Abstract
BACKGROUND:
Psychosis onset typically occurs in adolescence, and subclinical psychotic experiences peak in adolescence. Adolescence is also a time of critical neural and cognitive maturation. Using cross-sectional data from the Philadelphia Neurodevelopmental Cohort, we examined whether regional white matter (WM) development is disrupted in youths with psychosis spectrum (PS) features and whether WM maturation mediates the relationship between age and cognition in typically developing (TD) youths and youths with PS features.
METHODS:
We examined WM microstructure, as assessed via diffusion tensor imaging, in 670 individuals (age 10–22 years; 499 TD group, 171 PS group) by using tract-based spatial statistics. Multiple regressions were used to evaluate age × group interactions on regional WM indices. Mediation analyses were conducted on four cognitive domains—executive control, complex cognition, episodic memory, and social cognition—using a bootstrapping approach.
RESULTS:
There were age × group interactions on fractional anisotropy (FA) in the superior longitudinal fasciculus (SLF) and retrolenticular internal capsule. Follow-up analyses revealed these effects were significant in both hemispheres. Bilateral SLF FA mediated the relationship between age and complex cognition in the TD group, but not the PS group. Regional FA did not mediate the age-associated increase in any of the other cognitive domains.
CONCLUSIONS:
Our results showed aberrant age-related effects in SLF and retrolenticular internal capsule FA in youths with PS features. SLF development supports emergence of specific higher-order cognitive functions in TD youths, but not in youths with PS features. Future mechanistic explanations for these relationships could facilitate development of earlier and refined targets for therapeutic interventions.
Keywords: Cognition, Development, DTI, Mediation, Psychosis spectrum, Psychotic-like experiences, Subclinical psychosis, Superior longitudinal fasciculus
Schizophrenia (SZ) has a lifetime prevalence of 0.75% (1), but, consistent with our understanding of a psychosis continuum (2,3), psychotic-like experiences (PLEs) are reported more frequently (5%–8% of the population) (4,5). These subclinical symptoms are qualitatively similar to the hallmark psychotic symptoms of SZ but with decreased severity and frequency. Although below the clinical threshold, such symptoms are still distressing and are associated with lower reported happiness (6), increased suicidality (7,8), and significant occupational and social impairments (9). PLEs also share etiological risk factors, cognitive correlates, and symptom profiles with SZ (3) and can precede subsequent full-blown illness. Similar to transition rates for clinical high-risk youths (10), up to 25% of individuals who report PLEs convert to a diagnosed psychotic disorder by adulthood (11,12). In individuals who do not convert to a diagnosed psychotic disorder, approximately 30% to 40% continue to experience PLEs into early adulthood (13,14) and potentially throughout life (15).
The onset period for SZ begins in late adolescence, and similarly, subclinical psychotic symptoms peak during adolescence (3), coinciding with periods of dynamic change in cognition and brain connectivity, including white matter (WM) maturation. The typical linear increase in WM volume from childhood until early adulthood (16,17) is thought to reflect axon growth, organization of axons into bundles, and myelination (18). WM integrity is putatively reflected by the principal diffusion tensor imaging (DTI) measure, fractional anisotropy (FA) (19–22). DTI studies have shown regional age-related changes with the overarching trend of FA increasing into adulthood (23,24). Although both early-onset and chronic SZ have been associated with reduced FA [for review see (25,26)], whether and how developmental trajectories of WM in youths who develop SZ diverge from those in typically developing (TD) youths is unknown. In this study, we used TD and psychosis spectrum (PS) groups from the Philadelphia Neurodevelopmental Cohort (PNC) to explore how psychosis affects age-associated regional changes in WM. One previous study reported an age × group interaction on regional gray matter volume in this cohort (27); however, no studies have investigated such interactions on DTI-assessed WM indices.
As with SZ, PLEs have been associated with cognitive deficits, both generally and in specific domains (28–32). However, despite the demonstrated link between psychosis and cognition and the known relationship of WM to cognitive development (33–35), it is not yet established whether regional WM mediates the relationship between age and cognitive deficits associated with psychosis. To address these questions, we first evaluated whether the age-related pattern of regional WM development was disrupted in youths with elevated PLEs. We then conducted post hoc analyses on tracts with significant interactions to evaluate whether FA mediated the relationship between age and cognition and whether that relationship differed between TD youths and youths with PS features.
METHODS AND MATERIALS
Participants
The PNC is a publicly available population-based sample of more than 9500 individuals (age range: 8–22 years) [for description see (36)]. All subjects provided medical history, clinical, and cognitive data. A subset of participants underwent neuroimaging, with DTI scans acquired for 1312 individuals. All data presented here were collected at the University of Pennsylvania and analyzed at the University of California, Los Angeles. Psychopathology self-report and parent-report ratings and medical histories were obtained from the GOASSESS structured computerized instrument.
In addition to the PNC’s exclusion for medical problems impacting brain function (36), participants were excluded if they had more than 2.5 mm of total Euclidean distance movement during the DTI scan (n = 44), a history or current diagnosis of autism spectrum disorder (n = 13), or other neurological or nonpsychotic psychiatric diagnoses (n = 452) (see Supplement). Owing to the limited number of younger individuals with PS features, participants younger than 10 years of age (n = 122) were also excluded to match groups for age distribution. After excluding participants who met one or more of the aforementioned criteria, 707 individuals remained for analysis.
Subclinical Grouping
Psychosis screening and classification of individuals into groups were performed as described by Calkins et al. (37) (see Supplement). Although our primary analysis consisted of PS and TD groups, we included a third group, limited PS (LPS), with symptom severity between TD and PS in secondary analyses to allow for preliminary investigation of subtle dimensional changes. These individuals were included only in secondary analyses owing to the small sample size. In our sample of 707 youths, 499 were in the TD group, 171 were in the PS group, and 37 were in the LPS group.
Cognitive Data
Cognitive testing was administered via the Computerized Neurocognitive Battery developed at the University of Pennsylvania. Scores for complex cognition (language reasoning, nonverbal reasoning, and spatial ability), executive control (mental flexibility, attention, and working memory), episodic memory (verbal memory, face memory, and spatial memory), and social cognition (emotion identification, emotion differentiation, and age differentiation) were calculated as previously described (38). Our analyses focused on efficiency scores—obtained via the PNC data release and based on the entire neurocognitive sample (N = 9138)—which reflect the sum of Z scores for accuracy and speed. Analyses of covariance, with age and sex as covariates, were used to evaluate group differences in efficiency scores. A Bonferroni correction for each of the four domains was applied, resulting in a significance threshold of p < .05/4 = .0125.
DTI Processing
The PNC protocol divides a 64-direction diffusion-weighted imaging set into two independent 32-direction sequences (b value: 1000 s/mm2) [see Supplement and (36) for parameter details]. The two sequences were concatenated before applying standard preprocessing using tools from FMRIB Software Library’s Diffusion Toolbox. Specifically, Eddy Correct was used to correct for distortions due to eddy currents and head motion. Registered b0 and concatenated files were skull stripped and masked using Brain Extraction Tool. FA images were calculated by fitting a diffusion tensor model at each voxel with DTIFit. We then implemented tract-based spatial statistics (39) in accordance with the ENIGMA-DTI pipeline (http://enigma.ini.usc.edu/protocols/dti-protocols) (40) and extracted regions of interest (ROIs) from the Johns Hopkins University White-Matter Tractography Atlas (41–43). Bilateral ROIs were generated by averaging across both hemispheres. We tested the average FA for 25 total ROIs (Supplemental Figure S1; Supplemental Table S1).
FA was our principal measure of interest. Additional diffusivity measures were examined in follow-up analyses. Axial diffusivity (AD) reflects diffusivity parallel to the axon and is thought to reflect fiber organization (44). Radial diffusivity (RD) is perpendicular to axons and an indirect measure of myelination (44). Mean diffusivity (MD) is an average of diffusivity across all three axes (20). AD, MD, and FA were calculated by DTIFit, whereas RD was computed as the average of the second and third eigenvalue images. Non-FA DTI measures also underwent ENIGMA tract-based spatial statistics processing.
Statistical Analysis
Statistical analyses and graphical representations were performed using Stata 15 software (StataCorp LP, College Station, TX). For each tract, individuals whose FA exceeded 1.5 interquartile ranges (IQRs) either below the first quartile (Q1 − [1.5 × IQR]) or above the third quartile (Q3 + [1.5 × IQR]) were deemed outliers and excluded from subsequent analysis of that tract. The number of individuals used in analysis of each tract is reported in Supplemental Table S1. To test for an age × group interaction and main effect of group on regional FA, a regression model including age, sex, and group (TD and PS) was used. The regression was followed by evaluation of marginal effects, computed at 1-year age intervals, for interaction analyses. A corrected significance threshold for all regression analyses was set at p < .05/25 = .002, which corrects for the 25 tracts analyzed.
In tracts where significant age × group interactions for FA were detected, we conducted a series of follow-up analyses. To conduct preliminary exploration of whether the extent of PLEs affected age-associated FA changes, analyses including youths with LPS features as a third group were conducted to determine if an age × group (TD, LPS, and PS) interaction was still present. Post hoc contrasts compared the slope of marginal effects between groups to determine whether LPS represented an intermediate phenotype. We also tested for age × group interactions on hemispheric FA and non-FA measures. A significance threshold of p < .05/2 = .025 was applied to account for the two tracts undergoing follow-up analyses.
Post Hoc Mediation Analyses
For tracts with significant age × group interactions, we conducted post hoc mediation analyses to evaluate whether the relationship between age and any of the cognitive domains was mediated by FA and, if so, whether that mediation differed between TD and PS groups. Individuals missing a score for a given cognitive domain were excluded from mediation analyses for that particular domain.
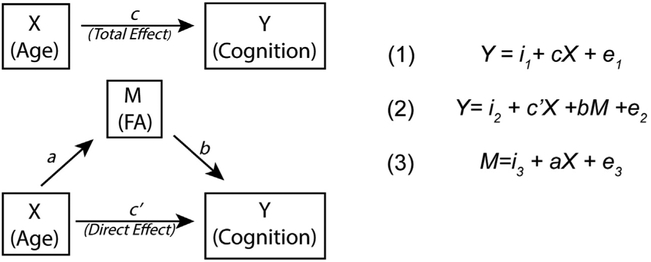
Mediation was tested using methods described by MacKinnon et al. (45). Path coefficients between the independent (X), dependent (Y), and mediator (M) variables were determined using three regression equations (Figure 1). We first determined whether initial criteria for mediation were met. Specifically, whether path coefficients a (effect of age on FA), b (effect of FA on cognition), and c (effect of age on cognition) all were significant (Figure 1). If these criteria were satisfied, we continued to test the significance of the indirect (i.e., mediated) effect, computed as the product of regression coefficients a and b (46). We used a bootstrapping approach, in which data were resampled with replacement 1000 times, to assess for significance of the indirect effect. Bootstrapping allows for confidence intervals to be built from resampled data without assumptions of normality. To reduce the chance of type I errors, we increased our confidence interval to 99%. Confidence intervals including 0 were deemed as nonsignificant. Finally, we determined the proportion of the total effect that was mediated by dividing the indirect effect (ab) by the total effect (c = c′ + ab).
Figure 1.
Schematic of a simple mediation model. We examined whether regional fractional anisotropy (FA) significantly mediated the effect of age on cognition. In a mediation model, the relationship between an independent variable (X) and a dependent variable (Y) is influenced by the (nonobservable) mediator variable (M). Intercepts and residuals for each equation are denoted by i and e, respectively. The total effect (c) is the sum of both the direct (c′) and mediated (ab) effects. The total effect, c, was determined with equation (1). Coefficients a and b were determined with equation (2) and equation (3), respectively. The direct effect (c′) was determined with equation (2). Mediation is determined by assessing the significance of the mediated effect (ab) with a bootstrapping approach.
For tracts that significantly mediated a cognitive domain, supplementary analyses were conducted to determine if non-FA measures mediated the age-cognition relationship and whether FA mediated cognition accuracy and speed (rather than the combined efficiency score) (see Supplement).
RESULTS
Participants
There were no significant differences between groups in age or sex distribution (Table 1).
Table 1.
Participants’ Demographic Information
| Group | Statistic | Significance (p) | |||
|---|---|---|---|---|---|
| TD | PS | LPS | |||
| Age, Years, Mean ± SD | 15.75 ± 3.36 | 15.83 ± 2.89 | 15.32 ± 3.13 | F2,706 = 0.371 | .690 |
| TD vs. PS | t1,668 = −0.28 | .784 | |||
| TD vs. LPS | t1,534 = 0.75 | .454 | |||
| PS vs. LPS | t1,206 = 0.95 | .342 | |||
| Number (% Female) | 499 (49.1%) | 171 (53.2%) | 37 (37.8%) | χ22,707 = 2.99 | .224 |
| TD vs. PS | χ21,670 = 0.864 | .353 | |||
| TD vs. LPS | χ21,536 = 1.75 | .186 | |||
| PS vs. LPS | χ21,208 = 2.88 | .09 | |||
| GAF, Mean ± SD | 79.81 ± 21.07 | 69.22 ± 19.7 | 73.22 ± 15.86 | F2,654 = 16.14 | 1.44 × 10−7 |
| TD vs. PS | t1,623 = 5.53 | 4.8 × 10−8 | |||
| TD vs. LPS | t1,499 = 1.74 | .083 | |||
| PS vs. LPS | t1,186 = −1.079 | .282 | |||
| Education, Years, Mean ± SD | 8.61 ± 3.27 | 8.13 ± 2.64 | 8.08 ± 3.06 | F2,704= 1.797 | .167 |
| TD vs. PS | t1,668 = 1.73 | .084 | |||
| TD vs. LPS | t1,534 = 0.949 | .343 | |||
| PS vs. LPS | t1,206 = 0.952 | .342 | |||
GAF, Global Assessment of Functioning Score; LPS, limited psychosis spectrum; PS, psychosis spectrum; TD, typically developing.
DTI Analysis
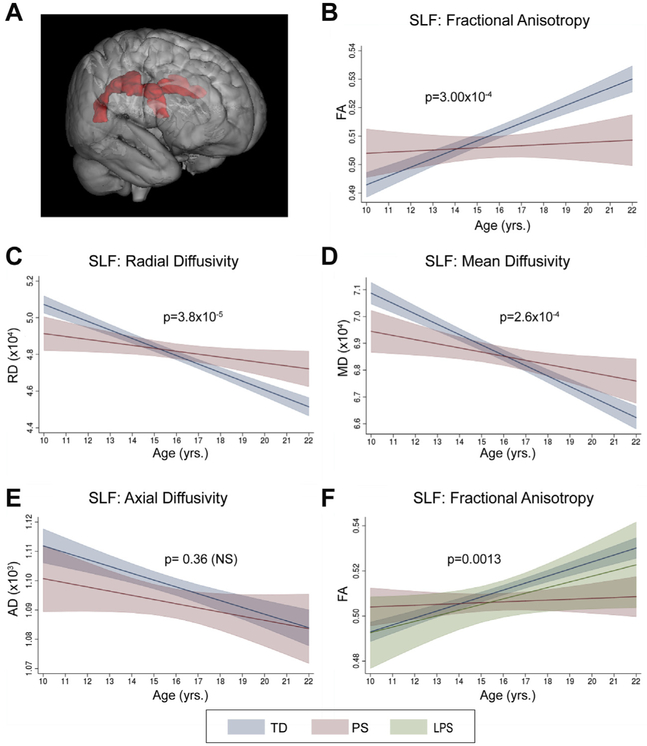
Multiple regression analyses, including age, sex, and group, for each of the 25 tracts revealed that only the superior longitudinal fasciculus (SLF) and retrolenticular internal capsule (RLIC) showed a significant age × group interaction (TD vs. PS; p < .002). The SLF (Figure 2A) was the only tract with a significant main effect of group (Supplemental Table S1). Additional tracts showed age × group interactions meeting the thresholds for trend-level significance (p < .004) and uncorrected significance (p < .05) (Supplemental Table S2).
Figure 2.
Age-associated changes in the superior longitudinal fasciculus (SLF). (A) Three-dimensional representation of SLF tract. In analyses of typically developing (TD) and psychosis spectrum (PS) groups, there was a significant (p < .002) interaction between age and group on SLF fractional anisotropy (FA) (B). Follow-up analyses revealed additional significant (p < .025) age × group interactions on radial diffusivity (RD) (C) and mean diffusivity (MD) (D), but not on axial diffusivity (AD) (E). (F) Follow-up analyses including a third group, limited psychosis spectrum (LPS), also revealed a significant age × group interaction on SLF FA. Graphs show predicted margins with 95% confidence intervals. Significance values represent corrected p values for age × group interactions. p < .025. NS, not significant.
Superior Longitudinal Fasciculus.
All three variables (age, sex, and group) in the regression model significantly explained SLF FA (R2 = .14, F4,660 = 26.86, p = 1.15 × 10−20), with a significant interaction between age and group (β = −.002, F1,660 = 13.20, p = 3.0 × 10−4) (Figure 2B) and each variable independently significantly contributing to the model (Supplemental Table S3). Evaluation of regression coefficients for each group revealed that whereas FA increased with age in the TD group (β = .003, p = 3.68 × 10−19), there was no significant relationship in the PS group (β = 4.9 × 10−4, p = .48). Confirmatory analyses indicated no significant group × sex (F1,660 = 2.04, p = .15) or age × group × sex (F1,657 = 1.18, p = .28) interactions on SLF FA.
Secondary analyses revealed significant age × group interactions in both the right (β = −.0032, F1,660 = 15.51, p = 1.0 × 10−4) and the left (β = −.0022, F1,660 = 8.51, p = .0036) SLF. Of the three non-FA SLF diffusivity measures, both RD (β = 3.30 × 10−6, F1,660 = 17.21, p = 3.8 × 10−5) (Figure 2C) and MD (β = 2.54 × 10−6, F1,660 = 13.46, p = 2.6 × 10−4)(Figure 2D) showed a significant age × group interaction. Youths in the TD group showed significant decreases in RD (β = −4.47 × 10−6, p = 3.8 × 10−32) and MD with age (β = −3.82 × 10−6, p = 6.8 × 10−32). Youths in the PS group also showed lower RD (β = −1.36 × 10−6) and MD (β = −0.13 × 10−6) with increasing age, but this association was not significant (p > .025). There was no significant interaction between age and group for AD (Figure 2E).
Analyses including youths with LPS features as a third group in the regression also resulted in a significant age × group interaction (F2,695 = 6.68, p = .0013) (Figure 2F). Post hoc contrasts revealed that the slope of the relationship between age and FA for the LPS group did not significantly differ from the TD (t1,695 = 0.43, p = .664) or the PS (t1,695 = −0.144, p = .149) group.
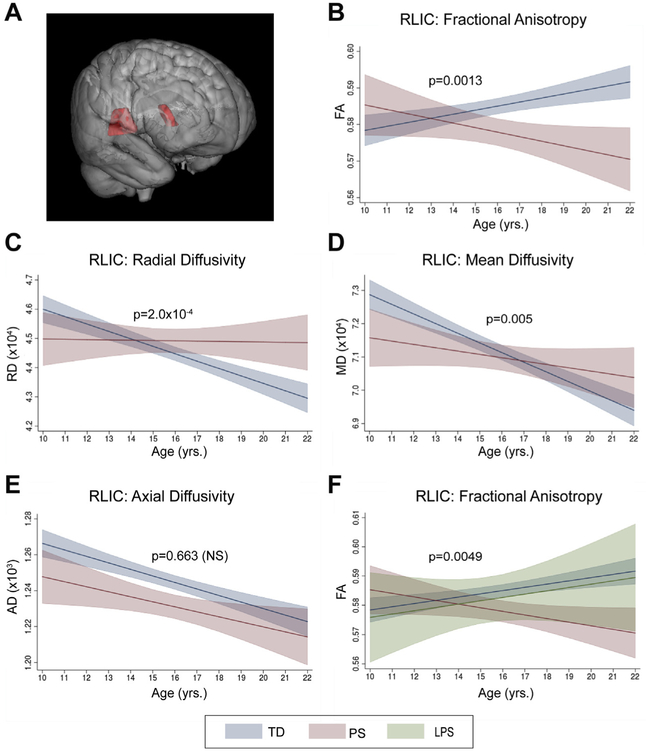
Retrolenticular Internal Capsule.
In the RLIC (Figure 3A), all three variables in the multiple regression analysis collectively explained FA (R2 = .037, F4,657 = 6.24, p = .0001), with a significant interaction between age and group on RLIC FA (β = −.002, F1,657 = 10.44, p = .0013) (Figure 3B) but no significant main effect of group (p > .002) (Supplemental Table S4). Evaluation of regression coefficients for each group indicated that TD and PS groups exhibited opposite patterns, with a gradual linear increase in RLIC FA across development for the TD group (β = .001, p = .001) and a nonsignificant decrease in the PS group (β = −.001, p = .062) with increasing age. Confirmatory analyses indicated no significant group × sex (F1,657 = 0.21, p = .644) or age × group × sex (F1,654 = 0.17, p = .681) interactions.
Figure 3.
Age-associated changes in the retrolenticular internal capsule (RLIC). (A) Three-dimensional representation of RLIC tract. In analyses of typically developing (TD) and psychosis spectrum (PS) groups, there was a significant interaction (p < .002) between age and group on RLIC fractional anisotropy (FA) (B). Follow-up analyses revealed significant age × group interactions (p < .025) on radial diffusivity (RD) (C) and mean diffusivity (MD) (D), but not on axial diffusivity (AD) (E). (F) Follow-up analyses including a third group, limited psychosis spectrum (LPS), also revealed a significant (p < .025) age × group interaction on RLIC FA. Graphs show predicted margins with 95% confidence intervals. Significance values represent corrected p values for age × group interactions. NS, not significant.
Secondary analyses of RLIC FA indicated there were significant age × group interactions in both the left (β = −.0021, F1,654 = 7.72, p = .0056) and the right (β = −.0024, F1,647 = 9.40, pROI = .0023) hemispheres. Of the three non-FA diffusivity measures, both RD (β = 2.92 × 10−6, F1,657 = 14.21, p = 2.00 × 10−4) (Figure 3C) and MD (β = 7.58 × 10−7, F1,656 = 7.90, p = .0051) (Figure 3D) showed a significant age × group interaction. TD youths showed a significant decrease in RLIC RD (β = −2.5 × 10−6, p = 9.1 × 10−13) and MD (β = −2.9 × 10−6, p = 3.4 × 10−16) with increasing age. In youths with PS features, there were no significant relationships between age and RLIC RD (β = 4.97 × 10−7, p = .50) or MD (β = −6.8 × 10−7, p = .39). Neither group showed a significant age × group interaction on AD (Figure 3E).
Inclusion of youths with LPS features as a third group in the regression model also resulted in a significant age × group interaction (F2,692 = 5.36, p = .0049) (Figure 3F). Post hoc contrasts revealed that the slope of the relationship between age and FA for the LPS group did not significantly differ from the TD (t1,692 = −0.02, p = .982) or the PS (t1,695 = −0.166, p = .097) group.
Neurocognitive Efficiency Results
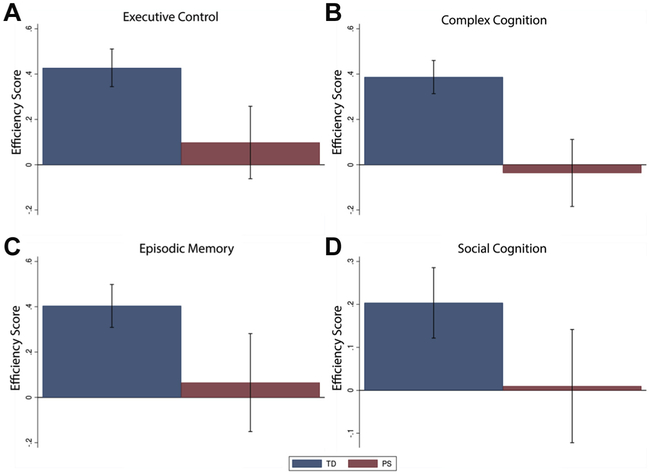
Youths in the PS groups had significantly (p < .0125) lower efficiency scores in all four cognitive domains (Figure 4; Supplemental Table S5).
Figure 4.
Group differences in cognitive domains. Youths in the psychosis spectrum (PS) group consistently showed significantly (p < .0125) lower efficiency scores in all four cognitive domains: (A) executive control, (B) complex cognition, (C) episodic memory, and (D) social cognition. Efficiency scores reflect the average Z score (sum of Z score for accuracy and −1 multiplied by Z score for speed) per group, such that higher scores indicate better performance. Error bars represent ± SE of the group average. TD, typically developing.
Post Hoc Mediation Analyses
Superior Longitudinal Fasciculus.
The PS group did not satisfy initial requirements for mediation of the effect of age on any cognitive domain, as both a (coefficient for effect of age on SLF) and b (coefficient for effect of SLF on cognition) were nonsignificant (p > .05) for all domains. However, in the TD group, path coefficients a and b were significant for complex cognition and episodic memory; scores increased with both age and SLF FA (Table 2). Evaluating mediation in TD with a bootstrapping approach indicated that only the relationship between age and complex cognition was significantly mediated by SLF FA, with SLF FA accounting for 27.6% of the total effect between age and complex cognition. (See Supplement for path coefficients of mediation analyses for executive control and social cognition [Supplemental Table S6], complex cognition by non-FA SLF measures [Supplemental Table S7], and complex cognition accuracy and speed by SLF FA [Supplemental Table S8]).
Table 2.
Mediation Analysis Results for TD Youths
| Dependent Variable | Path Coefficients (SE) | Total Effect Mediated (%) | Bootstrap 99% CI | ||||
|---|---|---|---|---|---|---|---|
| Age → SLF | SLF → Cognition | Direct Effect | Total Effect | Mediated Effect | |||
| a | b | c′ | c = ab + c′ | ab | |||
| Complex Cognitionc | 0.0030b (0.0003) | 6.377b (1.415) | 0.0492b (0.0113) | 0.0683b (0.0107) | 0.0189d (0.0047) | 27.6 | 0.0066, 0.0311 |
| Episodic Memorye | 0.0031b (0.0003) | 3.763a (1.85) | 0.0860b (0.0149) | 0.0975b (0.014) | 0.0115 (0.0058) | 11.8 | −0.0055, 0.0285 |
CI, confidence interval; SLF, superior longitudinal fasciculus; TD, typically developing.
p < .05.
p < .001.
n = 491.
Mediation effect significant at 99% CI.
n = 495.
Retrolenticular Internal Capsule.
In contrast to the SLF, the effect of age on RLIC FA (a) was significant for both TD (β = −3.75 × 10−6, F1,491 = 46.18, p = 3.13 × 10−11) and PS (β = −3.03 × 10−6, F1,164 = 4.99, p = .027) groups. However, RLIC FA did not significantly explain any cognitive score in either the TD group or the PS group (p > .05). Thus, criteria for the mediation of the relationship between age and each cognitive domain were not met, and no further analyses were conducted.
DISCUSSION
Our results provide compelling evidence that subclinical PLEs are associated with disrupted age-related changes of WM in the SLF and RLIC. We have also demonstrated that WM development in the SLF partially mediates the relationship between age and complex cognition in TD youths but not in youths with PS features. These findings suggest that alterations in WM neurodevelopment may potentially contribute to cognitive deficits in psychosis.
Psychosis has been increasingly conceptualized as having a neurodevelopmental component (47), with an age-related progression from high-risk and prodromal stages to first-episode and, eventually, chronic illness [for review see (48)]. Longitudinal imaging studies relate this evolution of illness in part to disruptions in traditional WM trajectories, particularly when the disease onset occurs in childhood or adolescence [for review see (49)]. Understanding the link between the trajectories of psychosis and WM at all stages of the disease is necessary for developing age-appropriate targeted interventions. We contribute to elucidating this relationship by identifying aberrant age-related effects in specific WM tracts.
Of the 25 tracts evaluated, only the SLF and RLIC reached the corrected threshold for a significant age × group interaction. The SLF is an associative tract with ipsilateral frontoparietal connections (50) that shows pronounced development across adolescence (24,51,52) and has a well-established role in fundamental cognitive processes, such as working memory, language, and attention (53–55). The RLIC is a projection tract containing motor and sensory fibers that carry projections from the thalamic pulvinar and lateral geniculate nuclei to association and visual cortices (56). Consistent with existing studies of WM maturation during typical development (24), we found a linear increase in FA in both the SLF and the RLIC in the TD group. The PS group, however, did not show any significant age-related changes in either SLF FA or RLIC FA, suggesting disrupted maturation. Together with our findings of age × group interactions for RD, these results collectively suggest that the altered relationships in youths with PS features may be driven by decreased regional myelination, rather than axonal degeneration or differences in tract organization (57–59).
Reduced SLF integrity has previously been reported in youths with risk factors for psychosis (60), subclinical symptoms (61,62), recent-onset SZ (47,63), and first-episode SZ (64,65) and adults with established SZ (66). Furthermore, a longitudinal study indicated that high-risk individuals who converted to psychotic disorders had reduced SLF FA at baseline relative to individuals who did not convert (67). Our results extend on these previous findings, collectively suggesting that age-associated alterations of SLF development as early as late childhood may be associated with subclinical psychosis and serve as a putative early biomarker for SZ, although this possibility awaits confirmation in longitudinal studies. Furthermore, these collective results suggest that abnormalities in SLF FA associated with SZ may be independent of illness confounds, such as medication.
FA of association tracts, such as the SLF, have previously been related to general cognitive function (68–72) as well as development of higher-level cognition during adolescence (33–35,73,74). However, the relationship between the SLF and cognition has been less frequently investigated in youths with psychosis. Furthermore, previous studies have typically evaluated the relationship between cognition and WM separately from that of cognition and age (75) and thus could not rule out that gains in neurocognition may only relate to FA because of a shared association with age.
Our analysis took the novel approach of testing the degree to which WM integrity mediates cognitive development from childhood through young adulthood and how this relationship differs in PS. We found a significant positive relationship of age with complex cognition and SLF FA in TD youths, but not in youths with PS features. Additionally, the association between age and complex cognition in TD youths was significantly mediated by SLF FA, highlighting the importance of the tract in healthy cognitive development. Complex cognition is a reflection of nonverbal reasoning, language reasoning, and spatial attention (38), making our finding consistent with existing studies in which frontoparietal structural connectivity, including the SLF, has been related to attention (76,77), language (77,78), spatial working memory (79,80), and reasoning ability (81) in TD youths. Absence of any significant age-associated changes in complex cognition and SLF FA, and thus any corresponding mediation, in youths with PS features suggests that the presence of subclinical PLEs disrupts maturation of the SLF, which, in turn, affects development of higher-level cognitive functions, as demonstrated by the overall significantly lower complex cognition observed in youths with PS features relative to TD youths. Critically, mediation studies evaluating changes across adulthood into elderly populations found that age-related reductions in SLF FA did not mediate corresponding cognitive decline (82,83), suggesting that its role as a mediator in TD youths may be specific to emergence rather than decline of higher-level cognitive function. The SLF has been divided into as many as five subcomponents (84) with functionally distinct roles. We used a predefined ROI that predominantly includes the frontoparietal portion [traditionally, SLF I, II, and III (85)] without extensive temporoparietal connections. Future tractography studies could extend our analyses to investigate these subcomponents individually.
Our findings also revealed an age × group interaction on RLIC FA. Reduced FA in the internal capsule of patients with chronic SZ has been consistently reported, with mixed findings in first-episode and medication-naïve patients [for review see (26,86)]. To our knowledge, there are no studies that focus specifically on the RLIC in relation to psychosis, though there is previous evidence for an association of reduced RLIC FA with psychosis. Nonhuman adolescent primates receiving daily exposure to ketamine (an N-methyl-D-aspartate antagonist used to mimic psychotic symptoms) showed reduced FA in multiple tracts, including the RLIC and SLF (87). Furthermore, a longitudinal study found that patients (20–40 years of age) with SZ showed an attenuated increase in RLIC FA across a 3-year follow-up relative to control subjects (88). Finally, a meta-analysis reported significantly reduced FA in the left RLIC (89), whereas another meta-analysis, using the same 25 ROIs, showed that adults with SZ had lower RLIC FA than healthy control subjects, though the effect size (d = −.13) did not meet criteria for corrected significance (90). Considered with our findings, these studies may suggest that abnormalities in RLIC FA are most pronounced during adolescent development, with a compensatory increase occurring later in adulthood, or that some other confound in adult patients, such as long-term medication, affects this measure. Further study of this tract in developmental and adult populations is needed to confirm these results.
Projection tracts such as the internal capsule (and here RLIC) are important for corticospinal and sensory communication and have been less frequently investigated in relation to psychiatric disease than associative tracts. Previous studies have postulated that reduced WM in the internal capsule may lead to functional dysconnectivity between the cortex and subcortical structures (91), with corresponding clinically relevant correlates such as increased positive symptoms (92) and impairments in emotional stability, motivation, and executive function (86,92–94). Although dysfunctional information processing has been increasingly implicated in SZ as a putative contributor to impairments in higher-level cognitive function (95), we did not find a relationship between RLIC FA and the cognitive measures used here. However, there is evidence that motor-related and sensory-related symptoms of psychosis emerge before deficits in higher-order cognition (96). The RLIC contains both motor and sensory fibers (97), and thus it is possible that RLIC FA mediates age-related changes in these systems rather than cognition.
Finally, our exploratory analysis in the LPS group supports the hypothesis that the extent of deviation from typical age-related patterns in RLIC and SLF FA relates to the severity of PLEs. The LPS group showed an intermediate pattern, with FA values falling between those of the TD and PS groups. This is further evidence that abnormalities in age-associated structural changes are emerging even at the lowest end of the psychosis spectrum. However, owing to the small LPS sample size, these findings are preliminary and require additional studies with larger samples.
Strengths of this study include the population-based cohort that allows us to eliminate confounds such as medication and illness chronicity. Our results also expand on our evidence of regionally specific FA disruptions by evaluating non-FA measures. Finally, there is a paucity of studies examining how regional WM measures may mediate the relationship between age and cognitive endophenotypes of SZ. This is likely attributable to the rarity of access to both neuroimaging and cognitive data for a sample size of this magnitude that spans such a broad age range. Future studies incorporating more precise measures of maturation, such as pubertal stage, could help to refine our understanding of these relationships. Additionally, future mediation analyses with tasks designed to investigate more focal areas of cognition implicated in psychosis, such as processing speed, could further elucidate the role of WM in cognitive development. Similarly, additional clinical measures, such as individual symptom scales, would be informative when exploring age-related patterns within subgroups. Finally, the PNC consists of cross-sectional data, which limits our ability to evaluate developmental trajectories. Prospective longitudinal data are essential to determine neural biomarkers that predict subsequent outcome.
Conclusions
In this population-based sample, youths with PLE showed disrupted development of regional WM connectivity and, in contrast to TD youths, did not show mediation of the relationship between age and cognition by SLF FA. These findings suggest that disturbances of age-related changes in SLF and RLIC WM may be an early biomarker for psychosis and that the SLF may contribute to cognitive deficits. Future studies should pursue longitudinal investigations to determine their predictive validity and preclinical experimental studies to provide mechanistic explanations and potentially identify critical temporal and anatomical targets for neurotherapeutic interventions.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute of Mental Health (Grant No. R01 MH107250 [to CEB] and Grant No. R01 MH101506 [to KHK]).
Footnotes
All data were obtained via the Database of Genotypes and Phenotypes platform (Project No. 6984, Karlsgodt, Releases 1 and 2).
This article was published as a preprint on bioRxiv (https://doi.org/10.1101/423574).
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2018.12.008.
REFERENCES
- 1.Moreno-Küstner B, Martín C, Pastor L (2018): Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One 13:e0195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss JS (1969): Hallucinations and delusions as points on continua function. Rating scale evidence. Arch Gen Psychiatry 21:581–586. [DOI] [PubMed] [Google Scholar]
- 3.DeRosse P, Karlsgodt KH (2015): Examining the psychosis continuum. Curr Behav Neurosci Rep 2:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linscott RJ, van Os J (2013): An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: On the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med 43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 5.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L (2009): A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med 39:179–195. [DOI] [PubMed] [Google Scholar]
- 6.Koyanagi A (2017): Psychotic-like experiences and happiness in the English general population. J Affect Disord 222:211–217. [DOI] [PubMed] [Google Scholar]
- 7.Jang JH, Lee YJ, Cho SJ, Cho IH, Shin NY, Kim SJ (2014): Psychotic-like experiences and their relationship to suicidal ideation in adolescents. Psychiatry Res 215:641–645. [DOI] [PubMed] [Google Scholar]
- 8.Fisher HL, Caspi A, Poulton R, Meier MH, Houts R, Harrington H, et al. (2013): Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: A birth cohort study. Psychol Med 43:2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correll CU, Hauser M, Auther AM, Cornblatt BA (2010): Research in people with psychosis risk syndrome: A review of the current evidence and future directions. J Child Psychol Psychiatry 51: 390–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. (2012): Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 69:220–229. [DOI] [PubMed] [Google Scholar]
- 11.David AS, Ajnakina O (2016): Psychosis as a continuous phenotype in the general population: The thin line between normality and pathology. World Psychiatry 15:129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H (2000): Children’s self-reported psychotic symptoms and adult schizophreniform disorder: A 15-year longitudinal study. Arch Gen Psychiatry 57:1053–1058. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J (2011): Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: An 8-year cohort study. Schizophr Bull 37:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escher S, Romme M, Buiks A, Delespaul P, Van Os J (2002): Independent course of childhood auditory hallucinations: A sequential 3-year follow-up study. Br J Psychiatry Suppl 43:s10–s18. [DOI] [PubMed] [Google Scholar]
- 15.Rössler W, Riecher-Rössler A, Angst J, Murray R, Gamma A, Eich D, et al. (2007): Psychotic experiences in the general population: A twenty-year prospective community study. Schizophr Res 92:1–14. [DOI] [PubMed] [Google Scholar]
- 16.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. (2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, et al. (2009): Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry 48:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB (2010): Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 20: 534–548. [DOI] [PubMed] [Google Scholar]
- 19.Thomason ME, Thompson PM (2011): Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol 7:63–85. [DOI] [PubMed] [Google Scholar]
- 20.Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basser PJ, Jones DK (2002): Diffusion-tensor MRI: Theory, experimental design and data analysis—a technical review. NMR Biomed 15:456–467. [DOI] [PubMed] [Google Scholar]
- 22.Chang EH, Argyelan M, Aggarwal M, Chandon TS, Karlsgodt KH, Mori S, et al. (2017): Diffusion tensor imaging measures of white matter compared to myelin basic protein immunofluorescence in tissue cleared intact brains. Data Brief 10:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- 24.Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, et al. (2012): White matter development in adolescence: Diffusion tensor imaging and meta-analytic results. Schizophr Bull 38: 1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A (2011): Dysconnectivity in schizophrenia: Where are we now? Neurosci Biobehav Rev 35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler AL, Voineskos AN (2014): A review of structural neuroimaging in schizophrenia: From connectivity to connectomics. Front Hum Neurosci 8:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satterthwaite TD, Wolf DH, Calkins ME, Vandekar SN, Erus G, Ruparel K, et al. (2016): Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 73:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krabbendam L, Myin-Germeys I, Hanssen M, van Os J (2005): Familial covariation of the subclinical psychosis phenotype and verbal fluency in the general population. Schizophr Res 74:37–41. [DOI] [PubMed] [Google Scholar]
- 29.Ziermans TB (2013): Working memory capacity and psychotic-like experiences in a general population sample of adolescents and young adults. Front Psychiatry 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelleher I, Clarke MC, Rawdon C, Murphy J, Cannon M (2013): Neurocognition in the extended psychosis phenotype: performance of a community sample of adolescents with psychotic symptoms on the MATRICS neurocognitive battery. Schizophr Bull 39:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martín-Santiago O, Suazo V, Rodríguez-Lorenzana A, Ruiz de Azúa S, Valcárcel C, Díez Á, et al. (2016): Relationship between subclinical psychotic symptoms and cognitive performance in the general population [article in Spanish]. Rev Psiquiatr Salud Ment 9:78–86. [DOI] [PubMed] [Google Scholar]
- 32.Barnett JH, Hachinski V, Blackwell AD (2013): Cognitive health begins at conception: Addressing dementia as a lifelong and preventable condition. BMC Med 11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moseley M, Bammer R, Illes J (2002): Diffusion-tensor imaging of cognitive performance. Brain Cogn 50:396–413. [DOI] [PubMed] [Google Scholar]
- 34.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (2005): Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Hum Brain Mapp 26: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C (2006): White matter growth as a mechanism of cognitive development in children. Neuroimage 33:936–946. [DOI] [PubMed] [Google Scholar]
- 36.Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. (2016): The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage 124:1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. (2014): The psychosis spectrum in a young U.S. community sample: Findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry 13:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC (2015): Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology 29:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. (2006): Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 40.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. (2013): Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA-DTI working group. Neuroimage 81:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori S, Wakana S, van Zijl P, Nagae-Poetscher LM (2005): MRI Atlas of Human White Matter. Amsterdam: Elsevier Science. [Google Scholar]
- 42.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. (2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. (2008): Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aung WY, Mar S, Benzinger TL (2013): Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med 5:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacKinnon DP, Fairchild AJ, Fritz MS (2007): Mediation analysis. Annu Rev Psychol 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacKinnon DP, Dwyer JH (1993): Estimation of mediated effects in prevention studies. Eval Rev 17:144–158. [Google Scholar]
- 47.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD (2008): Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry 63:512–518. [DOI] [PubMed] [Google Scholar]
- 48.Trotman HD, Holtzman CW, Ryan AT, Shapiro DI, MacDonald AN, Goulding SM, et al. (2013): The development of psychotic disorders in adolescence: A potential role for hormones. Horm Behav 64: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters BD, Karlsgodt KH (2015): White matter development in the early stages of psychosis. Schizophr Res 161:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmahmann JD, Smith EE, Eichler FS, Filley CM (2008): Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lebel C, Beaulieu C (2011): Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, et al. (2010): Longitudinal changes in grey and white matter during adolescence. Neuroimage 49:94–103. [DOI] [PubMed] [Google Scholar]
- 53.Mesulam MM (1998): From sensation to cognition. Brain 121(Pt 6): 1013–1052. [DOI] [PubMed] [Google Scholar]
- 54.Petrides M, Pandya DN (2002): Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16:291–310. [DOI] [PubMed] [Google Scholar]
- 55.Madhavan KM, McQueeny T, Howe SR, Shear P, Szaflarski J (2014): Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res 1562:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larry R, Berg D, Bloom F, Sascha D, Anirvan G, Nicholas C (2012): Fundamental Neuroscience, 4th ed Waltham, MA: Academic Press. [Google Scholar]
- 57.Beaulieu C, Allen PS (1994): Determinants of anisotropic water diffusion in nerves. Magn Reson Med 31:394–400. [DOI] [PubMed] [Google Scholar]
- 58.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- 59.Wozniak JR, Lim KO (2006): Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev 30:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeRosse P, Ikuta T, Peters BD, Karlsgodt KH, Szeszko PR, Malhotra AK (2014): Adding insult to injury: Childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Res 224:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Hanlon E, Leemans A, Kelleher I, Clarke MC, Roddy S, Coughlan H, et al. (2015): White matter differences among adolescents reporting psychotic experiences: A population-based diffusion magnetic resonance imaging study. JAMA Psychiatry 72:668–677. [DOI] [PubMed] [Google Scholar]
- 62.DeRosse P, Ikuta T, Karlsgodt KH, Peters BD, Gopin CB, Szeszko PR, et al. (2017): White matter abnormalities associated with subsyndromal psychotic-like symptoms predict later social competence in children and adolescents. Schizophr Bull 43:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatton SN, Lagopoulos J, Hermens DF, Hickie IB, Scott E, Bennett MR (2014): White matter tractography in early psychosis: Clinical and neurocognitive associations. J Psychiatry Neurosci 39: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng B, Ardekani BA, Tang Y, Zhang T, Zhao S, Cui H, et al. (2016): Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res 172:1–8. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Y, Liu J, Driesen N, Womer F, Chen K, Wang Y, et al. (2017): White matter integrity in genetic high-risk individuals and first-episode schizophrenia patients: Similarities and disassociations. Biomed Res Int 2017:3107845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viher PV, Stegmayer K, Giezendanner S, Federspiel A, Bohlhalter S, Vanbellingen T, et al. (2016): Cerebral white matter structure is associated with DSM-5 schizophrenia symptom dimensions. Neuroimage Clin 12:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bloemen OJ, de Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, et al. (2010): White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med 40:1297–1304. [DOI] [PubMed] [Google Scholar]
- 68.Felten DL, O’Banion MK, Maida MS (2016): Telencephalon. In: Netter’s Atlas of Neuroscience, 3rd ed New York: Elsevier, 463–477. [Google Scholar]
- 69.Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y, et al. (2009): Abnormal integrity of association fiber tracts in amnestic mild cognitive impairment. J Neurol Sci 278:102–106. [DOI] [PubMed] [Google Scholar]
- 70.Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA (2007): Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia 45:2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potapov AA, Goryainov SA, Zhukov VY, Pitskhelauri DI, Kobyakov GL, Pronin IN, et al. (2014): The long-associative pathway of the white matter: modern view from the perspective of neuroscience [article in English, Russian]. Zh Vopr Neirokhir Im N N Burdenko 78:66–77. discussion 77. [PubMed] [Google Scholar]
- 72.Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD (2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42:1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choudhury S, Blakemore SJ, Charman T (2006): Social cognitive development during adolescence. Soc Cogn Affect Neurosci 1: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luna B (2009): Developmental changes in cognitive control through adolescence. Adv Child Dev Behav 37:233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madden DJ, Bennett IJ, Song AW (2009): Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev 19:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilton LS, Levitt JG, O’Neill J, Alger JR, Luders E, Phillips OR, et al. (2008): Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport 19:1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J (2015): The superior longitudinal fasciculus in typically developing children and adolescents: Diffusion tensor imaging and neuropsychological correlates. J Child Neurol 30:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brauer J, Anwander A, Friederici AD (2011): Neuroanatomical pre-requisites for language functions in the maturing brain. Cereb Cortex 21:459–466. [DOI] [PubMed] [Google Scholar]
- 79.Nagy Z, Westerberg H, Klingberg T (2004): Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16:1227–1233. [DOI] [PubMed] [Google Scholar]
- 80.Vestergaard M, Madsen KS, Baaré WF, Skimminge A, Ejersbo LR, Ramsøy TZ, et al. (2011): White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci 23:2135–2146. [DOI] [PubMed] [Google Scholar]
- 81.Wendelken C, Ferrer E, Ghetti S, Bailey SK, Cutting L, Bunge SA (2017): Frontoparietal Structural connectivity in childhood predicts development of functional connectivity and reasoning ability: A large-scale longitudinal investigation. J Neurosci 37:8549–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, et al. (2009): Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salami A, Eriksson J, Nilsson LG, Nyberg L (2012): Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochim Biophys Acta 1822:408–415. [DOI] [PubMed] [Google Scholar]
- 84.Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM (2014): Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct 219:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hecht EE, Gutman DA, Bradley BA, Preuss TM, Stout D (2015): Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage 108:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vitolo E, Tatu MK, Pignolo C, Cauda F, Costa T, Andó A, et al. (2017): White matter and schizophrenia: A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res Neuroimaging 270:8–21. [DOI] [PubMed] [Google Scholar]
- 87.Li Q, Shi L, Lu G, Yu HL, Yeung FK, Wong NK, et al. (2017): Chronic ketamine exposure causes white matter microstructural abnormalities in adolescent cynomolgus monkeys. Front Neurosci 11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Domen P, Peeters S, Michielse S, Gronenschild E, Viechtbauer W, Roebroeck A, et al. (2017): Differential time course of microstructural white matter in patients with psychotic disorder and individuals at risk: A 3-year follow-up study. Schizophr Bull 43:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. (2011): Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 127:46–57. [DOI] [PubMed] [Google Scholar]
- 90.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2018): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry 23:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. (2005): DTI and MTR abnormalities in schizophrenia: Analysis of white matter integrity. Neuroimage 26:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yao L, Lui S, Deng W, Wu M, Chen L, Xiao Y, et al. (2014): Association of white matter deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: An optimized VBM study using 3T. MAGMA 27:283–290. [DOI] [PubMed] [Google Scholar]
- 93.Guo W, Liu F, Liu Z, Gao K, Xiao C, Chen H, et al. (2012): Right lateralized white matter abnormalities in first-episode, drug-naive para-noid schizophrenia. Neurosci Lett 531:5–9. [DOI] [PubMed] [Google Scholar]
- 94.Lyu H, Hu M, Eyler LT, Jin H, Wang J, Ou J, et al. (2015): Regional white matter abnormalities in drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Aust N Z J Psychiatry 49:246–254. [DOI] [PubMed] [Google Scholar]
- 95.Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC (2010): Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry 167:818–827. [DOI] [PubMed] [Google Scholar]
- 96.Forsyth JK, Lewis DA (2017): Mapping the consequences of impaired synaptic plasticity in schizophrenia through development: An integrative model for diverse clinical features. Trends Cogn Sci 21: 760–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ribas GC (2018): Applied Cranial-Cerebral Anatomy: Brain Architecture and Anatomically Oriented Microneurosurgery. Cambridge: Cambridge University Press, 15–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.