Summary
Silver selenide is considered as a promising room temperature thermoelectric material due to its excellent performance and high abundance. However, the silver selenide-based flexible film is still behind in thermoelectric performance compared with its bulk counterpart. In this work, the composition of paper-supported silver selenide film was successfully modulated through changing reactant ratio and annealing treatment. In consequence, the power factor value of 2450.9 ± 364.4 μW/(mK2) at 303 K, which is close to that of state-of-the-art bulk Ag2Se has been achieved. Moreover, a thermoelectric device was fabricated after optimizing the length of annealed silver selenide film via numerical simulation. At temperature difference of 25 K, the maximum power density of this device reaches 5.80 W/m2, which is superior to that of previous film thermoelectric devices. Theoretically and experimentally, this work provides an effective way to achieve silver-selenide-based flexible thermoelectric film and device with high performance.
Subject Areas: Electrochemical Materials Science, Materials Synthesis, Energy Materials
Graphical Abstract
Highlights
-
•
A remarkably improved power factor is achieved by annealing treatment
-
•
Optimal length of Ag-rich Ag2Se film is obtained via a numerical simulation
-
•
A high output power density at temperature difference of 25 K is realized
Electrochemical Materials Science; Materials Synthesis; Energy Materials
Introduction
Wearable electronic devices is expected to play an important role in addressing the issue of an individual's timely healthcare, real-time safety monitoring, and life improvement (Gao et al., 2017a, Hu et al., 2019). Indisputably, for the wearable electronic devices, being continuously operated, mechanically flexible, biocompatible, and easy to maintain is of critical importance. Nonetheless, most of the available wearable electronic devices are still powered by rigid batteries that require frequent charging or replacement, which leads to a lot of problems, such as poor flexibility, inconvenience, and environmental pollution. As a result, the self-powered flexible electronic devices that can work constantly without an external power supply are extremely in demand (Yang et al., 2018, Lou et al., 2017).
To date, in general, based on piezoelectric or triboelectric effect, photovoltaic effect, and thermoelectric effect, the mechanical energy, solar energy, and thermal energy can be accordingly converted into electrical energy for wearable electronic devices (Jeon et al., 2016, Zi and Wang, 2017, Kim et al., 2016). Compared with the piezoelectric, triboelectric, and photovoltaic energy harvesters, the flexible thermoelectric generators can directly generate electricity by utilizing the temperature difference between the skin and the ambient environment all-weather, regardless of the motion state of human body. Therefore, in recent ten years, flexible thermoelectric materials and generators have drawn greatly increasing attention (Du et al., 2018), and a variety of conductive polymers (Kroon et al., 2016), carbon materials (Gao et al., 2016), nano-sized inorganic semiconductors (Dun et al., 2015), and metals (Chen et al., 2017) have been applied to fabricate flexible thermoelectric films. So far, in spite of their relatively poor flexibility, the nano-sized inorganic semiconductors are superior to their counterparts because of their excellent thermoelectric performance.
Currently, the cost-effective and abundant metal sulfides and metal selenides are hot topics in the research of thermoelectric materials applied over from room temperature to moderate temperature (Ge et al., 2016, Zhao et al., 2014, Li et al., 2016), and due to its high power factor and low thermal conductivity at room temperature, the silver selenide is considered to be a promising substitute for commercial Bi2Te3-based alloy (Ferhat and Nagao, 2000). Day et al. predicted that by reducing carrier concentration to 1.6 × 1018 cm−3, a ZT value higher than 1 from 300 K to 600 K can be realized in Ag2+xSe (Day et al., 2013), and previous study reveals that the carrier concentration can be regulated by changing molar ratio of Ag/Se in the silver selenide samples (Lee et al., 2007, Mi et al., 2014, Perez-Taborda et al., 2018). For instance, Lee et al. investigated a series of samples with Ag/Se varying from 1.73 to 2.33, and it is found that the excess silver atoms promote the carrier concentration and diminish the Hall carrier mobility (Lee et al., 2007). Perez-Taborda et al. demonstrated that the PF value of accurately controlled stoichiometric Ag2Se thin film on glass substrate reached up to 2440 ± 192 μW m−1 K−2 at room temperature (Perez-Taborda et al., 2018). Besides the non-flexible silver selenide materials, the flexible silver selenide film with outstanding performance has also been reported very recently. Cai's group published two articles about nylon-membrane-supported Ag2Se-nanowire-based flexible films in succession (Ding et al., 2019, Lu et al., 2019), and an outstanding PF value of 2231.5 μWm−1K−2 at 300 K for flexible thermoelectric film was realized in CuAg4Se3 composite films.
Despite the fact that remarkable progress has been made in silver-selenide-based flexible thermoelectric film, there is still room for further improvement of their power factor. On the other hand, researches on bulk thermoelectric generators show that for the thermoelectric leg with a certain cross-sectional area, its output power increases first and then decreases with increasing length of thermoelectric leg, thus, the maximum output power is obtained in thermoelectric leg with ideal length (Ferreira-Teixeira and Pereira, 2018, Zhang et al., 2018). However, most of the studies on flexible thermoelectric films are confined to improving the power factor of materials, while ignoring the optimization of leg geometry configuration in flexible thermoelectric generators, which greatly determines the output voltage and output power density (Madan et al., 2015). In this work, by simply varying the molar ratio of AgNO3 to Se from 1.9 to 2.5, the silver selenide NPs with different content of Ag were synthesized via a solvothermal reaction. Subsequently, the flexible paper-supported silver selenide NPs films were fabricated by our previously reported cold-press method (Gao et al., 2017b). The highest PF value of 1674.1 μW/(mK2) at 303 K was achieved in the sample Ag2.3Se film (AgNO3/Se = 2.3). Then the composition of these films was further modulated by annealing the films in Ar/H2 atmosphere at 250°C for 2 h. According to the XRD spectra and EDX mapping images, after being annealed, for all samples, the crystallinity of Ag2Se phase was enhanced, the molar ratio of Ag/Se was greatly reduced, and the distribution of Ag in the film was more uniform. As a result, all of the annealed films showed significantly improved PF values, and the highest PF value of the annealed Ag2.3Se film reached up to 2450.9 ± 364.4 μW/(mK2) at 303 K, which is close to that of state-of-the-art bulk silver selenide materials. Moreover, according to the results of numerical simulation, the optimal length of single annealed Ag2.3Se film (the width is 5 mm) is calculated to be 13 mm. By series-connecting four pieces of annealed Ag2.3Se films with silver paste, a paper-supported thermoelectric module was fabricated. At temperature difference of about 25 K, the maximum output power density reaches up to 5.80 W/m2, which is superior to other film thermoelectric devices.
Results and Discussion
Phase and Morphology of Silver Selenide Nanoparticles and Films
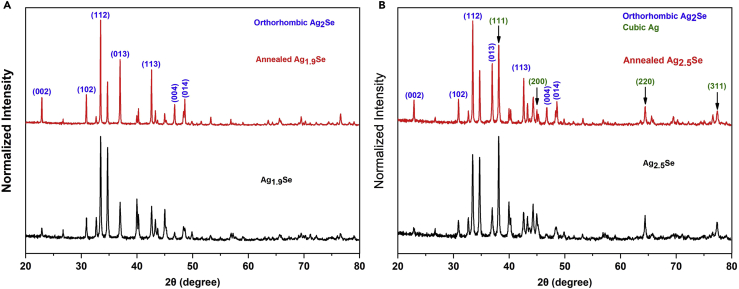
The XRD patterns of Ag1.9Se and Ag2.5Se films before and after being annealed are compared in Figure 1. As shown in Figure 1A, all peaks in XRD pattern of Ag1.9Se film can be indexed to the orthorhombic Ag2Se, and in Figure 1B, the XRD patterns of Ag2.5Se films show characteristic peaks of orthorhombic Ag2Se and cubic Ag. In addition, in the XRD spectra of annealed films, the relative intensity of peaks corresponding to (002), (102), (112), (013), (113), (004), and (014) planes of Ag2Se are obviously enhanced, whereas the relative intensity of peaks indexed to cubic Ag are weakened, which indicates that the content of Ag has been reduced in the annealed Ag2.5Se film. The XRD spectra of other films are displayed in Figure S1, and they exhibit the same change as that of Ag2.5Se films. On the other hand, as shown in Table S1, for all annealed silver selenide films, the full width at half maximum (FWHM) values of dominant peak [(112) plane] are reduced, implying that the annealing treatment leads to the enhanced crystallinity of Ag2Se phase.
Figure 1.
Phase Characterization of Silver Selenide Film before and after Being Annealed
XRD patterns of Ag1.9Se film (A) and Ag2.5Se film (B) before and after being annealed. This figure is related to Figure S1 and Table S1.
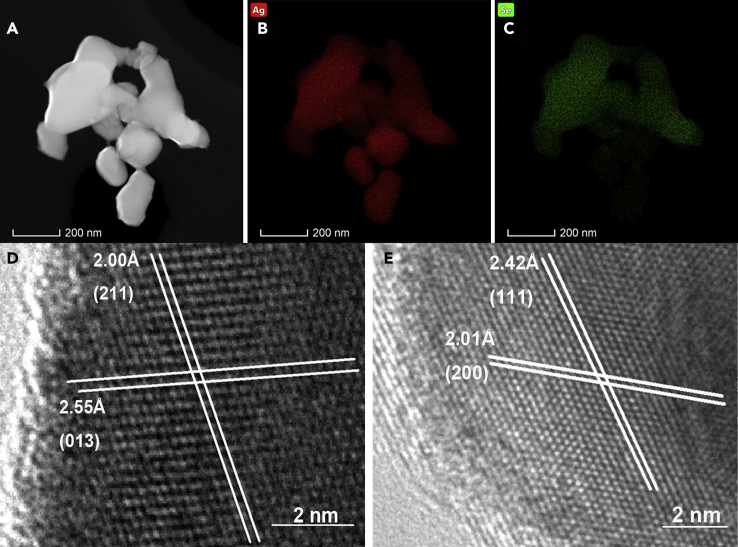
In Figure 2A, the TEM image clearly shows particles with different sizes and shapes. Further, the TEM-EDS maps in Figures 2B and 2C suggest that the small-sized near-spherical particles are Ag nanoparticles and the large-sized irregular particles are Ag2Se nanoparticles. Figure 2D displays the HRTEM image of Ag2Se nanoparticle, and the interplanar distances of 2.55 Å and 2.00 Å correspond to the lattice spaces of (013) and (211) planes. The HRTEM image of Ag nanoparticles in Figure 2E shows the (200) and (111) planes with interplanar distances of 2.01 Å and 2.42 Å.
Figure 2.
Composition and Crystal Lattice of Ag2.5Se Particles
TEM image (A), TEM-EDS maps (B and C) and HRTEM images (D and E) of Ag2.5Se particles. (D) and (E) reveal the HRTEM images of Ag2Se phase and Ag phase in Ag2.5Se particles, respectively. In (A), (B) and (C), the scale bar is 200 nm, and in (D) and (E), the scale bar is 2 nm.
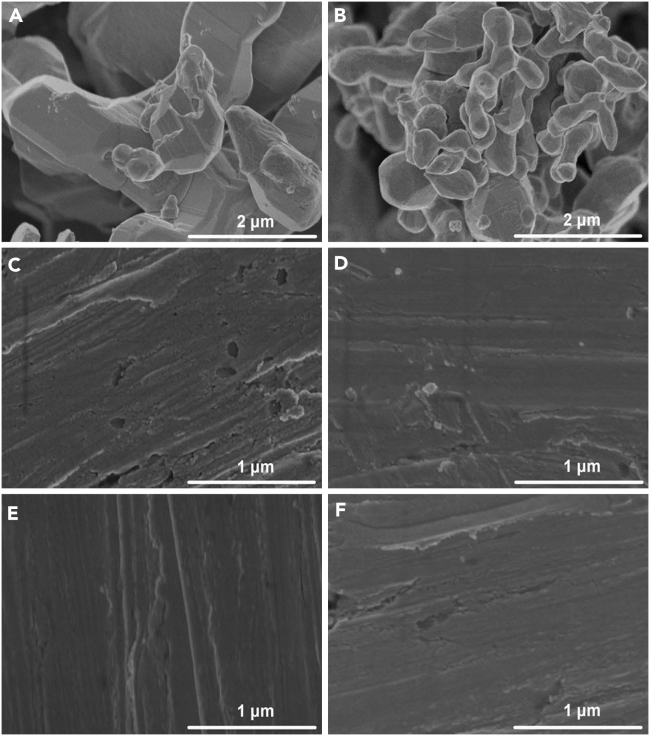
Figure 3 reveals the representative morphology of silver selenide particles and films, and more SEM images of silver selenide particles and films are presented in Figure S2. As shown in Figures 3A and 3B, the large-sized (1–2 μm) and small-sized (100–200 nm) particles with different shapes can be found in both of the two samples. Meanwhile, with increasing ratio of Ag/Se, more particles with small size are observed in the images, indicating that more Ag are contained in the sample. Figures 3C and 3D show the relatively smooth surface of paper-supported films, suggesting that the morphology of particles and the boundaries among particles have almost been destroyed due to the compression, and the similar result has been reported in our previous work (Gao et al., 2017b). As exhibited in Figures 3E and 3F, no obvious change can be found in the annealed films, which indicates that the annealing treatment has little impact on the morphology of silver selenide films. The cross-sectional SEM image of a piece of annealed Ag2.5Se film is shown in Figure S3, and it can be deduced from this figure that the thickness of silver selenide films is about 10 μm.
Figure 3.
Morphology of Silver Selenide Particles and Films
The representative SEM images of silver selenide particles and paper-supported films. Ag1.9Se and Ag2.5Se particles (A and B), Ag1.9Se and Ag2.5Se films (C and D), and annealed Ag1.9Se and Ag2.5Se films (E and F). The scale bar is 1 μm and this figure is related to Figures S2 and S3.
Compositional Modulation and Regulation of Carrier Transport Characteristics
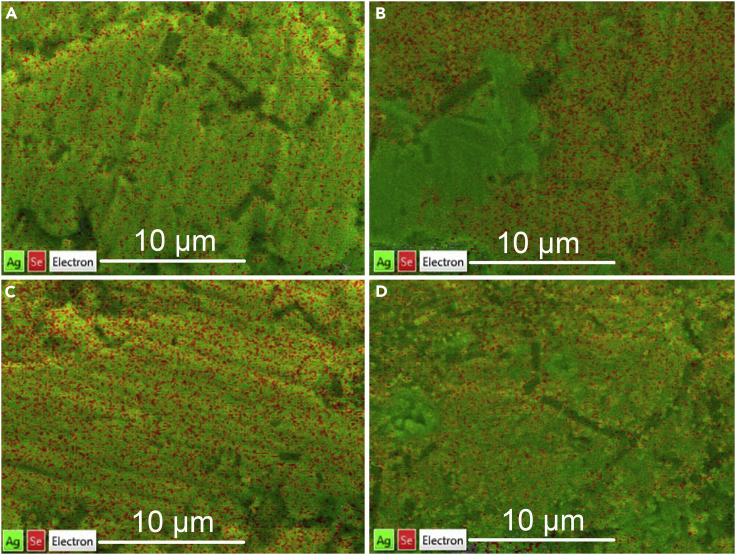
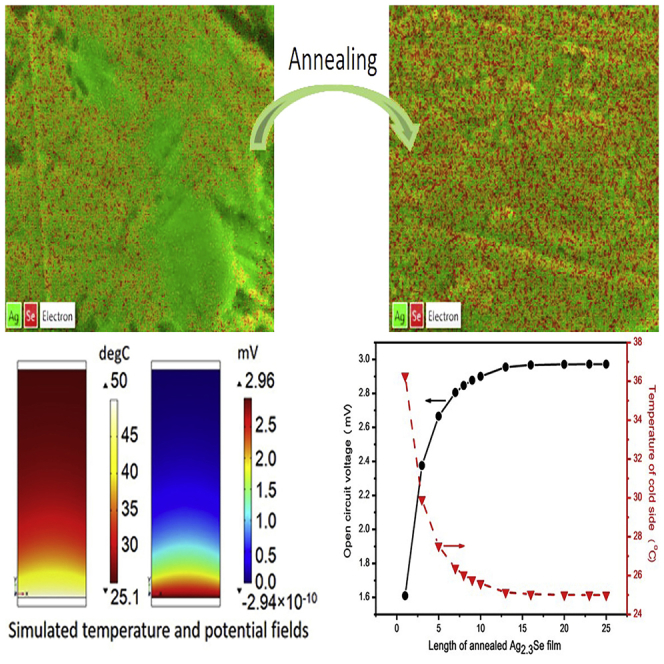
The SEM-EDS maps in Figure 4 illustrate the varied composition of silver selenide films before and after being annealed. As shown in Figure 4A, although some tiny Ag aggregations can be observed in limited regions, the Ag and Se elements are almost uniformly distributed in the Ag1.9Se film. On the contrary, Figure 4B clearly shows that Ag aggregation with size of micrometers can be found in the Ag2.5Se film. Then in the SEM-EDS maps of annealed films, the signal of Ag element is obviously reduced and the area of Ag aggregations in annealed Ag2.5Se films is greatly shrunk. These results can also be observed in SEM-EDS maps of other silver selenide films (Figure S4 in Supplemental Information). Despite the EDS maps can only provide semi-quantitative results, based on the SEM-EDS maps, the molar ratios of Ag/Se in silver selenide films before and after being annealed are calculated and listed in Table 1. This table reveals that all samples are Ag-rich silver selenide films, and the annealing treatment indeed leads to the decreased concentrations of Ag element in films, especially in the film containing more Ag element, such as Ag2.5Se film.
Figure 4.
Elemental Distribution in Silver Selenide Films before and after Being Annealed
The representative SEM-EDS maps of silver selenide films before and after being annealed. Ag1.9Se film (A), Ag2.5Se film (B), annealed Ag1.9Se film (C), and Ag2.5Se film (D). The scale bar is 10 μm and this figure is related to Figure S4.
Table 1.
The Molar Ratios of Ag/Se in Silver Selenide Films before and after Being Annealed
| Ag1.9Se Film | Annealed Ag1.9Se Film | Ag2.1Se Film | Annealed Ag2.1Se Film | Ag2.3Se Film | Annealed Ag2.3Se Film | Ag2.5Se Film | Annealed Ag2.5Se Film | |
|---|---|---|---|---|---|---|---|---|
| Ag | 69.92 | 68.69 | 72.94 | 71.66 | 75.96 | 73.76 | 78.13 | 74.06 |
| Se | 30.08 | 31.31 | 27.06 | 28.34 | 24.04 | 26.24 | 21.87 | 25.94 |
| Ag/Se | 2.32 | 2.19 | 2.70 | 2.53 | 3.16 | 2.81 | 3.57 | 2.85 |
This table is related to Figure S4.
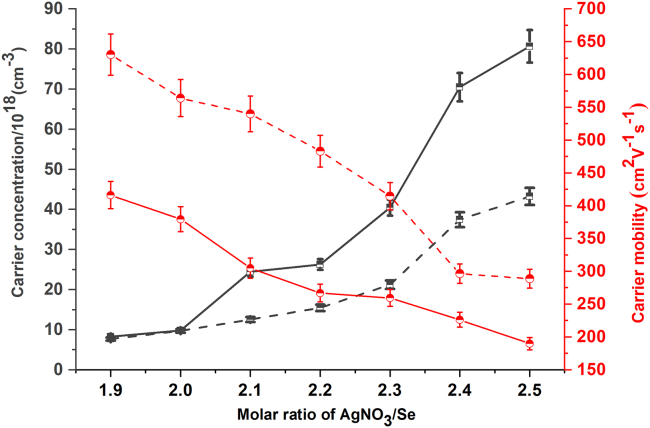
All in all, it can be concluded from XRD patterns and SEM-EDS maps that due to the annealing treatment, the compositions of silver selenide films have been modulated and the crystallinity of Ag2Se phase in the films has been improved. As a result, the carrier transport characteristics of silver selenide films have also been regulated. As shown in Figure 5, on the whole, the carrier concentrations of all films are diminished because of the annealing treatment, in particular those of the films with high content of Ag (Ag2.1Se to Ag2.5Se), and this result is in consistence with the reduction of Ag in the films, which has been verified by SEM-EDS maps. As shown in SEM-EDS maps, for the film with less Ag (Ag1.9Se film), the annealing treatment has less impact on the content of Ag in the film, whereas for the film with more Ag, for example, Ag2.5Se film, the content of Ag in the film is greatly reduced by annealing treatment. Accordingly, the carrier concentration of film with less Ag is slightly changed, whereas that of film with more Ag is obviously decreased due to the annealing treatment. Moreover, the shrinkage of Ag aggregation regions possibly increases the amount of nano-sized interfaces between Ag and Ag2Se in the film, whereas the energy filtering effect on the nano-sized interfaces also leads to the reduction of carrier concentration (Lu et al., 2019). On the other hand, after being annealed, all films show almost doubled carrier mobility, and this increased carrier mobility results from two reasons. First, the enhanced crystallinity of Ag2Se phase in the annealed films is benefit for increasing carrier mobility. Second, the decreased carrier concentration in annealed films leads to weaker scattering among carriers, which also contributes to the improvement of carrier mobility.
Figure 5.
Carrier Transport Characteristics of Silver Selenide Films before and after Being Annealed
The carrier concentration ( ) and mobility (
) and mobility ( ) of silver selenide films before (solid line) and after (dash line) being annealed, and both of the errors of carrier concentration and mobility are ±5%.
) of silver selenide films before (solid line) and after (dash line) being annealed, and both of the errors of carrier concentration and mobility are ±5%.
Thermoelectric Properties of Silver Selenide Films
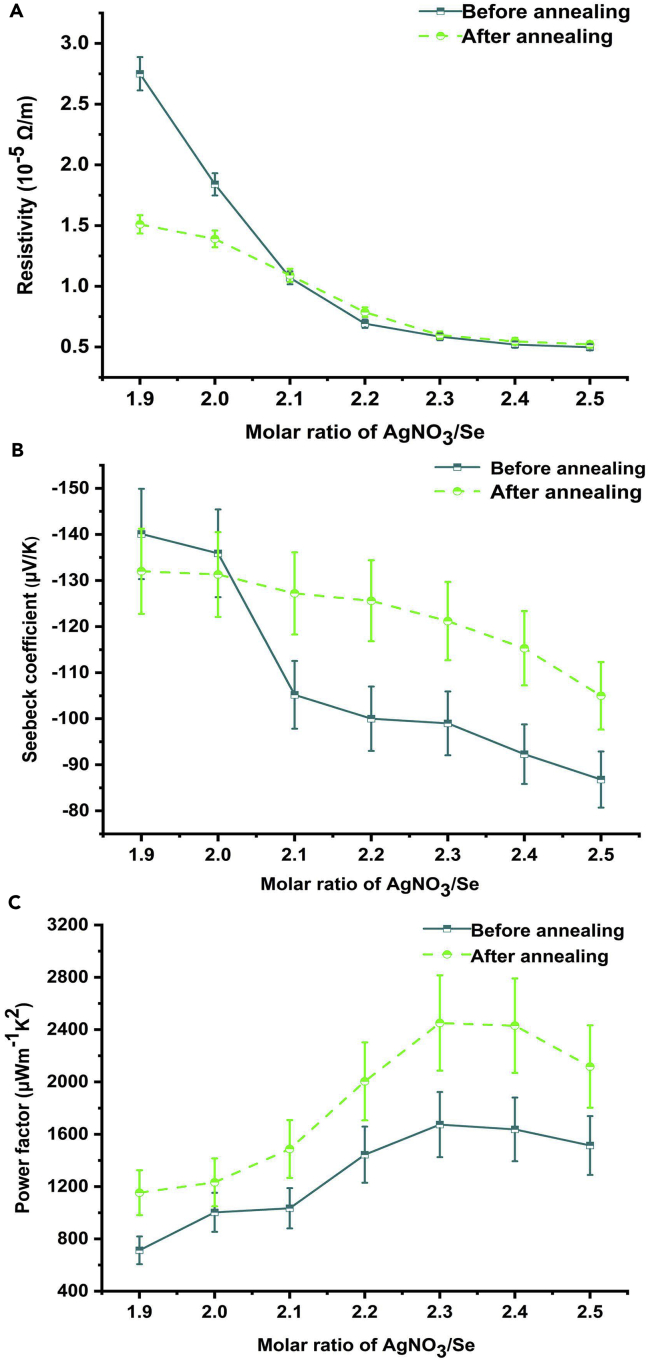
Figure 6 displays the thermoelectric properties at 303 K of silver selenide films before and after being annealed, and the trend of electrical resistivity in Figure 6A is in accordance with that of product of n and μ. The resistivity decreases with increasing molar ratio of AgNO3/Se and after being annealed, the Ag1.9Se and Ag2.0Se films show the markedly reduced resistivity, whereas other films show the slightly increased resistivity. Before being annealed, the Ag2.5Se film possesses the lowest resistivity of (0.5 ± 0.025) × 10−5 Ω/m and this value turns to be (0.52 ± 0.026) × 10−5 Ω/m for the annealed Ag2.5Se film. The Seebeck coefficients in Figure 6B show a similar changing tendency to that of electrical resistivity. As to the annealed films, the Seebeck coefficients of Ag1.9Se and Ag2.0Se films are reduced, whereas those of other films are raised. Before being annealed, the Ag1.9Se film shows the largest Seebeck coefficient of −140.1 ± 9.8 μV/K, which is very close to the value reported in previous work (Ding et al., 2019). For comparison, the Seebeck coefficients of films prepared with smaller ratios of AgNO3/Se (from 1.8 to 1.5) are measured and displayed in Figure S5. According to Figure S5, it can be concluded that the further reduction of AgNO3 is not helpful to improve the Seebeck coefficient of silver selenide film. In Figure 6C, the power factor increases and then decreases with the increasing molar ratio of AgNO3/Se, and all films show the enhanced power factors after being annealed. The highest power factor of films before being annealed is 1674.1 ± 248.9 μW/(mK2), which is realized in the Ag2.3Se film, and this value is remarkably improved to be 2450.9 ± 364.4 μW/(mK2) for the annealed Ag2.3Se film.
Figure 6.
The Thermoelectric Properties of Silver Selenide Films before and after Being Annealed
The electrical resistivity (A), Seebeck coefficient (B), and power factor (C) at 303 K of silver selenide films before and after being annealed, and the errors of these three parameters are ±5%, ±7%, and ±14%, respectively. This figure is related to Figure S5.
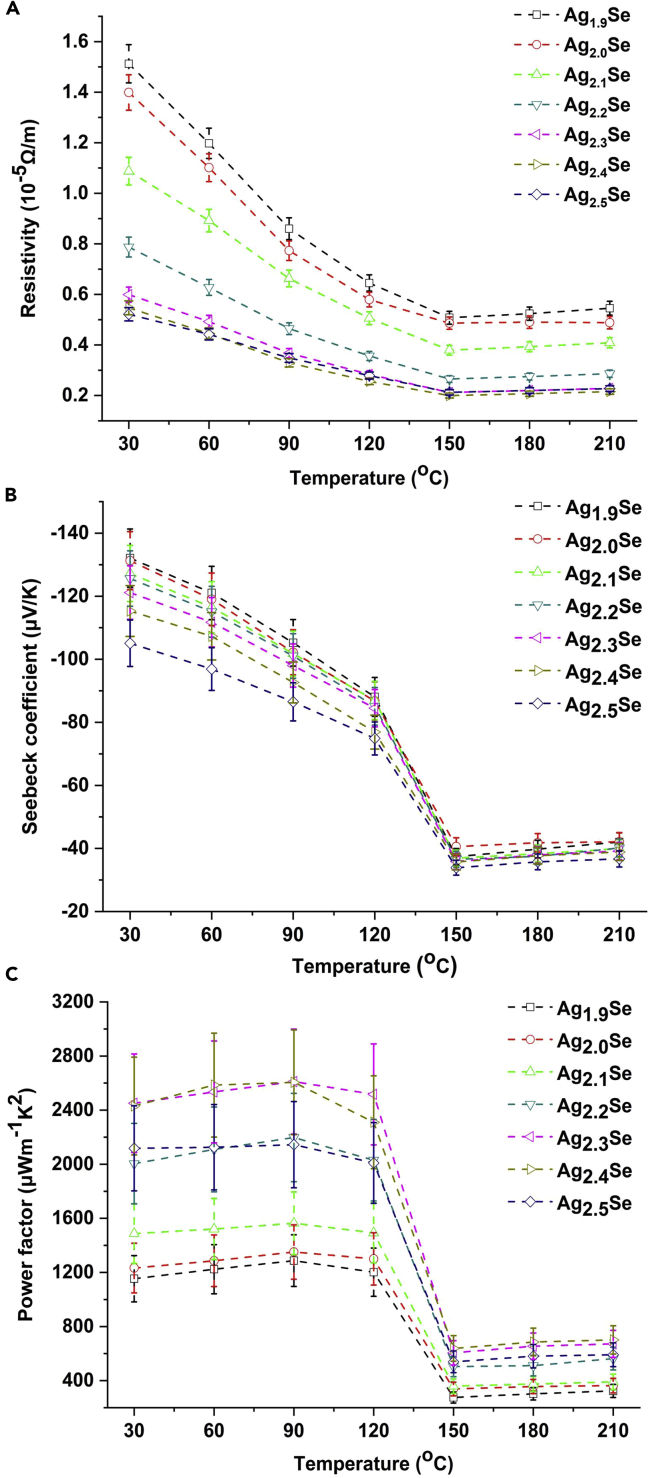
The thermoelectric properties of annealed silver selenide films at different temperatures are shown in Figure 7. In Figure 7A, the resistivity significantly decreases and then slightly increases with the temperature for measurement increasing from 30°C to 210°C, and this trend is attributed to the phase transition of Ag2Se. The phase transition temperature of Ag2Se is about 133°C (Xiao et al., 2012), and Ag2Se with orthorhombic structure (β-Ag2Se) is a semiconductor, so the resistivity of annealed films decreases with increasing temperature when the temperature is below 133°C. Then once the temperature for measurement is above 150°C, the Ag2Se has been transformed from orthorhombic structure to cubic structure (α-Ag2Se), which shows conductive character of metal. Similarly, as exhibited in Figure 7B, with the temperature increasing from 30°C to 150°C, the absolute value of Seebeck coefficient remarkably drops from over 100 μV/K to around 30 μV/K; when the temperature continues to rise from 150°C to 210°C, it turns to ascend slightly. This variation tendency of Seebeck coefficient also results from the phase transition of Ag2Se. Because of the variation of resistivity and Seebeck coefficient, all annealed films display the highest power factors at 90°C in Figure 7C, and the maximum of power factor is realized in the annealed Ag2.3Se film, reaching up to 2610.9 ± 388.0 μW/(mK2). The power factor at around 300 K of annealed Ag2.3Se film in this work is compared with those of representative bulk silver selenide materials, Ag2Se-based flexible films, and BiTeSe film (Table 2); it can be concluded from this table that among the Ag2Se-based flexible film, the power factor of annealed Ag2.3Se film in this work is closest to that of Ag2Se-based bulk materials.
Figure 7.
The Thermoelectric Properties of Annealed Silver Selenide Films at Different Temperatures
The electrical resistivities (A), Seebeck coefficients (B), and power factors (C) of annealed silver selenide films at different temperatures, and the errors of these three parameters are ±5%, ±7%, and ±14%, respectively.
Table 2.
The Power Factors at Around 300 K of Annealed Ag2.3Se Film in This Work and Representative Thermoelectric Bulk Materials and Flexible Films
| Sample | Power Factor (μWm−1K−2) |
|---|---|
| Flexible annealed Ag2.3Se film | 2451 ± 364 (this work) |
| 5%Te/Ag2Se bulk materials | 1800 (Lim et al., 2019) |
| Ag2Se1.06 bulk materials | 3010 (Mi et al., 2014) |
| Ag2Se film on glass substrate | 2440 ± 192 (Perez-Taborda et al., 2018) |
| Flexible nylon-supported Ag2Se film | 987 ± 104 (Ding et al., 2019) |
| Flexible nylon-supported Cu1Ag4Se3 film | 2232 (Lu et al., 2019) |
| Bi2Te2.7Se0.3 film on glass fiber | 2077 (Shin et al., 2017) |
It is very difficult to obtain the accurate in-plane thermal conductivity of a substrate-supported thin film due to the following reasons. First, the thickness of sample for the laser flash diffusivity apparatus is usually 1–2 mm, which is much larger than that of flexible film, thus it is almost impossible to obtain the cross-plane thermal diffusivity of thin film by a general commercial laser flash diffusivity apparatus. Second, for the film with high electrical conductivity (>500 S/cm), the anisotropy in thermal conductivity can be as large as 1:0.3 (in-plane: cross-plane) (Liu et al., 2015). Third, the substrate contribution must be removed (Bahk et al., 2015), and if the electrical conductivity and the thermal conductivity were measured from two different sets of samples, a large uncertainty in the ZT value would be resulted in (Weathers et al., 2015). As a reference, the thermal diffusivity and specific heat capacity of annealed Ag2.3Se pellet has been measured and displayed in Figure S6, and the thermal conductivity of annealed Ag2.3Se pellet is calculated to be 0.635 W/(mK) at 303 K. However, we find that the paper substrate might also play a role in determining the composition of silver selenide film due to the possible reaction between paper substrate and the Ag2Se or Ag particles in the film at the temperature for annealing treatment (250°C), and there is no paper substrate attached with the Ag2.3Se pellet during the annealing treatment, thus the composition and electrical and thermal properties of annealed Ag2.3Se film and Ag2.3Se pellet are likely to be different. Because the specific influence of paper substrate on silver selenide during the process of annealing treatment is remained to be investigated in our following work, it is reasonable to use the theoretic thermal conductivity of silver-rich silver selenide at room temperature (1.8 W/K) (Day et al., 2013) rather than the thermal conductivity of annealed Ag2.3Se pellet, and the thermal conductivity of paper substrate for numerical simulation is 0.8 W/(mK) (Lavrykov and Ramarao, 2012).
Optimal Length of Annealed Ag2.3Se Film by Numerical Simulation
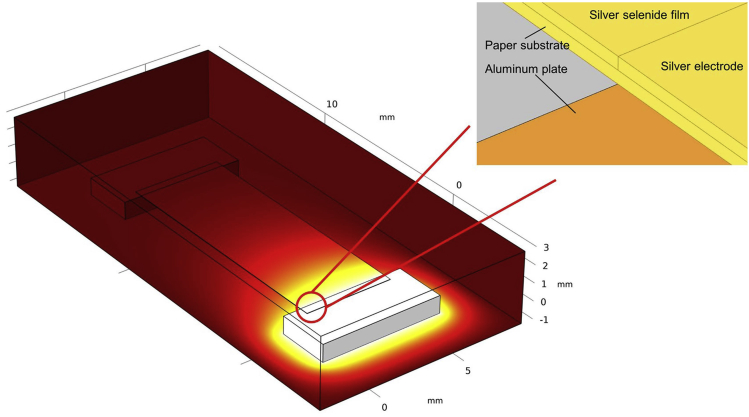
As reported in previous work concerning bulk thermoelectric device, the length of thermoelectric leg plays a key role in determining the output power of thermoelectric generator (Zhang et al., 2018). Generally, reducing the length of thermoelectric film may lead to a too small temperature difference between the two ends of film, and as result, the output voltage of thermoelectric film would be diminished. On the other hand, the electrical resistance of thermoelectric film with excessive length is too large. Most numerical simulations of thermoelectric generators focus on devices based on conventional bulk materials (Ferreira-Teixeira and Pereira, 2018, Qiu et al., 2019), and in this work, in order to obtain the ideal output voltage and power of flexible thermoelectric generator fabricated with the annealed Ag2.3Se film, the optimal length of single annealed Ag2.3Se film is investigated through numerical simulation using finite element analysis. As shown in Figure 8, the annealed Ag2.3Se film, silver electrodes, and paper-substrate are simulated to be thin cubes, and aluminum plates are placed on both ends of the film.
Figure 8.
The Three-Dimensional Model of Single Paper-Supported Ag2.3Se Film for Numerical Simulation
This figure is related to Figure S6.
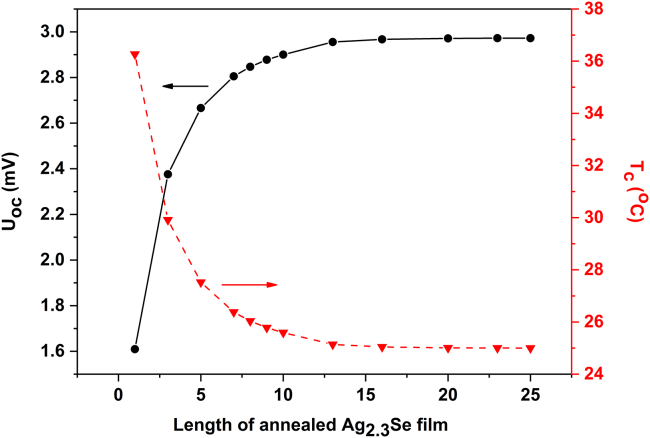
Based on the above model, the temperature and potential fields of annealed Ag2.3Se films with different lengths (from 1 mm to 25 mm) are obtained via numerical simulation, and the results are listed in Figure S7. As illustrated in Figure 9, the simulated temperature of cold side (Tc) decreases, whereas the open-circuit voltage (Uoc) increases with the ascending length of film and finally reaches a steady state once the length exceeds 13 mm. In the steady state, the temperature difference of hot and cold sides remains nearly unchanged at 25°C and the corresponding theoretic open-circuit voltage is up to about 2.97 mV. This value is a little higher than the experimental result (2.8 mV) due to two possible reasons. First, the actual temperature of hot side of annealed Ag2.3Se film is less than 50°C because of the thermal resistance at the interface between hot side of paper substrate and heating aluminum plate. Second, the actual voltage is measured by contacting probes to electrodes, which inevitably leads to the heat loss at hot side (Jin et al., 2018).
Figure 9.
The Simulated Electrical and Thermal Data of Single Annealed Ag2.3Se Film with Different Lengths
The numerically simulated Uoc (solid line) and Tc (dash line) of annealed Ag2.3Se films with different lengths. This figure is related to Figure S7.
Thermoelectric Performance of Paper-Supported Silver Selenide Thermoelectric Generator
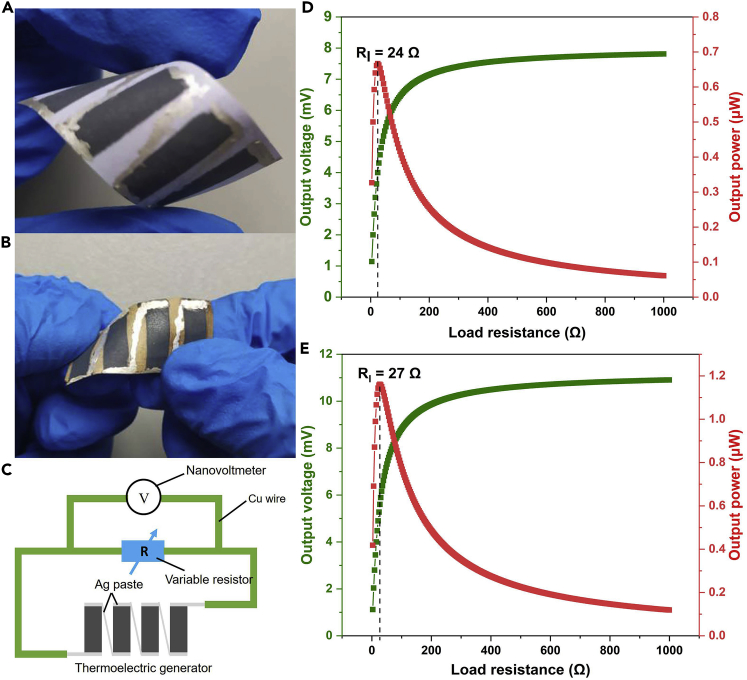
By series-connecting four pieces of Ag2.3Se films with length of 13 mm, the flexible paper-supported thermoelectric module is fabricated and the photographs of module before and after being annealed are shown in Figure 10A and 10B. The width of each film is 5 mm and the distance between two adjacent films is 3 mm. Figure 10C displays the method for measurement, and the temperatures of hot and cold side are 50°C and 25°C (environmental temperature), respectively. The output voltages and output powers of paper-supported Ag2.3Se thermoelectric generator before and after being annealed are shown in Figures 10D and 10E, and the output power is calculated by this formula: , where Pl is the output power, Ul is the voltage across the load variable resistor, namely output voltage, and Rl is the resistance of variable resistor (Madan et al., 2015). As is known to all, The output voltage is calculated by this formula: , where Uo is the open-circuit voltage of module, Rin is the internal resistance of module, and Rl is the load variable resistor. Thus, at a certain temperature difference, the output voltage will increase with the increasing load resistance and eventually infinitely approach the open-circuit voltage. On the other hand, the output power reaches the maximum value when the load resistance equals to the internal resistance of thermoelectric generator.
Figure 10.
The Actual Thermoelectric Devices and Corresponding Electrical Performance
The photograph of paper-supported Ag2.3Se thermoelectric generator before (A) and after (B) being annealed, the diagram of measuring output voltage of thermoelectric generator (C), and the output voltage and power of thermoelectric generator before (D) and after (E) being annealed at temperature difference of 25 K.
For the generator before being annealed, the internal resistance measured to be about 24 Ω, and as shown in Figure 10D, the maximum value of output power and the corresponding output voltage are 0.67 μW and 4.05 mV at temperature difference of 25 K. After being annealed, the generator shows a little higher internal resistance of 27 Ω. Figure 10E displays that the maximum value of output power and the corresponding output voltage of annealed generator are improved to be 1.16 μW and 5.60 mV, respectively. Obviously, this improvement of performance is resulted from the enhanced Seebeck coefficient of annealed Ag2.3Se film. The power density of annealed Ag2.3Se module can be calculated by the following equation: , where Pd is the power density, Pmax is the maximum value of output power, N is number of thermoelectric film, and S is the cross-sectional area of annealed Ag2.3Se film (Lu et al., 2019). And in this work, the N is 4 and the S is calculated by the equation , where t is the thickness of Ag2.3Se film without substrate (10 μm) and w is the width of Ag2.3Se film (5 mm). Thus, S is 0.05 mm2 and the Pd is calculated to be 5.80 W/m2. As displayed in Table 3, at similar temperature difference, the output power density of annealed Ag2.3Se-based module is much higher than that of previous representative film thermoelectric devices, and in the case that temperature difference is 1 K, the output power density of our annealed Ag2.3Se film is also the highest.
Table 3.
The Output Power Density of Annealed Ag2.3Se-Film-Based Device and Previous Representative Film Thermoelectric Devices
| Sample | Pd (W/m2) | ▵T (K) | Pd/▵T (Wm−2K−1) |
|---|---|---|---|
| Annealed Ag2.3Se film (this work) | 5.80 | 25 | 0.23 |
| Cu1Ag4Se3 film (Lu et al., 2019) | 5.42 2.37 |
45 28 |
0.12 0.08 |
| C60/TiS2 nanosheet hybrid film (Wang et al., 2018) | 1.68 | 20 | 0.08 |
| PANI/SWNT/Te composite film (Wang et al., 2017) | 0.62 | 40 | 0.02 |
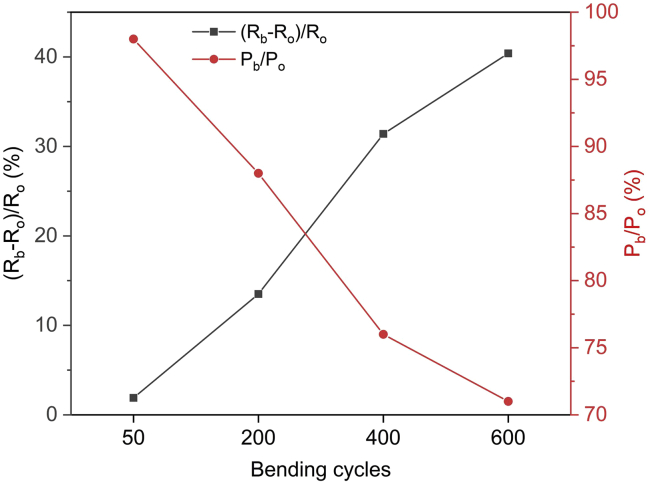
The flexibility of annealed Ag2.3Se thermoelectric device has been tested and the result is shown in Figure 11. Ro and Po represent the internal resistance and output power of original device, and Rb and Pb mean the internal resistance and output power of device has been bent for a certain of cycles. A short video (Video S1) is provided in Supplemental Information to show how we bend the device and the bending radius is about 1 cm. With increasing bending cycles, the internal resistance of device increases while the output power decreases. After 600 bending cycles, the internal resistance of device increases by 40.4% and the output power decreases by 29%. The flexibility test of thermoelectric device has barely been reported in previous articles, thus the result of this flexibility test is compared with that of other single paper-supported thermoelectric film in Table 4. Because the nanowires can form crisscrossed conductive network, the Ag2Te NWs film, which was reported in our previous work, showed a better flexibility than the annealed Ag2.3Se device. However, due to the tight bond between the Ag2.3Se film and paper substrate, the flexibility of annealed Ag2.3Se device is still superior to the paper-supported Bi2Te3 and Sb2Te3 films prepared by magnetron sputtering (Rojas et al., 2017).
Figure 11.
The Internal Resistance and Output Power Ratio as a Function of Bending Cycles
Ro and Po represent the internal resistance and output power of original device, and Rb and Pb mean the internal resistance and output power of device has been bent for a certain of cycles. This figure is related to Video S1.
Table 4.
The Comparison of Flexibility Test of Annealed Ag2.3Se Thermoelectric Device and Other Paper-Supported Thermoelectric Films
| Sample | Bending Cycles | (Rb-Ro)/Ro |
|---|---|---|
| Annealed Ag2.3Se device (this work) | 600 | 40.4% |
| Ag2Te NWs film (Gao et al., 2017b) | 500 | 21% |
| Bi2Te3 film (Rojas et al., 2017) | 400 | 140% |
| Sb2Te3 film (Rojas et al., 2017) | 400 | 50% |
It can be observed from this video that the bending radius is about 1 cm
Limitations of the Study
To improve the output power density of flexible film thermoelectric generator, the length of single thermoelectric film has been optimized by numerical simulation in this work. Although the actual output power density of our generator is superior to that of other flexible film thermoelectric generator, the thermal conductivity of annealed Ag2.3Se film for simulation is a theoretic value rather than an actually measured value because it is difficult to obtain the accurate thermal conductivity of thermoelectric film adhered on a substrate. In further work, we will try our best to obtain the actual thermal conductivity of flexible thermoelectric film for numerical simulation.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51572049, 51772056, 51961011), National Key Research and Development Program of China (No. 2017YFE9128000) and the Natural Science Foundation of Guangxi, China (Grant No. 2018GXNSFAA294135, AD19110020).
Author Contributions
J. G. conceived the idea and prepared the nanoparticles, films, and devices. J. G. and S. J. Z. measured the thermoelectric properties of the films and devices. J. G., Y. P., and X. Y. W. characterized the nanoparticles and films. H. J. L and H. F. C. completed the numerical simulation. J. G., L. M., and H. J. L. discussed and analyzed the data. J. G., L. M., H. J. L., and K. K. wrote and edited the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100753.
Supplemental Information
References
- Bahk J.-H., Fang H., Yazawa K., Shakouri A. Flexible thermoelectric materials and device optimization for wearable energy harvesting. J. Mater. Chem. C. 2015;3:10362–10374. [Google Scholar]
- Chen Y., He M., Liu B., Bazan G.C., Zhou J., Liang Z. Bendable n-type metallic nanocomposites with large thermoelectric power factor. Adv. Mater. 2017;29:1604752. doi: 10.1002/adma.201604752. [DOI] [PubMed] [Google Scholar]
- Day T., Drymiotis F., Zhang T., Rhodes D., Shi X., Chen L., Snyder G.J. Evaluating the potential for high thermoelectric efficiency of silver selenide. J. Mater. Chem. C. 2013;1:7568–7573. [Google Scholar]
- Ding Y., Qiu Y., Cai K., Yao Q., Chen S., Chen L., He J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019;10:841. doi: 10.1038/s41467-019-08835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Xu J., Paul B., Eklund P. Flexible thermoelectric materials and devices. Appl. Mater. Today. 2018;12:366–388. [Google Scholar]
- Dun C., Hewitt C.A., Huang H., Xu J., Zhou C., Huang W., Cui Y., Zhou W., Jiang Q., Carroll D.L. Flexible n-type thermoelectric films based on Cu-doped Bi2Se3 nanoplate and Polyvinylidene Fluoride composite with decoupled Seebeck coefficient and electrical conductivity. Nano Energy. 2015;18:306–314. [Google Scholar]
- Ferhat M., Nagao J. Thermoelectric and transport properties of β-Ag2Se compounds. J. Appl. Phys. 2000;88:813–816. [Google Scholar]
- Ferreira-Teixeira S., Pereira A.M. Geometrical optimization of a thermoelectric device: numerical simulations. Energy Convers. Manag. 2018;169:217–227. [Google Scholar]
- Gao J., Liu C., Miao L., Wang X., Peng Y., Chen Y. Enhanced power factor in flexible reduced graphene oxide/nanowires hybrid films for thermoelectrics. RSC Adv. 2016;6:31580–31587. [Google Scholar]
- Gao M., Li L., Song Y. Inkjet printing wearable electronic devices. J. Mater. Chem. C. 2017;5:2971–2993. [Google Scholar]
- Gao J., Miao L., Liu C., Wang X., Peng Y., Wei X., Zhou J., Chen Y., Hashimoto R., Asaka T., Koumoto K. A novel glass-fiber-aided cold-press method for fabrication of n-type Ag2Te nanowires thermoelectric film on flexible copy-paper substrate. J. Mater. Chem. A. 2017;5:24740–24748. [Google Scholar]
- Ge Z.-H., Zhao L.-D., Wu D., Liu X., Zhang B.-P., Li J.-F., He J. Low-cost, abundant binary sulfides as promising thermoelectric materials. Mater. Today. 2016;19:227–239. [Google Scholar]
- Hu X., Li F., Song Y. Wearable power source: a newfangled feasibility for perovskite photovoltaics. ACS Energy Lett. 2019;4:1065–1072. [Google Scholar]
- Jeon T., Jin H.M., Lee S.H., Lee J.M., Park H.I., Kim M.K., Lee K.J., Shin B., Kim S.O. Laser crystallization of organic–inorganic hybrid perovskite solar cells. ACS Nano. 2016;10:7907–7914. doi: 10.1021/acsnano.6b03815. [DOI] [PubMed] [Google Scholar]
- Jin W., Liu L., Yang T., Shen H., Zhu J., Xu W., Li S., Li Q., Chi L., Di C.-a., Zhu D. Exploring Peltier effect in organic thermoelectric films. Nat. Commun. 2018;9:3586. doi: 10.1038/s41467-018-05999-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Lee H.E., Choi H., Kim Y., We J.H., Shin J.S., Lee K.J., Cho B.J. High-performance flexible thermoelectric power generator using laser multiscanning lift-off process. ACS Nano. 2016;10:10851–10857. doi: 10.1021/acsnano.6b05004. [DOI] [PubMed] [Google Scholar]
- Kroon R., Mengistie D.A., Kiefer D., Hynynen J., Ryan J.D., Yu L., Müller C. Thermoelectric plastics: from design to synthesis, processing and structure–property relationships. Chem. Soc. Rev. 2016;45:6147–6164. doi: 10.1039/c6cs00149a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrykov S.A., Ramarao B.V. Thermal properties of copy paper sheets. Drying Technol. 2012;30:297–311. [Google Scholar]
- Lee C., Park Y.-H., Hashimoto H. Effect of nonstoichiometry on the thermoelectric properties of a Ag2Se alloy prepared by a mechanical alloying process. J. App. Phys. 2007;101:024920. [Google Scholar]
- Li W., Lin S., Ge B., Yang J., Zhang W., Pei Y. Low sound velocity contributing to the high thermoelectric performance of Ag8SnSe6. Adv. Sci. 2016;3:1600196. doi: 10.1002/advs.201600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.H., Wong K.W., Liu Y., Zhang Y., Cadavid D., Cabot A., Ng K.M. Critical role of nanoinclusions in silver selenide nanocomposites as a promising room temperature thermoelectric material. J. Mater. Chem. C. 2019;7:2646–2652. [Google Scholar]
- Liu J., Wang X., Li D., Coates N.E., Segalman R.A., Cahill D.G. Thermal conductivity and elastic constants of PEDOT: PSS with high electrical conductivity. Macromolecules. 2015;48:585–591. [Google Scholar]
- Lou Z., Li L., Wang L., Shen G. Recent progress of self-powered sensing systems for wearable electronics. Small. 2017;13:1701791. doi: 10.1002/smll.201701791. [DOI] [PubMed] [Google Scholar]
- Lu Y., Qiu Y., Cai K., Ding Y., Wang M., Jiang C., Yao Q., Huang C., Chen L., He J. Ultrahigh power factor and flexible silver selenide-based composite film for thermoelectric devices. Energ. Environ. Sci. 2019 [Google Scholar]
- Madan D., Wang Z., Wright P.K., Evans J.W. Printed flexible thermoelectric generators for use on low levels of waste heat. Appl. Energ. 2015;156:587–592. [Google Scholar]
- Mi W., Qiu P., Zhang T., Lv Y., Shi X., Chen L. Thermoelectric transport of Se-rich Ag2Se in normal phases and phase transitions. Appl. Phys. Lett. 2014;104:133903. [Google Scholar]
- Perez-Taborda J.A., Caballero-Calero O., Vera-Londono L., Briones F., Martin-Gonzalez M. High thermoelectric zT in n-type silver selenide films at room temperature. Adv. Energy Mater. 2018;8:1702024. [Google Scholar]
- Qiu P., Mao T., Huang Z., Xia X., Liao J., Agne M.T., Gu M., Zhang Q., Ren D., Bai S. High-efficiency and stable thermoelectric module based on liquid-like materials. Joule. 2019;3:1538–1548. [Google Scholar]
- Rojas J.P., Conchouso D., Arevalo A., Singh D., Foulds I.G., Hussain M.M. Paper-based origami flexible and foldable thermoelectric nanogenerator. Nano Energy. 2017;31:296–301. [Google Scholar]
- Shin S., Kumar R., Roh J.W., Ko D.-S., Kim H.-S., Kim S.I., Yin L., Schlossberg S.M., Cui S., You J.-M. High-performance screen-printed thermoelectric films on fabrics. Sci. Rep. 2017;7:7317. doi: 10.1038/s41598-017-07654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yao Q., Shi W., Qu S., Chen L. Engineering carrier scattering at the interfaces in polyaniline based nanocomposites for high thermoelectric performances. Mater. Chem. Front. 2017;1:741–748. [Google Scholar]
- Wang L., Zhang Z., Geng L., Yuan T., Liu Y., Guo J., Fang L., Qiu J., Wang S. Solution-printable fullerene/TiS2 organic/inorganic hybrids for high-performance flexible n-type thermoelectrics. Energy Environ. Sci. 2018;11:1307–1317. [Google Scholar]
- Weathers A., Khan Z.U., Brooke R., Evans D., Pettes M.T., Andreasen J.W., Crispin X., Shi L. Significant electronic thermal transport in the conducting polymer poly(3,4-ethylenedioxythiophene) Adv. Mater. 2015;27:2101–2106. doi: 10.1002/adma.201404738. [DOI] [PubMed] [Google Scholar]
- Xiao C., Xu J., Li K., Feng J., Yang J., Xie Y. Superionic phase transition in silver chalcogenide nanocrystals realizing optimized thermoelectric performance. J. Am. Chem. Soc. 2012;134:4287–4293. doi: 10.1021/ja2104476. [DOI] [PubMed] [Google Scholar]
- Yang H., Leow W.R., Chen X. 3D printing of flexible electronic devices. Small Methods. 2018;2:1700259. [Google Scholar]
- Zhang A.B., Wang B.L., Pang D.D., Chen J.B., Wang J., Du J.K. Influence of leg geometry configuration and contact resistance on the performance of annular thermoelectric generators. Energy Convers. Manag. 2018;166:337–342. [Google Scholar]
- Zhao L.-D., Lo S.-H., Zhang Y., Sun H., Tan G., Uher C., Wolverton C., Dravid V.P., Kanatzidis M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature. 2014;508:373. doi: 10.1038/nature13184. [DOI] [PubMed] [Google Scholar]
- Zi Y., Wang Z.L. Nanogenerators: an emerging technology towards nanoenergy. APL Mater. 2017;5:074103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
It can be observed from this video that the bending radius is about 1 cm