Increases in tick-borne disease prevalence and transmission are important public health issues. Efforts to control these emerging diseases are frustrated by the struggle to control tick populations and to detect and treat infections caused by the pathogens that they transmit. This review covers tick-borne infectious diseases of nonrickettsial bacterial, parasitic, and viral origins.
KEYWORDS: tick borne, babesiosis, blood-borne parasites, Babesia, Babesia microti, emerging disease, Anaplasma phagocytophilum, Ehrlichia chaffeensis, Ehrlichia ewingii, Borrelia miyamotoi, Lyme, Borrelia, Borrelia burgdorferi, Borrelia mayonii, Ixodes, Amblyomma, tick-borne encephalitis virus (TBEV), Powassan virus (POWV), deer tick virus (DTV), Crimean-Congo hemorrhagic fever virus (CCHFV)
SUMMARY
Increases in tick-borne disease prevalence and transmission are important public health issues. Efforts to control these emerging diseases are frustrated by the struggle to control tick populations and to detect and treat infections caused by the pathogens that they transmit. This review covers tick-borne infectious diseases of nonrickettsial bacterial, parasitic, and viral origins. While tick surveillance and tracking inform our understanding of the importance of the spread and ecology of ticks and help identify areas of risk for disease transmission, the vectors are not the focus of this document. Here, we emphasize the most significant pathogens that infect humans as well as the epidemiology, clinical features, diagnosis, and treatment of diseases that they cause. Although detection via molecular or immunological methods has improved, tick-borne diseases continue to remain underdiagnosed, making the scope of the problem difficult to assess. Our current understanding of the incidence of tick-borne diseases is discussed in this review. An awareness of the diseases that can be transmitted by ticks in specific locations is key to detection and selection of appropriate treatment. As tick-transmitted pathogens are discovered and emerge in new geographic regions, our ability to detect, describe, and understand the growing public health threat must also grow to meet the challenge.
INTRODUCTION
Tick populations are increasing, and their geographic ranges are expanding, as are suitable habitats for these arthropod vectors and the pathogens that they carry. Ticks can transmit bacterial, parasitic, and viral pathogens and often harbor more than one agent simultaneously. In this review on emerging tick-borne diseases, we discuss all three types of pathogens, including recently identified species of bacteria, new tick-borne viruses, as well as new locations or foci of endemicity. Rickettsioses were previously reviewed in this journal and are not included here (1).
For this review, we have drawn on an extensive examination of the literature as well as our basic research and public health experience with human tick-borne diseases (Table 1). While coinfections per se are outside the scope of this review, we know that not only do ticks carry more than one pathogen, they also can transmit more than one pathogen when taking a blood meal. Thus, taking a broader view is important when considering proper diagnosis and treatment. Here, we discuss the epidemiology and transmission of these agents and the clinical presentation in the patient, pointing out features that are unique to certain pathogens, such as the erythema migrans (EM) rash of Lyme disease, as well as less specific symptoms, such as malaise, headache, myalgia, or fever, that accompany many infections.
TABLE 1.
Tick-borne diseases discussed in this review
| Diseasea | Causative organism | Tick vector(s) | No. of reported casesb | Area(s) of endemicity |
|---|---|---|---|---|
| Lyme disease | Borrelia burgdorferi | Ixodes scapularis, Ixodes pacificus | 36,429/yr | Northeastern and northern Midwest U.S. |
| B. mayonii | B. mayonii | Ixodes scapularis | NA | Northern Midwest U.S. |
| B. miyamotoi | B. miyamotoi | Ixodes scapularis, I. pacificus, I. ricinus, I. persulcatus | NA | Northeastern and northern Midwest U.S., California, China, Japan, Russia |
| Tick-borne encephalitis | Tick-borne encephalitis virus (Flaviviridae: Flavivirus) | Ixodes ricinus, Ixodes persulcatus | >5,000–12,000/yr | Eurasia |
| Crimean-Congo hemorrhagic fever | Crimean-Congo hemorrhagic fever orthonairovirus (Nairoviridae: Orthonairovirus) | Hyalomma spp., Rhipicephalus sanguineus, among others | >5,000 | Africa, Asia, eastern Europe, the Middle East, the Indian subcontinent |
| HGAc | Anaplasma phagocytophilum | Ixodes scapularis, I. pacificus | 4,151/yr | Northeastern U.S. |
| Babesiosisd | Babesia microti | Ixodes scapularis, Ixodes pacificus | 1,910/yr | Northeastern and northern Midwest U.S. |
| Babesiosis | Babesia venatorum, B. microti, B. divergens | Ixodes ricinus | NA | Europe, northwestern China |
| HME | Ehrlichia chaffeensis | Amblyomma americanum, Dermacentor variabilis | 1,377/yr | Northeastern mid-Atlantic and Midwest U.S. |
| Severe fever with thrombocytopenia | Severe fever with thrombocytopenia virus (Phenuiviridae: Phlebovirus) | Haemaphysalis longicornis | >650 | China, Japan, South Korea |
| Heartland virus disease | Heartland virus (Phenuiviridae: Phlebovirus) | Amblyomma americanum | 40f | Midwest and southern U.S. |
| E. ewingii infection | E. ewingii | Amblyomma americanum and others | 22/yr | Midwest |
| Powassan encephalitise | Powassan virus lineages 1 and 2 (Flaviviridae: Flavivirus) | Ixodes scapularis, Ixodes cookei | 133 | Northeastern and northern Midwest U.S. |
| Bourbon virus disease | Bourbon virus (Orthomyxoviridae: Thogotovirus) | Amblyomma americanum | >5g | Midwest and southern U.S. |
HGA, human granulocytic anaplasmosis; HME, human monocytic ehrlichiosis.
Numbers of cases and geographic locations in the United States are from the CDC (66) unless otherwise noted. NA, data not available.
Formerly known as human granulocytic ehrlichiosis (HGE).
Includes cases reported from 24 of 33 states where babesiosis is reportable.
An excellent review of tick-borne viruses in the world can be found in reference 289.
Numbers of cases and geographic locations for Heartland virus can be found at https://www.cdc.gov/heartland-virus/statistics/index.html.
Information on Bourbon virus can be found in reference 285.
Given the nonspecific nature of the symptoms of many of the diseases, knowledge of the ticks that are found in a given area, the diseases that those ticks carry, and which life cycle stages can transmit are all pieces of the puzzle to determine the best course for diagnosis and treatment. In many cases, especially for newly identified pathogens or those that are just emerging in a new part of the world, public health and commercial reference laboratories are important diagnostic resources. Awareness is vital to mounting an appropriate public health response and motivating personal protective practices that minimize risk. An appreciation of the scope of the problem will help drive better solutions to decrease the tick population and human exposure.
BACTERIA (NONRICKETTSIAL)
Tick-borne bacteria are found throughout the world in ever-expanding ranges. The geographic spread of tick species caused by micro- and macroclimate changes, human behavior, land use, the vector population, population growth, and many other factors has allowed tick-borne bacterial diseases to follow in their wake. As we continue to discover new species of these bacteria, it is important to understand host transmission and to monitor the emergence of both new and existing pathogens.
Anaplasma phagocytophilum and Ehrlichia Species
Anaplasma and Ehrlichia bacteria are small, Gram-negative, obligately intracellular alphaproteobacteria in the order Rickettsiales, family Anaplasmataceae (2–4). They cause nonspecific febrile illnesses that are mostly self-limiting. However, in some cases, such as infection with Ehrlichia chaffeensis and Anaplasma phagocytophilum, illness can be severe, or even fatal, if left untreated. Older individuals, patients with comorbidities, or those who are immunocompromised have a greater risk of morbidity or mortality if treatment is not provided or is delayed. Since the symptoms are nonspecific, these infections can be challenging to diagnose and differentiate from each other. However, when the geographic locations of various pathogens are separate, the diagnosis is simplified. Infections may also be asymptomatic, which is one of the reasons why they are underreported (5, 6).
History.
(i) Anaplasma phagocytophilum.
A. phagocytophilum is a zoonotic tick-borne pathogen transmitted by infected Ixodes ticks (7). Ixodes ticks feed on deer, ruminants, equines, rodents, and other mammals. The major reservoir species are the white-tailed deer and the white-footed mouse (8). As with E. chaffeensis, the infection is maintained in the ticks transstadially, with nymphal and adult ticks infecting humans, which are dead-end hosts (9). Transstadial transmission, unlike transovarial transmission, precludes the transmission of infection by larval ticks. A. phagocytophilum resides in an intracytoplasmic inclusion body or morulae in human granulocytes (10). Anaplasma species are known as both human and veterinary pathogens; however, A. phagocytophilum is the primary species that infects humans. Thieler first identified Anaplasma marginale as the etiological agent of a devastating erythrocytic pathogen of cattle in 1910 (7). A. phagocytophilum was first discovered in humans in 1994 and was thought to be a new Ehrlichia species found in neutrophils, which garnered it the name Ehrlichia phagocytophilum, the causative agent of human granulocytic ehrlichiosis (HGE) (2, 3, 7). The bacteria resembled Ehrlichia equi, which also has a tropism for neutrophils and is a pathogen of horses. In 2001, E. phagocytophilum was officially renamed Anaplasma phagocytophilum. The disease was referred to as human granulocytic anaplasmosis (HGA) but now is more commonly referred to as anaplasmosis (11).
(ii) Ehrlichia chaffeensis.
E. chaffeensis is a zoonotic tick-borne pathogen transmitted by infected Amblyomma americanum ticks (7). A. americanum ticks feed on a large number of host species, but the major reservoir is considered the white-tailed deer (6). The bacteria are maintained in the ticks transstadially (from larva to nymph to adult); therefore, humans are infected by ticks only in the nymph and adult life stages. Since A. americanum larvae are active earlier in the spring, the period of higher risk for being exposed to this pathogen is longer than for those carried by Ixodes ticks. E. chaffeensis resides in phagosomes (morulae) of monocytes (4), while other species of Ehrlichia, such as Ehrlichia ewingii, reside in morulae in granulocytes (2, 4). E. chaffeensis is the most common species of Ehrlichia to infect humans.
Ehrlichia bacteria were originally classified in the genus Rickettsia in 1932 and credited with causing tick-borne fever (TBF), which is a devastating illness in ruminants. E. chaffeensis was discovered in humans in the United States in 1986 and became reportable to the CDC in 1994 (2, 7, 9). The first human case of E. chaffeensis in Europe was reported in Portugal in 1991 (12). The most recently discovered Ehrlichia species to infect humans is Ehrlichia muris subsp. eauclairensis, which is endemic in the upper Midwest of the United States. Other members of the genus are important veterinary pathogens (9).
(iii) Ehrlichia ewingii.
E. ewingii is an emerging Ehrlichia species that infects humans and is transmitted by infected larval and nymphal A. americanum ticks. The major reservoir for these bacteria has not yet been identified; however, E. ewingii has been found in deer, dogs, and goats (6, 10). It was first discovered in 1992 in the United States when it was implicated in canine disease (10). It was first reported in humans in 1999, when 16S sequencing of bacteria from 413 patients, collected from 1994 to 1998 in Missouri, revealed 4 patients carrying bacteria matching the E. ewingii sequence (13, 14). Infections with E. ewingii became reportable to the CDC in the United States in 2008 (6).
Epidemiology and ecology.
(i) Anaplasma phagocytophilum.
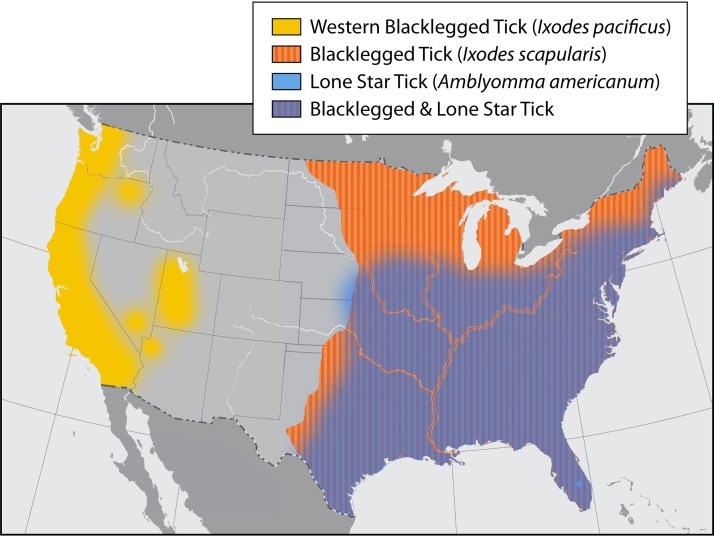
In North America, A. phagocytophilum is now found on the East Coast and in midwestern regions of the United States, where it is transmitted by Ixodes scapularis, and on the Pacific Coast, where it is transmitted by Ixodes pacificus (Fig. 1) (5). A. phagocytophilum became reportable to the CDC in 2000. By 2010, there were cases reported in 27 states, with the incidence of the disease increasing from 1.4 to 6.1 cases per million people (6). I. scapularis has greatly expanded its geographic range in the United States and Canada. Between 1996 and 2016, the number of U.S. counties with established populations of I. scapularis doubled to 44.7%. This expansion has been enabled by habitat and climate changes and the availability of hosts (especially deer) and Peromyscus leucopus, which is the major reservoir (15).
FIG 1.
Areas of the United States where I. scapularis, A. americanum, and I. pacificus are endemic. Pathogens that can be transmitted by I. scapularis include A. phagocytophilum, B. burgdorferi, B. miyamotoi, Babesia microti, Babesia divergens, and POWV. Pathogens that can be transmitted by I. pacificus include A. phagocytophilum, B. burgdorferi, B. miyamotoi, Babesia microti, and Babesia duncani. Pathogens that are transmitted by A. americanum include E. chaffeensis, E. ewingii, Heartland virus, and Bourbon virus. Where tick species ranges overlap, all pathogens need to be considered in making a diagnosis.
A. phagocytophilum is distributed over large portions of Europe and parts of central Asia, where it is vectored by Ixodes ricinus ticks. The range of Ixodes persulcatus ticks overlaps the range of I. ricinus ticks and extends coverage farther into Asia (Fig. 2) (2, 16, 17). The northward expansion of I. ricinus ticks in Sweden, Russia, and other parts of northern Europe has been attributed to milder winters. This climate difference can have multiple benefits for the ticks, including better host survival, better tick survival, and more plentiful host food sources. The northern expansion of I. persulcatus has also been linked to climate change (15).
FIG 2.
Areas where Ixodes species are endemic are at risk for transmission of Lyme borreliosis, A. phagocytophilum, and Babesia sp. Data presented in this map were collected through the VectorNet project. Countries and regions are displayed at different scales to facilitate visualization. Antic., anticipated; Obs., observed. (Adapted from European Centre for Disease Prevention and Control and European Food Safety Authority maps at https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps.)
Serologic evidence backs up data on the spread of A. phagocytophilum throughout the range of these ticks (4, 11, 16). The first serologic evidence of A. phagocytophilum was found in Switzerland in 1995, followed by a case in Slovenia in 1997, which was confirmed by serology, PCR, and sequencing (18). A. phagocytophilum cases have now been reported from Austria, Croatia, France, Italy, Latvia, the Netherlands, Norway, Poland, Slovenia, Spain, and Sweden (5, 9, 10, 16, 18). There have also been reports from several Asian countries, including Russia, China, and South Korea (6).
(ii) Ehrlichia chaffeensis and E. ewingii.
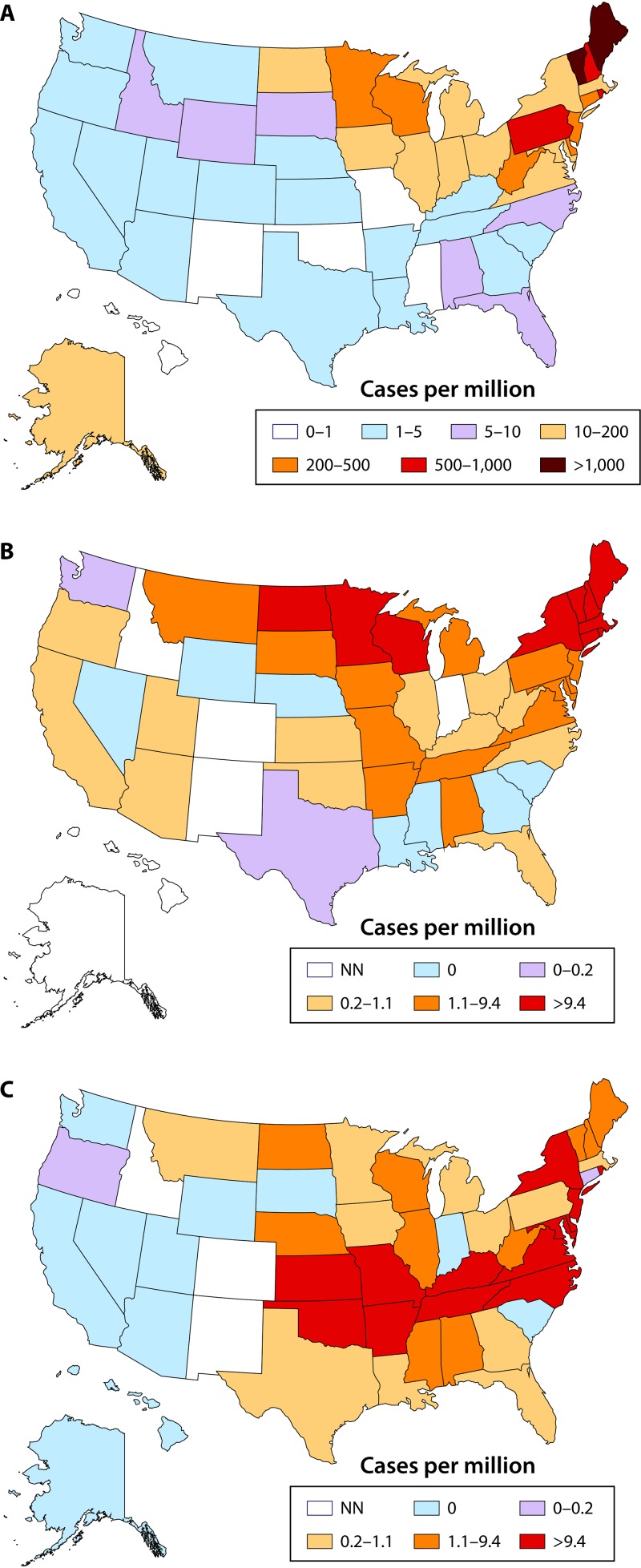
E. chaffeensis and E. ewingii are found in the southeastern, southern, central, and mid-Atlantic portions of the United States. These regions coincide with the range of the principal vector, A. americanum. As of 2008, E. chaffeensis had been reported in 29 states. By 2015, E. chaffeensis infection was reported in 35 states, with most cases coming from Missouri, Arkansas, New York, and Virginia (Fig. 3) (19). In 2008, the year when E. ewingii became reportable to the CDC, infections were reported in five states, with most cases coming from Missouri and Minnesota. In 2015, E. ewingii infections had expanded to only two more states; however, it is likely that infections with E. ewingii are underreported, as testing is not readily available (6).
FIG 3.
Incidence of bacterial tick-borne diseases in the United States in 2017. (A) Lyme disease. Note that the scale is different for Lyme disease due to the greater incidence. (B and C) Anaplasmosis (B) and ehrlichiosis (E. chaffeensis) (C). NN, not notifiable. (Based on data from the Centers for Disease Control and Prevention at https://www.cdc.gov/lyme/stats/tables.html [panel A], https://www.cdc.gov/anaplasmosis/stats/index.html [panel B], and https://www.cdc.gov/ehrlichiosis/stats/index.html [panel C].)
Serologic and molecular evidence of E. chaffeensis infection has come primarily from studies conducted in North America. There have been reports in the literature of human infection with E. chaffeensis in Mali, South Korea, Southeast Asia, and Peru, although serologic methods lack specificity to distinguish between E. chaffeensis and related species (10). The availability of diagnostic testing and the lack of awareness of the disease contribute to the lack of knowledge of the disease burden (20). In the European Union, E. chaffeensis is not a reportable disease. Only Borrelia sensu lato species (discussed below) are reported, and thus, it is difficult to determine the actual burden of disease in this region as well.
Clinical features.
(i) Anaplasma phagocytophilum and Ehrlichia chaffeensis.
The clinical presentations of infections with A. phagocytophilum and E. chaffeensis are very similar but have a few notable differences. The onset of symptoms occurs 1 to 2 weeks postexposure, with about 75% of people reporting a tick bite (5). Unlike the typical 36 to 72 h from tick bite to human transmission for Lyme disease, rodent models have shown that the transmission time for A. phagocytophilum can be within 24 h (21). This highlights the need for prompt tick checks to prevent disease transmission after engaging in activities, or visiting areas, where ticks may be encountered. Symptoms can consist of general cold-like symptoms or begin more abruptly as flu-like symptoms, with high fever, rigors, generalized myalgia, severe headache, and gastrointestinal symptoms. Central nervous system (CNS) involvement occurs more commonly with E. chaffeensis (5, 7, 9, 22). A generalized rash is an uncommon symptom for A. phagocytophilum but may be seen in E. chaffeensis, especially in children (6). When symptoms are mild, they may go unnoticed and resolve on their own. This accounts for seroprevalence studies suggesting that infection rates are higher than the number of reported cases (3). The case fatality rate of A. phagocytophilum is relatively low at 0.3% (based on data from 2008 to 2012), while the fatality rate for E. chaffeensis is higher at 3%. This higher observed fatality rate for E. chaffeensis is due to the greater severity of the disease, particularly if the patient is immunosuppressed (5, 23, 24). The initial laboratory findings in the acute phase of both anaplasmosis and ehrlichiosis are similar and include moderate leukopenia, thrombocytopenia, a 2- to 4-fold increase in liver enzymes, increased C-reactive protein, and an increased erythrocyte sedimentation rate. A. phagocytophilum is rarely transmitted by blood transfusion. Only 10 cases of transfusion-transmitted anaplasmosis have been reported, but they include 1 fatal case (25, 26). There have been no published reports of E. chaffeensis transmitted by blood products. However, there are two reports of transmission through organ transplantation associated with a single donor (27).
(ii) Ehrlichia ewingii.
The clinical symptoms and laboratory results for E. ewingii are very similar to those for E. chaffeensis or A. phagocytophilum. The most common symptoms are fever, headache, malaise, and myalgia (6). Laboratory results include thrombocytopenia, with or without leukopenia, and elevated liver enzymes (13, 28). It should be noted that in E. chaffeensis infections, morulae would be seen in monocytic cells seen on a Giemsa stain, while in E. ewingii infections, morulae would be seen in neutrophils. However, because the possibility of E. ewingii infection is not yet widely appreciated in clinical laboratories, morulae identified in neutrophils are likely to be attributed to A. phagocytophilum and not E. ewingii. In serological studies using indirect immunofluorescence, patient antibodies to E. ewingii cross-react with E. chaffeensis (13). These factors make the differentiation of E. ewingii from E. chaffeensis difficult, which may result in E. ewingii infections being missed or misclassified as E. chaffeensis infections (29).
No deaths have been attributed to E. ewingii, which causes less severe disease than E. chaffeensis (6, 30). There has been only one report of transfusion transmission of E. ewingii; this was attributed to platelet transfusion and occurred in 2011 (30).
Diagnosis.
E. ewingii is not cultivable (31); however, E. chaffeensis and A. phagocytophilum can be grown in tissue culture. While culture is the gold standard, it is not widely available, is resource-intensive, and takes more than 2 weeks to obtain results (10).
During the first 1 to 2 weeks of infection, real-time PCR is the most specific, sensitive, and widely available method for the detection and identification of E. chaffeensis, A. phagocytophilum, and E. ewingii. After the first 1 to 2 weeks, the infection rapidly wanes, and the likelihood of detection decreases, limiting the value of PCR (4). The sensitivity of PCR for detecting E. chaffeensis is 60 to 80%, and the sensitivity for detecting A. phagocytophilum is 67 to 90%, depending on the number of days since the infection was acquired (9, 10, 17). Giemsa-stained peripheral blood smears can be examined microscopically for morulae, but highly experienced microscopists are essential. This method is rapid but much less sensitive than PCR (6, 9, 10). Currently, when morulae are found in neutrophils, however, they are considered a diagnostic indicator of A. phagocytophilum rather than E. ewingii because E. ewingii is less well known by both physicians and laboratorians (5, 10, 22).
Whole blood is the most appropriate sample for PCR testing due to the presence of the organisms in peripheral blood leukocytes. While cerebrospinal fluid (CSF) can also be tested for A. phagocytophilum and E. chaffeensis, testing of this sample type may not be available in commercial laboratories. Recently, in addition to E. chaffeensis, E. ewingii and E. muris subsp. eauclairensis have been added to Ehrlichia species PCR assays available at several commercial reference laboratories. E. muris subsp. eauclairensis is a rare human pathogen that was found in 2009 in a very limited area in the northern Midwest of the United States. Unlike other Ehrlichia species, E. muris subsp. eauclairensis is transmitted by I. scapularis and not A. americanum ticks. Since E. muris subsp. eauclairensis cannot be cultured, PCR is the only definitive test available.
The A. phagocytophilum and E. chaffeensis antibody-specific IgG and IgM immunofluorescence (IFA) assay is the most frequently used confirmatory test (22). Since antibody is not usually present in the first week of illness when patients typically present, a diagnosis is made by collecting paired sera 2 to 6 weeks apart and showing a 4-fold difference in titers or an IgG titer of least 1:256. It should be noted that there are high rates of cross-reactivity between antibodies to A. phagocytophilum and those to E. chaffeensis, so generally, serologic testing for both pathogens should be performed. If the titer is much higher for one organism than the other, the one with the higher titer should be considered the likely causative agent. Antibodies to E. ewingii also cross-react, such that infections may be mistakenly diagnosed as E. chaffeensis or A. phagocytophilum infection. False-negative serology results may occur if a patient is immunocompromised or was treated very early in the disease (9, 22). Conditions that may cause false-positive results include infections such as Rocky Mountain spotted fever, typhus, Q fever, brucellosis, Lyme disease, Epstein-Barr virus infection, and several autoimmune conditions (10). When interpreting serology results, not only treatment and immune status but also other factors, such as the patient’s travel history, geographic location, medical history, and activities, should be considered. Health care providers should consider the geographic location of the patient and the range of tick species that are endemic in the area where the patient was likely to have become infected. Testing for Ehrlichia species should be considered for patients living in areas where A. americanum is endemic. Testing for A. phagocytophilum, Borrelia burgdorferi, Babesia microti, and Borrelia miyamotoi should be considered for patients living in or traveling through areas where infections caused by these pathogens are endemic. Where there is overlap of regions where specific infections are endemic (Fig. 3) or travel is uncertain, testing should be more comprehensive.
Treatment.
Currently, no vaccines are available to protect against infections with Anaplasma phagocytophilum or Ehrlichia species. Due to the inherent delay in obtaining confirmatory test results, empirical therapy is recommended when suspicion is high and in cases of severe illness. E. chaffeensis, E. ewingii, and A. phagocytophilum are universally susceptible to doxycycline (5, 9, 14, 32). There have been no studies addressing the duration of treatment, but most authorities advocate at least 3 days after defervescence and until there is evidence of clinical improvement, typically 5 to 7 days (6, 9). Prompt defervescence, within 48 h, is typical. If concurrent Lyme disease is suspected, treatment should be extended to 10 to 14 days (6, 33). Treatment relapses or antibiotic resistance has not been reported (4, 9, 34). (For additional details on treatment, see references 33 and 35–37.)
The best method to prevent becoming infected with A. phagocytophilum or E. chaffeensis is to limit exposure to ticks. These infectious agents can be transmitted within a few hours postattachment; therefore, even rapid removal may not prevent infection (10).
Borrelia burgdorferi and Lyme Disease
Borrelia burgdorferi is a highly motile, helix-shaped organism that can be visualized under dark-field microscopy and is grouped with two other pathogenic spirochete genera, Leptospira and Treponema. B. burgdorferi is in the order Spirochaetales, family Spirochaetaceae (38). The Borrelia genus is divided into the Lyme disease group (B. burgdorferi sensu lato complex) and relapsing fever Borrelia species (including B. hermsii, B. turicatae, B. parkeri, and B. miyamotoi). In 2015, Lyme disease-causing Borrelia species, which cause different symptomology and have a different ecology than relapsing fever Borrelia species, were separated into a new genus named Borreliella. The relapsing fever Borrelia species remained in the genus Borrelia. This nomenclature is officially recognized but is not yet widely used (39).
B. burgdorferi is a zoonotic tick-borne pathogen transmitted by the bite of an infected Ixodes tick (Fig. 1 and 2). It is highly invasive and infects more humans in Europe, Asia, Russia, and North America than any other tick-borne bacteria. Worldwide, there are 12 Borrelia genospecies within the B. burgdorferi sensu lato complex; however, not all of them cause human disease. In North America, B. burgdorferi sensu stricto was the sole species known to infect humans until 2016, when B. mayonii was identified as a new genospecies in the sensu lato complex (14, 35, 40, 41). In Europe, the main species that infect humans are B. burgdorferi, B. afzelii, and B. garinii, whereas in Asia, B. afzelii and B. garinii are considered the main species involved in human disease. B. mayonii is transmitted transstadially as well as transovarially, making it possible for ticks in all life stages to transmit disease (40). B. burgdorferi, B. afzelii, and B. garinii are considered to be transmitted transstadially only (42).
History.
Lyme disease, subsequently found to be caused by B. burgdorferi, was first recognized in the United States in Lyme, CT, in 1975 (43). However, descriptions of a Lyme disease-like syndrome were first reported in the literature in Europe by as early as 1883, and in the 1940s, antibiotics were already being used to treat patients with symptoms compatible with those of Lyme disease (44). B. burgdorferi was first cultured from I. scapularis in 1982 and then subsequently from human skin and cerebrospinal fluid (35). The disease became reportable to the CDC in 1990 (6). B. afzelii and B. garinii are the primary species responsible for causing Lyme borreliosis in Europe. B. garinii was officially recognized in the Journal of Systematic Bacteriology in 1992, and B. afzelii was differentiated from B. burgdorferi and B. garinii in 1993 (45).
Epidemiology and ecology.
Lyme disease is widely spread across the United States, Europe, Asia, and Japan (46, 47). It is the most commonly reported tick-borne disease in the United States and is highly endemic in the Northeast and northern Midwest regions of the United States (Fig. 3). I. scapularis expanded northward into eastern and central Canada in 2004, which was followed by the emergence of Lyme disease (15). Migratory birds have been considered a possible mode for that expansion (37). Of note, although I. scapularis is found throughout the southeastern United States, cases of Lyme disease are rare or unconfirmed. According to the CDC, there were 22,561 cases of Lyme disease in the United States in 2010, with the rate of disease increasing rapidly to a total of 28,453 confirmed cases in 2015. This number is considered severely underreported due to undiagnosed cases, inconsistency in reporting, and empirical treatment (36). The CDC estimates that the real number of new cases annually in the United States is approximately 300,000 (37). Borrelia mayonii is a newly described species that has been found only in the upper Midwest of the United States (14, 41).
B. afzelii and B. garinii are spread by I. ricinus in Europe and I. persulcatus in Asia, with the habitats of these two species overlapping in eastern Europe (Fig. 2) (35, 48). Cases of Lyme borreliosis have been reported in Austria, Belgium, Bosnia, Bulgaria, Croatia, the Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Norway, Russia, Sweden, and the United Kingdom (Fig. 2). Lyme disease in Europe is most widely reported in temperate zones, where approximately 85,000 cases were reported in 2016. However, as in the United States, the disease is likely underreported (48). Ixodes species continue to expand, or even shift their range, because of a dynamic, interwoven, and ever-changing combination of human behavior; modification of habitats; human, host, and tick population growth; and micro- and macroclimate changes (15).
Clinical features.
Signs and symptoms of Lyme disease are broken into three stages: early localized, early disseminated, and late disease. In the early stage, symptoms include fever, chills, headache, fatigue, muscle and joint aches, and one or more erythema migrans (EM) rashes (36, 38, 49). The classic EM rash occurs in approximately 70 to 80% of people and occurs at the site of the tick bite 3 to 30 days (average, 7 days) after the tick bite, typically before the onset of fever. Disease transmission from tick to human occurs within 36 to 72 h. Again, it should be stressed that checking for ticks immediately after being in an area where ticks may be present is important for preventing the chance of disease transmission.
The early disseminated stage occurs within days to weeks of initial symptoms. Patients may continue to have fever, fatigue, headache, myalgia, and arthralgia during this stage. More severe symptoms can include a variety of neurological manifestations, such as cranial nerve palsies, peripheral neuropathy, radiculopathy (Bannwarth syndrome), mononeuropathy multiplex, meningitis, and, more rarely, carditis (36, 38, 50). Carditis symptoms include palpitations, syncope, chest pain, and dyspnea in conjunction with other common Lyme disease symptoms. The carditis symptoms typically occur within 2 to 4 weeks but in rare cases have occurred up to 7 months after the initial infection. Carditis is a rare complication that occurs when the spirochetes invade heart tissue and cause various degrees of atrioventricular block. About 1% of cases reported to, and confirmed by, the CDC had second- or third-degree heart block (51). Death due to Lyme carditis is rare, and with proper treatment, the prognosis is excellent (37). (For the most up-to-date treatment recommendations, see the IDSA guidelines [52, 288].)
The most common type of nervous system involvement, especially in children, is facial nerve palsy or “Bell’s palsy,” which can be bilateral. The most common symptom of late-stage Lyme disease is intermittent or persistent arthritis in one or more large joints (Lyme arthritis) and, less commonly, subtle encephalopathy or neuropathy (5). A range of symptoms occurs in Europe as well as North America, although there are differences based on the species of Borrelia causing the infection. In Europe, B. afzelii is known for the skin infection acrodermatitis chronica atrophicans, and B. garinii is more neurotropic and related to Bannwarth syndrome (36–38). Patients infected with B. mayonii have symptoms similar to those of patients infected with bacteria in the B. burgdorferi sensu lato complex, which makes infections with B. mayonii clinically indistinguishable from Lyme disease caused by B. burgdorferi (41).
Diagnosis.
Clinical diagnosis begins with evaluating the epidemiological factors of the patient (lifestyle, line of work, travel history, and living in an area of endemicity) as well as clinical symptoms such as EM rash and arthritic joints. Most cases of Lyme disease are diagnosed and treated after the identification of an EM rash (37). Unfortunately, Borrelia species, with the possible exception of B. mayonii, are not reliably present in blood during acute infections. Thus, laboratory diagnosis of infection relies on the antibody response. Serologic testing is the preferred tool to support a diagnosis.
The current recommendation for serologic testing is a two-tier testing system (35). Standard two-tier testing (STTT) consists of an initial enzyme immunoassay (EIA) or a chemiluminescence immunoassay (CIA) (commonly used in large laboratories), followed by Western blot testing if the initial results show reactivity or are equivocal. There are two types of EIAs available, the whole-cell sonicate and the more specific C6 peptide EIAs (33, 36). A C6 CIA is also available (53). A multiplex microsphere assay utilizing the antigens VlsE1-IgG and pepC10-IgM (a conserved portion of OspC to which early IgM is directed) is another option that appears to provide an earlier diagnosis (54). The C6 EIA is more specific because it uses antigens specific to Borrelia and reduces cross-reactivity to related species. However, this test has recently been shown to cross-react with B. miyamotoi, so it is important to interpret the test result based on possible exposure to B. miyamotoi (55). It should also be noted that C6 testing is more valuable when European strains are suspected because the Western blots designed for use in the United States have poor sensitivity for European Borrelia strains (33). If a patient has a negative or equivocal EIA result, they may be in the early phase of the disease, or an alternative diagnosis might be considered. A patient with appropriate signs and symptoms, such as EM rash, may be treated empirically, and a convalescent-phase sample may be drawn in 2 to 4 weeks (37).
In second-tier testing with Western blotting, antibodies bind to a set of conserved B. burgdorferi protein antigens. Test guidelines in the United States are based on the number of proteins detected as well as reactivity to specific proteins. A patient in the first 30 days of symptoms (early phase) is considered to have a positive IgM blot if they have antibodies to at least two of the following three protein bands: 23, 39, and 41 kDa. However, false-positive Lyme IgM blots are not uncommon and can occur with a variety of other infections and with autoimmune disease. If symptoms have been present for more than 30 days, an IgG blot should also be positive. If not, a positive IgM blot is likely a false-positive result. A positive IgG blot can be observed in early disseminated and late-stage disease or can reflect past infection. A positive result requires the detection of 5 of the following 10 protein bands: 18, 23, 28, 30, 39, 41, 45, 58, 66, and 93 kDa (36, 37). Antibodies may not be present early in the course of infection. When no EM rash is detected and B. burgdorferi antibodies are not found or are not diagnostic in the acute phase, a convalescent-phase specimen should be drawn if Lyme disease is still suspected. False-negative serology results may also occur if a patient is immunocompromised or was treated very early in the course of the disease.
The FDA recently cleared a modified two-tier testing (MTTT) algorithm as an alternative to STTT. This algorithm utilizes two FDA-cleared first-tier EIAs, run concurrently or sequentially, and omits immunoblot testing. Both EIAs must be positive for a specimen to be considered positive. MTTT is an improvement compared with STTT, which is less sensitive for detecting early infections, more subjective to interpret, and more labor-intensive. The MTTT algorithm thus would simplify testing, lower costs, shorten the time to results, and potentially improve both sensitivity and specificity (54, 56, 57). However, for Lyme arthritis or for complex cases, Western blotting may provide more information and thus may retain a diagnostic role (A. Steere, personal communication).
In both early disseminated and late-stage neurological disease, PCR of CSF is often negative. Thus, the diagnosis of neuroborreliosis relies on documentation of local antibody production in the CSF. Currently, detection of antibodies in simultaneously collected serum and CSF, and calculation of an index to confirm intrathecal antibody production, is considered the preferred approach. However, recommended test algorithms may vary for early disseminated versus late-stage disease (35, 37, 58).
When positive, PCR can provide supporting evidence of B. burgdorferi infection. PCR is best applied for the diagnosis of Lyme arthritis (36). Testing of synovial fluid has >75% sensitivity in IgG-positive patients (33). PCR testing of blood and CSF is usually negative and of limited value due to the low number of bacteria in these specimen types (33, 35, 36). Testing blood for B. mayonii is an exception because of the high level of spirochetemia associated with the infection in the early stages of the disease (41). Culture is not recommended for B. burgdorferi since it is a slow-growing organism and the test is labor-intensive and is more suited to a research setting (36).
The newest technology being developed to improve the early diagnosis of Lyme disease is metabolomics (36). This method uses liquid chromatography-mass spectrometry to detect low-molecular-weight biomolecules that are produced by the body during illness. By developing a biosignature of early Lyme disease that is absent in healthy individuals or those with other diseases, it may be possible to establish a Lyme disease diagnosis using this approach. Studies by Molins et al. and Theel reported 88% sensitivity and 95% specificity for metabolomic testing (59, 60). This test is currently in the research phase and is not yet available for clinical testing.
Measurement of chemokine CXCL13 levels in CSF of adults and children is also being studied by many groups as a new potential tool for the diagnosis of acute Lyme neuroborreliosis (LNB). It has been shown to be highly sensitive and detectable from days to weeks before antibody production. Specificity should be taken into consideration, however, since CXCL13 levels are also elevated in other neuroinfectious and neuroinflammatory diseases. Further studies are being done to establish the cutoff values, differences for age ranges, and other variables to establish the diagnostic value of this assay. This is an important step toward finding a specific and sensitive biomarker to be used as an adjunct test along with signs and symptoms of early LNB (58, 61).
Tests that have not been validated or whose clinical validity is not established include T-lymphocyte transformation assays (38), cell sorting of cell wall-deficient or cystic forms of B. burgdorferi (38), quantitative CD57 lymphocyte assays (36), IgM or IgG tests interpreted with nonstandard methods (36), novel culture methods (33), and urine antigen testing (33).
Treatment.
B. burgdorferi is susceptible to several classes of antibiotics, including doxycycline, penicillin, amoxicillin, cefuroxime axetil, ceftriaxone, and azithromycin. Treatment of patients in the early stages of Lyme disease usually results in complete recovery. While doxycycline is most commonly used, a number of factors affect the antibiotic choice, including age, drug allergy, side effects, clinical disease manifestations, whether the patient is an outpatient or hospitalized, sun exposure, a differential diagnosis that includes cellulitis versus EM, as well as concern for coinfection with Anaplasma phagocytophilum or Ehrlichia muris subsp. eauclairensis. The treatment duration varies from 7 to 28 days depending on disease manifestations, the antibiotic used, and the route of administration. A detailed description of treatment recommendations is beyond the scope of this review but can be found elsewhere (33, 35, 37) and in the most recent clinical practice guidelines developed jointly by the Infectious Diseases Society of America, the American Academy of Neurology, and the American College of Rheumatology (52).
Borrelia miyamotoi and the Relapsing Fever Group
As discussed above, there are two major groups of Borrelia, the Lyme disease group and the relapsing fever group, which includes B. hermsii, B. parkeri, and B. turicatae (62). The relapsing fever group is transmitted by soft ticks of the genus Ornithodoros. Tick-borne relapsing fever (TBRF) is endemic in the western United States, Canada, and Mexico and is primarily characterized by recurrent bouts of fever, headaches, and malaise. Other species of the relapsing group, including B. duttonii, B. hispanica, and B. persica, are endemic in Africa, central Asia, the Middle East, and Central and South America. While TBRF is often a mild illness, severe sequelae and death can also occur (63, 64).
B. miyamotoi is distantly related to B. burgdorferi but is genetically more closely related to the relapsing fever group. While other TBRF agents are transmitted by soft ticks of the species Ornithodoros, B. miyamotoi is transmitted by infected Ixodes ticks. B. miyamotoi and B. burgdorferi (and other Ixodes-transmitted pathogens) can simultaneously infect ticks, reservoir hosts, and humans (62). Unlike B. burgdorferi, B. miyamotoi can be transmitted transstadially and transovarially, making it possible for ticks in all life stages to transmit disease (42). The genus name of this organism has caused some initial confusion among health care providers because they assume it to be within the genospecies and to be another agent of Lyme disease, although B. miyamotoi is really in the relapsing fever group.
History.
B. miyamotoi was discovered in I. persulcatus in Japan in 1995. It was subsequently discovered in other Ixodes species, but the first human cases were not identified until 2011 in Russia (65). The first cases in the United States were described in 2013 (65, 66).
Epidemiology and ecology.
Currently, B. miyamotoi prevalence has been studied more widely in ticks than in humans. In the United States, B. miyamotoi is found in I. scapularis (black-legged tick or deer tick) in the eastern and upper midwestern United States, in I. pacificus in California, in I. ricinus in Europe, and in I. persulcatus in Japan and Russia (62, 67). Studies have reported small numbers of cases in humans from China, southern regions of Russia, Europe, Japan, and the eastern and upper midwestern United States (65, 68–71). In a larger study by Molloy et al. in 2015, 11,515 blood samples collected from 2013 to 2014 in Massachusetts, Rhode Island, and New York were tested by real-time PCR. Ninety-seven samples were positive for B. miyamotoi, and of those patients, 51 had case reviews available that supported a diagnosis of B. miyamotoi infection (55).
Clinical features.
Symptoms of B. miyamotoi infection are similar to those associated with A. phagocytophilum. In areas where other rickettsial diseases are rare, a presumptive diagnosis of anaplasmosis is not uncommon. Symptoms include fever (may be relapsing), chills, myalgia, fatigue, arthralgia, lymphadenopathy, and possible EM rash (65, 67). As health care providers become more aware of B. miyamotoi and testing becomes more widely available, more will become known about the clinical presentation.
Diagnosis.
Initial laboratory test results for B. miyamotoi infections show leukopenia, thrombocytopenia, and elevated liver enzymes, similar to anaplasmosis. Spirochetes may be visualized by Giemsa stain in the acute phase, when there is high spirochetemia. Glycerophosphodiester phosphodiesterase (GlpQ) has been selected for clinical testing for B. miyamotoi. GlpQ was chosen as a target because it is not found in other Lyme disease-causing Borrelia species, therefore making it easily distinguishable from that group (68, 72). GlpQ is found in other relapsing fever Borrelia species; however, this makes specificity an issue in areas where other relapsing fever spirochetes are enzootic (e.g., the West Coast of the United States) (73).
Currently, PCR targeting B. miyamotoi GlpQ is the most specific test for B. miyamotoi but is available from only a few commercial laboratories and some public health departments. Blood is an appropriate specimen for PCR testing since there is high spirochetemia in the acute phase (74). CSF is also an appropriate specimen for PCR. Serology for testing of IgG antibodies to B. miyamotoi is performed by an indirect enzyme-linked immunosorbent assay (ELISA) and by a two-step ELISA and Western blot assay (72). Clinical testing for IgM and IgG antibodies to recombinant B. miyamotoi GlpQ protein is available on an even more limited basis through commercial laboratories. Acute-phase serum samples should be taken within 7 days of the onset of symptoms, and convalescent-phase serum should be drawn approximately 3 weeks after symptom onset. Since cross-reactivity between B. miyamotoi and other relapsing fever spirochetes can exist, the presence or absence of other endemic relapsing fever spirochetes has to be considered. Diagnosis of B. miyamotoi (as in other infections) is confirmed by a 4-fold rise in the antibody titer in acute- and convalescent-phase sera. B. miyamotoi is cultivable in liquid media, but this is performed only in research settings (75).
Treatment.
The typical treatment for B. miyamotoi is doxycycline for 14 days (65, 76). It has been suggested that if a patient diagnosed with presumed anaplasmosis and treated with doxycycline does not defervesce in the first 24 h, it may be due to a missed diagnosis of B. miyamotoi or B. burgdorferi (76).
PARASITES (BABESIA)
Both the geographic range of ticks carrying Babesia microti and the incidence of babesiosis have increased significantly over the last 20 years, primarily in the United States. The clinical symptoms are similar to those of malaria. Like malarial parasites, Babesia infects erythrocytes and therefore is a threat to the safety of the blood supply. In recognition of the increasing incidence of transfusion-transmitted babesiosis, the FDA issued recommendations in May 2019 for reducing risk. They include screening donated blood using a licensed antibody and nucleic acid amplification testing (NAT) or using pathogen reduction methods. They recommended that these approaches be used only for blood collected in specified areas of endemicity (Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, Wisconsin, and Washington, DC). In states that do not screen donations or use pathogen-reduced blood components, a donor questionnaire is recommended to guide donor deferral (77).
History
Babesiosis may date back to Biblical times; it is believed to be the plague described as attacking cattle that belonged to Pharaoh Ramses II. The first description of the parasite came from European pathologist and microbiologist Viktor Babes, for whom the pathogen was named. In 1888, he observed organisms within the erythrocytes of cattle exhibiting fever and hemoglobinuria. Cattle infections in the southern United States led to the first discovery of arthropods as a disease vector. Theobald Smith, a doctor from Albany, NY, and two veterinarians, Fred Kilbourne and Cooper Curtice, demonstrated that ticks from infected Texas cattle could transmit Babesia bigemina to previously uninfected northern cattle (78). Since then, more than 100 species of Babesia with the ability to infect animals have been identified (79, 80). Fortunately, only a few species are known to infect humans.
The first case of babesiosis in a human was described in 1957 when a 33-year-old farmer from Yugoslavia became infected after grazing his cattle in a tick-infested pasture. The farmer was asplenic and died within 2 weeks due to renal insufficiency (81). The parasite responsible was thought to be Babesia bovis. However, given the similarity in morphology and rarity of other reports of human infection, the species was likely to have been Babesia divergens. Twelve years later, the first species-confirmed case in the United States was identified in a 59-year-old woman living on Nantucket Island, MA (82). This was also the first reported case of babesiosis in someone who had an intact spleen. The patient recalled removing an embedded tick. She was treated with chloroquine and recovered, although low-level parasitemia could be observed 4 months after her initial treatment, and subsequent reports found that chloroquine is ineffective for the treatment of babesiosis. In this case, the parasite was identified as B. microti, the species that has subsequently become endemic in the northeastern and northern midwestern United States.
Epidemiology and Ecology
Ixodid ticks have long been identified as the vector for species of Babesia that cause disease in humans. In North America, I. scapularis is responsible for the transmission of B. microti, while either I. scapularis (83) or Dermacentor albipictus (84) may be the vector for Babesia duncani. In Europe, the primary vector for Babesia spp. is Ixodes ricinus. In China, I. persulcatus has also been identified as a vector for human infections. Other species of ticks have been shown to carry Babesia: Dermacentor reticulatus in Poland (85), Haemaphysalis concinna and Dermacentor nuttalli in China (86), and Amblyomma americanum and Dermacentor variabilis in the United States (87). However, the role of these species in transmission to humans is unknown.
Most species of Babesia are maintained in ticks by both transovarial and transstadial transmission. Thus, larval, nymphal, and adult ticks are all capable of transmitting the parasite to humans or other mammals. However, for B. microti, transovarial transmission has not been demonstrated (88). As a result, only nymphs and adults can transmit disease after first feeding on an infected mammal during the preceding life cycle stage.
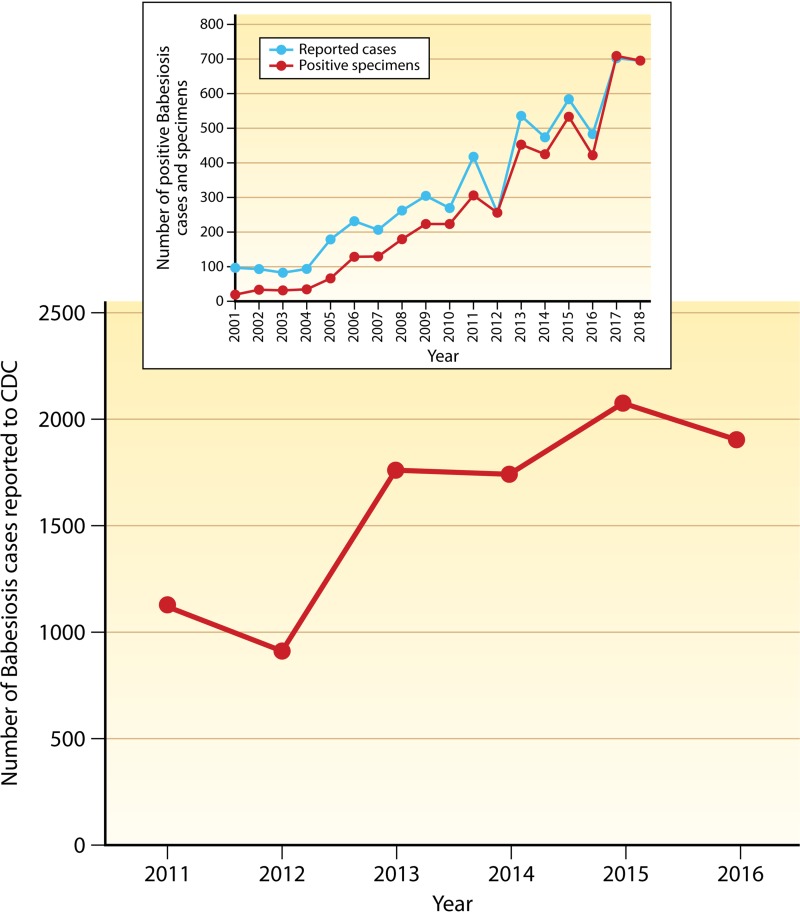
In the United States, the majority of babesiosis cases are caused by B. microti. Both the number of cases and the geographic range have increased in the United States over the last 20 years (89, 90). Due to the rapid increase in the number of cases, babesiosis became a nationally notifiable condition in 2011. Surveillance for 2011 resulted in 1,124 confirmed or probable cases (Fig. 4) (91). The most recent surveillance results are from 2016, when 1,910 cases were reported (92). The increase in the number of cases from 2011 to 2016 is consistent with the trend in the five northeastern states and two states in the upper Midwest where >90% of cases have occurred. An example of the dramatic rise in the number of cases over a longer period of time is shown in the inset of Fig. 4. In New York, babesiosis has been a reportable disease since 1986. National and New York data show that there tends to be an increase in the number of cases every other year, which may be influenced by the 2-year life cycle of the tick.
FIG 4.
Increase in the number of reported cases of babesiosis in the Unites States since the disease became nationally notifiable in 2011. The inset shows the number of cases in New York (where babesiosis has been a reportable disease since 1986) and the number of specimens that were positive for Babesia microti by RT-PCR at the Wadsworth Center, New York State Department of Health.
Infections with species other than B. microti have occurred sporadically in the United States, primarily in the 1990s. Namely, B. duncani (93, 94) and a Babesia sp. (95) closely related to B. duncani were identified as causes of infection in Washington and California (WA-1), while B. divergens or B. divergens-like/MO-1 (96, 97) parasites have caused infections in Missouri, Kentucky, Washington, Arkansas, and Michigan (98, 99).
A significantly smaller number of cases of babesiosis occurs in Europe, and the predominant species causing human infections has been B. divergens (100–102). Those most at risk are farmers, forestry workers, and others who have contact with livestock or spend considerable time outdoors in rural areas. The majority of cases (84%) have been reported in individuals who were splenectomized, and the case fatality rate was 40 to 60% (100, 102). However, outcomes have been better with supportive therapy and since treatment with atovaquone and azithromycin (103) or clindamycin and quinine (104, 105) has become standard. In severe cases, exchange transfusion has been utilized, although evidence for a beneficial effect is limited (106). The basis for exchange transfusion stems from its use in the treatment of severe cases of malaria. A thorough review of the literature failed to find supporting evidence for improved survival with exchange transfusion for babesiosis or malaria (107).
A number of recent seroprevalence studies in Europe suggest that B. microti and Babesia venatorum are now more prevalent than B. divergens. A seroprevalence survey of French forestry workers showed that 2.5% of those tested were positive for antibodies to B. microti, while only 0.1% had antibodies to B. divergens (108). Reactivity to B. venatorum was not tested in this study. A similar study in Italy found that the overall positivity for B. microti (4.6%) was slightly higher than for B. bovis (4.3%), B. divergens (3.9%), and B. canis (3.4%) (109). Reactivity to B. bovis was detected using an assay developed for use in cattle and likely represented cross-reactivity, as human cases of infection with B. bovis have been described only rarely. The Italian study showed that B. microti was more common in foresters, while the other three species were more common in livestock keepers and veterinary practitioners (109). As in the French study, seroreactivity to B. venatorum was not specifically tested. In Belgium, patients who had a history of a tick bite and exhibited symptoms were tested for antibodies to B. venatorum. The serosurvey results showed that 39.7% of patients were reactive to this species (110). Rates of reactivity to B. divergens and B. microti were 33.2% and 9%, respectively. A study of blood samples from patients in the Netherlands who reported tick bites or had EM detected DNA from B. divergens in only 3 of 626 blood samples. Although DNA from other tick-borne pathogens was detected, no other species of Babesia were found. Directly evaluating Babesia in ticks showed that B. venatorum was the species most commonly detected in several European countries (Norway, France, Denmark, the Netherlands, and Germany) over the last 12 years (111–114) as well as in mainland China (115). Taken together, data from the serosurveys as well as studies in ticks suggest that B. divergens is no longer the most prevalent species in France, Italy, and the Netherlands.
Human babesiosis has been reported in China, including cases of infection with B. microti (116) and B. venatorum (117), which is endemic in northwestern China. Although several studies have reported the incidence of B. divergens or B. microti in ticks and mammals in Japan (118–121), to date, only sporadic human cases have been reported (122). Similarly, India (123), Egypt (124), the Democratic Republic of the Congo (125), and Australia (126) have occasionally reported human cases.
Babesiosis has rarely been reported in South America. A small study of people living in rural northwestern Colombia indicated that 30.6% (127) had antibodies to B. microti. A 2013 study in rural Bolivia detected B. microti in 9 of 271 (3.3%) healthy volunteers, and the seroprevalence among all study participants was 45.7% (128). Thus, based on high seroprevalence, at least in rural areas, it is likely that the true incidence of infection is greater than the reported cases.
Clinical Features
The time required for transmission after tick attachment has not been studied in humans. Early studies in hamsters and white-footed mice suggest that the time period may be 36 to 54 h, similar to the time for transmission of B. burgdorferi (129). Symptoms of babesiosis depend on the species causing the infection and the immune competence of the patient (130). For transfusion transmission, symptoms can occur from 1 to 6 weeks after receiving the blood product, although the average incubation time is 36 days (130). The incubation period for tick-borne cases is between 1 and 4 weeks. However, most patients do not recall a tick bite, and about 25% of infections in adults and 50% in children are asymptomatic or cause only minor symptoms. For milder cases associated with parasitemia of <4%, the most common symptoms of infection with B. microti are fever (83 to 85%), chills (63 to 66%), and myalgia (64 to 68%) (91, 131). Individuals who are immunocompromised (people who have HIV infection, have a malignancy, are on immunosuppressive drugs, or are recipients of an organ transplant), have certain comorbid conditions (such as chronic obstructive pulmonary disease, congestive heart failure, or liver disease), have had a splenectomy, are neonates, or are elderly typically have more severe disease. Complications of babesiosis include acute respiratory distress, renal failure, congestive heart failure, shock, and disseminated intravascular coagulation. Complications are associated with severe anemia and high-level parasitemia (>10%). Between 2% and 9% of B. microti infections that require hospitalization are fatal (132–134), and the fatality rate in transfusion-transmitted cases can be as high as 20% (135).
Infection with B. divergens and B. venatorum in immunocompetent patients can cause flu-like symptoms similar to those associated with B. microti but more frequently include headache and arthralgia (115, 136). In people who have undergone prior splenectomy, Babesia infections and especially B. divergens infections have typically been severe, with a high fatality rate (137). One case of severe B. divergens infection in an immunocompetent patient has been reported (103). A small number of cases of B. duncani infection have been reported from the West Coast of the United States, with similar risk factors and clinical presentations (95).
The primary route of infection is through a tick bite from an Ixodes tick, but transmission by contaminated blood products and organ transplantation and congenital transmission can occur. Indeed, B. microti is the infectious agent with the highest number of reports of transfusion transmission and is associated with the most deaths due to pathogen contamination of blood products. From 1979 to 2009, there were 159 cases of transfusion-transmitted babesiosis in the United States (138), and from 2004 to 2015, there were 55 cases in New York State alone (139). Both reports noted that transfusion-associated transmission increased over the time period studied, which reflects the increase in the number of cases of babesiosis overall (91, 131). Congenital transmission has been clearly demonstrated, and while only a few cases have been reported (106, 140–142), underreporting is likely. Typically, the mother is unaware of the tick bite or the parasitic infection, and neonates develop symptoms at 2.5 to 7 weeks of age with high-level parasitemia and anemia leading to exchange transfusion. Thus, asymptomatic or mild cases in immunocompetent people can have severe consequences for both blood product recipients and newborns.
Diagnosis
The diagnosis of babesiosis is routinely made by microscopic examination of Giemsa-stained blood smears with the detection of the parasite in red blood cells. Especially for hospitalized patients, microscopic examination of blood smears is considered the preferred test, as it can be performed on-site with rapid results, can detect clinically relevant parasitemia, and provides quantification of parasitemia to guide therapy. While inexpensive to perform, microscopy is labor-intensive, requires skilled microscopists, and may fail to detect low-grade parasitemia, typically less than 0.1%. In countries where malaria is endemic, or for patients with a history of travel to an area where malaria is endemic, confusing Babesia spp. and Plasmodium falciparum is a concern (116, 125, 143). The identification of Babesia to the species level is not possible with microscopy. PCR assays for Babesia have been developed and are available in commercial and reference laboratories (144, 145). Nucleic acid-based methods are more sensitive than microscopy (145), less labor-intensive, and extremely specific. Multiplex PCR assays can also be used to simultaneously detect any tick-borne pathogen that might be present and thus avoid missing coinfections (50).
Indirect immunofluorescence assays (IFAs) can be used to detect IgM and/or IgG antibodies. However, serology may yield negative results in the very early stages of the infection (146) and is best performed with acute- and convalescent-phase serum samples to demonstrate a 4-fold rise in the IgG titer. A low IgG titer in an acute-phase sample usually represents past infection. IgM can be useful but, as with all IgM tests, can be falsely positive.
Treatment
Babesiosis is treated with a combination of an antiparasitic and an antibiotic. For mild to moderate disease, the combination of atovaquone plus azithromycin is the treatment of choice. The recommended treatment for severe disease is quinine plus clindamycin, with or without exchange transfusion (147, 148). The latter drug combination is less well tolerated. A study of 58 patients from the northeastern United States showed that 72% of patients receiving quinine plus clindamycin experienced side effects; in 33% of these patients, the side effects were severe (149). Adverse effects included auditory complaints of tinnitus and decreased hearing as well as vertigo, diarrhea, and rash. In comparison, only 15% of patients who received atovaquone plus azithromycin experienced side effects, which were primarily diarrhea and rash.
Despite side effects, the combination of quinine plus clindamycin is recommended when parasitemia is >10%, hemolysis is significant, or kidney, liver, or lung function is compromised. However, recent evidence suggests that atovaquone plus azithromycin can be just as effective (133, 150–152). Treatment for longer than the recommended 10-day course may be necessary, as symptoms and parasitemia may persist or recur for several weeks to months (146, 153) or longer, particularly for immunocompromised patients (154, 155).
Cases of relapsing babesiosis have been reported in immunocompromised hosts, especially those who have an impaired antibody response. Longer treatment regimens are typically effective in clearing the parasites (156). Nevertheless, there is evidence of the occasional development of resistance during prolonged therapy in immunocompromised hosts. Mutations in the cytochrome b (cytb) and ribosomal protein L4 (rpl4) genes emerged after 6 weeks of treatment in a patient who had previously been diagnosed with leukemia (157). A B. microti whole-genome study, which included isolates from 5 relapse cases, also identified mutations in cytb and rpl4 (79). The association of the mutations with resistance is strengthened by their role in resistance to atovaquone (cytb) and azithromycin (rpl4) in the related apicomplexan parasite P. falciparum (158, 159) and in Babesia gibsoni, which infects dogs (160).
VIRUSES
Currently, more than 35 species of viruses from six different virus families are transmitted by ticks. All tick-borne viruses, with only one exception (African swine fever virus, family Asfarviridae), are RNA viruses. These viruses are increasing in prevalence on a global scale as a consequence of anthropogenic changes bringing humans into greater contact with ticks as well as burgeoning tick populations. New tick-borne viruses are being identified regularly, increasing the risk of disease from tick bites. Virus-tick-vertebrate host relationships are highly specific, and <10% of all tick species (Argasidae and Ixodidae) are known to play a role as vectors of arboviruses. Understanding the basic biology, ecology, clinical features, and diagnosis of these agents is critical to public health and is addressed for selected viruses that cause disease in humans.
Flaviviridae: Tick-Borne Encephalitis Virus and Powassan Virus
There are four major virus groups within the Flavivirus genus (family Flaviviridae), which can be distinguished by their ecological, biological, and genetic characteristics. Tick-borne flaviviruses (TBFVs) comprise one of the groups, and mosquito-borne, no-known-vector, and mosquito-only viruses (i.e., viruses that infect only mosquitoes) comprise the other three. Among the 160 currently known tick-borne viruses are the mammalian, seabird, and Kadam flaviviruses, which include a number that pose significant global threats to human and animal health. The most important mammalian TBFVs (originally named the tick-borne encephalitis complex) are tick-borne encephalitis virus (TBEV) and Powassan virus (POWV) (161), which are discussed here. As the most widespread tick-borne virus and the most genetically diverse arbovirus, Crimean-Congo hemorrhagic fever virus (CCHFV) (Bunyavirales: Orthonairovirus) is also discussed. While other viruses in this group are not discussed, they also cause serious disease and are likely to be the focus of future reviews. These viruses include Omsk hemorrhagic fever virus (OHFV) and Kyasanur Forest disease virus (KFDV), both of which cause hemorrhagic fever, and Langat virus (LGTV) and louping ill virus (LIV), which infect the central nervous system.
Background.
TBEV includes three pathogenic subtypes, Far Eastern (previously Russian Spring-Summer encephalitis), Siberian (previously west-Siberian encephalitis), and Western European (previously central European encephalitis [CEE]); the variation in amino acid sequences between subtypes is 5 to 6% (162). Viruses antigenically related to TBEV were originally known as the TBEV serocomplex but have been renamed the mammalian group of tick-borne flaviviruses (161).
These viruses are prevalent in foci of endemicity across the Eurasian continent from Japan to France. An expansion of prevalence in northern Russia, Sweden, and Finland, as well as in Mongolia, northern China, Denmark, Kazakhstan at altitudes of 1,000 to 2,100 m, Kyrgyzstan, and isolated areas of endemicity in Armenia, Azerbaijan, and Uzbekistan, has been observed in recent years (163). The increased prevalence of these viral infections is likely due to improved diagnostics as well as increasing interest in tick-borne disease.
Powassan virus is the most genetically divergent member of the TBFV group (164) and exists as two lineages, POWV (lineage 1) and DTV (deer tick virus) (lineage 2), in which the E protein nucleotide and amino acid sequences differ by 14.6% and 4%, respectively (136, 137, 165). Thus, the two lineages are considered distinct genetic subtypes or genotypes that likely diverged and evolved independently into two distinct ecological niches from a single origin (166). DTV and POWV have coexisted throughout the historical range of POWV (167). Both lineages are responsible for human disease (167, 168). POWV is conserved over space and time (167, 169); e.g., virus isolated from Primorsky Krai, Russia, in 2006 (170) was 99.8% similar to the POWV LB strain isolated in Canada in 1958. This genetic homogeneity is true of all mammalian tick-borne viruses, which appear to have evolved as a complex of viruses, despite their distribution across a wide biogeographic area.
An apparent increase in case numbers of POWV/DTV has been observed in the United States. Among possible factors having an impact on this are improved diagnostics leading to increased detection, climate change affecting populations of I. scapularis (the predominant vector for DTV) (171, 172), as well as increased human-tick contact due to changes in recreational activities and landscape. POWV has a wide distribution, as evident by detection in its enzootic hosts in North America and Far East Asia (173) as well as the Nearctic zoogeographic region, including mainly Canada and the United States (174). Serologically positive wild mammals have been reported in the United States, British Columbia, Alberta, Ontario, Nova Scotia, and southeastern Siberia.
Epidemiology and ecology.
The transmission cycles of both prototype POWV and DTV involve ixodid ticks (hard ticks) and small mammals. Virus transmission can be accomplished through tick feeding on viremic vertebrates as well as cofeeding or nonviremic transmission. Cofeeding or nonviremic transmission occurs when uninfected ticks feed in close proximity to an infected tick without the need for the vertebrate host to have virus circulating in the blood. This mechanism of transmission was demonstrated in the laboratory with TBEV by Labuda and colleagues (175) as the predominant means of virus transmission of TBEV. It has been modeled for POWV for long-term maintenance in natural foci (176). A third mode of transmission is transovarial transmission through the egg, as has been documented in the laboratory with I. ricinus, Dermacentor reticulatus, Haemaphysalis longicornis (neumanni), and other ticks and various strains of TBEV (177, 178).
The principal vector of prototype POWV is Ixodes cookei (woodchuck or groundhog tick), especially in the northeastern United States and eastern Canada (179–186). This species extends from South Dakota to Texas northeasterly through the United States and eastern Canada. Little is known about the vector competence of this species because it is difficult to collect sufficient numbers of individuals to conduct such studies.
I. cookei is also known as the groundhog tick because of its propensity to feed on groundhogs (Marmota monax), but it will also feed on carnivores and occasionally humans (187–190). Morphologically, I. cookei is very similar to I. scapularis, but ecologically, it is quite different in more ways than its feeding habits alone. I. cookei is found mostly in woodchuck burrows, similar to nidicolous argasid ticks, and likely is transported with the woodchuck as it moves to new burrows by dispersal of young animals or adult woodchuck movement (189). Unlike I. scapularis, it does not quest and thus is generally collected not by dragging or flagging but rather by swiping woodchuck burrows. Humans rarely encounter I. cookei (187–190; New York State, unpublished data), which may explain the relatively low number of human cases of prototype POWV encephalitis.
I. scapularis is the most important vector of DTV; however, the virus has also been isolated from Dermacentor andersoni ticks in Colorado. I. scapularis has three hosts in its life cycle: larva, nymph, and adult. Each stage takes a blood meal from a separate distinct host (191). Peromyscus leucopus (white-footed mouse) is the preferred host of I. scapularis larvae, although they have been recorded feeding on numerous other species (192). Nymphs feed more indiscriminately on a wider range of hosts, including small mammals, birds, reptiles, and humans (187, 192–196). Larger animals such as deer and livestock constitute the main blood meals of adult females (187, 192, 193). The majority of tick-derived DTV isolates have obtained been from the adult stage of I. scapularis (166, 197–200) (New York State, unpublished data).
I. scapularis has been demonstrated experimentally to be a competent vector of POWV (lineage 1 [201] and lineage 2). Infection rates of 10%, 40%, and 57% were observed for larvae, nymphs, and adult females, respectively, after feeding on POWV lineage 1 viremic hosts (201). Transstadial (larva to nymph or nymph to adult) and transovarial (adult female to progeny) transmissions were also reported in this study. Transmission of DTV to naive P. leucopus by infected nymphal I. scapularis ticks in ≤15 min was demonstrated in a more recent study (202). Thus, POWV differs from other tick-borne pathogens, such as Borrelia, Ehrlichia, and Babesia, in lacking a grace period for the removal of an attached tick to prevent pathogen infection. This is likely because the virus is already in the salivary glands of the tick at the time of feeding. Transstadial transmission was also noted in the same study. Due to the indiscriminate feeding behavior and experimentally derived vector competence of I. scapularis, it is hypothesized that it may have provided the bridge for POWV to escape from the focal, enzootic cycle to become an emerging virus.
More than 14 species of ticks can be infected by TBEV, but I. ricinus and I. persulcatus are the principal vectors of the Western European subtype and the Siberian and Far Eastern subtypes, respectively (203). The main vector of TBEV in China is I. persulcatus; however, this virus has also been isolated from H. concinna, Haemaphysalis japonica, Dermacentor silvarum, and Ixodes ovatus (204).
The first reported case of POWV encephalitis occurred in Ontario, Canada, in 1958 (205), followed by 27 cases reported from 1958 to 1998 in North America. From 1999 to 2005, an additional nine cases were reported in the United States (206), and one was reported in Canada (207). POW encephalitis was reported in Russia in 1973, and 14 additional cases were reported between 1974 and 1989 (170). In the United States, a total of 98 cases have been reported, of which 88 were neuroinvasive, causing 11 deaths (208). The number of POWV cases reported strongly suggests an increase in the incidence of this disease in recent years (206, 209).
Clinical features.
TBEV causes clinical disease in more than 10,000 to 20,000 humans in Europe and Asia per year. An increased incidence of TBE has been noted in Europe, as a consequence of climate and socioeconomic changes (163, 210, 211), and the areas of endemicity for TBEV are shifting northward. This group of viruses produces a wide range of disease symptoms as well as subclinical infections depending on the subtype. The European and Siberian subtypes of TBEV generally cause biphasic fever characterized by an influenza-like prodromal phase followed by an asymptomatic period and, in one-third of cases, a second phase with aseptic meningitis, encephalitis, or meningoencephalitis (211, 212). Up to 50% of patients have long-term sequelae (213) Asymptomatic and subclinical infections constitute approximately 70 to 95% of all TBEV infections (213). Following an outbreak of the Far Eastern subtype of TBEV in China in 1952, approximately one-third of the patients had sequelae, and approximately one-third of the patients died (204).
Similarly, POWV also produces disease symptoms that range from mild to severe (encephalitis) as well as asymptomatic infection. Unlike TBEV, POWV has not led to major outbreaks. DTV had been thought to cause milder illness than prototype POWV (178); however, at least two recent cases of DTV were fatal (168, 214), demonstrating that this virus has the potential to be highly virulent. A comparative experimental infection of mice should be able to shed light on this question. Regardless, severe disease is rare (215), but encephalitis in humans may be associated with significant neurological sequelae.
The incubation period of POWV extends from 8 to 34 days (216, 217). A prodrome of nonspecific symptoms lasting 1 to 3 days may include sore throat, drowsiness, generalized malaise, nausea, headache, myalgia, and disorientation (216). Early symptoms include a sudden onset of fever with a temperature of up to 40°C and convulsions (216, 217). Severe disease manifests as encephalitis, meningoencephalitis, and aseptic meningitis with vomiting, respiratory distress, prolonged fever, stupor, and convulsions. Patients may experience generalized weakness, ataxia, tremor, and ocular symptoms (206). In some cases, a fine macular erythematous rash has been reported (138, 168, 216–220), as have muscle weakness or rigidity and some degree of paralysis in some patients (168, 206, 217, 218, 221–224). In the most severe cases, patients become comatose, and a fatality rate of approximately 10% has been reported (197, 225).
It is nearly impossible to differentiate the nonspecific symptoms of POWV from those caused by other arboviruses. A seroprevalence of up to 3% of the population in certain northern Ontario communities was noted in 1962 (185). Thus, it is clear that, as with other arboviral infections, subclinical infections occur. More recent large-scale serosurveys have not been conducted.
Diagnosis.
Since POWV is endemic in North America and symptoms are nonspecific, it should be included in the differential diagnosis when arboviral encephalitis is suspected, especially when a tick bite is recalled. An in-depth patient history should include questions on travel history, recent outdoor activities, contact with animals, tick bites, and vaccinations. Since I. scapularis is able to transmit POWV within 15 min of attachment (202), finding an attached tick should serve as a warning to be alert to symptoms.
Neurologic patients whose blood work suggests viral infection are often tested by PCR of blood or CSF. But with TBEV, this assay is useful only during the viremic period before antibody production begins and prior to CNS symptoms. Therefore, PCR has no diagnostic value after viremia drops and is not a suitable method for the detection of TBEV in CSF (212).
For both suspected TBE and POWV infection, serologic assays are generally the principal diagnostic method because of the short-lived viremia. These assays include IgM antibody capture enzyme-linked immunosorbent assays (MAC-ELISAs) and indirect IgG ELISAs (226, 227). Alternative assays, such as the indirect fluorescent-antibody test, may be used but are less sensitive than ELISAs and not suitable for high-throughput testing (228). Newer assays have been developed, such as fluorescent-microsphere immunoassays (MIAs), which employ the virus envelope to measure flavivirus antibodies (229, 230). POWV-positive results are generally confirmed by a PRNT (plaque reduction neutralization test) in biosafety level 3 (BSL-3) containment facilities (231). A PRNT is required because the possibility of cross-reaction with other flaviviruses is common. TBEV confirmation by a PRNT must be conducted in a BSL-4 facility, placing a severe limitation on the number of laboratories that can perform this test.
Treatment.
Treatment is supportive only, but vaccination is used prophylactically against TBEV because of the high incidence of infection in Europe. A number of TBEV vaccines have been developed, including TBEV vaccines that contain inactivated viral antigens of strains of Far Eastern (Sofjin and 205) or European (Neudorfl and К23) TBEV subtypes (232, 233). TBEV vaccine Moscow (against Sofjin) has been tested in mice against a range of TBEV variants. It successfully protected against these variants but did not protect mice against 10 doses at 50% lethality (LD50) of POWV (234). An additional problem encountered is vaccine breakthroughs, mainly in older individuals >60 years old. In these individuals, the vaccine-induced immune responses, while present, did not appear to be sufficiently protective to prevent infection (235). Recently, a lipid nanoparticle (LNP)-encapsulated modified mRNA vaccine carrying the POWV prM and E genes was developed, which was protective in mice after one or two doses against lethal challenge with both lineage 1 and 2 POWV strains. In addition, the vaccine induced cross-neutralizing antibodies against multiple other tick-borne flaviviruses, including the distantly related Langat virus (236).
Bunyavirales: Crimean-Congo Hemorrhagic Fever Virus
The bunyaviruses, more than 300 in number, were reclassified in 2016 by the International Committee on Taxonomy of Viruses (237). Most members of the former family Bunyaviridae have been elevated to a new order, Bunyavirales, which is subdivided into 9 families (Feraviridae, Fimoviridae, Hantaviridae, Jonviridae, Nairoviridae, Peribunyaviridae, Phasmaviridae, Phenuiviridae, and Tospoviridae) and 13 genera. The hantaviruses are rodent-borne viruses, while three families (Nairoviridae, Phenuiviridae, and Peribunyaviridae) include tick-borne viruses, and the tospoviruses infect plants.
Background.
Crimean-Congo hemorrhagic fever virus (CCHFV) is the causative agent of the most widespread tick-borne viral infection of humans, causing severe viral hemorrhagic fever outbreaks with a case fatality rate of 5 to 30% (238, 239). The first definitive cases of CCHF were recognized in 1944 during a large outbreak among agricultural workers in the Crimean peninsula (240), but recent phylogenetic analysis indicates that the virus dates back more than 2,500 years (241, 242). CCHFV has been reported in more than 30 countries over a wide geographic area, including Asia, Africa, southeastern Europe, and the Middle East. This range is similar to that of its major vector and reservoir, Hyalomma species ticks (Fig. 5) (243, 244). Although the virus appears to be expanding its range, genetic analysis indicates that it has been present in many locations, circulating without being noticed, for more than 1,000 years. Most recent outbreaks result from environmentally favorable conditions that result from changes in agricultural practices, transport of livestock, migration of human populations, as well as climate (245). CCHFV must also be considered a potential agent for bioterrorism (246) because of the ease of amplifying the virus to high titers in large volumes, infectiousness for humans, the severity of the disease, the ability to be transmitted by aerosol, and the lack of measures available for its control (247).
FIG 5.
Geographical distribution of Crimean-Congo hemorrhagic fever (CCHF). Source data for the map are from the World Health Organization. The boundaries and names shown and designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any county, territory, city, or area or of its authorities or concerning the delimitation of its frontiers or boundaries. (Modified from the World Health Organization map at http://www.who.int/emergencies/diseases/crimean-congo-haemorrhagic-fever/en/.)
Epidemiology and ecology.
Humans most often become infected through the bite of a tick, by exposure to a highly infected patient with CCHF during the acute phase of infection, or by contact with blood or tissues of infected livestock, especially during slaughtering (248). CCHFV is maintained in nature by ixodid ticks predominantly of the genus Hyalomma. Although CCHFV has been detected in other tick species, these ticks appear to be incapable of transmission and probably acquire the virus through cofeeding or feeding on viremic hosts (249, 250). Hyalomma species ticks are considered to be the sole reservoir of CCHFV. Blood meals taken by larvae and nymphs from small mammals such as hedgehogs, hares, and ground-feeding birds and by adults from large ungulates result in brief periods of viremia but no disease (251). Although small and large mammals are not considered reservoirs of the virus, they play an important role in supporting the tick vector population through blood-feeding and cofeeding transmission (251). The establishment of a focus of endemicity of CCHFV relies on adequate densities of both Hyalomma species ticks and vertebrate hosts (251). It is important to note that the low levels of viremia in vertebrate hosts nonetheless result in CCHFV seropositivity, which serves as an important tool in mapping regions of endemicity and the potential for future outbreaks (243, 244). Once the virus is acquired, each tick remains infected with CCHFV for its lifetime, and the detection of virus in eggs and unfed larvae (evidence of transovarial transmission) and transstadial passage mean that an initial infection can last for generations (250).
The emergence of new CCHF outbreaks due to bird migration or the transport of livestock carrying ticks has been proposed but not yet proven (239, 252). Climate change can influence the spread of CCHFV by altering tick seasonal behavior and growth patterns and redirecting bird migration routes (253).
Clinical features.
Like most arboviral infections, CCHFV infections initially present as nonspecific febrile illness (238); it has been estimated that 88% of infections are subclinical (254). Four progressive stages of CCHF infections have been identified: incubation, prehemorrhagic, hemorrhagic, and convalescent (240, 255). The onset of disease is rapid, with an incubation period of 1 to 7 days, during which the virus replicates and disseminates. The prehemorrhagic period, identified by the rapid onset of symptoms, lasts an average of 3 days and ends when bleeding from various sites commences. During this stage, viremia is peaking, and reverse transcription-PCR (RT-PCR) can be used to detect circulating virus (256–259). Virus isolation is also possible during this period.
The hemorrhagic stage typically begins on days 3 to 5 of illness and lasts 2 to 3 days (239). It is usually marked by the appearance of petechiae caused by bleeding from broken capillaries. Severe cases present with disseminated intravascular coagulation (DIC), bleeding from multiple sites (212), and shock (255). Compared to other hemorrhagic fevers, CCHF results in the most severe bleeding and ecchymosis (256). In fatal cases, death generally occurs on days 5 to 14, resulting from hemorrhage, multiorgan failure, and shock (238).
The convalescence stage for survivors occurs about 10 to 20 days after illness (260, 261). Laboratory test results usually return to normal levels within 5 to 9 days, but surviving patients often experience a variety of health problems, which may not resolve for a year (238, 239, 262). Relapse of infection has not been observed (238, 239).
Case fatality rates for CCHF have ranged from 5 to 80%, but the higher rates reported may be due to the low case numbers in an outbreak and the probability that less severe cases were not identified and included in the denominator (240).
Diagnosis.
Suspected cases are evaluated based on clinical symptoms, patient history, and diagnostic laboratory tests (239). Nonspecific laboratory tests should be conducted first, such as hematological and biochemical measurements, including complete blood counts and liver enzyme tests, followed by more specific testing of serum, utilizing RT-PCR early in infection and an ELISA later in infection. A positive PCR or positive IgM result is considered confirmatory.
Since other hemorrhagic fevers exhibit early symptoms similar to those of CCHFV, it is important to consider the other viruses circulating in each region for an accurate differential diagnosis. CCHFV covers a vast geographic range where other agents causing hemorrhagic fever are endemic, so each region will have specific agents to consider for differential diagnosis (263). For example, in China, CCHF needs to be differentiated from TBE. Other diseases with similar symptoms include brucellosis, rickettsial infections, babesiosis, spirochetosis, Q fever, Japanese encephalitis, poliomyelitis, dengue hemorrhagic fever, Lyme disease, malaria, mumps, and other viral hemorrhagic infections (204).
As soon as CCHF is suspected, precautionary measures should be activated, including isolation of the patient, barrier precautions to prevent nosocomial transmission, and communication to colleagues and staff, since transmission of CCHFV from patients to health care workers may be controlled effectively if universal precautions are adopted (264, 265).
The development of IgM antibody occurs approximately 5 to 7 days after the onset of symptoms and persists for 4 months. IgG may be detected in as early as 7 days and can persist for 5 years (239). IgM and IgG are usually detected by an ELISA in paired acute- and convalescent-phase sera, which is more specific and sensitive than immunofluorescence assays (266). IgM is confirmatory for diagnosis, but antibodies are often not detected in fatal cases (239). The presence of IgG is not considered confirmation of acute illness, as it may represent past infection (263). A review of available ELISAs has recently been published (266).
Treatment.
During the prehemorrhagic period when viremia is most pronounced, the first phase of treatment is often with ribavirin, a guanosine analog that has been used to treat CCHF patients since 1985 (267). Although results of treatment with ribavirin are mixed (249, 268), the available literature provides convincing evidence that prompt administration of ribavirin can be effective (263, 269, 270). Recently, the antiviral drug T-705 (favipiravir), which directly inhibits viral RNA polymerases and is approved in Japan to treat influenza infection, has been shown to be more potent than ribavirin against CCHFV in vitro and in vivo (271). Antiviral treatment is not effective after the onset of the hemorrhagic period due to the decrease in the viral load (255).
With or without the administration of antivirals, supportive care is essential for the control of CCHV infection. Based on fluid and electrolyte balance and blood cell counts, thrombocytes, fresh-frozen plasma, and erythrocyte preparations are administered as replacement therapy (255). Platelet transfusions are used to prevent bleeding caused by thrombocytopenia or platelet dysfunction. The use of immunoglobulin therapy with anti-CCHFV hyperimmunoglobulin has been limited, and more data are needed (249).
A licensed vaccine for CCHF is not available, but ongoing research is promising (252). One of the major limitations is the lack of an animal model. Nonhuman primates do not develop disease, perhaps due to the inability to deplete the host’s innate interferon (IFN)-mediated defense mechanism (272). Results from a number of studies have shown that both antibody and T cells are required to prevent lethal CCHFV infection (252, 273). One promising vaccine candidate uses a modified vaccinia virus Ankara (MVA) vector expressing the CCHF viral glycoprotein, which induces both humoral and cellular immunogenicity (273, 274).
Recently Emerged Tick-Borne Viruses: Severe Fever with Thrombocytopenia Syndrome, Heartland, and Bourbon Viruses
New tick-borne viruses have emerged in the past decade. Two are in the order Bunyavirales, i.e., severe fever with thrombocytopenia syndrome virus (SFTSV) and Heartland virus (HRTV) (species phlebovirus, genus Phlebovirus, and family Phenuiviridae). Illness from SFTSV was first confirmed in China in 2009 (275). It was retrospectively identified in South Korea in 2010 (276) and 2012 (277) and in the western regions of Japan in 2013 (278). The vector most commonly associated with SFTSV is Haemaphysalis longicornis (279). Virus infection can cause hemorrhagic symptoms with leukopenia and thrombocytopenia as well as neurological symptoms and multiorgan failure.
Heartland virus is related to SFTSV and was first reported in the United States. This virus was first isolated from two farmers aged 57 and 67 years in Missouri in 2009. Since 2012, more than 35 cases have been reported (280). Six confirmed Heartland virus disease cases were identified in 2012 to 2013 (281, 282). All were Missouri residents, except one who was a Tennessee resident. All were males aged 50 to 80 years (median age, 66 years). Symptoms were noted from May to September. All had fever, thrombocytopenia, and leukopenia when first evaluated. All of them reported fatigue and anorexia, and most reported headache, nausea, myalgia, and arthralgia. Four of the five were hospitalized. One patient with multiple comorbidities died. All cases reported spending time outdoors, and most of them reported tick bites in the 2 weeks prior to the illness (283). Three fatal cases have been reported. The second death was a 68-year-old man who had been in good health until being bitten by an infected tick and who received very aggressive care. On autopsy, the virus was found systemically throughout his body.
Diagnosis requires laboratory tests since individuals with acute HRTV disease have clinical signs also seen with tick-borne ehrlichiosis, such as thrombocytopenia at presentation and leukopenia in some cases. Unlike HRTV, ehrlichiosis can be treated successfully with antibiotics. Since viruses do not respond to antibiotic treatment, a lack of a response to such treatment is indicative of a viral etiology (280). Two critical needs are improved diagnosis and treatment of HRTV.
The predominant vector of HRTV appears to be Amblyomma americanum, the Lone Star tick. Transmission can occur transstadially from one stage to the next, vertically from adult to offspring, and by cofeeding infection. SFTV can also be transmitted from person to person or from infected animal to person through infected blood. These routes of infection have not been observed with the few cases of HRTV that have been reported (282).
Another new tick-borne virus in the United States, Bourbon virus (BRBV) (Orthomyxoviridae: Thogotovirus), has been detected in humans. Bourbon virus was first discovered in 2014 in Bourbon County, KS, in a previously healthy 50-year-old male presenting with flu-like symptoms who died after being bitten by ticks (284). Symptoms included fever, anorexia, chills, headache, myalgia, and arthralgia. Other viruses of the genus Thogotovirus are tick borne and have only rarely been shown to cause human illnesses. Very little is known about this virus since there have been only five known cases of Bourbon virus infection. The symptoms that have been reported are high fever, headache, decreased appetite, muscle aches, joint pain, fatigue, malaise, nausea, vomiting, diarrhea, and a maculopapular rash on the abdomen, chest, and back. Following acute infection, acute respiratory distress syndrome and multiple-organ failure may develop (285).
The vector of BRBV is thought to be Amblyomma americanum (L.) ticks on the basis of virus isolation from three pools of adults and nymphs in northwestern Missouri at a location near Bourbon County, KS (286). It should be noted that Dhori virus, an Old World virus from Europe, North Africa, and western and central Asia that is closely related to BRBV, has also been isolated from mosquitoes as well as ticks (286). Also, of note is that a pool of male adult A. americanum ticks was positive for both HRTV and BRBV. Because this was a pool, it is more likely that two ticks collected from the same location were infected, one with each virus, but it could possibly be a coinfection. Until more is known about these two viruses, we can only speculate on their ecology.
TICK CONTROL AND TICK BITE PREVENTION
Personal protective strategies can help in the prevention of tick-borne disease. Avoid spending time in high-risk areas that include places with high grass, dense woods, and low-lying brush and stay to the center of cleared walking paths. Wear long-sleeved shirts and long pants; tuck the shirt into the pants and the pants into socks. Wear closed-toed shoes or boots. Clothing should be light in color, not to repel ticks but to make them easier to spot. Wear repellants, such as DEET or other products that are approved by the Environmental Protection Agency and can be applied to the skin, or 0.5% permethrin, which can be used to treat clothing, shoes, and camping gear. Prevention of tick attachment will prevent transmission and can be facilitated with daily showers, tick checks, and prompt tick removal. Exposure to ticks may also result from exposure to domestic and companion animals that bring ticks into the house. Tick prevention in pets using repellents and tick checks after domestic animal exposure may assist in prevention (287). Vigilance for unexplained fevers or flu-like symptoms when living in or visiting areas where ticks are endemic can help in early detection, which is key to early treatment and optimal outcomes. Finally, environmental controls such as keeping grass mowed short, keeping brush and leaf litter away from frequented areas, keeping children’s swing sets in sunny areas and away from forested areas, and keeping stacks of wood away from houses and off the ground are methods that can help prevent exposure to ticks.
ACKNOWLEDGMENTS
S.M.-A. thanks the Parasitology Laboratory for their work with the ever-increasing number of Babesia specimens and for affording her the time to work on this review.
Biographies

Susan Madison-Antenucci is the Director of Parasitology at the Wadsworth Center, New York State Department of Health. Dr. Madison-Antenucci received a B.S. in chemistry from the University of Rochester and a Ph.D. in biochemistry from Duke University. She worked with parasites as a postdoctoral fellow at the University of Alabama—Birmingham before joining the Wadsworth Center. Under her direction, the Parasitology Laboratory has developed numerous molecular assays to detect and perform species-level identification of parasites and uses next-generation sequencing to investigate drug resistance genes in P. falciparum. She has been involved in waterborne and foodborne outbreak investigations and tracebacks to identify donors for transfusion-transmitted babesiosis. She is a member of the Association of Public Health Laboratories Global Health Committee, where she worked with the Botswana Public Health Laboratory in a partnering relationship. She is also on the faculty of the Biomedical Sciences Department, School of Public Health, University of Albany.

Laura D. Kramer, Director of the Arbovirus Laboratories, Wadsworth Center, New York State Department of Health, and Professor of Biomedical Sciences, School of Public Health, SUNY Albany, is also an Adjunct Professor in the Biology Department, Ecology and Evolution of Infectious Diseases, SUNY Albany. She has more than 50 years of experience studying arboviruses, using both experimental and observational approaches and classical and molecular tools. Her research focuses on how the interactions between arthropod vectors, viruses, and vertebrate hosts are affected by biotic and abiotic factors and how these interactions impact the intensity of viral transmission and, subsequently, viral evolution and adaptation. Her work has resulted in more than 215 publications, 30 book chapters, and more than 100 invited presentations in national and international settings. She also serves as a Virology moderator for ProMED (Program for Monitoring Emerging Diseases, International Society for Infectious Diseases) and is a virology editor for the Merck Manual.

Linda L. Gebhardt, B.S., M.T. (A.S.C.P.), has been the supervisor in the Tick-Borne Bacteria Laboratory at the New York State Department of Health, Wadsworth Center, for the last 10 years. The laboratory is a reference laboratory focused on the identification of tick-borne bacteria as well as the development of new assays for emerging infectious agents in New York State. She is also involved in a collaborative research project that monitors the expansion of several tick-borne diseases in both humans and ticks in New York State. Her work at the Wadsworth Center has focused on maintaining quality assurance standards and implementing new regulations in the Division of Infectious Diseases. She is a reviewer for the New York State Clinical Laboratory Evaluation Program. She has mentored more than 20 students. She is a member of the American Society for Microbiology (ASM) and is the current treasurer for the Eastern New York Branch of ASM.

Elizabeth Kauffman is a research scientist at the Arbovirus Laboratories, Wadsworth Center, New York State Department of Health. She received a Ph.D. from the State University of New York in Albany in the field of cell biology. She joined Virogenetics, Inc., in 1986 and worked for 14 years on the development of human and animal vaccines that utilize genetically engineered poxvirus vectors to express select antigens of infectious diseases, such as dengue (DEN) virus, yellow fever virus (YFV), rabies (RAB) virus, HIV, and malaria. Dr. Kauffman joined the Arbovirus Laboratory shortly after the outbreak of West Nile virus (WNV) in New York in 1999 and has been involved in surveillance of mosquito and tick vectors collected throughout New York and neighboring states for the presence of arboviruses. Her research efforts have focused on the role of arthropod vectors in the transmission of flavivirus and bunyavirus diseases.
REFERENCES
- 1.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier PE, Raoult D. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansfield KL, Cook C, Ellis RJ, Bell-Sakyi L, Johnson N, Alberdi P, de la Fuente J, Fooks AR. 2017. Tick-borne pathogens induce differential expression of genes promoting cell survival and host resistance in Ixodes ricinus cells. Parasit Vectors 10:81. doi: 10.1186/s13071-017-2011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken JS. 1998. The discovery of human granulocytotropic ehrlichiosis. J Lab Clin Med 132:175–180. doi: 10.1016/S0022-2143(98)90165-2. [DOI] [PubMed] [Google Scholar]
- 4.Ganguly S, Mukhopadhayay SK. 2008. Tick-borne ehrlichiosis infection in human beings. J Vector Borne Dis 45:273–280. [PubMed] [Google Scholar]
- 5.Bakken JS, Dumler JS. 2006. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci 1078:236–247. doi: 10.1196/annals.1374.042. [DOI] [PubMed] [Google Scholar]
- 6.Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Folk SM, Kato CY, Lash RR, Levin ML, Massung RF, Nadelman RB, Nicholson WL, Paddock CD, Pritt BS, Traeger MS. 2016. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recommend Rep 65:1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 7.Walker DH, Dumler JS. 1996. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis 2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakken JS, Dumler JS, Chen SM, Eckman MR, Van Etta LL, Walker DH. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212–218. doi: 10.1001/jama.1994.03520030054028. [DOI] [PubMed] [Google Scholar]
- 9.Ismail N, McBride JW. 2017. Tick-borne emerging infections: ehrlichiosis and anaplasmosis. Clin Lab Med 37:317–340. doi: 10.1016/j.cll.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Thomas RJ, Dumler JS, Carlyon JA. 2009. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther 7:709–722. doi: 10.1586/eri.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 12.Morais JD, Dawson JE, Greene C, Filipe AR, Galhardas LC, Bacellar F. 1991. First European case of ehrlichiosis. Lancet 338:633–634. doi: 10.1016/0140-6736(91)90644-5. [DOI] [PubMed] [Google Scholar]
- 13.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med 341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 14.Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. 2011. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wikel SK. 2018. Ticks and tick-borne infections: complex ecology, agents, and host interactions. Vet Sci 5:E60. doi: 10.3390/vetsci5020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edouard S, Koebel C, Goehringer F, Socolovschi C, Jaulhac B, Raoult D, Brouqui P. 2012. Emergence of human granulocytic anaplasmosis in France. Ticks Tick Borne Dis 3:403–405. doi: 10.1016/j.ttbdis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Salinas LJ, Greenfield RA, Little SE, Voskuhl GW. 2010. Tickborne infections in the southern United States. Am J Med Sci 340:194–201. doi: 10.1097/MAJ.0b013e3181e93817. [DOI] [PubMed] [Google Scholar]
- 18.Blanco JR, Oteo JA. 2002. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect 8:763–772. doi: 10.1046/j.1469-0691.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 19.Adams DA, Thomas KR, Jajosky RA, Foster L, Baroi G, Sharp P, Onweh DH, Schley AW, Anderson WJ, Nationally Notifiable Infectious Conditions Group. 2017. Summary of notifiable infectious diseases and conditions—United States, 2015. MMWR Morb Mortal Wkly Rep 64:1–143. doi: 10.15585/mmwr.mm6453a1. [DOI] [PubMed] [Google Scholar]
- 20.Koh FX, Kho KL, Kisomi MG, Wong LP, Bulgiba A, Tan PE, Lim YAL, Nizam QNH, Panchadcharam C, Tay ST. 2018. Ehrlichia and Anaplasma infections: serological evidence and tick surveillance in peninsular Malaysia. J Med Entomol 55:269–276. doi: 10.1093/jme/tjx204. [DOI] [PubMed] [Google Scholar]
- 21.Larson SR, Lee X, Paskewitz SM. 2018. Prevalence of tick-borne pathogens in two species of Peromyscus mice common in northern Wisconsin. J Med Entomol 55:1002–1010. doi: 10.1093/jme/tjy027. [DOI] [PubMed] [Google Scholar]
- 22.Paddock CD, Childs JE. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev 16:37–64. doi: 10.1128/cmr.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, Jardine CM. 2017. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One 12:e0189393. doi: 10.1371/journal.pone.0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumler JS, Madigan JE, Pusterla N, Bakken JS. 2007. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis 45(Suppl 1):S45–S51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 25.Fine AB, Sweeney JD, Nixon CP, Knoll BM. 2016. Transfusion-transmitted anaplasmosis from a leukoreduced platelet pool. Transfusion 56:699–704. doi: 10.1111/trf.13392. [DOI] [PubMed] [Google Scholar]
- 26.Proctor MC, Leiby DA. 2015. Do leukoreduction filters passively reduce the transmission risk of human granulocytic anaplasmosis? Transfusion 55:1242–1248. doi: 10.1111/trf.12976. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev SH, Joshi V, Cox ER, Amoroso A, Palekar S. 2014. Severe life-threatening Ehrlichia chaffeensis infections transmitted through solid organ transplantation. Transpl Infect Dis 16:119–124. doi: 10.1111/tid.12172. [DOI] [PubMed] [Google Scholar]
- 28.Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, Davidson WR. 2002. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus). Emerg Infect Dis 8:668–671. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paddock CD, Folk SM, Shore GM, Machado LJ, Huycke MM, Slater LN, Liddell AM, Buller RS, Storch GA, Monson TP, Rimland D, Sumner JW, Singleton J, Bloch KC, Tang YW, Standaert SM, Childs JE. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis 33:1586–1594. doi: 10.1086/323981. [DOI] [PubMed] [Google Scholar]
- 30.Regan J, Matthias J, Green-Murphy A, Stanek D, Bertholf M, Pritt BS, Sloan LM, Kelly AJ, Singleton J, McQuiston JH, Hocevar SN, Whittle JP. 2013. A confirmed Ehrlichia ewingii infection likely acquired through platelet transfusion. Clin Infect Dis 56:e105–e107. doi: 10.1093/cid/cit177. [DOI] [PubMed] [Google Scholar]
- 31.Singu V, Liu H, Cheng C, Ganta RR. 2005. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect Immun 73:79–87. doi: 10.1128/IAI.73.1.79-87.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuen S, Granquist EG, Silaghi C. 2013. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez E, Vannier E, Wormser GP, Hu LT. 2016. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branger S, Rolain JM, Raoult D. 2004. Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother 48:4822–4828. doi: 10.1128/AAC.48.12.4822-4828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore A, Nelson C, Molins C, Mead P, Schriefer M. 2016. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis 22:1169–1177. doi: 10.3201/eid2207.151694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiltske B, Johnson BJB, Schriefer ME. 2006. Borrelia, p 971–986. In Murray P, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed), Manual of clinical microbiology, 9th ed, vol1 ASM Press, Washington, DC. [Google Scholar]
- 39.Oren A, Garrity GM. 2015. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol 65:3763–3767. doi: 10.1099/ijsem.0.000632. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelmsson P, Lindgren PE. 2016. Detection of a novel Lyme borreliosis pathogen. Lancet Infect Dis 16:511–512. doi: 10.1016/S1473-3099(15)00483-1. [DOI] [PubMed] [Google Scholar]
- 41.Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, Schiffman E, Hoang Johnson DK, Davis JP, Paskewitz SM, Boxrud D, Deedon A, Lee X, Miller TK, Feist MA, Steward CR, Theel ES, Patel R, Irish CL, Petersen JM. 2016. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol Microbiol 66:4878–4880. doi: 10.1099/ijsem.0.001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollend L, Fish D, Childs JE. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis 4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM. 1977. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 44.Falkenbach A, Wigand R, Weber B, Gottschalk R, Doerr HW, Kaltwasser JP. 1993. Incidence of Lyme borreliosis in a rheumatologic patient sample. Study of 153 patients of an internal medicine-rheumatologic ambulatory clinic. Fortschr Med 111:377–379. (In German.) [PubMed] [Google Scholar]
- 45.Guner ES, Hashimoto N, Takada N, Kaneda K, Imai Y, Masuzawa T. 2003. First isolation and characterization of Borrelia burgdorferi sensu lato strains from Ixodes ricinus ticks in Turkey. J Med Microbiol 52:807–813. doi: 10.1099/jmm.0.05205-0. [DOI] [PubMed] [Google Scholar]
- 46.Oda R, Kutsuna S, Sekikawa Y, Hongo I, Sato K, Ohnishi M, Kawabata H. 2017. The first case of imported Borrelia miyamotoi disease concurrent with Lyme disease. J Infect Chemother 23:333–335. doi: 10.1016/j.jiac.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Jaenson TG, Lindgren E. 2011. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis 2:44–49. doi: 10.1016/j.ttbdis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Lindgren E, Jaenson TGT. 2006. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. WHO Regional Office for Europe, Copenhagen, Denmark. [Google Scholar]
- 49.Molins CR, Ashton LV, Wormser GP, Andre BG, Hess AM, Delorey MJ, Pilgard MA, Johnson BJ, Webb K, Islam MN, Pegalajar-Jurado A, Molla I, Jewett MW, Belisle JT. 2017. Metabolic differentiation of early Lyme disease from southern tick-associated rash illness (STARI). Sci Transl Med 9:eaal2717. doi: 10.1126/scitranslmed.aal2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. 2016. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Negl Trop Dis 10:e0005042. doi: 10.1371/journal.pntd.0005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. 2013. Three sudden cardiac deaths associated with Lyme carditis—United States, November 2012–July 2013. MMWR Morb Mortal Wkly Rep 62:993–996. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6249a1.htm. [PMC free article] [PubMed] [Google Scholar]
- 52.Infectious Diseases Society of America. 2019. Clinical practice guidelines. Infectious Diseases Society of America, Arlington, VA: https://www.idsociety.org/practice-guideline/alphabetical-guidelines/. Accessed 10 October 2019. [Google Scholar]
- 53.Ledue TB, Collins MF, Young J, Schriefer ME. 2008. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 15:1796–1804. doi: 10.1128/CVI.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porwancher RB, Hagerty CG, Fan J, Landsberg L, Johnson BJ, Kopnitsky M, Steere AC, Kulas K, Wong SJ. 2011. Multiplex immunoassay for Lyme disease using VlsE1-IgG and pepC10-IgM antibodies: improving test performance through bioinformatics. Clin Vaccine Immunol 18:851–859. doi: 10.1128/CVI.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molloy PJ, Telford SR III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. 2015. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med 163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 56.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. 2011. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 53:541–547. doi: 10.1093/cid/cir464. [DOI] [PubMed] [Google Scholar]
- 57.Pegalajar-Jurado A, Schriefer ME, Welch RJ, Couturier MR, MacKenzie T, Clark RJ, Ashton LV, Delorey MJ, Molins CR. 2018. Evaluation of modified two-tiered testing algorithms for Lyme disease laboratory diagnosis using well-characterized serum samples. J Clin Microbiol 56:e01943-17. doi: 10.1128/JCM.01943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Remy MM, Schobi N, Kottanattu L, Pfister S, Duppenthaler A, Suter-Riniker F. 2017. Cerebrospinal fluid CXCL13 as a diagnostic marker of neuroborreliosis in children: a retrospective case-control study. J Neuroinflammation 14:173. doi: 10.1186/s12974-017-0948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molins CR, Ashton LV, Wormser GP, Hess AM, Delorey MJ, Mahapatra S, Schriefer ME, Belisle JT. 2015. Development of a metabolic biosignature for detection of early Lyme disease. Clin Infect Dis 60:1767–1775. doi: 10.1093/cid/civ185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theel ES. 2016. The past, present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol 54:1191–1196. doi: 10.1128/JCM.03394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rupprecht TA, Manz KM, Fingerle V, Lechner C, Klein M, Pfirrmann M, Koedel U. 2018. Diagnostic value of cerebrospinal fluid CXCL13 for acute Lyme neuroborreliosis. A systematic review and meta-analysis. Clin Microbiol Infect 24:1234–1240. doi: 10.1016/j.cmi.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. 2004. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis 10:1661–1664. doi: 10.3201/eid1009.040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cutler SJ. 2015. Relapsing fever borreliae: a global review. Clin Lab Med 35:847–865. doi: 10.1016/j.cll.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Forrester JD, Kjemtrup AM, Fritz CL, Marsden-Haug N, Nichols JB, Tengelsen LA, Sowadsky R, DeBess E, Cieslak PR, Weiss J, Evert N, Ettestad P, Smelser C, Iralu J, Nett RJ, Mosher E, Baker JS, Van Houten C, Thorp E, Geissler AL, Kugeler K, Mead P, Centers for Disease Control and Prevention. 2015. Tickborne relapsing fever—United States, 1990–2011. MMWR Morb Mortal Wkly Rep 64:58–60. [PMC free article] [PubMed] [Google Scholar]
- 65.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. 2013. Human Borrelia miyamotoi infection in the United States. N Engl J Med 368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wroblewski D, Gebhardt L, Prusinski MA, Meehan LJ, Halse TA, Musser KA. 2017. Detection of Borrelia miyamotoi and other tick-borne pathogens in human clinical specimens and Ixodes scapularis ticks in New York State, 2012-2015. Ticks Tick Borne Dis 8:407–411. doi: 10.1016/j.ttbdis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jahfari S, Herremans T, Platonov AE, Kuiper H, Karan LS, Vasilieva O, Koopmans MP, Hovius JW, Sprong H. 2014. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect 2:144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, Callister SM. 2016. Borrelia miyamotoi infection in patients from upper midwestern United States, 2014–2015. Emerg Infect Dis 22:1471–1473. doi: 10.3201/eid2208.151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang BG, Jia N, Jiang JF, Zheng YC, Chu YL, Jiang RR, Wang YW, Liu HB, Wei R, Zhang WH, Li Y, Xu XW, Ye JL, Yao NN, Liu XJ, Huo QB, Sun Y, Song JL, Liu W, Cao WC. 2018. Borrelia miyamotoi infections in humans and ticks, northeastern China. Emerg Infect Dis 24:236–241. doi: 10.3201/eid2402.160378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siński E, Welc-Falęciak R, Zajkowska J. 2016. Borrelia miyamotoi: a human tick-borne relapsing fever spirochete in Europe and its potential impact on public health. Adv Med Sci 61:255–260. doi: 10.1016/j.advms.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D, Tick Borne Diseases Group. 2014. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krause PJ, Carroll M, Fedorova N, Brancato J, Dumouchel C, Akosa F, Narasimhan S, Fikrig E, Lane RS. 2018. Human Borrelia miyamotoi infection in California: serodiagnosis is complicated by multiple endemic Borrelia species. PLoS One 13:e0191725. doi: 10.1371/journal.pone.0191725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karan L, Makenov M, Kolyasnikova N, Stukolova O, Toporkova M, Olenkova O. 2018. Dynamics of spirochetemia and early PCR detection of Borrelia miyamotoi. Emerg Infect Dis 24:860–867. doi: 10.3201/eid2405.170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. 2014. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors 7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, Sterling SL, Telford SR III. 2013. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med 159:21–27. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 77.Anonymous. 2019. Recommendations for reducing the risk of transfusion-transmitted babesiosis. Center for Biologics Evaluation and Research, Food and Drug Administration, Department of Health and Human Services, Rockville, MD: https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. [Google Scholar]
- 78.Schultz M. 2008. Theobald Smith. Emerg Infect Dis 14:1940–1942. doi: 10.3201/eid1412.081188. [DOI] [Google Scholar]
- 79.Lemieux JE, Tran AD, Freimark L, Schaffner SF, Goethert H, Andersen KG, Bazner S, Li A, McGrath G, Sloan L, Vannier E, Milner D, Pritt B, Rosenberg E, Telford S III, Bailey JA, Sabeti PC. 2016. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol 1:16079. doi: 10.1038/nmicrobiol.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yabsley MJ, Shock BC. 2013. Natural history of zoonotic Babesia: role of wildlife reservoirs. Int J Parasitol Parasites Wildl 2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skrabalo Z, Deanovic Z. 1957. Piroplasmosis in man; report of a case. Doc Med Geogr Trop 9:11–16. [PubMed] [Google Scholar]
- 82.Western KA, Benson GD, Gleason NN, Healy GR, Schultz MG. 1970. Babesiosis in a Massachusetts resident. N Engl J Med 283:854–856. doi: 10.1056/NEJM197010152831607. [DOI] [PubMed] [Google Scholar]
- 83.Scott JD. 2017. First record of locally acquired human babesiosis in Canada caused by Babesia duncani: a case report. SAGE Open Med Case Rep 5:2050313X17725645. doi: 10.1177/2050313X17725645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swei A, O’Connor KE, Couper LI, Thekkiniath J, Conrad PA, Padgett KA, Burns J, Yoshimizu MH, Gonzales B, Munk B, Shirkey N, Konde L, Ben Mamoun C, Lane RS, Kjemtrup A. 2019. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int J Parasitol 49:95–103. doi: 10.1016/j.ijpara.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wójcik-Fatla A, Zając V, Sawczyn A, Cisak E, Dutkiewicz J. 2015. Babesia spp. in questing ticks from eastern Poland: prevalence and species diversity. Parasitol Res 114:3111–3116. doi: 10.1007/s00436-015-4529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou X, Xia S, Huang JL, Tambo E, Zhuge HX, Zhou XN. 2014. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasit Vectors 7:509. doi: 10.1186/s13071-014-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shock BC, Moncayo A, Cohen S, Mitchell EA, Williamson PC, Lopez G, Garrison LE, Yabsley MJ. 2014. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis 5:373–380. doi: 10.1016/j.ttbdis.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Gray J, von Stedingk LV, Gürtelschmid M, Granström M. 2002. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J Clin Microbiol 40:1259–1263. doi: 10.1128/jcm.40.4.1259-1263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stein E, Elbadawi LI, Kazmierczak J, Davis JP. 2017. Babesiosis surveillance—Wisconsin, 2001–2015. MMWR Morb Mortal Wkly Rep 66:687–691. doi: 10.15585/mmwr.mm6626a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB. 2014. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley region, New York State. J Med Entomol 51:226–236. doi: 10.1603/me13101. [DOI] [PubMed] [Google Scholar]
- 91.Herwaldt BL, Montgomery S, Woodhall D, Bosserman EA. 2012. Babesiosis surveillance—18 states, 2011. MMWR Morb Mortal Wkly Rep 61:505–509. [PubMed] [Google Scholar]
- 92.Anonymous. 2017. 2016 annual tables of infectious disease data. CDC Division of Health Informatics and Surveillance, Atlanta, GA: https://wonder.cdc.gov/nndss/static/2016/annual/2016-table1.html. [Google Scholar]
- 93.Quick RE, Herwaldt BL, Thomford JW, Garnett ME, Eberhard ML, Wilson M, Spach DH, Dickerson JW, Telford SR III, Steingart KR, Pollock R, Persing DH, Kobayashi JM, Juranek DD, Conrad PA. 1993. Babesiosis in Washington State: a new species of Babesia? Ann Intern Med 119:284–290. doi: 10.7326/0003-4819-119-4-199308150-00006. [DOI] [PubMed] [Google Scholar]
- 94.Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR III, Herwaldt BL. 2006. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol 36:779–789. doi: 10.1016/j.ijpara.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Persing DH, Herwaldt BL, Glaser C, Lane RS, Thomford JW, Mathiesen D, Krause PJ, Phillip DF, Conrad PA. 1995. Infection with a Babesia-like organism in northern California. N Engl J Med 332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 96.Herwaldt B, Persing DH, Precigout EA, Goff WL, Mathiesen DA, Taylor PW, Eberhard ML, Gorenflot AF. 1996. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med 124:643–650. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 97.Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, Fritsche TR, Persing DH, Limaye AP. 2004. Babesia divergens-like infection, Washington State. Emerg Infect Dis 10:622–629. doi: 10.3201/eid1004.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burgess MJ, Rosenbaum ER, Pritt BS, Haselow DT, Ferren KM, Alzghoul BN, Rico JC, Sloan LM, Ramanan P, Purushothaman R, Bradsher RW. 2017. Possible transfusion-transmitted Babesia divergens-like/MO-1 infection in an Arkansas patient. Clin Infect Dis 64:1622–1625. doi: 10.1093/cid/cix216. [DOI] [PubMed] [Google Scholar]
- 99.Herc E, Pritt B, Huizenga T, Douce R, Hysell M, Newton D, Sidge J, Losman E, Sherbeck J, Kaul DR. 2018. Probable locally acquired Babesia divergens-like infection in woman, Michigan, USA. Emerg Infect Dis 24:1558–1560. doi: 10.3201/eid2408.180309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brasseur P, Gorenflot A. 1992. Human babesiosis in Europe. Mem Inst Oswaldo Cruz 87(Suppl 3):131–132. doi: 10.1590/s0074-02761992000700019. [DOI] [PubMed] [Google Scholar]
- 101.Gray J, von Stedingk L-V, Granström M. 2002. Zoonotic babesiosis. Int J Med Microbiol 291(Suppl 33):108–111. doi: 10.1016/s1438-4221(02)80021-2. [DOI] [PubMed] [Google Scholar]
- 102.Kjemtrup AM, Conrad PA. 2000. Human babesiosis: an emerging tick-borne disease. Int J Parasitol 30:1323–1337. doi: 10.1016/s0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez LM, Rojo S, Gonzalez-Camacho F, Luque D, Lobo CA, Montero E. 2014. Severe babesiosis in immunocompetent man, Spain, 2011. Emerg Infect Dis 20:724–726. doi: 10.3201/eid2004.131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hildebrandt A, Gray JS, Hunfeld KP. 2013. Human babesiosis in Europe: what clinicians need to know. Infection 41:1057–1072. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- 105.Morch K, Holmaas G, Frolander PS, Kristoffersen EK. 2015. Severe human Babesia divergens infection in Norway. Int J Infect Dis 33:37–38. doi: 10.1016/j.ijid.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 106.Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, Horowitz HW, Aguero-Rosenfeld ME, Moore JM, Abramowsky C, Wormser GP. 2012. Vertical transmission of Babesia microti, United States. Emerg Infect Dis 18:1318–1321. doi: 10.3201/eid1808.110988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan KR, Wiegand RE, Arguin PM. 2013. Exchange transfusion for severe malaria: evidence base and literature review. Clin Infect Dis 57:923–928. doi: 10.1093/cid/cit429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rigaud E, Jaulhac B, Garcia-Bonnet N, Hunfeld KP, Femenia F, Huet D, Goulvestre C, Vaillant V, Deffontaines G, Abadia-Benoist G. 2016. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clin Microbiol Infect 22:735.e1–735.e9. doi: 10.1016/j.cmi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 109.Gabrielli S, Calderini P, Cassini R, Galuppi R, Tampieri MP, Pietrobelli M, Cancrini G. 2014. Human exposure to piroplasms in central and northern Italy. Vet Ital 50:41–47. doi: 10.12834/VetIt.1302.13. [DOI] [PubMed] [Google Scholar]
- 110.Lempereur L, Shiels B, Heyman P, Moreau E, Saegerman C, Losson B, Malandrin L. 2015. A retrospective serological survey on human babesiosis in Belgium. Clin Microbiol Infect 21:96.e1–96.e7. doi: 10.1016/j.cmi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Oines O, Radzijevskaja J, Paulauskas A, Rosef O. 2012. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasit Vectors 5:156. doi: 10.1186/1756-3305-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, Chirico J, van der Wal FJ, Sprong H, Boye Pihl TP, Klitgaard K, Bødker R, Fach P, Moutailler S. 2014. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol 4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paul RE, Cote M, Le Naour E, Bonnet SI. 2016. Environmental factors influencing tick densities over seven years in a French suburban forest. Parasit Vectors 9:309. doi: 10.1186/s13071-016-1591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schorn S, Pfister K, Reulen H, Mahling M, Silaghi C. 2011. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasit Vectors 4:135. doi: 10.1186/1756-3305-4-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, Sun Y, Jia N, Wang YW, Ma L, Liu HB, Chu YL, Ni XB, Liu K, Song YD, Yao NN, Wang H, Sun T, Cao WC. 2015. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis 15:196–203. doi: 10.1016/S1473-3099(14)71046-1. [DOI] [PubMed] [Google Scholar]
- 116.Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, Ge HX, Chen JH, Hu W. 2013. Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty 2:24. doi: 10.1186/2049-9957-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, Wang Z, Yang D, Wang S, Chai T. 2011. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res 109:241–245. doi: 10.1007/s00436-011-2382-8. [DOI] [PubMed] [Google Scholar]
- 118.Chen XR, Ye LI, Fan JW, Li C, Tang F, Liu W, Ren LZ, Bai JY. 2017. Detection of Kobe-type and Otsu-type Babesia microti in wild rodents in China’s Yunnan Province. Epidemiol Infect 145:2704–2710. doi: 10.1017/S0950268817001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moustafa MAM, Taylor K, Nakao R, Shimozuru M, Sashika M, Rosa R, Thu MJ, Rizzoli A, Tsubota T. 2016. Dynamics, co-infections and characteristics of zoonotic tick-borne pathogens in Hokkaido small mammals, Japan. Ticks Tick Borne Dis 7:922–928. doi: 10.1016/j.ttbdis.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 120.Zamoto-Niikura A, Morikawa S, Hanaki KI, Holman PJ, Ishihara C. 2016. Ixodes persulcatus ticks as vectors for the Babesia microti U.S. lineage in Japan. Appl Environ Microbiol 82:6624–6632. doi: 10.1128/AEM.02373-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zamoto-Niikura A, Tsuji M, Qiang W, Morikawa S, Hanaki KI, Holman PJ, Ishihara C. 2018. Babesia divergens Asia lineage is maintained between Ixodes persulcatus and sika deer in Hokkaido, Japan. Appl Environ Microbiol 84:e02491-17. doi: 10.1128/AEM.02491-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, Arai S, Kamiyama T, Hioki K, Ishihara C. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol 38:4511–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marathe A, Tripathi J, Handa V, Date V. 2005. Human babesiosis—a case report. Indian J Med Microbiol 23:267–269. [PubMed] [Google Scholar]
- 124.El-Bahnasawy MM, Morsy TA. 2008. Egyptian human babesiosis and general review. J Egypt Soc Parasitol 38:265–272. [PubMed] [Google Scholar]
- 125.Gabrielli S, Bellina L, Milardi GL, Katende BK, Totino V, Fullin V, Cancrini G. 2016. Malaria in children of Tshimbulu (Western Kasai, Democratic Republic of the Congo): epidemiological data and accuracy of diagnostic assays applied in a limited resource setting. Malar J 15:81. doi: 10.1186/s12936-016-1142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Senanayake SN, Paparini A, Latimer M, Andriolo K, Dasilva AJ, Wilson H, Xayavong MV, Collignon PJ, Jeans P, Irwin PJ. 2012. First report of human babesiosis in Australia. Med J Aust 196:350–352. doi: 10.5694/mja11.11378. [DOI] [PubMed] [Google Scholar]
- 127.Buelvas F, Alvis N, Buelvas I, Miranda J, Mattar S. 2008. A high prevalence of antibodies against Bartonella and Babesia microti has been found in villages and urban populations in Cordoba, Colombia. Rev Salud Publica (Bogota) 10:168–177. (In Spanish.) . doi: 10.1590/s0124-00642008000100016. [DOI] [PubMed] [Google Scholar]
- 128.Gabrielli S, Totino V, Macchioni F, Zuniga F, Rojas P, Lara Y, Roselli M, Bartoloni A, Cancrini G. 2016. Human babesiosis, Bolivia, 2013. Emerg Infect Dis 22:1445–1447. doi: 10.3201/eid2208.150195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Piesman J, Spielman A. 1982. Babesia microti: infectivity of parasites from ticks for hamsters and white-footed mice. Exp Parasitol 53:242–248. doi: 10.1016/0014-4894(82)90065-0. [DOI] [PubMed] [Google Scholar]
- 130.Hunfeld KP, Hildebrandt A, Gray JS. 2008. Babesiosis: recent insights into an ancient disease. Int J Parasitol 38:1219–1237. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 131.Gray EB, Herwaldt BL. 2016. Surveillance for babesiosis—United States, 2014. Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 132.Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. 2001. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 32:1117–1125. doi: 10.1086/319742. [DOI] [PubMed] [Google Scholar]
- 133.Kletsova EA, Spitzer ED, Fries BC, Marcos LA. 2017. Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann Clin Microbiol Antimicrob 16:26. doi: 10.1186/s12941-017-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.White DJ, Talarico J, Chang HG, Birkhead GS, Heimberger T, Morse DL. 1998. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med 158:2149–2154. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 135.Levin AE, Krause PJ. 2016. Transfusion-transmitted babesiosis: is it time to screen the blood supply? Curr Opin Hematol 23:573–580. doi: 10.1097/MOH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, Jouglin M, Chauvin A, De Briel D. 2011. Babesiosis in immunocompetent patients, Europe. Emerg Infect Dis 17:114–116. doi: 10.3201/eid1701.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosner F, Zarrabi MH, Benach JL, Habicht GS. 1984. Babesiosis in splenectomized adults. Review of 22 reported cases. Am J Med 76:696–701. doi: 10.1016/0002-9343(84)90298-5. [DOI] [PubMed] [Google Scholar]
- 138.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. 2011. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 155:509–519. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 139.Linden JV, Prusinski MA, Crowder LA, Tonnetti L, Stramer SL, Kessler DA, White J, Shaz B, Olkowska D. 2018. Transfusion-transmitted and community-acquired babesiosis in New York, 2004 to 2015. Transfusion 58:660–668. doi: 10.1111/trf.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saetre K, Godhwani N, Maria M, Patel D, Wang G, Li KI, Wormser GP, Nolan SM. 2018. Congenital babesiosis after maternal infection with Borrelia burgdorferi and Babesia microti. J Pediatr Infect Dis Soc 7:e1–e5. doi: 10.1093/jpids/pix074. [DOI] [PubMed] [Google Scholar]
- 141.Sethi S, Alcid D, Kesarwala H, Tolan RW Jr. 2009. Probable congenital babesiosis in infant, New Jersey, USA. Emerg Infect Dis 15:788–791. doi: 10.3201/eid1505.070808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aderinboye O, Syed SS. 2010. Congenital babesiosis in a four-week-old female infant. Pediatr Infect Dis J 29:188. doi: 10.1097/INF.0b013e3181c3c971. [DOI] [PubMed] [Google Scholar]
- 143.Warren T, Lau R, Ralevski F, Rau N, Boggild AK. 2015. Fever in a visitor to Canada: a case of mistaken identity. J Clin Microbiol 53:1783–1785. doi: 10.1128/JCM.00269-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. 2012. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol 50:903–908. doi: 10.1128/JCM.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang G, Wormser GP, Zhuge J, Villafuerte P, Ip D, Zeren C, Fallon JT. 2015. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis 6:376–382. doi: 10.1016/j.ttbdis.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 146.Moritz ED, Tonnetti L, Hewins ME, Berardi VP, Dodd RY, Stramer SL. 2017. Description of 15 DNA-positive and antibody-negative “window-period” blood donations identified during prospective screening for Babesia microti. Transfusion 57:1781–1786. doi: 10.1111/trf.14103. [DOI] [PubMed] [Google Scholar]
- 147.Anonymous. 2013. Drugs for parasitic infections. Treat Guidel Med Lett 11:e1–e31. [Google Scholar]
- 148.Saifee NH, Krause PJ, Wu Y. 2016. Apheresis for babesiosis: therapeutic parasite reduction or removal of harmful toxins or both? J Clin Apher 31:454–458. doi: 10.1002/jca.21429. [DOI] [PubMed] [Google Scholar]
- 149.Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR III, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. 2000. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 343:1454–1458. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 150.Man SQ, Qiao K, Cui J, Feng M, Fu YF, Cheng XJ. 2016. A case of human infection with a novel Babesia species in China. Infect Dis Poverty 5:28. doi: 10.1186/s40249-016-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Panduranga V, Kumar A. 2014. Severe babesiosis presenting as acute respiratory distress syndrome in an immunocompetent patient. Conn Med 78:289–291. [PubMed] [Google Scholar]
- 152.Wudhikarn K, Perry EH, Kemperman M, Jensen KA, Kline SE. 2011. Transfusion-transmitted babesiosis in an immunocompromised patient: a case report and review. Am J Med 124:800–805. doi: 10.1016/j.amjmed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 153.Wang G, Villafuerte P, Zhuge J, Visintainer P, Wormser GP. 2015. Comparison of a quantitative PCR assay with peripheral blood smear examination for detection and quantitation of Babesia microti infection in humans. Diagn Microbiol Infect Dis 82:109–113. doi: 10.1016/j.diagmicrobio.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR III, Christianson D, Dardick K, Coleman M, Girotto JE, Spielman A. 2008. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 46:370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 155.Krause PJ, Spielman A, Telford SR III, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH. 1998. Persistent parasitemia after acute babesiosis. N Engl J Med 339:160–165. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 156.Wormser GP, Prasad A, Neuhaus E, Joshi S, Nowakowski J, Nelson J, Mittleman A, Aguero-Rosenfeld M, Topal J, Krause PJ. 2010. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis 50:381–386. doi: 10.1086/649859. [DOI] [PubMed] [Google Scholar]
- 157.Simon MS, Westblade LF, Dziedziech A, Visone JE, Furman RR, Jenkins SG, Schuetz AN, Kirkman LA. 2017. Clinical and molecular evidence of atovaquone and azithromycin resistance in relapsed Babesia microti infection associated with rituximab and chronic lymphocytic leukemia. Clin Infect Dis 65:1222–1225. doi: 10.1093/cid/cix477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 44:2100–2108. doi: 10.1128/aac.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. 1999. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol Microbiol 33:704–711. doi: 10.1046/j.1365-2958.1999.01515.x. [DOI] [PubMed] [Google Scholar]
- 160.Matsuu A, Miyamoto K, Ikadai H, Okano S, Higuchi S. 2006. Short report: cloning of the Babesia gibsoni cytochrome B gene and isolation of three single nucleotide polymorphisms from parasites present after atovaquone treatment. Am J Trop Med Hyg 74:593–597. doi: 10.4269/ajtmh.2006.74.593. [DOI] [PubMed] [Google Scholar]
- 161.Grard G, Moureau G, Charrel RN, Lemasson JJ, Gonzalez JP, Gallian P, Gritsun TS, Holmes EC, Gould EA, de Lamballerie X. 2007. Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 361:80–92. doi: 10.1016/j.virol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 162.Ecker M, Allison SL, Meixner T, Heinz FX. 1999. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol 80(Part 1):179–185. doi: 10.1099/0022-1317-80-1-179. [DOI] [PubMed] [Google Scholar]
- 163.Dobler G, Gniel D, Petermann R, Pfeffer M. 2012. Epidemiology and distribution of tick-borne encephalitis. Wien Med Wochenschr 162:230–238. doi: 10.1007/s10354-012-0100-5. [DOI] [PubMed] [Google Scholar]
- 164.Calisher CH, Gresikova M. 1989. Tick-borne encephalitis, p 177–202. In Monath TP. (ed), The arboviruses: epidemiology and ecology, 1st ed, vol 4 CRC Press, Boca Raton, FL. [Google Scholar]
- 165.Beasley DW, Suderman MT, Holbrook MR, Barrett AD. 2001. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res 79:81–89. doi: 10.1016/s0168-1702(01)00330-6. [DOI] [PubMed] [Google Scholar]
- 166.Telford SR III, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A. 1997. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuno G, Artsob H, Karabatsos N, Tsuchiya KR, Chang GJ. 2001. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg 65:671–676. doi: 10.4269/ajtmh.2001.65.671. [DOI] [PubMed] [Google Scholar]
- 168.Tavakoli NP, Wang H, Dupuis M, Hull R, Ebel GD, Gilmore EJ, Faust PL. 2009. Fatal case of deer tick virus encephalitis. N Engl J Med 360:2099–2107. doi: 10.1056/NEJMoa0806326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pesko KN, Torres-Perez F, Hjelle BL, Ebel GD. 2010. Molecular epidemiology of Powassan virus in North America. J Gen Virol 91:2698–2705. doi: 10.1099/vir.0.024232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leonova GN, Sorokina MN, Krugliak SP. 1991. The clinico-epidemiological characteristics of Powassan encephalitis in the southern Soviet Far East. Zh Mikrobiol Epidemiol Immunobiol 1991:35–39. (In Russian.) [PubMed] [Google Scholar]
- 171.Levy S. 2014. Ticking time bomb? Climate change and Ixodes scapularis. Environ Health Perspect 122:A168. doi: 10.1289/ehp.122-A168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J. 2014. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect 122:631–638. doi: 10.1289/ehp.1307799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mandl CW, Holzmann H, Kunz C, Heinz FX. 1993. Complete genomic sequence of Powassan virus: evaluation of genetic elements in tick-borne versus mosquito-borne flaviviruses. Virology 194:173–184. doi: 10.1006/viro.1993.1247. [DOI] [PubMed] [Google Scholar]
- 174.Clarke DH. 1964. Further studies on antigenic relationships among the viruses of the group B tick-borne complex. Bull World Health Organ 31:45–56. [PMC free article] [PubMed] [Google Scholar]
- 175.Labuda M, Nuttall PA, Kozuch O, Eleckova E, Williams T, Zuffova E, Sabo A. 1993. Non-viraemic transmission of tick-borne encephalitis virus: a mechanism for arbovirus survival in nature. Experientia 49:802–805. doi: 10.1007/bf01923553. [DOI] [PubMed] [Google Scholar]
- 176.Nonaka E, Ebel GD, Wearing HJ. 2010. Persistence of pathogens with short infectious periods in seasonal tick populations: the relative importance of three transmission routes. PLoS One 5:e11745. doi: 10.1371/journal.pone.0011745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Naumov RL, Gutova VP, Chunikhin SP. 1980. Ixodid ticks and the causative agent of tick-borne encephalitis. 2. The genera Dermacentor and Haemaphysalis. Med Parazitol (Mosk) 49:66–69. (In Russian.) [PubMed] [Google Scholar]
- 178.Danielova V, Holubova J, Pejcoch M, Daniel M. 2002. Potential significance of transovarial transmission in the circulation of tick-borne encephalitis virus. Folia Parasitol (Praha) 49:323–325. doi: 10.14411/fp.2002.060. [DOI] [PubMed] [Google Scholar]
- 179.Artsob H, Spence L, Th’ng C, Lampotang V, Johnston D, MacInnes C, Matejka F, Voigt D, Watt I. 1986. Arbovirus infections in several Ontario mammals, 1975-1980. Can J Vet Res 50:42–46. [PMC free article] [PubMed] [Google Scholar]
- 180.McLean DM, Cobb C, Gooderham SE, Smart CA, Wilson AG, Wilson WE. 1967. Powassan virus: persistence of virus activity during 1966. Can Med Assoc J 96:660–664. [PMC free article] [PubMed] [Google Scholar]
- 181.McLean DM, Smith PA, Livingstone SE, Wilson WE, Wilson AG. 1966. Powassan virus: vernal spread during 1965. Can Med Assoc J 94:532–536. [PMC free article] [PubMed] [Google Scholar]
- 182.McLean DM, Macpherson LW, Walker SJ, Funk G. 1960. Powassan virus: surveys of human and animal sera. Am J Public Health Nations Health 50:1539–1544. doi: 10.2105/ajph.50.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McLean DM, Best JM, Mahalingam S, Chernesky MA, Wilson WE. 1964. Powassan virus: summer infection cycle, 1964. Can Med Assoc J 91:1360–1362. [PMC free article] [PubMed] [Google Scholar]
- 184.McLean DM, Devos A, Quantz EJ. 1964. Powassan virus: field investigations during the summer of 1963. Am J Trop Med Hyg 13:747–753. doi: 10.4269/ajtmh.1964.13.747. [DOI] [PubMed] [Google Scholar]
- 185.McLean DM, McQueen EJ, Petite HE, Macpherson LW, Scholten TH, Ronald K. 1962. Powassan virus: field investigations in northern Ontario, 1959 to 1961. Can Med Assoc J 86:971–974. [PMC free article] [PubMed] [Google Scholar]
- 186.Whitney E, Jamnback H. 1965. The first isolations of Powassan virus in New York State. Proc Soc Exp Biol Med 119:432–435. doi: 10.3181/00379727-119-30202. [DOI] [PubMed] [Google Scholar]
- 187.Cohen SB, Freye JD, Dunlap BG, Dunn JR, Jones TF, Moncayo AC. 2010. Host associations of Dermacentor, Amblyomma, and Ixodes (Acari: Ixodidae) ticks in Tennessee. J Med Entomol 47:415–420. doi: 10.1603/me09065. [DOI] [PubMed] [Google Scholar]
- 188.Anderson JF, Magnarelli LA. 1980. Vertebrate host relationships and distribution of ixodid ticks (Acari: Ixodidae) in Connecticut, USA. J Med Entomol 17:314–323. doi: 10.1093/jmedent/17.4.314. [DOI] [PubMed] [Google Scholar]
- 189.Campbell BS, Bowles DE. 1994. Human tick bite records in a United States Air Force population, 1989–1992: implications for tick-borne disease risk. J Wilderness Med 5:405–412. doi: 10.1580/0953-9859-5.4.405. [DOI] [Google Scholar]
- 190.Hall JE, Amrine JW Jr, Gais RD, Kolanko VP, Hagenbuch BE, Gerencser VF, Clark SM. 1991. Parasitization of humans in West Virginia by Ixodes cookei (Acari: Ixodidae), a potential vector of Lyme borreliosis. J Med Entomol 28:186–189. doi: 10.1093/jmedent/28.1.186. [DOI] [PubMed] [Google Scholar]
- 191.Barbour AG, Fish D. 1993. The biological and social phenomenon of Lyme disease. Science 260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 192.Keirans JE, Hutcheson HJ, Durden LA, Klompen JS. 1996. Ixodes (Ixodes) scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol 33:297–318. doi: 10.1093/jmedent/33.3.297. [DOI] [PubMed] [Google Scholar]
- 193.Smith RP Jr, Rand PW, Lacombe EH, Morris SR, Holmes DW, Caporale DA. 1996. Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease. J Infect Dis 174:221–224. doi: 10.1093/infdis/174.1.221. [DOI] [PubMed] [Google Scholar]
- 194.Apperson CS, Levine JF, Nicholson WL. 1990. Geographic occurrence of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) infesting white-tailed deer in North Carolina. J Wildl Dis 26:550–553. doi: 10.7589/0090-3558-26.4.550. [DOI] [PubMed] [Google Scholar]
- 195.Apperson CS, Levine JF, Evans TL, Braswell A, Heller J. 1993. Relative utilization of reptiles and rodents as hosts by immature Ixodes scapularis (Acari: Ixodidae) in the coastal plain of North Carolina, USA. Exp Appl Acarol 17:719–731. [DOI] [PubMed] [Google Scholar]
- 196.Levine JF, Apperson CS, Howard P, Washburn M, Braswell AL. 1997. Lizards as hosts for immature Ixodes scapularis (Acari: Ixodidae) in North Carolina. J Med Entomol 34:594–598. doi: 10.1093/jmedent/34.6.594. [DOI] [PubMed] [Google Scholar]
- 197.Brackney DE, Brown IK, Nofchissey RA, Fitzpatrick KA, Ebel GD. 2010. Homogeneity of Powassan virus populations in naturally infected Ixodes scapularis. Virology 402:366–371. doi: 10.1016/j.virol.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD. 2008. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg 79:971–973. doi: 10.4269/ajtmh.2008.79.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ebel GD, Campbell EN, Goethert HK, Spielman A, Telford SR III. 2000. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg 63:36–42. doi: 10.4269/ajtmh.2000.63.36. [DOI] [PubMed] [Google Scholar]
- 200.Ebel GD, Foppa I, Spielman A, Telford SR III. 1999. A focus of deer tick virus transmission in the northcentral United States. Emerg Infect Dis 5:570–574. doi: 10.3201/eid0504.990423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Costero A, Grayson MA. 1996. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari: Ixodidae). Am J Trop Med Hyg 55:536–546. doi: 10.4269/ajtmh.1996.55.536. [DOI] [PubMed] [Google Scholar]
- 202.Ebel GD, Kramer LD. 2004. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 71:268–271. doi: 10.4269/ajtmh.2004.71.3.0700268. [DOI] [PubMed] [Google Scholar]
- 203.Valarcher JF, Hagglund S, Juremalm M, Blomqvist G, Renstrom L, Zohari S, Leijon M, Chirico J. 2015. Tick-borne encephalitis. Rev Sci Tech 34:453–466. doi: 10.20506/rst.34.2.2371. [DOI] [PubMed] [Google Scholar]
- 204.Xing Y, Schmitt HJ, Arguedas A, Yang J. 2017. Tick-borne encephalitis in China: a review of epidemiology and vaccines. Vaccine 35:1227–1237. doi: 10.1016/j.vaccine.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 205.McLean DM, Donohue WL. 1959. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 206.Hinten SR, Beckett GA, Gensheimer KF, Pritchard E, Courtney TM, Sears SD, Woytowicz JM, Preston DG, Smith RP Jr, Rand PW, Lacombe EH, Holman MS, Lubelczyk CB, Kelso PT, Beelen AP, Stobierski MG, Sotir MJ, Wong S, Ebel G, Kosoy O, Piesman J, Campbell GL, Marfin AA. 2008. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis 8:733–740. doi: 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- 207.Ford-Jones EL, Fearon M, Leber C, Dwight P, Myszak M, Cole B, Greene PB, Artes S, McGeer A, D’Cunha C, Naus M, Ontario West Nile Virus Working Group. 2002. Human surveillance for West Nile virus infection in Ontario in 2000. CMAJ 166:29–35. [PMC free article] [PubMed] [Google Scholar]
- 208.Centers for Disease Control and Prevention. 2016. Powassan virus, statistics and maps. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/powassan/statistics.html. [Google Scholar]
- 209.Ebel GD. 2010. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- 210.Godfrey ER, Randolph SE. 2011. Economic downturn results in tick-borne disease upsurge. Parasit Vectors 4:35. doi: 10.1186/1756-3305-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bogovic P, Strle F. 2015. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases 3:430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Taba P, Schmutzhard E, Forsberg P, Lutsar I, Ljøstad U, Mygland Å, Levchenko I, Strle F, Steiner I. 2017. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol 24:1214–e61. doi: 10.1111/ene.13356. [DOI] [PubMed] [Google Scholar]
- 213.Kaiser R. 2008. Tick-borne encephalitis. Infect Dis Clin North Am 22:561–575. doi: 10.1016/j.idc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 214.Cavanaugh CE, Muscat PL, Telford SR III, Goethert H, Pendlebury W, Elias SP, Robich R, Welch M, Lubelczyk CB, Smith RP. 2017. Fatal deer tick virus infection in Maine. Clin Infect Dis 65:1043–1046. doi: 10.1093/cid/cix435. [DOI] [PubMed] [Google Scholar]
- 215.Hoang Johnson DK, Staples JE, Sotir MJ, Warshauer DM, Davis JP. 2010. Tick-borne Powassan virus infections among Wisconsin residents. WMJ 109:91–97. [PubMed] [Google Scholar]
- 216.Smith R, Woodall JP, Whitney E, Deibel R, Gross MA, Smith V, Bast TF. 1974. Powassan virus infection. A report of three human cases of encephalitis. Am J Dis Child 127:691–693. doi: 10.1001/archpedi.1974.02110240077010. [DOI] [PubMed] [Google Scholar]
- 217.Rossier E, Harrison RJ, Lemieux B. 1974. A case of Powassan virus encephalitis. Can Med Assoc J 110:1173–1174. [PMC free article] [PubMed] [Google Scholar]
- 218.Partington MW, Thomson V, O’Shaughnessy MV. 1980. Powassan virus encephalitis in southeastern Ontario. Can Med Assoc J 123:603–606. [PMC free article] [PubMed] [Google Scholar]
- 219.Embil JA, Camfield P, Artsob H, Chase DP. 1983. Powassan virus encephalitis resembling herpes simplex encephalitis. Arch Intern Med 143:341–343. doi: 10.1001/archinte.1983.00350020167030. [DOI] [PubMed] [Google Scholar]
- 220.Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, Jamieson F, Blaser S, Gold R, Otsubo H, Heurter H, MacGregor D. 1998. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994-1995. Clin Infect Dis 26:398–409. doi: 10.1086/516301. [DOI] [PubMed] [Google Scholar]
- 221.Gholam BI, Puksa S, Provias JP. 1999. Powassan encephalitis: a case report with neuropathology and literature review. CMAJ 161:1419–1422. [PMC free article] [PubMed] [Google Scholar]
- 222.Lessell S, Collins TE. 2003. Ophthalmoplegia in Powassan encephalitis. Neurology 60:1726–1727. doi: 10.1212/01.wnl.0000064167.16083.02. [DOI] [PubMed] [Google Scholar]
- 223.Goldfield M, Austin SM, Black HC, Taylor BF, Altman R. 1973. A non-fatal human case of Powassan virus encephalitis. Am J Trop Med Hyg 22:78–81. doi: 10.4269/ajtmh.1973.22.78. [DOI] [PubMed] [Google Scholar]
- 224.Jackson AC. 1989. Leg weakness associated with Powassan virus infection—Ontario. Can Dis Wkly Rep 15:123–124. [PubMed] [Google Scholar]
- 225.Artsob H. 1989. Powassan encephalitis, p 29–49. In Monath TP. (ed), The arboviruses: epidemiology and ecology, 1st ed, vol4 CRC Press, Boca Raton, FL. [Google Scholar]
- 226.Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol 38:1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 38:1823–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gubler DJ, Campbell GL, Nasci R, Komar N, Petersen L, Roehrig JT. 2000. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunol 13:469–475. doi: 10.1089/vim.2000.13.469. [DOI] [PubMed] [Google Scholar]
- 229.Wong SJ, Boyle RH, Demarest VL, Woodmansee AN, Kramer LD, Li H, Drebot M, Koski RA, Fikrig E, Martin DA, Shi PY. 2003. Immunoassay targeting nonstructural protein 5 to differentiate West Nile virus infection from dengue and St. Louis encephalitis virus infections and from flavivirus vaccination. J Clin Microbiol 41:4217–4223. doi: 10.1128/jcm.41.9.4217-4223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Johnson AJ, Noga AJ, Kosoy O, Lanciotti RS, Johnson AA, Biggerstaff BJ. 2005. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin M antibodies. Clin Diagn Lab Immunol 12:566–574. doi: 10.1128/CDLI.12.5.566-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lindsey HS, Calisher CH, Mathews JH. 1976. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol 4:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fritz R, Orlinger KK, Hofmeister Y, Janecki K, Traweger A, Perez-Burgos L, Barrett PN, Kreil TR. 2012. Quantitative comparison of the cross-protection induced by tick-borne encephalitis virus vaccines based on European and Far Eastern virus subtypes. Vaccine 30:1165–1169. doi: 10.1016/j.vaccine.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 233.Fulcher LJ, Bozatzi P, Tachie-Menson T, Wu KZL, Cummins TD, Bufton JC, Pinkas DM, Dunbar K, Shrestha S, Wood NT, Weidlich S, Macartney TJ, Varghese J, Gourlay R, Campbell DG, Dingwell KS, Smith JC, Bullock AN, Sapkota GP. 2018. The DUF1669 domain of FAM83 family proteins anchor casein kinase 1 isoforms. Sci Signal 11:eaao2341. doi: 10.1126/scisignal.aao2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chernokhaeva LL, Rogova YV, Vorovitch MF, Romanova L, Kozlovskaya LI, Maikova GB, Kholodilov IS, Karganova GG. 2016. Protective immunity spectrum induced by immunization with a vaccine from the TBEV strain Sofjin. Vaccine 34:2354–2361. doi: 10.1016/j.vaccine.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 235.McAuley AJ, Sawatsky B, Ksiazek T, Torres M, Korva M, Lotric-Furlan S, Avsic-Zupanc T, von Messling V, Holbrook MR, Freiberg AN, Beasley DWC, Bente DA. 2017. Cross-neutralisation of viruses of the tick-borne encephalitis complex following tick-borne encephalitis vaccination and/or infection. NPJ Vaccines 2:5. doi: 10.1038/s41541-017-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.VanBlargan LA, Himansu S, Foreman BM, Ebel GD, Pierson TC, Diamond MS. 2018. An mRNA vaccine protects mice against multiple tick-transmitted flavivirus infections. Cell Rep 25:3382–3392. doi: 10.1016/j.celrep.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.International Committee on Taxonomy of Viruses. 2017. Virus taxonomy: 2017 release. https://talk.ictvonline.org/taxonomy/.
- 238.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. 2013. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 239.Ergonul O. 2006. Crimean-Congo haemorrhagic fever. Lancet Infect Dis 6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hoogstraal H. 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol 15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- 241.Carroll SA, Bird BH, Rollin PE, Nichol ST. 2010. Ancient common ancestry of Crimean-Congo hemorrhagic fever virus. Mol Phylogenet Evol 55:1103–1110. doi: 10.1016/j.ympev.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 242.Grard G, Drexler JF, Fair J, Muyembe J-J, Wolfe ND, Drosten C, Leroy EM. 2011. Re-emergence of Crimean-Congo hemorrhagic fever virus in central Africa. PLoS Negl Trop Dis 5:e1350. doi: 10.1371/journal.pntd.0001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, Gibson H, Robinson TP, Gilbert M, Wint GRW, Nuttall PA, Gething PW, Myers MF, George DB, Hay SI. 2015. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg 109:503–513. doi: 10.1093/trstmh/trv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Spengler JR, Bergeron E, Rollin PE. 2016. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis 10:e0004210. doi: 10.1371/journal.pntd.0004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Estrada-Pena A, Jameson L, Medlock J, Vatansever Z, Tishkova F. 2012. Unraveling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: a gap analysis for the western Palearctic. Vector Borne Zoonotic Dis 12:743–752. doi: 10.1089/vbz.2011.0767. [DOI] [PubMed] [Google Scholar]
- 246.Bronze MS, Huycke MM, Machado LJ, Voskuhl GW, Greenfield RA. 2002. Viral agents as biological weapons and agents of bioterrorism. Am J Med Sci 323:316–325. doi: 10.1097/00000441-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 247.Sidwell RW, Smee DF. 2003. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res 57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 248.Whitehouse CA. 2004. Crimean-Congo hemorrhagic fever. Antiviral Res 64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 249.Papa A, Mirazimi A, Koksal I, Estrada-Pena A, Feldmann H. 2015. Recent advances in research on Crimean-Congo hemorrhagic fever. J Clin Virol 64:137–143. doi: 10.1016/j.jcv.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Watts D, Ksiazek T, Linthicum K, Hoogstraal H. 1988. Crimean-Congo hemorrhagic fever, p 177–260. In Monath TP. (ed), The arboviruses: epidemiology and ecology, 1st ed, vol2 CRC Press, Boca Raton, FL. [Google Scholar]
- 251.Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA. 2017. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antiviral Res 144:93–119. doi: 10.1016/j.antiviral.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dowall SD, Carroll MW, Hewson R. 2017. Development of vaccines against Crimean-Congo haemorrhagic fever virus. Vaccine 35:6015–6023. doi: 10.1016/j.vaccine.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Purnak T, Selvi NA, Altundag K. 2007. Global warming may increase the incidence and geographic range of Crimean-Congo hemorrhagic fever. Med Hypotheses 68:924–925. doi: 10.1016/j.mehy.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 254.Bodur H, Akinci E, Ascioglu S, Onguru P, Uyar Y. 2012. Subclinical infections with Crimean-Congo hemorrhagic fever virus, Turkey. Emerg Infect Dis 18:640–642. doi: 10.3201/eid1804.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ergonul O. 2008. Treatment of Crimean-Congo hemorrhagic fever. Antiviral Res 78:125–131. doi: 10.1016/j.antiviral.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 256.Cevik MA, Erbay A, Bodur H, Gülderen E, Baştuğ A, Kubar A, Akinci E. 2008. Clinical and laboratory features of Crimean-Congo hemorrhagic fever: predictors of fatality. Int J Infect Dis 12:374–379. doi: 10.1016/j.ijid.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 257.Garrison AR, Alakbarova S, Kulesh DA, Shezmukhamedova D, Khodjaev S, Endy TP, Paragas J. 2007. Development of a TaqMan minor groove binding protein assay for the detection and quantification of Crimean-Congo hemorrhagic fever virus. Am J Trop Med Hyg 77:514–520. doi: 10.4269/ajtmh.2007.77.514. [DOI] [PubMed] [Google Scholar]
- 258.Drosten C, Gottig S, Schilling S, Asper M, Panning M, Schmitz H, Gunther S. 2002. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol 40:2323–2330. doi: 10.1128/jcm.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Drosten C, Kummerer BM, Schmitz H, Gunther S. 2003. Molecular diagnostics of viral hemorrhagic fevers. Antiviral Res 57:61–87. doi: 10.1016/s0166-3542(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 260.Schwarz TF, Nsanze H, Ameen AM. 1997. Clinical features of Crimean-Congo haemorrhagic fever in the United Arab Emirates. Infection 25:364–367. doi: 10.1007/bf01740819. [DOI] [PubMed] [Google Scholar]
- 261.Ergönül O, Celikbaş A, Dokuzoguz B, Eren S, Baykam N, Esener H. 2004. Characteristics of patients with Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clin Infect Dis 39:284–287. doi: 10.1086/422000. [DOI] [PubMed] [Google Scholar]
- 262.Swanepoel R, Shepherd AJ, Leman PA, Shepherd SP, McGillivray GM, Erasmus MJ, Searle LA, Gill DE. 1987. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am J Trop Med Hyg 36:120–132. doi: 10.4269/ajtmh.1987.36.120. [DOI] [PubMed] [Google Scholar]
- 263.Ergonul O. 2012. Crimean-Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol 2:215–220. doi: 10.1016/j.coviro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 264.Ergonul O, Zeller H, Celikbas A, Dokuzoguz B. 2007. The lack of Crimean-Congo hemorrhagic fever virus antibodies in healthcare workers in an endemic region. Int J Infect Dis 11:48–51. doi: 10.1016/j.ijid.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 265.Mardani M, Keshtkar-Jahromi M, Ataie B, Adibi P. 2009. Crimean-Congo hemorrhagic fever virus as a nosocomial pathogen in Iran. Am J Trop Med Hyg 81:675–678. doi: 10.4269/ajtmh.2009.09-0051. [DOI] [PubMed] [Google Scholar]
- 266.Emmerich P, Mika A, von Possel R, Rackow A, Liu Y, Schmitz H, Gunther S, Sherifi K, Halili B, Jakupi X, Berisha L, Ahmeti S, Deschermeier C. 2018. Sensitive and specific detection of Crimean-Congo hemorrhagic fever virus (CCHFV)-specific IgM and IgG antibodies in human sera using recombinant CCHFV nucleoprotein as antigen in mu-capture and IgG immune complex (IC) ELISA tests. PLoS Negl Trop Dis 12:e0006366. doi: 10.1371/journal.pntd.0006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.van de Wal BW, Joubert JR, van Eeden PJ, King JB. 1985. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital. Part IV. Preventive and prophylactic measures. S Afr Med J 68:729–732. [PubMed] [Google Scholar]
- 268.Leblebicioglu H, Ozaras R, Irmak H, Sencan I. 2016. Crimean-Congo hemorrhagic fever in Turkey: current status and future challenges. Antiviral Res 126:21–34. doi: 10.1016/j.antiviral.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 269.Bausch DG, Hadi CM, Khan SH, Lertora JJ. 2010. Review of the literature and proposed guidelines for the use of oral ribavirin as postexposure prophylaxis for Lassa fever. Clin Infect Dis 51:1435–1441. doi: 10.1086/657315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mardani M, Jahromi MK, Naieni KH, Zeinali M. 2003. The efficacy of oral ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Iran. Clin Infect Dis 36:1613–1618. doi: 10.1086/375058. [DOI] [PubMed] [Google Scholar]
- 271.Oestereich L, Rieger T, Neumann M, Bernreuther C, Lehmann M, Krasemann S, Wurr S, Emmerich P, de Lamballerie X, Ölschläger S, Günther S. 2014. Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (favipiravir) in a mouse model for Crimean-Congo hemorrhagic fever. PLoS Negl Trop Dis 8:e2804. doi: 10.1371/journal.pntd.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Akira S. 1999. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 17:138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 273.Dowall SD, Graham VA, Rayner E, Hunter L, Watson R, Taylor I, Rule A, Carroll MW, Hewson R. 2016. Protective effects of a modified vaccinia Ankara-based vaccine candidate against Crimean-Congo haemorrhagic fever virus require both cellular and humoral responses. PLoS One 11:e0156637. doi: 10.1371/journal.pone.0156637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Buttigieg KR, Dowall SD, Findlay-Wilson S, Miloszewska A, Rayner E, Hewson R, Carroll MW. 2014. A novel vaccine against Crimean-Congo haemorrhagic fever protects 100% of animals against lethal challenge in a mouse model. PLoS One 9:e91516. doi: 10.1371/journal.pone.0091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kim YR, Yun Y, Bae SG, Park D, Kim S, Lee JM, Cho NH, Kim YS, Lee KH. 2018. Severe fever with thrombocytopenia syndrome virus infection, South Korea, 2010. Emerg Infect Dis 24:2103–2105. doi: 10.3201/eid2411.170756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. 2013. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Saito T, Fukushima K, Umeki K, Nakajima K. 2015. Severe fever with thrombocytopenia syndrome in Japan and public health communication. Emerg Infect Dis 21:487–489. doi: 10.3201/eid2103.140831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, Xue ZF, Ma DQ, Zhang XS, Ding SJ, Lei XY, Yu XJ. 2015. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis 21:1770–1776. doi: 10.3201/eid2110.150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Brault AC, Savage HM, Duggal NK, Eisen RJ, Staples JE. 2018. Heartland virus epidemiology, vector association, and disease potential. Viruses 10:E498. doi: 10.3390/v10090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pastula DM, Turabelidze G, Yates KF, Jones TF, Lambert AJ, Panella AJ, Kosoy OI, Velez JO, Fisher M, Staples E, Centers for Disease Control and Prevention. 2014. Notes from the field: Heartland virus disease—United States, 2012-2013. MMWR Morb Mortal Wkly Rep 63:270–271. [PMC free article] [PubMed] [Google Scholar]
- 282.Wormser GP, Pritt B. 2015. Update and commentary on four emerging tick-borne infections: Ehrlichia muris-like agent, Borrelia miyamotoi, deer tick virus, Heartland virus, and whether ticks play a role in transmission of Bartonella henselae. Infect Dis Clin North Am 29:371–381. doi: 10.1016/j.idc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 283.Esguerra EM. 2016. Heartland virus: a new virus discovered in Missouri. Mo Med 113:256–257. [PMC free article] [PubMed] [Google Scholar]
- 284.Kosoy OI, Lambert AJ, Hawkinson DJ, Pastula DM, Goldsmith CS, Hunt DC, Staples JE. 2015. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg Infect Dis 21:760–764. doi: 10.3201/eid2105.150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Anonymous. 2015. Bourbon viruses. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/ncezid/dvbd/bourbon/index.html. [Google Scholar]
- 286.Savage HM, Burkhalter KL, Godsey MS Jr, Panella NA, Ashley DC, Nicholson WL, Lambert AJ. 2017. Bourbon virus in field-collected ticks, Missouri, USA. Emerg Infect Dis 23:2017–2022. doi: 10.3201/eid2312.170532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jones EH, Hinckley AF, Hook SA, Meek JI, Backenson B, Kugeler KJ, Feldman KA. 2018. Pet ownership increases human risk of encountering ticks. Zoonoses Public Health 65:74–79. doi: 10.1111/zph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 289.Bartikova P, Holikova V, Kazimirova M, Stibraniova I. 2017. Tick-borne viruses. Acta Virol 61:413–427. doi: 10.4149/av_2017_403. [DOI] [PubMed] [Google Scholar]