Abstract
Most odors are not composed of a single volatile chemical species, but rather are mixtures of many different volatile molecules, the perception of which is dependent on the identity and relative concentrations of the components. Changing either the identity or ratio of components can lead to shifts between configural and elemental perception of the mixture. For example, a 30/70 ratio of ethyl isobutyrate (odorant A, a strawberry scent) and ethyl maltol (odorant B, a caramel scent) is perceived as pineapple by humans - a configural percept distinct from the components. In contrast, a 68/32 ratio of the same odorants is perceived elementally, and is identified as the component odors. Here, we examined single-unit responses in the anterior and posterior piriform cortex (aPCX and pPCX) of mice to these A and B mixtures. We first demonstrate that mouse behavior is consistent with a configural/elemental perceptual shift as concentration ratio varies. We then compared responses to the configural mixture to those evoked by the elemental mixture, as well as to the individual components. Hierarchical cluster analyses suggest that in the mouse aPCX, the configural mixture was coded as distinct from both components, while the elemental mixture was coded as similar to the components. In contrast, mixture perception did not predict pPCX ensemble coding. Similar electrophysiological results were also observed in rats. The results suggest similar perceptual characteristics of the AB mixture across species, and a division in the roles of aPCX and pPCX in the coding of configural and elemental odor mixtures.
Keywords: piriform cortex, odor mixtures, configural perception, elemental perception, single-unit recording
1. INTRODUCTION
The vast majority of odors in the natural environment are not composed of a single volatile chemical species, but rather are mixtures of two to many hundreds of different volatile molecules (Thomas-Danguin et al., 2014). In some cases these mixtures are the result of specific metabolic processes that result in a specific set of molecules in a specific ratio to evoke adaptive behaviors in the receiver who is especially tuned to receive that mixture (Riffell, 2012). Examples include plant volatiles which attract pollinators (Szyszka and Stierle, 2014) or animal pheromones which attract mates (Brown et al., 2004; Deisig et al., 2014; Martin et al., 2013; Picimbon et al., 1997). Processing of these kinds of mixtures is species- and potentially sex-specific and is the result of highly adapted olfactory receptors and/or olfactory central circuits (Wyatt, 2014). Importantly, these species-specific mixtures require the right odorants at the right proportion to evoke a response in the given species. A single component alone, even if it is the dominant component in the mixture, is insufficient to evoke the appropriate response. Thus, the mixture is perceived (i.e., drives behavior) as a synthetic configuration, distinct from its components.
In contrast to these species-specific odor mixtures, there is increasing evidence of species-non-specific configural odor processing. That is, some combinations of odorants, at a specific ratio of concentrations, are perceived configurally across several species, including lagomorphs and humans. For example, a 30/70 ratio of ethyl isobutyrate (odorant A a strawberry scent) and ethyl maltol (odorant B, a caramel scent) is perceived as pineapple by humans – a configural percept distinct from the components in this AB mixture. Conversely, a 68/32 ratio of the same odorants (A’B’ mixture) is not perceived configurally, and is not identified as pineapple scent (Le Berre et al., 2008a). Data from a variety of behavioral assays, either involving explicit training or not, suggest a similar configural (or at least weak, i.e., partial configural (Kay et al., 2005)) perception of this same 30/70 ratio of ethyl isobutyrate and ethyl maltol AB mixture when tested in newborn rabbits (Coureaud et al., 2008; Coureaud et al., 2009; Coureaud et al., 2011; Coureaud et al., 2014b; Coureaud et al., 2019; Schneider et al., 2016; Sinding et al., 2011). Data using a six-component mixture are showing a similar species-non-specific configural processing effect in human adults and newborn rabbits (Romagny et al., 2014; Romagny et al., 2018; Sinding et al., 2013).
Processing of odor mixtures begins at the olfactory receptors and continues throughout the olfactory pathway in both invertebrates (Clifford and Riffell, 2013; Derby and Ache, 1984; Riffell et al., 2009; Schubert et al., 2014) and vertebrates (Duchamp-Viret et al., 2003; Grabenhorst et al., 2007) In mammals, antagonistic interactions can occur at the receptor between ligands (Singh et al., 2019), and mixture interactions, especially mixture suppression, can be observed in single-unit responses in the olfactory bulb and olfactory cortex (Davison and Katz, 2007; Davison and Ehlers, 2011; Kadohisa and Wilson, 2006; Tabor et al., 2004). In the anterior piriform cortex (aPCX), evidence from both single-units and single-unit ensembles suggests configural processing, with odor mixtures coded as distinct from their components (Wilson, 2003). In both humans (Gottfried et al., 2006) and rats (Kadohisa and Wilson, 2006), mixture coding in the aPCX aligns well with the perceived odorant/mixture identity, while coding in the posterior piriform cortex (pPCX) may be more consistent with general odor quality (e.g., floral, fruitiness, etc.).
Here, we present initial behavioral results in mice suggesting that AB and A’B’ are perceived as configural and elemental mixtures, respectively, in this rodent species. We then take advantage of this odor set, well characterized in multiple species, to examine single-unit and single-unit ensemble responses in the aPCX and pPCX of both mice and rats to the AB mixture known to be perceived configurally in humans, and in a manner consistent with configural perception in rabbits (Coureaud and Wilson, 2019; Coureaud et al., in prep.). We compared responses to the apparent configural mixture with those evoked by the same chemical mixture presented at a different ratio, supposed to evoke an elemental percept, as well as to the individual chemical components. Based on results from hierarchical cluster analyses, in both the mouse and rat aPCX, the presumed configural AB mixture was coded as distinct from both components, while the presumed elemental A’B’ mixture was coded as very similar to the components, suggesting that aPCX ensemble coding matched known odor perceptual characteristics. However, behavioral mixture perception did not predict pPCX ensemble coding, further demonstrating the distinction between odor coding in the aPCX and pPCX.
2. RESULTS
2.1. Behavioral results
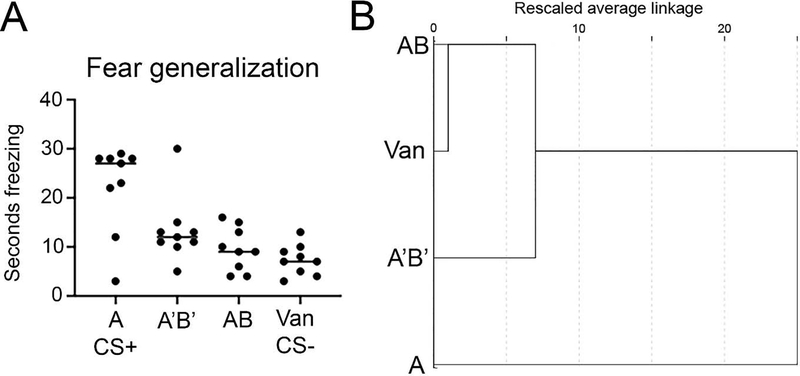
Mice (n=6) were trained in a discriminatory odor mixture cued threat task (CS+ odor + shock, CS− odor with no shock). Odor cue testing in a novel environment was performed 24 hrs post-conditioning and included the CS+, CS− and two novel odors. The CS+ was component A (ethyl isobutyrate) of the binary AB mixture (ethyl isobutyrate + ethyl maltol) known in humans and rabbits to be perceived configurally at v/v A/B component ratio 30/70 and elementally at A/B ratio of 68/32 (A’B’ mixture) (Coureaud et al., 2008; Le Berre et al., 2008b). The CS− was vanilla. During testing, odor-evoked freezing was quantified for the CS+ (A), the CS− (vanilla), and both the presumed configural and elemental mixtures. As shown in Fig. 1A mice froze significantly more to the CS+ than the CS−, and more to the CS+ than either mixture, though there was a trend toward generalization to the elemental A’B’ mixture compared to the configural AB mixture (ANOVA: F(3,32) = 9.88, p < 0.0001, post-hoc Tukey tests revealed freezing to A was significantly greater than to all other odors). Hierarchical cluster analysis (HCA) was then performed to determine how similar behavioral responses were to the different odors during testing. As shown in Fig. 1B, mice behaved distinctly different to the CS+ odor A compared to all other odors. However, the AB odor was more similar to the CS− vanilla odor, while the odor A’B’ was more similar to the CS+ A odor. It is important to note that this clustering does not imply that animals could not discriminate between vanilla and AB. Rather, the data suggest that behavioral responses to AB were more similar to the CS− while responses to the mixture A’B’ were more similar to the CS+ odor A, consistent with a configural perception of AB and elemental perception of A’B’.
Figure 1.
Mouse conditioned freezing behavior to the CS+ (odor A), the CS− (vanilla) and the two odor mixtures AB and A’B’. A) Mice froze significantly more (ANOVA, p < 0.001) to the CS+ than to any other odor, though showed a trend toward generalizing between the A’B’ mixture and A. B) Hierarchical cluster analyses of odor-evoked freezing across animals suggest that behavior toward mixture AB were clustered closely to vanilla (CS−), while the mixture A’B’ evoked behavior more similar to A (CS+). These results are consistent with an elemental perception of A’B’ and a novel configural perception of AB. See text for details.
2.2. Mouse electrophysiology data
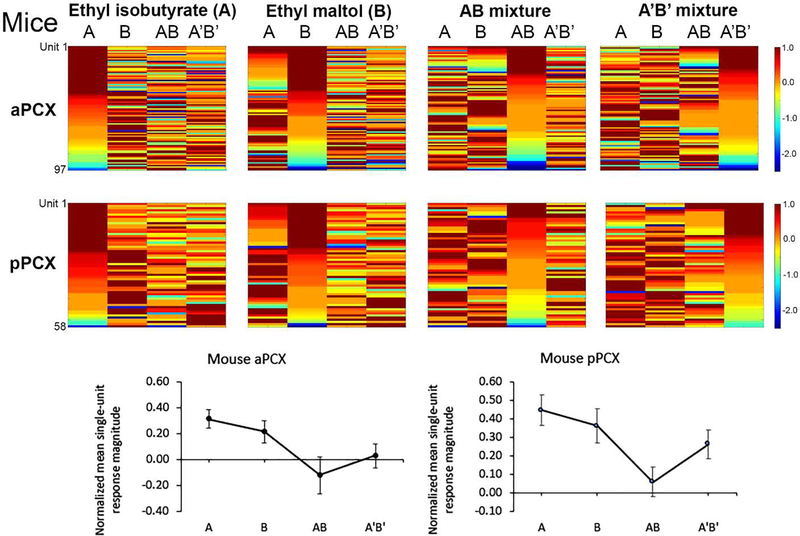
A total of 97 single-units were recorded in aPCX and 58 units in pPCX from n=11 B6SJLF1/J mice. Each of the different odorants were effective at driving unit activity, with different cells maximally tuned to different component and mixture stimuli (Fig. 2, Top). Average normalized response magnitudes also varied across components and mixtures, with mean responses to components significantly higher than responses to the binary mixtures (Fig. 2, Bottom; aPCX: 1-way ANOVA for odor, F (3, 365) = 3.459, p = 0.017; pPCX: F (3, 228) = 4.012, p = 0.008). This outcome is consistent with mixture suppression in both aPCX and pPCX in mouse, as has been previously reported in PCX (Kadohisa and Wilson, 2006).
Figure 2.
(Top) Pseudocolor plots of mouse single-unit responses to two odor mixtures and their components. As in Fig. 1, each row is data from a single-unit color coded to reflect the normalized (maximal response = 1) to the four odors, sorted for cells showing their strongest response to odor A, odor B, AB or A’B’. As can be seen there are a subset of cells that are maximally responsive to each of the four different odors in both the aPCX and pPCX. (Bottom) Mean normalized response magnitude to each odor in the mouse aPCX and pPCX. Units in both regions showed significant mixture suppression compared to their response to the components (ANOVA, p < 0.05). Note that the configural AB mixture showed the strongest mixture suppression in both aPCX and pPCX.
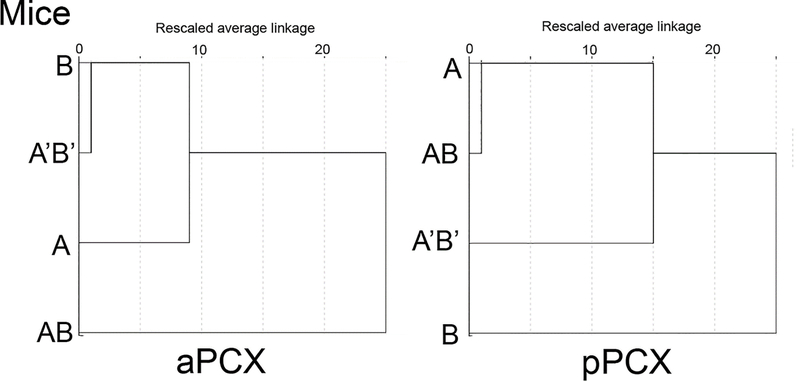
In addition to analysis of single-unit responses, we also merged all units recorded in a given region and analyzed the activity of this pseudo-ensemble using HCA. This analysis allowed extraction of how ensemble activity categorized the different monomolecular and mixture stimuli, and how distinct each stimulus was from the others. As shown in Fig. 3, HCA of mouse aPCX ensemble activity revealed two primary clusters. One included the monomolecular components, A and B, together with the mixture A’B’. In contrast, the AB mixture formed its own, distinct cluster, as might be predicted from the perceptual data previously published in other species (Coureaud et al., 2014b; Schneider et al., 2016; Sinding et al., 2011) and our behavioral results in mice, due to its presumed configural perception. HCA of pPCX ensemble activity roughly mirrored that obtained in mouse aPCX, with configural mixture AB and elemental mixture A’B’ separated into two separate clusters, though the clustering of the monomolecular components is more complex than that observed in aPCX and did not align with the behavioral data. The underlying bases of the mouse pPCX HCA outcome (e.g., perceptual, molecular, etc.) is unclear, but nonetheless it is distinct from that in mouse aPCX, and isolates AB and A’B’ from each other.
Figure 3.
Hierarchical cluster analyses of mouse single-unit ensembles in aPCX and pPCX to A, B, AB and A’B’. In aPCX, the component odors clustered closely with the elemental A’B’ mixture, while the configural AB mixture formed its own cluster, similar to that observed in the rat. In pPCX, while A’B’ and AB occupied distinct clusters, the association with the components was less clearly organized than in aPCX.
2.2. Rat electrophysiology data
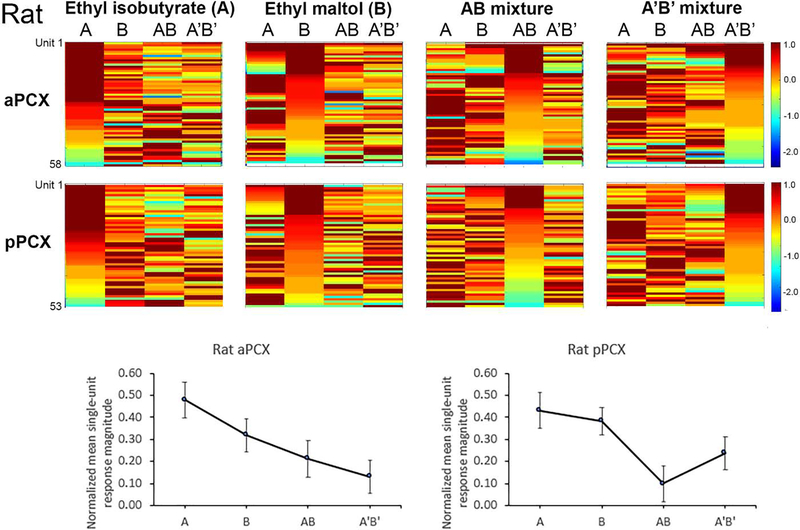
Although no behavioral data were collected in rats, for comparison of PCX odor coding between rats and mice a total of 58 single-units were recorded in aPCX and 53 in pPCX from n=9 Long Evans rats. As in the mice, each of the different odorants was effective at driving unit activity, with different cells maximally tuned to different stimuli (Fig. 4, Top). As shown in Fig. 4 (Bottom), the mean of the proportional response magnitudes across cells for the different stimuli shows that units were most commonly maximally responsive to a given component than to the mixtures. In both aPCX and pPCX, across all units, response magnitudes were thus stronger to the components than to the mixtures (Fig. 4, aPCX: 1-way ANOVA for odor, F (3, 228) = 3.688, p = 0.013; pPCX: F (3, 208) = 3.993, p = 0.009), again suggesting mixture suppression.
Figure 4.
(Top) Pseudocolor plots of rat single-unit responses to two odor mixtures and their components. Each row is data from a single-unit color coded to reflect the normalized (maximal response = 1) to the four odors. The same data are replotted but sorted for cells showing their strongest response to odor A, odor B, AB or A’B’. As can be seen there are a subset of cells that are maximally responsive to each of the four different odors in both the aPCX and pPCX. (Bottom) Mean normalized response magnitude to each odor in the rat aPCX and pPCX. Units in both regions showed significant mixture suppression compared to their response to the components (ANOVA, p < 0.05).
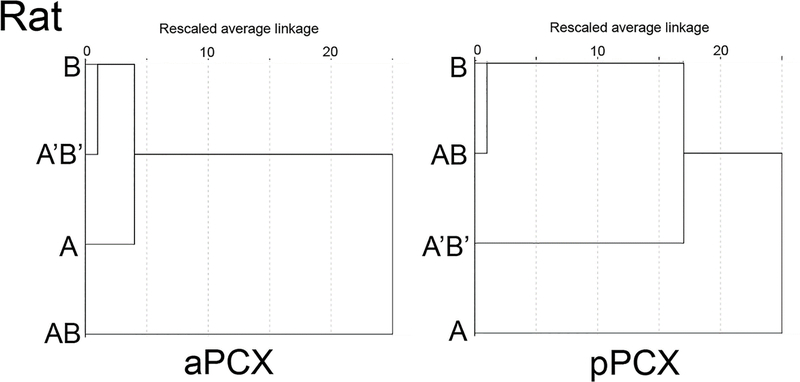
HCA in rat revealed remarkably similar results to those obtained in mouse. As shown in Fig. 5, HCA analyses of single-unit ensemble activity revealed distinctly different stimulus categorization outcomes between rat aPCX and pPCX. In aPCX, based on average linkage distances, the four odor stimuli were segregated into two primary clusters, with monomolecular components, A and B, together with the mixture A’B’ in one cluster and the AB mixture forming its own, distinct cluster.
Figure 5.
Hierarchical cluster analyses of rat single-unit ensembles in aPCX and pPCX to A, B, AB and A’B’. In aPCX, the component odors clustered closely with the elemental A’B’ mixture, while the configural AB mixture formed its own cluster. In pPCX, while A’B’ and AB occupied distinct clusters, the association with the components was less clearly organized than in aPCX.
In rat pPCX, however, clustering did not align with supposed perceptual similarities of these odors and their mixtures (Fig. 5). In pPCX, ensemble activity was described by three clusters, with component B clustered together with AB, and A and mixture A’B’ each forming their own clusters. Thus, in pPCX the presumed elemental mixture A’B’ and configural mixture AB lie in different clusters from each other, although the clustering of mixtures with components is more complex than in aPCX. As in mouse, the underlying bases of the rat pPCX HCA outcome is unclear, but nonetheless strongly differentiates coding of AB and A’B’.
3. DISCUSSION
The present results suggest that, similar to humans and infant rabbits (Coureaud et al., 2008; Le Berre et al., 2008b), mice perceive (i.e., behaviorally respond to) the component odor A as more similar to the mixture A’B’ than it is to the mixture AB. These data are consistent with the interpretation that mixture A’B’ is perceived elementally while mixture AB is perceived configurally, and thus more distinct from its components. Furthermore, single-unit ensemble coding of odor mixtures and their components in the mouse and rat aPCX aligns with known cross-species perceptual characteristics of those stimuli. A 68/32 ratio mixture of components EI and EM, which is perceived elementally (i.e., sharing perceptual qualities of the two components) across several species (Coureaud et al., 2018; Coureaud and Wilson, 2019; Coureaud et al., in prep.) is encoded by aPCX ensembles as similar to those components. In contrast, a 30/70 ratio of the same components, which is perceived configurally and different from those components, is categorized as distinct from both the components and elemental mixture by aPCX ensembles. Thus, aPCX ensemble activity predicts the known perceptual qualities of these mixtures. In contrast, ensemble activity in the pPCX, while differentially categorizing the configural and elemental mixtures, demonstrated a more complex relationship between the mixtures and their components, presumably reflecting additional qualities, other features of the stimuli, or their associations (Coureaud et al., in prep.; Courtiol et al., 2019; Gottfried et al., 2006; Kadohisa and Wilson, 2006).
The AB mixture used here has been well characterized behaviorally in multiple species (Coureaud et al., 2018; Coureaud et al., in prep.). Thus, in human adults, the odor of the AB mixture was rated as more typical of pineapple than its A (strawberry) and B (caramel) components; this was not observed for the A’B’ mixture or another binary mixture combining a fruity odor and a caramel odor (Barkat et al., 2012); (Le Berre et al., 2008a). The configural perception of the AB mixture can be altered by perceptual experience (pre-exposure to the elements) and attentional processes (Le Berre et al., 2008b). In newborn rabbits, after conditioning for instance to odorant A (by pairing with the mammary pheromone; similar results for odorant B) the pups display a typical orocephalic response (usually displayed to localize and grasp the maternal nipples) in response to A, not to B and not to AB. In contrast, conditioning to A is followed by strong responsiveness to A’B’ and another binary mixture including A, the AC mixture. Thus, rabbit neonates perceive something special in AB, distinct from the component odors, while they perceive the elements but no configuration in A’B’ and AC (e.g., Coureaud et al., 2008, Coureaud et al., 2019a). It should also be noted that analysis of c-fos activity patterns in the rabbit PCX suggest a differential encoding of AB and A’B’ in both the aPCX and pPCX (Schneider et al., 2016). Use of reconsolidation and pharmacology allowed demonstrating that they actually perceive and memorize in the AB mixture a specific odor for AB - a configuration - in addition to the odors of A and B, i.e., their perception of the AB mixture is weak configural (Coureaud et al., 2014a; Coureaud et al., 2014b). As in humans, previous experience of the elements or the mixture can modulate the spontaneous perception of AB in rabbit pups (Sinding et al., 2011). In adult mice (B6sJLF/J), habituation/cross-habituation assays showed that habituation to AB is more rapid than to A’B’ and that mice are able to discriminate between the two mixtures (Coureaud et al., 2018; Coureaud and Wilson, 2019). Habituation is delayed in response to complex stimuli compared to more simple stimuli (Caron and Caron, 1969; Cohen et al., 1975; Oakes, 2010), thus, the rapid habituation to AB is consistent with a single, configural percept, distinct from a more complex, elemental percept of A and B in the A’B’ mixture (Coureaud et al., 2018). One may note that in honeybees, conditioning of the proboscis extension response and negative patterning procedure highlighted that the AB mixture is clearly much more differentiated by bees compared to its components, than other binary mixtures, pinpointing that the configural perception of AB is also displayed in a non-mammalian species (Coureaud et al., in prep.).
The results in rodents presented here provide a potential neural basis for these very different perceptual outcomes as component ratios change. The shift between elemental and configural coding in aPCX may be due to several underlying processes. The aPCX is hypothesized to serve an important role in recognition of patterns of activity projected from the olfactory bulb (Haberly, 2001; Wilson and Sullivan, 2011). Olfactory bulb output is determined by a variety of factors. First, ligand interactions with olfactory receptors and ligand-ligand interactions at those receptors determine the identity and temporal pattern of olfactory sensory neuron activated by a given stimulus. Agonistic and antagonistic interactions between odorants (Duchamp-Viret et al., 2003; Singh et al., 2019) may be especially relevant in the initial stages of configural processing, help mask component selective input, and may be expected to be dependent on the ratio of component concentrations (Coureaud et al., 2011) in a standard receptor pharmacology manner. It should be noted that responses to the configural AB mixture showed the strongest mixture suppression in aPCX and pPCX of mouse and pPCX of rat, potentially consistent with antagonistic effects at the receptor. A second factor influencing olfactory bulb output is inhibitory interactions within the glomerular layer and also via granule cells. These interactions can mediate lateral inhibition and changes in temporal coding that may differentially affect bulb output depending on the identity and ratio of mixture components (Linster and Cleland, 2004). Third, the olfactory bulb network is under heavy top-down and neuromodulatory influences that can shape odor responses depending on internal state, context and expectation (Kapoor et al., 2016; Linster and Cleland, 2016; Markopoulos et al., 2012; Ogg et al., 2018). However, given that the recordings here were in anesthetized animals, these affects are expected to be stable across stimuli.
Within the PCX, olfactory bulb output is hypothesized to be synthesized into odor objects via convergence of heterogeneous mitral cell input onto individual PCX neurons and extensive auto-associative, intracortical fibers (Haberly, 2001; Wilson and Sullivan, 2011). Previous work has emphasized the merging of features in aPCX into odor objects distinct from their components, similar to the configural AB mixture (Wilson, 2000; Wilson, 2003). Interestingly however, the A’B’ mixture maintains an elemental perceptual quality, where perception of the components is not lost. While active training could help an organism enhance its ability to identify components within mixtures, the A’B’ mixture used here and in previous behavioral work (Coureaud et al., 2009; Coureaud et al., 2014a; Coureaud et al., 2018; Sinding et al., 2011) was novel to the subjects. Understanding mechanisms of elemental odor perception will require additional research and is the focus on ongoing work in our labs.
A variety of differences in odor coding and function have been previously described between the aPCX and pPCX (Calu et al., 2007; Chabaud et al., 2000; Chapuis et al., 2009; Gottfried et al., 2006; Kadohisa and Wilson, 2006; Roesch et al., 2007; Schneider et al., 2016; Wang et al., 2019). For example, in contrast to the aPCX’s presumed role in coding odor identity, previous work has suggested that pPCX activity is more indicative of odor qualities and associations than aPCX (Gottfried et al., 2006; Kadohisa and Wilson, 2006; Schneider et al., 2016). This difference in function is most likely shaped by anterior-posterior gradients in PCX cell populations, variations in local circuit anatomy, and differences in the relative strength of olfactory bulb and top-down inputs to these areas (Large et al., 2018; Litaudon et al., 2003; Majak et al., 2004; Mouly et al., 1998; Neville and Haberly, 2004; Young and Sun, 2009). In line with these anterior-posterior differences, pPCX ensembles encoded the mixtures and components differently than aPCX according to HCA. HCA of aPCX ensemble activity matched perceptual data while pPCX activity did not. Further work is required to understand how pPCX activity contributes to odor mixture perception. Coding in pPCX also differed between species, with for example A and AB clustering tightly in the mouse, but B and AB clustering tightly in the rat. Further work examining the specific perceptual qualities of these mixtures and components in rats and mice may provide some rationale for this differential clustering. Alternatively, these components may have differential biological meaning in these two species.
In summary, using a well-characterized, simple binary odor mixture that shifts between elemental and configural perceptual characteristics as component proportions vary, we have identified a cross-species signature of elemental and configural coding in ensembles of PCX single-units. Future work will further clarify how this signature is expressed to other mixtures and in other species as a way more closely align odor perception with cortical odor coding.
4. EXPERIMENTAL PROCEDURE
4.1. Subjects
B6SJLF1/J mice (Jackson Labs, 20–50g, n = 14; 7 female) and Long-Evans hooded rats obtained from Envigo Lab animals (200–400g, n= 9 male) were used as subjects. All procedures were approved by the Institutional Animal Care and Use Committee of the Nathan Kline Institute for Psychiatric Research and were in compliance with NIH guidelines. Testing was performed during the light phase and animals had ad lib food and water prior to data collection.
4.2. Fear conditioning
Mice (n = 6, 3 males) were trained in a differential cued threat conditioning task (chamber 8 cm wide X 21 cm long X 20 cm high with shock grid floor) wherein a 15 sec CS+ odor ethyl isobutyrate (odorant A; CAS 97–62-1; Sigma; stock solution 100.5mg in 10mL of 100% ethanol) preceded and co-terminated with a 0.5mA, 1 sec, footshock and a CS− odor (vanilla extract, McCormick) predicted no shock. CS+ and CS− trials were randomly interspersed with a mean 1 min inter-stimulus interval, and 10 presentations of the CS+ and 30 presentations of the CS−, as well as 20 random non-odor-related valve click auditory stimuli to minimize the value of auditory cues. Following training, animals were returned to their homecage until testing the following day. Odor-evoked freezing tests were performed in a novel context (13 cm wide X 30 cm long X 18 cm high, glass aquarium), and included presentations of the CS+, CS−, the AB mixture (ethyl isobutyrate [odorant A] + ethyl maltol [odorant B; CAS 4940–11-8; Sigma; stock solution 100mg in 10mL of 100% ethanol at a component ratio of 30/70), and the A’B’ mixture (same components as AB but at a component ratio of 68/32). For further details of the stimuli and mixtures see (Coureaud et al., 2008; Le Berre et al., 2008b). Stimuli were 15 sec in duration with a 1–3 min inter-stimulus interval. Each stimulus was presented 3 times randomly interspersed with the other test odors. Behavior was videotaped and scored offline.
4.3. Behavioral analyses
Stimulus-evoked freezing during testing was quantified for each stimulus and total time freezing summed across all presentations of the same odor. Thus, each animal contributed freezing data for each of the four odors. Differences between odor-evoked freezing was compared across odors with a one-way repeated measures ANOVA and post-hoc Tukey tests. In addition, similarity of odor-evoked behavior across the stimuli was determined with hierarchical cluster analysis (HCA). For HCA of behavioral similarity to the different stimuli, standard HCA routines in SPSS were used. An agglomerative protocol was used to determine clustering and squared-euclidian distance was used to determine distance between clusters. This analysis does not determine discriminability between odors, but rather how similar behavioral responses were to the distinct stimuli.
4.4. Electrophysiology
For electrophysiology, naive animals were anesthetized with urethane (0.8g/kg mice, 1.5g/kg rats) and placed in a stereotaxic apparatus. The scalp was resected and holes drilled in the skull overlying either the aPCX (mouse: 2mm anterior, 2.5 mm lateral, rat: coordinates: 1mm anterior, 5mm lateral to Bregma) or pPCX (mouse: 1mm posterior, 3.5 mm lateral, rat: coordinates: 3mm posterior, 6.5mm lateral). Tungsten microelectrodes (5Mohm; A-M Systems) were directed toward Layer II/III of PCX and single-unit activity recorded. Recordings were amplified (500x), band-pass filtered (0.3–3kHz), and digitized at 10kHz for data collection and analyses with Spike2 software (CED, Inc.). Local field potentials (0.3–3kHz; 200x amplification, 1kHz sample rate) were recorded simultaneously to monitor brain state during the recordings. Odor responses were obtained during fast-wave states when the PCX is known to be most responsive to odors (Murakami et al., 2005; Wilson, 2010).
Once units were isolated, their basal activity rates (3 sec pre-odor onset) and response to odor (3 sec post-odor onset) were assessed. Single-units had at least 4:1 signal:noise ratio and at least 2 ms refractory period in an interval histogram. Odorant stimulation was a 2 sec pulse at 0.5 LPM directed to the nose of the freely breathing animal, with at least 30 sec between stimuli. Each stimulus was repeated three times in random order for each unit. As noted above, stimuli included ethyl isobutyrate (odorant A; CAS 97–62-1; Sigma; stock solution 100.5mg in 10mL of 100% ethanol), ethyl maltol (odorant B; CAS 4940–11-8; Sigma; stock solution 100mg in 10mL of 100% ethanol), a binary mixture AB at a component ratio of 30/70 (A/B stock solutions), or a binary mixture A’B’ at a component ratio of 68/32. For further details of the stimuli and mixtures see (Coureaud et al., 2008; Le Berre et al., 2008b).
4.5. Electrophysiology data analyses
Cumulative stimulus-evoked single-unit spike counts (number of spikes during a 3 sec period post odor onset – number of spikes during the 3 sec pre-odor onset) formed the primary dataset. Data were organized and presented as both normalized odor receptive fields and hierarchical cluster analysis (SPSS) of ensemble unit activity for each region in each species. Normalization involved expressing number of evoked spikes for a given single-unit as a proportion of the maximal response to the ‘best’ stimulus for that unit. The average response magnitude to a given odor was the mean of the proportional responses across cells for that odor. Thus, if all cells respond maximally (response mag. = 1.0) to A the mean proportional score for that odor would be 1.0.
For HCA of how single-unit ensembles organized their activity (i.e., odor-evoked spike counts) to the different stimuli, standard HCA routines in SPSS were used as described above for behavioral analyses. HCA was performed on data obtained from single-units merged across animals in each brain region in each species.
4.6. Histology
Following the termination of recording, animals were overdosed with urethane (3g/kg) and perfused transcardially with phosphate buffered saline and 4% paraformaldehyde. Brains were sectioned, stained with cresyl violet, and electrode placements verified with light microscopy.
HIGHLIGHTS.
Perception of odor mixtures can be either configural or elemental.
Single-unit ensemble responses to these mixtures were assessed in aPCX and pPCX.
Anterior PCX coding matched known odor mixture perceptual characteristics.
In contrast, mixture perception did not predict posterior PCX ensemble coding.
Acknowledgments
FUNDING: Funding was provided by National Institutes of Health, DC003906 (USA) and CNRS-PICS (Projet International de Coopération Scientifique) CONFODOUR n°269867 (France)
Footnotes
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barkat S, Le Berre E, Coureaud G, Sicard G, Thomas-Danguin T, 2012. Perceptual blending in odor mixtures depends on the nature of odorants and human olfactory expertise. Chem Senses. 37, 159–66. [DOI] [PubMed] [Google Scholar]
- Brown GE, Poirier J-F, Adrian JC, 2004. Assessment of local predation risk: the role of subthreshold concentrations of chemical alarm cues. Behav Ecol. 15, 810–815. [Google Scholar]
- Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G, 2007. Associative Encoding in Posterior Piriform Cortex during Odor Discrimination and Reversal Learning. Cereb Cortex. 17, 1342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron RF, Caron AJ, 1969. Degree of stimulus complexity and habituation of visual fixation in infants. Psychonomic Science. 14, 78–79. [Google Scholar]
- Chabaud P, Ravel N, Wilson DA, Mouly AM, Vigouroux M, Farget V, Gervais R, 2000. Exposure to behaviourally relevant odour reveals differential characteristics in rat central olfactory pathways as studied through oscillatory activities. Chem Senses. 25, 561–73. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, Ravel N, 2009. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci. 29, 10287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford MR, Riffell JA, 2013. Mixture and odorant processing in the olfactory systems of insects: a comparative perspective. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 199, 911–28. [DOI] [PubMed] [Google Scholar]
- Cohen LB, DeLoache JS, Rissman MW, 1975. The effect of stimulus complexity on infant visual attention and habituation. Child Dev. 46, 611–7. [PubMed] [Google Scholar]
- Coureaud G, Thomas-Danguin T, Le Berre E, Schaal B, 2008. Perception of odor blending mixtures in the newborn rabbit. Physiol Behav. 95, 194–9. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Hamdani Y, Schaal B, Thomas-Danguin T, 2009. Elemental and configural processing of odour mixtures in the newborn rabbit. J Exp Biol. 212, 2525–31. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Gibaud D, Le Berre E, Schaal B, Thomas-Danguin T, 2011. Proportion of odorants impacts the configural versus elemental perception of a binary blending mixture in newborn rabbits. Chem Senses. 36, 693–700. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Thomas-Danguin T, Datiche F, Wilson DA, Ferreira G, 2014a. Differential memory persistence of odor mixture and components in newborn rabbits: competition between the whole and its parts. Front Behav Neurosci. 8, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureaud G, Thomas-Danguin T, Wilson DA, Ferreira G, 2014b. Neonatal representation of odour objects: distinct memories of the whole and its parts. Proc Biol Sci. 281, 20133319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureaud G, Fleming G, Thomas-Danguin T, Wilson DA, 2018. Cross-species configural perception of binary odor mixtures. Chem Senses. 43, 110. [Google Scholar]
- Coureaud G, Letagneaux C, Thomas-Danguin T, Romagny S, 2019. Developmental changes in elemental and configural perception of odor mixtures in young rabbits. Developmental Psychobiology. doi: 10.1002/dev.21929. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Coureaud G, Wilson DA, 2019. Configural perception of odor mixtures: Functional early in life, convergent between species. Chemical Senses. 44, e74. [Google Scholar]
- Coureaud G, Thomas-Danguin T, Sandoz JC, Wilson DA, in prep. Biological constraints on configural odour mixture perception. [DOI] [PMC free article] [PubMed]
- Courtiol E, Buonviso N, Litaudon P, 2019. Odorant features differentially modulate beta/gamma oscillatory patterns in anterior versus posterior piriform cortex. Neuroscience. 409, 26–34. [DOI] [PubMed] [Google Scholar]
- Davison IG, Katz LC, 2007. Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J Neurosci. 27, 2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison IG, Ehlers MD, 2011. Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron. 70, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisig N, Dupuy F, Anton S, Renou M, 2014. Responses to pheromones in a complex odor world: Sensory processing and behavior. Insects. 5, 399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby CD, Ache BW, 1984. Quality coding of a complex odorant in an invertebrate. J Neurophysiol. 51, 906–24. [DOI] [PubMed] [Google Scholar]
- Duchamp-Viret P, Duchamp A, Chaput MA, 2003. Single olfactory sensory neurons simultaneously integrate the components of an odour mixture. Eur J Neurosci. 18, 2690–6. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ, 2006. Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron. 49, 467–79. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Margot C, da Silva MA, Velazco MI, 2007. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. J Neurosci. 27, 13532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB, 2001. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 26, 551–76. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA, 2006. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci U S A. 103, 15206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor V, Provost AC, Agarwal P, Murthy VN, 2016. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels. Nat Neurosci. 19, 271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Crk T, Thorngate J, 2005. A redefinition of odor mixture quality. Behav Neurosci. 119, 726–33. [DOI] [PubMed] [Google Scholar]
- Large AM, Vogler NW, Canto-Bustos M, Friason FK, Schick P, Oswald AM, 2018. Differential inhibition of pyramidal cells and inhibitory interneurons along the rostrocaudal axis of anterior piriform cortex. Proc Natl Acad Sci U S A. 115, E8067–E8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre E, Beno N, Ishii A, Chabanet C, Etievant P, Thomas-Danguin T, 2008a. Just noticeable differences in component concentrations modify the odor quality of a blending mixture. Chem Senses. 33, 389–95. [DOI] [PubMed] [Google Scholar]
- Le Berre E, Thomas-Danguin T, Beno N, Coureaud G, Etievant P, Prescott J, 2008b. Perceptual processing strategy and exposure influence the perception of odor mixtures. Chem Senses. 33, 193–9. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA, 2004. Configurational and elemental odor mixture perception can arise from local inhibition. J Comput Neurosci. 16, 39–47. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA, 2016. Neuromodulation of olfactory transformations. Curr Opin Neurobiol. 40, 170–177. [DOI] [PubMed] [Google Scholar]
- Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N, 2003. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur J Neurosci. 17, 2457–61. [DOI] [PubMed] [Google Scholar]
- Majak K, Ronkko S, Kemppainen S, Pitkanen A, 2004. Projections from the amygdaloid complex to the piriform cortex: A PHA-L study in the rat. J Comp Neurol. 476, 414–28. [DOI] [PubMed] [Google Scholar]
- Markopoulos F, Rokni D, Gire DH, Murthy VN, 2012. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 76, 1175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JP, Lei H, Riffell JA, Hildebrand JG, 2013. Synchronous firing of antennal-lobe projection neurons encodes the behaviorally effective ratio of sex-pheromone components in male Manduca sexta. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 199, 963–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly AM, Litaudon P, Chabaud P, Ravel N, Gervais R, 1998. Spatiotemporal distribution of a late synchronized activity in olfactory pathways following stimulation of the olfactory bulb in rats. Eur J Neurosci. 10, 1128–35. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kashiwadani H, Kirino Y, Mori K, 2005. State-dependent sensory gating in olfactory cortex. Neuron. 46, 285–296. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly L, 2004. Olfactory cortex In The synaptic organization of the brain. Vol., Shepherd GM, ed.êds. Oxford University Press, New York, pp. 415–454. [Google Scholar]
- Oakes LM, 2010. Using habituation of looking time to assess mental processes in infancy. J Cogn Dev. 11, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg MC, Ross JM, Bendahmane M, Fletcher ML, 2018. Olfactory bulb acetylcholine release dishabituates odor responses and reinstates odor investigation. Nat Commun. 9, 1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picimbon JF, Gadenne C, Becard JM, Clement JL, Sreng L, 1997. Sex pheromone of the french black cutworm moth, Agrotis ipsilon (Lepidoptera: Noctuidae): Identification and regulation of a multicomponent blend. Journal of Chemical Ecology. 23, 211–230. [Google Scholar]
- Riffell JA, Lei H, Christensen TA, Hildebrand JG, 2009. Characterization and coding of behaviorally significant odor mixtures. Curr Biol. 19, 335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell JA, 2012. Olfactory ecology and the processing of complex mixtures. Curr Opin Neurobiol. 22, 236–42. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Stalnaker TA, Schoenbaum G, 2007. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 17, 643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagny S, Thomas-Danguin T, Coureaud G, 2014. Newborn rabbit perception of 6-odorant mixtures depends on configural processing and number of familiar elements. PLoS One. 9, e107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagny S, Coureaud G, Thomas-Danguin T, 2018. Key odorants or key associations? Insights into elemental and configural odour processing in humans. Flav. Frag. J 33, 97–105. [Google Scholar]
- Schneider NY, Datiche F, Wilson DA, Gigot V, Thomas-Danguin T, Ferreira G, Coureaud G, 2016. Brain processing of a configural vs elemental odor mixture in the newborn rabbit. Brain Struct Funct. 221, 2527–39. [DOI] [PubMed] [Google Scholar]
- Schubert M, Hansson BS, Sachse S, 2014. The banana code-natural blend processing in the olfactory circuitry of Drosophila melanogaster. Front Physiol. 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinding C, Thomas-Danguin T, Crepeaux G, Schaal B, Coureaud G, 2011. Experience influences elemental and configural perception of certain binary odour mixtures in newborn rabbits. J Exp Biol. 214, 4171–8. [DOI] [PubMed] [Google Scholar]
- Sinding C, Thomas-Danguin T, Chambault A, Beno N, Dosne T, Chabanet C, Schaal B, Coureaud G, 2013. Rabbit neonates and human adults perceive a blending 6-component odor mixture in a comparable manner. PLoS One. 8, e53534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Murphy NR, Balasubramanian V, Mainland JD, 2019. Competitive binding predicts nonlinear responses of olfactory receptors to complex mixtures. Proc Natl Acad Sci U S A. 116, 9598–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P, Stierle JS, 2014. Mixture processing and odor-object segregation in insects. Prog Brain Res. 208, 63–85. [DOI] [PubMed] [Google Scholar]
- Tabor R, Yaksi E, Weislogel JM, Friedrich RW, 2004. Processing of odor mixtures in the zebrafish olfactory bulb. J Neurosci. 24, 6611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Danguin T, Sinding C, Romagny S, El Mountassir F, Atanasova B, Le Berre E, Le Bon AM, Coureaud G, 2014. The perception of odor objects in everyday life: a review on the processing of odor mixtures. Front Psychol. 5, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liu P, Mao X, Zhou Z, Cao T, Xu J, Sun C, Li A, 2019. Task-demand-dependent neural representation of odor information in the olfactory bulb and posterior piriform cortex. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, 2000. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol. 84, 3036–42. [DOI] [PubMed] [Google Scholar]
- Wilson DA, 2003. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 90, 65–72. [DOI] [PubMed] [Google Scholar]
- Wilson DA, 2010. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci. 30, 1760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, 2011. Cortical processing of odor objects. Neuron. 72, 506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TD, 2014. Introduction to chemical signaling in vertebrates and invertebrates. In Neurobiology of Chemical Communication. Frontiers in Neuroscience, Vol., Mucignat-Care C, ed.êds., Boca Raton (FL). [PubMed] [Google Scholar]
- Young A, Sun QQ, 2009. GABAergic inhibitory interneurons in the posterior piriform cortex of the GAD67-GFP mouse. Cereb Cortex. 19, 3011–29. [DOI] [PMC free article] [PubMed] [Google Scholar]