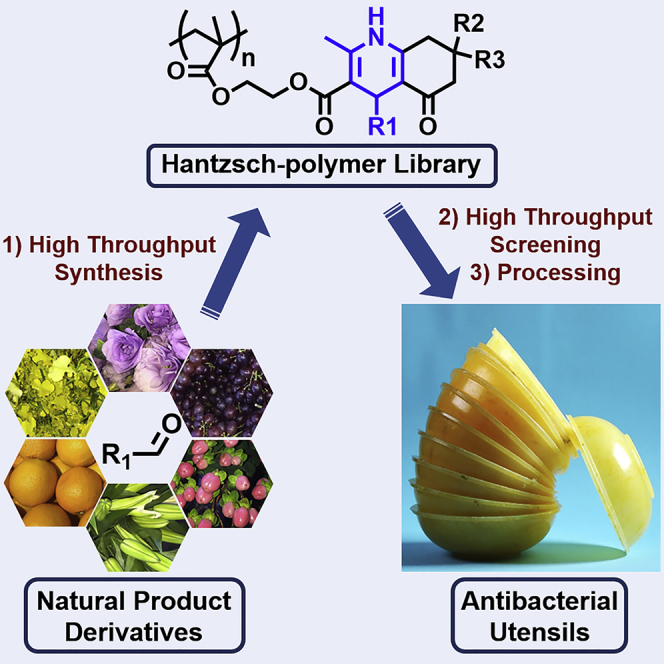
Summary
The Hantzsch and free-radical polymerization reactions were combined in a one-pot high-throughput (HTP) system to simultaneously prepare 30 unique polymers in parallel. Six aldehydes derived from natural products were used as the starting materials to rapidly prepare the library of 30 poly(1,4-dihydropyridines). From this library, HTP evaluation methods led to the identification of an antibacterial polymer. Mechanistic studies revealed that the dihydropyridine group in the polymer side-chain structure plays an important role in resisting bacterial attachment to the polymer surface, thus leading to the antibacterial function of this polymer. This research demonstrates the value of multicomponent reactions (MCRs) in interdisciplinary fields by discovering functional polymers for possible practical applications. It also provides insights to further developing new functional polymers using MCRs and HTP methods with important implications in organic chemistry, polymer chemistry, and materials science.
Subject Areas: Organic Chemistry, Natural Product Chemistry, Polymers
Graphical Abstract
Highlights
-
•
A one-pot high-throughput (HTP) system was developed to prepare a polymer library
-
•
A new antibacterial polymer was selected from this library via HTP measurements
-
•
This antibacterial polymer was mixed in polyethylene to get antibacterial utensils
-
•
Mechanism study revealed that the Hantzsch reaction leads to new antibacterial polymers
Organic Chemistry; Natural Product Chemistry; Polymers
Introduction
High-throughput (HTP) synthesis methods enable the rapid production of hundreds to even millions of samples for screening and identifying products with the desired properties/functions (Briceno et al., 1995, Danielson et al., 1997, Hanak, 1970, Lehn and Eliseev, 2001, Rademann and Jung, 2000, Xiang et al., 1995). This effectively accelerates the pace of research, significantly increasing productivity and resulting in remarkable benefits. In polymer chemistry, HTP strategies have been successfully applied to the discovery of new functional polymers. Many polymers have been synthesized in parallel and characterized via HTP methods for detailed studies of the subtle relationships between polymer structures and properties. As a result, many new functional polymers have been explored and used in interdisciplinary fields as sensors, gene/drug carriers, antibacterial surfaces, tissue engineering scaffolds, (stem)cell factories, etc.(Akinc et al., 2003, Algahtani et al., 2014, Anderson et al., 2004, Anderson et al., 2005, Anderson et al., 2006, Bosman et al., 2001, Celiz et al., 2014, Chapman et al., 2016, Goldberg et al., 2008, Gupta et al., 2010, Hook et al., 2012, Khan et al., 2010, Lynn et al., 2001, Mao et al., 2018, Mei et al., 2010, Meier et al., 2004a, Meier et al., 2004b, Neumann et al., 2017, Ting et al., 2015, Ting et al., 2016). This has opened new research directions in polymer science and expanded the application scope of polymers beyond traditional plastics and rubbers. Thus, the development of new HTP methods to synthesize, screen, and isolate new polymers with application values is important from both academic and industrial points of view.
Multicomponent reactions (MCRs) are defined as reactions where three or more reactants combine to effectively generate a single product. Recently, MCRs have aroused widespread attention in polymer chemistry because Meier and coworkers reported the synthesis of polycondensates via the tricomponent Passerini reaction (Kreye et al., 2011). Since that report, more and more MCRs have been exploited for the preparation of elegant polymers containing multicomponent main chains and side chains. Such MCRs include the Passerini, Ugi, Mannich, Biginelli, Hantzsch, and Kabachnik-Fields reactions, as well as metal-catalyzed and thiolactone-based MCRs (Blasco et al., 2017, Deng et al., 2012, Espeel et al., 2011, Kakuchi and Theato, 2014, Kreye et al., 2011, Lee et al., 2013, Liu et al., 2014, Llevot et al., 2017, Sehlinger et al., 2013, Siamaki et al., 2011, Theato, 2015, Wu et al., 2017a, Zhang et al., 2014, Zhang et al., 2016, Zhao et al., 2016).
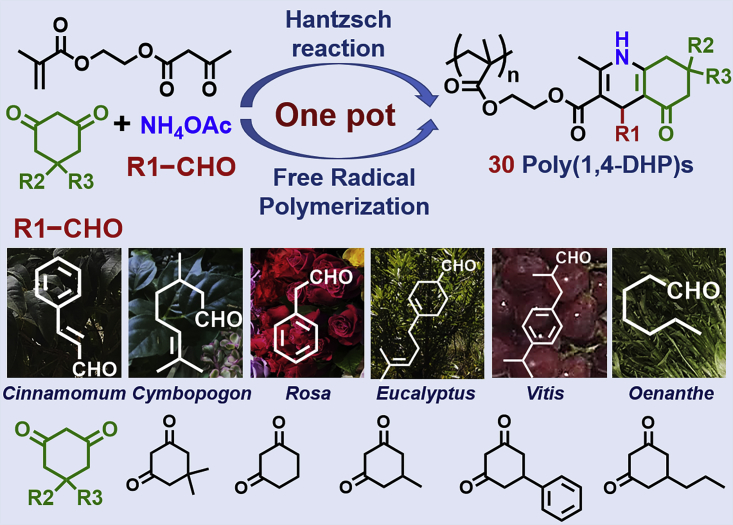
MCRs have been applied to the construction of polymer libraries in an HTP manner (Mao et al., 2018, Wu et al., 2017b, Xue et al., 2016). MCRs quickly increase the number and diversity of polymer samples, owing to their multicomponent nature, effectively improving the synthesis efficiency for constructing polymer libraries. Selected robust MCRs have been carried out during controlled radical polymerization (CRP) to successfully prepare well-defined polymers with multicomponent pendant/chain end groups in a one-pot manner (Yang et al., 2015, Zhang et al., 2014, Zhang et al., 2015, Zhu et al., 2013). This greatly simplifies the preparation of new polymers by avoiding the tedious separation and purification steps during a new monomer/initiator synthesis. HTP methods, MCRs, and a one-pot strategy straightforwardly improve the efficiency of polymer preparation; thus, the combination of HTP methods, MCRs, and a one-pot strategy might lead to a new methodology for facilely constructing polymer libraries. Herein, we report a one-pot HTP method that can quickly establish a library of poly(1,4-dihydropyridine)s (poly(1,4-DHP)s) via the Hantzsch reaction (a typical MCR) and free-radical polymerization (FRP) (Scheme 1).
Scheme 1.
One-Pot HTP Method for Preparing a Library of Poly(1,4-DHP)s
The Hantzsch reaction, reported by Arthur R. Hantzsch in 1881(Hantzsch, 1881), consists of four common components (aldehyde, β-ketoester, 1,3-diketone, and NH4OAc) and effectively produces 1,4-dihydropyridines (1,4-DHPs), which are candidate drugs for treating cardiovascular diseases (Loev et al., 1974, Stout and Meyers, 1982). The Hantzsch reaction has been broadly studied in organic chemistry and pharmaceutical chemistry; only recently has this venerable reaction been explored in polymer chemistry. Well-defined polymers with 1,4-DHP side groups have been efficiently prepared by the combination of the Hantzsch reaction and reversible addition-fragmentation chain transfer (RAFT) polymerization in a one-pot fashion (Zhang et al., 2015). Here, we chose FRP instead of CRP to further simplify the polymerization process. Aldehydes derived from natural products were reacted with a commercially available monomer, 2-(acetoacetoxy) ethyl methacrylate (AEMA), via the Hantzsch reaction during the FRP process. The polymers were synthesized in parallel using one-pot reactions containing different combinations of six aldehydes (A(X)) and five 1,3-cyclohexanedione compounds (B(Y)) to simultaneously create 6 × 5 = 30 unique polymers (P(X) (Y)).
The resulting polymers were fully characterized by NMR, gel permeation chromatography (GPC), thermal gravimetric analysis (TGA), and differential scanning calorimetry (DSC) to determine the polymerization conversions and investigate polymer properties. These polymers were then screened via HTP measurement techniques to ultimately identify the polymer with the best antibacterial ability. This polymer was then blended with commodity polymers (polyethylene [PE], poly(propene) [PP], poly(ethylene terephthalate) [PET], poly(methyl methacrylate) [PMMA], and polyamide [PA66]) to dramatically improve the antibacterial ability of these general polymers without impairing the mechanical strength of the overall polymer system. This research used the Hantzsch reaction and HTP methods to develop antibacterial polymers via a green chemistry approach by utilizing aldehydes derived from natural sources instead of oil-based products. This demonstrates the utility of synthesis strategies in polymer chemistry to explore functional polymers for practical applications.
Results and Discussion
One-Pot HTP Preparation of Polymers via the Hantzsch Reaction and FRP
In a representative procedure, A(X), B(Y), AEMA, and NH4OAc were added to centrifuge tubes; the molar ratio of each component was A(X): B(Y): AEMA: NH4OAc = 1:1:1:1.5. Solvents (acetonitrile and 1,4-dioxane [1/1, v/v]) were then added followed by glycine (10 mol% with respect to aldehydes) and 2,2ʹ-azobisisoheptonitrile (ABVN, 2 mol% to AEMA) as the catalyst for the Hantzsch reaction and the FRP initiator, respectively. The 30 charged tubes were purged with bubbling nitrogen for 20 min and then immersed in a 75°C oil bath for 12 h. After quenching the polymerization in an ice-water bath, aliquots (20–50 μL) were taken for 1H NMR and GPC analyses. For every tube, AEMA was polymerized in high conversions (95%–99%), and the peaks associated with β-ketoester groups of AEMA nearly completely disappeared (92%–99%) (Figure S1 [P(X) (1) as a typical example], Table S1). All poly(1,4-DHP)s had high molecular weights (Mn(GPC): 16,000–28,000 g mol−1, Figure S2 [P(X) (1) as a typical example], Table S1). It is noticed that P(5) (Y) have broader polydispersity indices (PDIs) than others. This might be attributed to the iso-propylbenzene moieties in P(5) (Y). The iso-propylbenzene group is capable of producing radical during polymerization (Gregg and Mayo, 1947, Okamura and Katagiri, 1958). The iso-propylbenzene radical in polymer structures might link other polymer chains leading to broad PDIs.
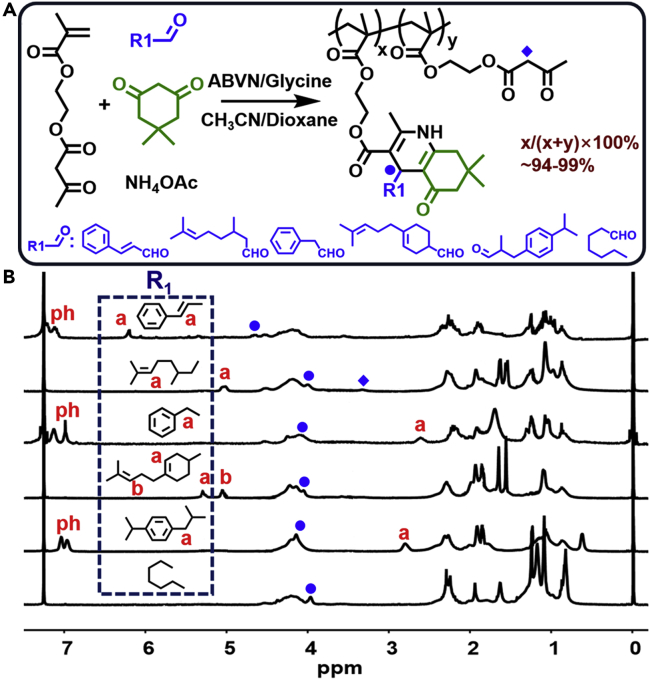
The P(X) (Y) were purified through simple precipitation in water followed by re-precipitation from tetrahydrofuran by diethyl ether. As a typical example, the 1H NMR spectra of P(X) (1) are presented in Figure 1.
Figure 1.
Preparation of P(X)(1)
(A) Reaction conditions: acetonitrile/dioxane (1/1, v/v), glycine (10% to aldehydes), ABVN (2% to AEMA), 75°C for 12 h. A(X): B(1): AEMA: NH4OAc = 1:1:1:1.5.
(B) 1H NMR spectra (CDCl3, 400M) of P(X) (1).
The specific peaks of the 1,4-DHP moieties (R1-CH) can be found at 3.95–4.55 ppm, whereas the peaks of the AEMA vinyl group (6.07, 5.55 ppm) have completely disappeared. The unreacted β-ketoesters (∼3.48 ppm) in P(X) (1) were calculated as ∼1%–6% (Table S1). Other P(X) (Y) polymers showed similar results (Figures S3–S6, Table S1). Thus, the selected aldehydes and 1,3-cyclohexanedione derivatives were suitable components for the Hantzsch reaction and highly compatible with FRP.
To compare the effectiveness of the one-pot HTP method with the traditional monomer polymerization approach, P(X) (1) with 100% 1,4-DHP side groups were prepared in a two-step process (Figures S7–S12). As a typical example, cinnamaldehyde (A(1)) was reacted with AEMA and dimedone (B(1)) via the Hantzsch reaction to obtain the target monomer M(1) (1) in 73.6% yield after column chromatography purification. M(1) (1) was polymerized through FRP to obtain the desired “pure” polymer P(1) (1). 1H NMR spectra indicated that this polymer was nearly identical to that obtained by the one-pot HTP method (Figure S7). Similar results were observed when other aldehydes were used to prepare pure monomers and polymers (Figures S8–S12). This suggests the feasibility of this one-pot HTP strategy to quickly build a library of target polymers in a time-saving and labor-saving manner.
HTP Analyses of the Antibacterial Capabilities of P(X) (Y)
1,4-DHP derivatives have been reported as potential antimicrobial agents, antioxidants, and anti-inflammatory agents (Vijesh et al., 2011). The selected aldehydes in this study have been widely employed in the cosmetic, food, and pharmaceutical industries as flavor agents, antibacterial agents, and antioxidants (Aziz and Karboune, 2018, Gyawali and Ibrahim, 2014). Thus, this library of poly(1,4-DHP)s might contain new functional polymers with bioactive properties. To test this hypothesis, the antibacterial capability of the resulting polymers was evaluated via HTP measurement techniques.
Preliminary Screening
The resulting polymers were mixed with poly(ethylene) (PE) to prepare mixed hot-pressed samples. The weight ratio of P(X) (Y) in P(X) (Y)-PE was 33%, and the actual polymer concentrations on PE surface were found to range from 27.0% to 39.9% by X-ray photoelectron spectroscopy (XPS) analyses (Table S2). Escherichia coli and Staphylococcus aureus were used as representative Gram-negative and Gram-positive bacteria, respectively. Both bacteria were transfected with red fluorescent protein (RFP) for easy observation.
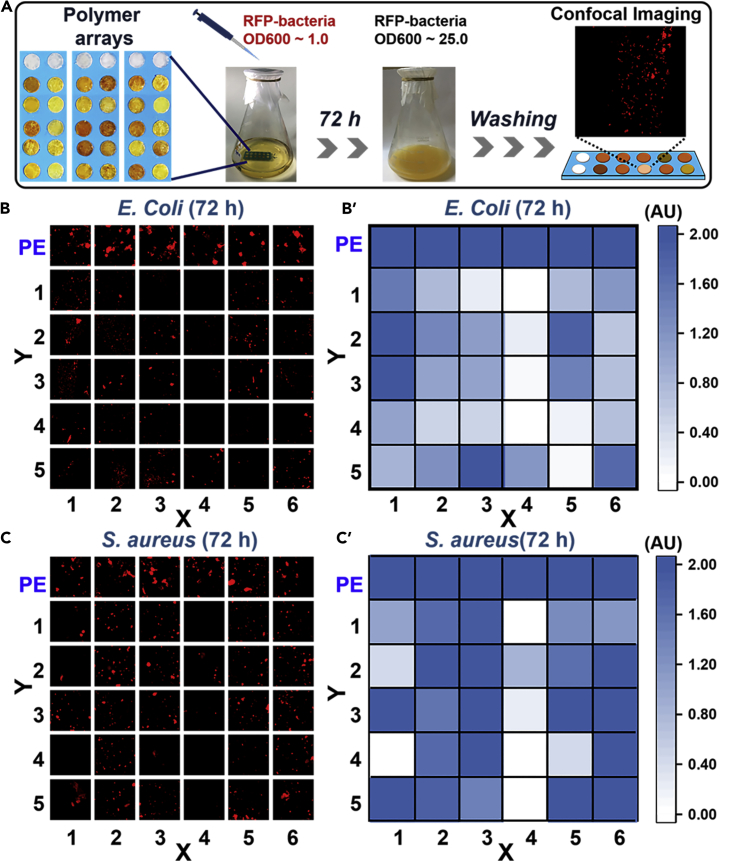
Briefly, 30 disk samples of P(X) (Y)-PE (diameter: 5 mm, thickness: 0.5 mm) were attached to three pieces of glass to form three mini-arrays (Figure 2A, 10 samples/piece). Pure PE disks served as the control. These polymer arrays were sterilized by a 75% ethanol aqueous solution and UV light irradiation (254 nm, 40 w, 30 min), then put into a Luria-Bertani (LB) broth (200 mL) followed by the addition of a suspension of planktonic E. coli or S. aureus (10 μL). The optical density of this suspension at UV ∼ 600 nm (OD600) was approximately 1.0. The polymer arrays were incubated with bacteria for 72 h before observation by laser scanning confocal microscopy (LSCM) (Figure 2A). The fluorescence intensity at each sample disk reflected the number of attached bacteria.
Figure 2.
HTP analysis of the antibacterial capabilities of P(X) (Y)
(A) Schematic of the polymer arrays and the test procedure.
(B) LSCM images and (B’) intensity map of E. coli on sample disks after 72-h incubation. PE served as the control; each image is 200 × 200 μm. Three replicate samples were tested.
(C) LSCM images and (C’) intensity map of S. aureus on sample disks after 72-h incubation. PE served as the control; each image is 200 × 200 μm. Three replicate samples were tested.
From the LSCM images (Figures 2B and 2C), the P(X) (Y)-PE samples contained far fewer bacteria than the pure PE sample. This was interpreted as the antibacterial capability of these 1,4-DHP-containing polymers. The quantitative fluorescence intensity maps of the samples indicated almost no E. coli on P(4) (1), P(4) (3), P(4) (4), and P(5) (5) (Figure 2B’) and almost no S. aureus on P(1) (4), P(4) (1), P(4) (3), P(4) (4), and P(4) (5) (Figure 2C’). Meanwhile, mature biofilm might begin to disperse (Morimatsu et al., 2012, Steinberger et al., 2002); thus, these polymer samples have been tested with shorter culture time and/or lower nutrient medium (Figure S13: normal medium/24 h culture; Figure S14: 20% nutrient medium/72 h culture; Figure S15: 20% nutrient medium/24 h culture). More samples demonstrated excellent antibacterial capability to E. coli and S. aureus suggesting negligible biofilm generated during the mini-array experiment. Then, the antibacterial efficiency of all poly(1,4-DHP)s was calculated by comparing the fluorescence intensity on P(X) (Y)-PE and PE (Table S3, normal medium/72 h). P(4) (1), P(4) (3), and P(4) (4) demonstrated better antibacterial efficiency to both E. coli and S. aureus (>95%) than the other poly(1,4-DHP)s; thus, these three polymers were selected as the top three broad-spectrum antibacterial polymers for the next round of screening.
Secondary Screening
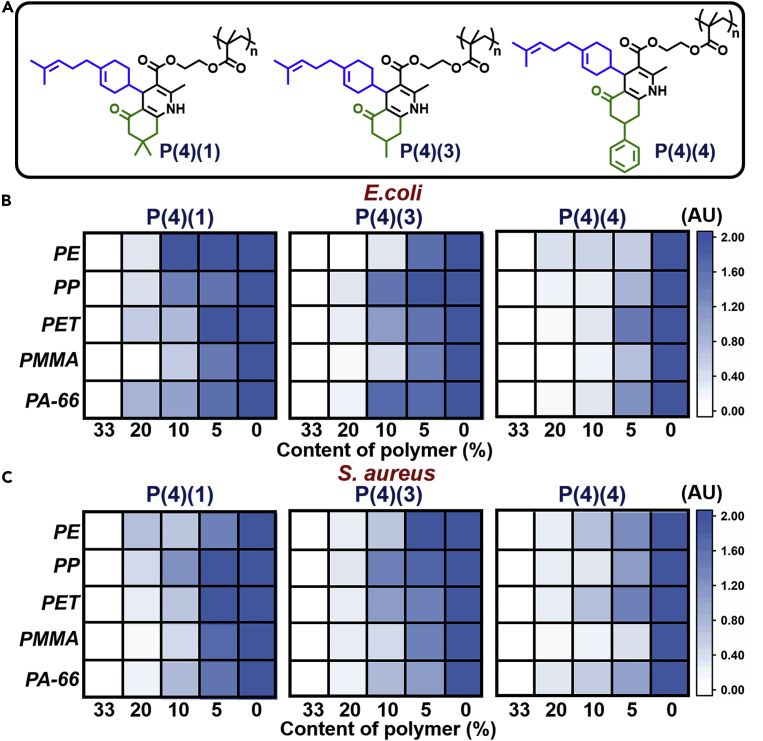
Commodity polymers are indispensable to everyday life. If items composed from these polymers can be imbued with antibacterial properties, the value of the items produced with such polymers would be increased greatly. Thus, the three best polymers from preliminary testing (Figure 3A) were blended into five commonly found commodity polymers (PE, PP, PET, PMMA, and PA66) in different ratios (33%, 20%, 10%, and 5%).
Figure 3.
HTP analysis of the antibacterial capabilities of selected polymers
(A) The three poly(1,4-DHP)s selected for further study after preliminary tests.
(B and C) Intensity map of the bacteria on sample disks (commodity polymers + P(X) (Y)), 72 h culture. Three replicate samples were tested, and pure commodity polymer disks served as the controls.
The antibacterial capabilities of these P(4) (1,3,4)-polymer samples were subjected to a similar test as before. Pure commodity polymer disks were used as the respective controls.
The fluorescence intensity maps (Figures 3B and 3C; Table S4) were obtained according to the LSCM images (Figure S16). The greater the P(4) (1,3,4) content, the fewer the bacteria found on the P(4) (1,3,4)-polymer blends, indicative of a concentration-dependent antibacterial property. When the poly(1,4-DHP) to commodity polymer blend was 10 wt.%, P(4) (4) offered the best antibacterial properties. Thus, P(4) (4) was selected for further research.
Development of a Model Antibacterial Utensil
For items made from these commodity polymer-antibacterial polymer blends to remain practical, the basic mechanical properties of the base commodity polymer must not be meaningfully compromised. Thus, the mechanical strength of the P(4) (4)-polymer samples (33 wt.% P(4) (4)) was tested using PE, PP, PET, PMMA, and PA66 (Figure S17). All five 33 wt.% P(4) (4)-polymer samples yielded the similar mechanical strength results as the pure-polymer controls. This suggests that commodity polymers can be easily blended with antibacterial P(4) (4) without a loss of mechanical strength.
Next, we applied the injection molding technique to form antibacterial plastic products from a blended P(4) (4) and PE mixture. PE was chosen as a representative sample owing to its easy machining and broad applicability as a packaging material. P(4) (4)-PE (33 wt.% of P(4) (4)) and PE were injection molded into plastic bowls (Figure 4A, diameter: 44 mm, height: 14 mm).
Figure 4.
Antibacterial bowls made from P(4)(4)-PE
(A) Bowls made from PE and P(4) (4)-PE.
(B) Commercially available beverages (5 mL) for testing and the experimental setup.
(C) Photographic images of the plate-streaking experiment: 100 μL sample on LB agar (15 g/L), 24-h culture.
An experiment was designed to evaluate the antibacterial ability of these bowls by simulating the actual application. Briefly, these bowls were sterilized by a 75% ethanol aqueous solution and air dried on a laboratory bench for 24 h. Then, various commonly consumed beverages (water, orange juice, milk, red wine, coffee, and black tea) (5 mL) were placed in the PE and P(4) (4)-PE bowls (Figure 4B). Glass covers were placed on the bowls, and the beverages were incubated at 37°C. Aliquots were obtained at specified time points.
A plate-streaking experiment was performed to test for the presence of viable bacteria. After 24 h, beverage aliquots (100 μL) from the different bowls were taken and evenly coated on the surface of LB agar-coated petri dishes (diameter: 90 mm, thickness: 6 mm, LB: 1.5 g/L). These petri dishes were incubated at 37°C before observation, and the original beverages were used as the controls (Figure 4C).
Commercially available beverages are typically sterile; thus, almost no bacterial colonies were observed (Figure 4C, control). However, aliquots of water, orange juice, milk, and coffee from the pure PE bowls clearly contained bacteria, as demonstrated by the visible colonies grown on the LB agar petri dishes (Figure 4C, PE). This suggests that the PE bowls were contaminated with germs when stored in the open air, leading to the contamination of these beverages inside. Notably, red wine and black tea samples from the pure PE bowls yielded similar results to the control during the LB agar growth test. This was attributed to the antibacterial compounds contained in the parent beverages, such as tannin, ethanol, and tea polyphenols (Figure 4C, PE). On the other hand, only milk in the P(4) (4)-PE bowl resulted in a small bacterial colony on the LB agar surface (Figure 4C, P(4) (4)-PE). This suggests that only milk contained sufficient nutrients to encourage growth of the small amount of bacteria present after 24 h of open-air storage and overcome the inherent antibacterial properties of the P(4) (4)-PE bowls.
Additional samples were obtained after 48 and 72 h to repeat the plate-streaking experiments (Figure S18). Denser and denser bacterial colonies were observed in the pure PE group over time, indicating propagation of bacteria in beverages incubated in the pure PE bowls. The inherent antibacterial defenses of tea and red wine expired after 48 and 72 h, respectively. Nevertheless, all beverages, except milk, in the P(4) (4)-PE bowls remained bacteria-free after 48 h of incubation (Figure S18A). Red wine and tea in P(4) (4)-PE bowls remained sterile even after 72-h incubation (Figure S18B). These results agreed well with the quantitative OD600 analyses (Figure S19).
Furthermore, a quantitative experiment was performed according to a standard method (QB/T2591-2003) to evaluate the antibacterial capability of these bowls. The bowls were incubated with S. aureus (an approved model bacterium, 5 mL, LB medium, OD600 ∼1.0) for 24 h at 37°C, then washed by sterilized PBS followed by addition of beverages (5 mL). A plate-streaking experiment and OD600 analyses were performed at different time points to test viable bacteria (Figures S20 and S21). The antibacterial results obtained by this way are similar to those obtained by the previous method, suggesting great potential for the P(4) (4)-PE blended polymer as a new antibacterial material for utensils and food applications.
Mechanism Study
The possible mechanisms for antibacterial action of P(4) (4) were studied. Several polymers were used according to the different evaluation protocols.
Bactericidal Ability
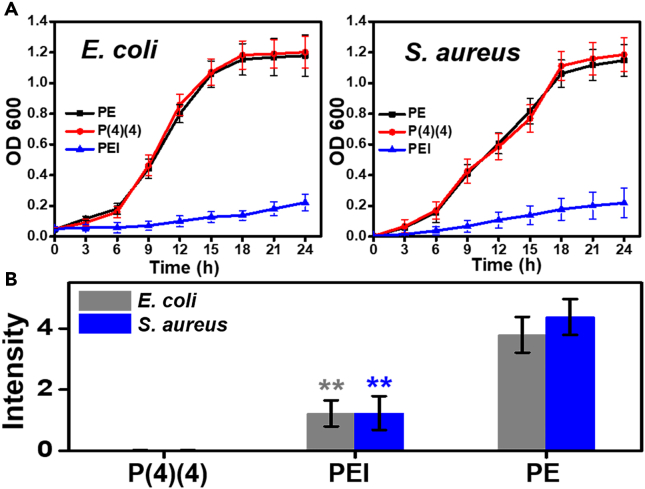
Killing bacteria is a general strategy in developing antibacterial materials. Some 1,4-DHPs have been reported to effectively inhibit bacterial growth. Thus, the bactericidal ability of P(4) (4) was evaluated. Polyetherimide (PEI), a well-known antibacterial polymer that is lethal to bacteria(Gibney et al., 2012), was used as the control. P(4) (4)-PE (33 wt.% P(4) (4)) and PEI-PE (33 wt.% PEI) disks were prepared as mentioned earlier. These two samples were attached to the bottom of a multi-well plate and covered with LB broth (200 μL). Then, a suspension of planktonic E. coli or S. aureus (10 μL, OD600∼1.0) was added. The plate was incubated at 37°C for 24 h. The OD600 values were tested at different time intervals, and a pure PE disk was used as the blank control.
From the OD600 curves over time (Figure 5A), the rate of bacterial growth was slower with PEI-PE than with P(4) (4)-PE and PE, confirming the ability of PEI to kill bacteria. There was no observable difference in bacterial growth rates between P(4) (4)-PE and PE. This suggests that P(4) (4) has negligible bactericidal effects. Thus, P(4) (4) likely achieves its antibacterial function by preventing bacterial adhesion or biofilm formation instead of killing the bacteria outright. Moreover, P(4) (4) has weaker interaction than PE with lipopolysaccharide and peptidoglycan, two polysaccharides on the bacterial surface (Table S5). This suggests that the interaction of P(4) (4) with the cell membrane may play a role in its ability to prevent biofilm formation.
Figure 5.
Evaluation of bactericidal ability of P(4)(4)-PE
(A) OD600 values versus time in the presence of different polymers, 24-h culture. Data are represented as mean ± SD, n = 5.
(B) Intensity data of bacteria on sample disks after 72-h incubation. Data are represented as mean ± SD, n = 3; **p < 0.01, compared with PE.
Then, the antibacterial abilities of samples were evaluated through the polymer-array method. After 72-h incubation, PEI effectively killed bacteria, resulting in much less bacteria on the PEI-PE surface than on PE (Figure 5B, p < 0.01 compared with PE; Figure S22). However, almost no bacteria was detected on the P(4) (4)-PE surface despite the poor sterilization ability of P(4) (4) (Figures 5B and S22).
Molecular Structures
Polymer functions are determined by their molecular structures. Thus, the relationship between the structure of P(4) (4) and its antibacterial ability was studied.
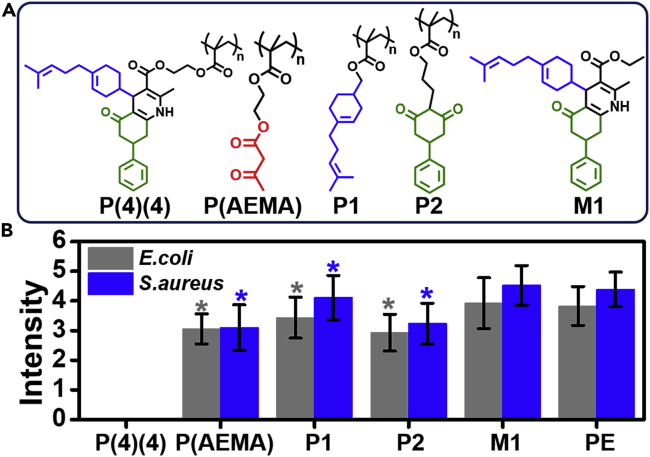
The Hantzsch ring in P(4) (4) is composed of a long-branched olefin and aromatic groups. There are still some β-ketoester moieties (∼2%) left in P(4) (4). Thus, two polymers containing only the long-branched olefin and aromatic groups were prepared (Figures 6A, P1, P2; S23, and S24). A homopolymer of AEMA was also prepared (Figures 6A, P(AEMA) and S25). Meanwhile, a small molecule with similar Hantzsch ring as the side group of P(4) (4) was prepared (Figure 6A, M1). These polymers (P1, P2, P(AEMA)) and M1 were mixed with PE to prepare samples, respectively. The antibacterial capabilities of these samples were studied via the polymer-array method. The sample composed of pure PE served as the blank.
Figure 6.
Relationship between the structure of P(4)(4) and its antibacterial ability
(A) P(4) (4), P(AEMA), P1 (polymer containing a long-branched olefin), P2 (polymer containing an aromatic group), and M1.
(B) Intensity data of bacteria on sample disks after a 72-h culture. Data are represented as mean ± SD, n = 3; *p < 0.05, compared with PE.
The fluorescence signals of E. coli and S. aureus on the polymer samples were recorded (Figure S26). Quantitative data from the fluorescence intensity were calculated, and the number of bacteria present on the samples followed the order P(4) (4) << P(AEMA) ≈ P2 < P1 < M1 ≈ PE (Figure 6B). M1 concentration on the PE surface was tested by XPS as 29.5% (Table S2), and M1-PE had almost no antibacterial ability (p = 0.118 [E. coli], 0.137 [S. aureus], compared with PE), suggesting that direct addition of free 1,4-DHP molecules to bulk commodity polymers would not be effective. P1, P2, and P(AEMA) demonstrated poor antibacterial capability (p < 0.05, compared with PE), whereas P(4) (4) had the best antibacterial ability among all poly(1,4-DHP)s. These results suggest the polymer chain and 1,4-DHP pendant group both play key roles for the antibacterial function of P(4) (4); the long-branched olefin and aromatic groups enhance the antibacterial ability of P(4) (4) via a possible synergistic effect.
Limitation of the Study
Here, a simple polymer (random structure with a broad PDI) with potential antibacterial applications was developed via a very simple HTP method (small sample pool and semiquantitative measurements). This highlights the validity of HTP methods and MCRs in exploring new functional polymers. However, in addition to side-chain groups, the core polymer structure can also be important for polymer functions. Recent studies have reported that the antibacterial capability of polymers can be remarkably improved by tuning the polymer structures (i.e., monomer sequences and topology structures) (Judzewitsch et al., 2018, Kuroki et al., 2017, Lam et al., 2016, Namivandi-Zangeneh et al., 2018). Currently, well-defined polymers can be rapidly prepared via modern technologies in CRP, including single electron transfer-atom transfer radical polymerization (SET-ATRP), sulfur-free RAFT emulsion polymerization, photoinduced ATRP, and photoinduced electron/energy transfer-RAFT (PET-RAFT) (Anastasaki et al., 2014, Anastasaki et al., 2016, Boyer et al., 2016, Carmean et al., 2017, Chen et al., 2016, Engelis et al., 2017, Fors and Hawker, 2012, Gormley et al., 2018, Rosen and Percec, 2009, Xu et al., 2016, Zhang et al., 2013). The future combination of these modern CRP techniques with the method used here might offer new polymer libraries with different side groups, molecular weights, monomer sequences, topology structures, etc. This will be helpful for developing new polymers with improved antibacterial capabilities and other bioactive functions.
Conclusions
In summary, we have developed a one-pot HTP method by simultaneously carrying out the Hantzsch reaction and FRP in an HTP manner. Using this method, six aldehydes derived from natural products were used to rapidly synthesize 30 poly(1,4-DHP)s in one step. This highlighted the advantage of MCRs in amplifying molecular diversity and the superiority of a one-pot strategy in improving synthesis efficiency. The resulting poly(1,4-DHP)s were screened via HTP measurement methods to ultimately identify a polymer that could effectively resist bacterial attachment due to its unique 1,4-DHP side group. This opens the door to opportunities for developing antibacterial compounds via the Hantzsch reaction. The developed one-pot method highlighted the value of HTP methods, natural product derivatives, and MCRs for exploring new functional polymers with potential applications in the real world. These results might prompt a broad study of MCRs and HTP methods in polymer science for developing other new functional polymers for interdisciplinary applications.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgment
This research was supported by the National Science Foundation of China (21574073, 21971141).
Author Contributions
L.T. developed the concept and conceived the experiments. G.L., Y.L., H.W., and Y.Z. performed the laboratory experiments. L.T., G.L., Q.Z., and X.W. contributed to the experimental analyses. L.T. and G.L. wrote the manuscript. L.T. and Y.W. provided financial support to the research.
Declaration of Interests
The authors declare no competing financial interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100754.
Supplemental Information
References
- Akinc A., Lynn D.M., Anderson D.G., Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J. Am. Chem. Soc. 2003;125:5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- Algahtani M.S., Scurr D.J., Hook A.L., Anderson D.G., Langer R.S., Burley J.C., Alexander M.R., Davies M.C. High throughput screening for biomaterials discovery. J. Control Release. 2014;190:115–126. doi: 10.1016/j.jconrel.2014.06.045. [DOI] [PubMed] [Google Scholar]
- Anastasaki A., Nikolaou V., Nurumbetov G., Wilson P., Kempe K., Quinn J.F., Davis T.P., Whittaker M.R., Haddleton D.M. Cu(0)-Mediated living radical polymerization: a versatile tool for materials synthesis. Chem. Rev. 2016;116:835–877. doi: 10.1021/acs.chemrev.5b00191. [DOI] [PubMed] [Google Scholar]
- Anastasaki A., Nikolaou V., Zhang Q., Burns J., Samanta S.R., Waldron C., Haddleton A.J., McHale R., Fox D., Percec V. Copper(II)/Tertiary amine synergy in photoinduced living radical polymerization: accelerated synthesis of omega-functional and alpha,omega-heterofunctional poly(acrylates) J. Am. Chem. Soc. 2014;136:1141–1149. doi: 10.1021/ja411780m. [DOI] [PubMed] [Google Scholar]
- Anderson D.G., Peng W.D., Akinc A., Hossain N., Kohn A., Padera R., Langer R., Sawicki J.A. A polymer library approach to suicide gene therapy for cancer. Proc. Natl. Acad. Sci. U S A. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.G., Putnam D., Lavik E.B., Mahmood T.A., Langer R. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Anderson D.G., Tweedie C.A., Hossain N., Navarro S.M., Brey D.M., Van Vliet K.J., Langer R., Burdick J.A. A combinatorial library of photocrosslinkable and degradable materials. Adv. Mater. 2006;18:2614–2618. [Google Scholar]
- Aziz M., Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: a review. Crit. Rev. Food Sci. 2018;58:486–511. doi: 10.1080/10408398.2016.1194256. [DOI] [PubMed] [Google Scholar]
- Blasco E., Sims M.B., Goldmann A.S., Sumerlin B.S., Barner-Kowollik C. 50th anniversary perspective: polymer functionalization. Macromolecules. 2017;50:5215–5252. [Google Scholar]
- Bosman A.W., Heumann A., Klaerner G., Benoit D., Frechet J.M.J., Hawker C.J. High-throughput synthesis of nanoscale materials: structural optimization of functionalized one-step star polymers. J. Am. Chem. Soc. 2001;123:6461–6462. doi: 10.1021/ja010405z. [DOI] [PubMed] [Google Scholar]
- Boyer C., Corrigan N.A., Jung K., Nguyen D., Nguyen T.K., Adnan N.N.M., Oliver S., Shanmugam S., Yeow J. Copper-mediated living radical polymerization (atom transfer radical polymerization and copper(0) mediated polymerization): from fundamentals to bioapplications. Chem. Rev. 2016;116:1803–1949. doi: 10.1021/acs.chemrev.5b00396. [DOI] [PubMed] [Google Scholar]
- Briceno G., Chang H.Y., Sun X.D., Schultz P.G., Xiang X.D. A class of cobalt oxide magnetoresistance materials discovered with combinatorial synthesis. Science. 1995;270:273–275. [Google Scholar]
- Carmean R.N., Becker T.E., Sims M.B., Sumerlin B.S. Ultra-high molecular weights via aqueous reversible-deactivation radical polymerization. Chem. 2017;2:93–101. [Google Scholar]
- Celiz A.D., Smith J.G.W., Langer R., Anderson D.G., Winkler D.A., Barrett D.A., Davies M.C., Young L.E., Denning C., Alexander M.R. Materials for stem cell factories of the future. Nat. Mater. 2014;13:570–579. doi: 10.1038/nmat3972. [DOI] [PubMed] [Google Scholar]
- Chapman R., Gormley A.J., Stenzel M.H., Stevens M.M. Combinatorial low-volume synthesis of well-defined polymers by enzyme degassing. Angew. Chem. Int. Ed. 2016;55:4500–4503. doi: 10.1002/anie.201600112. [DOI] [PubMed] [Google Scholar]
- Chen M., Zhong M.J., Johnson J.A. Light-controlled radical polymerization: mechanisms, methods, and applications. Chem. Rev. 2016;116:10167–10211. doi: 10.1021/acs.chemrev.5b00671. [DOI] [PubMed] [Google Scholar]
- Danielson E., Golden J.H., McFarland E.W., Reaves C.M., Weinberg W.H., Wu X.D. A combinatorial approach to the discovery and optimization of luminescent materials. Nature. 1997;389:944–948. [Google Scholar]
- Deng X.X., Li L., Li Z.L., Lv A., Du F.S., Li Z.C. Sequence regulated poly(ester-amide)s based on Passerini reaction. ACS Macro. Lett. 2012;1:1300–1303. doi: 10.1021/mz300456p. [DOI] [PubMed] [Google Scholar]
- Engelis N.G., Anastasaki A., Nurumbetov G., Truong N.P., Nikolaou V., Shegiwal A., Whittaker M.R., Davis T.P., Haddleton D.M. Sequence-controlled methacrylic multiblock copolymers via sulfur-free RAFT emulsion polymerization. Nat. Chem. 2017;9:171–178. doi: 10.1038/nchem.2634. [DOI] [PubMed] [Google Scholar]
- Espeel P., Goethals F., Du Prez F.E. One-pot multistep reactions based on thiolactones: extending the realm of thiol-ene chemistry in polymer synthesis. J. Am. Chem. Soc. 2011;133:1678–1681. doi: 10.1021/ja1098098. [DOI] [PubMed] [Google Scholar]
- Fors B.P., Hawker C.J. Control of a living radical polymerization of methacrylates by light. Angew. Chem. Int. Ed. 2012;51:8850–8853. doi: 10.1002/anie.201203639. [DOI] [PubMed] [Google Scholar]
- Gibney K.A., Sovadinova I., Lopez A.I., Urban M., Ridgway Z., Caputo G.A., Kuroda K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012;12:1279–1289. doi: 10.1002/mabi.201200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Mahon K., Anderson D. Combinatorial and rational approaches to polymer synthesis for medicine. Adv. Drug Deliver. Rev. 2008;60:971–978. doi: 10.1016/j.addr.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley A.J., Yeow J., Ng G., Conway O., Boyer C., Chapman R. An oxygen-tolerant PET-RAFT polymerization for screening structure-activity relationships. Angew. Chem. Int. Ed. 2018;57:1557–1562. doi: 10.1002/anie.201711044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R.A., Mayo F.R. Chain transfer in the polymerisation of styrene iii. The reactivities of hydrocarbons toward the styrene radical. Discuss. Faraday Soc. 1947;2:328–342. [Google Scholar]
- Gupta N., Lin B.F., Campos L., Dimitriou M.D., Hikita S.T., Treat N.D., Tirrell M.V., Clegg D.O., Kramer E.J., Hawker C.J. A versatile approach to high-throughput microarrays using thiol-ene chemistry. Nat. Chem. 2010;2:138–145. doi: 10.1038/nchem.478. [DOI] [PubMed] [Google Scholar]
- Gyawali R., Ibrahim S.A. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. [Google Scholar]
- Hanak J.J. Multiple-sample-concept in materials research - synthesis, compositional analysis and testing of entire multicomponent systems. J. Mater. Sci. 1970;5:964–971. [Google Scholar]
- Hantzsch A. Condensationprodukte aus Aldehydammoniak und Ketonartigen Verbindungen. Ber. Dtsch. Chem. Ges. 1881;14:1637–1638. [Google Scholar]
- Hook A.L., Chang C.Y., Yang J., Luckett J., Cockayne A., Atkinson S., Mei Y., Bayston R., Irvine D.J., Langer R. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol. 2012;30:868–875. doi: 10.1038/nbt.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judzewitsch P.R., Nguyen T.K., Shanmugam S., Wong E.H.H., Boyer C. Towards sequence-controlled antimicrobial polymers: effect of polymer block order on antimicrobial activity. Angew. Chem. Int. Ed. 2018;57:4559–4564. doi: 10.1002/anie.201713036. [DOI] [PubMed] [Google Scholar]
- Kakuchi R., Theato P. Efficient multicomponent postpolymerization modification based on kabachnik-fields reaction. ACS Macro. Lett. 2014;3:329–332. doi: 10.1021/mz500139c. [DOI] [PubMed] [Google Scholar]
- Khan F., Tare R.S., Kanczler J.M., Oreffo R.O.C., Bradley M. Strategies for cell manipulation and skeletal tissue engineering using high-throughput polymer blend formulation and microarray techniques. Biomaterials. 2010;31:2216–2228. doi: 10.1016/j.biomaterials.2009.11.101. [DOI] [PubMed] [Google Scholar]
- Kreye O., Toth T., Meier M.A.R. Introducing multicomponent reactions to polymer science: Passerini reactions of renewable monomers. J. Am. Chem. Soc. 2011;133:1790–1792. doi: 10.1021/ja1113003. [DOI] [PubMed] [Google Scholar]
- Kuroki A., Sangwan P., Qu Y., Peltier R., Sanchez-Cano C., Moat J., Dowson C.G., Williams E.G.L., Locock K.E.S., Hartlieb M. Sequence control as a powerful tool for improving the selectivity of antimicrobial polymers. ACS Appl. Mater. Interface. 2017;9:40117–40126. doi: 10.1021/acsami.7b14996. [DOI] [PubMed] [Google Scholar]
- Lam S.J., O'Brien-Simpson N.M., Pantarat N., Sulistio A., Wong E.H.H., Chen Y.Y., Lenzo J.C., Holden J.A., Blencowe A., Reynolds E.C. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016;1:16162. doi: 10.1038/nmicrobiol.2016.162. [DOI] [PubMed] [Google Scholar]
- Lee I.H., Kim H., Choi T.L. Cu-catalyzed multicomponent polymerization to synthesize a library of poly(N-sulfonylamidines) J. Am. Chem. Soc. 2013;135:3760–3763. doi: 10.1021/ja312592e. [DOI] [PubMed] [Google Scholar]
- Lehn J.M., Eliseev A.V. Chemistry - dynamic combinatorial chemistry. Science. 2001;291:2331–2332. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Gao M., Lam J.W.Y., Hu R.R., Tang B.Z. Copper-catalyzed polycoupling of diynes, primary amines, and aldehydes: a new one-pot multicomponent polymerization tool to functional polymers. Macromolecules. 2014;47:4908–4919. [Google Scholar]
- Llevot A., Boukis A.C., Oelmann S., Wetzel K., Meier M.A.R. An update on isocyanide-based multicomponent reactions in polymer science. Top. Curr. Chem. 2017;375:127–155. doi: 10.1007/s41061-017-0153-4. [DOI] [PubMed] [Google Scholar]
- Loev B., Goodman M.M., Snader K.M., Tedeschi R., Macko E. Hantzsch-type dihydropyridine hypotensive agents .3. J. Med. Chem. 1974;17:956–965. doi: 10.1021/jm00255a010. [DOI] [PubMed] [Google Scholar]
- Lynn D.M., Anderson D.G., Putnam D., Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of degradable polymer library. J. Am. Chem. Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- Mao T.F., Liu G.Q., Wu H.B., Wei Y., Gou Y.Z., Wang J., Tao L. High throughput preparation of UV-protective polymers from essential oil extracts via the Biginelli reaction. J. Am. Chem. Soc. 2018;140:6865–6872. doi: 10.1021/jacs.8b01576. [DOI] [PubMed] [Google Scholar]
- Mei Y., Saha K., Bogatyrev S.R., Yang J., Hook A.L., Kalcioglu Z.I., Cho S.W., Mitalipova M., Pyzocha N., Rojas F. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M.A.R., Gohy J.F., Fustin C.A., Schubert U.S. Combinatorial synthesis of star-shaped block copolymers: host-guest chemistry of unimolecular reversed micelles. J. Am. Chem. Soc. 2004;126:11517–11521. doi: 10.1021/ja0488481. [DOI] [PubMed] [Google Scholar]
- Meier M.A.R., Hoogenboom R., Schubert U.S. Combinatorial methods, automated synthesis and high-throughput screening in polymer research: the evolution continues. Macromol. Rapid Commun. 2004;25:21–33. [Google Scholar]
- Morimatsu K., Eguchi K., Hamanaka D., Tanaka F., Uchino T. Effects of temperature and nutrient conditions on biofilm formation of Pseudomonas putida. Food Sci. Technol. Res. 2012;18:879–883. [Google Scholar]
- Namivandi-Zangeneh R., Kwan R.J., Nguyen T.K., Yeow J., Byrne F.L., Oehlers S.H., Wong E.H.H., Boyer C. The effects of polymer topology and chain length on the antimicrobial activity and hemocompatibility of amphiphilic ternary copolymers. Polym. Chem. 2018;9:1735–1744. [Google Scholar]
- Neumann K., Conde-Gonzalez A., Owens M., Venturato A., Zhang Y.C., Geng J., Bradley M. An approach to the high-throughput fabrication of glycopolymer microarrays through thiol-ene chemistry. Macromolecules. 2017;50:6026–6031. [Google Scholar]
- Okamura S., Katagiri K. Chain transfer to telomer. Makromolekul. Chem. 1958;28:177–184. [Google Scholar]
- Rademann J., Jung G. Drug discovery - integrating combinatorial synthesis and bioassays. Science. 2000;287:1947–1948. doi: 10.1126/science.287.5460.1947. [DOI] [PubMed] [Google Scholar]
- Rosen B.M., Percec V. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chem. Rev. 2009;109:5069–5119. doi: 10.1021/cr900024j. [DOI] [PubMed] [Google Scholar]
- Sehlinger A., Kreye O., Meier M.A.R. Tunable polymers obtained from Passerini multicomponent reaction derived acrylate monomers. Macromolecules. 2013;46:6031–6037. [Google Scholar]
- Siamaki A.R., Sakalauskas M., Arndtsen B.A. A palladium-catalyzed multicomponent coupling approach to pi-conjugated oligomers: assembling imidazole-based materials from imines and acyl chlorides. Angew. Chem. Int. Ed. 2011;50:6552–6556. doi: 10.1002/anie.201100558. [DOI] [PubMed] [Google Scholar]
- Steinberger R.E., Allen A.R., Hansma H.G., Holden P.A. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa-unsaturated biofilms. Microb. Ecol. 2002;43:416–423. doi: 10.1007/s00248-001-1063-z. [DOI] [PubMed] [Google Scholar]
- Stout D.M., Meyers A.I. Recent advances in the chemistry of dihydropyridines. Chem. Rev. 1982;82:223–243. [Google Scholar]
- Theato P. Vol. 269. Springer; 2015. (Multi-Component and Sequential Reactions in Polymer Synthesis). [Google Scholar]
- Ting J.M., Nayale T.S., Jones S.D., Bates F.S., Reineke T.M. Deconstructing HPMCAS: excipient design to tailor polymer-drug interactions for oral drug delivery. ACS Biomater. Sci. Eng. 2015;1:978–990. doi: 10.1021/acsbiomaterials.5b00234. [DOI] [PubMed] [Google Scholar]
- Ting J.M., Tale S., Purchel A.A., Jones S.D., Widanapathirana L., Tolstyka Z.P., Guo L., Guillaudeu S.J., Bates F.S., Reineke T.M. High-throughput excipient discovery enables oral delivery of poorly soluble pharmaceuticals. ACS Cent. Sci. 2016;2:748–755. doi: 10.1021/acscentsci.6b00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijesh A.M., Isloor A.M., Peethambar S.K., Shivananda K.N., Arulmoli T., Isloor N.A. Hantzsch reaction: synthesis and characterization of some new 1,4-dihydropyridine derivatives as potent antimicrobial and antioxidant agents. Eur. J. Med. Chem. 2011;46:5591–5597. doi: 10.1016/j.ejmech.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Wu H.B., Wang Z.M., Tao L. The Hantzsch reaction in polymer chemistry: synthesis and tentative application. Polym. Chem. 2017;8:7290–7296. [Google Scholar]
- Wu H.B., Yang L., Tao L. Polymer synthesis by mimicking nature's strategy: the combination of ultra-fast RAFT and the Biginelli reaction. Polym. Chem. 2017;8:5679–5687. [Google Scholar]
- Xiang X.D., Sun X.D., Briceno G., Lou Y.L., Wang K.A., Chang H.Y., Wallacefreedman W.G., Chen S.W., Schultz P.G. A combinatorial approach to materials discovery. Science. 1995;268:1738–1740. doi: 10.1126/science.268.5218.1738. [DOI] [PubMed] [Google Scholar]
- Xu J.T., Shanmugam S., Fu C.K., Aguey-Zinsou K.F., Boyer C. Selective photoactivation: from a single unit monomer insertion reaction to controlled polymer architectures. J. Am. Chem. Soc. 2016;138:3094–3106. doi: 10.1021/jacs.5b12408. [DOI] [PubMed] [Google Scholar]
- Xue H.D., Zhao Y., Wu H.B., Wang Z.L., Yang B., Wei Y., Wang Z.M., Tao L. Multicomponent combinatorial polymerization via the Biginelli reaction. J. Am. Chem. Soc. 2016;138:8690–8693. doi: 10.1021/jacs.6b04425. [DOI] [PubMed] [Google Scholar]
- Yang B., Zhao Y., Ren X., Zhang X.Y., Fu C.K., Zhang Y.L., Wei Y., Tao L. The power of one-pot: a hexa-component system containing pi-pi stacking, Ugi reaction and RAFT polymerization for simple polymer conjugation on carbon nanotubes. Polym. Chem. 2015;6:509–513. [Google Scholar]
- Zhang Q., Wilson P., Li Z.D., McHale R., Godfrey J., Anastasaki A., Waldron C., Haddleton D.M. Aqueous copper-mediated living polymerization: exploiting rapid disproportionation of CuBr with Me6TREN. J. Am. Chem. Soc. 2013;135:7355–7363. doi: 10.1021/ja4026402. [DOI] [PubMed] [Google Scholar]
- Zhang Q.D., Zhang Y.L., Zhao Y., Yang B., Fu C.K., Wei Y., Tao L. Multicomponent polymerization system combining Hantzsch reaction and reversible addition-fragmentation chain transfer to efficiently synthesize well-defined poly(1,4-dihydropyridine)s. ACS Macro. Lett. 2015;4:128–132. doi: 10.1021/mz500734c. [DOI] [PubMed] [Google Scholar]
- Zhang X.J., Wang S.X., Liu J., Xie Z.G., Luan S.F., Xiao C.S., Tao Y.H., Wang X.H. Ugi reaction of natural amino acids: a general route toward facile synthesis of polypeptoids for bioapplications. ACS Macro. Lett. 2016;5:1049–1054. doi: 10.1021/acsmacrolett.6b00530. [DOI] [PubMed] [Google Scholar]
- Zhang Y.L., Zhao Y., Yang B., Zhu C.Y., Wei Y., Tao L. 'One pot' synthesis of well-defined poly(aminophosphonate)s: time for the Kabachnik-Fields reaction on the stage of polymer chemistry. Polym. Chem. 2014;5:1857–1862. [Google Scholar]
- Zhao Y., Wu H.B., Wang Z.L., Wei Y., Wang Z.M., Tao L. Training the old dog new tricks: the applications of the Biginelli reaction in polymer chemistry. Sci. China. Chem. 2016;59:1541–1547. [Google Scholar]
- Zhu C.Y., Yang B., Zhao Y.A., Fu C.K., Tao L., Wei Y. A new insight into the Biginelli reaction: the dawn of multicomponent click chemistry? Polym. Chem. 2013;4:5395–5400. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.