Abstract
Background:
Inflammation and focal atrophy are common features adjacent to prostate tumors. Limited evidence exists on whether these features have prognostic significance.
Methods:
In the Health Professionals Follow-Up Study and Physicians’ Health Study, we studied 1,035 men diagnosed with prostate cancer. A genitourinary pathologist centrally reviewed tumor and normal areas of hematoxylin and eosin slides from prostate cancer specimens for the presence of acute and chronic inflammation, and four subtypes of focal atrophy. Cox proportional hazards models adjusted for potential confounders were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of these features with lethal prostate cancer, defined as development of metastatic disease or death during follow-up.
Results:
During a median of 12 years of follow-up, 153 men developed lethal prostate cancer. Eighty-four percent of men had histologic evidence of chronic inflammation and 30% had acute inflammation. Both chronic and acute inflammation were inversely associated with lethal prostate cancer in age- and lifestyle-adjusted models. Chronic inflammation remained inversely associated with lethal prostate cancer after additionally adjusting for prognostic clinical features (HR=0.45, 95% CI 0.30 to 0.69 for mild, HR=0.51, 95% CI 0.33 to 0.80 for moderate to severe). None of the atrophic lesions were associated with lethal prostate cancer.
Conclusions:
Our data suggest that the presence of inflammation, particularly chronic inflammation, in prostate cancer tissue is associated with better prognosis among prostate cancer patients.
Impact:
This is the largest prospective cohort study to examine the association between inflammation, focal atrophy, and lethal prostate cancer.
Keywords: inflammation, focal atrophy, prostate cancer, prognosis
Introduction
Acute and chronic inflammation are commonly found in both normal prostate and prostate cancer tissue. The inflammatory infiltrate of chronic inflammation has been hypothesized to be involved in prostate cancer initiation or progression through the induction of oxidative stress and generation of reactive oxygen species (1, 2). Along with their pro-tumorigenic effects, tumor-infiltrating immune cells may also suppress tumor development and growth through tumor immune surveillance mechanisms, and current immunotherapeutic approaches are being developed to amplify the immune response against tumor cells (3, 4).
Focal atrophy of the prostate gland, which has been put forth previously as a potential precursor of prostate cancer, often occurs in close association with chronic inflammation (5). It is highly proliferative compared with matched normal-appearing epithelium and, together with inflammation, frequently occurs in the peripheral zone where prostate cancer most commonly develops (6–8). The term “proliferative inflammatory atrophy” (PIA) was proposed to designate foci of proliferative glandular epithelium with the morphological appearance of simple atrophy (SA) or postatrophic hyperplasia (PAH), two forms of focal atrophy, occurring in association with inflammation (5).
Few studies have investigated the association of inflammation in prostate tumor tissue with prostate cancer outcomes (9–11). Only one study (11) to date has examined the relationship between histologic measures of atrophy and inflammation and prostate cancer-specific mortality. In this population-based nested case-control study of men diagnosed with localized prostate cancer, a positive association was observed between chronic inflammation and lethal prostate cancer, although this finding was not statistically significant.
In this study, we characterized intraprostatic inflammation and focal atrophy in prostate tumor tissue among over 1,000 men diagnosed with prostate cancer from two prospective studies, the Physicians’ Health Study (PHS) and the Health Professionals Follow-up Study (HPFS), and investigated the associations between these histologic features and the development of lethal (metastatic or fatal) prostate cancer.
Materials and Methods
Study Population
We included 1,035 men from the PHS (n=73) or HPFS (n=962) who were diagnosed with prostate cancer from 1983–2010, and had hematoxylin and eosin (H&E) stained slides available for pathological review. H&E slides were primarily from prostatectomy specimens (n=926, 89.5%). Other tissue specimens included transurethral resection of the prostate (TURP, n=69, 6.7%), biopsy (n=37, 3.6%), and benign prostate hyperplasia (BPH) adenomectomy (n=2, 0.2%). The PHS was a randomized trial of aspirin and beta-carotene for the prevention of cardiovascular disease and cancer among 22,071 male physicians aged 40 to 84 years at enrollment. Information on lifestyle and medical history was ascertained at baseline and updated annually through questionnaires(12). The HPFS is an ongoing cohort study of 51,529 male health professionals aged 40 to 75 years at enrollment. Information on demographics, lifestyle, medical history, and diet was collected at baseline and updated biannually through questionnaires, except that diet information was updated every four years by a validated semi-quantitative food frequency questionnaire(13).
Identification of prostate cancer cases and outcome ascertainment
In both PHS and HPFS, self-reported prostate cancer diagnoses on follow-up questionnaires were subsequently confirmed through medical record and pathology report review. Age and year of diagnosis, clinical and pathological stage, prostate-specific antigen (PSA) level at diagnosis, and prostate cancer progression (i.e., distant metastases and biochemical recurrence) after diagnosis were collected by medical records and questionnaires sent to prostate cancer survivors and their attending physicians. Details of the prostate cancer survivor cohort within these two studies are available elsewhere (14–16). In both PHS and HPFS, vital status was ascertained by reports from family members, autopsy reports, and searches of the National Death Index. The underlying cause of death was determined by the endpoint review committee, consisting of trained clinicians who have the credentials for outcome adjudication and were blinded to any exposure information, based on all available data including medical history, medical records, registry information, and death certificates. Lethal prostate cancer was defined as cancer that progressed to distant metastases or death from prostate cancer as the underlying cause during follow-up. Fatal prostate cancer was defined as death from prostate cancer as the underlying cause (i.e., prostate cancer-specific mortality).
Assessment of intraprostatic Inflammation, focal atrophy, and other histologic features
For each patient all available H&E slides with prostate tumor foci were centrally reviewed by a single experienced genitourinary pathologist (M.F.) blinded to disease outcome and other clinical data to confirm cancer status; to determine Gleason patterns; to evaluate histologic features such as perineural invasion (PNI), acute and chronic inflammation, and classes of focal atrophy (17); and to identify areas of interest for tissue microarray (TMA) construction (at least 3 cores each). Both the tumor and adjacent normal areas were included when scoring inflammation. Acute inflammation was characterized by the presence of neutrophils and scored as absent vs. present. Chronic inflammation was characterized by the presence of mononuclear cells, e.g., lymphocytes and macrophages, and graded as absent, mild (≤10% of the microscopically benign area), moderate (11-19%), or severe (≥20%).
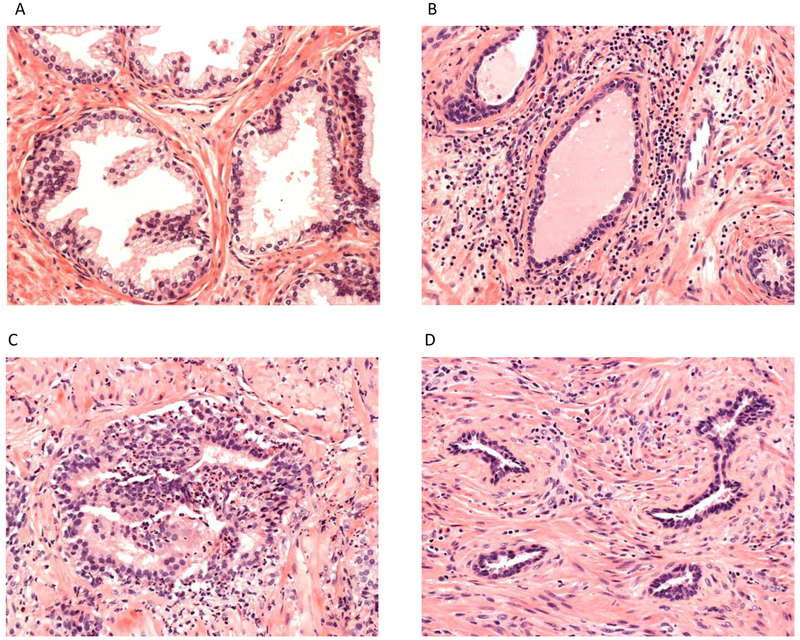
Focal atrophy was characterized according to the classification scheme proposed in 2006 by the Working Group for Histologic Classification of Prostate Atrophy Lesions with the following subtypes: SA, simple atrophy with cyst formation (SACF), PAH, and partial atrophy(17). Figure 1 shows a typical pathological view of normal prostate glands, inflammation, and PAH on H&E slides. Details of the major characteristics of the four classes of focal atrophy are described elsewhere (11, 17).
Figure 1.
Pathological view of normal prostate glands, typical intraprostatic inflammation and post-atrophic hyperplasia on hematoxylin and eosin slide. A) Normal prostate glands. B) Peri-glandular chronic inflammation. C) Intra-glandular acute inflammation. D) Post-atrophic hyperplasia.
The presence of PNI was defined as the existence of complete circumferential encirclement of nerve structures by malignant glands. Patients with non-circumferential PNI or a single focus of PNI among multiple tumor slides were deemed as PNI-absent (18).
PTEN gene loss and the TMPRSS2:ERG gene fusion (measured by ERG protein expression) were assessed by immunohistochemistry on TMAs using validated antibodies. The markers were scored blinded to disease outcome and other clinical data. PTEN loss was considered if the intensity of cytoplasmic and nuclear staining was markedly decreased or entirely lost across all TMA cores compared with surrounding benign glands and/or stroma(19). We classified tumors as ERG fusion positive if at least one TMA core stained positive for ERG, and ERG fusion negative if all cores stained negative for ERG (20).
Statistical analyses
We used χ2 tests and Fisher’s exact tests to examine the associations between measures of inflammation and focal atrophy. Hazard ratios (HRs) and 95% confidence intervals (CIs) of the associations of inflammation and focal atrophy with lethal prostate cancer were estimated using Cox proportional hazards regression models. Person-time for progression to lethal outcome was calculated from the date of cancer diagnosis to the earliest date among the following: development of distant metastases, deaths due to any cause, or the end of follow-up (June 2015 for PHS and January 2014 for HPFS). For analyses using lethal prostate cancer as the endpoint, prostate cancer cases with M1 stage at diagnosis were excluded. In the secondary analysis, we examined the associations of inflammation and focal atrophy with fatal prostate cancer.
To control for potential confounding(13), we considered three models: 1) adjusted for age at diagnosis (years, continuous); 2) additionally adjusted for BMI at diagnosis (<25, 25-29, ≥30 kg/m2), regular aspirin use at diagnosis (yes, no; defined as >twice per week in HPFS and >3 days per week in PHS), and history of diabetes at diagnosis (yes, no); and 3) additionally adjusted for clinical or pathological tumor stage (T1b-T3a, T3b/T4/N1/M1), Gleason score (≤6, 7(3+4), 7(4+3), 8(4+4), 9-10), and PSA at diagnosis (<10, 10-20, >20 ng/mL). For all models, the proportional hazards assumption was evaluated and satisfied by plotting Schoenfeld residuals of the exposure against follow-up time and found to be satisfied. We also explored which specific factors in Model 3 were responsible for the attenuation of the association of acute inflammation with lethal prostate cancer.
As chronic inflammation and focal atrophy often occur concurrently (5) and our primary analysis showed men with chronic inflammation were more likely to have SA and PAH compared to men who did not have chronic inflammation, we assessed whether the association between chronic inflammation and lethal prostate cancer varied according to the presence of SA and PAH. We also performed a stratification analysis within strata of Gleason score categories (≤7[3+4] vs. ≥7[4+3]) and tumor stage (T1b-T3a vs. T3b/T4/N1/M1) for the association between chronic inflammation and lethal prostate cancer. We further conducted two separate sensitivity analyses examining chronic inflammation and lethal prostate cancer, restricting to 1) cases with prostatectomy tissue type and 2) cases diagnosed in the PSA era (i.e., 1993 onward).
Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC), and all statistical tests were two-sided, with P values below 0.05 considered statistically significant.
This research project was approved by the institutional review board at the Harvard T.H. Chan School of Public Health. Written informed consent was obtained from each study participant.
Results
Table 1 shows the frequency of individual pathological features and their associations among the 1,035 study participants. More than 80% of the prostate cancer cases had chronic inflammation (mild 51%, moderate to severe 33%) (Table 1). Thirty percent of the cases had acute inflammation (Table 1). Acute and chronic inflammations were positively associated (P<0.0001). Among the four types of focal atrophy, SA was the most common (74%), followed by PAH (21%), SACF (19%), and partial atrophy (2%). Men with chronic or acute inflammation were more likely to have SA, PAH, and SACF than men without inflammation (Table 1).
Table 1.
Associations between inflammation and focal atrophy among men diagnosed with prostate cancer between 1983 and 2010 in the Health Professionals Follow-up Study and the Physicians’ Health Study.
| All men, n(%) | Men according to inflammation and atrophy lesions, n (%)a |
|||||
|---|---|---|---|---|---|---|
| Acute inflammation | SA | PAH | SACF | Partial atrophy | ||
| Acute inflammation | ||||||
| Yes | 299 (29.8) | 266 (89.0) | 93 (31.1) | 79 (26.4) | 8 (2.7) | |
| No | 703 (70.2) | 473 (67.3) | 120 (17.1) | 107 (15.2) | 15 (2.1) | |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P = 0.59 | |||
| Chronic inflammation | ||||||
| Moderate/Severe | 339 (33.3) | 208 (61.7) | 302 (89.4) | 118 (34.9) | 67 (19.8) | 9 (2.7) |
| Mild | 516 (50.6) | 85 (16.7) | 375 (73.8) | 90 (17.8) | 103 (20.3) | 12 (2.4) |
| None | 164 (16.1) | 5 (3.3) | 62 (40.3) | 5 (3.3) | 16 (10.4) | 2 (1.3) |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P = 0.02 | P = 0.71b | ||
| SA | ||||||
| Yes | 740 (73.7) | 187 (25.3) | 176 (23.8) | 21 (2.8) | ||
| No | 264 (26.3) | 27 (10.2) | 10 (3.8) | 2 (0.8) | ||
| P < 0.0001 | P < 0.0001 | P = 0.06b | ||||
| PAH | ||||||
| Yes | 214 (21.3) | 49 (22.9) | 10 (4.7) | |||
| No | 789 (78.7) | 137 (17.4) | 13 (1.7) | |||
| P = 0.06 | P = 0.008 | |||||
| SACF | ||||||
| Yes | 186 (18.5) | 5 (2.7) | ||||
| No | 818 (81.5) | 18 (2.2) | ||||
| P = 0.69 | ||||||
| Partial atrophy | ||||||
| Yes | 23 (2.3) | |||||
| No | 980 (97.7) | |||||
Abbreviations: SA, simple atrophy; PAH, postatrophic hyperplasia; SACF, simple atrophy with cyst formation.
Note: Among 1035 men diagnosed with prostate cancer, acute inflammation was missing for 33 men; chronic inflammation was missing for 16 men; SA was missing for 31 men; PAH was missing for 32 men; SACF was missing for 31 men; partial atrophy was missing for 32 men.
Row percentage restricted to patients positive for features including chronic inflammation, acute inflammation, SA, PAH, SACF and partial atrophy.
P-values based on the Fisher’s exact test; all other p-values based on the chi-square test.
The patient characteristics overall and with respect to the histological features of inflammation are shown in Table 2. More than half of the patients were older than 65 years (61%), and were diagnosed during the PSA era (after 1993, 71%). Most men had localized cancer (stage T1b-T3a, 84%), and about half had low grade disease (Gleason score ≤7/3+4, 52%). Approximately 15% of tumors showed complete PTEN loss. Forty-eight percent of tumors were ERG positive.
Table 2.
Tumor, clinical, and lifestyle characteristics among men diagnosed with prostate cancer between 1983 and 2010 in the Health Professionals Follow-up Study and the Physicians’ Health Study overall and according to inflammation status.
| Chronic Inflammation, n(%)a |
||||
|---|---|---|---|---|
| Overall, n (%) (n=1,035) | Acute inflammation, n(%)a (n=299) | Mild (n=516) | Moderate / Severe (n=339) | |
| Cohort | ||||
| HPFS | 962 (93.0) | 274 (91.6) | 477 (92.4) | 320 (94.4) |
| PHS | 73 (7.0) | 25 (8.4) | 39 (7.6) | 19 (5.6) |
| Age at diagnosis, years | ||||
| <65 | 401 (38.7) | 120 (40.1) | 208 (40.3) | 126 (37.2) |
| 65–69 | 345 (33.3) | 101 (33.8) | 168 (32.5) | 120 (35.4) |
| 70–74 | 212 (20.5) | 55 (18.4) | 104 (20.2) | 67 (19.7) |
| ≥75 | 77 (7.5) | 23 (7.7) | 36 (7.0) | 26 (7.7) |
| Year of diagnosis | ||||
| Before 1990 (pre-PSA era) | 68 (6.6) | 15 (5.0) | 29 (5.6) | 21 (6.2) |
| 1990-1993 (peri-PSA era) | 237 (22.9) | 59 (19.7) | 125 (24.2) | 75 (22.1) |
| After 1993 (PSA era) | 730 (70.5) | 225 (75.3) | 362 (70.2) | 243 (71.7) |
| Stageb | ||||
| T1b–T3a | 873 (84.3) | 268 (89.6) | 451 (87.4) | 288 (85.0) |
| T3b/T4/N1/M1 | 158 (15.3) | 31 (10.4) | 63 (12.2) | 50 (14.7) |
| Unknown | 4 (0.4) | 0 (0.0) | 2 (0.4) | 1 (0.3) |
| Gleason score | ||||
| ≤6 | 172 (16.6) | 61 (20.4) | 83 (16.1) | 67 (19.8) |
| 7 (3+4) | 371 (35.9) | 108 (36.1) | 198 (38.4) | 113 (33.3) |
| 7 (4+3) | 199 (19.2) | 62 (20.8) | 108 (20.9) | 60 (17.7) |
| 8 | 100 (9.6) | 27 (9.0) | 42 (8.1) | 32 (9.4) |
| 9–10 | 188 (18.2) | 38 (12.7) | 82 (15.9) | 65 (19.2) |
| Unknown | 5 (0.5) | 3 (1.0) | 3 (0.6) | 2 (0.6) |
| PSA at diagnosis, ng/ml | ||||
| <10 | 668 (64.5) | 200 (66.9) | 350 (67.8) | 205 (60.5) |
| 10–20 | 151 (14.6) | 49 (16.4) | 71 (13.8) | 59 (17.4) |
| >20 | 82 (7.9) | 20 (6.7) | 33 (6.4) | 28 (8.2) |
| Unknown | 134 (13.0) | 30 (10.0) | 62 (12.0) | 47 (13.9) |
| Specimen type | ||||
| Prostatectomy | 926 (89.5) | 273 (91.3) | 467 (90.5) | 306 (90.5) |
| TURP | 69 (6.7) | 20 (6.7) | 36 (7.0) | 25 (7.4) |
| Biopsy or BPH adenomectomy | 39 (3.8) | 6 (2.0) | 13 (2.5) | 7 (2.1) |
| Perineural invasion | ||||
| Yes | 470 (45.4) | 123 (41.1) | 235 (45.5) | 146 (43.1) |
| No | 562 (54.3) | 176 (58.9) | 280 (54.3) | 192 (56.6) |
| Unknown | 3 (0.3) | 0 (0.0) | 1 (0.2) | 1 (0.3) |
| Complete PTEN lossc | ||||
| Yes | 102 (14.8) | 17 (9.7) | 52 (14.9) | 31 (13.5) |
| No | 587 (85.2) | 158 (90.3) | 296 (85.1) | 198 (86.5) |
| ERG expressiond | ||||
| Positive | 399 (48.2) | 90 (43.3) | 202 (48.9) | 127 (46.0) |
| Negative | 429 (51.8) | 118 (56.7) | 211 (51.1) | 149 (54.0) |
| BMI at diagnosis, kg/m2 | ||||
| <25 | 428 (41.3) | 117 (39.1) | 207 (40.1) | 134 (39.5) |
| 25–29 | 506 (48.9) | 153 (51.2) | 254 (49.2) | 167 (49.3) |
| ≥30 | 101 (9.8) | 29 (9.7) | 55 (10.7) | 38 (11.2) |
| Smoking status at diagnosise | ||||
| Never | 438 (42.3) | 120 (40.1) | 229 (44.4) | 138 (40.7) |
| Past | 420 (40.6) | 130 (43.5) | 203 (39.3) | 143 (42.2) |
| Current | 65 (6.3) | 12 (4.0) | 26 (5.0) | 25 (7.4) |
| Unknown | 112 (10.8) | 37 (12.4) | 58 (11.3) | 33 (9.7) |
| Regular aspirin use at diagnosise | ||||
| Yes | 462 (44.6) | 138 (46.2) | 221 (42.8) | 164 (48.4) |
| No | 567 (54.8) | 158 (52.8) | 291 (56.4) | 175 (51.6) |
| Unknown | 6 (0.6) | 3 (1.0) | 4 (0.8) | 0 (0.0) |
| History of diabetes at diagnosise | ||||
| Yes | 52 (5.0) | 14 (4.7) | 27 (5.2) | 16 (4.7) |
| No | 983 (95.0) | 285 (95.3) | 489 (94.8) | 323 (95.3) |
Abbreviations: HPFS, Health Professionals Follow-up Study; PHS, Physicians’ Health Study; TURP, transurethral resection of the prostate.
Column percentage restricted to patients who had acute or chronic inflammation.
Pathological stage as primary, clinical stage as secondary if pathological stage is missing.
PTEN status was missing for 346 men.
ERG status was missing for 207 men.
Smoking, aspirin use, and diabetes information was obtained from questionnaire at prostate cancer diagnosis or the most recent questionnaire prior to prostate cancer diagnosis.
A higher percentage of localized stage (T1b-T3a) disease was observed among cases with acute or chronic inflammation compared to cases without inflammation (Table 2). The prevalence of low grade (Gleason score ≤ 7/3+4) disease was slightly higher among tumors with acute or chronic inflammation compared to tumors without inflammation (Table 2). The majority of patients has a PSA at diagnosis <10 ng/ml, irrespective of inflammation status (Table 2). PNI, complete PTEN loss, and positive ERG expression were less often observed among tumors with acute inflammation compared to tumors without acute inflammation (Table 2). Similar relationships were generally observed for the four types of focal atrophy lesions with respect to the clinical and pathologic features described (Supplemental Table 1).
During a median follow-up of 12.0 years, 153 lethal prostate cancer events occurred. The associations between inflammation and focal atrophy features and lethal prostate cancer are shown in Table 3. Overall, we found that the presence of chronic inflammation was associated with a reduced risk of lethal prostate cancer after adjusting for age, BMI, diabetes, aspirin use, disease stage, tumor grade, and PSA (HR 0.45, 95% CI 0.30 to 0.69 for mild; HR 0.51, 95% CI 0.33 to 0.80 for moderate-to-severe inflammation), compared to cases without chronic inflammation. Presence of acute inflammation was associated with a reduced risk of lethal prostate cancer after adjusting for age, BMI, diabetes, and aspirin use (HR 0.66, 95% CI 0.45 to 0.99). The association was attenuated and became not significant after additional adjustment for disease stage, tumor grade, and PSA (HR 0.91, 95% CI 0.61 to 1.37). No statistically significant associations were observed between any of the four classes of focal atrophy and lethal prostate cancer (Table 3).
Table 3.
Associations of inflammation and focal atrophy with lethal prostate cancer among men diagnosed with prostate cancer in the Health Professionals Follow-up Study and the Physicians’ Health Study, 1983-2015.
| Lethal Prostate Cancer (HR, 95%CI) | |||||
|---|---|---|---|---|---|
| N lethal (N total)a | Person-years | Model 1b | Model 2c | Model 3d | |
| Inflammation | |||||
| Acute inflammation | |||||
| Absent | 107 (679) | 8141.42 | ref | ref | ref |
| Present | 31 (293) | 3601.92 | 0.66 (0.44 to 0.99) | 0.66 (0.45 to 0.99) | 0.91 (0.61 to 1.37) |
| Chronic inflammation | |||||
| Absent | 39 (150) | 1620.58 | ref | ref | ref |
| Mild | 61 (501) | 6283.25 | 0.41 (0.28 to 0.62) | 0.42 (0.28 to 0.63) | 0.45 (0.30 to 0.69) |
| Moderate to Severe | 46 (332) | 3959.58 | 0.49 (0.32 to 0.75) | 0.49 (0.32 to 0.76) | 0.51 (0.33 to 0.80) |
| Focal atrophy | |||||
| SA | |||||
| Absent | 43 (242) | 2894.75 | ref | ref | ref |
| Present | 96 (732) | 8862.00 | 0.73 (0.51 to 1.05) | 0.74 (0.52 to 1.07) | 0.75 (0.51 to 1.09) |
| PAH | |||||
| Absent | 113 (761) | 9204.17 | ref | ref | ref |
| Present | 26 (212) | 2541.25 | 0.88 (0.57 to 1.35) | 0.89 (0.58 to 1.36) | 1.12 (0.72 to 1.74) |
| SACF | |||||
| Absent | 115 (790) | 9520.83 | ref | ref | ref |
| Present | 24 (184) | 2235.92 | 0.84 (0.54 to 1.31) | 0.83 (0.53 to 1.30) | 0.94 (0.60 to 1.48) |
| Partial atrophy | |||||
| Absent | 136 (950) | 11437.83 | ref | ref | ref |
| Present | 3 (23) | 298.08 | 0.95 (0.30 to 2.99) | 0.98 (0.31 to 3.09) | 1.75 (0.55 to 5.61) |
Abbreviations: CI, confidence interval; HR, Hazard ratio; SA, simple atrophy; PAH, postatrophic hyperplasia; SACF, simple atrophy with cyst formation.
Note: Among 153 lethal prostate cancer events, acute inflammation was missing for 15 men; chronic inflammation was missing for 7 men; SA was missing for 14 men; PAH was missing for 14 men; SACF was missing for 14 men; partial atrophy was missing for 14 men.
N=number of events
Model 1: adjusted for age at diagnosis (years, continuous).
Model 2: adjusted for age at diagnosis (years, continuous), body mass index at diagnosis (<25, 25-29, ≥30 kg/m2), regular aspirin use at diagnosis (yes, no), and history of diabetes at diagnosis (yes, no).
Model 3: adjusted for age at diagnosis (years, continuous), body mass index at diagnosis (<25, 25-29, ≥30 kg/m2), regular aspirin use at diagnosis (yes, no), history of diabetes at diagnosis (yes, no), stage (T1b-T3a vs. T3b/T4/N1), Gleason score (≤6, 7(3+4), 7(4+3), 8(4+4), 9-10) and PSA at diagnosis (<10, 10-20, >20 ng/mL).
When restricting only to patients with prostatectomy specimens (n=926), the association between chronic inflammation and lethal prostate cancer adjusted for clinical and lifestyle factors was similar to the full analysis (HR 0.45, 95% CI 0.28 to 0.74 for mild; HR 0.50, 95% CI 0.30 to 0.85 for moderate-to-severe inflammation, data not tabulated). When restricting to patients diagnosed in the PSA era (n=730), the association between chronic inflammation and lethal prostate cancer adjusted for clinical and lifestyle factors was slightly attenuated but remained inverse (HR 0.49, 95% CI 0.27 to 0.88 for mild; HR 0.63, 95% CI 0.34 to 1.15 for moderate-to-severe inflammation, data not tabulated).
When using 151 fatal prostate cancer events accrued during a mean follow-up of 13 years, we found similar associations to what was observed using lethal prostate cancer as the outcome, except that the presence of SA became inversely associated with fatal prostate cancer (HR 0.68, 95% CI 0.46 to 0.99, adjusting for age, BMI, diabetes, aspirin use, disease stage, tumor grade, and PSA, Supplemental Table 2).
The association between chronic inflammation and lethal prostate cancer did not statistically differ according to the presence of SA or PAH (Supplemental Table 3).When stratifying by stage at diagnosis, the inverse association between chronic inflammation and lethal prostate cancer appeared to be stronger among patients with localized tumor stage (T1b-T3a) compared to those with regional tumor stage (T3b/T4/N1). However, estimates for regional tumor stage were limited by small sample size rendering wide confidence intervals (Supplemental Table 4).
We also explored which specific factors in Model 3 (Table 3) were responsible for the attenuation of the association of acute inflammation with lethal prostate cancer. Gleason score and tumor stage at diagnosis were the predominant factors for the attenuation (Supplemental Table 5).
Discussion
In this large prospective study of lethal prostate cancer, we found the presence of chronic inflammation to be inversely associated with progression to lethal disease, independent of prognostic clinical and lifestyle factors. Acute inflammation was also inversely associated with lethal prostate cancer, but this association was attenuated when adjusting for clinical factors. Our data showed none of the focal atrophy lesions were associated with lethal prostate cancer.
Relatively few studies have examined the association between intraprostatic inflammation and prostate cancer aggressiveness (9–11). Klink et al.(9) and Irani et al. (10) both reported that inflammation (not distinguishing chronic or acute) was positively associated with biochemical recurrence among men treated with radical prostatectomy, yet the association became non-significant after adjusting for pathologic features. In a case-control study among men diagnosed with stage T1a-b prostate cancer through TURP (11), neither acute or chronic inflammation was found to be significantly associated with prostate cancer-specific death, but there was a suggestion of a positive association for chronic inflammation.
In the current study, we found presence of acute and chronic inflammation to be inversely associated with progression to lethal prostate cancer. There are several potential reasons to explain the different findings in our study. We evaluated inflammation comprehensively in both tumor and adjacent normal tissue, whereas the positive association reported by Klink et al. (9) and Irani et al. (10) both restricted inflammation within the tumor. Moreover, our study used lethal prostate cancer as the endpoint compared to biochemical recurrences which was used by Klink et al. (9) and Irani et al(10). While the study by Davidsson et al.(11) used the same inflammation evaluation and lethal endpoint as our study, the tumor specimens were from TURP arising primarily in the transitional zone, compared with the majority of RP tumor specimens in our study. The difference in study population may also play a role in the differences observed; the study by Davidsson et al(11) only included T1a or T1b tumors from an active surveillance cohort, while our study included men with advanced stage tumors treated by RP. Interestingly, when restricting to cases with localized stage in our study, the inverse association between chronic inflammation and lethal prostate became even stronger.
Our findings provide supportive evidence that innate and adaptive immune cells may play an anti-tumorigenic role at some time point in the continuum of tumor development and progression. Biologically, it is plausible that a robust immune response in the tumor microenvironment may play a role in preventing prostate cancer growth. Previous research has suggested specific immune cells are important in the anti-tumorigenic immune response(21); further studies are needed to understand which immune cells are influencing our findings. In a recent study by Hempel et al., the presence of intratumoral mast cells was found to be inversely associated with prostate cancer recurrence after prostatectomy taking into account prognostic factors (22).
Studies from the Finnish prostate cancer screening trial (23) and Reduction by Dutasteride of prostate cancer Events study (REDUCE)(24) reported that men who were biopsy negative for prostate cancer had a lower prostate cancer risk if inflammation was present in their biopsy. However, Platz et al. (25) showed a positive association between benign tissue inflammation and prostate cancer risk in their prospective study of men without biopsy indication (regardless of PSA level) and argued that the Finnish and REDUCE studies could be biased by detection as inflammation might lead to a higher PSA concentration. As both the Finnish and REDUCE studies required men to have had a negative biopsy following an elevated PSA, it is possible those men with elevated PSA leading to a negative biopsy may indeed be more likely to have a smaller risk for cancer on a follow-up biopsy if their initial biopsies showed inflammation because the main determinant of the PSA rise in these men was the inflammation and not cancer. Similarly, for prostate cancer progression, it is possible that men with more intraprostatic inflammation throughout their prostate would be more likely to be detected early due to a PSA rise resulting from the inflammation, and as such have longer survival. However, our study is not likely to be subject to such detection bias, as the association between chronic inflammation and lethal prostate cancer remained inverse after adjusted for PSA at diagnosis or restricting patients to those diagnosed in the PSA era when the majority of participants in HPFS and PHS were routinely screened.
Although it has been suggested that focal atrophy may give rise to prostate cancer both through or independently of high grade prostatic intraepithelial neoplasia (hgPIN) (6, 26), studies evaluating the relationship between focal atrophy and prostate cancer risk or aggressiveness have shown inconsistent findings. A morphologic transition from PIA to hgPIN and to prostate cancer has been observed histologically (26), yet other studies have not observed an association between focal atrophy and prostate cancer risk (27, 28). With respect to outcomes in men with prostate cancer, a cross-sectional study nested in the REDUCE cohort found that baseline focal atrophy is associated with lower Gleason score (29) and lower prostate cancer volumes (30). In our data, all four types of focal atrophy were more often detected in prostate cancer with localized stage disease or with a low PSA level at diagnosis. Neither Davidsson et al.(11) or our study observed an association between focal atrophy in prostate cancer specimens and progression to lethal prostate cancer. However, our data suggested an inverse relationship between SA and fatal prostate cancer.
ERG, PTEN, and PNI are important biomarkers in prostate cancer. The ERG gene fusion is the most common somatic event in prostate cancer, and there is compelling evidence to suggest the fusion may play a role in prostate cancer progression through its cooperation with other events or alterations(19, 20). In addition, both presence of PNI and PTEN loss has been associated with worse prostate cancer outcomes(18, 19). Interestingly, in our samples we found presence of acute inflammation was inversely associated with complete PTEN loss, and previous animal study reported that loss of PTEN in tumor cells decreases T-cell trafficking into tumors(31). We did not observe any other associations between these molecular subtypes and inflammation or focal atrophy. This may be limited by statistical power, as PTEN or ERG information were only available for 689 and 828 men respectively.
The strengths of this study include long-term follow-up, prospectively monitored and validated prostate cancer outcomes, and central pathological review for all morphological features, which reduces measurement error and inter-observer variations. We also used distant metastases and prostate cancer-specific death to define outcomes, which are the most clinically relevant endpoints for prostate cancer. Moreover, using prostatectomy specimens provides a more comprehensive review of the tumor and therefore better accuracy in characterizing the tumor compared to biopsy or TURP specimens. However, our study has limitations to consider. First, although this is the largest study of inflammation and lethal prostate cancer to date, we had a modest number of lethal events despite long-term follow-up, limiting the statistical power to evaluate effect modifications. Second, our results may not be generalizable to other racial/ethnic groups, as white men primarily comprise the PHS and HPFS cohorts, and racial difference in inflammatory cell composition are evident (32). In particular, black men are more likely to have tumors arising in the transitional zone, which may have different types or causes of inflammation. Third, sampling bias is possible since not all slides were available for review. However, if an advanced tumor is more likely to be classified as having inflammation due to more slides reviewed, and subsequently more likely to develop lethal prostate cancer, we would expect a positive association between inflammation and lethal prostate cancer. We observed an inverse association between inflammation and lethal prostate cancer, therefore the sampling bias is likely to pull the true association towards the null. Fourth, as our pathologist was blinded to patients’ information, non-differential misclassification of inflammation or atrophy was possible, and it would also bias the association towards null. Also, as data for several potential confounders (e.g., family history) were not available, a possibility of residual confounding exists. Lastly, we were only able to characterize inflammation on H&E slides according to the presence and percentage of overall lymphocytes. Future studies may want to classify inflammation with more detailed information, such as location and specific cell types present.
In summary, this study adds to evidence that the presence of inflammation, particularly chronic inflammation, in prostate cancer tissue may be associated with better prostate cancer-specific survival, while none of the atrophic lesions were associated with lethal prostate cancer. Our findings support the inclusion of chronic inflammation as a standardized component of pathologic review of prostate tissue specimens, and provide evidence for the use of these markers in prognostic prediction and treatment decisions if future studies with greater sample size confirm our results.
Supplementary Material
Acknowledgments
This project was supported in part by funding from Cancer Center Support Grants from the National Cancer Institute: P30 CA006516 and P30 CA006973. This project was also supported by funding from Emory, Harvard and University of Washington Prostate Cancer Biomarker Center: A176870. The Health Professionals Follow-up Study is supported by grant number U01 CA167552. We would like to thank the participants and staff of the PHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
ABBREVIATIONS LIST
- BPH
benign prostate hyperplasia
- CI
confidence interval
- H&E
hematoxylin and eosin
- hgPIN
high grade prostatic intraepithelial neoplasia
- HPFS
Health Professionals Follow-Up Study
- HR
harzard ratio
- PAH
postatrophic hyperplasia
- PHS
Physicians’ Health Study
- PIA
proliferative inflammatory atrophy
- PNI
perineural invasion
- PSA
prostate-specific antigen
- REDUCE
Reduction by Dutasteride of prostate cancer Events study
- SA
simple atrophy
- SACF
simple atrophy with cyst formation
- TMA
tissue microarray
- TURP
transurethral resection of the prostate
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors declare no potential conflicts of interest.
References
- 1.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology 2012;60(1):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 2011;60(1):106–17. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009;115(16):3670–9. [DOI] [PubMed] [Google Scholar]
- 5.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative Inflammatory Atrophy of the Prostate. The American Journal of Pathology 1999;155(6):1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology 2000;56(5):828–32. [DOI] [PubMed] [Google Scholar]
- 7.Franks LM. Atrophy and hyperplasia in the prostate proper. J Pathol Bacteriol 1954;68(2):617–21. [DOI] [PubMed] [Google Scholar]
- 8.McNeal JE. Regional morphology and pathology of the prostate. Am J Clin Pathol 1968;49(3):347–57. [DOI] [PubMed] [Google Scholar]
- 9.Klink JC, Banez LL, Gerber L, Lark A, Vollmer RT, Freedland SJ. Intratumoral inflammation is associated with more aggressive prostate cancer. World J Urol 2013;31(6):1497–503. [DOI] [PubMed] [Google Scholar]
- 10.Irani J, Goujon JM, Ragni E, Peyrat L, Hubert J, Saint F, et al. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology 1999;54(3):467–72. [DOI] [PubMed] [Google Scholar]
- 11.Davidsson S, Fiorentino M, Andren O, Fang F, Mucci LA, Varenhorst E, et al. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev 2011;20(10):2280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. New England Journal of Medicine 1996;334(18):1145–1149. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer 2007;121(7):1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platz EA, Drake CG, Wilson KM, Sutcliffe S, Kenfield SA, Mucci LA, et al. Asthma and risk of lethal prostate cancer in the Health Professionals Follow-Up Study. Int J Cancer 2015;137(4):949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Aspirin use after a prostate cancer diagnosis and cancer survival in a prospective cohort. Cancer Prev Res (Phila) 2012;5(10):1223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flavin R, Pettersson A, Hendrickson WK, Fiorentino M, Finn S, Kunz L, et al. SPINK1 protein expression and prostate cancer progression. Clin Cancer Res 2014;20(18):4904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Marzo AM, Platz EA, Epstein JI, Ali T, Billis A, Chan TY, et al. A working group classification of focal prostate atrophy lesions. Am J Surg Pathol 2006;30(10):1281–91. [DOI] [PubMed] [Google Scholar]
- 18.Zareba P, Flavin R, Isikbay M, Rider JR, Gerke TA, Finn S, et al. Perineural Invasion and Risk of Lethal Prostate Cancer. Cancer Epidemiol Biomarkers Prev 2017;26(5):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, et al. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J Natl Cancer Inst 2016;108(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21(9):1497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sfanos KS, Hempel HA, De Marzo AM. The role of inflammation in prostate cancer. Adv Exp Med Biol 2014;816:153–81. [DOI] [PubMed] [Google Scholar]
- 22.Hempel HA, Cuka NS, Kulac I, Barber JR, Cornish TC, Platz EA, et al. Low Intratumoral Mast Cells Are Associated With a Higher Risk of Prostate Cancer Recurrence. Prostate 2017;77(4):412–424. [DOI] [PubMed] [Google Scholar]
- 23.Yli-Hemminki TH, Laurila M, Auvinen A, Maattanen L, Huhtala H, Tammela TL, et al. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU Int 2013;112(6):735–41. [DOI] [PubMed] [Google Scholar]
- 24.Moreira DM, Nickel JC, Gerber L, Muller RL, Andriole GL, Castro-Santamaria R, et al. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: results from the REDUCE study. Cancer 2014;120(2):190–6. [DOI] [PubMed] [Google Scholar]
- 25.Platz EA, Kulac I, Barber JR, Drake CG, Joshu CE, Nelson WG, et al. A Prospective Study of Chronic Inflammation in Benign Prostate Tissue and Risk of Prostate Cancer: Linked PCPT and SELECT Cohorts. Cancer Epidemiol Biomarkers Prev 2017;26(10):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Bergh A, Damber JE. Morphological transition of proliferative inflammatory atrophy to high-grade intraepithelial neoplasia and cancer in human prostate. Prostate 2009;69(13):1378–86. [DOI] [PubMed] [Google Scholar]
- 27.Billis A, Freitas LL, Magna LA, Ferreira U. Inflammatory atrophy on prostate needle biopsies: is there topographic relationship to cancer? Int Braz J Urol 2007;33(3):355–60; discussion 361-3. [DOI] [PubMed] [Google Scholar]
- 28.Postma R, Schroder FH, van der Kwast TH. Atrophy in prostate needle biopsy cores and its relationship to prostate cancer incidence in screened men. Urology 2005;65(4):745–9. [DOI] [PubMed] [Google Scholar]
- 29.Moreira DM, Bostwick DG, Andriole GL, Peterson BL, Cohen HJ, Castro-Santamaria R, et al. Baseline Prostate Atrophy is Associated with Reduced Risk of Prostate Cancer in Men Undergoing Repeat Prostate Biopsy. J Urol 2015;194(5):1241–6. [DOI] [PubMed] [Google Scholar]
- 30.Moreira DM, Andriole GL, Castro-Santamaria R, Freedland SJ. Baseline prostate atrophy is associated with lower tumor volume in men with prostate cancer on repeat biopsy. Prostate Cancer Prostatic Dis 2018;21(1):106–112. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 2001;14(5):523–34. [DOI] [PubMed] [Google Scholar]
- 32.Vidal AC, Howard LE, de Hoedt A, Cooperberg MR, Kane CJ, Aronson WJ, et al. Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control 2018;29(6):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.