Abstract
Objective
Understanding the placebo response is critical to interpreting treatment efficacy, particularly for agents with a ceiling to their therapeutic effect, where an increasing placebo response makes it harder to detect potential benefit. The objective of this study is to assess the change in placebo responses over time in RA randomised placebo control trials (RCT) for drug licencing authorisation.
Methods
The Cochrane Controlled Trials Register database was searched to identify RCTs of biological or targeted synthetic DMARDs in RA. Studies were excluded if patients were: csDMARD naïve, not receiving background csDMARD therapy or were biologic experienced. Meta-regression model was used to evaluate changes in ACR20, ACR50 and ACR70 treatment response over time.
Results
There were 32 trials in total; anti-TNF therapy (n=15), tocilizumab (n=4), abatacept (n=2), rituximab (n=2) and JAK inhibitors (n=6). From 1999 to 2018, there was no significant trend in the age or gender of patients in the placebo arm. Disease duration, swollen joint count and DAS28-ESR at baseline all significantly reduced over time. There was a statistically significant increase in placebo ACR50 and ACR70 responses (ACR50 β=0.41, 95 CI 0.09 to 0.74, p=0.01; ACR70 β=0.18, 95 CI 0.04 to 0.31, p=0.01), that remained significant after controlling for potential confounders.
Conclusion
There has been a rise in the placebo response in RA clinical trials over the last two decades. Shifting RA phenotype, changes in trial design and expectation bias are possible explanations for this phenomenon. This observation has important implications when evaluating newer novel agent against established therapies.
Keywords: Rheumatoid arthritis, Systematic review, Study design, Outcome measures, American College of Rheumatology response, Placebo
Introduction
Novel therapies in Rheumatoid Arthritis (RA) are coming to market with increasing regularity. It is a challenge for clinicians to comprehend how different drugs compare with each other, particularly as few head-to-head trials are conducted. This has led to a growing reliance on network meta-analyses that rely on indirect comparisons linking multiple interventions to a fixed common comparator, typically placebo. The assumption is that results from different trials are sufficiently homogenous in their patient characteristics, settings, and outcomes to allow pooling of the data. [1].
Placebos are not inert. They cannot shrink tumours or heal fractures, but they do have an effect on symptoms modulated by the brain, particularly the perception of disease. A placebo may be very effective in improving pain and modifying mood. Randomised control trials (RCT) in inflammatory arthritis use the disease activity score (DAS-28) or American College of Rheumatology (ACR) response as key outcome measures. These are composite scores combine objective evidence of inflammation, which is unaffected by placebo and subjective measures of disease activity, which may be more amenable.
In antidepressant and antihypertensive drug trials, the magnitude of placebo response is trending upwards [2–5]. It is important to appreciate this when interpreting treatment efficacy, particularly for agents with a ceiling to their therapeutic effect, where no matter how efficacious the drug, there is a maximum number of people who will achieve disease control. In this circumstance, an increasing placebo response will make it harder to detect quantifiable benefit. This phenomenon is apparent when looking across targeted drugs trials in RA, where therapeutic improvements have largely plateaued.
The aim of this study was to assess if placebo response is rising in RA randomised control trials (RCT) used for drug licencing authorisation.
Methods
The study was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis guidelines [6]. The systematic review was registered with the international prospective register of systematic reviews (registration number: CRD4201810521). Ethics board approval was not required for this study.
The Cochrane Controlled Trials Register databases was searched systematically for all biological or targeted synthetic Disease Modifying Anti Rheumatic Drugs (bDMARD, tsDMARD) that are licensed for the treatment of RA in the UK. The search terms were ‘rheumatoid arthritis’ and either ‘infliximab’, ‘adalimumab’, ‘etanercept’, ‘certolizumab’, ‘golimumab’, ‘abatacept’, ‘tocilizumab’, ‘rituximab’, ‘tofacitinib’, ‘baricitinib’ or ‘upadacitinib’. The search was undertaken in June 2017 and re-run prior to the final analysis to identify further studies that could be retrieved for analysis.
English language publications of phase II and III randomised control trials (RCT) published by July 2018 were sought. Conference abstracts were excluded. RCTs were included if they met the following criteria: (1) the study provided a placebo comparator, (2) the placebo comparator were not conventional synthetic DMARD (csDMARD) naïve at enrolment and were receiving background csDMARD therapy during follow-up study and (3) less than 15% of participants were biologic experienced. Studies presenting duplicate data were excluded. No restrictions were applied by the length of follow-up. Titles and abstracts of studies retrieved using the search strategy detailed above were screened independently. The full text of the potential studies for inclusion were retrieved and assessed for eligibility.
The primary outcome of interest was treatment response, measured using the American College of Rheumatology (ACR) Criteria, defined as 20, 50 or 70% improvement in both tender and swollen joint count, and in 3 of the 5 core measures; patient assessment, physician assessment, pain scale, disability/functional questionnaire, and acute phase reactant (ESR or CRP). Analyses were undertaken using Stata 14. Meta-regression was used to evaluate changes in ACR20, ACR50 and ACR70 treatment response over time. A multivariate model was applied adjusting for age, gender, disease duration, baseline tender joint count, swollen joint count, CRP at baseline and time to primary outcome.
Results
1.1. Study characteristics
The literature search identified 1828 trials in total, of which 149 were either phase II of III RCTs. 115 studies were excluded as they enrolled patients that were csDMARD naive, had no background csDMARD therapy during follow up, a high percentage of previous biologic exposure, or did not include a placebo comparator. All Japanese bridging studies were excluded.
There were 32 trials in total; 15 RCTs evaluating anti-TNF therapy; adalimumab (n=3), etanercept (n=3), infliximab (n=2) certolizumab (n=3) and golimumab (n=4) (table 1). The remaining RCTS evaluated tocilizumab (n=4), abatacept (n=2), rituximab (n=2) and JAK inhibitors (n=8). Studies were published from 1999 to 2018, with a median time to primary outcome of 24 weeks, (range of 8 to 52 weeks). This duration on placebo has shortened over the last 20 years (β= -0.44, 95 CI -0.87 to -0.004, p=0.048). On average, assessment visits were 4 weeks apart, with half of the studies arranging more frequent visits at study initiation. There were no trends in the frequency of study visits across the time period. All studies recruited from North America and or Europe. From 2008 onwards, a greater number of studies recruited patients from Latin America and South East Asia.
Table 1. Published randomised placebo-controlled trial of biologics and JAK inhibitors in rheumatoid arthritis between 1999 and 2018.
| Year | Author | Drug | PBO (N) | Recruitment site (visit\week) | Age Mean (SD) | Female) (%) | Duration Mean (SD) | TJC 68 Mean (SD) | SJC 66 Mean (SD) | DAS28 Mean | ACR20 (%) | ACR50 (%) | ACR70 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | Weinblatt [16] | ETN | 30 | US CAN. 4 | 53 | 73 | 13 | 28 | 17 | - | 27 | 3 | 0 |
| 1999 | Maini [17] | IFX | 88 | US CAN EU 4 | 50(11) | 80 | 10(7) | 27(24) | 20(11) | - | 20 | 5 | 0 |
| 2003 | Weinblatt [18] | ADA | 62 | US CAN 2† | 56(11) | 82 | 11(8) | 29(15) | 17(10) | - | 15 | 8 | 5 |
| 2003 | Kremer [19] | ABT | 119 | US CAN EU AUS SA 4 | 54(11) | 66 | 9(8) | 29(13) | 22(9) | - | 35 | 12 | 2 |
| 2003 | Furst [20] | ADA | 318 | US CAN 4† | 56(12) | 79 | 12(10) | 28(14) | 21(11) | - | 35 | 11 | 4 |
| 2004 | Keystone [21] | ETN | 53 | US CAN 8 | 55(15) | 72 | 12(10) | 25(20) | 19(18) | - | 19 | 6 | 2 |
| 2004 | Keystone [22] | ADA | 200 | US CAN 4† | 56(12) | 73 | 11(9) | 28(14) | 19(10) | - | 30 | 10 | 3 |
| 2004 | Edwards [23] | RTX | 40 | EU CAN AUS ISR 4† | 54(11) | 80 | 11(7) | 32(13) | 19(10) | 6.9 | 38 | 13 | 5 |
| 2006 | Kremer [24] | ABT | 219 | US CAN EU MEX 4† | 50(12) | 82 | 9(7) | 32(14) | 22(9) | 6.4 | 40 | 17 | 7 |
| 2006 | Maini [25] | TOC | 49 | EU 2 | 51 | 78 | 0.9 | 16 * | 12 * | 6.8 | 41 | 29 | 16 |
| 2008 | Smolen [26] | TOC | 204 | Worldwide 4 | 51(12) | 78 | 8(7) | 33(16) | 21(12) | 6.8 | 26 | 11 | 2 |
| 2008 | Kay [27] | GOL | 35 | US CAN EU AUS 4† | 55(11) | 74 | 6(2) | 26(17) | 14(6) | 6.5 | 37 | 6 | 0 |
| 2008 | Schiff [28] | IFX | 165 | Worldwide 4 | 49(12) | 87 | 7(6) | 32(15) | 20(8) | 6.8 | 42 | 20 | 9 |
| 2008 | Genovese [29] | TOC | 413 | Worldwide 4 | 54(13) | 84 | 10(9) | 29(15) | 19(11) | 6.6 | 25 | 9 | 3 |
| 2008 | Keystone [30] | CZP | 199 | Worldwide 2† | 52(11) | 84 | 6(4) | 30(15) | 21(10) | 7.0 | 14 | 8 | 3 |
| 2009 | Keystone [31] | GOL | 133 | Worldwide 4 | 51(12) | 82 | 7(2) | 20(8) | 13(8) | 6.0 | 33 | 10 | 4 |
| 2009 | Smolen [32] | CZP | 127 | EU 2† | 52(12) | 84 | 6(4) | 30(13) | 22(10) | 6.8 | 9 | 3 | 1 |
| 2010 | Kremer [33] | GOL | 129 | Worldwide 4 | 50 | 80 | 7 | 28 | 16 | 25 | 9 | 3 | |
| 2010 | Emery [34] | RTX | 172 | Worldwide 4-8 | 52(12) | 86 | 8(8) | 30(16) | 21(11) | 6.5 | 23 | 9 | 5 |
| 2011 | Kremer [35] | TOC | 393 | Worldwide 4† | 51(12) | 83 | 10(7) | 28(15) | 17(9) | 6.5 | 27 | 9 | 1 |
| 2012 | van Vollen-hoven [36] | TOF | 56 | Worldwide | 56(14) | 77 | 7 | 27 | 17 | 6.6 | 28 | 16 | 5 |
| 2012 | Kremer [37] | TOF | 69 | Worldwide 4 | 53(13) | 81 | 9 | 22 | 16 | 6.1 | 33 | 17 | 6 |
| 2012 | Choy [38] | CZP | 121 | US EU 4† | 56(12) | 66 | 10(8) | 31(13) | 22(10) | 6.3 | 23 | 6 | 2 |
| 2012 | Moreland [39] | ETN | 255 | US 6 | 49(13) | 69 | 0.2 | 14(7)* | 13(6)* | 5.8 | 40 | 22 | 4 |
| 2013 | Weinblatt [40] | GOL | 197 | US CAN 4† | 51(11) | 80 | 7(7) | 26(14) | 15(9) | 5.9* | 32 | 13 | 5 |
| 2013 | Kremer [41] | TOF | 79 | Worldwide 4† | 51(11) | 80 | 11(8) | 27(17) | 15(10) | 6.4 | 31 | 13 | 3 |
| 2013 | van der Heijde [42] | TOF | 81 | Worldwide 4-8 | 53(12) | 80 | 11(9) | 23(13) | 14(8) | 6.3 | 25 | 8 | 2 |
| 2015 | Keystone [43] | BARI | 98 | US CAN MEX IND 4† | 49(12) | 87 | 5(4) | 22(12) | 16(9) | 6.3 | 41 | 9 | 5 |
| 2016 | Genovese [44] | UPA | 50 | US EU SA 2 | 55(12) | 76 | 6(5) | 29(16) | 19(12) | 5.6* | 46 | 18 | 6 |
| 2017 | Dougados [45] | BARI | 228 | Worldwide 4† | 51(13) | 83 | 7(8) | 24(15) | 13(7) | 6.2 | 39 | 13 | 3 |
| 2017 | Taylor [46] | BARI | 488 | Worldwide 4† | 53 | 78 | 10(9) | 23(14) | 16(9) | 6.4 | 40 | 17 | 5 |
| 2018 | Burmester [47] | UPA | 221 | Worldwide 4† | 56(12) | 75 | 7(8) | 25(15) | 15(9) | 5.6* | 36 | 15 | 6 |
ETN = Etanercept; IFX = Infliximab; ADA = Adalimumab; GOL = Golimumab; CTZ = Certolizumab; RTX = Rituximab; ABT = Abatacept; TOF = Tofacitinib; BARI = Baricitinib; UPA = Upadacitinib. US = United states of America; CAN = Canada; EU = Europe; AUS = Australia; SA = South Africa; ISR = Israel; MEX = Mexico; IND = India; † = visits initially weeks 1 and 2 followed by either 2 or 4 weekly as indicated; * = 28 joint count; * = DAS-CRP
1.2. Patient characteristics
The median number of patients in placebo arms was 128 (IQR 66-212). The mean age was 53 years (SD 2), and 79% (SD 5%) of patients were female. From 1999 to 2018, there was no significant trend in the age or gender of patients in the placebo arm (age β= -0.05, 95 CI -0.23 to 0.12, p=0.56 and gender β= 0.16, 95 CI -0.21 to 0.52, p=0.39). Excluding the two studies that recruited patients with early RA (duration disease <1 year) [Maini 2006, Moreland 2012], the mean duration of disease was 8.7 years (SD 2). This fell significantly across the time period studied (β= -0.22, 95 CI -0.35 to 0.10, p=0.001).
There were no significant trends in csDMARD exposure. The median methotrexate dose was 16mg (IQR 15mg -17mg). Over two thirds of the studies reported data on glucocorticoid exposure, which was administered in 58% (50%-69%) of patents and had fallen across the time period studied (β= - 1.00, 95 CI -1.94 to -0.06, p=0.04). More recent studies included a greater proportion of patients with prior biologic exposure. Prior to 2008, the average percentage exposure was less than 1 % compared with 4% from 2008 onwards. There were significant trends in baseline disease activity over time, with falling tender joint counts [median 28 (IQR 24-30) β= -0.26, 95 CI -0.46 to -0.05, p=0.02] swollen joint counts [median 17 (IQR15-21) β= -0.26, 95 CI -0.42 to -0.09, p=0.003] and DAS28-ESR, despite this variable not being reported in any study prior to 2004 [mean DAS28-ESR 6.47 (SD 0.31), β= -0.05, 95 CI -0.08 to -0.02, p=0.001]. There was no trend in patient or physician global assessment (β= -0.07, 95 CI -0.14 to -0.14, p=0.48 and β= -0.04, 95 CI -0.31 to -0.22, p=0.75 respectively).
1.3. Changing placebo responses
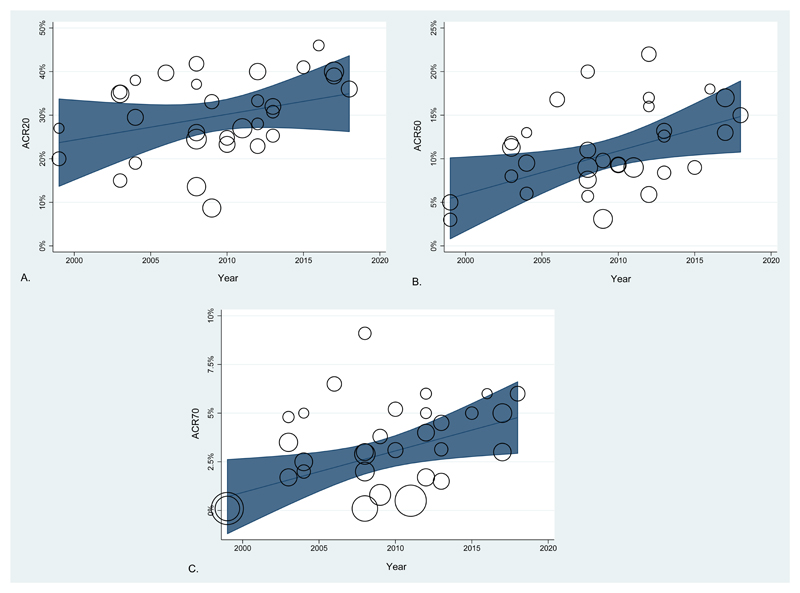
ACR responses are shown in figure 1. The percentage of patients in placebo arms achieving ACR response was; ACR20 31% (25-39), ACR50 10% (8-16), ACR70 3% (2-5). Considering placebo arm size, there was a statistically significant increase in placebo ACR50 and ACR70 responses from 1999 to 2018; (ACR50 β= 0.39, 95 CI 0.04 to 0.75, p=0.03) and (ACR70 β=0.17, 95 CI 0.02 to 0.32, p=0.02). There was no statistically significant change in ACR20 response.
Figure 1.
Adjusted ACR responses in the placebo arm of published RCTs of biologics and JAK inhibitors in rheumatoid arthritis between 1999 and 2018; A: ACR20, B. ACR 50, C: ACR70.
One trial had an outlier ACR70 response (Maini 2006 Tocilizumab, see table 1). Excluding this study did not alter the findings with comparable changes in ACR response; (ACR50 β= 0.41, 95 CI 0.09 to 0.74, p=0.01) and (ACR70 β= 0.18, 95 CI 0.04 to 0.31, p=0.01) although the trend in ACR20 responses become statistically significant (β= 0.70, 95 CI 0.03 to 1.38, p=0.04). For each additional year there is around a 0.5 percentage point increase in ACR50 treatment response, which over 10 years equates to a 5% increase in ACR50 response. The changes in ACR50 and ACR70 responses remained significant after adjustment for age, gender, disease duration, baseline tender joint count, swollen joint count, CRP and time to primary outcome.
We considered other factors which may influence or explain the placebo response. This included looking in parallel at treatment response in the therapeutic arm over time, which did not change. We explored RA disease duration which did have an effect on placebo ACR50 response (β= -0.84, 95 CI -1.4 to -0.19, p=0.01) but not ACR20 or ACR70. Finally, we examined the inclusion of CRP or ESR at recruitment, however there were inadequate data to draw firm conclusions.
Discussion
This analysis confirms significant increases in both ACR50 and ACR70 treatment responses in patients in the placebo arm of RA RCTs from 1999 to 2018. This remained statistically significant after controlling for potential confounders. These results have important clinical implications and should be acknowledged when comparing efficacy between emerging and established therapies.
There are several possible explanations for the rise in placebo response. RA severity has decreased over time, a reflection of the emphasis on early diagnosis and improvements in pharmacological therapies [7, 8]. This has reduced the pool of potential patients who meet eligibility criteria, which may result in investigators inflating baseline disease scores to enable entry into a study. This is particularly relevant for industry funded trials where clinical units are financially compensated for study participation. The course of RA has also changed over time. Patients sustain lower disease activity levels, interspersed with episodes of increased activity defined as ‘flares’. It is plausible that a proportion of patients are recruited during a flare which spontaneously resolves, and consequently their follow up disease score reflects a significant improvement from baseline.
Changes in trial design may account for the rise in placebo response. There has been a shift in the geographical distribution of RA trial sites, with greater recruitment from Latin America and Eastern Europe. In resource poor countries, trial participation would improve adherence to background csDMARDs amplifying placebo response. An analysis of 981 placebo subjects across worldwide RA trials, reported a consistently higher placebo response in patient recruited from Latin America. The same study also identified a higher odd of ACR20 response in Asian patients compared to Caucasian [9]. A shift in the recruitment of patients with different cultural beliefs may have contributed to an increased response to the Hawthorne effect. This is defined as an additional clinical response resulting from increased attention provided by participation in the clinical trial, a phenomenon described in RA studies [10].
The rise in placebo response may also related to recent changes in the use of background csDMARDs, with recommendations for combination therapy early in the disease. As maximal response to csDMARD is seen at 6 months, RCTs requiring only 3 months of background therapy may be associated with higher placebo effect [11]. The formulation of a placebo may also influence response. Research has suggested that patient perceptions of placebo is influenced by its colour, size, and form; injections elicit a stronger placebo effect than oral medications, whilst capsules are perceived to be ‘stronger’ than tablets [12]. Interestingly, the more recent studies in this analysis assess oral JAK inhibitors and thus use an oral placebo comparator. This is in contrast to the earlier biologic RCTs that evaluated injectable placebo, which one would expect to elicit a stronger placebo effect. Lastly, the desire for the new treatments to succeed can result in implicit bias in both subject and investigator-controlled outcomes.
Expectation bias, the awareness that a new drug being administered imparts an expectation benefit to both the investigators and the recipients, may also contribute to the rising placebo response. Outcome expectation is based on patients’ understanding of the treatment offered, their own illness, and experiences with past treatments. In antidepressant clinical trials, patient expectancy is a chief mechanism for placebo response. Perceived prestige, credibility, and sophistication of a treatment can significantly increase expectations of improvement [13]. It would be unusual for this to affect objective biological responses, but it is plausible that expectation bias influences subjective measures of disease activity. With the decline over time in the severity of objective markers of inflammation, the impact of expectation bias on subjective measures of disease activity may be substantial.
The identification of biomarkers of a placebo response would be a powerful tool in improving the interpretability of trials and assisting in stratifying populations and adjusting effect sizes. Measuring expectation benefit to identify participants susceptible to a placebo effect would be valuable, although no fully validated method exists [14].
We did not demonstrate a significant increase in ACR20 treatment responses in patients in the placebo arm of RA RCTs from 1999 to 2018. A possibility explanation for this, is that despite its high specificity, unlike ACR50 and ACR70, the ACR20 has demonstrated only modest sensitivity for patient-reported improvement [15]. This suggests that patients who judged themselves to have improved, do not demonstrate an associated ACR20 response, and may explain the absence of an increase over time.
Our goal was to understand changing placebo responses over time. There is a growing number of RCTs recruiting patients with previous biologics exposure. However, there is a noticeable difference in treatment effect between patients who are biologic naive versus those who have failed one, or perhaps even multiple biologics. The restricted search criteria increased homogeneity among the placebo patients and facilitated a cohort that was representative of current practice. However, we could not control for all differences in the study populations and trends in study quality.
In this study, the restricted search criteria increased homogeneity among the placebo patients and facilitated a cohort that was representative of current practice. However, we could not control for all differences in the study populations and trends in study quality. Unfortunately, there is very little published data on the socio-economic or educational level of the patient populations included in each RCT. It is acknowledge that these factors influence placebo responses, although substantial research has not yet identified a consistent demographic characteristic that predict placebo response [16].The results are potentially influenced by publication bias, with under-sampling of placebo responses from failed trials. If a trial had a large placebo response, it is likely they failed to demonstrate a positive therapeutic advantage and therefore less likely to be published. We did not consider the impact of the nocebo effect, a phenomenon where patients’ concerns and expectations about the value of a therapeutic intervention negatively influence adherence and treatment response. This has been considered in patients switching biologics from bio-originators to biosimilars, to explain a deterioration in therapeutic benefit [17]. How the nocebo effect influences RA trials over time has not been explored and is an area for potential further study.
In conclusion, this study has demonstrated an increase in treatment response in the placebo arm of RA trials. It is essential that we improve our understanding of the mechanisms behind this phenomenon. A rising placebo response has important implications when comparing the efficacy of treatments across clinical trials, including in network meta-analyses. Estimates of drug efficacy within a trial are unlikely to be confounded by the placebo response, as this is expected to be equal in both the placebo and active comparator arm. However, in trials where there is a therapeutic ceiling effect, as seen in RA, an increasing placebo response rate will result in a reduced treatment effect size. This will impact on comparisons between established and novel agents and should be considered by clinicians when evaluating the efficacy of different therapies.
Role of the Funding source
This work was primarily supported by Medical Research Council as a Clinical Training Research Fellowship to KB (CTRF-MR/R001332/1 to K Bechman)
Footnotes
Katie Bechman: Orcid 0000-0001-7552-046
Mark Yates: Orcid 0000-0001-5449-5211
Sam Norton: Orcid 0000-0003-1714-9963
Andrew Cope: Orcid 0000-0001-6735-5496
James B Galloway: Orcid 0000-0002-1230-2781
Competing interest: None declared by authors.
Disclosure statement: J.B.G. has received honoraria for speaking or attending conferences from Pfizer, Bristol-Myers Squibb, UCB and Celgene.
References
- 1.Fleischmann R, Landewe, Smolen JS. Review of head-to-head study designs in rheumatoid arthritis. Semin Arthritis Rheum. 2016;46:279–285. doi: 10.1016/j.semarthrit.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 2.K Arif, FM Kaysee, F Jim, KS Shirin, B WA. Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987-2013. World Psychiatry. 2017;16:181–192. doi: 10.1002/wps.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A, Fahl Mar K, Schilling J, Brown WA. Does the rising placebo response impact antihypertensive clinical trial outcomes? An analysis of data from the Food and Drug Administration 1990-2016. PLOS ONE. 2018;13:e0193043. doi: 10.1371/journal.pone.0193043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh B, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: Variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 5.Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Meta-analysis of the placebo response in antidepressant trials. J Affective Disord. 2009;118:1–8. doi: 10.1016/j.jad.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlig T, Kvien TK. Is rheumatoid arthritis really getting less severe? Nat Rev Rheumatol. 2009;5:461–464. doi: 10.1038/nrrheum.2009.140. [DOI] [PubMed] [Google Scholar]
- 8.Aga A-B, Lie E, Uhlig T, Olsen IC, Wierød A, Kalstad S, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000–2010. Ann Rheum Dis. 2015;74:381–388. doi: 10.1136/annrheumdis-2013-204020. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, D B, Hsu CH, Hu C, Lei C, Song J, Lu J, Beutler A. Physician/Site Staff Assessments Contribute to High Placebo Response in Rheumatoid Arthritis Clinical Trials. Arthritis Rheumatol. 2016;68(10) [abstract] [Google Scholar]
- 10.Wolfe F, Michaud K. The Hawthorne effect, sponsored trials, and the overestimation of treatment effectiveness. J Rheumatol. 2010 Nov;37(11):2216–2220. doi: 10.3899/jrheum.100497. [DOI] [PubMed] [Google Scholar]
- 11.Strand V, Sokolove J. Randomized controlled trial design in rheumatoid arthritis: the past decade. Arthritis Res Ther. 2009;11:205–205. doi: 10.1186/ar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford BR, Wall MM, Glass A, Stewart JW. The Role of Patient Expectancy in Placebo and Nocebo Effects in Antidepressant Trials. J Clin Psychiatry. 2014;75:1040–1046. doi: 10.4088/JCP.13m08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mestre TA, Lang AE. Placebos in clinical trials: unravelling a complex phenomenon. Lancet Neurol. 2017;16:28–29. doi: 10.1016/S1474-4422(16)30349-0. [DOI] [PubMed] [Google Scholar]
- 15.Ward MM, Guthrie LC, Alba MI. Rheumatoid arthritis response crtiteria and patient-reported improvement in arthritis activity: Is an ACR20 Response Meaningful to Patients? Arthritis Rheumatol. 2014;66:2339–2343. doi: 10.1002/art.38705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coste J, Montel S. Placebo-related effects: a meta-narrative review of conceptualization, mechanisms and their relevance in rheumatology. Rheumatology. 2017;56:334–343. doi: 10.1093/rheumatology/kew274. [DOI] [PubMed] [Google Scholar]
- 17.Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology. 2010;49:924–928. doi: 10.1093/rheumatology/kep458. [DOI] [PubMed] [Google Scholar]
- 18.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–9. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 19.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 20.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 21.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 22.Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- 23.Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:353–363. doi: 10.1002/art.20019. [DOI] [PubMed] [Google Scholar]
- 24.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 25.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 26.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 27.Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 28.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 29.Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:964–975. doi: 10.1002/art.23383. [DOI] [PubMed] [Google Scholar]
- 30.Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 32.Keystone E, Heijde D, Mason D, Jr, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–3329. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 33.Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–796. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68:797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremer J, Ritchlin C, Mendelsohn A, Baker D, Kim L, Xu Z, et al. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2010;62:917–928. doi: 10.1002/art.27348. [DOI] [PubMed] [Google Scholar]
- 36.Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69:1629–1635. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–621. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- 38.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. New Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 39.Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–981. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 40.Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology. 2012;51:1226–1234. doi: 10.1093/rheumatology/ker519. [DOI] [PubMed] [Google Scholar]
- 41.Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64:2824–2835. doi: 10.1002/art.34498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinblatt ME, Bingham CO, 3rd, Mendelsohn AM, Kim L, Mack M, Lu J, et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis. 2013;72:381–389. doi: 10.1136/annrheumdis-2012-201411. [DOI] [PubMed] [Google Scholar]
- 43.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 44.van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 45.Keystone EC, Taylor PC, Drescher E, Schlichting DE, Beattie SD, Berclaz PY, et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis. 2015;74:333–340. doi: 10.1136/annrheumdis-2014-206478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391:2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 47.Dougados M, van der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76:88–95. doi: 10.1136/annrheumdis-2016-210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. New Engl J Med. 2017;376:652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 49.Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]