Summary
T lymphocytes are critical for effective immunity, and the ability to study their behavior in vitro can facilitate major insights into their development, function, and fate. However, the composition of human plasma differs from conventional media, and we hypothesized that such differences could impact immune cell physiology. Here, we showed that relative to the medium typically used to culture lymphocytes (RPMI), a physiologic medium (human plasma-like medium; HPLM) induced markedly different transcriptional responses in human primary T cells and in addition, improved their activation upon antigen stimulation. We found that this medium-dependent effect on T cell activation is linked to Ca2+, which is six-fold higher in HPLM than in RPMI. Thus, a medium that more closely resembles human plasma has striking effects on T cell biology, further demonstrates that medium composition can profoundly affect experimental results, and broadly suggests that physiologic media may offer a valuable way to study cultured immune cells.
Subject Areas: Biological Sciences, Immunology, Immunological Methods
Graphical Abstract
Highlights
-
•
Activation of T lymphocytes in plasma-like medium is much more efficient
-
•
RPMI is severely hypocalcemic relative to human plasma and this impacts activation
-
•
Non-physiological levels of nutrients in RPMI alter lymphocyte metabolism
Biological Sciences; Immunology; Immunological Methods
Introduction
Adaptive immune systems involve the movement of immune cells throughout the internal milieu of the blood and interstitial spaces to recognize foreign agents and respond to ensure the health of the host. T lymphocytes are central effectors of immunity in infectious diseases, autoimmunity, and cancer. T cell activation by cognate antigen is a complex process influenced by both intrinsic and extrinsic factors. Ligation of the αβ-T cell receptor by peptide antigens bound to major histocompatibility complex (MHC) class I/II initiates a number of signaling events culminating in the activation of a robust transcriptional program, which is key for an appropriate response.
The extracellular environment of the T lymphocyte has increasingly been recognized as a major influence on the characteristics and magnitude of the immune response. Namely, the availability of nutrients/second messengers in the environment, and the consequent changes in the metabolic status of the cell, are critical for shaping an appropriate T cell response. Recent publications have highlighted the influence of lipids, glucose, and amino acid metabolism on both the magnitude and characteristics of T cell responses (Pearce et al., 2009, van der Windt and Pearce, 2012, Sinclair et al., 2013, O'Sullivan et al., 2014, Buck et al., 2017, Ma et al., 2017, Werner et al., 2017, Jacobs et al., 2018). In addition to metabolic changes, ligation of the T cell receptor also induces the influx of extracellular calcium ions via the STIM/Orai channel, leading to activation of downstream transcription factors such as NFAT (Smith-garvin et al., 2010, Derler et al., 2016). Although a complete deficiency of this process has been studied in multiple contexts, the influence of subtler variations in extracellular calcium levels on T cell activation remain unclear (Feske et al., 2006, Prakriya et al., 2006).
Formulations of common cell culture media such as RPMI-1640 were developed in the mid-20th century to optimize in vitro growth of cell lines and have since undergone remarkably little change (Eagle, 1955, McCoy et al., 1959, Moore et al., 1966). Despite a growing focus on the effects of metabolic changes during T cell activation and proliferation, culture conditions that more closely resemble the in vivo milieu have not been studied. Recently, several studies in non-immune cells have described the use of modified traditional media or new systematically constructed synthetic media designed to either improve growth in cell culture or to better model the in vivo environment (Favaro et al., 2012, Schug et al., 2015, Pan et al., 2016, Cantor et al., 2017, Vande Voorde et al., 2019). Among these is human plasma-like medium (HPLM), which contains a cocktail of 31 components that are absent from the defined formulations of RPMI and other commonly used basal culture media (Cantor et al., 2017). HPLM further contains at physiologically relevant concentrations other typical media components such as glucose, amino acids, and salt ions. It is worth noting that all of these defined components may be otherwise present at non-physiological levels in fetal bovine serum (FBS), the most widely used tissue culture supplement (Cantor et al., 2017). And therefore, HPLM is instead supplemented with 10% dialyzed FBS (HPLMdFBS). Here, we asked how HPLMdFBS influences gene expression and activation of cultured primary human T lymphocytes.
Results
Transcriptome Analysis Reveals Extensive Differences in T Lymphocytes Activated in HPLMdFBS Compared with RPMIdFBS
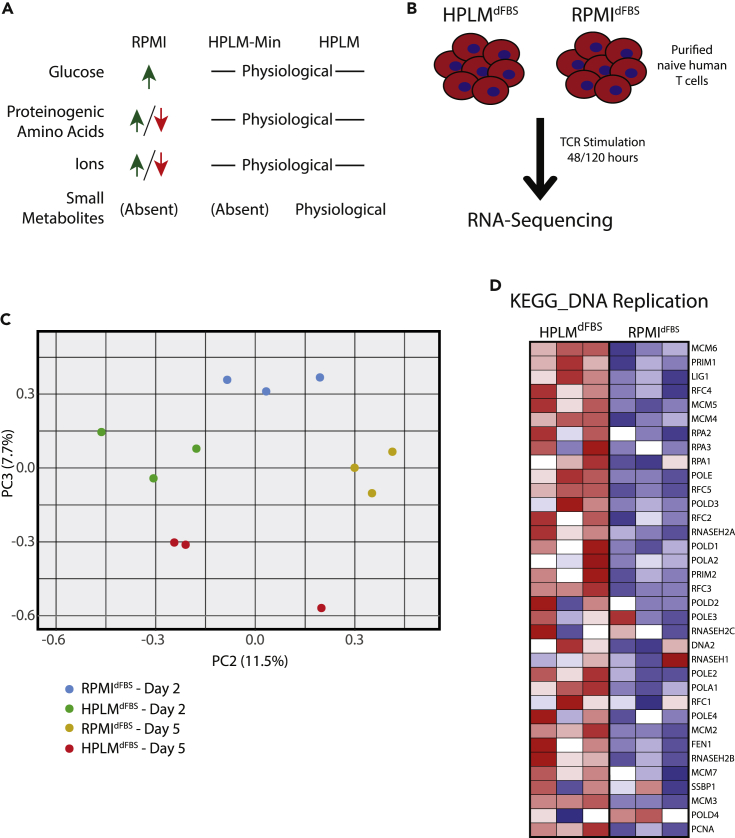
T lymphocytes in vivo undergo broad transcriptional re-programming following TCR activation, and this process occurs in the context of a rich internal milieu containing high levels of amino acids, lipids, and a variety of small organic metabolites (Crabtree, 1989). In contrast, typical in vitro methods used to study these same processes are based on T cells cultured in RPMI, which contains a collection of nutrients at non-physiologic concentrations (Moore et al., 1967). Therefore, we evaluated activation in naive CD4/CD8+ T cells stimulated in HPLMdFBS compared with RPMI analogously supplemented with 10% dialyzed serum (RPMIdFBS) to restrict our downstream analysis of potential phenotypic differences to defined media components only (Figure 1A, Table S1). We then activated purified human naive T lymphocytes from three individual donors with plate-bound anti-CD3/CD28 antibodies for 48 or 120 h in either HPLMdFBS or RPMIdFBS, isolated polyadenylated mRNAs, and characterized the transcriptional differences between these two conditions via deep sequencing. Principal component analysis revealed changes between 48 and 120 h of activation independently of the medium used. Nonetheless, the 2nd and 3rd principal components divided each group of samples (RPMIdFBS-48 h, RPMIdFBS-120 h, HPLMdFBS-48 h, and HPLMdFBS-120 h) into clear clusters revealing the transcriptional differences between HPLMdFBS and RPMIdFBS (Figure 1C). We next used gene set enrichment analysis (GSEA) to identify statistically significant differences in 29 different Kyoto encyclopedia of genes and genomes (KEGG) pathways (Kanehisa and Goto, 2000, Kanehisa et al., 2019). Nine pathways were significantly different at 48 h, 19 significantly different at 120 h, and one pathway was shared between both timepoints (Figure S1). Among these we observed a striking enrichment of pathways involved in DNA replication and cell cycle in HPLMdFBS at 120 h post-activation and an enrichment of pathways involved in T cell activation at 48 h (Figure S1). In particular, essentially every gene in the KEGG DNA replication pathway exhibited increased expression in HPLMdFBS relative to RPMIdFBS (Figure 1D). Thus, T cell activation in HPLMdFBS was superior to RPMIdFBS, and this difference was readily apparent as early as 48 h post activation.
Figure 1.
Transcriptomic Analysis Reveals Major Differences between Human Lymphocytes Activated in HPLMdFBS Relative to RPMIdFBS
(A) Schematic of the comparative compositions of RPMIdFBS, HPLMdFBS, and HPLMdFBS-Min. Detailed composition can be found in Table S1.
(B) Experimental outline for T cell activation in either HPLMdFBS or RPMIdFBS and downstream transcriptome analysis.
(C) Transcriptional differences between primary naive human mixed CD4+ and CD8+ T cells from three different donors activated in HPLMdFBS or RPMIdFBS as depicted in (B) were measured via RNA-sequencing. These data were used to generate PCA plots showing principal components 2 and 3 at the indicated timepoints.
(D) Heatmap showing the Log2(fold change) in transcript abundance for genes involved in the KEGG DNA replication pathway using the RNA-sequencing data generated as described in (B).
There were also marked medium-dependent transcriptional differences across several metabolic pathways (Table 1). For instance, we found that HPLMdFBS induced large increases in the expression of genes involved in amino acid metabolism, including the KEGG annotated pathways: arginine/proline metabolism; glycine/threonine/serine metabolism; and alanine, aspartate, and glutamate metabolism. These differences may be driven as a result of the roughly 2–10 fold differences in availability of arginine, aspartate, serine, and glutamate between the two media (Table S1). We also found that HPLMdFBS induced increased relative expression of genes involved in the p53 signaling pathway as well as in various nucleic acid metabolism pathways (e.g. DNA repair, pyrimidine metabolism, RNA polymerase, spliceosome, and homologous recombination). In contrast, HPLMdFBS also induced a relative decrease in the expression of genes associated with several other pathways, including glycerophospholipid and cytochrome p450 metabolism, cell adhesion, allograft rejection, graft vs. host disease, type 1 diabetes, cytokine receptor interactions, and autoimmune thyroid disease. Taken together, these results suggest that HPLMdFBS promotes a T cell expression signature that is more to promote proliferation and to restrain autoimmunity.
Table 1.
Transcriptional Differences in Metabolic Genes in T Cells Cultured in HPLMdFBS
| Fold Change in Expression in HPLMdFBS | |
|---|---|
| Serine Biosynthesis | |
| PHGDH | 6.10 |
| PSAT1 | 5.30 |
| PSPH | 1.25 |
| Glycine, Arginine, Proline Biosynthesis | |
| SHMT1 | 1.80 |
| SHMT2 | 1.33 |
| PYCR1 | 4.32 |
| ASS1 | 81.00 |
| Aspartate Biosynthesis | |
| GOT1 | 1.45 |
| Cholesterol Biosynthesis | |
| HMGCS1 | 0.38 |
| HMGCR | 0.49 |
| MVD | 0.48 |
| FDFT1 | 0.47 |
HPLMdFBS Is Superior to RPMIdFBS in Supporting Naive Human CD4/CD8+ T Cell Activation
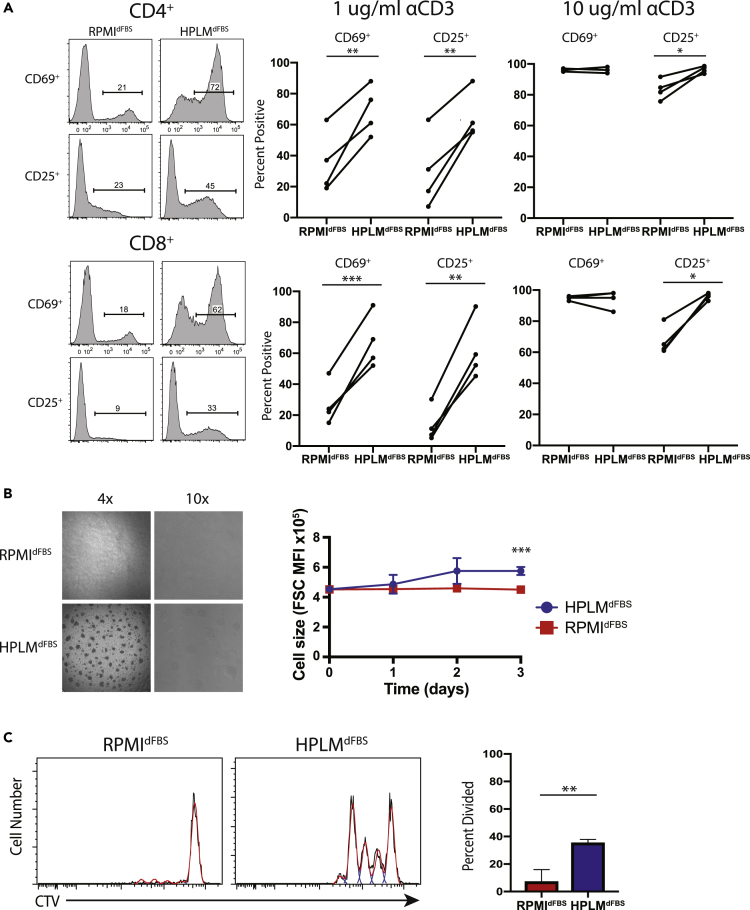
Guided by our RNA-Seq data, we hypothesized that T cell activation and proliferation would be more efficient in HPLMdFBS. Thus, we went on to measure markers of activation in antigen-stimulated naive human T cells (Figure 1). In both CD4+ and CD8+ T cells from five different healthy donors, we observed a significant increase in CD25 and CD69 in HPLMdFBS compared with RPMIdFBS (Figure 2A). We also carried out time courses spanning multiple days and the differences in this case were robust at every time point examined (Figure S2A). In addition, the use of increased anti-CD3/CD28 antibody concentrations during the activation led to a nearly equivalent maximal response rate in both conditions (i.e. >90% positive for both CD25 and CD69) (Figure 2A). This result suggests that HPLMdFBS effectively reduces the activation threshold, as further supported both by the relatively unique appearance of large activated T cell clusters in the HPLMdFBS cultures, as well as a marked difference in cell size between the two conditions (Figure 2B). Given that the T cells activated in RPMIdFBS exhibited both a bimodal size distribution and much larger absolute size within the unstimulated population, this relative cell size difference was likely driven by defective activation. Furthermore, as anticipated from the transcriptional differences described earlier, we also found that the rapid proliferation of activated T cells in HPLMdFBS was far less apparent in RPMIdFBS (Figure 2C). Lastly, although HPLM was not designed to mimic the in vivo milieu of mice, we also tested activation of murine T cells in HPLMdFBS versus RPMIdFBS, as this is a popular model used in the field. Again, we observed greatly improved activation in HPLMdFBS, suggesting that the mechanism responsible is evolutionarily conserved. In addition, these results would suggest that HPLM is better suited to the in vitro culture of both murine and human T cells.
Figure 2.
Primary Human T Cell Activation Is Superior in HPLMdFBS Compared with RPMIdFBS
(A) Flow cytometric measurement of T cell activation markers CD25 and CD69 on purified naive human T cells following stimulation with either 1 or 10 μg of plate-bound anti-CD3 (αCD3)/CD28 in HPLMdFBS or RPMIdFBS for 16 h. Data shown are representative of four different experiments each conducted with cells isolated from a different healthy donor (Student's t test; paired; two-tails; *p < 0.05, **p < 0.01, ***p < 0.001).
(B) Bright-field microscopy images of naive T cells stimulated with 1 μg of plate-bound anti-CD3/CD28 in either HPLMdFBS or RPMI for 16 h.
(C) Flow cytometry histograms of CellTrace Violet staining of naive T cells stimulated in either HPLMdFBS or RPMIdFBS (left) with quantitation of the fraction of CD4+ T cells that have undergone at least one division (right). Columns represent the mean of three experiments, each done with cells isolated from a different healthy donor (Student's t test; unpaired; two-tails; *p < 0.05). Error bars represent the SEM.
Physiological Calcium Availability Augments T Cell Activation
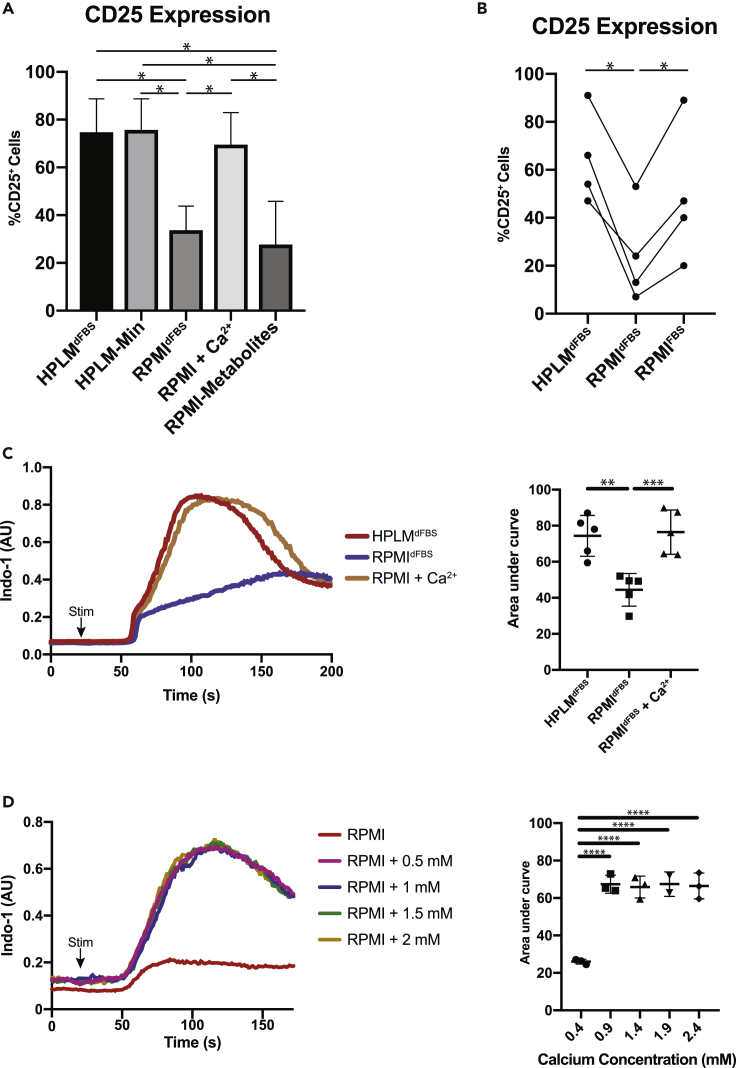
We next asked which component(s) of HPLMdFBS dictated the relative differences in T cell activation. To do so, we first compared T cell activation HPLMdFBS, HPLM-Min (which contains only the defined amino acids, glucose, vitamins, salts, and 10% dialyzed serum; see Table S1), and (Cantor et al., 2017) and RPMIdFBS (Figure 1A, Table S1). Further, we also included a medium derivative in which we supplemented all HPLM-specific polar metabolites to RPMIdFBS (RPMI-Metabolites). We ultimately observed an equivalent extent of activation in the HPLMdFBS and HPLM-Min conditions and a significantly reduced relative extent of activation in both RPMIdFBS and RPMI-metabolites (Figure 3A). These results suggest that in this context, the relative differences in activation between HPLMdFBS and RPMIdFBS were instead due to concentration differences in amino acids, glucose, or salt ions. And indeed, one striking concentration difference among these is that of calcium (Ca2+), which is provided by HPLM at a defined concentration of 2.4 mM but is instead present in RPMI at a defined level of 0.4 mM, (Table S1), which is markedly hypocalcemic relative to the in vivo milieu (2–2.5 mM) (Goldstein, 1990). In turn, we in fact found that supplementing RPMIdFBS with 2 mM CaCl2 (RPMI+Ca2+) was sufficient to achieve T cell activation to an extent equivalent to that seen in HPLMdFBS (Figure 3A).
Figure 3.
RPMIdFBS Is Severely Hypocalcemic Relative to Human Plasma and HPLMdFBS
(A) Measurement of activation marker CD25 on CD4+ T cells comparing RPMIdFBS and HPLMdFBS-min supplemented with various metabolite components unique to HPLMdFBS. Columns represent the mean with error bars showing the standard error (one-way ANOVA comparing other conditions to HPLMdFBS with p values calculated by Dunnett test; *p < 0.05).
(B) Measurement of activation marker CD25 on CD4+ T cells activated in HPLMdFBS, RPMIdFBS, or RPMIcFBS. Experiment was repeated four times with each repeat using a different healthy human donor (one-way ANOVA; Tukey's test; *p < 0.05).
(C) Flow cytometric plots of calcium flux following primary stimulation of isolated human CD8+ T lymphocytes in either RPMIdFBS or HPLMdFBS (left) as well as quantification of the area under the curve (right). Quantification shows data from five experiments each done with a different individual donor, and error bars show standard error (one-way ANOVA; Tukey's test; **p < 0.01, ***p < 0.001).
(D) Flow cytometric plots of calcium flux following primary stimulation of isolated human CD8+ T lymphocytes in RPMIdFBS supplemented with the indicated concentrations of calcium chloride. Quantification shows data from five experiments each done with a different individual donor, and error bars show standard error (one-way ANOVA; Tukey's test; **p < 0.01, ***p < 0.001).
It is worth noting that basal media used to culture T lymphocytes (often RPMI) are instead typically supplemented with 10% unmodified FBS (RPMIFBS), and so we next wanted to determine the Ca2+ concentration of RPMIFBS and ask how this particular complete medium influenced relative T lymphocyte activation as well. We found that [Ca2+] was approximately 3.9 mM in our typical stock FBS, and thus, a 10% supplement to basal RPMI would result in a RPMIFBS Ca2+ concentration of ∼0.8 mM, a value that is still considered hypocalcemic although is closer to physiologic levels. We next found that TCR stimulation of naive T cells in RPMIFBS induced activation to an extent nearly equivalent to that in HPLMdFBS (Figure 3B), suggesting that 10% unmodified FBS provides RPMI sufficient Ca2+ to achieve comparable activation. We next examined Ca2+ flux following TCR stimulation in RPMIdFBS, RPMICa2+, and HPLMdFBS. It is worth noting that most calcium flux protocols are carried out in Hank's balanced salt solution or Ringer's solution; however, some studies have described a decreased Ca2+ flux in RPMI relative to these solutions (Prakriya et al., 2006, Gwack et al., 2008, Bertin et al., 2014). We observed a striking decrease in the amount of Ca2+ entering T cells following activation in RPMIdFBS, as compared with either HPLMdFBS or RPMICa2+ (Figure 3C). Taken together, our results suggest that the relatively greater threshold for T cell activation in RPMIdFBS resulted from lower Ca2+ availability.
Our results also raise the possibility that in vivo Ca2+ levels can influence T cell activation and that hypocalcemic (or hypercalcemic) patients may be more prone to immunodeficiency (or autoimmunity), and in fact others had previously shown that extracellular [Ca2+] can influence cytokine production in murine T cells (Zimmermann et al., 2015). Moreover, our observations indicated that RPMIdFBS poorly recapitulates in vivo conditions, given the hypocalcemic conditions it provides to cells. Thus, we next titrated Ca2+ levels in basal RPMI in 0.5 mM increments, up to a maximum of 2.2 mM, and then measured TCR-induced Ca2+ flux. We again observed that basal RPMI induced a meager Ca2+ flux, but the supplementation of [Ca2+] from 0.7 to 2.2 mM yielded Ca2+ fluxes that were virtually identical in both magnitude and kinetics (Figure 3D), suggesting that even at the lower end of physiologically reported levels, the Ca2+ flux rates can reach achieve maximum levels and that greater Ca2+ levels likely do not affect the propensity of in vivo T cell activation. Nonetheless, the [Ca2+] in basal RPMI is insufficient to promote full Ca2+ flux or T cell activation.
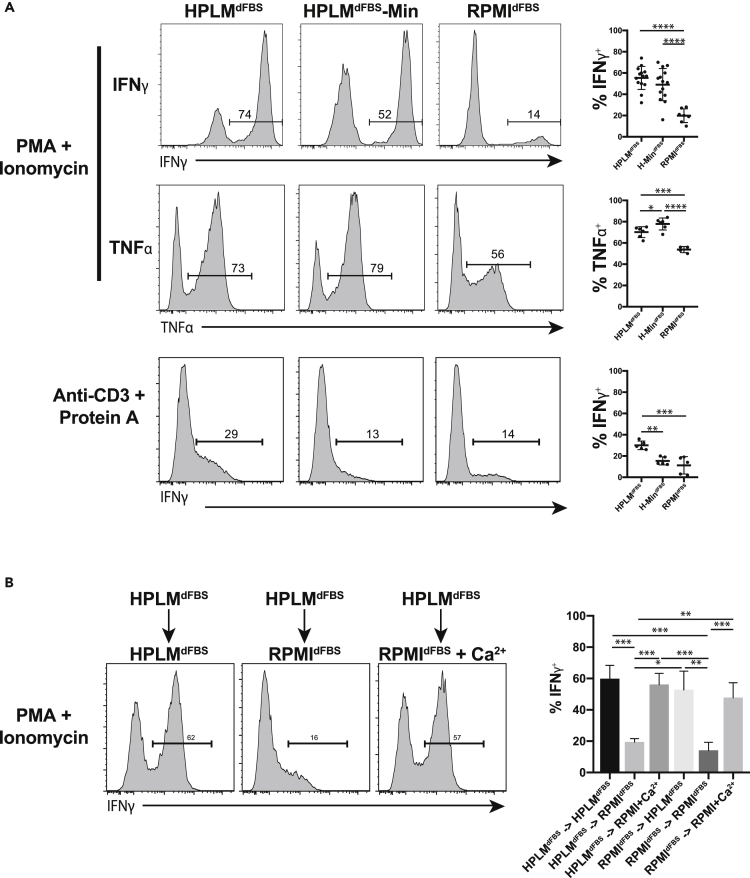
HPLMdFBS Promotes CD8+ T Cell Effector Cytokine Production
We next evaluated cytokine production in CD8+ T cells activated in HPLMdFBS or HPLMdFBS-Min. We activated T cells in RPMIdFBS and grew them for 14 to 21 days supplemented with IL-2 prior to restimulation and measurement of cytokine production. We find that TNFα and IFNγ production was similar in HPLMdFBS or HPLMdFBS-Min but substantially greater than RPMIdFBS (Figure 4A). Similar results were obtained with phorbol myristate acetate (PMA) and ionomycin or anti-CD3 antibodies cross-linked with protein A. IFNγ production was slightly reduced in HPLMdFBS compared with HPLMdFBS-min although this did not reach statistical significance. We next examined whether this difference was due to the media composition during the initial stimulation or the subsequent restimulation by activating and expanding the cells in HPLMdFBS or RPMIdFBS and then switching them to other media just prior to restimulation (Figure 4B). Under these conditions, we observed robust cytokine production in cells that were activated in HPLMdFBS or RPMIdFBS and then transferred to HPLMdFBS or RPMIdFBS supplemented with 2mM Ca2+ (Figure 4B). However, irrespective of the initial activation medium, cells instead transferred to RPMIdFBS exhibited reduced cytokine production levels, suggesting that [Ca2+] is a critical factor at the time of restimulation.
Figure 4.
T Cells Activated in HPLMdFBS Produce Higher Levels of Effector Cytokines
(A) Levels of cytokines produced in primary human T lymphocytes following restimulation after being expanded in the indicated medium. T lymphocytes from 5 to 14 individuals were tested across two to three experiments, with the error bars representing standard deviation (one-way ANOVA; Tukey's test; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
(B) Quantification of cytokine production in primary human T lymphocytes expanded in the indicated medium, switched to fresh medium of the indicated type, and then restimulated. Columns represent the mean of measurements from three experiments each with two individuals, with the error bars representing the standard error (one-way ANOVA; Tukey's test; *p < 0.05, **p < 0.01, ***p < 0.001).
CD19 CAR-T Cell Transduction Efficiency in HPLMdFBS Is Similar to Other Commonly Used Media
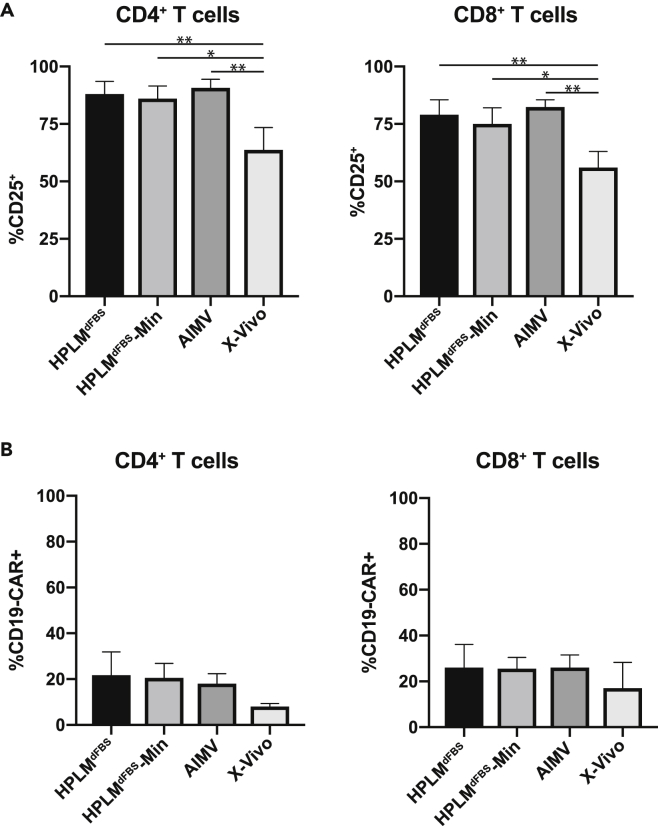
T lymphocytes have become a central focus in the cancer immunotherapy field, and many clinical protocols require in vitro culture and expansion of human T cells engineered with chimeric antigen receptors (CAR-T cell) (Newick et al., 2018). Therefore, we wanted to ask whether pan naive T cells cultured in HPLMdFBS exhibit improved transduction efficiency for lentiviruses that express CAR-T cell receptors. To do so, we first evaluated how HPLMdFBS affected activation relative to that in two other synthetic media commonly used in clinical CAR-T expansion protocols: a mixture of AIM V and RPMI (supplemented with 5% human serum, referred to as AIM V here) and X-VIVO 15 (serum free). Following stimulation with plate bound anti-CD3/CD28 antibodies, we observed equivalent CD25 expression levels in HPLMdFBS, HPLMdFBS-Min, and AIM V, which were ultimately greater than those induced by culture in X-VIVO 15 (Figure 5A). We then observed that the lentiviral transduction efficiencies of both CD4+ and CD8+ T cells were equivalent in HPLMdFBS, HPLMdFBS-Min, and AIM V media and greater than those in X-VIVO 15 (Figure 5B). Taken together, HPLMdFBS performs comparably or better than commonly used culture media that have been used to generate CAR-expressing T cells.
Figure 5.
Lentiviral Transduction Rates Are Equivalent in T Cells Cultured in HPLMdFBS and Conventional Culture Media
(A) Quantification of levels of activation markers in CD4+ (left panel) and CD8+ T cells following activation in the indicated medium. Columns represent the mean of measurements from three experiments each conducted with cells from a different individual, with error bars representing the standard error (one-way ANOVA; Tukey's test; *p < 0.05, **p < 0.01).
(B) Quantification of transduction efficiency human CD4+ (left panel) and CD8+ T cells (right panel) with CD19-CAR expressing lentiviruses activated in the indicated media. Columns represent the mean of measurements from three experiments each conducted with cells from a different individual, with error bars representing the standard error (one-way ANOVA; Tukey's test).
Discussion
The development of cell culture techniques in the mid 20th century heralded an enormous advance in life sciences research by permitting tissue-free in vitro studies of cell physiology. However, media formulations developed in the 1960s remain almost universally used today. Here, we took advantage of a recent effort to systematically develop a new cell culture medium that was designed to more closely model human plasma (HPLM) by extending the use of HPLM to the study of primary human lymphocytes. We show that compared to RPMIdFBS, HPLMdFBS induces a much more robust activation of naive T cells and a marked increase in the section of effector cytokines. Further, comparative RNA-Seq methods revealed a large number of medium-dependent T cell transcriptional differences across several metabolic pathways.
Through the use of HPLM, we also find that calcium is a rate-limiting component for lymphocyte activation, a result that corroborates recent work that describes the induction of acute Ca2+ flux following TCR engagement (Zhang et al., 2005, Prakriya et al., 2006). Others have also described a relative increase in effector cytokine secretion for murine T cells cultured in IMDM ([Ca2+] = 1.5 mM) or calcium supplemented-RPMIdFBS compared with basal RPMI (Zimmermann et al., 2015). Similarly, we found in primary human T cells that relative activation, proliferation, and effector cytokine secretion are reduced in RPMIdFBS lacking any additional Ca2+ supplementation. It is likely that the use of 10% FBS as a common supplement to basal media sufficiently augments the defined calcium concentration of RPMI, as we measured the Ca2+ levels of stock FBS as approximately 3.9 mM. Although our current study suggests that the resultant RPMIFBS Ca2+ concentration (∼0.8 mM), which still corresponds to a severely hypocalcemic condition, is sufficient to achieve maximal TCR-induced Ca2+ flux and activation, it is now clear that calcium level is an independent parameter that should be considered in studies of T cell biology. For instance, it is possible that the cross-linking of anti-CD3 antibodies used in the calcium flux methods masks an underlying defect that would be present in response to physiological antigens. Further, the calcium flux assay only assesses calcium changes for the first several minutes of a response, whereas the activation process is much more dynamic and can extend over the course of h/day in vivo.
Although our results comparing HPLMdFBS with a derivative containing only amino acids, glucose, vitamins, salts, and 10% dialyzed serum (HPLM-min) did not suggest an induction of dramatically different phenotypic outputs, a caveat of our study is that prior work with HPLMdFBS described a much more robust serum dialysis procedure (Cantor et al., 2017). Thus, it is possible that our commercial source of this supplement was far less stringently dialyzed, and in turn, perhaps still contained appreciable levels of several polar metabolites, which would mask any putative differences between the two HPLM derivatives.
This study was primarily focused on the impact of the extracellular milieu on early T cell activation events. As we observed significant differences in the activation efficiency of human T lymphocytes cultured in either HPLMdFBS or RPMIdFBS, we restricted our subsequent analyses to these early timeframes. We would speculate that culturing T lymphocytes in physiologic medium for longer time frames would have major impacts on the proliferation, activation, and metabolic state of the T cells. Similarly, metabolic status has been shown to have an impact on T cell differentiation and type of effector cytokines being secreted (Buck et al., 2015). It is likely that culture in HPLM would impact these processes as well; however, it was outside the purview of our study.
We also found that HPLMdFBS had a large relative impact on the transcriptional response of activated human T cells, revealing major differences that led to our subsequent focus on extracellular [Ca2+]. However, many of the most significant differentially regulated genes in our dataset were associated with metabolic pathways. In particular, we found that HPLMdFBS induced large increases in the expression of genes involved in several amino acid metabolism pathways, including significant upregulation of genes that encode various rate-limiting enzymes in such pathways (ASS1, PHGDH, PYCR1, GOT1). It is likely that such differences result from marked differences in the availability of several amino acids between HPLMdFBS and RPMIdFBS. For instance, arginine, which is present at nearly 10-fold greater levels in basal RPMI compared with human blood and (HPLM), have been suggested to be critical for T cell proliferation, differentiation, and survival, and previous studies have described improved T cell survival upon arginine supplementation to 3 mM (Rodriguez et al., 2007, Geiger et al., 2016). Thus, it is likely that in the in vivo environment arginine is even more limiting than previously thought.
Our data broadly highlight the fact that commonly used conditions used to culture and examine T lymphocytes in vitro may not be ideal for metabolic studies, and we also specifically identify one extracellular component (calcium) that can be easily considered by others in the field. Our approach also further demonstrates the value of using HPLM to improve the modeling capacity of in vitro cell culture systems.
Limitations of the Study
In this work we characterize for the first time the impact of physiologic media on the metabolism and activation of human T cell in vitro culture and highlight the drawbacks of conventional media formulations. Although we did observe significant transcriptional changes in multiple critical metabolic pathways, we did not explore the mechanisms behind this nor the downstream impacts on longevity, memory/effector differentiation or differentiation to different subsets such as Th1, Th2, Tregs, and others. It is known that the metabolic state has a critical impact on these processes (van der Windt and Pearce, 2012, Berod et al., 2014, Lochner et al., 2015), and it is likely that physiologic media is a superior model of the in vivo condition; however this hypothesis remains to be tested. In addition to this, our analysis was restricted to the transcriptome and effector/proliferative functions of T lymphocytes rather than direct investigation of the metabolic state of primary cells cultured in HPLM relative to conventional media. Lastly, although we focused exclusively on T lymphocytes, it is likely that B cells, myeloid cells, and other lineages are likely to be similarly affected, although again this remains to be tested.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
M.L.G. was a student in the NIH-University of Pennsylvania graduate partnership program and the authors would like to acknowledge the mentorship and guidance provided. We thank Ann Park, Xijin Xu, other members of the Molecular Development of the Immune System Section at the NIH, John Wherry, Sara Cherry, Pamela Schwartzberg, and Igor Brodsky for helpful discussions as well as technical guidance. We are grateful for financial support from the Lupus Foundation of America and Merck, Inc. The authors would also like to thank the Clinical Cancer Research Sequencing Facility for assistance in performing the RNA-Sequencing experiments and subsequent analysis, in particular; Justin Lack, Jyoti Shetty, and Bao Tran. The authors would like to thank Ryan Kissinger for assistance in creating the graphical abstract. Lastly, the authors thank Tori Yamamoto for the kind gift of the CAR-T cell lentivirus. J.R.C. is supported in part by the NIH/NCI (K22CA225864). This research was supported in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Author Contributions
M.L.G., H.C.S., and M.J.L. initiated the project and designed the research. M.L.G. performed the experiments and analyzed the data with input from J.R.C., H.C.S., and M.J.L. J.R.C. prepared the HPLM media. A.B. performed bioinformatic analysis. M.L.G., H.C.S., J.R.C., and M.L. wrote the manuscript and all authors edited.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100759.
Data and Code Availability
The RNA-Seq data from this study were uploaded to GEO and may be accessed at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE135936.
Supplemental Information
References
- Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bähre H. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- Bertin S., Aoki-Nonaka Y., de Jong P.R., Nohara L.L., Xu H., Stanwood S.R., Srikanth S., Lee J., To K. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4+ T cells. Nat. Immunol. 2014;15:1055–1063. doi: 10.1038/ni.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M.D., Sowell R.T., Kaech S.M., Pearce E.L. Metabolic instruction of immunity. Cell. 2017;169:570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M.D., O’Sullivan D., Pearce E.L. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J.R., Abu-Remaileh M., Kanarek N., Freinkman E., Gao X., Louissaint A., Jr., Lewis C.A., Sabatini D.M. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell. 2017;169:258–272.e17. doi: 10.1016/j.cell.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G.R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Derler I., Jardin I., Romanin C. Molecular mechanisms of STIM/Orai communication. Am. J. Physiol. Cell Physiol. 2016;310:C643–C662. doi: 10.1152/ajpcell.00007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H. The specific amino acid requirements of a mammalian cell (Strain L) in tissue culture. J. Biol. Chem. 1955;214:839–852. [PubMed] [Google Scholar]
- Favaro E., Bensaad K., Chong M.G., Tennant D.A., Ferguson D.J., Snell C., Steers G., Turley H., Li J.L., Günther U.L. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012;16:751–764. doi: 10.1016/j.cmet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S.H., Tanasa B., Hogan P.G., Lewis R.S., Daly M., Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Geiger R., Rieckmann J.C., Wolf T., Basso C., Feng Y., Fuhrer T., Kogadeeva M., Picotti P., Meissner F., Mann M. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D.A. Serum calcium. In: Walker H., Hall W., Hurst J., editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Third Edition. Butterworths; 1990. pp. 677–679. [PubMed] [Google Scholar]
- Gwack Y., Srikanth S., Oh-Hora M., Hogan P.G., Lamperti E.D., Yamashita M., Gelinas C., Neems D.S., Sasaki Y., Feske S. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., Rathmell J.C. Glucose uptake is limiting in T cell activation and requires CD28-Mediated Akt-Dependent and independent pathways. J. Immunol. 2018;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y., Furumichi M., Morishima K., Tanabe M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019;47:D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M., Berod L., Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Ma E.H., Bantug G., Griss T., Condotta S., Johnson R.M., Samborska B., Mainolfi N., Suri V., Guak H., Balmer M.L. Serine is an essential metabolite for effector t cell expansion. Cell Metab. 2017;25:345–357. doi: 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- McCoy T.A., Maxwell M., Kruse P.F. The amino acid requirements of the jensen sarcoma in vitro. Cancer Res. 1959;19:591–595. [PubMed] [Google Scholar]
- Moore G.E., Ito E., Ulrich K., Sandberg A.A. Culture of human leukemia cells. Cancer. 1966;19:713–723. doi: 10.1002/1097-0142(196605)19:5<713::aid-cncr2820190518>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Moore G., Gerner R., Franklin H.A. Culture of normal human leukocytes. JAMA. 1967;199:519–524. [PubMed] [Google Scholar]
- Newick K. CAR T-cell therapy for solid tumors? Cancer Discov. 2018;8:1341. doi: 10.1158/2159-8290.CD-ND2018-008. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D., van der Windt G.J., Huang S.C., Curtis J.D., Chang C.H., Buck M.D., Qiu J., Smith A.M., Lam W.Y., DiPlato L.M. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Reid M.A., Lowman X.H., Kulkarni R.P., Tran T.Q., Liu X., Yang Y., Hernandez-Davies J.E., Rosales K.K., Li H. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat. Cell Biol. 2016;18:1090–1101. doi: 10.1038/ncb3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., Jones R.G., Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Rodriguez P.C., Quiceno D.G., Ochoa A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1574. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug Z.T., Peck B., Jones D.T., Zhang Q., Grosskurth S., Alam I.S., Goodwin L.M., Smethurst E., Mason S., Blyth K. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair L.V., Rolf J., Emslie E., Shi Y.B., Taylor P.M., Cantrell D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-garvin J.E., Koretzky G.A., Jordan M.S. T cell activation. Immunology. 2010;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Voorde J., Ackermann T., Pfetzer N., Sumpton D., Mackay G., Kalna G., Nixon C., Blyth K., Gottlieb E., Tardito S. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 2019;5:eaau7314. doi: 10.1126/sciadv.aau7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Koschke M., Leuchtner N., Luckner-Minden C., Habermeier A., Rupp J., Heinrich C., Conradi R., Closs E.I., Munder M. Reconstitution of T cell proliferation under arginine limitation: activated human T cells take up citrulline via L-type amino acid transporter 1 and use it to regenerate arginine after induction of argininosuccinate synthase expression. Front. Immunol. 2017;8:864. doi: 10.3389/fimmu.2017.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt G.J.W., Pearce E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.L., Yu Y., Roos J., Kozak J.A., Deerinck T.J., Ellisman M.H., Stauderman K.A., Cahalan M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J., Radbruch A., Chang H.D. A Ca2+ concentration of 1.5 mM, as present in IMDM but not in RPMI, is critical for maximal response of Th cells to PMA/ionomycin. Eur. J. Immunol. 2015;45:1270–1273. doi: 10.1002/eji.201445247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-Seq data from this study were uploaded to GEO and may be accessed at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE135936.