Summary
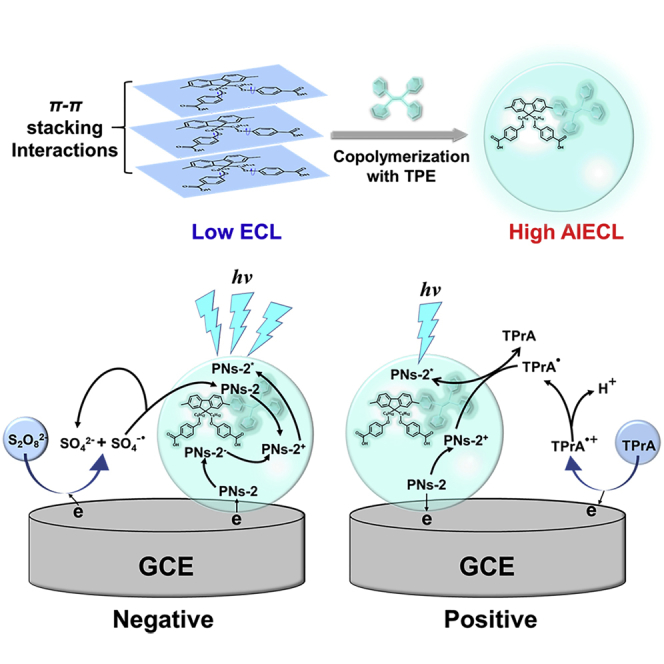
The aggregation-induced electrochemiluminescence (AIECL) of polyfluorene derivative nanoparticles containing tetraphenylethylene (TPE) in aqueous media is reported in this work. The TPE unit limits the intramolecular free rotation of phenyl rings, as well as the π-π stacking interactions of molecules, which significantly enhances ECL signal of the polyfluorene nanoparticles. With co-reactants of tri-n-propylamine (TPrA) and S2O82−, the copolymer nanoparticles show visualized ECL emissions at both positive and negative potentials. The ECL efficiency of copolymer nanoparticles in solid state is 163% compared with that of standard ECL species, Ru(bpy)32+. And at negative potential, the ECL intensity of copolymer nanoparticles is even stronger with 6.5 times compared with that at positive potential. The ECL generation mechanisms are analyzed detailed by annihilation and co-reactant route transient ECL test (millisecond scale). This work provides a reference for the organic structure design for AIECL and shows promising potential in luminescent device and biological applications.
Subject Areas: Polymer Chemistry, Nanoparticles, Molecular Electrochemistry
Graphical Abstract
Highlights
-
•
AIECL of polyfluorene nanoparticles containing tetraphenylethylene is reported
-
•
TPE unit limits the stacking interactions of the molecular to enhance the ECL intensity
-
•
The ECL efficiency of copolymer nanoparticles is higher than that of Ru(bpy)32+
-
•
Annihilation and co-reactant transient tests clarified the ECL generation mechanisms
Polymer Chemistry; Nanoparticles; Molecular Electrochemistry
Introduction
A novel concept of aggregation-induced electrochemiluminescence (AIECL) was officially proposed by De Cola's team in 2017, which indicated aggregation-induced emission (AIE) was also present in ECL system and achieved significant ECL signal amplification from platinum (II) complex (Carrara et al., 2017). In this system, the changed HOMO and LUMO energies by self-assembly led to the generation of the ECL excited state. This discovery has opened up a new field of ECL research and enkindled research enthusiasm for seeking luminescent molecules with AIECL properties. Recently, iridium-based redox polymers have been reported with unusually intense luminescence under the aggregate state, which were significantly greater than the analogous ruthenium-based materials (Carrara et al., 2018, Gao et al., 2018). Our group first reported that the carborane structure could enhance the ECL signal of carbazole molecule and established the relation between AIE organic structures and ECL properties (Wei et al., 2019a, Wei et al., 2019b). So far, research on AIECL is still in its infancy, focusing on the discovery of new molecules with AIECL properties (Han et al., 2019, Wei et al., 2019a, Wei et al., 2019b, Jiang et al., 2019a, Jiang et al., 2019b). It is urgent to develop the ultra-efficient biocompatible AIECL luminophores with strong potential for biological applications (Valenti et al., 2017, Voci et al., 2018, Zhang et al., 2019).
Polyfluorene is a promising semiconductor π-conjugated polymer with high photoluminescence (PL) efficiency and good chemical stability (Scherf and List, 2002, Knaapila and Monkman, 2013, O'Carroll et al., 2008). Meanwhile the effective overlap of π orbitals of fluorene provides electronic coupling to promote electron and hole transfer (Josh et al., 2010, Qi et al., 2012, Voityuk, 2010), indicating that it is also a potential ECL emitter with the high efficiency. The electrochemistry and ECL properties of polyfluorene and its derivatives in organic solution have been reported in previous studies, and further works have been made to prepare aqueous phase dispersed polyfluorene nanoparticles (PNs) (Wong et al., 2002, Honmou et al., 2014, Chang et al., 2008). However, to our knowledge, the ECL efficiencies of all PNs reported were much lower than ruthenium and iridium complexes, mainly due to the aggregation quenching caused by the π-π stacking interactions of molecules (Omer et al., 2011, Suk et al., 2011a, Suk et al., 2011b), which seriously limited the wide application of PNs in aqueous phase ECL systems (Guo et al., 2018, Deng and Ju, 2013).
Here, we synthesized the polyfluorene derivative by copolymerization of fluorene with tetraphenylethylene (TPE), and the prepared copolymer nanoparticles exhibited strong aggregation-induced ECL in aqueous solution. The introduction of TPE suppressed the intermolecular interactions and limited the intramolecular free rotation of phenyl rings (Zhao et al., 2011, Zhao et al., 2012), which solved the problem of aggregation quenching and provided much higher ECL efficiency than that of Ru(bpy)32+. More crucially, the novel PNs could stably generate high-strength ECL signals at both positive and negative potentials with proper co-reactants. And the mechanism of luminescence was explained by transient ECL test. The new idea of improving ECL efficiency by linking organic molecules with special unit (TPE) has been proposed for the first time, and its universality could be used to improve the ECL efficiency of organic nanoparticles.
Results and Discussion
Synthesis
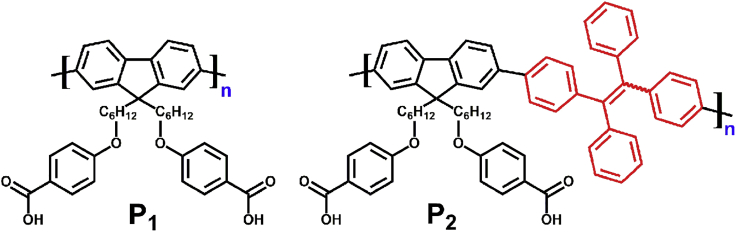
Scheme 1 showed the structure of polyfluorene derivative containing TPE (P2) and its control molecule, P1. The synthesis routes were detailed in Scheme S1, and the NMR characterization of all molecules and corresponding monomers were given in Figures S1–S6. Briefly, the compounds were synthesized through Suzuki coupling polymerization using Pd as the catalyst. The yields for P1 and P2 were 75% and 60%, respectively. The terminal carboxyl groups provide sites for the future bio-orthogonal binding. Furthermore, the corresponding organic nanoparticles PNs-1 and PNs-2 were prepared in aqueous solution using the reprecipitation method (Feng et al., 2016, Suk et al., 2011a, Suk et al., 2011b).
Scheme 1.
Chemical Structures of Researched Molecule P2 and Control Molecule P1
See also Scheme S1 and Figures S1–S6.
Spectroscopic Characterization of Polymers and PNs
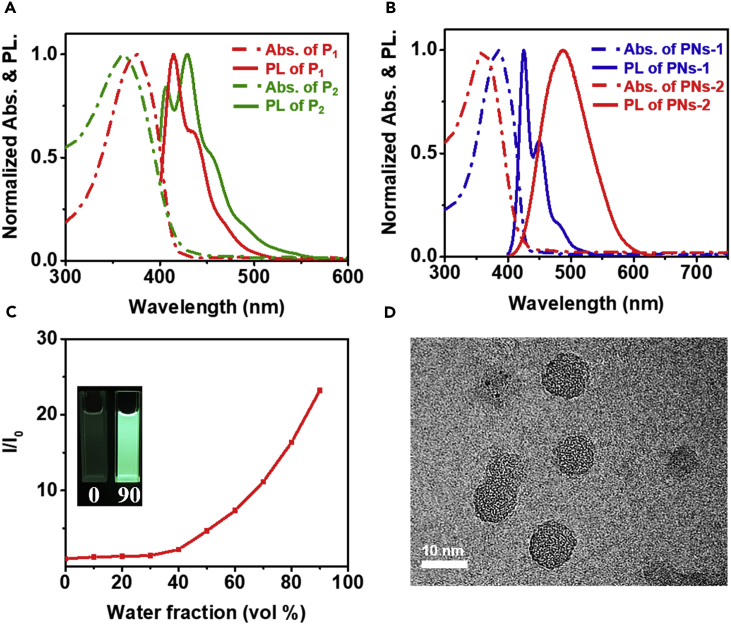
The optical properties of P1, P2, and the corresponding organic nanoparticles were systematically characterized and shown in Figures 1A and 1B. For UV−vis absorption spectra, the absorption peaks at 376 and 362 nm belonging to P1 and P2, respectively, which could be attributed to π−π∗ transition of the entire conjugated skeleton. The PL spectra of P1 showed the emission maxima at 414 nm, whereas the PL spectra of P2 showed the emission peak at 429 nm and a shoulder at 455 nm. For the optical properties of PNs-1 and PNs-2, it was noteworthy that the Stokes shift increasing from 4,314.3 cm−1 (P2) to 7,090.4 cm−1 (PNs-2), but it barely showed obvious change between those of P1 and PNs-1. The larger Stokes shift could be ascribed to the conformational change (Omer et al., 2011). In addition, the PL lifetime of PNs-2 was longer than that of P2 (Figure S7). Details of all the optical properties were shown in Table 1.
Figure 1.
The Spectroscopic and Orphological Characterizations of the Polymers and PNs
(A) Absorbance (dashed curve) and PL spectra (solid curve) of P1 (red) and P2 (green) in a solution of MeCN:benzene = 1:1. λexc = 350 nm.
(B) Absorbance (dashed curve) and PL spectra (solid curve) of PNs-1 (blue) and PNs-2 (red) in aqueous solution. λexc = 350 nm.
(C) AIE effect of P2. Plots of I/I0 vs water fractions in THF/water mixtures (10 μM), where I0 and I are the PL intensities in pure THF and THF/water mixtures, respectively. Inset: Photographs of P2 in THF/water mixtures (0, 90%) taken under 365 nm UV lamp.
(D) High-resolution TEM image of 9 nm PNs-2. Scale bar, 10 nm. Data are represented as mean ± TEM.
See also Figures S7–S12.
Table 1.
Photophysical Properties of P1 and P2 in Organic Solvent and PNs-1 and PNs-2 in Aqueous Solution
| λabs (nm) | λPL (nm)a | Stoke's Shifts Δλst (cm−1) | Absolute QYPLb (%) | τPL (ns) | |
|---|---|---|---|---|---|
| P1 | 376 | 414, 434(sh) | 2441.2 | 81.8 | 0.92 |
| P2 | 362 | 407, 429, 455(sh) | 4314.3 | 3.5 | 1.08 |
| PNs-1 | 384 | 425, 450(sh), 481 | 2512.3 | 21.2 | 0.81 |
| PNs-2 | 362 | 487 | 7090.4 | 45 | 1.84 |
λexc = 350 nm.
Measured by integrated sphere in dichloromethane; sh: shoulder.
AIE Effect
The difference of fluorescence performance exhibited by P1 and P2 in aggregate state was examined. The PL spectra of P1 and P2 in tetrahydrofuran (THF)/water mixtures with different water fractions were shown in Figure S8. With increase water fraction, the PL intensity of P2 increased remarkably (Figure 1C). I0 and I are the PL intensities of P2 dissolved in pure THF and THF/water mixtures, respectively. It was worth noting that when the water faction was 90%, the PL intensity of P2 was 23.2 times that of pure organic phase, indicating an AIE property (Hong et al., 2009, Hu et al., 2014, Liang et al., 2015). On the contrary, P1 showed aggregation caused quenching (ACQ) effect (Figure S9). The absolute fluorescence quantum efficiencies (QYPL) also proved the ACQ and AIE properties of P1 and P2 (Figure S10 and Table 1). It means that in aggregate state, π-π stacking interactions of polyfluorene molecule normally cause ACQ. However, TPE unit could prevent this from happening. In addition, intramolecular rotation is restricted in aggregate state, which blocks the nonradiative pathway and enhances the radiative decay (Zhao et al., 2011, Zhao et al., 2012). Figures S11A and 1D showed the HRTEM images of PNs-1 and PNs-2. The morphologies of both PNs are similar with the average diameter of about 9 nm. The dynamic light scattering (DLS) in Figure S12 revealed the similar hydrodynamic diameter of PNs-1 (10.13 nm) and PNs-2 (10.82 nm). And PNs-2 is negatively charged with the zeta potential of −33.1 mV, which is attributed to the carboxyl functional group.
Electrochemistry
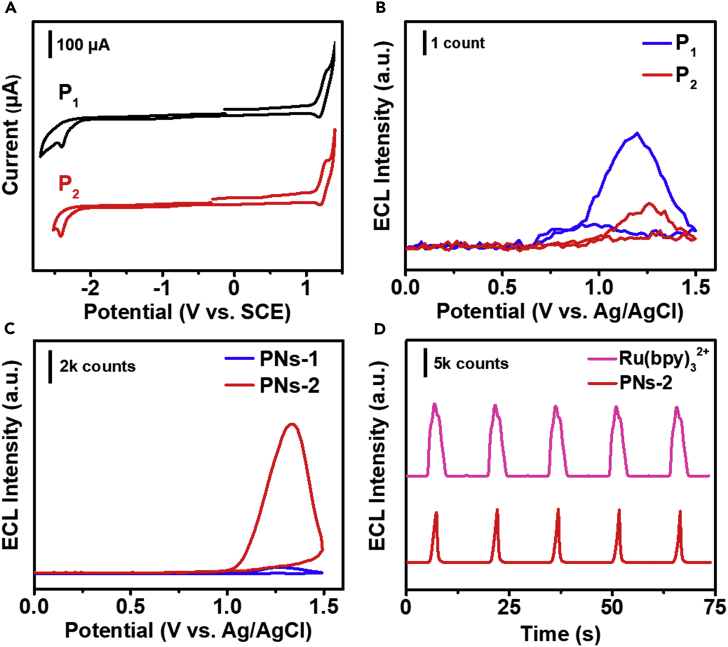
The electrochemical and ECL performances of P1, P2, and corresponding organic nanoparticles were investigated. Figure 2A displayed the cyclic voltammograms (CVs) of P1 and P2 in acetonitrile and benzene-mixed solution containing 0.1 M TBAPF6 as the supporting electrolyte. At the scan rate of 0.1 V/s, both P1 and P2 showed a pair of well-defined quasi-reversible redox waves, with the similar formal potentials at +1.25 V and +1.27 V vs. SCE, respectively, which could be ascribed to the P/P+ redox reactions. Upon scanning toward the negative direction, CVs showed irreversible reduction peaks at −2.36 V (P1) and −2.38 V (P2) vs. SCE, which belong to the reduction of fluorene and suggested that the anionic radicals were less stable than the cationic radicals (Prieto et al., 2001).
Figure 2.
The Electrochemical and ECL Performances of the Polymers and PNs
(A) CVs of 0.5 mM P1 and P2 in Bz:MeCN (v:v = 1:1) containing 0.1 M TBAPF6 as the supporting electrolyte with scan rate of 0.1 V/s.
(B) ECL intensity-potential profiles of 20 μM P1 and P2 in THF containing 0.1 M TBAPF6 as supporting electrolyte, upon addition of 75 mM TPrA as co-reactant.
(C) ECL intensity-potential profiles of 20 μM PNs-1 and PNs-2 in water containing 0.1 M LiClO4 as the supporting electrolyte, upon addition of 75 mM TPrA as co-reactant.
(D) ECL intensity-time curves of 20 μM PNs-2 and Ru(bpy)32+ in water containing 0.1 M LiClO4 as the supporting electrolyte with 75 mM TPrA as the co-reactant. PMT = 500 V.
See also Figures S13 and S14.
AIECL Effect
In order to verify the AIECL effect of polyfluorene derivative containing TPE, we measured ECL intensity of P1 and P2 dissolved in organic solvent and the corresponding PNs dispersed in aqueous solution, using tri-n-propylamine (TPrA) as the co-reactant. As shown in Figure 2B, ECL intensities of P1 and P2 were extremely low, whereas the ECL intensity of P1 was slightly higher than P2. We examined the ECL intensities of P1 and P2 under the different water fractions. As shown in Figure S13, the ECL intensity of P2 increased dramatically along with the increased water fraction, which was significantly different from that of P1. After they were prepared into PNs, the ECL intensity of PNs-2 increased dramatically, which was 24 times that of PNs-1, as shown in Figure 2C. Moreover, the ECL intensity of PNs-2 was 4,000 times that of P2 dissolved in organic solvent. The highly efficient ECL was attributed to the limitation of intramolecular rotation and π-π stacking interactions caused by TPE unit (Jiang et al., 2019a, Jiang et al., 2019b, Liu et al., 2019a, Liu et al., 2019b). Therefore, the most commonly used Ru(bpy)32+/TPrA system was selected as the standard for evaluating ECL performance of PNs-2 in aqueous solution. The ECL intensity-time curves for the same concentration of Ru(bpy)32+ and PNs-2 were shown in Figure 2D. The ECL intensity of PNs-2 was slightly lower but quite stable compared with Ru(bpy)32+ under the condition of 75 mM TPrA as a co-reactant. The effect of TPrA concentration was also investigated and shown in Figure S14. It is worth noting that when the concentration of TPrA decreased below 50 mM, the ECL intensity of PNs-2 began to exceed Ru(bpy)32+. And the spectral response of the PMT should be considered when the relative ECL efficiency of PNs-2 compared with Ru(bpy)32+ was calculated. The cathode radiant sensitivity and quantum efficiency vs. wavelength of the PMT applied in this work were shown in Figure S15. At the maximum emission wavelengths of PNs-2 (λmax = 481 nm) and Ru(bpy)32+ (λmax = 620 nm), the quantum efficiencies of the PMT are very close. Therefore, the ECL intensities of PNs-2 and Ru(bpy)32+ were not further corrected. This result indicates that PNs-2 dispersed in aqueous solution is definitely a potential high-quality ECL luminophore. Moreover, it also proves that it is quite an effective method to link organic molecules with TPE unit to improve the ECL intensity of organic nanoparticles.
Annihilation ECL
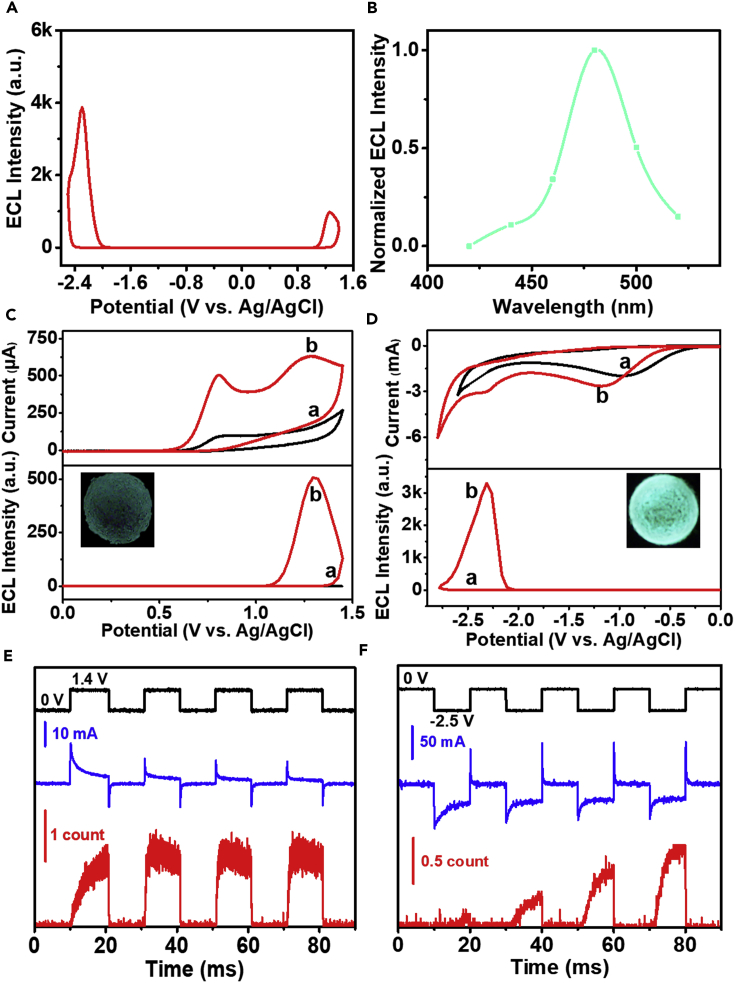
In order to clarify the ECL generation mechanism of PNs-2, the particles were modified on the surface of glassy carbon electrode (GCE) and first performed annihilation ECL experiment without co-reactant (Yang et al., 2019). As shown in Figure 3A, when the applied electrode potential was cyclically scanned between +1.4 V and −2.5 V vs. Ag/AgCl, PNs-2 could produce annihilation ECL signals at both positive and negative potentials of +1.25 V and −2.3 V, respectively. Further, the annihilation ECL signal intensity at −2.3 V was significantly higher than that at +1.25 V. And the transient ECL profile was captured using homemade setup by stepping the potential from −2.5 V to +1.4 V vs. Ag/AgCl with the pulse width of 10 ms. As shown in Figure S16, the ECL signal was not obtained when the potential turned from −2.5 V to +1.4 V but showed on the reversal step. In the classic self-annihilation pathway, the excited state PNs-2∗ was produced by PNs-2+ and PNs-2− generated at oxidation and reduction potentials, respectively (Richter, 2004, Miao, 2008). Therefore, one conclusion could be drawn that the PNs-2− obtained by the reduction step was quite unstable and might decompose slowly (Prieto et al., 2001), which become unavailable to maintain the anodic ECL signal. On the other hand, it could be determined that PNs-2+ was relatively stable. And it was a reasonable explaination for the much higher ECL intensity at −2.3 V than that at +1.25 V. Figures S11B and 3B revealed the ECL spectra of PNs-1 and PNs-2 obtained by the annihilation routes. The ECL peaks centered at 427 nm (PNs-1) and 481 nm (PNs-2), respectively, which were similar to those of the fluorescence spectra, indicating that the excited state of PNs were caused by bandgap transition (Liu et al., 2013).
Figure 3.
The Annihilation and Co-reactant Route ECL Test of PNs-2
(A) Annihilation ECL intensity-potential curve of PNs-2 modified on GCE surface in water with 0.1 M LiClO4 as supporting electrolyte. Scan rate = 0.10 V s−1. PMT = 500 V.
(B–F) (B) Normalized ECL spectrum of PNs-2 generated through annihilation route by pulsing potential from approximately 100 mV past the peak potentials. CV and ECL intensity-potential profiles of (a) bare GCE and (b) PNs-2 modified GCE in 0.10 M PBS containing (C) 75 mM TPrA and (D) 100 mM K2S2O8 as the co-reactant. PMT = 200 V. Inset: ECL photographs of PNs-2 fixed on GCE surface. Transient profiles of current (blue), ECL (red), and applied voltage (black) of PNs-2 modified GCE in 0.10 M PBS containing (E) 75 mM TPrA and (F) 100 mM K2S2O8 as co-reactant. The pulse width was 10 ms. PMT = 900 V (E) and 600 V (F).
See also Figures S15–19.
Co-reactant ECL
Relative to the annihilation reaction, PNs-2 could produce stronger ECL signals in the presence of co-reactants. The ECL generation mechanisms of PNs-2 with TPrA and K2S2O8 as the co-reactants at oxidative and reductive potentials were researched in detail. As shown in Figure 3C, both bare GCE and PNs-2 modified GCE had an irreversible oxidation peak at +0.80 V vs. Ag/AgCl, which was attributed to the oxidation of TPrA to produce TPrA⋅+ and then underwent a rapid deprotonation process to form TPrA⋅ (Miao et al., 2002, Sentic et al., 2014). As the potential scan increased, another new oxidation peak appeared at +1.29 V, which belonged to the oxidation of PNs-2 to produce PNs-2+. Meanwhile, strong ECL emission peaked at the same electrode potential. This meant that excited state PNs-2∗ was generated from the reaction between PNs-2+ and TPrA⋅. Then the excited state relaxed back to ground state with light radiation. Equations 1, 2, 3, 4, and 5 described the reaction routes using TPrA as the co-reactant.
| TPrA – e → TPrA⋅+ | (Equation 1) |
| TPrA⋅+ → TPrA⋅ + H+ | (Equation 2) |
| PNs-2 – e → PNs-2+ | (Equation 3) |
| PNs-2+ + TPrA⋅ → PNs-2∗ + Pr2N+ = CHCH2CH3 | (Equation 4) |
| PNs-2∗ → PNs-2 + hv | (Equation 5) |
The ECL photograph of PNs-2 modified GCE was taken by camera (inset of Figure 3C). The strong cyan anode ECL emission could even be observed directly with the naked eyes. More importantly, it was easily distinguishable with Ru(bpy)32+ by wavelength and could be used in combination in cell imaging and immunoassays (Li et al., 2019). The transient oxidative-process-initiated ECL signal was captured by stepping the potential from 0 V to +1.4 V vs. Ag/AgCl with the pulse width of 10 ms (Figure 3E). The first and last 10 ms data provided a baseline, as the applied voltage was zero and no electrode reaction occurred. Approximately equal ECL signals were observed at each pulse period, indicating that PNs-2 could stably emit over this timescale. The transient ECL signal was obtained immediately when the potential was stepped to +1.4 V, indicating the rapid homogeneous electron transfers rates among the radical ions (Miao et al., 2002). Obviously, as PNs-2 was immobilized on GCE surface, the AIECL effect in solid state greatly enhanced. And the ECL intensity of PNs-2 was 4.5 times that of Ru(bpy)32+ modified electrode at the same concentration (Figure S17B). Furthermore, the ECL efficiencies were calculated and compared with those of Ru(bpy)32+ modified electrode with different concentrations of TPrA (Hesari et al., 2014); the corresponding results were shown in Table S1. It is worth noting that the high relative ECL efficiency of PNs-2 was 163% with 75 mM TPrA. When the concentration of TPrA was reduced to 25 mM, its ECL efficiency was almost three times that of Ru(bpy)32+. Hence, it could be confirmed that PNs-2 with outstanding ECL efficiency in the positive region has the potential to replace Ru(bpy)32+ as the best anodic ECL material in aqueous media.
In addition, PNs-2 could also react with K2S2O8 to produce a stronger cathodic ECL signal, which deserved our attention. As shown in Figure 3D, the reduction peak of bare GCE at −0.95 V vs. Ag/AgCl was attributed to the reduction of K2S2O8 to generate strong oxidant intermediate SO4-⋅ (Liu et al., 2017). On PNs-2 modified GCE, it was slightly moved to −1.2 V and the reduction current in CV curve became larger. This phenomenon was caused by the reaction between immediately generated strong oxidant intermediate SO4⋅ and PNs-2 to produce positively charged PNs-2+. Another new reduction peak appeared at −2.3 V belonged to the reduction of PNs-2 to produce PNs-2−. Meanwhile, the strong cathodic ECL emission peaked at the same potential. There are two possible generation mechanisms of the cathodic ECL. First, SO4-⋅ may directly react with PNs-2− to produce the excite state of PNs-2∗ (Ding et al., 2002, Zhang et al., 2015). Second, SO4-⋅ could firstly oxidize PNs-2 to produce PNs-2+, and then the excited state PNs-2∗ was produced by the annihilation reaction between PNs-2+ and PNs-2− (Tan et al., 2017). To figure out the real ECL generation mechanism, transient reductive-process-initiated ECL profile was captured by stepping the potential from 0 V to −2.5 V vs. Ag/Agcl with the pulse width of 10 ms. Similarly, the first and last 10 ms data provided the baseline, as no electrode reaction occurred. As shown in Figure 3F, almost no significant ECL signal was observed when the potential was turned to −2.5 V at the first step. Interestingly, the transient ECL intensity continuously increased during the following steps. As previously mentioned, PNs-2+ was a quite stable species, but PNs-2− was clearly not. Considering the fact that the transient ECL signal raised continuously rather than gradually decreased during the cycles, it could be confirmed that the ECL generation mechanism followed the second route. And Equations 6, 7, 8, 9, and 10 described the reaction route between PNs-2 and K2S2O8.
| S2O82− + e → SO42− + SO4−⋅ | (Equation 6) |
| PNs-2 + SO4−⋅ → PNs-2+ + SO42− | (Equation 7) |
| PNs-2 + e → PNs-2− | (Equation 8) |
| PNs-2+ + PNs-2− → PNs-2∗ | (Equation 9) |
| PNs-2∗ → PNs-2 + hv | (Equation 10) |
This is because the cationic radicals generated in each cycle were sufficiently stable to accumulate and resulted in increasing the ECL signal intensity. On the other hand, if the ECL mechanism followed the first route, there would not be continuously enhanced responses. More critically, the reductive initiated ECL signal of PNs-2 was significantly stronger than that of the oxidative progress. It was approximately 6.5 times higher under the optimal experiment conditions (Figure S18). As shown in the inset of Figure 3D, the cathode ECL emission was also bright cyan. As far as we know, this is the first report of water-soluble nanoparticles modified on GCE surface with ultra-high ECL intensity and efficiency at both positive and negative potentials and showed excellent stability (Figure S19).
Conclusion
In summary, we successfully synthesized tetraphenylethylene-containing polyfluorene nanoparticles, which exhibited strong AIECL in aqueous solution. The TPE units limit the stacking interactions of molecules, thus enhancing the ECL signal of nanoparticles. And this TPE copolymerization method perfectly solved the problem of easy quenched and low ECL efficiency of the organic nanoparticles under the aqueous condition. With the suitable co-reactants (TPrA and S2O82−), the copolymer nanoparticles showed visualized ECL emission at both positive and negative potentials. The ECL efficiency is far more than traditional Ru(bpy)32+ and showed excellent stability. The transient oxidative and reductive initiated ECL profiles were recorded to clarify the mechanism of ECL reactions. This work provides a reference for molecular design of subsequent aqueous-phase ECL emitters and shows promising potential in the development of luminescent device and research of bioanalysis.
Limitations of the Study
Since the AIECL effect depends on the preparation process of the nanoparticles, if the experimental steps given by us are not strictly followed, the nanoparticles with significant enhancement effect will not be obtained.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21535003, 21674046) and the Excellent Research Program of Nanjing University (ZYJH004).
Author Contributions
S.J., J.X., and H.C. designed the project; H.G. synthesized the polymers; J.P. and C.X. built the ECL transient instrument; S.J. performed the optical characterization and ECL tests; S.J., Z.W., Y.Q., and J.X. analyzed and discussed the data; S.J. and W.Z. wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100774.
Contributor Information
Cong-Hui Xu, Email: chxu@nju.edu.cn.
Yi-Wu Quan, Email: quanyiwu@nju.edu.cn.
Jing-Juan Xu, Email: xujj@nju.edu.cn.
Supplemental Information
References
- Carrara S., Aliprandi A., De Cola L. Aggregation-induced electrochemiluminescence of platinum (II) complexes. J. Am. Chem. Soc. 2017;139:14605–14610. doi: 10.1021/jacs.7b07710. [DOI] [PubMed] [Google Scholar]
- Carrara S., Stringer B., Hogan C.F. Unusually strong electrochemiluminescence from iridium-based redox polymers immobilized as thin layers or polymer nanoparticles. ACS Appl. Mater. Interfaces. 2018;10:37251–37257. doi: 10.1021/acsami.8b12995. [DOI] [PubMed] [Google Scholar]
- Chang Y.L., Bard A.J., Barbara P.F. Electrogenerated chemiluminescence of single conjugated polymer nanoparticles. J. Am. Chem. Soc. 2008;130:8906–8907. doi: 10.1021/ja803454x. [DOI] [PubMed] [Google Scholar]
- Deng S.Y., Ju H.X. Electrogenerated chemiluminescence of nanomaterials for bioanalysis. Analyst. 2013;138:43–61. doi: 10.1039/c2an36122a. [DOI] [PubMed] [Google Scholar]
- Ding Z.F., Korgel B.A., Bard A.J. Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science. 2002;296:1293–1297. doi: 10.1126/science.1069336. [DOI] [PubMed] [Google Scholar]
- Feng Y.Q., Ju H.X., Cheng Y.X. Silole-containing polymer nanodot: an aqueous low-potential electrochemiluminescence emitter for biosensing. Anal. Chem. 2016;88:845–850. doi: 10.1021/acs.analchem.5b03391. [DOI] [PubMed] [Google Scholar]
- Gao T.B., Jiang D.C., Ye D.J. Aggregation-induced electrochemiluminescence from a cyclometalated iridium (III) complex. Inorg. Chem. 2018;57:4310–4316. doi: 10.1021/acs.inorgchem.7b03093. [DOI] [PubMed] [Google Scholar]
- Guo W.L., Su B., Shao Y.H. Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample. J. Am. Chem. Soc. 2018;140:15904–15915. doi: 10.1021/jacs.8b09422. [DOI] [PubMed] [Google Scholar]
- Han Z.G., Yang Z.F., Lu X.Q. Electrochemiluminescence platforms based on small water-insoluble organic molecules for ultrasensitive aqueous-phase detection. Angew. Chem. Int. Ed. 2019;131:5976–5980. doi: 10.1002/anie.201814507. [DOI] [PubMed] [Google Scholar]
- Hesari M., Workentin M.S., Ding Z.F. Highly efficient electrogenerated chemiluminescence of Au38 nanoclusters. ACS Nano. 2014;8:8543–8553. doi: 10.1021/nn503176g. [DOI] [PubMed] [Google Scholar]
- Hong Y.N., Lam J.W.Y., Tang B.Z. Aggregation-induced emission: phenomenon, mechanism and applications. Chem. Commun. (Camb.) 2009;29:4332–4353. doi: 10.1039/b904665h. [DOI] [PubMed] [Google Scholar]
- Honmou Y., Iyoda T., Vacha M. Single-molecule electroluminescence and photoluminescence of polyfluorene unveils the photophysics behind the green emission band. Nat. Commun. 2014;5:4666. doi: 10.1038/ncomms5666. [DOI] [PubMed] [Google Scholar]
- Hu R.R., Leung N.L.C., Tang B.Z. AIE macromolecules: syntheses, structures and functionalities. Chem. Soc. Rev. 2014;43:4494–4562. doi: 10.1039/c4cs00044g. [DOI] [PubMed] [Google Scholar]
- Jiang H., Liu L., Wang X.M. Aggregation-induced electrochemiluminescence by metal-binding protein responsive hydrogel scaffolds. Small. 2019;15:1901170. doi: 10.1002/smll.201901170. [DOI] [PubMed] [Google Scholar]
- Jiang M.H., Zhuo Y., Yuan R. Electrochemiluminescence enhanced by restriction of intramolecular motions (RIM): tetraphenylethylene microcrystals as a novel emitter for mucin 1 detection. Anal. Chem. 2019;91:3710–3716. doi: 10.1021/acs.analchem.8b05949. [DOI] [PubMed] [Google Scholar]
- Josh V.W., Ratner M.A., Wasielewski M.R. Crossover from single-step tunneling to multistep hopping for molecular triplet energy transfer. Science. 2010;328:1547–1550. doi: 10.1126/science.1189354. [DOI] [PubMed] [Google Scholar]
- Knaapila M., Monkman A.P. Methods for controlling structure and photophysical properties in polyfluorene solutions and gels. Adv. Mater. 2013;25:1090–1108. doi: 10.1002/adma.201204296. [DOI] [PubMed] [Google Scholar]
- Li C.P., Wang S.S., Jin Y.D. Nanoengineered metasurface immunosensor with over 1000-Fold electrochemiluminescence enhancement for ultra-sensitive bioassay. iScience. 2019;17:267–276. doi: 10.1016/j.isci.2019.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Tang B.Z., Liu B. Specific light-up bioprobes based on AIEgen conjugates. Chem. Soc. Rev. 2015;44:2798–2811. doi: 10.1039/c4cs00444b. [DOI] [PubMed] [Google Scholar]
- Liu S.F., Zhang X., Zou G.Z. Bandgap engineered and high monochromatic electrochem-iluminescence from dual-stabilizers-capped CdSe nanocrystals with practical application potential. Biosens. Bioelectron. 2013;55:203–208. doi: 10.1016/j.bios.2013.11.078. [DOI] [PubMed] [Google Scholar]
- Liu H.W., Qi H.L., Gao Q. Aggregation-induced enhanced electrochemiluminescence from organic nanoparticles of donor-acceptor based coumarin derivatives. ACS Appl. Mater. Interfaces. 2017;9:44324–44331. doi: 10.1021/acsami.7b15434. [DOI] [PubMed] [Google Scholar]
- Liu J.L., Chai Y.Q., Yuan R. Near-infrared aggregation-induced enhanced electrochemiluminescence from tetraphenylethylene nanocrystals: a new generation of ECL emitters. Chem. Sci. 2019;10:4497–4501. doi: 10.1039/c9sc00084d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.L., Chai Y.Q., Yuan R. BSA stabilized tetraphenylethylene nanocrystals as aggregation-induced enhanced electrochemiluminescence emitters for ultrasensitive microRNA assay. Chem. Commun. (Camb.) 2019;55:9959–9962. doi: 10.1039/c9cc04660g. [DOI] [PubMed] [Google Scholar]
- Miao W.J. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008;108:2506–2553. doi: 10.1021/cr068083a. [DOI] [PubMed] [Google Scholar]
- Miao W.J., Choi J.P., Bard A.J. Electrogenerated chemiluminescence 69: the tris(2,2‘-bipyridine) ruthenium (II), (Ru(bpy)32+)/tri-n-propylamine (TPrA) system revisited a new route involving TPrA⋅+ cation radicals. J. Am. Chem. Soc. 2002;124:14478–14485. doi: 10.1021/ja027532v. [DOI] [PubMed] [Google Scholar]
- Omer K.M., Ku S.Y., Bard A.J. Electrochemistry and electrogenerated chemiluminescence of a spirobifluorene-based donor (triphenylamine)-acceptor (2,1,3-benzothiadiazole) molecule and its organic nanoparticles. J. Am. Chem. Soc. 2011;133:5492–5499. doi: 10.1021/ja2000825. [DOI] [PubMed] [Google Scholar]
- O'Carroll D., Iacopino D., Redmond G. Poly(9,9-dioctylfluorene) nanowires with pronounced β-phase morphology: synthesis, characterization, and optical properties. Adv. Mater. 2008;20:42–48. [Google Scholar]
- Prieto I., Teetsov J., Bard A.J. A study of excimer emission in solutions of poly(9,9-dioctylfluorene) using electrogenerated chemiluminescence. J. Phys. Chem. A. 2001;105:520–523. [Google Scholar]
- Qi H.L., Rathore R., Bard A.J. Electrochemistry and electrogenerated chemiluminescence of π-stacked poly(fluorenemethylene) oligomers. multiple, interacting electron transfers. J. Am. Chem. Soc. 2012;134:16265–16274. doi: 10.1021/ja3057997. [DOI] [PubMed] [Google Scholar]
- Richter M.M. Electrochemiluminescence (ECL) Chem. Rev. 2004;104:3003–3036. doi: 10.1021/cr020373d. [DOI] [PubMed] [Google Scholar]
- Scherf U., List E.J.W. Semiconducting polyfluorenes towards reliable structure property relationships. Adv. Mater. 2002;14:477–487. [Google Scholar]
- Sentic M., Milutinovic M., Sojic N. Mapping electrogenerated chemiluminescence reactivity in space: mechanistic insight into model systems used in immunoassays. Chem. Sci. 2014;5:2568–2572. [Google Scholar]
- Suk J., Cheng J.Z., Bard A.J. Synthesis, electrochemistry, and electrogenerated chemiluminescence of Azide-BTA, a D–A−π–A–D species with benzothiadiazole and N,N-Diphenylaniline, and its nanoparticles. J. Phys. Chem. C. 2011;115:14960–14968. [Google Scholar]
- Suk J., Wang L., Bard A.J. Electrochemistry, electrogenerated chemiluminescence, and excimer formation dynamics of intramolecular π-stacked 9-naphthylanthracene derivatives and organic nanoparticles. J. Am. Chem. Soc. 2011;133:14675–14685. doi: 10.1021/ja203731n. [DOI] [PubMed] [Google Scholar]
- Tan X., Zhang B., Zou G. Electrochemistry and electrochemiluminescence of organometal halide perovskite nanocrystals in aqueous medium. J. Am. Chem. Soc. 2017;139:8772–8776. doi: 10.1021/jacs.7b05073. [DOI] [PubMed] [Google Scholar]
- Valenti G., Scarabino S., Sojic N. Single cell electrochemiluminescence imaging: from the proof-of-concept to disposable device-based analysis. J. Am. Chem. Soc. 2017;139:16830–16837. doi: 10.1021/jacs.7b09260. [DOI] [PubMed] [Google Scholar]
- Voci S., Goudeau B., Sojic N. Surface-confined electrochemiluminescence microscopy of cell membranes. J. Am. Chem. Soc. 2018;140:14753–14760. doi: 10.1021/jacs.8b08080. [DOI] [PubMed] [Google Scholar]
- Voityuk A.A. Triplet excitation energy transfer through fluorene π stack. J. Phys. Chem. C. 2010;114:20236–20239. [Google Scholar]
- Wei X., Yan H., Xu J.J. Aggregation-induced electrochemiluminescence of carboranyl carbazoles in aqueous media. Angew. Chem. Int. Ed. 2019;58:3162–3166. doi: 10.1002/anie.201900283. [DOI] [PubMed] [Google Scholar]
- Wei X., Yan H., Xu J.J. Recent advances in aggregation-induced electrochemiluminescence. Chem. Eur. J. 2019;25:1–14. doi: 10.1002/chem.201902465. [DOI] [PubMed] [Google Scholar]
- Wong K.T., Chien Y.Y., Wu C.C. Ter(9,9-diarylfluorene)s: highly efficient blue emitter with promising electrochemical and thermal stability. J. Am. Chem. Soc. 2002;124:11576–11577. doi: 10.1021/ja0269587. [DOI] [PubMed] [Google Scholar]
- Yang L.Q., Zhang B., Zou G.Z. Efficient and monochromatic electrochemiluminescence of aqueous soluble Au nanoclusters via host–guest recognition. Angew. Chem. Int. Ed. 2019;131:6975–6979. doi: 10.1002/anie.201900115. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Y., Feng Q.M., Xu J.J. Silver nanoclusters for high-efficiency quenching of CdS nanocrystal electrochemiluminescence and sensitive detection of microRNA. ACS Appl. Mater. Interfaces. 2015;7:26307–26314. doi: 10.1021/acsami.5b09129. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Jiang D.C., Chen H.Y. Electrochemiluminescence-based capacitance microscopy for label-free imaging of antigens on the cellular plasma membrane. J. Am. Chem. Soc. 2019;141:10294–10299. doi: 10.1021/jacs.9b03007. [DOI] [PubMed] [Google Scholar]
- Zhao Z.J., Chen S.M., Tang B.Z. Construction of efficient solid emitters with conventional and AIE luminogens for blue organic light-emitting diodes. J. Mater. Chem. 2011;21:10949–10956. [Google Scholar]
- Zhao Z.J., Chan C.Y.K., Tang B.Z. Using tetraphenylethene and carbazole to create efficient luminophores with aggregation-induced emission, high thermal stability, and good hole-transporting property. J. Mater. Chem. 2012;22:4527–4534. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.