Summary
Active and highly stable electrocatalysts for oxygen evolution reaction (OER) in acidic media are currently in high demand as a cleaner alternative to the combustion of fossil fuels. Herein, we report a Co-doped nanorod-like RuO2 electrocatalyst with an abundance of oxygen vacancies achieved through the facile, one-step annealing of a Ru-exchanged ZIF-67 derivative. The compound exhibits ultra-high OER performance in acidic media, with a low overpotential of 169 mV at 10 mA cm−2 while maintaining excellent activity, even when exposed to a 50-h galvanostatic stability test at a constant current of 10 mA cm−2. The dramatic enhancement in OER performance is mainly attributed to the abundance of oxygen vacancies and modulated electronic structure of the Co-doped RuO2 that rely on a vacancy-related lattice oxygen oxidation mechanism (LOM) rather than adsorbate evolution reaction mechanism (AEM), as revealed and supported by experimental characterizations as well as density functional theory (DFT) calculations.
Subject Areas: Catalysis, Electrochemical Energy Production, Nanomaterials
Graphical Abstract

Highlights
-
•
A Co-doped RuO2 electrocatalyst with an abundance of oxygen vacancies was synthesized
-
•
The compound exhibits ultra-high OER performance in acidic media
-
•
The oxygen vacancies contribute to the high OER performance
Catalysis; Electrochemical Energy Production; Nanomaterials
Introduction
Hydrogen is considered an ideal clean energy carrier to store electrical energy derived from renewable, intermittent power sources that has evoked significant interest and stimulated intensive investigations in the scientific literature. Furthermore, the conversion of hydrogen back into electrical energy can be efficiently performed in fuel cells to effectively harness the energy initially stored. Electrochemical water splitting, including hydrogen evolution reaction and oxygen evolution reaction (OER), provide an efficient and environmentally friendly way for large-scale hydrogen production with high purity. To date, alkaline water splitting technologies have been well established and are commercially available for industrial H2 production (Jin et al., 2016, Suen et al., 2017, Zheng et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Zhuang et al., 2019, Zhou et al., 2019). Nevertheless, compared with alkaline water splitting, acidic water splitting using proton exchange membrane (PEM) electrolyzer offers great advantages such as higher ionic conductivity, fewer unfavorable reactions, high voltage efficiency, and faster system response (Nong et al., 2015, Sardar et al., 2014). However, the scarcity of highly active and stable OER electrocatalysts in acidic condition greatly hinders the widespread commercialization of acidic water splitting. Ir oxide-based materials are considered as the state-of-the-art electrocatalysts for OER under acidic condition. For example, Ir-based double perovskites (Diaz-Morales et al., 2016), IrOx/SrIrO3 hybrid (Seitz et al., 2016), and pyrochlore-structured Ir-based oxide (Kim et al., 2017) were recently reported to be active and stable OER electrocatalysts in acidic condition. Unfortunately, their potential for commercialization is greatly hindered by the low production and high price of Ir. A possible alternative to Ir is the much cheaper Ru, which also shows high OER catalytic activity in acidic condition, such as the typical rutile-structured RuO2 (Lee et al., 2012). However, many Ru-based electrocatalysts suffer from low stability in acidic condition owing to the over-oxidation of Ru into soluble RuO4 moieties under the demanding oxidative environment (Kotz et al., 1983). Therefore, elaborate design or optimization of Ru-based systems with improved activity and stability is a matter of utmost urgency and a prospective area of research for the development of efficient and economically attractive acidic water splitting. To address this problem, in our previous work, we focused on altering RuO2 surface atom arrangement and/or electronic structures that are pivotal to the catalytic performances. Accordingly, we fabricated hollow porous RuO2 polyhedra (Su et al., 2018) and CrO2-RuO2 solid solution (Lin et al., 2019) materials that exhibit excellent OER performance. Specifically, CrO2-RuO2 solid solution possess a very low overpotential of 178 mV at 10 mA cm−2 and galvanostatic stability test of 10 h at the same current density in 0.5 M H2SO4. Recently, Lotsch and coworkers reported RuO2 nanosheets with enhanced acidic OER activity and stability ascribing the increase in reactivity to the surface edges of RuO2 (Laha et al., 2019).
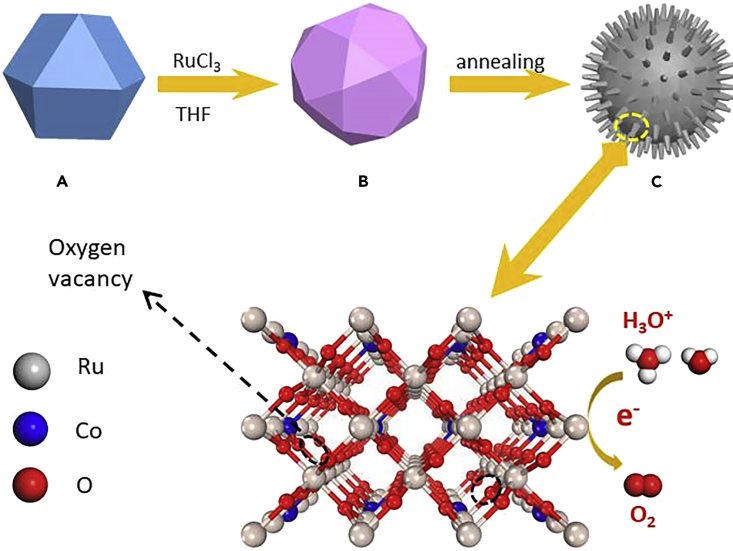
Besides metal doping and morphological control, oxygen vacancies in transition metal oxides have been reported to enhance the electrocatalytic process. For example, Xu et al. created oxygen vacancies on Co3O4 nanosheets via a plasma-engraving strategy, revealing that the vacancies can significantly enhance the alkaline OER activity of the nanosheets (Xu et al., 2016, Xu et al., 2018). The authors proposed that the oxygen vacancies on the Co3O4 nanosheets surface could improve the electronic conductivity and create more active sites for OER. Very recently, Wang et al. (Huang et al., 2019) and Kolpak et al. (Yoo et al., 2018) proposed a vacancy-related lattice oxygen oxidation mechanism (LOM), which could bypass the limitation of the most commonly reported adsorbate evolution mechanism (AEM) having a minimum theoretical overpotential of 0.37 eV. In light of these studies, we attempted to synthesize RuO2-based electrocatalyst that has abundant oxygen vacancies with optimized electronic structure for enhanced OER performance in acidic media. To achieve this target, we proposed to use some alien metal ions with different valence state to replace the Ru ions, which can simultaneously create oxygen vacancies and alter the electronic structures. Herein we chose Co because the lower oxidation states of Co dopants in the RuO2 lattice require less O2− ions to appropriately balance the charge, thus yielding oxygen vacancies. Metal-organic frameworks (MOFs) have been demonstrated to be versatile templates or precursors to prepare highly active electrocatalysts (Liu et al., 2017), such as N-doped porous carbon (Ma et al., 2016, Xia et al., 2016), metal oxides nanocomposites (Cai et al., 2017, Salunkhe et al., 2017), and carbon-coated nanosized metal alloys (Su et al., 2017). Based on this platform, we have successfully prepared a Co-doped RuO2 nanorod electrocatalyst by annealing a Ru-exchange ZIF-67 amorphous composite. We selected ZIF-67 as a candidate because of its high surface area, accessible porosity that can facilitate ion exchange, and ease of synthesis (Banerjee et al., 2008, Gross et al., 2012, Qian et al., 2012). The synthetic route is briefly illustrated in Scheme 1. The resulting Co-doped RuO2 nanorod exhibits an extremely low overpotential of 169 mV for OER in acidic environment at 10 mA cm−2 and excellent stability with a chronopotentiometry performance of over 50 h at the same current, outperforming the most active OER electrocatalysts reported to date. Based on the density functional calculations, we propose that the OER process on the compound undergoes a vacancy-related LOM, which enhances both the activity and stability.
Scheme 1.
Illustration of the Synthesis of Co-doped RuO2 Nanorods
Results and Discussions
Synthesis and Characterization of Ru-ZIF-67 and Co-Doped RuO2 Nanorods
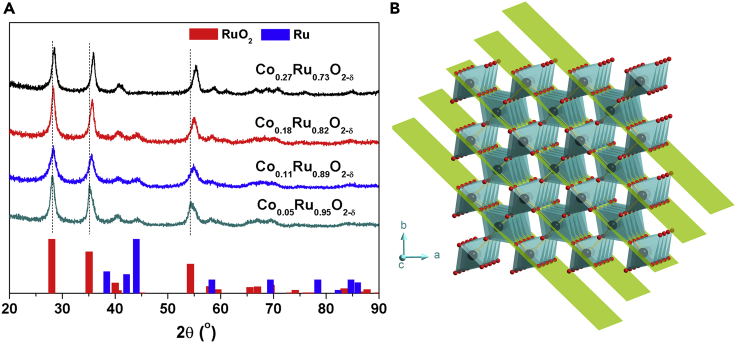
ZIF-67 was synthesized via a facile procedure previously reported (Feng and Carreon, 2015) (see details in Methods section). The powder X-ray pattern (PXRD) and scanning electron microscope (SEM) image of the as-prepared product confirms that a pure ZIF-67 phase was obtained (Figures S1 and S2). The Co ions in ZIF-67 were exchanged by Ru ion (RuCl3 was used as the Ru ion source) in THF solvent at room temperature to obtain Ru-exchanged ZIF-67 (Ru-ZIF-67). The Ru content in the resulting product can be varied by controlling the amount of RuCl3 reactant used for the exchange reaction. The resulting Ru-ZIF-67 is amorphous as revealed by the PXRD pattern and SEM image (Figures S3 and S4), which could be attributed to the significantly different coordination manner and environment of Ru and Co. In fact, crystal structure is not necessary for the following annealing treatment, which is associated with pyrolysis and atom rearrangements. Co-doped RuO2 nanorods with different Co/Ru ratio can be obtained through further thermal decomposition of the as-prepared Ru-ZIF-67 powders in air at 350°C. Based on inductively coupled plasma-mass spectroscopy (ICP-MS) measurements, the formulas of the as-prepared RuO2 samples are Co0.27Ru0.73O2-δ, Co0.18Ru0.82O2-δ, Co0.11Ru0.89O2-δ, and Co0.04Ru0.96O2-δ (δ is used to balance the valence), respectively. Figure 1A shows the PXRD patterns of these samples, in which the major phase can be identified as rutile RuO2 (JCPDS. No. 43-1027). Notably, the PXRD signals that belong to RuO2 shift to the right when the Co content in the sample increases, suggesting that Co atoms have been successfully incorporated into the RuO2 crystal lattice to form a rutile Co-doped RuO2 phase with a concomitant lattice shrink. Figures 1B and S5 show the planes of RuO2 corresponding to the labeled PXRD peaks. The d spaces of these planes decrease after the insertion of Co atoms that have a smaller size than that of the Ru atom. We further varied the annealing temperature for Co0.11Ru0.89O2-δ to investigate the structural evolution of Co-doped RuO2 nanocrystals. As shown in Figure S6, the content of metallic Ru can be decreased by increasing the annealing temperature and becomes negligible when the annealing temperature is increased to 500°C. The higher annealing temperatures lead to better crystallinity for the Co-doped RuO2 as seen from the higher peak intensity and increased sharpness of the PXRD signals.
Figure 1.
Structure Characterization of Co-doped RuO2
(A) PXRD patterns of Co-doped RuO2 samples with different Co content annealed at 350°C.
(B) Crystal structure of rutile RuO2 viewed along the c axis. Yellow planes represent the (110) facets. Red ball, O; gray ball, Ru.
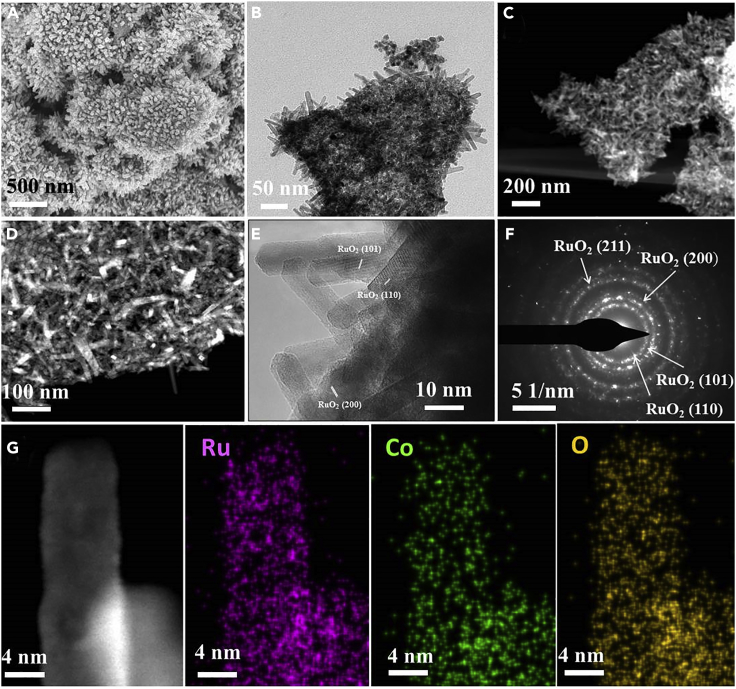
Transmission electron microscopy (TEM) was employed to characterize the morphology and structure of the Co-doped RuO2 powders. As shown in Figures 2A–2D and S7–S9, all of the as-prepared Co-doped RuO2 samples display nanorod-like shape morphology with a diameter of ∼8 nm and length of ∼20 nm. High-resolution TEM (HR-TEM) image (Figure 2E) clearly shows lattice fringes, indicating the high crystallinity of the Co-doped RuO2 samples. Both the HR-TEM and the selected area electron diffraction (SAED) characterization (Figure 2F) confirm the rutile RuO2 structure of Co-doped RuO2 nanorods. High-angle annular dark-field scanning transmission microscopy (HAADF-STEM) was employed to analyze the elemental distribution over a single nanorod. As shown in Figure 2G, the elements of Ru, Co, and O were uniformly distributed over an entire single nanorod crystal, demonstrating the formation of rutile Co-doped RuO2 phase (the mapping images for a wider region are shown in Figure S10), consistent with the finding from PXRD characterization. In addition, Co0.11Ru0.89O2-δ (350) displays a typical type II isotherm with an H1 type hysteresis loop (Figure S11), which is characteristic for the aggregation of small particles. The calculated surface area for Co0.11Ru0.89O2-δ (350) is 31.3 m2 g−1, larger than that of RuO2 (8.9 m2 g−1).
Figure 2.
Morphology, Structure, and Element Mapping Images of Co0.11Ru0.89O2 Annealed at 350°C
(A) SEM image.
(B) TEM image.
(C and D) Dark-field TEM images with small (C) and large (D) magnification levels.
(E) HR-TEM image.
(F) SAED image.
(G) HAADF-STEM image and the corresponding mapping images.
OER Activity in Strong Acidic Media
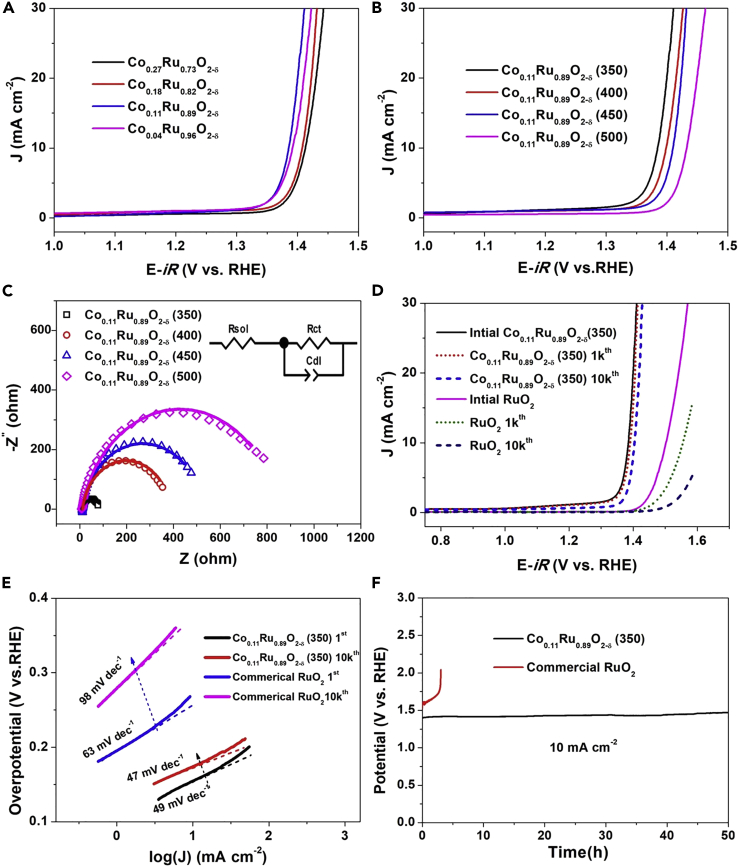
The OER activity of Co-doped RuO2 powders was investigated in a strong acidic media (0.5 M H2SO4) by use of a typical three-electrode electrochemical system containing a Pt wire as counter-electrode and an Hg/Hg2SO4 reference electrode (see details in Methods section). The Co-doped RuO2-based electrodes were prepared by drop-casting a water/ethanol and Nafion-based ink of Co-doped RuO2 on glassy carbon disk. We measured linear sweep voltammetry (LSV) curves at a scan rate of 5 mV s−1. The Ohmic potential drop (iR) correction was performed to all experimental data to eliminate the effect of solution resistance, and the potentials were calibrated to the reversible hydrogen electrode (RHE) for comparison. Initially, we investigated the OER performance of Co-doped RuO2 powders with different Co content to emphasize the effects of Co content on the OER catalysis of RuO2 and to obtain a sample with optimum OER activity for further exploration. Figure 3A presents the LSV curves of Co0.27Ru0.73O2-δ, Co0.18Ru0.82O2-δ, Co0.11Ru0.89O2-δ, and Co0.03Ru0.97O2-δ annealed at 350°C, where the rising current indicates the evolution of oxygen. As seen in Figure 3A, the OER activity is inversely proportional to Co content when the Co/Ru ratio is higher than 0.11:0.89, suggesting that the Ru atom is the catalytic center. Reasonably, a higher Co content will decrease the number of exposed catalytic Ru sites. However, when the Co/Ru ratio reduces to 0.04:0.96, the OER activity becomes relatively poor, thus emphasizing the significant role of Co dopant, which can induce oxygen vacancy and modify the electronic structure of Ru. Clearly, Co0.11Ru0.89O2-δ (350) exhibited the best OER activity among the samples, with an overpotential of 169 mV at 10 mA cm−2. We thus further altered the annealing temperature of Co0.11Ru0.89O2-δ to realize the best annealing temperature for Co0.11Ru0.89O2-δ to maximize the OER performance. As shown in Figure 3B, along with the rise of annealing temperature, the OER activity decreases successively. This decrease can be assigned to the lattice strain effects and/or reduced active crystal defects under higher annealing temperature and is supported by the increase in PXRD intensity and sharpness. Electrochemical impedance spectroscopy (EIS) measurements were also performed to assess the electrical resistance of Co0.11Ru0.89O2-δ samples annealed at different temperature. As shown in Figure 3C, all the Nyquist plots display a depressed semicircle, suggesting a charge-transfer process during the OER. These Nyquist plots were further fitted to analyze the electrical resistance by a simple equivalent electrical circuit as shown in the inset in Figure 3C (Audichon et al., 2016, Harrington and van den Driessche, 2011). Co0.11Ru0.89O2-δ (350) exhibits the smallest semicircle radius compared with other catalysts, implying the smallest electric resistance and fastest charge transfer rate OER kinetics at the interface.
Figure 3.
OER Performance of Co-doped RuO2
(A) LSV curves of CoxRu1-xO2-δ annealed at 350°C.
(B) LSV curves of Co0.11Ru0.89O2-δ annealed at different temperature.
(C) Impedances of Co0.11Ru0.89O2-δ annealed at different temperature.
(D) LSV curves of Co0.11Ru0.89O2-δ (350) and commercial RuO2 before and after CV cycling measurements.
(E) Tafel plots of Co0.11Ru0.89O2-δ (350) and commercial RuO2 before and after CV cycling measurements.
(F) Chronopotentiometry test of Co0.11Ru0.89O2-δ (350) and commercial RuO2.
As demonstrated by the above results and discussion, Co0.11Ru0.89O2-δ (350) is the optimized material as it possesses the highest OER performance among those samples with varying Co content or annealing temperatures. We further tested the stability of Co0.11Ru0.89O2-δ (350) by cycling the catalyst between 1.2 and 1.6 V at a sweep rate of 100 mV s−1 in 0.5 M H2SO4 for 10,000 cycles. As shown in Figure 3D, after 10,000 cycles, the overpotential of Co0.11Ru0.89O2-δ (350) was 184 mV, only a 15-mV increase relative to the initial overpotential. For further comparison, the OER performance of commercial RuO2 with a particle size of ∼30 nm was also tested at identical conditions. RuO2 displays much lower OER activity, with an overpotential up to 273 mV to drive the current density of 10 mA cm−2. Moreover, after 10,000 cycles, the OER activity of RuO2 is dramatically reduced and becomes negligible compared with the initial OER activity, revealing poor stability for OER in acidic media. These results for commercial RuO2 are consistent with those seen in the literature (Kim et al., 2017, Audichon et al., 2016, Li et al., 2017). In addition, considering that the OER activity is correlated to the number of exposed sites, we further plotted the LSV of Co0.11Ru0.89O2-δ (350) and RuO2 with respect to the calculated surface areas (Figure S12). The results show that the enhanced OER performance of Co0.11Ru0.89O2-δ (350) is not merely increased by the surface area. In contrast, the enhanced intrinsic activity arises from oxygen vacancies and the Co dopant plays a much more important role.
Figure 3E shows the Tafel plots of Co0.11Ru0.89O2-δ (350) and RuO2 before and after 10,000 cycles. The Tafel slope for RuO2 of the initial cycle is 63 mV dec−1 and dramatically rises to 98 mV dec−1 after 10,000 cycles. For Co0.11Ru0.89O2-δ (350), the Tafel slope slightly decreases from 49 to 47 mV dec−1 after 10,000 cycles, implying that the OER kinetics of Co0.11Ru0.89O2-δ (350) after 10,000 cycles is slightly faster than that of initial Co0.11Ru0.89O2-δ (350). The smaller and unchanged Tafel slope of Co0.11Ru0.89O2-δ (350) suggests a faster and much more stable OER kinetic rate than RuO2. Ultimately, to further confirm the big difference in stability of Co0.11Ru0.89O2-δ (350) and RuO2, chronopotentiometry was examined under a constant current density of 10 mA cm−2. Figure 3F presents the corresponding potential change for both Co0.11Ru0.89O2-δ (350) and RuO2. Clearly, RuO2 loses OER activity in less than 3 h; on the contrary, Co0.11Ru0.89O2-δ (350) remains essentially stable throughout the 50-h galvanostatic stability test. We also measured the chronopotentiometric curve under a higher current density of 50 mA cm−2. As shown in Figure S13, Co0.11Ru0.89O2-δ (350) remains essentially stable throughout the 8-h galvanostatic stability test. Furthermore, as shown in Figure S14, the Co/Ru ratio before cyclic voltammetry (CV) is 0.124/0.876, generally consistent with the ICP result (Co/Ru = 0.11/0.89). After CV cycles, the Co/Ru ratio shows a very slight decrease, with a value of 0.107/0.893 (Figure S15), indicating the high stability of Co0.11Ru0.89O2 (350). In addition, the morphology of Co0.11Ru0.89O2 (350) after 10,000 CV cycles remains unchanged (Figure S16). These results demonstrate that Co0.11Ru0.89O2-δ (350) is stable in 0.5 M H2SO4 at the oxidizing potential during the OER process. For comparison, we summarized the reported materials with high OER activity in acidic media in Table S1. Notably, Co0.11Ru0.89O2-δ (350) presents a record low overpotential and outperforms IrO2-based catalysts, which represent the state-of-the-art electrocatalyst for OER in acidic media.
Origin of OER Activity and Stability
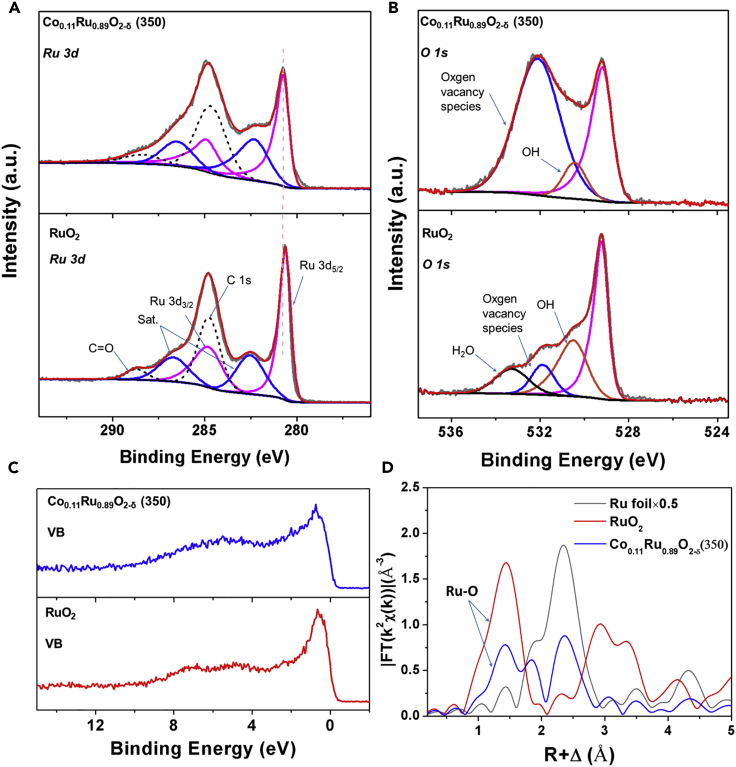
We first carried out X-ray photoelectron spectroscopy (XPS) analysis to assess the surface chemical state of Co0.11Ru0.89O2-δ (350). For comparison, XPS of commercial RuO2 was also measured. As shown in Figure 4A, the XPS peaks for Ru 3d of Co0.11Ru0.89O2-δ (350) and RuO2 can be deconvoluted into two sets of doublet peaks for Ru 3d5/2, 3d3/2, their satellite peaks, and two single peaks for C1s (arising from corrosion carbon) (Morgan, 2015). For RuO2, the primary Ru 3d5/2 and 3d3/2 peaks of RuO2 center at 280.6 and 284.8 eV, respectively, consistent with those reported in the literature (Sarma and Rao, 1980, Shen et al., 1991). As for Ru 3d of Co0.11Ru0.89O2-δ (350), a small shift to higher binding energy can be clearly observed, which may be attributed to a higher electron density at the Ru sites. As mentioned earlier, there is a small portion of metallic Ru in our sample as determined by PXRD characterizations. However, the corresponding XPS peaks for metallic Ru are nearly invisible possibly because the majority of species in the outer surface are aggregated Co0.11Ru0.89O2-δ (350) particles in the RuO2 phase. In the annealing process, the outer surface of the sample is directly exposed to air largely increasing the probability of generating metal oxides. The Co 2p spectrum of Co0.11Ru0.89O2-δ (350) is shown in Figure S17. For comparison, we also prepared a sample by annealing pure ZIF-67 using the same procedure for preparing Co0.11Ru0.89O2-δ (350). The structure of the resulting powder is identified as Co3O4 (Figure S18). As shown in Figure S14, the main peak of Co 2p is shifted toward higher binding energy state compared with that of Co3O4 derived from ZIF-67, suggesting a relatively higher oxidation state of Co doped into Co0.11Ru0.89O2-δ (350) attributed to the electron withdrawing effect of Ru4+ in the lattice. O 1s spectra for Co0.11Ru0.89O2-δ (350) and RuO2 are shown in Figure 4B. For RuO2, the O 1s XPS peak can be deconvoluted into four peaks, corresponding to different type of O species. The main peak centered at 529.2 eV corresponds to the Ru-O in the lattice, and the shoulder peak centered at 530.5 eV can be assigned to the OH− ions that integrated into the outer surface of RuO2. The higher binding energy peak centered at 531.9 eV is attributed to the surface oxygen vacancies species, which is often observed on metal oxides (Dupin et al., 2000, Uhlenbrock et al., 1992). The small peak centered at 533.3 eV originates from the adsorbed H2O molecules (Dupin et al., 2000). For Co0.11Ru0.89O2-δ (350), the O 1s peak can be deconvoluted into three peaks, corresponding to the Ru-O, OH−, and oxygen vacancy species, respectively. We can find that the peak for oxygen vacancy species is much stronger than that of RuO2, suggesting that a larger number of oxygen vacancies exist on the Co0.11Ru0.89O2-δ (350) surface. The increase of oxygen vacancies on Co0.11Ru0.89O2-δ (350) arises from the lower oxidation state of Co dopants in the RuO2 lattice that require less O2− ions to appropriately balance the charge. In many cases, oxygen vacancies species can significantly enhance the OER activity by dramatically improving the electronic conductivity that leads to the creation of more active sites (Xu et al., 2016). In addition, compared with RuO2, Co0.11Ru0.89O2-δ (350) shows a broadened valence band (VB) spectra (Figure 4C), suggesting that the incorporation of Co dopants not only introduces the oxygen vacancies but also modulates the electronic structure of RuO2. The XPS wide-scan spectrum of Co0.11Ru0.89O2-δ (350) is shown in Figure S19. In addition, we also measured the XPS spectra for Co and Ru elements of Co0.11Ru0.89O2-δ (350) after stability test. As shown in Figures S20 and S21, the XPS spectra of Co and Ru after stability test remained generally unchanged in both position and shape, indicating the high stability of Co0.11Ru0.89O2-δ (350).
Figure 4.
Chemical State and Structure Analysis of Co0.11Ru0.89O2-δ (350)
(A) Ru 3d XPS profiles of Co0.11Ru0.89O2-δ (350) and RuO2.
(B) O 1s XPS profiles of Co0.11Ru0.89O2-δ (350) and RuO2.
(C) Valence band XPS profiles of Co0.11Ru0.89O2-δ (350) and RuO2.
(D) Fourier transformed EXAFS spectra of Ru K-edge for Co0.11Ru0.89O2-δ (350), RuO2, and Ru foil.
To investigate the local structure of Co0.11Ru0.89O2-δ (350), X-ray absorption spectroscopy (XAS) characterization was further employed (Figure S22). The Fourier transformed (FT) radial structure based on the k2-weighted extended X-ray absorption fine structure (EXAFS) is displayed in Figure 4D. The peak at ∼1.5 Å corresponds to Ru-O bonds. Interestingly, compared with RuO2, the intensity of this peak for Co0.11Ru0.89O2-δ (350) distinctly decreased, which is ascribed to the coordination deficiency from Ru, revealing the existence of abundant oxygen vacancies in Co0.11Ru0.89O2-δ (350). Note that the Ru-Ru peak is also present, which is consistent with the PXRD results.
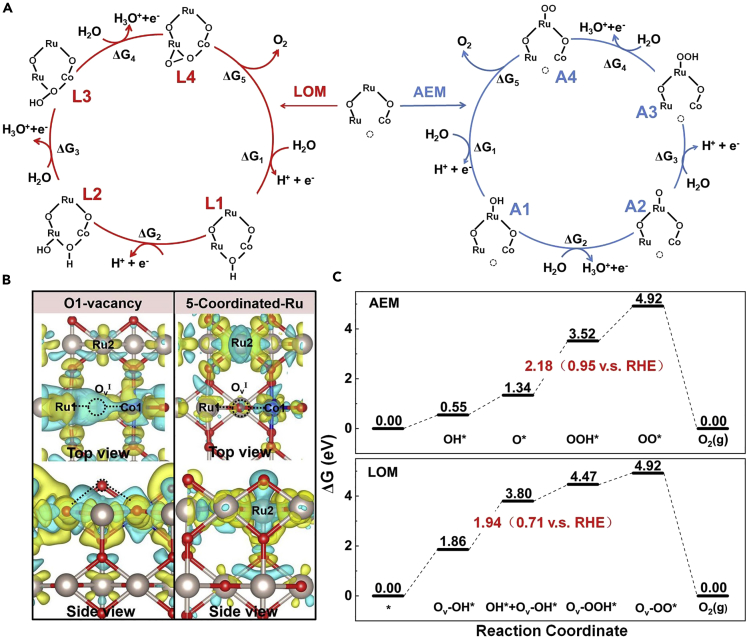
Finally, we carried out density functional theory (DFT) calculations to gain more insights into the excellent OER activity of Co-RuO2. Based on previous studies that RuO2 (110) was identified as the most stable surface with the lowest surface energy among various facets (Su et al., 2018, Fang and Liu, 2010, Wei and Liu, 2011), we constructed a (110) surface model of CoxRu1-xO2-x (x = 0.1) to simulate the experimentally obtained material. O vacancy is created to maintain the total charge balance induced by different valence states of Co and Ru atoms. As shown in Figure S23, two possible types of O vacancies were located on different sites: OvI formed by the substitution of 6-coordinated Ru and OvII formed by the substitution of 5-coordinated Ru. The comparison of the formation energies indicates that OvI vacancy sites are dominant and mainly participate in the subsequent OER process owing to its 0.50 eV lower formation energy than that of OvII sites.
To address the role of oxygen vacancies in OER, the activity of the neighbored 5-coordinated Ru is definitely inevitable to be compared. Therefore, two possible competing OER processes via OvI in LOM and 5-coordinated Ru in AEM were comparatively investigated to explore the preferred lower free energy path. As demonstrated in Figure 5A, five elementary steps are taken into account involving four electrochemical electron transfer steps (ΔG1 ∼ ΔG4) and one non-electrochemical O2 desorption step (ΔG5) in both processes. For the initial adsorption of upcoming H2O molecule, the intermediate L1 (OvI-OH) in LOM and intermediate A1 (Ru-OH) in AEM were formed on OvI and 5-coordinated Ru sites, respectively. The lower adsorption energy (0.71 eV) of L1 than A1 reveals that H2O preferred to react with OvI vacancy rather than the Ru site. It is well reasonable because OvI vacancy between the two unsaturated cations (Ru1 and Co1 in Figure S24) on the surface possesses lower electron depletion (blue contour) in Figure 5B to bind with H2O molecules, thus yielding lower adsorption energy of L1 to proceed the following OER steps in LOM mechanism.
Figure 5.
Comparison of OER Mechanisms with the Different Local Configurations
(A) Proposed LOM and AEM mechanisms. L1 is thermally favorable energetically than A2.
(B) The charge energy difference of A1 and L1 to illustrate the lower electron depletion on OvI than Ru2, which is attributed to the higher activity of OvI in OER process.
(C) The free energy diagrams of the two mechanisms of LOM and AEM. The rate-determining barriers together with that versus RHE are denoted.
Another important factor to evaluate the effect of O vacancy on OER process is free energy variation of the rate-determining step (RDS) in both LOM and AEM, as plotted in Figure 5C. Along the LOM reaction path, the formation of Ru(OH)-OvI(OH) after the second H2O attacking was identified as RDS, which yielded a free energy barrier of 0.71 eV versus RHE. The RDS in AEM occurred on the formation of ∗OOH on the 5-coordinated Ru with yielding a free energy barrier of 0.95 eV versus RHE. Clearly, the corresponding 0.24 eV lower energy barrier of RDS in LOM suggested that the OER would preferentially undertake O vacancy site rather than the 5-coordinated Ru site. Moreover, we believe that the enhanced stability is also associated with the LOM pathway. The participation of O vacancy can effectively avoid the over-oxidation of Ru to the soluble RuO4, which is deemed as the major reason for the instability of RuO2 electrocatalyst governed by AEM in the acidic conditions.
The density of states of RuO2 and Co-doped RuO2 were compared and provided in Figure S25 to further understand the inherent electron variation on O vacancies. The binding region between −2.0 eV and the Fermi level was obviously broadened in the Co-doped RuO2, which was well consistent with the observed XPS VB results. Actually, both IrO2 and Cu-doped RuO2 electrocatalysts were previously found to promote OER activity by broadening the binding regions (Su et al., 2018, Sun et al., 2015). It is well accepted that the p-band center of O is an effective descriptor to evaluate OER performance (Grimaud et al., 2013). Our calculated p-band centers were −4.57 eV in RuO2 and −3.03 eV in Ru1–xCoxO2–x, showing a higher energy shift toward the Fermi level upon the creation of O vacancy by Co dopant. According to p-band center theory, Ru1-xCoxO2-x with p-band center closer to the Fermi level exhibits higher OER catalytic activity.
Conclusion
In summary, by use of Co-based MOF, we have developed a nanorod-like Co-doped RuO2 electrocatalyst with superior OER performance in acidic condition. Impressively, this compound outperforms the state-of-the-art IrO2-based electrocatalyst and the currently reported RuO2-based electrocatalysts by demonstrating an ultralow overpotential (169 mV) and excellent stability. The abundance of oxygen vacancies together with the modulated electronic structure contribute to the superior OER activity, as revealed by both experimental characterizations and DFT calculations. We show that the OER preferentially proceeds via the LOM pathway by passing a lower RDS barrier (0.24 eV) in the assistance of O vacancies than reacting on 5-coordinated Ru. This OER enhancement is attributed to larger charge depletion on O vacancies and higher energy shift toward the Fermi level of p-band center. Our study puts forward the potential of using doped RuO2-based materials to confront the challenges in acidic OER process.
Limitations of the Study
Based on the combination of XPS, XAS, and DFT, oxygen vacancy has been interpreted as a key contributor to the excellent acidic OER activity and stability. However, to get an in-depth sight of the effect of oxygen vacancy, an in situ characterization of the oxygen vacancy is still needed but is very challenging.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (Nos. 51602320 and 51872306), the aided program for science and technology innovative research team of Ningbo municipality (Nos. 2015B11002 and 2016B10005), and Ningbo S&T Innovation 2025 Major Special Program (No.2018B10016).
Author Contributions
L.C. and Y.L. designed the project and wrote the manuscript; Y.T. and Y.L. carried out the experiments; S.W. and Q.Z. carried out DFT calculations; Q.Z. wrote the computational part of the manuscript; L.Z. performed the XAS experiments and data analysis. E.V., L.C., and X.L. provided helpful suggestions and polished the manuscript. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100756.
Contributor Information
Yichao Lin, Email: yclin@nimte.ac.cn.
Qiuju Zhang, Email: zhangqj@nimte.ac.cn.
Liang Chen, Email: chenliang@nimte.ac.cn.
Supplemental Information
References
- Audichon T., Napporn T.W., Canaff C., Morais C., Comminges C., Kokoh K.B. IrO2 Coated on RuO2 as efficient and stable electroactive nanocatalysts for electrochemical water splitting. J. Phys. Chem. C. 2016;120:2562–2573. [Google Scholar]
- Banerjee R., Phan A., Wang B., Knobler C., Furukawa H., O'Keeffe M., Yaghi O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science. 2008;319:939–943. doi: 10.1126/science.1152516. [DOI] [PubMed] [Google Scholar]
- Cai D.P., Zhan H.B., Wang T.H. MOF-derived porous ZnO/ZnFe2O4 hybrid nanostructures as advanced anode materials for lithium ion batteries. Mater. Lett. 2017;197:241–244. [Google Scholar]
- Diaz-Morales O., Raaijman S., Kortlever R., Kooyman P.J., Wezendonk T., Gascon J., Fu W.T., Koper M.T. Iridium-based double perovskites for efficient water oxidation in acid media. Nat. Commun. 2016;7:12363. doi: 10.1038/ncomms12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin J.C., Gonbeau D., Vinatier P., Levasseur A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000;2:1319–1324. [Google Scholar]
- Fang Y.H., Liu Z.P. Mechanism and Tafel lines of electro-oxidation of water to oxygen on RuO2(110) J. Am. Chem. Soc. 2010;132:18214–18222. doi: 10.1021/ja1069272. [DOI] [PubMed] [Google Scholar]
- Feng X.H., Carreon M.A. Kinetics of transformation on ZIF-67 crystals. J. Cryst. Growth. 2015;418:158–162. [Google Scholar]
- Grimaud A., May K.J., Carlton C.E., Lee Y.L., Risch M., Hong W.T., Zhou J.G., Shao-Horn Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013;4:2439. doi: 10.1038/ncomms3439. [DOI] [PubMed] [Google Scholar]
- Gross A.F., Sherman E., Vajo J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012;41:5458–5460. doi: 10.1039/c2dt30174a. [DOI] [PubMed] [Google Scholar]
- Harrington D.A., van den Driessche P. Mechanism and equivalent circuits in electrochemical impedance spectroscopy. Electrochim. Acta. 2011;56:8005–8013. [Google Scholar]
- Huang Z.F., Song J.J., Du Y.H., Xi S.B., Dou S., Nsanzimana J.M.V., Wang C., Xu Z.C.J., Wang X. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy. 2019;4:329–338. [Google Scholar]
- Jin Y.S., Wang H.T., Li J.J., Yue X., Han Y.J., Shen P.K., Cui Y. Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting. Adv. Mater. 2016;28:3785–3790. doi: 10.1002/adma.201506314. [DOI] [PubMed] [Google Scholar]
- Kim J., Shih P.C., Tsao K.C., Pan Y.T., Yin X., Sun C.J., Yang H. High-Performance Pyrochlore-type yttrium ruthenate electrocatalyst for oxygen evolution reaction in acidic media. J. Am. Chem. Soc. 2017;139:12076–12083. doi: 10.1021/jacs.7b06808. [DOI] [PubMed] [Google Scholar]
- Kotz R., Lewerenz H.J., Stucki S. XPS studies of oxygen evolution on Ru and RuO2 anodes. J. Electrochem. Soc. 1983;130:825–829. [Google Scholar]
- Laha S., Lee Y., Podjaski F., Weber D., Duppel V., Schoop L.M., Pielnhofer F., Scheurer C., Muller K., Starke U. Ruthenium oxide nanosheets for enhanced oxygen evolution catalysis in acidic medium. Adv. Energy Mater. 2019;9:1803795. [Google Scholar]
- Lee Y., Suntivich J., May K.J., Perry E.E., Shao-Horn Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012;3:399–404. doi: 10.1021/jz2016507. [DOI] [PubMed] [Google Scholar]
- Li G.Q., Li S.T., Ge J.J., Liu C.P., Xing W. Discontinuously covered IrO2-RuO2@Ru electrocatalysts for the oxygen evolution reaction: how high activity and long-term durability can be simultaneously realized in the synergistic and hybrid nano-structure. J. Mater. Chem. A. 2017;5:17221–17229. [Google Scholar]
- Lin Y.C., Tian Z.Q., Zhang L.J., Ma J.Y., Jiang Z., Deibert B.J., Ge R.X., Chen L. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat. Commun. 2019;10:162. doi: 10.1038/s41467-018-08144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhu D., Guo C., Vasileff A., Qiao S.-Z. Design strategies toward advanced MOF-Derived electrocatalysts for energy-conversion reactions. Adv. Energy Mater. 2017;7:1700518. [Google Scholar]
- Ma X., Zhou Y.X., Liu H., Li Y., Jiang H.L. A MOF-derived Co-CoO@N-doped porous carbon for efficient tandem catalysis: dehydrogenation of ammonia borane and hydrogenation of nitro compounds. Chem. Commun. 2016;52:7719–7722. doi: 10.1039/c6cc03149h. [DOI] [PubMed] [Google Scholar]
- Morgan D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015;47:1072–1079. [Google Scholar]
- Nong H.N., Oh H.S., Reier T., Willinger E., Willinger M.G., Petkov V., Teschner D., Strasser P. Oxide-supported IrNiO(x) core-shell particles as efficient, cost-effective, and stable catalysts for electrochemical water splitting. Angew. Chem. Int. Ed. 2015;54:2975–2979. doi: 10.1002/anie.201411072. [DOI] [PubMed] [Google Scholar]
- Qian J.F., Sun F.A., Qin L.Z. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012;82:220–223. [Google Scholar]
- Salunkhe R.R., Kaneti Y.V., Yamauchi Y. Metal-Organic Framework-Derived Nanoporous metal oxides toward supercapacitor applications: progress and prospects. ACS Nano. 2017;11:5293–5308. doi: 10.1021/acsnano.7b02796. [DOI] [PubMed] [Google Scholar]
- Sardar K., Petrucco E., Hiley C.I., Sharman J.D., Wells P.P., Russell A.E., Kashtiban R.J., Sloan J., Walton R.I. Water-splitting electrocatalysis in acid conditions using ruthenate-iridate pyrochlores. Angew. Chem. Int. Ed. 2014;53:10960. doi: 10.1002/anie.201406668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D.D., Rao C.N.R. Xpes studies of oxides of 2nd-row and 3rd-row transition-metals including rare-earths. J. Electron Spectrosc. 1980;20:25–45. [Google Scholar]
- Seitz L.C., Dickens C.F., Nishio K., Hikita Y., Montoya J., Doyle A., Kirk C., Vojvodic A., Hwang H.Y., Norskov J.K., Jaramillo T.F. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science. 2016;353:1011. doi: 10.1126/science.aaf5050. [DOI] [PubMed] [Google Scholar]
- Shen J.Y., Adnot A., Kaliaguine S. An ESCA study of the interaction of oxygen with the surface of ruthenium. Appl. Surf. Sci. 1991;51:47–60. [Google Scholar]
- Su J.W., Yang Y., Xia G.L., Chen J.T., Jiang P., Chen Q.W. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017;8:14969. doi: 10.1038/ncomms14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J.W., Ge R.X., Jiang K.M., Dong Y., Hao F., Tian Z.Q., Chen G.X., Chen L. Assembling ultrasmall copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: highly robust electrocatalysts for oxygen evolution in acidic Media. Adv. Mater. 2018;30:1801351. doi: 10.1002/adma.201801351. [DOI] [PubMed] [Google Scholar]
- Suen N.T., Hung S.F., Quan Q., Zhang N., Xu Y.J., Chen H.M. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 2017;46:337–365. doi: 10.1039/c6cs00328a. [DOI] [PubMed] [Google Scholar]
- Sun W., Song Y., Gong X.Q., Cao L.M., Yang J. An efficiently tuned d-orbital occupation of IrO2 by doping with Cu for enhancing the oxygen evolution reaction activity. Chem. Sci. 2015;6:4993–4999. doi: 10.1039/c5sc01251a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbrock S., Scharfschwerdt C., Neumann M., Illing G., Freund H.J. The influence of defects on the Ni-2p and O-1s XPS of Nio. J. Phys. Condens. Mater. 1992;4:7973–7978. [Google Scholar]
- Wang X., Zhuang L.Z., Jia Y., Liu H.L., Yan X.C., Zhang L.Z., Yang D.J., Zhu Z.H., Yao X.D. Plasma-triggered synergy of exfoliation, phase transformation, and surface engineering in cobalt diselenide for enhanced water oxidation. Angew. Chem. Int. Ed. 2018;130:16659–16663. doi: 10.1002/anie.201810199. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhuang L.Z., He T.W., Jia Y., Zhang L.Z., Yan X.C., Gao M.R., Du A.J., Zhu Z.H., Yao X.D., Yu S.-H. Grafting cobalt diselenide on defective graphene for enhanced oxygen evolution reaction. iScience. 2018;7:145–153. doi: 10.1016/j.isci.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G.F., Liu Z.P. Towards active and stable oxygen reduction cathodes: a density functional theory survey on Pt2M skin alloys. Energy Environ. Sci. 2011;4:1268–1272. [Google Scholar]
- Xia B.Y., Yan Y., Li N., Wu H.B., Lou X.W., Wang X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy. 2016;1:15006. [Google Scholar]
- Xu L., Jiang Q.Q., Xiao Z.H., Li X.Y., Huo J., Wang S.Y., Dai L.M. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2016;55:5277–5281. doi: 10.1002/anie.201600687. [DOI] [PubMed] [Google Scholar]
- Xu L., Zou Y.Q., Xiao Z.H., Wang S.Y. Transforming Co3O4 nanosheets into porous N-doped CoxOy nanosheets with oxygen vacancies for the oxygen evolution reaction. J. Energy Chem. 2018;35:24–29. [Google Scholar]
- Yoo J.S., Rong X., Liu Y.S., Kolpak A.M. Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites. ACS Catal. 2018;8:4628–4636. [Google Scholar]
- Zheng Y., Jiao Y., Zhu Y.H., Li L.H., Han Y., Chen Y., Jaroniec M., Qiao S.Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 2016;138:16174–16181. doi: 10.1021/jacs.6b11291. [DOI] [PubMed] [Google Scholar]
- Zhou P., He J.Y., Zou Y.Q., Wang Y.Y., Xie C., Chen R., Zang S.Q., Wang S.Y. Single-crystalline layered double hydroxides with rich defects and hierarchical structure by mild reduction for enhancing the oxygen evolution reaction. Sci. China Chem. 2019;62 [Google Scholar]
- Zhuang L.Z., Jia Y., Liu H.L., Wang X., Hocking R.K., Liu H.W., Chen J., Ge L., Zhang L.Z., Li M.R. Defect-induced Pt–Co–Se coordinated sites with highly asymmetrical electronic distribution for boosting oxygen-involving electrocatalysis. Adv. Mater. 2019;31:1805581. doi: 10.1002/adma.201805581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.