Abstract
Context
The gastrointestinal hormone ghrelin stimulates growth hormone secretion and appetite, but recent studies indicate that ghrelin also stimulates the secretion of the appetite-inhibiting and insulinotropic hormone glucagon-like peptide-1 (GLP-1).
Objective
To investigate the putative effect of ghrelin on GLP-1 secretion in vivo and in vitro.
Subjects and Methods
A randomized placebo-controlled crossover study was performed in eight hypopituitary subjects. Ghrelin or saline was infused intravenously (1 pmol/min × kg) after collection of baseline sample (0 min), and blood was subsequently collected at time 30, 60, 90, and 120 minutes. Mouse small intestine was perfused (n = 6) and GLP-1 output from perfused mouse small intestine was investigated in response to vascular ghrelin administration in the presence and absence of a simultaneous luminal glucose stimulus. Ghrelin receptor expression was quantified in human (n = 11) and mouse L-cells (n = 3) by RNA sequencing and RT-qPCR, respectively.
Results
Ghrelin did not affect GLP-1 secretion in humans (area under the curve [AUC; 0–120 min]: ghrelin infusion = 1.37 ± 0.05 min × nmol vs. saline infusion = 1.40 ± 0.06 min × nmol [P = 0.63]), but induced peripheral insulin resistance. Likewise, ghrelin did not stimulate GLP-1 secretion from the perfused mouse small intestine model (mean outputs during baseline/ghrelin infusion = 19.3 ± 1.6/25.5 ± 2.0 fmol/min, n = 6, P = 0.16), whereas glucose-dependent insulinotropic polypeptide administration, used as a positive control, doubled GLP-1 secretion (P < 0.001). Intraluminal glucose increased GLP-1 secretion by 4-fold (P < 0.001), which was not potentiated by ghrelin. Finally, gene expression of the ghrelin receptor was undetectable in mouse L-cells and marginal in human L-cells.
Conclusions
Ghrelin does not interact directly with the L-cell and does not directly affect GLP-1 secretion.
Glucagon-like peptide-1 (GLP-1) is a hormone secreted from the gastrointestinal mucosa in response to nutrient intake. GLP-1 lowers postprandial blood glucose levels by potentiating glucose-stimulated insulin secretion as well as reducing gastric emptying and food intake (1–3). Because of its insulinotropic effect, GLP-1 is classified as an incretin hormone and accounts, together with the other incretin hormone glucose-dependent insulinotropic polypeptide (GIP), for an important part of postprandial insulin secretion (4). Because of these actions, GLP-1-based drugs have been developed to treat type 2 diabetes and obesity (5). This has stimulated renewed interest in understanding GLP-1 physiology, including the stimulus:sensor coupling that links food intake to GLP-1 secretion (6). It is well established that several macronutrients stimulate GLP-1 secretion (7) in addition to certain hormones including GIP (8, 9).
Recently, in two studies using mice and/or the GLP-1 secreting murine cell line GLUTag, ghrelin exposure was reported to potentiate glucose-stimulated GLP-1 secretion (10, 11), which was also reported in a human study (12). Notwithstanding, the dynamics of postprandial plasma ghrelin and GLP-1 concentrations are inversely related (13), and the physiological actions of ghrelin with respect to gastric emptying, satiety and glucose-stimulated insulin secretion are opposite to those of GLP-1 (2, 14, 15). Moreover, it is unclear whether the suggested stimulatory effect of exogenous ghrelin on GLP-1 secretion is a result of direct actions on the L-cells, which would require L-cells to express the ghrelin receptor (GHS-R) type 1a (16).
To test whether ghrelin has direct actions on GLP-1 secretion in humans, we infused (acyl)ghrelin at a supraphysiological rate in patients with hypopituitarism. The patients were on stable replacement therapy with hydrocortisone and growth hormone (GH), hence minimizing any confounding effect of ghrelin-induced GH and adrenocorticotropic hormone (ACTH) secretion on GLP-1 output, which would have occurred in individuals with intact pituitary function since ghrelin is an endogenous ligand for the GH secretagogue receptor (16). Furthermore, to investigate the direct effect of ghrelin on GLP-1 secretion, as well as potential enhancing effects of ghrelin on glucose-stimulated GLP-1 secretion, we infused (acyl)ghrelin at a supraphysiological concentration (100 times the plasma concentration) to isolated perfused mouse intestine preparations in presence or absence of a simultaneous luminal glucose stimulus and quantified GLP-1 output. Finally, we examined GHSR/Ghsr expression in human and mouse L-cells to investigate the likelihood of direct interaction of ghrelin with the L-cell.
Materials and Methods
Ethical considerations
Human study.
Studies were approved by the local ethics committee system and by the Danish Medicines Agency (protocol no. 2602-974) and conducted in accordance with the Helsinki Declaration. All subjects gave informed oral and written consent to participate, and the study protocol was registered at Clinicaltrials.gov (NCT01209416) before onset of enrollment (17).
Animals.
Mouse perfusion studies were conducted with permission from the Danish Animal Experiments Inspectorate (2018-15-0201-01397) and the local ethical committee in accordance with the guidelines of Danish legislation governing animal experimentation (1987) and the National Institutes of Health (publication no. 85-23).
Expression analysis studies on human and mouse L-cells were conducted in Cambridge, UK and were conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice. Human ethics approvals were given by Cambridge Central and South Research Ethics Committees (ref: 09/H0308/24, 16/EE/0338, 15/EE/0152) and the INSERM ethics committee and Agence de la Biomédecine (ref: PFS16–004). Animal work was regulated under the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 and conducted following ethics review by the University of Cambridge Animal Welfare and Ethical Review Body.
Intravenous ghrelin infusion to hypopituitary men
Samples used for the present study derived from a study designed to investigate direct effects of ghrelin on insulin sensitivity (17). In brief, eight hypopituitary men on stable replacement GH therapy (daily subcutaneous injection) and oral hydrocortisone for >6 months participated in the study (means ± standard error of the mean [SEM], span: age = 53 ± 5 years, 26–68 years; body mass index [BMI]: 30 ± 5 kg/m2, 26.0–42.2 kg/m2. Information about participants’ age, BMI, diagnosis, diagnostic test, GH peak during GH-stimulation test, and daily dose of GH replacement, HbA1c, and deficiencies of the hormones of the pituitary axes are provided in the original study (17). None of the participants had diabetes or other chronic disease.
All subjects were examined on four occasions in a 2 × 2 factorial design separated by minimum 2 weeks. The studies were conducted in a quiet and thermoneutral indoor environment. Each subject underwent 4 randomized interventions, using a double-blinded and placebo-controlled crossover study design: (i) ghrelin infusion, (ii) saline infusion, (iii) ghrelin infusion and intake of acipimox capsules, and (iv) saline infusion and intake of acipimox capsules. Only blood samples in the basal state from intervention arms 1 (ghrelin) and 2 (saline) are included in this study. The studies were performed between 8:00 am and 10:00 am (0–120 min) after an overnight fast. One intravenous (IV) cannula was inserted into an antecubital region for infusion, and one IV cannula was positioned in a dorsal hand vein for blood sampling. The hand was placed in a heat pad to arterialize the venous blood. At time 0 min (immediately after collection of the 0 min sample), an infusion of acyl-ghrelin or placebo (saline) was given via the catheter in the antecubital vein. Ghrelin was infused at a rate of 1 pmol/min × kg, based on experience from a previous experiment where this dose increased free-fatty acid levels in healthy young men (18). The ghrelin solution consisted of synthetic human acyl ghrelin (GMP-grade human acyl ghrelin; Bachem, Weil am Rhein, Germany) which was dissolved in isotonic saline immediately before infusion. The infusion solution was formulated by the hospital pharmaceutical services and complied with good distribution practice and good clinical practice guidelines. Arterialized blood was withdrawn at time points 0, 30, 60, 90, and 120 minutes. Samples for plasma ghrelin measurement were collected into 2 mL acidified EDTA tubes containing the protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF, 20 µl; 200 mg/mL; cat. no. A8456, Sigma Aldrich, Brøndby, Denmark), whereas plasma for GLP-1 (total) measurements were collected into prechilled EDTA-coated tubes (BA Vacutainer® K2E, Becton Dickinson, Plymouth, UK). DPP-IV inhibitor or protease inhibitors are not required for these measurements and were not added to the tubes. Samples were immediately centrifuged (2500 g, 10 min, 4°C) and supernatant was instantly after collected, transferred into fresh tubes, and stored at –80°C until analysis.
Isolated perfused mouse intestine
Male C57BL/6J mice (25 ± 2 g, 9–12 weeks of age, 6 mice/group) were obtained from Janvier (Le Genest-Saint-Isle, France) and housed in an animal facility at University of Copenhagen (Denmark) under standard conditions: 8/cage with ad libitum access to standard chow and water, following a 12:12 hour light:dark cycle. Mice were acclimatized for at least a week before experiment. At the day of experiment, animals were anaesthetized with intraperitoneal injection of ketamine/xylazine: Ketamine 90 mg/kg (Ketaminol Vet.; MSD Animal Health, Madison, NJ, US)/Xylazine: 10 mg/kg (Rompun Vet; Bayer Animal Health, Leverkusen, Germany). Following this, the abdominal cavity was opened by a midline incision, and the large intestine and distal half of the small intestine were removed after tying off the supplying vasculature, leaving ~12 cm of the proximal intestine. The spleen and stomach were excised and the renal stalks were tied off. A tube was inserted into the proximal part of the intestine and was flushed and perfused with saline (0.9%; 0.31 Osmol/l NaCl) at a flow rate of 0.035 mL/min throughout the experiment. The abdominal aorta was tied off just below the diaphragm, immediately after which a catheter (BD Insyte Autoguard, 24 GA 0.75 IN, 0.7 × 19 mm, Becton Dickinson, Lyngby, Denmark) was inserted retrogradely into the abdominal aorta just proximal to the renal arteries. The proximal part of the small intestine, supplied by the mesenteric artery, was perfused (2.5 mL/min) with a modified Krebs-Ringer buffer gassed with 95% O2 and 5% CO2 and passed through a UP100 Universal Perfusion System from Hugo Sachs (Harvard Apparatus, March Hugstetten, Germany) and thereby heated to 37°C. As soon as perfusion was evident, a similar catheter as the one used for the aorta was inserted into the portal vein for collection of venous effluent. Immediately after, the mouse was euthanized by diaphragm perforation, and the preparation was left to stabilize for 30 minutes before collection of the first effluent sample. Perfusate was collected each minute, instantly transferred onto ice and stored at –20°C until analysis within a week. Test stimulants consisted of acyl-ghrelin (mouse; ghrelin trifluoroacetate salt, cat. no. 4033076, Bachem, Bubendorf, Switzerland), glucose-dependent insulinotropic peptide (mouse GIP 1–42, cat. no. 027-27, Phenix Pharmaceuticals, Burlingame, CA, US) both administered at a final concentration of 100 nM, 20% (w/v) D-glucose (Sigma-Aldrich, cat. no. G8270) dissolved in 0.9% NaCl and bombesin (positive control, Bachem, cat. no. H-2155). Detailed information about the isolated perfused small intestine model has recently been published (19).
Biochemical measurements
Plasma glucose was quantified bedside using a glucose meter employing the glucose-oxidase method (YSI 2300 STAT Plus, YSI Life Sciences, Yellow Springs, OH, US). Plasma ghrelin was measured using a commercial ELISA kit for human acyl-ghrelin (cat. no. A05106, Bertin Pharma, Montigny-le-Bretonneux, France).
Total GLP-1 concentrations in human plasma and mouse small intestine effluents were quantified by in-house radioimmunoassay, employing a C-terminal directed antibody targeting the amidated C-terminus of GLP-1 x-36 amide (20). The assay reacts equally with intact 7–36 amide, the primary metabolite 9–36 amide as well as potential products resulting from mid-sequence cleavage, meaning that measurements are not influenced by presence or absence of protease inhibitors or DPP-4 inhibitors. We chose to target the amidated GLP-1 rather than glycine-extended GLP-1 isoforms (x-37) because humans and mice primarily store and secrete amidated GLP-1 (20–22).
L-cell expression of the ghrelin receptor
Expression of the ghrelin-receptor (GHSR) in human L-cells and non-L-cells (isolated from 11 donors) was determined using RNA-sequencing data of fluorescence-activated cell-sorted enteroendocrine cells published by Frank Reimann and Fiona M. Gribble and colleagues (National Center for Biotechnology Information Gene Expression Omnibus repository; human; GSE114853) (23). Donors consisted of 3 females and 8 males with a mean age and BMI ( ± SEM) of 53 ± 5 years and 33 ± 3 kg/m2 (23). Raw read counts were normalized using DESeq2 calculating size factors based on the median of ratios using all samples (24). Normalized read counts for gene of interests (GHSR and GIPR) were plotted for each sample in their respective population.
Ghsr expression in mouse was carried out by RT-qPCR in sorted L-cells and non-L-cells from the top and bottom 10 cm of the small intestine (duodenum and ileum) from 3 different GLU-Venus mice as previously described (23, 25). Briefly, epithelial cells were digested in 1mg/mL Collagenase XI and single cell sorted by fluorescence-activated cell-sorting at the phenotyping hub based on VENUS fluorescence (Cambridge, UK). RNA from 3 to 10 000 cells was isolated using a microplus RNeasy kit (Qiagen) and reverse transcribed using Superscript III (Invitrogen) following supplier recommendation. Gene expression was measured on an Abi QuantStudio 7 (Applied Biosystem) using fast Taqman master mix and Taqman primers specific for each gene (Ghs-r: Mm00616415_m1, Gipr: Mm01316344_m1, and β-Actin: Mm02619580_g1) in duplicate for each sample. Data are expressed as 2-ΔCt expression relative to β-Actin, where reactions in which the test gene was undetectable were assigned a Ct-value of 40; 2-∆Ct values ~2 × 10–5 are therefore close to the detection limit of the assay.
Statistical analysis
Results are expressed as means ± SEM. Statistical analysis was carried out using GraphPad Prism versus 7 (La Jolla, CA, US). In the human study, statistical significance between saline and ghrelin infusion day was tested at each time point (0, 30, 60, and 120 min) by two-way analysis of variance (ANOVA) for repeated measurements followed by Bonferroni multiple comparisons test. Significance between AUCs (0–120 min) from saline and ghrelin infusion days was tested by paired t test. For mouse intestine perfusions, significance was tested by one-way ANOVA for repeated measurements followed by Bonferroni multiple comparisons test, testing mean GLP-1 outputs (fmol/min) during acyl-ghrelin/GIP infusion (taken over entire stimulus administration period; 15 min) against each other or against preceding total baseline outputs (means of preceding 10/15 min outputs. For all tests, P < 0.05 was considered significant.
Results
Intravenous ghrelin infusion in hypopituitary patients
Plasma glucose.
The postabsorptive glucose levels were all in the normal range and did not change in response to either time or treatment (ghrelin vs. saline; P = 0.88–P > 0.99, n = 8) These data are a representation of previously published data (17).
Plasma acyl-ghrelin.
Baseline concentration of acyl-ghrelin was quantified in a subset of samples to control for appropriate infusion rate of acyl-ghrelin. At time 0 min (before start of acyl-ghrelin infusion), plasma concentrations were 50 ± 13 pg/mL on the acyl-ghrelin infusion day and 52 ± 21 pg/mL on the saline infusion day (P > 0.99, n = 3; data not shown). As expected, plasma acyl-ghrelin was markedly higher on the ghrelin infusion day compared to saline infusion day; at 60 minutes, 1015 ± 43 pg/mL versus 44.92 ± 17 (P = 0.0001), and at 120 minutes, 1066 ± 99 pg/mL versus 40.96 ± 15 (P < 0.0001; n = 3; data not shown). Acyl-ghrelin AUCs (0–120 min) were 94 ± 5.2 min × ng/mL and 6.0 ± 2.2 min × ng/mL on the ghrelin and saline infusion days, respectively (P < 0.0001; data not shown). These data are a representation of previously published data (17).
Total plasma GLP-1.
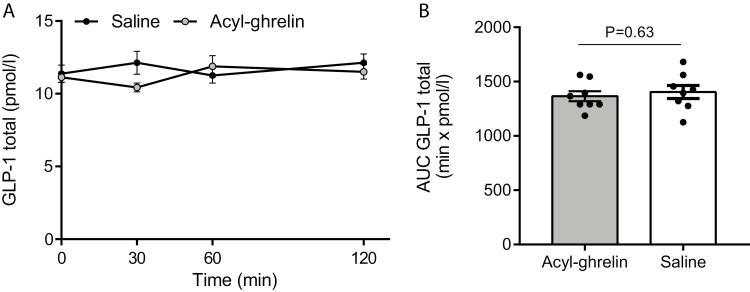
Baseline concentration was 11 ± 0.4 pmol/L on acyl-ghrelin infusion day and 11 ± 0.6 pmol/L at saline infusion day (P > 0.99, n = 8, Fig. 1A) and did not exceed 13 pmol/min at any time points and was not statistically different between treatments at any time points (P = 0.20–P > 0.99, n = 8, Fig. 1B). Total GLP-1 AUCs (1–120 min, mean ± SEM) were acyl-ghrelin = 1.4 ± 0.1 min × nmol, saline = 1.4 ± 0.1 min × nmol (P = 0.63, n = 8, Fig. 1B).
Figure 1.
Effects of intravenous ghrelin infusion on GLP-1 secretion. Plasma GLP-1 (total) concentrations are shown in response to intravenous acyl-ghrelin infusion (started after collection of 0 min sample; 1 pmol/min × kg; grey dots/bar) or saline infusion (black dots/white bar). Data are presented as means ± SEM. A: plasma GLP-1 total (pmol/l)and B: plasma GLP-1 total AUC (min × pmol/min). AUCs are from 0 to 120 min. n = 8 (A,B). Statistical significance was tested by paired t test. P-values **P < 0.01. P-values over 0.05 are indicated by respective pars. Individual values in B are indicated with dots. Plasma glucose and acyl-ghrelin data have been published previously as mentioned in the material and method section.
Effects of intra-arterial ghrelin infusion on GLP-1 output from isolated perfused mouse small intestine
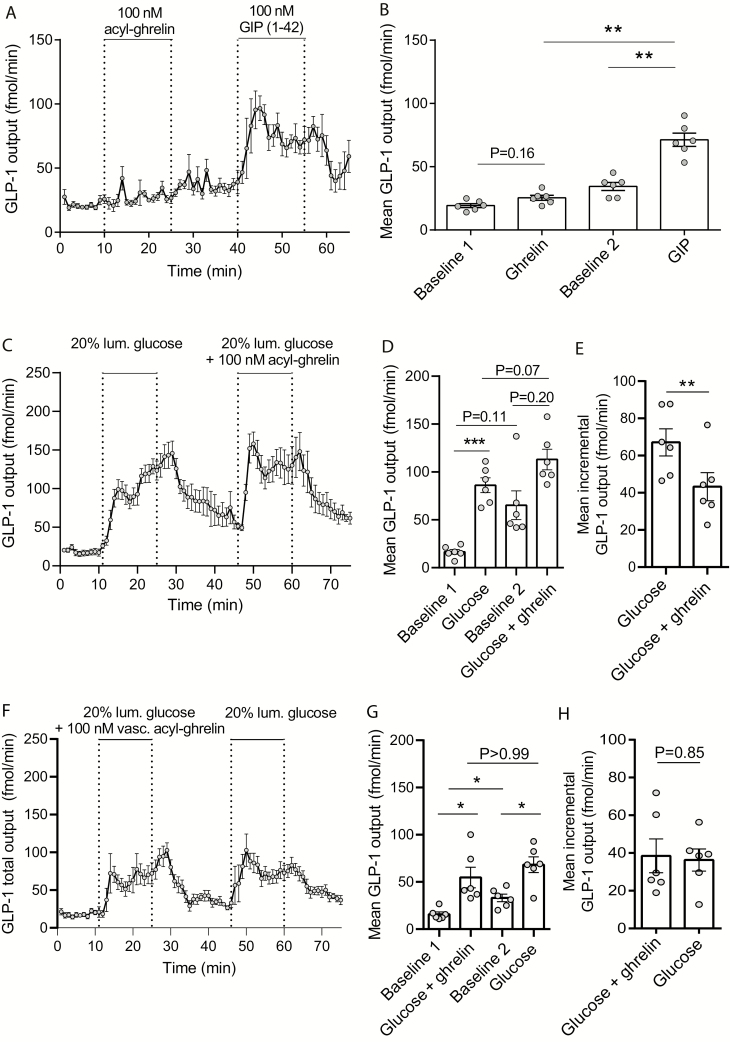
Intravascular acyl-ghrelin administration (100 nM) had no effects on GLP-1 secretion (preceding baseline output = 19 ± 1.6 fmol/min, output during acyl-ghrelin administration = 25 ± 2.0 fmol/min, P = 0.16, n = 6), whereas intravascular GIP administration at a matched dose (positive control) increased secretion by 2 fold (preceding mean baseline output = 34 ± 3.2 fmol/min, mean output under GIP infusion = 61 ± 5.3 fmol/min, P = 0.01; Fig. 2A and B). Consequently, mean output during GIP infusion was significantly higher than the mean output during ghrelin infusion (P < 0.001, n = 6; Fig. 2A and B).
Figure 2.
Effects of acyl-ghrelin and glucose-dependent insulinotropic polypeptide on GLP-1 secretion from isolated perfused mouse small intestine. A: GLP-1 output (fmol/min) in response to intravascular acyl-ghrelin or intra-vascular glucose-dependent insulinotropic peptide 1–42 (GIP; both 200 nM). B: Mean GLP-1 outputs (fmol/min) within ghrelin and GIP administration periods or at preceding time periods (baseline 1 and 2). C: GLP-1 output (fmol/min) in response to intraluminal glucose (20% w/v) administration with or without simultaneously intravascular acyl ghrelin infusion (100 nM). D: Mean GLP-1 outputs (fmol/min) at within respective stimulus periods or at time periods leading up to the stimulations (baseline 1 and 2). E: Mean incremental GLP-1 outputs (fmol/min) in response to luminal glucose or luminal glucose+ghrelin. F-H: as (C-E) but reversed order of glucose/glucose+ghrelin administration. In both experiments glucose concentration in the perfusion buffer was 3.5 mM. Statistical significance was tested by one-way analysis of variance for repeated measurements followed by Bonferroni multiple comparison test (B, D and G) or by paired t test (E and H). Data are presented as means ± SEM; dots in B, D, and E indicate values from different animals. Non-significant P-values are indicated above respective bars. **P < 0.05, **P < 0.01, ***P < 0.001. n (A-E) = 6.
As the reported potentiating effects of ghrelin on glucose-stimulated GLP-1 secretion could be mediated by gastrointestinal effects downstream of gastric emptying; for example, by direct interaction of ghrelin with the L-cell or increased intestinal motility and glucose absorption, we investigated in separate experiments whether ghrelin affected glucose-stimulated GLP-1 secretion from perfused mouse small intestine. Intraluminal glucose administration (20% w/v) increased secretion by a factor of 4 (mean outputs: baseline = 17 ± 2.5 fmol/min, during glucose administration = 86 ± 7.8 fmol/min, P < 0.001, n = 6, Fig. 2C, D), but co-stimulation with intravascular acyl-ghrelin (100 nmol/L) did not potentiate glucose-stimulated GLP-1 secretion (Fig. 2C and D). GLP-1 output did not return completely to preceding baseline levels after the first luminal glucose stimulation (17 ± 2.5 vs. 65 ± 15 fmol/min, P = 0.11). However, as this difference was only a trend, we based our comparison of secretory outputs during glucose and glucose+ghrelin administration on both unadjusted mean outputs and on baseline subtracted mean outputs. There was no significant difference in unadjusted mean outputs (P = 0.07) and the incremental outputs were 35% to 50% lower during glucose+ghrelin administration compared to administration of glucose alone (mean incremental outputs: glucose administration = 67 ± 7.3 fmol/min, glucose + ghrelin administration = 43 ± 7.5 fmol/min, P < 0.01) (Fig. 2E). In a separate line of experiments, we reversed the order of stimulations so glucose stimulations were first given together with acyl-ghrelin whereas the second glucose stimulation was without simultaneous ghrelin infusion. Again, baseline levels did not return completely to preceding baseline values after the first stimulation, but more important, co-stimulation with acyl-ghrelin did not potentiate glucose-stimulated GLP-1 secretion: expressed as unadjusted mean outputs the responses were (glucose + ghrelin administration = 55 ± 11 fmol/min, glucose administration = 68 ± 8.3 fmol/min, P > 0.99, n = 6) or as baseline adjusted mean outputs (glucose + ghrelin administration = 39 ± 9.0 fmol/min, glucose administration = 36 ± 5.9 fmol/min, P = 0.84, n = 6; Fig. 2E, F).
Human and mouse L-cell expression of the ghrelin receptor
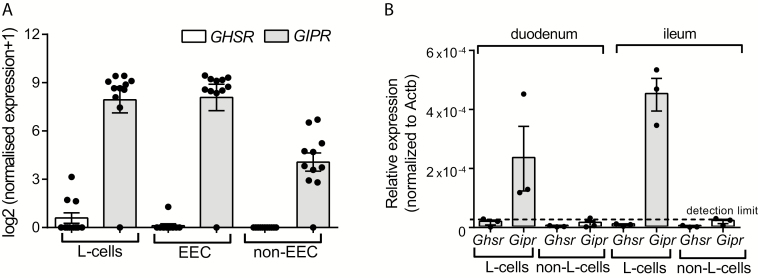
In human L-cells, GHSR expression was undetectable in L-cells from 8/11 donors and expression was marginal in L-cells from the 3 positive donors. GHSR expression was undetectable in non-L-cell enterendocrine cells in 10/11, and in all nonenterendocrine cells (presumably consisting of ~99% enterocytes). For comparison, we investigated GIPR expression. GIPR was highly expressed in 10/11 donors in both L-cells, non-L-cell enterendocrine cells and in nonenterendocrine cells (Fig. 3A). L-cells isolated from mice (based on proglucagon-directed expression of the fluorescent protein Venus) contained no detectable Ghsr, whereas Gipr was expressed at detectable levels and considerably enriched in L-cells compared to non-L-cells (Fig. 3B).
Figure 3.
Expression of growth hormone secretagogue receptor (ghrelin receptor) and glucose-dependent insulinotropic polypeptide receptor in L-cells. Expression is shown in A: human L-cells, enteroendocrine cells (EEC, not including L-cells) and nonenteroendocrine cells. Growth hormone secretagogue receptor (GHSR) expression is shown with white bars and glucose-dependent insulinotropic polypeptide receptor (GIPR) with grey bars. B: Ghsr and Gipr expression in L-cells and non-L-cells from mouse duodenum and ileum. Ghsr expression are shown with white bars; Gipr with grey bars. Data are presented as mean values ± SEM and are (A) log2 transformed normalized read counts and (B) – relative gene expression to β-actin (Actb) expression. Dots are values from cell populations sorted from different individuals/mice; a value of zero indicates lack of detection. Dotted line in (B) indicates detection limit. n (A) = 11, n (B) = 3.
Discussion
Ghrelin has been suggested to be a GLP-1 secretagogue based on one study in healthy young humans and a few studies in mice and in the GLP-1 secreting cell line GLUTag (10–12). However, the mechanism(s) whereby ghrelin potentiates glucose-stimulated GLP-1 secretion in the previously discussed experiments is not clear, and it is not obvious that endogenous ghrelin should act as a GLP-1 secretagogue under physiological circumstances since the plasma profiles of the 2 hormones show no clear relationship during fasting and are reciprocal after meal intake (13, 26–29). One possible mechanism for the potential stimulatory effect of ghrelin on GLP-1 secretion is that ghrelin interacts directly with the GLP-1 secreting L-cell by binding to the GHSR, which is predominately G-αq-linked (30, 31) and thus in turn would be expected to result in increased intracellular calcium concentrations and GLP-1 secretion, as is the case with, for example, the activation of the G-αq-linked bombesin receptor (32). Indeed, activation of different secretory pathways in the L-cell have been found to act synergistically on secretion (33), and since glucose-stimulated GLP-1 secretion is primarily driven by (sodium-glucose transporter-1-mediated) L-cell depolarization (34–38), simultaneous GHSR activation at the level of the L-cell would thus be expected to increase GLP-1 secretion. However, our RNA-sequencing-based expression analysis does not support this hypothesis, since GHSR expression was undetectable in L-cells from the mouse and negligible in human L-cells. Alternatively, the potentiating effects of systemic ghrelin administration after glucose intake found in the previously mentioned studies may be an effect of ghrelin-stimulated increase in gastric emptying and/or intestinal motility. Indeed, IV ghrelin infusions increases both parameters in humans (39–41), and nutrient-stimulated GLP-1 secretion clearly depends on intestinal nutrient delivery rate (42) and intestinal absorption (34, 35). In agreement with this, gastric bypass operated individuals show both exaggerated glucose absorption and glucose-stimulated GLP-1 secretion (43) with the absorption rate thought to drive secretion (44). However, unlike GLP-1, plasma ghrelin concentrations are decreased rather than increased following gastric bypass operation (both at fasting and postprandial) (45, 46), suggesting that ghrelin, at least in these individuals, does not regulate GLP-1 secretion. Moreover, as ghrelin secretion under physiological circumstances is inhibited by meal intake (29, 47), increasing plasma ghrelin concentrations by IV infusion during the active phase of glucose absorption is a rather artificial situation and may result in responses that do not occur under normal physiological circumstances.
We tested if ghrelin directly stimulates GLP-1 secretion in humans by IV infusion of a relatively high dose of acyl-ghrelin (1 pmol/min × kg) to fasted hypopituitary patients. The infusion resulted in a stable plasma concentration during the infusion of around 1000 pg/mL acyl-ghrelin and 350 pg/mL deacyl-ghrelin (17), which is slightly supra-physiological because total concentrations usually are 100 to 800 pg/mL (26, 29, 46). The infusion, however, resulted in an increased acyl:desacyl ratio compared to the physiological ratio, meaning that potential effects of the infusion could be attributable to a supra-physiological concentration of acyl-ghrelin. Hypopituitary patients were studied to minimize potential confounding effects of ghrelin on the secretion of ACTH and GH, which would have occurred since ghrelin is an endogenous ligand for the GH secretagogue receptor (16). However, at this infusion rate, there was no effect on plasma GLP-1 (total) concentrations, even though the same infusion induced peripheral insulin resistance (17). Of note, the ghrelin infusions employed in the human study showing an effect on postprandial GLP-1 secretion resulted in plasma concentrations of acyl-ghrelin of either 700 to 800 pg/mL or around 4000 pg/mL (infusion rates were either 0.26 or 2.0 µg/kg × h), and potentiating effects on postprandial GLP-1 secretion were only observed in response to the high infusion rate (12). Whether a higher infusion rate of acyl-ghrelin would have resulted in different results in our study is uncertain, but since our aim was to investigate whether ghrelin directly regulates GLP-1 secretion under physiological circumstances (at a physiological relevant plasma concentration) a higher infusion rate was considered irrelevant. Furthermore, the lack of GHSR expression by human L-cells seems to preclude a direct effect of ghrelin on the L-cell, even at supra-physiological concentrations. Besides the difference in acyl-ghrelin infusion rate, our discrepant results may relate to differences in the study design. Most notably, Tong and colleagues (12) studied effects of acyl-ghrelin on postprandial GLP-1 secretion while we studied effects on fasting GLP-1 secretion. Ghrelin is a well-known stimulator of gastric emptying and intestinal motility (39, 40), and since GLP-1 secretion is highly influenced by both of these, IV ghrelin infusion during the active phase of absorption will almost certainly give rise to increased GLP-1 secretion due to the effects on intestinal nutrient delivery rate. Supporting this notation, Tong et al. found no effects of acyl-ghrelin infusion on GLP-1 secretion following an IV glucose bolus during IV glucose tolerance test (12). To discriminate between direct versus indirect effects of ghrelin on GLP-1 secretion, we therefore studied the effects of IV ghrelin in the fasting state. The limitation of this approach is obviously that indirect effects of ghrelin on postprandial L-cell secretion are not detected, but given that ghrelin secretion is either unaltered or inhibited during the active phase of absorption, one may question the physiological relevance of such indirect effects. Ghrelin injections have also been shown to potentiate glucose-stimulated GLP-1 secretion in mice (10, 11) and to directly stimulate GLP-1 secretion from the GLP-1 producing murine cell line GLUTag (11). While the potentiating effects on glucose-stimulated secretion could be related to ghrelin’s effects on gastric emptying and intestinal motility as previously discussed, the effects on GLP-1 secretion from GLUTag cells are not easily explainable as we found Ghsr/GHSR to be undetectable in mouse L-cells and marginal in human L-cells The lack of GHSR expression by human L-cells, however, do not support that ghrelin should interact directly with the L-cell, even at supraphysiological ghrelin concentrations. Furthermore, some limitations apply to our study, which should be recognized. Most important, the number of participants was relatively small, consisted exclusively of males and their variation in BMI (26–42 kg/m2) and age (26–68 years) was relatively large. However, as our study was a cross-over design, potential interindividual differences would be expected to be present at both experiment days, ensuring that the lack of effects of ghrelin on GLP-1 secretion is not a result of differences in study populations. The use of hypopituitary patients may also be considered as a study limitation, although we primarily consider it a strength of our design since it minimizes the confounding effects of ACTH and GH secretion. We cannot, however, rule out that the difference between the Tong et al (12) study and our study may partly be related to that they conducted their study on subjects with normal pituitary function.
Little is known about the concentration of ghrelin in the submucosal plexus of the upper small intestine where ghrelin is also expressed in substantial amounts (23). Therefore, the concentration of ghrelin reached after IV infusion may theoretically have been insufficient to emulate the local ghrelin concentration within the upper small intestine during the active phase of secretion. To address this, we infused a supraphysiological concentration of ghrelin (100 nmol/l; 300–1000 fold higher than usual reference concentrations in human plasma (26, 48)) to the isolated perfused mouse intestine, which also failed to stimulate GLP-1 secretion. As a proof of the model, we infused GIP, a widely accepted stimulator of GLP-1 secretion (8, 9, 49), to the same preparations at the same dose, which resulted in a doubling of GLP-1 output. In separate perfusion experiments, we tested whether acyl-ghrelin potentiated glucose-stimulated GLP-1 secretion. Intraluminal glucose administration robustly stimulated GLP-1 secretion (3–4 fold) but simultaneous acyl-ghrelin infusion (100 nmol/L) had no effect on the glucose response, regardless of whether co-administered during the first or second glucose stimulation. Our combined data therefore clearly demonstrate that ghrelin is not a direct stimulator of GLP-1 secretion, which is supported by our GHS-R expression data.
Acknowledgments
Financial Support: REK was supported by a postdoctoral grant from Lundbeck foundation (Lundbeckfonden, R264-2017-3492) and by a grant from A.P. Møller Foundation (Lægefonden). SLJ was supported by a postdoctoral grant from the European Research Council (Grant no. 695069). Mouse perfusion studies were supported by an unrestricted grant to JJH from the Novo Nordisk Center for Basic Metabolic Research (Novo Nordisk Foundation, Denmark) and by a grant to JJH from the European Research Council (Grant no. 695069). The human study was supported by grants to JOLJ and ETV from the Danish Council for Independent Research (Medical Sciences) (11-105283) and grants from the Riisfort Fonden and the A.P. Moller Foundation. Expression studies on human and mouse L-cells were carried out in the laboratory of FMG and FR, supported by the Medical Research Council (Grant no. MRC_MC_UU_12012/3) and Wellcome Trust (grant no. 106262/Z/14/Z and 106263/Z/14/Z).
Clinical Trial Information: The study protocol was registered at Clinicaltrials.gov under the number: NCT01209416. Studies were approved by the local ethics committee system and by the Danish Medicines Agency (protocol nr. 2602-974) and conducted in accordance with the Helsinki Declaration. All subjects gave informed oral and written consent to participate.
Author Contributions: REK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. REK, SLJ, ETV, JJH, and JOLJ were involved in the conception and design of study. ETV and JLL wrote the protocol for the human study, conducted the study, and were responsible investigators for the study. REK and SLJ designed the mouse intestine perfusion study. SLJ performed the experiments and quantified GLP-1 in perfusion effluents. PL, FMG, and FR extracted, analyzed, and interpreted expression data in mouse and human L-cells and non-L-cells. REK drafted the manuscript. SLJ, ETV, PL, FR, FMG, JJH, and JLL critically revised it and provided intellectual content. All authors approved the final version of the manuscript.
Additional Information
The authors of this work declare no potential conflicts of interest relevant to this article.
Disclosure Summary: The authors of this work declare no potential conflicts of interest relevant to this article.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Baggio LL, Drucker DJ. Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2. Best Pract Res Clin Endocrinol Metab. 2004;18(4):531–554. [DOI] [PubMed] [Google Scholar]
- 2. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. [DOI] [PubMed] [Google Scholar]
- 3. Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68(3):525–530. [DOI] [PubMed] [Google Scholar]
- 4. Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492–498. [DOI] [PubMed] [Google Scholar]
- 5. Wilding JPH. Medication use for the treatment of diabetes in obese individuals. Diabetologia. 2018;61(2):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albrechtsen NJW, Kuhre RE, Deacon CF, Holst JJ. Targeting the intestinal L-cell for obesity and type 2 diabetes treatment. Expert Rev Endocrinol Metab. 2014;9(1):61–72. [DOI] [PubMed] [Google Scholar]
- 7. Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–299. [DOI] [PubMed] [Google Scholar]
- 8. Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133(1):233–240. [DOI] [PubMed] [Google Scholar]
- 9. Hansen L, Holst JJ. The effects of duodenal peptides on glucagon-like peptide-1 secretion from the ileum. A duodeno–ileal loop? Regul Pept. 2002;110(1):39–45. [DOI] [PubMed] [Google Scholar]
- 10. Lindqvist A, Shcherbina L, Fischer AT, Wierup N. Ghrelin is a regulator of glucagon-like peptide 1 secretion and transcription in mice. Front Endocrinol (Lausanne). 2017;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. Ghrelin is a novel regulator of GLP-1 secretion. Diabetes. 2015;64(5):1513–1521. [DOI] [PubMed] [Google Scholar]
- 12. Tong J, Davis HW, Gastaldelli A, D’Alessio D. Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. J Clin Endocrinol Metab. 2016;101(6):2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Djurhuus CB, Hansen TK, Gravholt C, Ørskov L, Hosoda H, Kangawa K, Jørgensen JO, Holst JJ, Schmitz O. Circulating levels of ghrelin and GLP-1 are inversely related during glucose ingestion. Horm Metab Res. 2002;34(7):411–413. [DOI] [PubMed] [Google Scholar]
- 14. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4(6):437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. [DOI] [PubMed] [Google Scholar]
- 16. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 17. Vestergaard ET, Jessen N, Møller N, Jørgensen JO. Acyl ghrelin induces insulin resistance independently of GH, cortisol, and free fatty acids. Sci Rep. 2017;7:42706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vestergaard ET, Buhl M, Gjedsted J, Madsen M, Jessen N, Nielsen S, Gaylinn BD, Liu J, Thorner MO, Moller N, Jorgensen JO. Acute peripheral metabolic effects of intraarterial ghrelin infusion in healthy young men. J Clin Endocrinol Metab. 2011;96(2):468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuhre RE, Holst JJ. Mechanisms underlying gut hormone secretion using the isolated perfused rat small intestine. JoVE. 2019;(144):e58533. [DOI] [PubMed] [Google Scholar]
- 20. Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43(4):535–539. [DOI] [PubMed] [Google Scholar]
- 21. Kuhre RE, Albrechtsen NW, Windeløv JA, Svendsen B, Hartmann B, Holst JJ. GLP-1 amidation efficiency along the length of the intestine in mice, rats and pigs and in GLP-1 secreting cell lines. Peptides. 2014;55:52–57. [DOI] [PubMed] [Google Scholar]
- 22. Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Toräng S, Rehfeld JF, Poulsen SS, Holst JJ. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156(3):847–857. [DOI] [PubMed] [Google Scholar]
- 23. Roberts GP, Larraufie P, Richards P, Kay RG, Galvin SG, Miedzybrodzka EL, Leiter A, Li HJ, Glass LL, Ma MKL, Lam B, Yeo GSH, Scharfmann R, Chiarugi D, Hardwick RH, Reimann F, Gribble FM. Comparison of human and murine enteroendocrine cells by transcriptomic and peptidomic profiling. Diabetes. 2019;68(5):1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol. 2005;152(6):845–850. [DOI] [PubMed] [Google Scholar]
- 27. Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31(7):665–670. [DOI] [PubMed] [Google Scholar]
- 28. Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88(6):2706–2713. [DOI] [PubMed] [Google Scholar]
- 29. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. [DOI] [PubMed] [Google Scholar]
- 30. Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor–identification of a potent inverse agonist. Mol Endocrinol. 2003;17(11):2201–2210. [DOI] [PubMed] [Google Scholar]
- 31. Smith RG, Leonard R, Bailey AR, Palyha O, Feighner S, Tan C, Mckee KK, Pong SS, Griffin P, Howard A. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14(1):9–14. [DOI] [PubMed] [Google Scholar]
- 32. Svendsen B, Pais R, Engelstoft MS, Milev NB, Richards P, Christiansen CB, Egerod KL, Jensen SM, Habib AM, Gribble FM, Schwartz TW, Reimann F, Holst JJ. GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J Endocrinol. 2016;228(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hauge M, Ekberg JP, Engelstoft MS, Timshel P, Madsen AN, Schwartz TW. Gq and Gs signaling acting in synergy to control GLP-1 secretion. Mol Cell Endocrinol. 2017;449:64–73. [DOI] [PubMed] [Google Scholar]
- 34. Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61(1):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuhre RE, Frost CR, Svendsen B, Holst JJ. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64(2):370–382. [DOI] [PubMed] [Google Scholar]
- 36. Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. Plos One. 2014;9(2):e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55(9):2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun EW, de Fontgalland D, Rabbitt P, Hollington P, Sposato L, Due SL, Wattchow DA, Rayner CK, Deane AM, Young RL, Keating DJ. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes. 2017;66(8):2144–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ejskjaer N, Vestergaard ET, Hellström PM, Gormsen LC, Madsbad S, Madsen JL, Jensen TA, Pezzullo JC, Christiansen JS, Shaughnessy L, Kosutic G. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29(11):1179–1187. [DOI] [PubMed] [Google Scholar]
- 40. Levin F, Edholm T, Schmidt PT, Grybäck P, Jacobsson H, Degerblad M, Höybye C, Holst JJ, Rehfeld JF, Hellström PM, Näslund E. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–3302. [DOI] [PubMed] [Google Scholar]
- 41. Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55(3):327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holst JJ, Gribble F, Horowitz M, Rayner CK. Roles of the gut in glucose homeostasis. Diabetes Care. 2016;39(6):884–892. [DOI] [PubMed] [Google Scholar]
- 43. Jacobsen SH, Bojsen-Møller KN, Dirksen C, Jørgensen NB, Clausen TR, Wulff BS, Kristiansen VB, Worm D, Hansen DL, Holst JJ, van Hall G, Madsbad S. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56(10):2250–2254. [DOI] [PubMed] [Google Scholar]
- 44. Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, Naver LS, Worm D, Holst JJ, Madsbad S, Hansen DL, Madsen JL. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346–e255. [DOI] [PubMed] [Google Scholar]
- 45. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–1630. [DOI] [PubMed] [Google Scholar]
- 46. Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu JL, Ramshaw BJ, Papanicolaou DA, Ziegler TR, Smith CD. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139(7):780–784. [DOI] [PubMed] [Google Scholar]
- 47. Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24(6):RC19–RC21. [DOI] [PubMed] [Google Scholar]
- 48. Rigamonti AE, Agosti F, De Col A, Marazzi N, Lafortuna CL, Cella SG, Muller EE, Sartorio A. Changes in plasma levels of ghrelin, leptin, and other hormonal and metabolic parameters following standardized breakfast, lunch, and physical exercise before and after a multidisciplinary weight-reduction intervention in obese adolescents. J Endocrinol Invest. 2010;33(9):633–639. [DOI] [PubMed] [Google Scholar]
- 49. Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140(4):1687–1694. [DOI] [PubMed] [Google Scholar]