuroimaging Clin N Am. 2018;28:303–317. doi: 10.1016/j.nic.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Som PM, Brandwein-Gensler MS. Anatomy and pathology of the salivary glands. In: Som PM, Curtin HD, editors. Head and neck imaging. 5th ed. St. Louis: Elsevier; 2011. pp. 2468–2474. [Google Scholar]
- 3.Capaccio P, Torretta S, Ottavian F, Sambataro G, Pignataro L. Modern management of obstructive salivary diseases. Acta Otorhinolaryngol Ital. 2007;27:161–172. [PMC free article] [PubMed] [Google Scholar]
- 4.Kalk WW, Vissink A, Spijkervet FK, Möller JM, Roodenburg JL. Morbidity from parotid sialography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:572–575. doi: 10.1067/moe.2001.117300. [DOI] [PubMed] [Google Scholar]
- 5.Oh SH, Kang JH, Choi YJ, Kim BY, Lee SR, Lee SH, et al. Ultrasound-guided sialo-irrigation with a saline-air mixture as the contrast medium. Oral Radiol. 2019;35:84–89. doi: 10.1007/s11282-018-0331-2. [DOI] [PubMed] [Google Scholar]
- 6.Babcock DS, Patriquin H, LaFortune M, Dauzat M. Power Doppler sonography: basic principles and clinical applications in children. Pediatr Radiol. 1996;26:109–115. doi: 10.1007/BF01372087. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Tokumori K, Okamura K, Chikui T, Yoshiura K, Kanda S. Possibility of sialographic sonography: a Doppler phantom study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:719–727. doi: 10.1067/moe.2001.113832. [DOI] [PubMed] [Google Scholar]
- 8.Funakoshi M, Hamada T, Kawamura Y. The effect of pressure within the submandibular gland on the glandular blood circulation. Jpn J Physiol. 1967;17:21–29. [Google Scholar]
- 9.Reed RK, Rubin K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res. 2010;87:211–217. doi: 10.1093/cvr/cvq143. [DOI] [PubMed] [Google Scholar]
- 10.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 11.Rubin JM, Bude RO, Carson PL, Bree RL, Adler RS. Power Doppler US: a potentially useful alternative to mean frequency-based color Doppler US. Radiology. 1994;190:853–856. doi: 10.1148/radiology.190.3.8115639. [DOI] [PubMed] [Google Scholar]