Abstract
Background:
“Mirror Syndrome” (Ballantyne’s Syndrome) refers to the association of fetal hydrops with placentomegaly and severe maternal edema. Preeclampsia occurs in approximately 50% of the cases. Soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), an anti-angiogenic factor, has been implicated in the pathophysiology of preeclampsia (PE).
Objective:
The objective of this study was to determine if the maternal plasma concentration of sVEGFR-1 is elevated in patients with “Mirror Syndrome”.
Study Design:
This case-control study included patients with uncomplicated pregnancies (n=40) and patients with “Mirror Syndrome” (n=4) matched for gestational age. “Mirror Syndrome” was defined as fetal hydrops and severe maternal edema. Maternal plasma sVEGFR-1 concentrations were determined using specific ELISA. Immunohistochemistry of sVEGFR-1 on villous trophoblasts was also performed in samples from one patient with “Mirror Syndrome” and compared with those from a patient with spontaneous preterm delivery matched by gestational age. Non-parametric statistics were used for analysis (p < 0.05).
Results:
1) The median maternal plasma concentration of sVEGFR-1 was significantly higher in patients with “Mirror Syndrome” than in the control group (median: 3,974 pg/mL, range: 3,083–10,780 vs. median: 824 pg/mL, range: 260–4712, respectively; p < 0.001); and 2) all patients with “Mirror Syndrome” had sVEGFR-1 concentrations above the 95th percentile for gestational age. Syncytiotrophoblast, especially syncytial knots, showed strong staining with antibodies against sVEGFR-1 in placental samples from the patient with “Mirror Syndrome”, but not in those from the patient with spontaneous preterm delivery.
Conclusion:
High maternal plasma concentrations of sVEGFR-1 were observed in “Mirror Syndrome”. We propose that this anti-angiogenic factor may participate in the pathophysiology of this syndrome. Thus, maternal plasma determination of sVEGFR-1 may help to identify the hydropic fetus that places the mother at risk for preeclampsia.
Keywords: mirror syndrome, Ballantyne’s syndrome, sVEGFR-1, preeclampsia, villous edema
INTRODUCTION
More than 100 years ago, John H. Ballantyne used the term “general dropsy of the fetus” to refer to fetal hydrops and concomitant edema of the placenta. Moreover, he reported that marked maternal edema and uterine distention were observed far more commonly with hydrops fetalis than in normal fetuses, and that albuminuria was associated with maternal edema in these cases [1]. The term “Mirror Syndrome” was introduced by O’Driscoll in 1956, who reported that in cases of fetal hydrops, “the mother to some degree mirrors the edema of the fetus” [2]. Ballantyne’s Syndrome was initially reported in cases of rhesus isoinmunization [2–7]. However, it has since been described as well in other pregnancy complications associated with fetal hydrops including: cytomegalovirus [8] and Parvovirus B 19 [9] infections, Ebstein’s anomaly [10], aneurysm of the vein of Galen [11], fetal supraventricular tachycardia [12], and placental chorioangioma [13].
“Mirror Syndrome” is also referred to as pseudotoxemia; however, hypertension and proteinuria consistent with the clinical diagnosis of preeclampsia is present in about half of the cases of “Mirror Syndrome” [1–16]. Recently, the soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), an antiangiogenic factor, has been associated with the pathogenesis of preeclampsia [17–19]. Thus, the objective of this study was to determine if the maternal plasma concentration of sVEGFR-1 is elevated among patients with “Mirror Syndrome”.
MATERIAL AND METHODS
Study design:
This case-control study included patients with “Mirror Syndrome” (n=4) and gestational age-matched controls (n=40). “Mirror Syndrome” was defined as fetal hydrops and severe maternal edema [1,2]. Preeclampsia was diagnosed in the presence of systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart, and proteinuria (≥300 mg in a 24-hour urine collection or one dipstick measurement ≥2+). Severe preeclampsia was defined as severe hypertension (diastolic blood pressure ≥110 mmHg) plus mild proteinuria, or mild hypertension plus severe proteinuria (a 24-hour urine sample containing 5 g protein or urine specimen ≥3+ protein by dipstick measurement). Patients with an abnormal liver function test (aspartate aminotransferase >70 IU/L) plus thrombocytopenia (platelet count <100,000/cm3) were also classified as having severe preeclampsia. Control cases consisted of patients who had a normal pregnancy and delivered a neonate that was appropriate for gestational age. These patients were matched for gestational age with the “Mirror Syndrome” cases.
All women provided written informed consent prior to the collection of plasma samples. The collection and utilization of the samples was approved for research purposes by the Institutional Review Board of the National Institutes of Child Health and Human Development.
Sample collection and human sVEGFR-1 immunoassay:
Venipuncture was performed and the blood was collected into tubes containing EDTA. The samples were centrifuged at 4°C for 10 minutes and stored at –70°C until assay. The concentrations of sVEGFR-1 were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) as previously described [18]. The inter- and intra-assay coefficients of variation (CVs) for human sVEGFR-1 immunoassay in our laboratory were 4.8% and 6.9%, respectively. The sensitivity of the assay was 17.8 pg/mL.
Histological examination:
Placental samples for histological examination were available in one patient with “Mirror Syndrome”. The histological findings were compared with that from a patient with spontaneous preterm delivery matched by gestational age. Immunohistochemistry of sVEGFR-1 on villous trophoblasts was also performed in the samples from the patient with “Mirror Syndrome”. Deparaffination of 5-μm-thick tissue slides, rehydration, and antigen retrieval were followed by immunohistochemistry using an automatic immunostainer (Ventana Discovery, Ventana Medical Systems, Inc., Tucson, AZ). Antigen retrieval was performed using Proteinase 2 solution for 8 minutes. Sections were treated with 10% normal horse blocking serum for 20 minutes. Subsequently, sections were incubated with primary antibody (1:300 in dilution, polyclonal goat anti-VEGF-R1, R&D Systems, Minneapolis, MN). Biotin-conjugated horse anti-goat antibody (1:200 in dilution, Jackson Immuno Research Laboratories, Inc., West Grove, PA) was applied as the secondary antibody. A DAB MAP kit (Ventana Medical Systems Inc., Tucson, AZ) was used for staining and Hematoxylin was used as a counter stain. A blocking peptide (1:25, R&D Systems, Minneapolis, MN) mixed with the primary antibody was used as a negative control.
Statistical analysis:
Plasma sVEGFR-1 concentrations were not normally distributed; therefore, non-parametric statistics were used for analysis. The 5th and 95th percentiles for the maternal plasma concentration of sVEGFR-1 were calculated for each gestational age in the normal cases, and the plasma concentrations of sVEGFR-1 of the four “Mirror Syndrome” cases were compared to the normal values. The statistical package used was SPSS v.12.0 (SPSS Inc., Chicago, IL). A p-value <0.05 was considered significant.
RESULTS
The clinical characteristics of the patients with “Mirror Syndrome” are displayed in Table I. One patient developed preeclampsia five weeks after sample collection, the other three patients had preeclampsia at the time of sample collection. One of these patients developed eclampsia during labor. All four cases delivered before 35 weeks of gestation.
Table I.
Clinical characteristics of the patients with “Mirror Syndrome”
| Cases | GA at blood draw (weeks) |
GA at diagnosis of PE (weeks) |
sVEGFR-1 plasma concentration (pg/mL) |
GA at delivery (weeks) |
Birthweight (g) |
Main diagnosis |
|---|---|---|---|---|---|---|
| 1 | 28.6 | 28.6 | 10,780.000 | 28.6 | 1080 | Non-immune hydrops fetalis, eclampsia |
| 2 | 28.7 | 34.1 | 3,083.640 | 34.1 | 2340 | Non-immune hydrops fetalis, severe PE |
| 3 | 32.4 | 32.4 | 3,912.658 | 34.0 | 3380 | Non-immune hydrops fetalis, PE, multiple fetal anomalies |
| 4 | 30.1 | 30.1 | 4,037.571 | 30.1 | 2190 | Rh (−) isoimmunization, polyhydramnios, gestational diabetes, severe PE |
GA: gestational age, PE: preeclampsia, sVEGFR-1: soluble vascular endothelial growth factor receptor-1
The median maternal plasma concentration of sVEGFR-1 was significantly higher in patients with “Mirror Syndrome” than that of women in the control group (median: 3,974 pg/mL, range: 3083–10,780 vs. median: 824 pg/mL, range: 260–4712, respectively; p < 0.001). All patients with “Mirror Syndrome” had sVEGFR-1 concentrations above the 95th percentile for gestational age (Figure 1).
Figure 1.

Maternal plasma concentration of sVEGFR-1 in patients with “Mirror Syndrome” and in those with uncomplicated pregnancies. The lower and upper lines represent the 5th and 95th percentiles of the plasma sVEGFR-1 concentrations among patients with normal pregnancies.
Histological examination of the placenta in a case of “Mirror Syndrome” revealed immature intermediate villi with edematous changes (Figure 2a), increased syncytial knots, increased intervillous fibrin, and multifocal villous calcifications (Figure 2b). In contrast, histological examination of the placenta, in the case with spontaneous preterm delivery without “Mirror Syndrome”, did not show these histological findings (Figure 2c). Syncytiotrophoblast, especially syncytial knots, showed strong staining with antibodies against sVEGFR-1 in the placental samples from the patient with “Mirror Syndrome” (Figure 3a and 3B), but not in those from the patient with spontaneous preterm delivery (Figure 3c).
Figure 2.

Histological examination of placental samples with Hematoxylin and Eosin (H&E) demonstrated immature intermediate villi with edematous changes (Figure 2a) and increased syncytial knots, increased intervillous fibrin, and multifocal villous calcifications (Figure 2b). In contrast, examination of the placental samples from the spontaneous delivery case did not show these histological findings (Figure 2c).
Figure 3.
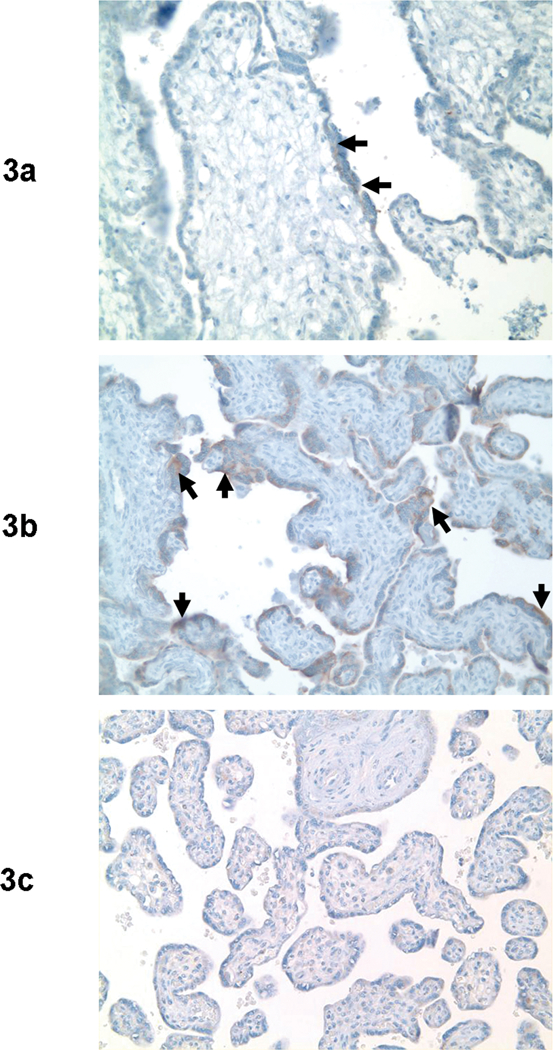
Syncytiotrophoblast, especially syncytial knots, showed strong staining with antibodies against sVEGFR-1 in the placental samples from a patient with “Mirror Syndrome” (Figure 3a and 3B), but not in those from the patient with spontaneous preterm delivery (Figure 3c).
DISCUSSION
Principal finding of this study:
“Mirror Syndrome” is associated with a high maternal plasma concentration of sVEGFR-1.
A role for the fetus:
“Mirror Syndrome” is characterized by the combination of fetal hydrops and severe maternal edema. Hypertension and proteinuria consistent with the clinical diagnosis of preeclampsia has been described in approximately half of the cases of “Mirror Syndrome” reported thus far (Table II). The observations that preeclampsia associated with “Mirror Syndrome” can resolve after the treatment of fetal anemia with an intrauterine transfusion in cases of Parvovirus B19 infection [9], correction of severe fetal tachycardia [12], or following the death of the hydropic fetus in twin pregnancies [14,16] indicates that the fetus plays an important role in the pathogenesis of “Mirror Syndrome”.
Table II.
Summary of the reports of “Mirror Syndrome” cases
| Author (s) | Cases (#) |
GA at diagnosis (weeks) |
Diagnosis | Highest BP |
Proteinuria | Peripheral edema |
Placentomegaly | Comments |
|---|---|---|---|---|---|---|---|---|
| Ordorica SA et al[11] | 1 | 30 | Aneurysm of the vein of Galen | 140/100 | 3.5 grms/24 hrs | 4+ | yes | |
| Duthie SJ et al[9] | 1 | 25 | Parvovirus B19 infection | 165/100 | 8.5 grams/24 hrs | None repoted | yes | Resolved after intrauterine transfusion |
| Quagliarello et al[8] | 1 | 30 | Cytomegalovirus infection | 140/100 | 1.3 grms/24 hrs | Gross pitting edema | yes | IUFD |
| Heyborne KD et al[14] | 1 | 16 | Twins discordant for fetal hydrops | 147/80 | 1+ | Peripheral edema | not reported | Resolved after selective feticide |
| Pirhonen JP et al[16] | 1 | 28 | Twins discordant for fetal hydrops | 130/90 | 1+ | Marked edema |
Grossly hydropic in the affected twin | Resolved after spontaneous fetal demise |
| Vidaeff AC et al[15] | 1 | 31 | Severe fetal ascitis | 136/64 | not reported | Yes | not reported | |
| Midgley DY et al[12] | 1 | 27 | Supraventricular tachycardia | 130/90 | 0.89 gr/24 hrs | Yes | not reported | Resolved following flecainide treatment |
| O’Driscoll DT et al[2] | 3 | 32–36 | RH isoimmunization | 130/90 | no | Yes | yes | Perinatal death |
| Cohen A[3] | 1 | 29 | RH isoimmunization | 140/90 | not reported | Yes | yes | IUFD |
| John AH et a[6]l | 20 | 28–40 | RH isoimmunization | 140/90 or more | 65% (13/20) | 85% (17/20) | yes | IUFD or perinatal death |
| Scott JS[7] | 26 | not reported | RH isoimmunization molar pregnancy | 140/90 or more | yes | yes | yes | |
| Hirsch MR et al[5] | 1 | 28 | RH isoimmunization | 130/80 | 4+ | Yes | yes | IUFD |
| Goodlin RC[4] | 10 | 32–34 | RH isoimmunization | 40% (4/10) had BP ≥ 140/90 | 40% 4/10) had 1+ or more | Yes | not reported | IUFD in all cases |
| Kaiser IH[1] | 1 | not reported | Lung sequestration | 140/90 | 59 mg/24 hrs | Yes | Placental weight: 1,020 grams | Perinatal death |
| Dorman SL et al[13] | 1 | 19 | Placental chorioangioma | 220/126 | 4+ | Gross pitting edema | Placental chorioangioma | Termination of pregnancy |
| Carbillon, L et al[10] | 1 | 31 | Ebstein’s anomaly | 180/110 | not reported | Edema of the face and hands | Grossly edematous | Stillborn |
Preeclampsia as an anti-angiogenic state:
Accumulating clinical and experimental evidence indicates that a subset of mothers with preeclampsia and/or SGA fetuses has an anti-angiogenic state. The following observations support this view: 1) serum from pregnant women with preeclampsia has anti-angiogenic properties demonstrated in the tube-formation assay [17]; 2) high maternal plasma concentrations of sVEGFR-1, a potent anti-angiogenic agent, have been reported at the time of the diagnosis [17–19] and before the development of preeclampsia [19,20]; sVEGFR-1 binds to the free form of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) reducing their bioavailability and their pro-angiogenic effect [17,21]; 3) VEFGR-1 mRNA is upregulated in placentas from preeclamptic patients [17]; 4) patients with preeclampsia have lower concentrations of PlGF and free VEGF than normal pregnant women [17,21]; and 5) the administration of adenovirus, expressing the sVEGFR-1 gene, to pregnant animals induces the clinical presentation of preeclampsia [17]. Collectively, this evidence indicates that sVEGFR-1 participates in the pathophysiology of preeclampsia. It seems that not all women with preeclampsia have elevated anti-angiogenic factors or low concentrations of angiogenic cytokines (free VEGF, PlGF, or angiogenin, among others). Moreover, an anti-angiogenic state is not specific to preeclampsia, because mothers with small-for-gestational-age fetuses (SGA) also have higher median maternal plasma concentrations of sVEGFR-1 than patients with normal pregnancy [18], but lower than patients with preeclampsia. This finding suggests that an anti-angiogenic state during pregnancy has a broad spectrum of severity ranging from mild to severe and that it can be expressed with different phenotypes: SGA [18], fetal death of unknown etiology, and preeclampsia. Of interest, other obstetrical syndromes, such as preterm labor and preterm rupture of membranes, are not characterized by an elevation of the maternal plasma concentration of sVEGFR-1 (unpublished observations).
Why do mothers of fetuses with hydrops develop edema and/or preeclampsia?
Hydrops is frequently a manifestation of fetal cardiac failure [22] and is associated with villous edema [1]. This condition is characterized by high intracellular water content and high total placental water [23]. Compression of the villous blood vessels by edematous villi or a thicker interface may impair oxygen exchange [24,25]; the greater the severity of villous edema, the lower the umbilical artery cord pH [25]. It has also been proposed that edematous villi may reduce the intervillous space and the intervillous blood flow with subsequent reduction in the fetal oxygen supply [26].
We propose that hypoxia of the villous trophoblast in cases of villous edema leads to increased production and release of sVEGFR-1 (and, perhaps, other anti-angiogenic factors) into the maternal circulation. Excessive concentrations of these products would then be responsible for maternal edema in “Mirror Syndrome” and for the endothelial cell dysfunction in those cases complicated with preeclampsia. Evidence in support of this includes: 1) cytotrophoblast [27] and trophoblast cells [28] cultured under hypoxic conditions upregulate the mRNA expression and production of sVEGFR-1 in the supernatant; and 2) the observations of the current study, in which the maternal plasma concentrations of sVEGFR-1 are increased in mothers with “Mirror Syndrome”.
Why do some mothers with hydropic fetuses develop “Mirror Syndrome” and others do not?
The excessive production of anti-angiogenic factors may be a function of the severity of the villous edema, as well as the genetic factors responsible for the production, metabolism and functional control of pro- and anti-angiogenic factors. Some mothers may be more susceptible than others to a given concentration of anti-angiogenic factors.
Two distinct placental lesions (hypoperfusion and villous edema), preeclampsia, and sVEGFR-1
The placental lesions frequently associated with preeclampsia include maternal abnormalities (failure of physiologic transformation of the spiral arteries, acute atherosis, thrombosis or decidual necrosis) [29,30] and fetal abnormalities (intervillous thrombi, localized ischemic villous necrosis, thrombotic occlusion of the fetal stem villous artery and decreased number of terminal villi) [29,30], which have been characterized as reflecting placental hypoperfusion. Yet, in “Mirror Syndrome”, the cardinal histologic finding is villous edema. We interpret this as indicating that two different pathologic processes lead to the development of a maternal anti-angiogenic state and the syndrome of preeclampsia. This suggests that the syndrome has a common pathway that includes the development of an anti-angiogenic state. A limitation of this study is that placental samples were available only from one case of “Mirror Syndrome”.
Further studies
Longitudinal studies of mothers with hydropic fetuses who develop “Mirror Syndrome” with and without preeclampsia are required to characterize the profile of pro-angiogenic and anti-angiogenic factors. Moreover, serial observations in patients in whom “Mirror Syndrome” resolves spontaneously after fetal treatment or the death of a hydropic co-twin can provide insights into the mechanism of disease. We welcome the opportunity to collaborate with investigators with appropriately collected samples with informed consent and IRB approval to address these issues.
Conclusion
We propose that maternal edema and preeclampsia in cases of fetal hydrops is the result of an anti-angiogenic state.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
REFERENCES
- 1.Kaiser IH. Ballantyne and triple edema. Am J Obstet Gynecol 1971; 110: 115–120. [DOI] [PubMed] [Google Scholar]
- 2.O’DRISCOLL DT. A fluid retention syndrome associated with severe iso-immunization to the rhesus factor. J Obstet Gynaecol Br Emp 1956; 63: 372–374. [DOI] [PubMed] [Google Scholar]
- 3.COHEN A Maternal syndrome in Rh iso-immunization: report of a case. J Obstet Gynaecol Br Emp 1960; 67: 325–327. [DOI] [PubMed] [Google Scholar]
- 4.Goodlin RC. Impending Fetal Death in Utero Due to Isoimmunization. Obstet Gynecol 1957; 10: 299–302. [Google Scholar]
- 5.HIRSCH MR, MARK MS. Pseudotoxemia and Erythroblastosis. Report of a Case. Obstet Gynecol 1964; 24: 47–48. [PubMed] [Google Scholar]
- 6.JOHN AH, DUNCAN AS. The Maternal Syndrome Associated with Hydrops Foetalis. J Obstet Gynaecol Br Commonw 1964; 71: 61–65. [DOI] [PubMed] [Google Scholar]
- 7.SCOTT JS. Pregnancy toxaemia associated with hydrops foetalis, hydatidiform mole and hydramnios. J Obstet Gynaecol Br Emp 1958; 65: 689–701. [DOI] [PubMed] [Google Scholar]
- 8.Quagliarello JR, Passalaqua AM, Greco MA, Zinberg S, Young BK. Ballantyne’s triple edema syndrome: prenatal diagnosis with ultrasound and maternal renal biopsy findings. Am J Obstet Gynecol 1978; 132: 580–581. [DOI] [PubMed] [Google Scholar]
- 9.Duthie SJ, Walkinshaw SA. Parvovirus associated fetal hydrops: reversal of pregnancy induced proteinuric hypertension by in utero fetal transfusion. Br J Obstet Gynaecol 1995; 102: 1011–1013. [DOI] [PubMed] [Google Scholar]
- 10.Carbillon L, Oury JF, Guerin JM, Azancot A, Blot P. Clinical biological features of Ballantyne syndrome and the role of placental hydrops. Obstet Gynecol Surv 1997; 52: 310–314. [DOI] [PubMed] [Google Scholar]
- 11.Ordorica SA, Marks F, Frieden FJ, Hoskins IA, Young BK. Aneurysm of the vein of Galen: a new cause for Ballantyne syndrome. Am J Obstet Gynecol 1990; 162: 1166–1167. [DOI] [PubMed] [Google Scholar]
- 12.Midgley DY, Harding K. The Mirror syndrome. Eur J Obstet Gynecol Reprod Biol 2000; 88: 201–202. [DOI] [PubMed] [Google Scholar]
- 13.Dorman SL, Cardwell MS. Ballantyne syndrome caused by a large placental chorioangioma. Am J Obstet Gynecol 1995; 173: 1632–1633. [DOI] [PubMed] [Google Scholar]
- 14.Heyborne KD, Chism DM. Reversal of Ballantyne syndrome by selective second-trimester fetal termination. A case report. J Reprod Med 2000; 45: 360–362. [PubMed] [Google Scholar]
- 15.Vidaeff AC, Pschirrer ER, Mastrobattista JM, Gilstrap LC III, Ramin SM. Mirror syndrome. A case report. J Reprod Med 2002; 47: 770–774. [PubMed] [Google Scholar]
- 16.Pirhonen JP, Hartgill TW. Spontaneous reversal of mirror syndrome in a twin pregnancy after a single fetal death. Eur J Obstet Gynecol Reprod Biol 2004; 116: 106–107. [DOI] [PubMed] [Google Scholar]
- 17.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol 2004; 190: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 19.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350: 672–683. [DOI] [PubMed] [Google Scholar]
- 20.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005; 17: 3–18. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669–676. [DOI] [PubMed] [Google Scholar]
- 22.Huhta JC. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr Cardiol 2004; 25: 274–286. [DOI] [PubMed] [Google Scholar]
- 23.Barker G, Boyd RD, D’Souza SW, Donnai P, Fox H, Sibley CP. Placental water content and distribution. Placenta 1994; 15: 47–56. [DOI] [PubMed] [Google Scholar]
- 24.Naeye RL, Maisels MJ, Lorenz RP, Botti JJ. The clinical significance of placental villous edema. Pediatrics 1983; 71: 588–594. [PubMed] [Google Scholar]
- 25.Kovalovszki L, Villanyi E, Benko G. Placental villous edema: a possible cause of antenatal hypoxia. Acta Paediatr Hung 1990; 30: 209–215. [PubMed] [Google Scholar]
- 26.Alvarez H, Sala MA, Benedetti WL. Intervillous space reduction in the edematous placenta. Am J Obstet Gynecol 1972; 112: 819–820. [DOI] [PubMed] [Google Scholar]
- 27.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004; 145: 4838–4845. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 2005; 26: 210–217. [DOI] [PubMed] [Google Scholar]
- 29.Benirschke K, Kaufmann P. Maternal diseases complicating pregnancy: diabetes, tumors, preeclampsia, lupus anticoagulant In; Benirschke K, Kaufmann P, editors. Pathology of the human placenta. New York: Springer-Verlag; 2000. Pages 523–589. [Google Scholar]
- 30.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol 2003; 189: 1173–1177. [DOI] [PubMed] [Google Scholar]


