Summary
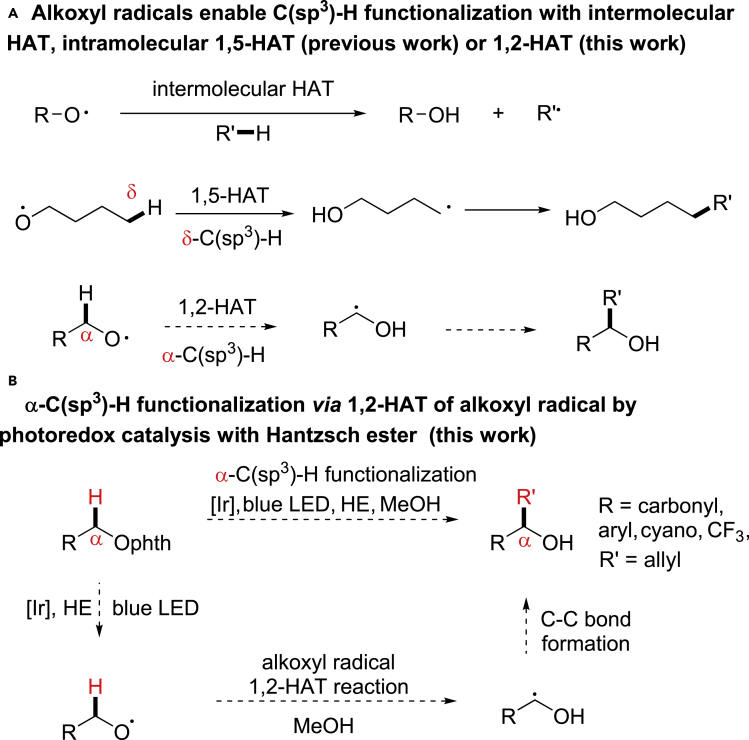
The alkoxyl radical is an essential reactive intermediate in mechanistic studies and organic synthesis with hydrogen atom transfer (HAT) reactivity. However, compared with intramolecular 1,5-HAT or intermolecular HAT of alkoxyl radicals, the intramolecular 1,2-HAT reactivity has been limited to theoretical studies and rarely synthetically utilized. Here we report the first selective 1,2-HAT of alkoxyl radicals for α-C(sp3)-H bond allylation of α-carbonyl, α-cyano, α-trifluoromethyl, and benzylic N-alkoxylphthalimides. The mechanistic probing experiments, electron paramagnetic resonance (EPR) studies, and density functional theory (DFT) calculations confirmed the 1,2-HAT reactivity of alkoxyl radicals, and the use of protic solvents lowered the activation energy by up to 10.4 kcal/mol to facilitate the α-C(sp3)-H allylation reaction.
Subject Areas: Organic Chemistry, Organic Reaction, Physical Organic Chemistry
Graphical Abstract
Highlights
-
•
1,2-Hydrogen atom transfer (HAT) of alkoxyl radical enables α-C(sp3)-H allylation
-
•
α-Carbonyl, α-cyano, α-trifluoromethyl, and benzylic C(sp3)-H bonds are applicable
-
•
Mechanistic and electron paramagnetic resonance (EPR) studies confirmed 1,2-HAT
-
•
DFT calculations explained the methanol acceleration of alkoxyl radical 1,2-HAT
Organic Chemistry; Organic Reaction; Physical Organic Chemistry
Introduction
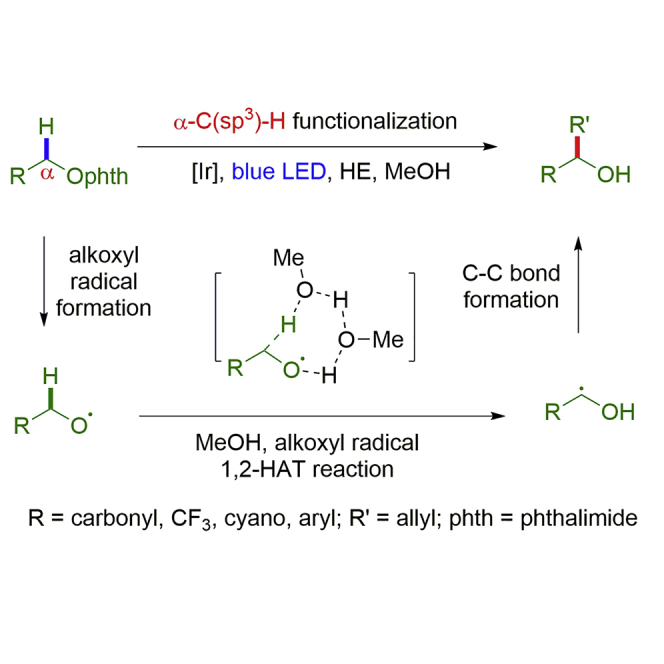
The selective inert C(sp3)-H bond activation for new C-C bond formation is very desirable in organic synthesis (Chen et al., 2009, Lyons and Sanford, 2010, Prier et al., 2013, Gensch et al., 2016, Yi et al., 2017). The hydroxyl groups are ubiquitous in organic molecules, and the use of hydroxyl derivatives provides an effective tool to differentiate chemically indistinguishable C-H bonds (Holmes et al., 2018, Engle et al., 2012, Ren et al., 2012, Wappes et al., 2017, Espino et al., 2001, Simmons and Hartwig, 2012a, Chen et al., 2008, Chen et al., 2015, Karmel et al., 2018). The alkoxyl radical is an essential reactive intermediate in mechanistic studies and organic synthesis, and its highly reactive character enables unactivated C-H bond functionalization with the hydrogen atom transfer (HAT) reactivity (Čeković, 2003, Čeković, 2005, Hartung, 2001, Chiba and Chen, 2014, Lundgren et al., 2006, Salamone et al., 2011, Salamone et al., 2012, Salamone et al., 2013, Salamone et al., 2014a, Salamone et al., 2014b, Salamone et al., 2016a, Salamone et al., 2016b, Bietti and Salamone, 2014, Salamone and Bietti, 2015). When intramolecular δ-C-H bonds are present within the molecule, the 1,5-HAT reaction of alkoxyl radicals preferentially occurs to abstract the δ-C-H; otherwise, the intermolecular HAT reaction dominates (Scheme 1A) (Dorigo and Houk, 1988, Robertson et al., 2001, Weavers, 2001, Burke et al., 1988, Petrovic et al., 2004, Zhu et al., 2009, Zhu et al., 2015, Rueda-Becerril et al., 2011, Hu et al., 2018, Wu et al., 2018a, Wu et al., 2018b, Guan et al., 2018). In contrast, the intramolecular C-H abstraction at positions other than δ-position by alkoxyl radicals has been less reported owing to the unfavorable transition states and high activation energies (Čeković, 2003, Čeković, 2005, Hartung, 2001, Chiba and Chen, 2014). Currently, there are only a few reports on the 1,2-HAT reactivity of alkoxyl radicals in theoretical or biological studies, and the synthetic utilization of 1,2-HAT for new C-C bond formation remains elusive (Buszek et al., 2011, Elford and Roberts, 1996, Fernández-Ramos and Zgierski, 2002, Konya et al., 2000, Gilbert et al., 1976, Che et al., 2016, Che et al., 2018). Here we report the first visible-light-induced α-C(sp3)-H allylation reaction enabled by the selective 1,2-HAT of alkoxyl radicals, which is facilitated by protic solvents and applicable to various α-carbonyl, α-cyano, α-trifluoromethyl, and benzylic C(sp3)-H bonds (Scheme 1B).
Scheme 1.
Selective C(sp3)-H Functionalization via Hydrogen Atom Transfer of Alkoxyl Radicals
phth, Phthalimide. (A) Alkoxyl radicals enable C(sp3)-H functionalization with intermolecular HAT, intramolecular 1,5-HAT or 1,2-HAT. (B) α-C(sp3)-H allylation via 1,2-HAT of alkoxyl radical by photoredox catalysis with Hantzsch ester.
Results and Discussion
Optimization of the Reaction Conditions
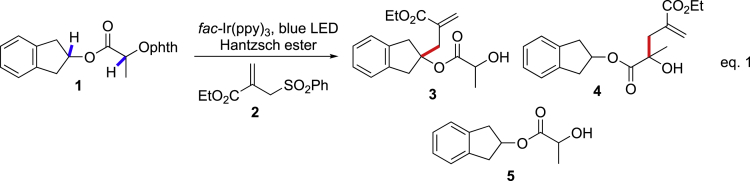
Our investigation was initiated by the serendipitous discovery with N-alkoxylphthalimide 1 as the alkoxyl radical precursor, which can be readily prepared from alcohols and are bench-stable (Scheme 2) (Zhu et al., 2009, Kim et al., 1998, Zhang et al., 2016, Zhang et al., 2017, Wang et al., 2016, Ito et al., 2018, Han et al., 2019, Deng et al., 2019, Shi et al., 2019). Under the reaction conditions of fac-Ir(ppy)3 and Hantzsch ester known to generate alkoxyl radicals (Zhang et al., 2016, Zhang et al., 2017, Wang et al., 2016, Ito et al., 2018, Han et al., 2019, Deng et al., 2019, Shi et al., 2019), the ester-derived N-alkoxylphthalimide 1 gave no δ-C(sp3)-H allylation adduct 3 with allyl sulfone 2 under blue LED irradiation. Instead, the α-C(sp3)-H allylation adduct 4 was observed in 41% yield, together with the hydrogenation adduct alcohol 5 in 52% yield (entry 1 in Table 1) (Zhang et al., 2016). These results were in sharp contrast with our previous observation on the reactivity of alkoxyl radicals under photocatalysis conditions (Zhang et al., 2016, Zhang et al., 2017). We then tested the addition of acids or bases to the reaction and found the outcomes of the reaction were not significantly affected (entries 2–3). The further screen of different Hantzsch ester derivatives has little effect on the reaction (entries 4–6) (Chen et al., 2016). Significantly, the use of ethanol or methanol as solvents dramatically improved the α-C(sp3)-H allylation adduct to 93%–97% yields (93% isolated yield, entries 7–8) and minimalized the hydrogenation adduct alcohol 5 formations. The mixed protic solvents were also beneficial that the addition of methanol or water improved the reaction of dioxane from 41% to 52%–66% yields (entries 9–10) (see Tables S2 and S3).
Scheme 2.
1,2-HAT Reaction of N-alkoxylphthalimide 1
Table 1.
Discovery and Optimization of the 1,2-HAT of Alkoxyl Radicals for α-C(sp3)-H Allylation
| Entry | Conditionsa | 4 Yield (%)b | 5 Yield (%)b |
|---|---|---|---|
| 1 | Dioxane | 41 | 52 |
| 2 | Entry 1, 2.0 equiv. Na2CO3 | 56 | 42 |
| 3 | Entry 1, 2.0 equiv. HCO2H | 40 | 56 |
| 4 | Entry 1, COOMe-HE | 43 | 56 |
| 5 | Entry 1, COOiPr-HE | 47 | 52 |
| 6 | Entry 1, COOtBu-HE | 45 | 54 |
| 7 | EtOH | 93 | 7 |
| 8 | MeOH | 97 (93) | <5 |
| 9 | MeOH/dioxane = 1:9 | 52 | 45 |
| 10 | H2O/dioxane = 1:9 | 66 | 33 |
Reaction conditions: 1 (0.10 mmol, 1.0 equiv.), 2 (0.30 mmol, 3.0 equiv.), fac-Ir(ppy)3 (0.001 mmol, 1%), and Hantzsch ester (0.15 mmol, 1.5 equiv.) in 1.0 mL solvent under nitrogen with 4 W blue LED irradiation at ambient temperature for 3 h, conversion was >95%, unless otherwise noted.
Conversion and yields were determined by 1H NMR analysis, and isolated yields are in parentheses.
Scope
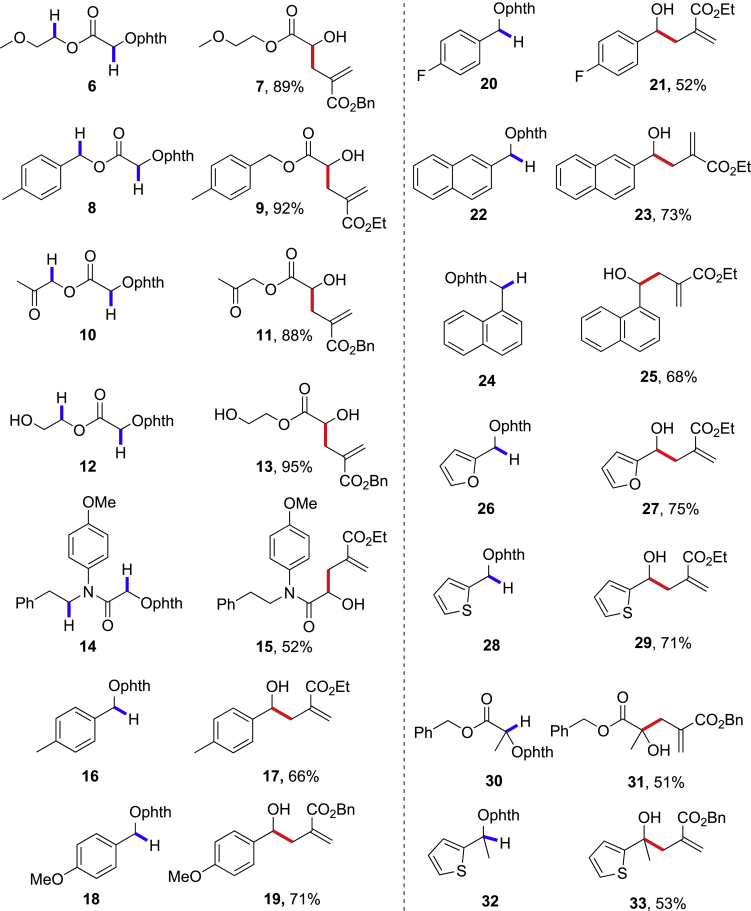
We next explored the scope of this 1,2-HAT reaction for other substrates (Scheme 3). The glycol-derived 6 without ring strain at the δ-C-H bonds afforded 7 in 89% yield, without the observation of the δ-C-H allylation adducts. The benzyl ester 8 with the activated benzylic δ-C-H bonds gave the α-C(sp3)-H allylation adduct 9 in 92% yield. The N-alkoxylphthalimides 10 and 12 provided 88% and 95% yields of α-C-H allylation adducts, successfully, with the ketone or the free hydroxyl group unaffected. The N-alkoxylphthalimide 14 with the amide linkage provided the 1,2-HAT adduct 15 smoothly in 52% yield, together with 17% yield of hydrogenation adduct as the side product (See Figures S1–S26).
Scheme 3.
Substrate Scope of the 1,2-HAT Reactions
Reaction condition is in entry 8 in Table 1, and isolated yields are reported.
The 1,2-HAT reaction is also applicable to N-alkoxylphthalimides without the ester or amide linkages. The N-alkoxylphthalimide 16 with benzyl C(sp3)-H bonds gave the α-C(sp3)-H allylation adduct 17 in 66% yield (Scheme 3). The incorporation of electron-rich methoxyl group on the phenyl ring slightly improved the reaction to give 19 in 71% yield, whereas the electron-deficient fluorides decreased the reaction to give 21 in 52% yield. The α- and β-substituted naphthalenes reacted nicely to give 23 and 25 in 68%–73% yields. The heterocyclic furans and thiophenes reacted to provide 27 and 29 in 71%–75% yields. The secondary-alcohol-derived N-alkoxylphthalimides 30 and 32 gave the corresponding tertiary homoallylic alcohols 31 and 33 in 51% and 53% yields, respectively (See Figures S27–S64).
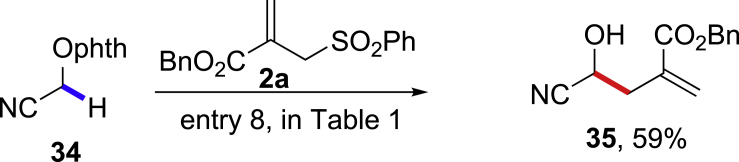
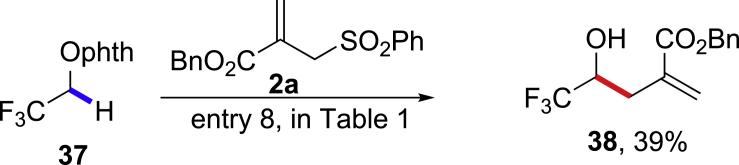
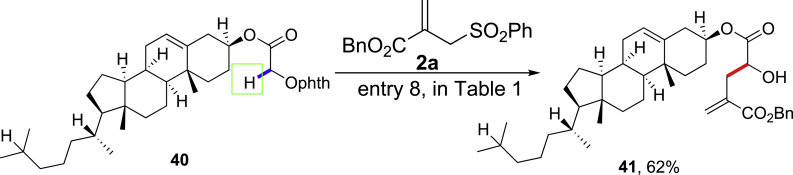
This reaction is particularly valuable for the homoallylic alcohol synthesis when the corresponding aldehydes are inaccessible by the nucleophilic addition methods (Yamamoto and Asao, 1993, Yus et al., 2011). The cyano-substituted homoallylic alcohol 35 can be obtained from the stable N-alkoxylphthalimide 34 by 1,2-HAT reaction smoothly in 59% yield, and the corresponding formyl cyanide 36 is unstable and cannot be synthetically utilized (Scheme 4) (Lewis-Bevan et al., 1992). Similarly, the trifluoromethyl-substituted homoallylic alcohol 37 can be prepared from the stable N-alkoxylphthalimide 38 in 39% yield, whereas the trifluoromethyl aldehyde 39 is very unstable and volatile (Scheme 5) (Ishikawa et al., 1984, Loh and Li, 1999). We also tested a structurally complexed steroid derivative 40 with multiple tertiary and allylic C-H bonds, which are challenging substrates to differentiate the targeted α-C-H bonds by intermolecular HAT reactions (Scheme 6) (Roberts, 1999, Chu and Rovis, 2018). Gratifyingly, the α-C(sp3)-H allylation adduct 41 was selectively obtained in 62% yield, leaving other six tertiary C-H and four allylic C-H bonds untouched (see Figures S65–S78).
Scheme 4.
1,2-HAT Reaction of N-alkoxylphthalimide 34
Scheme 5.
1,2-HAT Reaction of N-alkoxylphthalimide 38
Scheme 6.
1,2-HAT Reaction of N-alkoxylphthalimide 40
Mechanistic Investigations
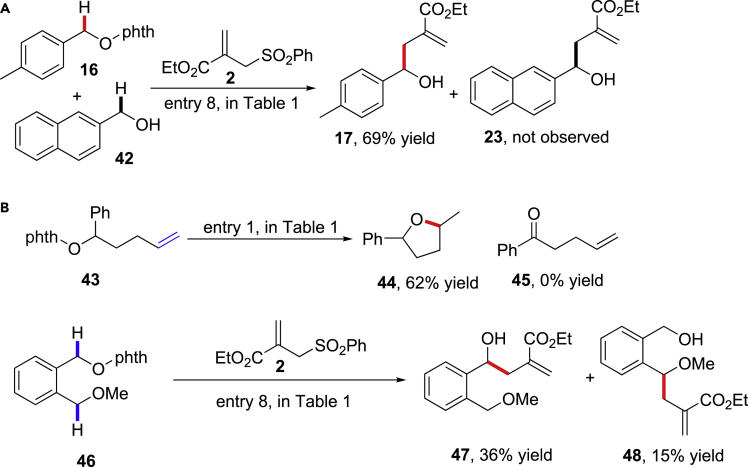
We next carried out mechanistic investigations (Scheme 7). The possible intermolecular hydrogen atom transfer pathway instead of the 1,2-HAT was first evaluated by crossover experiments (Schemes 7A and S1). With N-alkoxylphthalimide 16 and a structurally similar alcohol 42, the reaction with allylsulfone 2 gave the exclusive homoallylic alcohol 17 from 16 in 69% yield, whereas the formation of 23 from 42 was not observed. This result together with the chemoselective α-C(sp3)-H allylation demonstrated in Scheme 6 excluded the intermolecular HAT reaction pathway. We then compared the 1,2-HAT with other potential reaction pathways of alkoxyl radicals in different N-alkoxylphthalimides (Scheme 7B). With benzyl alcohol-derived N-alkoxylphthalimide 43 bearing a pendant alkene at the δ-position, the tetrahydrofuran 44 was obtained via the preferential 5-exo cyclization of alkoxyl radicals, whereas neither the α-C(sp3)-H allylation adduct nor the oxidized ketone adduct 45 was observed (Zlotorzynska and Sammis, 2011). With benzyl alcohol-derived N-alkoxylphthalimide 46 bearing activated δ-C-H bonds, the α-C(sp3)-H allylation adduct 47 was observed in 36% yield, together with the δ-C(sp3)-H allylation adduct 48 in 15% yield (see Scheme S3 for details). These results confirmed the presence of alkoxyl radicals and suggested other alkoxyl radical reaction pathways may be favored over 1,2-HAT pathway in certain substrates (the KIE (kH/kD) with deuterated N-alkoxylphthalimide was measured to be 0.87, suggesting the cleavage of the C-H bond was not the rate-determining step, see Table S1, Scheme S2 and Simmons and Hartwig, 2012b) (see Figures S79–S96).
Scheme 7.
Mechanistic Investigations of the 1,2-HAT of Alkoxyl Radicals
(A) The crossover experiment with N-alkoxylphthalimide 16 and alcohol 42. (B) The investigation of potential reaction pathways of alkoxyl radicals.
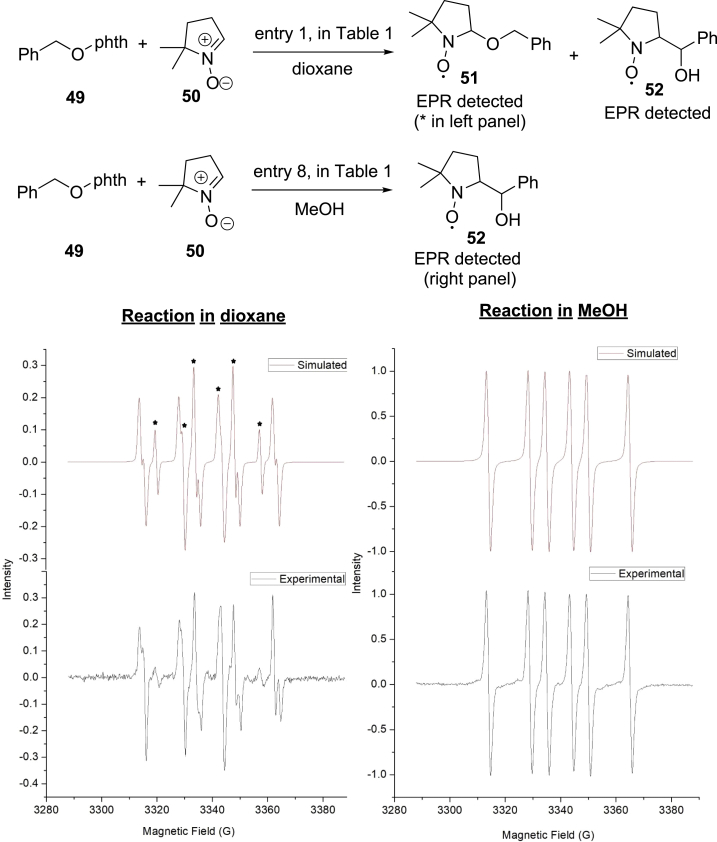
We further investigated the radical intermediates in the reaction by the electron paramagnetic resonance measurements (EPR) using 5,5-dimethyl-pyrroline N-oxide (DMPO) 50 as the radical spin trap. Scheme 8 illustrates the EPR spectrum from the addition of DMPO to the reaction of N-alkoxylphthalimide 49 (see Schemes S11–S13). The spectrum can be fit as the admixture of a triplet of doublets (aN = 14.1 G, aH = 9.6 G) and a triplet of doublets (aH = 14.2 G, aH = 19.7 G) in dioxane. The first triplet of doublets is attributed to DMPO-trapped alkoxyl radical 51 (asterisk ∗ signals in the left panel), and the second triplet of doublets is attributed to DMPO-trapped ketyl radical 52. However, only the ketyl radical trapping adduct 51 (right panel) could be observed in methanol. These results were consistent with the increased hydrogenation adduct from alkoxyl radicals in dioxane compared with in methanol, which indicated that the 1,2-HAT process of alkoxyl radicals to yield ketyl radicals was accelerated in methanol (the Stern-Volmer plots suggest the Hantzsch ester quenched the photoexcited fac-Ir(ppy)3 more effectively than N-alkoxylphthalimides and allyl sulfones; see Schemes S4–S8 for details).
Scheme 8.
EPR Studies of the 1,2-HAT of Alkoxyl Radicals
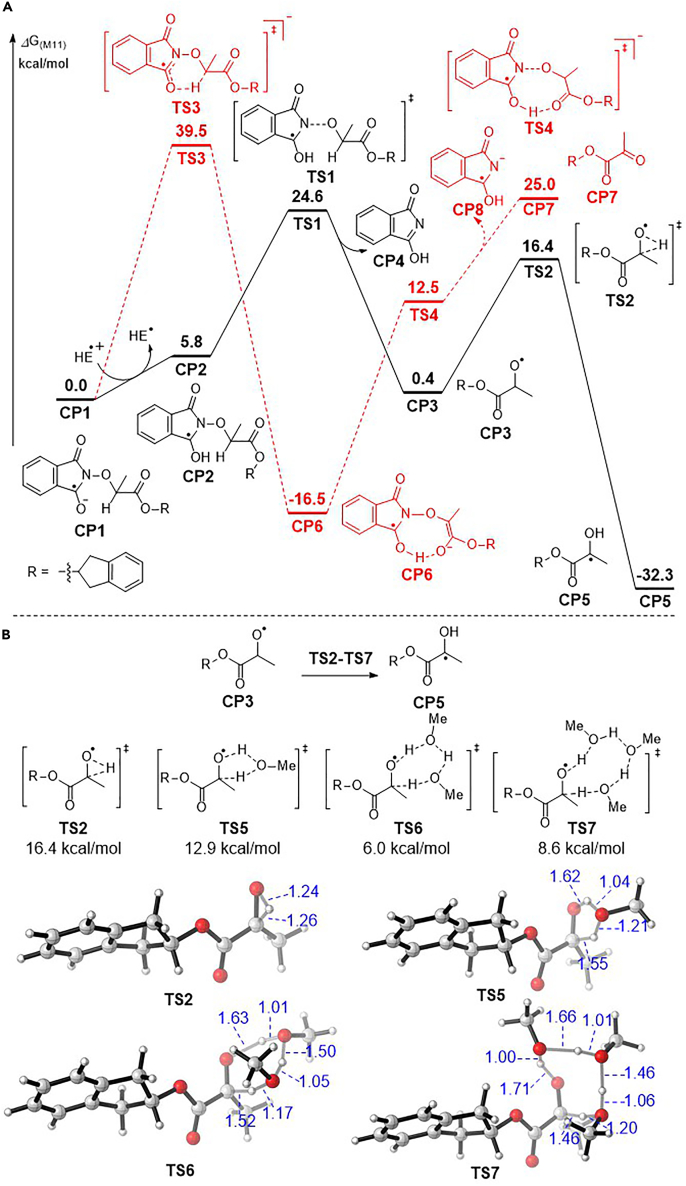
We then performed density functional theory (DFT) calculations to investigate the free energy profiles of the alkoxyl radical generation (Scheme 9A). From computational studies, the N-alkoxylphthalimide 1 first undergoes single electron reduction to generate the radical anion CP1. After protonation by the Hantzsch ester radical cation, the alkyl radical intermediate CP2 was formed with 5.8 kcal/mol endothermically (black line) (Azizi et al., 2015, McSkimming and Colbran, 2013, Zheng and You, 2012, Turovska et al., 2008, Zhu et al., 1999). The N-O bond was then homolytically cleaved to form the alkoxyl radical CP3 via the transition state TS1 with an activation energy of 18.8 kcal/mol. Alternatively, the radical anion CP1 may form the intermediate CP6 via the transition state TS3 with 39.5 kcal/mol of activation energy, and the following redox fragmentation generates the ketoester CP7 (red line) (Qi and Chen, 2016). However, the prohibitively high 39.5 kcal/mol of activation energy of TS3 excludes it as the major reaction pathway, which is consistent with the experimental observation of the preferential formation of alkoxyl radicals in different substrates.
Scheme 9.
DFT Calculation of the 1,2-HAT of Alkoxyl Radicals
(A) The free energy profile of two reaction pathways of N-alkoxylphthalimides. (B) The free energy profile of methanol-assisted 1,2-HAT of alkoxyl radicals.
The methanol-assisted 1,2-HAT of alkoxyl radicals was next investigated by computational studies (Scheme 9B). The direct hydrogen atom transfer to form the ketyl radical CP5 was calculated to have 16.4 kcal/mol of activation energy in TS2. In contrast, the involvement of methanol dramatically affects the energy diagram with hydrogen bonds. With one molecule of methanol participation, a 3.5-kcal/mol decrease of activation energy can be obtained in TS5. Significantly, two methanol molecules reduce the activation energy by 10.4 kcal/mol to merely 6.0 kcal/mol in TS6 with multiple hydrogen bond formation, and three methanol molecules can lower the activation energy by 7.8 kcal/mol in TS7. From the computational studies mentioned above, the methanol facilitates the alkoxyl radical CP3 rearrangement to ketyl radical CP5 with hydrogen bonds, and an up to 10.4 kcal/mol decrease of activation energy can be obtained with the methanol assistance (the involvement of one methanol and one water molecule decreased the activation barrier to 6.9 kcal/mol; see Scheme S14. The α-C-H functionalization product distribution in different solvents is not only determined by the 1,2-HAT reactivity, but also affected by the alkoxyl radical generation) (see Tables S4, S5, S6, and S7).
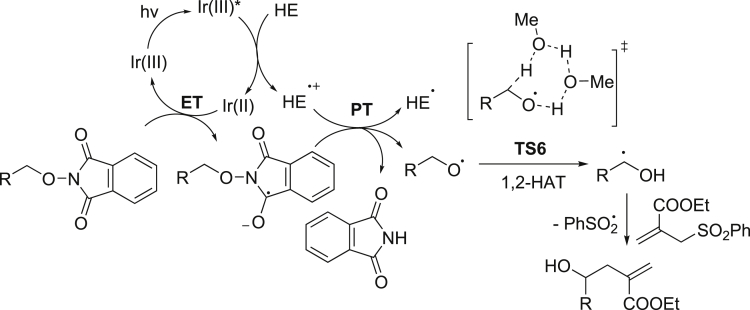
With mechanistic experiments and DFT calculations mentioned above, we propose the reaction is initiated from the reductive quenching of the photoexcited Ir(III)∗ to Ir(II) by Hantzsch ester, and Ir(II) subsequently reduces the N-alkoxylphthalimides to the radical anion (Scheme 10). The radical anion undergoes proton transfer with Hantzsch ester radical cation and subsequent N-O bond cleavage to form the alkoxyl radical (Fukuzumi et al., 1983, Lackner et al., 2015, Pratsch et al., 2015, Taylor et al., 2018, and see Schemes S4–S10 for details). Two methanol molecules then assist the 1,2-HAT reaction with hydrogen bonds at the α-carbonyl, α-cyano, α-trifluoromethyl, or benzylic C(sp3)-H bonds to form ketyl radicals for new C-C bond formations (Poutsma, 2007, Nechab et al., 2014).
Scheme 10.
Mechanistic Proposals
Conclusions
In conclusion, we have developed the first regioselective α-C(sp3)-H functionalization enabled by 1,2-HAT of alkoxyl radicals using photoredox catalysis. The 1,2-HAT of alkoxyl radicals was confirmed by various mechanistic investigations including EPR studies and was useful for the new C-C bond formation of α-carbonyl, α-cyano, α-trifluoromethyl, and benzylic N-alkoxylphthalimides. The computational studies indicate the assistance of protic solvents significantly facilities the 1,2-HAT reaction of alkoxyl radicals for new C-C bond formations. Further investigations are ongoing to explore this new 1,2-HAT reactivity of alkoxyl radicals.
Limitations of the Study
The 1,2-HAT pathway is not always favored for alkoxyl radicals; other alkoxyl radical reaction pathways may complete over 1,2-HAT pathway in different substrates. The existence of the carbonyl intermediate cannot be completely excluded; however, it is not the main reaction pathway from the performed computational and experimental studies.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Financial support was provided by the National Natural Science Foundation of China 21622207, 91753126, 21472230 and the Strategic Priority Research Program of the Chinese Academy of Sciences XDB20020200 to Y.C.; National Natural Science Foundation of China 21822303, 21772020, and Fundamental Research Funds for the Central Universities (Chongqing University) 2018CDXZ0002, 2018CDPTCG0001/4 to Y.L. We thank Dr. Yanxia Zhang (SIOC) for her help on EPR studies.
Author Contributions
J.Z., D,L., Y.L., and Y.C. designed the research, analyzed the data, and wrote the manuscript. J.Z., D.L., and Y.G. performed the experimental studies. S.L. performed the computational studies.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100755.
Contributor Information
Yu Lan, Email: lanyu@cqu.edu.cn.
Yiyun Chen, Email: yiyunchen@sioc.ac.cn.
Supplemental Information
References
- Azizi S., Ulrich G., Guglielmino M., le Calve S., Hagon J.P., Harriman A., Ziessel R. Photoinduced proton transfer promoted by peripheral subunits for some Hantzsch esters. J. Phys. Chem. A. 2015;119:39–49. doi: 10.1021/jp5078246. [DOI] [PubMed] [Google Scholar]
- Bietti M., Salamone M. Reaction pathways of alkoxyl radicals. The role of solvent effects on C–C bond fragmentation and hydrogen atom transfer reactions. Synlett. 2014;25:1803–1816. [Google Scholar]
- Burke S.D., Silks L.A., Strickland S.M.S. Remote functionalization and molecular modeling: observations relevant to the barton and hypoiodite reactions. Tetrahedron Lett. 1988;29:2761–2764. [Google Scholar]
- Buszek R.J., Sinha A., Francisco J.S. The isomerization of methoxy radical: intramolecular hydrogen atom transfer mediated through acid catalysis. J. Am. Chem. Soc. 2011;133:2013–2015. doi: 10.1021/ja1039874. [DOI] [PubMed] [Google Scholar]
- Čeković Ž. Reactions of δ-carbon radicals generated by 1,5-hydrogen transfer to alkoxyl radicals. Tetrahedron. 2003;59:8073–8090. [Google Scholar]
- Čeković Ž. Reactions of carbon radicals generated by 1,5-transposition of reactive centers. J. Serb. Chem. Soc. 2005;70:287–318. [Google Scholar]
- Che C., Huang Q., Zheng H., Zhu G. Copper-catalyzed cascade annulation of unsaturated α-bromocarbonyls with enynals: a facile access to ketones from aldehydes. Chem. Sci. 2016;7:4134–4139. doi: 10.1039/c5sc04980f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che C., Qian Z., Wu M., Zhao Y., Zhu G. Intermolecular oxidative radical addition to aromatic aldehydes: direct access to 1,4- and 1,5-diketones via silver-catalyzed ring-opening acylation of cyclopropanols and cyclobutanols. J. Org. Chem. 2018;83:5665–5673. doi: 10.1021/acs.joc.8b00666. [DOI] [PubMed] [Google Scholar]
- Chen K., Richter J.M., Baran P.S. 1,3-Diol synthesis via controlled, radical-mediated C-H functionalization. J. Am. Chem. Soc. 2008;130:7247–7249. doi: 10.1021/ja802491q. [DOI] [PubMed] [Google Scholar]
- Chen X., Engle K.M., Wang D.H., Yu J.Q. Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wang B., Zhang J., Yu W., Liu Z., Zhang Y. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2015;2:1107–1295. [Google Scholar]
- Chen W., Liu Z., Tian J., Li J., Ma J., Cheng X., Li G. Building congested ketone: substituted Hantzsch ester and nitrile as alkylation reagents in photoredox catalysis. J. Am. Chem. Soc. 2016;138:12312–12315. doi: 10.1021/jacs.6b06379. [DOI] [PubMed] [Google Scholar]
- Chiba S., Chen H. sp3 C–H oxidation by remote H-radical shift with oxygen- and nitrogen-radicals: a recent update. Org. Biomol. Chem. 2014;12:4051–4060. doi: 10.1039/c4ob00469h. [DOI] [PubMed] [Google Scholar]
- Chu J.C.K., Rovis T. Complementary strategies for directed C(sp3 )-H functionalization: a comparison of transition-metal-catalyzed activation, hydrogen atom transfer, and carbene/nitrene transfer. Angew. Chem. Int. Ed. 2018;57:62–101. doi: 10.1002/anie.201703743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Nguyen M.D., Zou Y., Houk K.N., Smith A.B., III Generation of dithianyl and dioxolanyl radicals using photoredox catalysis: application in the total synthesis of the danshenspiroketallactones via radical relay chemistry. Org. Lett. 2019;21:1708–1712. doi: 10.1021/acs.orglett.9b00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo A.E., Houk K.N. The relationship between proximity and reactivity. An ab initio study of the flexibility of the OH· + CH4 hydrogen abstraction transition state and a force-field model for the transition states of intramolecular hydrogen abstractions. J. Org. Chem. 1988;53:1650–1664. [Google Scholar]
- Elford P.E., Roberts B.P. EPR studies of the formation and transformation of isomeric radicals [C3H5O]˙. Rearrangement of the allyloxyl radical in non-aqueous solution involving a formal 1,2-hydrogen-atom shift promoted by alcohols. J. Chem. Soc. 1996;2:2247–2256. [Google Scholar]
- Engle K.M., Mei T.S., Wasa M., Yu J.Q. Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions. Acc. Chem. Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino C.G., Wehn P.M., Chow J., Du Bois J. Synthesis of 1,3-difunctionalized amine derivatives through selective C−H bond oxidation. J. Am. Chem. Soc. 2001;123:6935–6936. [Google Scholar]
- Fernández-Ramos A., Zgierski M.Z. Theoretical study of the rate constants and kinetic isotope effects of the 1,2-hydrogen-atom shift of methoxyl and benzyloxyl radicals assisted by water. J. Phys. Chem. A. 2002;106:10578–10583. [Google Scholar]
- Fukuzumi S., Hironaka K., Tanaka T. Photoreduction of alkyl halides by an NADH model compound. An electron-transfer chain mechanism. J. Am. Chem. Soc. 1983;105:4722–4727. [Google Scholar]
- Gensch T., Hopkinson M.N., Glorius F., Wencel-Delord J. Mild metal-catalyzed C-H activation: examples and concepts. Chem. Soc. Rev. 2016;45:2900–2936. doi: 10.1039/c6cs00075d. [DOI] [PubMed] [Google Scholar]
- Gilbert B.C., Holmes R.G.G., Laue H.A.H., Norman R.O.C. Electron spin resonance studies. Part L. Reactions of alkoxyl radicals generated from alkyl hydroperoxides and titanium(III) ion in aqueous solution. J. Chem. Soc. 1976;2:1047–1052. [Google Scholar]
- Guan H., Sun S., Mao Y., Chen L., Lu R., Huang J., Liu L. Iron(II)-catalyzed site-selective functionalization of unactivated C(sp3 )-H bonds guided by alkoxyl radicals. Angew. Chem. Int. Ed. 2018;57:11413–11417. doi: 10.1002/anie.201806434. [DOI] [PubMed] [Google Scholar]
- Han J.B., Guo A., Tang X.Y. Alkylation of allyl/Alkenyl sulfones by deoxygenation of alkoxyl radicals. Chem. Eur. J. 2019;25:2989–2994. doi: 10.1002/chem.201806138. [DOI] [PubMed] [Google Scholar]
- Hartung J. Stereoselective construction of the tetrahydrofuran nucleus by alkoxyl radical cyclizations. Eur. J. Org. Chem. 2001;2001:619–632. [Google Scholar]
- Holmes M., Schwartz L.A., Krische M.J. Intermolecular metal-catalyzed reductive coupling of dienes, allenes, and enynes with carbonyl compounds and imines. Chem. Rev. 2018;118:6026–6052. doi: 10.1021/acs.chemrev.8b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A., Guo J.J., Pan H., Tang H., Gao Z., Zuo Z. δ-Selective functionalization of alkanols enabled by visible-light-induced ligand-to-metal charge transfer. J. Am. Chem. Soc. 2018;140:1612–1616. doi: 10.1021/jacs.7b13131. [DOI] [PubMed] [Google Scholar]
- Ishikawa N., Moon G.K., Kitazume T., Sam K.C. Preparation of trifluoromethylated allylic alcohols from trifluoroacetaldehyde and organometallic compounds. J. Fluor. Chem. 1984;24:419–430. [Google Scholar]
- Ito Y., Kimura A., Osawa T., Hari Y. Photoredox-catalyzed deformylative 1,4-addition of 2'-Deoxy-5'- O-phthalimidonucleosides for synthesis of 5'-carba analogs of nucleoside 5'-phosphates. J. Org. Chem. 2018;83:10701–10708. doi: 10.1021/acs.joc.8b00637. [DOI] [PubMed] [Google Scholar]
- Karmel C., Li B., Hartwig J.F. Rhodium-catalyzed regioselective silylation of alkyl C-H bonds for the synthesis of 1,4-diols. J. Am. Chem. Soc. 2018;140:1460–1470. doi: 10.1021/jacs.7b11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee T.A., Song Y. Facile Generation of alkoxy radicals from N-alkoxyphthalimides. Synlett. 1998;1998:471–472. [Google Scholar]
- Konya K.G., Paul T., Lin S.Q., Lusztyk J., Ingold K.U. Laser flash photolysis studies on the first superoxide thermal source. First direct measurements of the rates of solvent-assisted 1,2-hydrogen atom shifts and a proposed new mechanism for this unusual rearrangement. J. Am. Chem. Soc. 2000;122:7518–7527. [Google Scholar]
- Lackner G.L., Quasdorf K.W., Pratsch G., Overman L.E. Fragment coupling and the construction of quaternary carbons using tertiary radicals generated from tert-alkyl N-phthalimidoyl oxalates by visible-light photocatalysis. J. Org. Chem. 2015;80:6012–6024. doi: 10.1021/acs.joc.5b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Bevan W., Gaston R.D., Tyrrell J., Stork W.D., Salmon G.L. Formyl cyanide: a stable species. Experimental and theoretical studies. J. Am. Chem. Soc. 1992;114:1933–1938. [Google Scholar]
- Loh T.-P., Li X.-R. A simple and practical synthesis of α-trifluoromethylated alcohols in water. Tetrahedron. 1999;55:5611–5622. [Google Scholar]
- Lundgren C.V., Koner A.L., Tinkl M., Pischel U., Nau W.M. Reaction of singlet-excited 2,3-diazabicyclo[2.2.2]oct-2-ene and tert-butoxyl radicals with aryl-substituted benzofuranones. J. Org. Chem. 2006;71:1977–1983. doi: 10.1021/jo052440k. [DOI] [PubMed] [Google Scholar]
- Lyons T.W., Sanford M.S. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSkimming A., Colbran S.B. The coordination chemistry of organo-hydride donors: new prospects for efficient multi-electron reduction. Chem. Soc. Rev. 2013;42:5439–5488. doi: 10.1039/c3cs35466k. [DOI] [PubMed] [Google Scholar]
- Nechab M., Mondal S., Bertrand M.P. 1,n-hydrogen-atom transfer (HAT) reactions in which n≠5: an updated inventory. Chem. Eur. J. 2014;20:16034–16059. doi: 10.1002/chem.201403951. [DOI] [PubMed] [Google Scholar]
- Petrovic G., Saicic R., Dosen-Micovic L., Čeković Ž. Stereoselective free radical phenylsulfenylation of a nonactivated δ-carbon atom. J. Serb. Chem. Soc. 2004;69:737–747. [Google Scholar]
- Poutsma M.L. Evaluation of the kinetic data for intramolecular 1,x-hydrogen shifts in alkyl radicals and structure/reactivity predictions from the carbocyclic model for the transition state. J. Org. Chem. 2007;72:150–161. doi: 10.1021/jo061815e. [DOI] [PubMed] [Google Scholar]
- Pratsch G., Lackner G.L., Overman L.E. Constructing quaternary carbons from N-(acyloxy)phthalimide precursors of tertiary radicals using visible-light photocatalysis. J. Org. Chem. 2015;80:6025–6036. doi: 10.1021/acs.joc.5b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prier C.K., Rankic D.A., MacMillan D.W. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L., Chen Y. Polarity-reversed allylations of aldehydes, ketones, and imines enabled by Hantzsch ester in photoredox catalysis. Angew. Chem. Int. Ed. 2016;55:13312–13315. doi: 10.1002/anie.201607813. [DOI] [PubMed] [Google Scholar]
- Ren Z., Mo F., Dong G. Catalytic functionalization of unactivated sp3 C-H bonds via exo-directing groups: synthesis of chemically differentiated 1,2-diols. J. Am. Chem. Soc. 2012;134:16991–16994. doi: 10.1021/ja3082186. [DOI] [PubMed] [Google Scholar]
- Roberts B.P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]
- Robertson J., Pillai J., Lush R.K. Radical translocation reactions in synthesis. Chem. Soc. Rev. 2001;30:94–103. [Google Scholar]
- Rueda-Becerril M., Leung J.C., Dunbar C.R., Sammis G.M. Alkoxy radical cyclizations onto silyl enol ethers relative to alkene cyclization, hydrogen atom transfer, and fragmentation reactions. J. Org. Chem. 2011;76:7720–7729. doi: 10.1021/jo200992m. [DOI] [PubMed] [Google Scholar]
- Salamone M., Bietti M. Tuning reactivity and selectivity in hydrogen atom transfer from aliphatic C-H bonds to alkoxyl radicals: role of structural and medium effects. Acc. Chem. Res. 2015;48:2895–2903. doi: 10.1021/acs.accounts.5b00348. [DOI] [PubMed] [Google Scholar]
- Salamone M., Anastasi G., Bietti M., DiLabio G.A. Diffusion controlled hydrogen atom abstraction from tertiary amines by the benzyloxyl radical. The importance of C-H/N hydrogen bonding. Org. Lett. 2011;13:260–263. doi: 10.1021/ol102690u. [DOI] [PubMed] [Google Scholar]
- Salamone M., Martella R., Bietti M. Hydrogen abstraction from cyclic amines by the cumyloxyl and benzyloxyl radicals. The role of stereoelectronic effects and of substrate/radical hydrogen bonding. J. Org. Chem. 2012;77:8556–8561. doi: 10.1021/jo3015352. [DOI] [PubMed] [Google Scholar]
- Salamone M., Milan M., DiLabio G.A., Bietti M. Reactions of the cumyloxyl and benzyloxyl radicals with tertiary amides. Hydrogen abstraction selectivity and the role of specific substrate-radical hydrogen bonding. J. Org. Chem. 2013;78:5909–5917. doi: 10.1021/jo400535u. [DOI] [PubMed] [Google Scholar]
- Salamone M., Amorati R., Menichetti S., Viglianisi C., Bietti M. Structural and medium effects on the reactions of the cumyloxyl radical with intramolecular hydrogen bonded phenols. The interplay between hydrogen-bonding and acid-base interactions on the hydrogen atom transfer reactivity and selectivity. J. Org. Chem. 2014;79:6196–6205. doi: 10.1021/jo5009367. [DOI] [PubMed] [Google Scholar]
- Salamone M., Milan M., DiLabio G.A., Bietti M. Absolute rate constants for hydrogen atom transfer from tertiary amides to the cumyloxyl radical: evaluating the role of stereoelectronic effects. J. Org. Chem. 2014;79:7179–7184. doi: 10.1021/jo5013459. [DOI] [PubMed] [Google Scholar]
- Salamone M., Carboni G., Bietti M. Fine control over site and substrate selectivity in hydrogen atom transfer-based functionalization of aliphatic C-H bonds. J. Org. Chem. 2016;81:9269–9278. doi: 10.1021/acs.joc.6b01842. [DOI] [PubMed] [Google Scholar]
- Salamone M., Mangiacapra L., Carboni G., Bietti M. Hydrogen atom transfer from tertiary alkanamides to the cumyloxyl radical. The role of substrate structure on alkali and alkaline earth metal ion induced C–H bond deactivation. Tetrahedron. 2016;72:7757–7763. [Google Scholar]
- Shi J.L., Wang Z., Zhang R., Wang Y., Wang J. Visible-light-promoted ring-opening alkynylation, alkenylation, and allylation of cyclic hemiacetals through b-scission of alkoxy radicals. Chem. Eur. J. 2019;25:8992–8995. doi: 10.1002/chem.201901762. [DOI] [PubMed] [Google Scholar]
- Simmons E.M., Hartwig J.F. Catalytic functionalization of unactivated primary C-H bonds directed by an alcohol. Nature. 2012;483:70–73. doi: 10.1038/nature10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons E.M., Hartwig J.F. On the interpretation of deuterium kinetic isotope effects in C−H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- Taylor M.T., Nelson J.E., Suero M.G., Gaunt M.J. A protein functionalization platform based on selective reactions at methionine residues. Nature. 2018;562:563–568. doi: 10.1038/s41586-018-0608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turovska B., Goba I., Turovskis I., Grinberga S., Belyakov S., Stupnikova S., Liepinsh E., Stradins J. Electrochemical oxidation of 4-monoalkyl-substituted 1,4-dihydropyridines. Chem. Heterocycl. Comp. 2008;44:1483–1490. [Google Scholar]
- Wang C., Harms K., Meggers E. Catalytic asymmetric Csp3-H functionalization under photoredox conditions by radical translocation and stereocontrolled alkene addition. Angew. Chem. Int. Ed. 2016;55:13495–13498. doi: 10.1002/anie.201607305. [DOI] [PubMed] [Google Scholar]
- Wappes E.A., Nakafuku K.M., Nagib D.A. Directed β C-H amination of alcohols via radical relay chaperones. J. Am. Chem. Soc. 2017;139:10204–10207. doi: 10.1021/jacs.7b05214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers R.T. Laurenanes: fenestranes with a twist. J. Org. Chem. 2001;66:6453–6461. doi: 10.1021/jo015734o. [DOI] [PubMed] [Google Scholar]
- Wu X., Wang M., Huan L., Wang D., Wang J., Zhu C. Tertiary-alcohol-directed functionalization of remote C(sp3 )-H bonds by sequential hydrogen atom and heteroaryl migrations. Angew. Chem. Int. Ed. 2018;57:1640–1644. doi: 10.1002/anie.201709025. [DOI] [PubMed] [Google Scholar]
- Wu X., Zhang H., Tang N., Wu Z., Wang D., Ji M., Xu Y., Wang M., Zhu C. Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)-H bonds. Nat. Commun. 2018;9:3343–3350. doi: 10.1038/s41467-018-05522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Asao N. Selective reactions using allylic metals. Chem. Rev. 1993;93:2207–2293. [Google Scholar]
- Yi H., Zhang G., Wang H., Huang Z., Wang J., Singh A.K., Lei A. Recent advances in radical C-H activation/radical cross-coupling. Chem. Rev. 2017;117:9016–9085. doi: 10.1021/acs.chemrev.6b00620. [DOI] [PubMed] [Google Scholar]
- Yus M., Gonzalez-Gomez J.C., Foubelo F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Y., Zhang F., Hu C., Chen Y. Generation of alkoxyl radicals by photoredox catalysis enables selective C(sp3)-H functionalization under mild reaction conditions. Angew. Chem. Int. Ed. 2016;55:1872–1875. doi: 10.1002/anie.201510014. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Y., Xu R., Chen Y. Donor-Acceptor complex enables alkoxyl radical generation for metal-free C(sp3)-C(sp3) cleavage and allylation/alkenylation. Angew. Chem. Int. Ed. 2017;56:12619–12623. doi: 10.1002/anie.201707171. [DOI] [PubMed] [Google Scholar]
- Zheng C., You S.L. Transfer hydrogenation with Hantzsch esters and related organic hydride donors. Chem. Soc. Rev. 2012;41:2498–2518. doi: 10.1039/c1cs15268h. [DOI] [PubMed] [Google Scholar]
- Zhu X.-Q., Liu Y.-C., Cheng J.-P. Which hydrogen atom is first transferred in the NAD(P)H model Hantzsch ester mediated reactions via one-step and multistep hydride transfer? J. Org. Chem. 1999;64:8980–8981. [Google Scholar]
- Zhu H., Wickenden J.G., Campbell N.E., Leung J.C., Johnson K.M., Sammis G.M. Construction of carbo- and heterocycles using radical relay cyclizations initiated by alkoxy radicals. Org. Lett. 2009;11:2019–2022. doi: 10.1021/ol900481e. [DOI] [PubMed] [Google Scholar]
- Zhu H., Leung J.C., Sammis G.M. Strategies to control alkoxy radical-initiated relay cyclizations for the synthesis of oxygenated tetrahydrofuran motifs. J. Org. Chem. 2015;80:965–979. doi: 10.1021/jo502499a. [DOI] [PubMed] [Google Scholar]
- Zlotorzynska M., Sammis G., M. Photoinduced Electron-Transfer- Promoted Redox Fragmentation of N-Alkoxyphthalimides. Org. Lett. 2011;13:6264–6267. doi: 10.1021/ol202740w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.