Abstract
Morphogens are signaling molecules produced by a localized source, specifying cell fate in a graded manner. The source secretes morphogens into the extracellular milieu to activate various target genes in an autocrine or paracrine manner. Here we describe various secreted forms of two canonical morphogens, the lipid-anchored Hedgehog (Hh) and Wnts, indicating the involvement of multiple carriers in the transport of these morphogens. These different extracellular secreted forms are likely to have distinct functions. Here we evaluate newly identified mechanisms that morphogens use to traverse the required distance to activate discrete paracrine signaling.
Multiple Carriers for Hh and Wingless (Wg) Transport
Morphogens play a major role in tissue patterning by activating various target genes in a spatiotemporally regulated manner [1]. They are predominantly produced by a localized source and function in a concentration-dependent manner. Interpretation of these concentration gradients has received increased attention [2]. Morphogen-mediated cell-fate determination requires coordination between several cellular processes such as morphogen production and secretion by the source, transport across several cell diameters, efficient receptor binding, and processing by signal-receiving cells [3,4].
Lipid-modified Hh and Wg are membrane-anchored morphogens that play major roles in tissue patterning and maintenance and in disease progression. First identified in Drosophila, these morphogens are conserved in metazoans [5,6]. In Drosophila they activate short- and long-range paracrine signaling to pattern the larval imaginal discs (e.g., wing discs; Figure 1A,B), which ultimately give rise to the adult body parts [7,8]. In vertebrates Hh [i.e., Sonic hedgehog (Shh)] and the Wg homologs (Wnts) act at short and long range to pattern the developing neural tube [9] (Figure 1C), limbs [10], craniofacial features [11,12], and endocrine system [13,14]. In adult systems these morphogens play a major role in stem cell maintenance [15], tissue repair [16], neural connection rewiring [17], and metabolism [18]. They are also hyperactivated in several carcinomas [19].
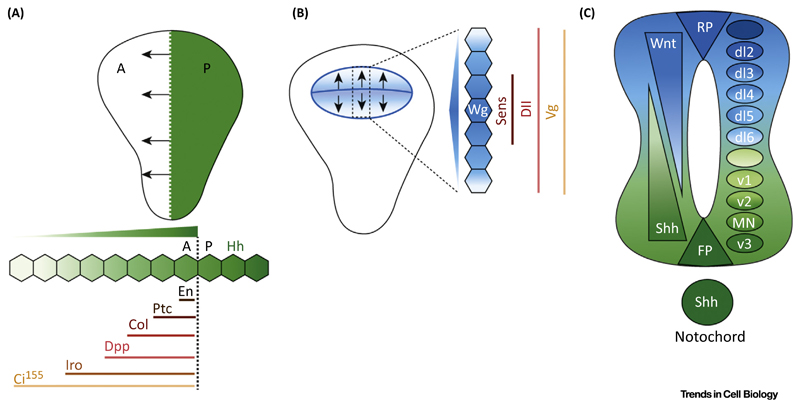
Figure 1. Hedgehog (Hh) and Wnt Signaling in the Drosophila Wing Disc and Vertebrate Neural Tube.
(A) Cartoon representing Hh signaling in wing discs. Hh is produced in the posterior domain (green; P) and generates a concentration gradient (arrows) along the anterior domain (white; A). Engrailed (En) and Patched (Ptc) are short-range targets of Hh, expressed by up to three or four cells abutting the AP boundary. Collier (Col) requires intermediate Hh levels, whereas Decapentaplegic (Dpp) and Iroquois (Iro) are long-range targets, expressed ∼10–15 cells away along the AP boundary. Stabilization of cubitus interruptus (Ci155) requires the lowest levels of Hh. Higher levels of this protein are seen near the AP boundary, while cells away from the AP boundary see lower levels of stabilized Ci. (B) Cartoon representing Wingless (Wg) signaling in wing discs. Wg expression is seen at the dorsoventral (DV) boundary in a narrow strip of cells and at the boundary of the wing pouch (blue). Wg forms an extracellular gradient on either side of the DV boundary (arrows) and activates targets in a concentration-dependent manner. Senseless (Sens) requires high Wg levels and Distalless (Dll) intermediate levels with graded expression, while Vestigial (Vg) is a lowest-threshold target. (C) Sonic hedgehog (Shh) (green) and Wnt (blue) during vertebrate neural tube pattering. These emanate from two sources: dorsally (BMPs/Wnts from the epidermis) and ventrally (Shh from the notochord). The roof plate (RP) and floor plate (FP) form the secondary signaling centers, which also secrete BMP/Wnt and Shh, respectively. They form reciprocal gradients to specify specific neural progenitors. Wnt/BMPs specify the dorsal sensory neurons (dI1–dI5) while Shh specifies the ventral motor neurons; highest to lowest: v3, MN, v2, v1, and v0.
Hh-family proteins undergo a series of post-translational modifications in the secretory pathway en route to the plasma membrane (PM), to generate a cholesterol- and palmitate-modified signaling protein [20–22]. Similarly, Wg and most Wnts are modified by palmitate or palmitoleic acid [23–25]. Despite being lipidated and membrane tethered, these proteins activate signals several cell diameters away from their source (Figure 1). This has raised two questions. (i) How is a lipid-anchored molecule released from the PM? (ii) How are the hydrophobic lipid tails of this molecule shielded in the aqueous milieu, enabling its transport in the extracellular space? Over the years several groups have made attempts to address these issues.
Extracellular Hh- and Wg-family proteins have been identified in association with various carriers, such as exovesicles or exosomes [26–32], lipoproteins [33,34], and cytonemes [31,35–39]. Hh proteins are also released by matrix metalloproteases (MMPs) in a lipid-free form [40]; such a mechanism for secretion of Wg proteins has not yet been reported except in the context of WntD, a naturally non-lipid-modified homolog [41]. Extracellular Hh and Wg are classified into three main forms: exovesicles (exosomes or microvesicles) and lipoprotein-associated and soluble forms. Several molecular players, such as Dispatched [42], Scube2 [43], and flotillin-2 [44] for Hh and Evenness interrupted (Evi)/Wntless [27,29], Swim [45], and Afamin [46] for Wg secretion, have also been identified as involved in the efficient release of these morphogens. Once they have been secreted into the extracellular milieu, cell-surface heparan sulfate proteoglycans (HSPGs) are known to facilitate their transport to neighboring signaling-competent cells [47]. However, a composite understanding of how these players function with respect to each other or with respect to the various secreted forms is missing. Here we discuss whether the different modes of release of membrane-anchored morphogens generate distinct extracellular forms with different signaling capacities.
Exosomes as Morphogen Carriers
Exosomes are secreted vesicles of 30–100 nm in size. Exosome-based intercellular interactions have been implicated in immunological processes, neuron–glia and tumor–stroma crosstalk [48], and morphogen-based paracrine signaling [26–32]. Exosomes are usually generated by the sorting of endocytic cargo into multivesicular bodies (MVBs). These proceed via an endosomal sorting complex required for transport (ESCRT)-dependent or -independent mechanism [49] (Box 1).
Box 1. Mechanisms of Exovesicle Biogenesis.
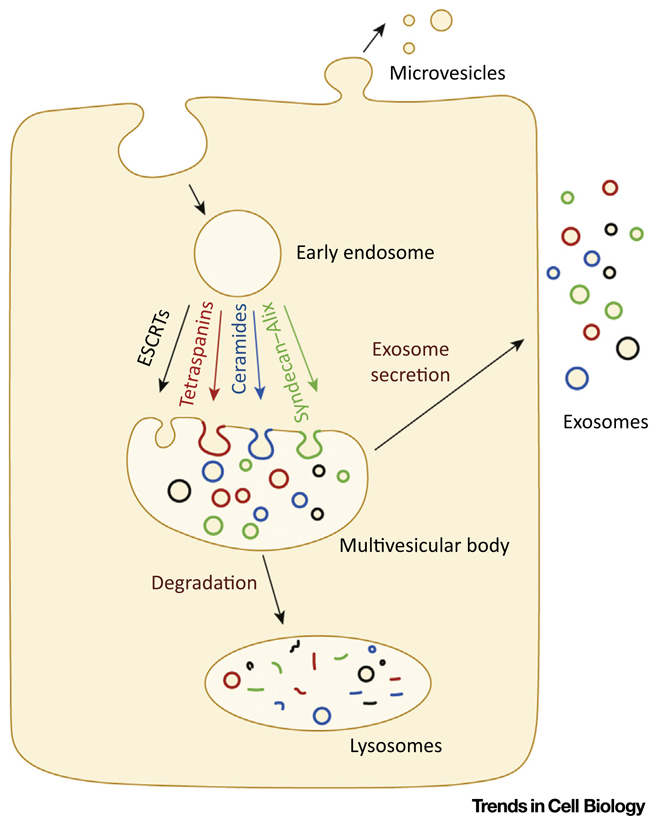
Conventionally, MVB biogenesis requires components of the ESCRT machinery. In brief, ESCRTs comprise four distinct protein complexes (i.e., ESCRT-0, -I, -II, and -III) that act in a sequential manner to generate ILVs by budding and scission of the MVB-limiting membrane [48]. HGS and signal transducing adaptor molecule (STAM) 1 and 2 form the ESCRT-0 complex. HGS loads onto endosomes via an interaction between its FYVE domain and endosomal lipid phosphatidylinositol 3-phosphates [88]. HGS and STAM are known to be critical in sorting ubiquitinated cargoes, via their ubiquitin-recognition domains, into clathrin-coated microdomains [89,90]. Loading of ESCRT-0, leads to a cascade of events to recruit the subsequent ESCRTs (i.e., -I, -II, and -III) [48]. Loading of the ESCRT-III component Shrub/Vps32 is important in generating membrane curvature and hence ILV formation [91]. ESCRTs are further assisted by accessory proteins such as Alix and Vps4 in ILV formation [48]. Vps4, an AAA-ATPase, is necessary for the dissociation of ESCRT-III assemblies from the endosome for subsequent cycles [92]. Alix can potentially link ESCRT-I and ESCRT-III subunits, contribute to ubiquitin-dependent and -independent modes of cargo sorting, and aid in ILV formation by interactions with ESCRT-III components [93]. Besides ubiquitinated proteins, several non- ubiquitinated proteins have also been identified in exosomes. However, unlike ubiquitinated proteins, much less is known about the mechanisms for sorting non-ubiquitinated proteins into exosomes. Several players have been implicated in this process. It has been demonstrated that non-ubiquitinated proteolipid protein (PLP) is secreted on exosomes by an oligoden-droglial precursor cell line in an ESCRT-independent, ceramide-dependent manner [94]. Tetraspanins have also been implicated in the regulation of exosomal secretion of proteins such as b-catenin [95] and premelanosome protein (PMEL) [96]. It has been proposed that tetraspanins can interact with each other as well as with several other cellular proteins (e.g., integrins, ICAM-1, VCAM-1) to form domains/webs that can assist the sorting of proteins into ILVs and hence exosomes [97,98]. Similarly, syndecan clustering along with syntenin–Alix interactions has also been implicated in the sorting of proteins into exosomes [99,100] (Figure I).
Microvesicles are generated by outward budding of the PM. Microvesicles are mainly reported for their secretion of viruses. Microvesicle biogenesis is known to involve ESCRT-I, -II, and -III and Vps4 function [57].
Figure I. Cartoon Representing Different Molecular Mechanisms of Exovesicle Biogenesis.
Recently, exosomes have been identified as carriers of Hh and Wg [26–32]. While it has been proposed that Wg secretion in Drosophila cells does not require the canonical players involved in MVB formation [i.e., ESCRT, Alix, or ceramide function [29] (unpublished data in [50])], Wnt-3 secretion by HEK cells [28] requires ESCRT function. Regardless, these molecules are first sorted into intraluminal vesicles (ILVs) in MVBs for their exosomal secretion. Since most of the cargo that accumulates in the MVB is destined for degradation [51] (Box 1), this raises the question of how the cells distinguish between the secretory and the degradative fate of these ILVs/MVBs. Given that exosomes ferry morphogens and play a significant role in disease progression via molecules on and in them [52], it will be valuable to identify these mechanisms and regulators to better understand morphogen secretion.
Exovesicles as Hh Carriers
The first evidence for secretion of Hh on exosomes came from work done in Caenorhabditis elegans. Here it was demonstrated that Hh-related peptides required for larval cuticle patterning are detectable on exosomes and require V0-ATPase activity [53]. A significant body of work has subsequently highlighted that cell lines and tissues secrete functional Hh molecules on exosomes [26,30–32]. Exovesicular release of Shh has also been reported in mouse embryos [54]. In wing discs, ESCRT proteins such as Tsg101 (ESCRT-I), Vps22 (ESCRT-II), Vps32 (ESCRT-III), and Vps4 are involved in Hh release and target gene activation [26,31]. Further, Hh proteins are sorted into at least two types of exosomal pools using differential ultracentrifugation from a Drosophila cell line, a human cell line, and primary chick notochord cells in culture. These biochemically distinct pools have different sedimentation capabilities and have distinct diffusion properties as monitored by fluorescence correlation spectroscopy as well as different compositions (proteins and RNAs) [30,32]. While the classical exosomal pool contains proteins capable of sorting in cholesterol–sphingolipid-rich microdomains, such as flotillins, annexins, and integrins; the newly identified pool of exovesicles appears to be devoid of these proteins [30,32]. Therefore, these two different types of exovesicle might be a result of microdomain segregation on the MVB-limiting membrane [30]. Notably, these vesicles activate different differentiation programs during ventral neuronal progenitor specification [30]. Here, only the classical exosomal pool was able to activate Shh-target genes (ventral neuronal progenitors), while the newly identified pool of exovesicular Shh failed to activate Shh-target genes. However, the mechanism and molecular players involved in generating these distinct pools have not been identified and hence their ability to travel or signal across developing tissues has not been resolved. To summarize, Hh is secreted on different types of exovesicles across different cell types and this difference has functional significance.
Microvesicles or Exosomes?
Besides exosomes, Hh molecules may be released on microvesicles that are generated by budding directly from the PM (Figure 2 and Box 1). Distinguishing PM budding from MVB-mediated exosomal release of Hh molecules is not straightforward since the core ESCRT machinery (ESCRT-I, -II, and -III) and Vps4 [55,56] are also implicated in microvesicle formation [57]. However, regulators of endocytosis such as Rab proteins are not involved in microvesicle biogenesis but regulate exosomal release of cargo by facilitating their sorting into MVBs.
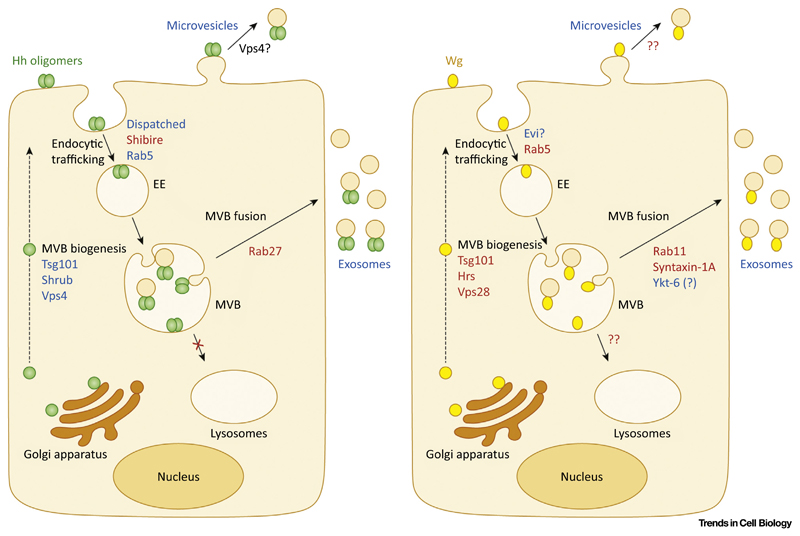
Figure 2. Molecular Players Involved in Exovesicular Release of Hedgehog (Hh) and Wingless (Wg).
Hh (in green) and Wg (in yellow) are secreted from the Golgi apparatus and anchored on the cell membrane via their lipid modifications. Exosomal release requires endocytosis of Hh (via Shibire, Dispatched) and Wg (maybe mediated by Evi; precise role unclear) with sorting in Rab5-containing early endosomes (EEs). From EEs, Hh and Wg are sorted onto intraluminal vesicles (ILVs) by inward budding of multivesicular body (MVB)-limiting membrane. The endosomal sorting complex required for transport (ESCRT) machinery is required for release of Sonic hedgehog (Shh) (Tsg101, Shrub, and Vps4) and Wg (Hrs, Tsg101, and Vps28) on exosomes. Fusion of the MVB with the cell surface results in the release of ILVs in the form of exosomes: Rab27 for Hh, while for Wg, Rab11, syntaxin-1A, and Ykt-6 are thought to be involved in MVB fusion. Endocytosed Hh fails to populate tubular lysosomes, which are known for their role in the degradation of endocytic cargo; in the case of Wg, this possibility has not been probed (marked ??). Besides exosomes, Hh and Wg can also be secreted on microvesicles by plasma membrane budding. In the case of Hh, cell-surface accumulation of Hh on the expression of a dominant-negative form of Vps4 has led to the proposition of microvesicle-mediated Hh secretion; however, for Wg exovesicles this mode of secretion has not yet been proposed (marked ??). Molecular players involved in exovesicular secretion and identified in vitro and in vivo/in vivo, are highlighted in blue text; molecules identified in specific cell types or in cell-based assays only are in red text.
Although several lines of evidence suggest that Hh exovesicles are derived from endocytic processing, an alternative possibility of secretion via microvesicles has also been proposed [38]. Evidence for the role of exosomes may be summarized as follows. (i) In S2 cells, Hh molecules are endocytosed into Rab7+Lamp+ endosomes by producing cells [32] and sorted onto ILVs (immune-electron microscope (EM)-based analysis; [31,32]) suggesting that they traffic to specific MVBs. This endocytosed Hh appears to be actively sorted away from degradation as it does not traffic towards tubular lysosomes [32], where degradation of endocytosed cargo occurs [58], nor is its trafficking affected by loss of function of a key lysosomal regulator, Deep orange, in wing discs [42,59]. Sorting of Hh-GFP as well as of endogenous Hh into the MVBs of Hh-producing cells has also been demonstrated in wing discs [31,42]. (ii) In cell lines, perturbing endocytosis by downregulation of Rab5, a regulator of early endocytosis, as well as Shibire, a Drosophila dynamin, and of Rab27, a regulator of MVB fusion with the PM, quantitatively affects the secretion of Hh on exovesicles [30,32]. Similarly, in wing discs perturbation of Rab5 function in Hh-producing cells results in intracellular accumulation of endogenous Hh, and reduced Hh signaling range [42,59]. Furthermore, a mutant of Hh (HhK132D) that is defective in oligomerization and long-range signaling [60] was defective in endocytosis and exovesicular secretion [32]. Last, overexpression of Dispatched (Disp), a regulator of Hh secretion, caused it to localize along with Hh on endosomes in the posterior domain of wing discs [42] and mediate endocytosis of Hh [59].
An alternative model suggests that Disp-dependent endocytosis of Hh followed by Rab4-dependent recycling is necessary for a secondary secretion event [59], which could be Hh release on microvesicles via PM budding. This idea is supported by the surface accumulation of Hh on the expression of a dominant-negative form of the key player involved in scission of the vesicles in wing discs, Vps4 [26] (Figure 2). In separate studies RNAi-mediated down-regulation of Vps4 as well as an ESCRT-III (Shrub) not only affected exovesicular secretion of Hh but also resulted in its intracellular accumulation in Hrs+ and Rab7+ endosomes in wing discs [32]. This suggests a MVB-dependent route for exosomal secretion of Hh. This observed discrepancy may be attributed to a difference in how the ESCRT machinery was perturbed (compare [26] and [32] for details). Experiments to track cell-surface Hh colocalization with various players, such as endogenously tagged Rab proteins [61], will be necessary to further dissect and understand the cellular events and molecular players involved in Hh secretion.
The need for such a complicated process for Hh release following endocytosis remains to be uncovered. A potential clue comes from studies with a Hh isoform that is incapable of forming higher-order oligomers (HhK132D) [60]. This isoform is also incapable of being efficiently packaged into exovesicles due to its poor endocytic capacity [32]. It is conceivable that endocytosis may be necessary to ensure that Hh molecules are organized and packaged with correct binding partners to generate exovesicles with efficient signaling and cell-fate determination capabilities [30,52].
Wnts and Exovesicles
Wg was the first morphogen found to be associated with and transported on extracellular vesicles that are similar to exosomes, termed argosomes [62]. However, later it was suggested that argosomes could be circulating lipoprotein particles (Lpps) on which Wg, Hh, and GPI-anchored proteins can be loaded [34]. One of the first pieces of evidence of exosomal release of Wg was obtained from studies of the Drosophila neuromuscular junction (NMJ). Evi, an eight-pass transmembrane protein necessary for Wg secretion, was found to be important for trans-synaptic transfer of Wg at the NMJ [63]. Evi was secreted on exosome-like vesicles at the NMJ and from cultured S2 cells that required Rab11 and syntaxin-1A [50]. Subsequently, cell-based assays showed the presence of Wg on exosome-like vesicles, secretion of which also required Rab11 function [28,29]. In wing discs, however, RNAi-mediated depletion of Rab11 failed to perturb the Wg gradient or target gene activation [29]. These results suggested that, unlike at the NMJ and in cell lines, Rab11 does not regulate the secretion of Wg on Evi exosomes in wing discs [29].
Evi and Wg independently colocalize with CD63-GFP (a marker of exosomes) in wing discs and cofractionate in biochemical assays; however, colocalization between Evi and Wg is extremely low, suggesting that they are not secreted on the same exosomes [28,29]. It is likely that Wg exosomes produced in wing discs and at the NMJ are distinct. Alternatively, Evi might be involved in the trafficking of Wg from the Golgi to the PM [64]. In both fly and mammalian cell lines, Wg localizes in internal compartments containing ESCRT-0 and -I [28,29] and knockdown of ESCRT-0 components [hepatocyte growth factor-regulated tyrosine kinase substrate (HGS)] affects exosomal Wg secretion [28]. Secretion of Wg exosomes proceeds via a MVB-dependent pathway since blocking of endosomal acidification (using NH4Cl or bafilomycin A1) and preventing its maturation affects the proper sorting of Wg into ILVs and blocks its secretion in both cell lines and wing discs [28]. In addition, the soluble NSF attachment protein receptor (SNARE) protein Ykt6 affected secretion of Wg, CD63, and CD81 – known exosomal markers in wing discs [28]. Wg-producing cells expressing Ykt6 RNAi in wing discs also resulted in intracellular trapping of Wg, reduction of its extracellular levels, and alterations in its spreading [28]. Thus, it appears that different molecular regulators govern exosomal release of Wg at the larval NMJ, in cell lines, and in wing discs (Figure 2).
Soluble Forms of Hh
The release of soluble forms of vertebrate Hh molecules via disintegrin and MMP domain protein (ADAM)-17 activity suggests that ADAM-17 is involved in the generation of a lipid-free, soluble, and biologically active pool of Hh proteins from cell lines [40]. ADAM-17 not only removes cholesterol and palmitate modifications but also cleaves the CW domain from the N terminus of Hh [65]. While the MMP-cleaved lipid-free Shh is signaling competent in a cell-based assay – namely, C3H10T1/2 osteoblast precursor cell differentiation and chick chondrocyte differentiation assay – it is unproven whether this form of Shh also exists under physiologically relevant conditions and contributes to tissue patterning and maintenance. However, it is tempting to speculate that this form of Hh might be unable to travel long distances in a directed fashion since it lacks the CW domain required for interaction with HSPGs and long-range transport. Soluble Hh molecules are also found in vivo in Drosophila hemolymph and in wing discs [66]. However, this is a non-sterol-modified but palmitoylated, lipoprotein-free form called HhN* (sterol-free form). Based on relative differences in the electrophoretic mobility of these forms, it has been speculated that HhN* might be distinct from the MMP-cleaved lipid-free-form [66]. Secretion of HhN* in the hemolymph was facilitated by expression of Hh in the fat body and simultaneous suppression of lipoprotein production. HhN* is monomeric/dimeric and signaling competent [66]. A soluble form of Hh is also detected when Hh is expressed in cell lines grown in the presence of serum factors [32]. How HhN* is generated remains unknown and its mechanism would clarify how many types of signaling-competent, soluble forms of Hh exist.
Hh proteins have also been secreted as multimeric complexes from Drosophila salivary glands, a Drosophila cell line (Cl8 cells), and vertebrate cell lines (CHO, STO, HeLa, HEK293T) [67–70]. Genetic evidence suggests that multimeric forms of Hh could represent complexes bearing different ratios of HSPG and Hh molecules [71]. However, the nature and origin of these complexes remain elusive.
Lipid-less forms of Wg/Wnt proteins have not been identified yet; however, proteins such as Swim, a Lipocalin family of extracellular transport proteins, and Afamin, a serum glycoprotein (a-albumin), are known to bind lipidated Wnt and generate soluble and active forms of Wg/Wnt proteins [45,46].
Lipoproteins as Morphogen Carriers
Hh and Wg are found on Lpps. The presence of Hh on Lpps and their role in ferrying Hh molecules in Drosophila wing discs has been explored [34,66]. Unlike the role of Disp, Lpp-mediated Hh release from producing cells does not require its cholesterol anchor; GPI-anchored GFP as well as Wg is transported on Lpps to a similar extent [34]. Using biochemical characterization and immunostaining studies of Drosophila hemolymph as well as mammalian-cell-based assays, it has been confirmed that a subset of secreted Hh and Shh is associated with lipoproteins.
When Hh is overexpressed in fat bodies (tissue that generates Lpps), it is efficiently released on Lpps but is only partly functional. Overexpression of Hh resulted in expansion of the Hh-protein gradient and stabilization of the full-length cubitus interruptus (Ci) across wing discs but failed to trigger target gene activation [66]. Lpp Hh competes with endogenous Hh produced locally by the wing discs, resulting in repression of transcriptional targets of Hh signaling (i.e., Col [66]). These results suggest that Lpp Hh in circulation is incapable of eliciting a complete Hh signaling response. However, under physiological conditions Lpp Hh, present in hemolymph, is mainly derived from enterocytes present in the midgut [72]. Hh secretion in the hemolymph is dependent on the nutritional status of the cells and is upregulated on starvation. Larval growth rate and timing of pupariation are controlled by the levels of circulating Hh [72]. Circulating Hh signals to the fat body cells to control larval development and to the cells of the prothoracic gland to regulate pupariation. Here, high levels of Hh inhibit the production of ecdysone and makisterone A, delaying pupariation. While this study ascribes a novel function to Lpp Hh, the precise regulation of circulating Hh and its influence on developmental stages remains unclear. Under physiological conditions Lpp Hh might play a hormonal role, and it does not seem to be involved in target gene activation for tissue patterning. Thus, Lpp Hh seems to be involved in noncanonical signaling, while sterol-free Hh (i.e., HhN*) can activate canonical signaling. Taken together these studies indicate that the different forms of Hh influence its signaling abilities.
Morphogens Associated with Cytonemes
In 1999 cytoneme-based morphogen transport was proposed for the first time [73]. Cytonemes are long, filopodium-like structures that assist in the transport of not only membraneanchored Notch, Wg, and Hh [31,35,39] but also secreted morphogens such as Decapentaplegic (Dpp), fibroblast growth factor (FGF), and epidermal growth factor (EGF) [74].
It has been demonstrated that endogenous Hh or Hh-GFP puncta organize on long, thread-like projections perpendicular to the AP boundary, resembling cytonemes [35]. Other overexpressed Hh-pathway components, such as Interference Hh (Ihog), Brother of interference hedgehog, Disp, and Dally-like protein (Dlp), also localize on these structures [35,75]. Cytonemes fail to cross mutant clones deficient in HSPG (cell-surface or extracellular matrix proteoglycans) production [35], suggesting that interaction between HSPGs and cytonemes is necessary for cytonemal extension. Interestingly, HSPGs are also necessary for long-range transport of Hh. HSPGs form visible clusters at the cell surface [60] and since Dlp decorates cytonemes, it is possible that homotypic interactions between HSPGs on the cytonemes and those in the extracellular matrix could determine the stability, extension, and localization of cytonemes.
Cytoneme-based transport of Hh molecules is likely to be conserved and has also been implicated in Shh transport in chick embryos [36]. Thus, cytonemes and HSPGs might work together to transport morphogens across cell layers. CD63-GFP (an exosomal marker) [31] and Ihog-CFP (Hh coreceptor) [35] were visualized on cytonemes as punctate structures; Ihog-CFP in wing discs was also identified on cytoplasmic protrusions and on exovesicles in the extracellular space in an ultrastructural study [35]. Thus, exosomes might use cytonemes as tracks to travel across tissue. Cytonemes have also been demonstrated in the transport of Wg/Wnt molecules across developing tissues [39,76]. In zebrafish live embryos as well as in fibroblast cells, overexpressed Wnt-8a-GFP was observed on cytonemes. Variations in filopodial length and frequency could influence the distribution of Wnt-8a and neural plate patterning [76]. In Drosophila, flight muscle progenitors (myoblasts) extend cytonemes containing Frizzled (Wg receptor) directly to Wg-producing cells in the wing disc [39]. This cytoneme-mediated Wg signaling in myoblasts can indirectly influence air sac primordium patterning [39].
Most studies pertaining to cytoneme visualization and correlation with the presence of morphogens and their receptors or coreceptors on these membrane extensions are performed under overexpression conditions. Hence, it remains to be seen whether this is the preferred mode of morphogen transport under physiological conditions.
Distinct Functions for Different Carriers?
The lipid-anchored nature of some forms of Hh and Wg raised the question of how these proteins signal over long distances and what carriers they may use. Hh and Wg signaling employ similar carriers for their transport across cell boundaries, such as Lpps, flotillin-based carriers, cytonemes, and exosomes. Are specific extracellular forms involved in short- versus long-range delivery of morphogens? There appears to be no correlation between signaling range and type of carrier in the available literature. For example, it has been observed that downregulation of Ykt6 (V-SNARE) affects exosomal secretion of Wg and reduces expression of Senseless (Sens), a short-range signaling target, suggesting that exosomes are important for the mediation of short-range Wg signaling [28]. By contrast, loss of flotillin, which reduces Wg carriers (identified to be distinct from exosomes based on their density on a sucrose gradient), specifically affect the expression range of Distalless (Dll) (a long-range target) without affecting the expression of Sens [44], suggesting that there may be at least two types of carrier with different functional consequences.
Similarly, downregulation of Shrub (ESCRT-III) in Hh-producing cells can perturb the endogenous-Hh gradient and reduce the expression of its long-range target gene Dpp. However, no effect on the expression of short [Patched (Ptc)] or intermediate (Col) target genes was seen [26,32]. Flotillin-based carriers [44] are also involved in mediating long-range signaling of Hh without affecting its short-range target genes. Although flotillins are present in exovesicular fractions [30] and are independently known to regulate exovesicular secretion [77], their role in regulating Hh and Wg release is unclear. It remains to be seen whether Flotillin-based carriers regulate Wg and/or Hh exosome production, influence the composition of exosomes and hence their range/functional abilities, or represent a distinct form. Studies appear to highlight an important difference between the roles of Wg and Hh exosomes; unlike Wg, exosomal Hh is involved in long-range signaling but not for short or intermediate target gene activation (Figure 3).
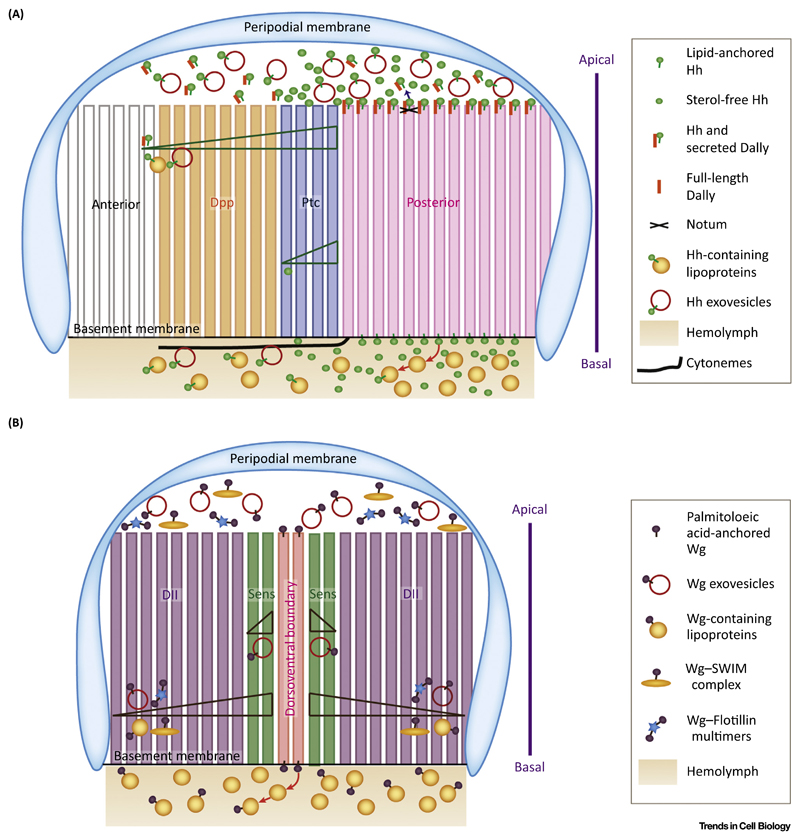
Figure 3. Transport of Morphogens Across Tissue: Multiplicity of Carriers.
(A) Cartoon representing the various extracellular forms of Hedgehog (Hh) and their polarized transport. Hh secretion and transport are seen on both the apical and the basolateral side of the Drosophila wing imaginal disc. On the apical side, Hh is secreted on exovesicles in the lumen, which is necessary for its long-range transport. Additionally, a carrier involving Hh and Dally (cleaved by Notum) is also secreted in the apical lumen and is necessary for long-range signaling by Hh. Basally, Hh-containing exovesicles are seen predominantly on cytonemes, which are filopodial structures. The hemolymph is in contact with the wing imaginal disc on the basal side. Hemolymph contains circulating lipoprotein particles. Hh interacts with lipoproteins, which in turn act as carriers for long-range transport. The identity of a short-range carrier involved in Hh signaling remains elusive and we speculate that the soluble forms of Hh could be crucial for transporting Hh at short distances to activate high-threshold targets like Patched (Ptc). (B) Cartoon representing the various extracellular forms of Wingless (Wg). Wg is also ferried by multiple carriers to facilitate its long-range spread. Secreted complexes of Wg with Flotillin and Swim are necessary for its long-range transport. Wg is also transported basolaterally on lipoprotein particles, which help it to spread over long distances. Exovesicles carrying Wg are necessary for its short- as well as long-range transport, thus differentiating them from exovesicles, which are uniquely employed only for long-range Hh spread.
Besides exosomes, Lpps mediate long-range signaling in wing discs. Ubiquitous expression of Lpp RNAi resulted in extracellular accumulation close to the source, suggesting that Lpp-based carriers could mediate long-range Wg and Hh transport. While the range of Dpp was reduced on Lpp RNAi, expression of Patched and Collier, which are short- and intermediate-range targets of Hh signaling, remained unaffected. Similarly, Dll expression was affected on Lpp RNAi but its effects on Sens expression were not tested [34].
Perturbations of proteins involved in the regulation of actin structure and dynamics, such as Capping protein, SCAR, and Pico affects the length of cytonemes that emanate from Hh-producing cells as well as the long-range spread of Hh and target gene activation [35]. Thus, to date, the forms of Hh that mediate its short-range signaling have not been identified. As observed for Wg, it is possible that exosomes could also be employed for signaling locally. The inability to identify the players involved in short-range Hh signaling might be due to inefficient depletion of the molecular players involved and/or compensation by other molecular players or extracellular forms.
Alternatively, soluble forms of Hh may facilitate short-range Hh signaling, because the MMP-derived lipid-free form of Hh has not been identified in vivo and the mechanism of generation of HhN* has not been identified. The signaling ability and range of endogenous soluble forms of Hh remain to be evaluated. Thus, while various forms of Hh have been identified and significant work has been done to evaluate their roles, it is not entirely clear whether these have differential signaling abilities or ranges. Several functional aspects of these different forms remain unclear (see Outstanding Questions).
Outstanding Questions.
Do different carriers of Hh and Wg represent tissue-specific mechanisms for their release and/or do they have different targets?
Do the accompanying constituents of exosomes that carry morphogens influence their signaling range or efficiency? Are the different morphogens involved in selecting at least some of these partners?
Do the extracellular forms and/or partners of morphogens change under normal versus disease conditions?
Compositional Heterogeneity of Hh and Wg Carriers
Exosomes also carry other functional signaling proteins and regulatory RNA molecules. Whether the exosomal form of morphogen release is also involved in the targeted delivery of other signaling molecules for synergistic signaling and cell-fate determination remains an exciting possibility to probe [52]. A recent study demonstrates that, besides target gene activation, interaction of Hh with Boc is also required for the activation of JNK and ABL kinases to facilitate neuronal differentiation and neurite growth [78]. Here it remains to be seen whether a specific form of extracellular Hh is involved in the process. It was demonstrated recently that Smo activation requires membrane cholesterol [79,80]. The cholesterol-sensing cysteine-rich domain of Smo is present extracellularly [81]; could this domain be involved in sensing the lipids in Hh carriers as well?
Disease Implications of the Various Forms of Hh and Wg
Hh and Wnt signaling is activated in several cancers [19]. Some Hh/Wnt ligand-dependent cancers exhibit increased autocrine, paracrine (tumor cells to stroma), or reverse paracrine (stroma to tumor cells) signaling abilities [82,83]. Hh and Wnt signaling are implicated in tumor progression and metastasis, relapse, and drug resistance. The focus so far has been on identifying the signaling pathways responsible for cancer progression as well as on evaluating the efficacy of various signaling inhibitors as druggable targets [82–85]. However, despite the encouraging preclinical data [82–85], it remains unclear whether a signaling inhibitor drug can be made efficacious with acceptable toxicity and without compromising the signaling’s role during tissue homeostasis and repair. The identification of extracellular forms and/or partners of Hh and Wg that are important for disease progression will be extremely valuable not only in improving our understanding of morphogens in cancer but also in identifying effective drug targets.
Concluding Remarks
Morphogens are reported to be secreted in more than one form. There is compelling evidence supporting the release of Hh and Wg on exosomes [26,28,29,31,32] and lipoproteins [33,34,66]. However, given that Hh can also be secreted in a soluble/sterol-free signaling-competent form, it remains to be resolved whether exosomes or lipoproteins have roles other than activating Hh target genes. Exosomes contain lipids as well as many different proteins and cellular RNAs [30,86]. Do these constituents contribute to the cell-fate determination of target cells? Like Hh and Wg proteins, even secreted morphogens such as vascular endothelial growth factor (VEGF), FGF, EGF, and Dpp are present on exosomes [87] and/or are ferried via cytonemes [74]. Do these different morphogen carriers provide unique abilities to these morphogens? While it is tempting to hypothesize that different secreted forms of morphogens perform non-overlapping roles, compelling evidence for this hypothesis requires definition of the proficiency of the various forms of extracellular morphogens and their precise mechanisms of formation.
Trends.
The membrane-anchored morphogens Hedgehog (Hh) and Wingless (Wg) play major roles in tissue patterning, tissue maintenance, and disease progression.
Hh and Wg must be secreted for their paracrine signaling function. In addition to being secreted on exovesicles, Hh and Wg are found on circulating lipoproteins and decorating cytonemes as well as in soluble forms.
The mechanism of Hh exovesicle generation involves its endocytosis from the cell surface regulated by Shibire (Drosophila dynamin) and Rab5-mediated endosomal progression, followed by sorting to intraluminal vesicles (ILVs) located in multivesicular bodies (MVBs) in an endosomal sorting complex required for transport (ESCRT)-dependent manner and subsequent release on exovesicles.
In addition to the morphogens, exovesicles carry other targeting and regulatory molecules including cellular RNAs. Besides activating their direct downstream signaling targets, exosomes can deliver cargo that may influence the cell-fate decisions ascribed to these morphogens.
Acknowledgments
The authors apologize to all colleagues whose work they could not include due to space constraints. They acknowledge Lee Fradkin for his critical feedback on the manuscript. This work was supported by an Early Career Fellowship from the Wellcome Trust DBT India Alliance to N.V., support to N.V. from a DST-ECR award grant (grant no. ECR/2016/000251), core support from the St John’s Research Institute, and a J.C. Bose Fellowship and a Wellcome Trust DBT-Alliance Margadarshi Fellowship (IA/M/15/1/502018) (S.M.).
References
- 1.Turing AM. The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 3.Teleman AA, et al. Shaping morphogen gradients. Cell. 2001;105:559–562. doi: 10.1016/s0092-8674(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 4.Christian JL. Morphogen gradients in development: from form to function. Wiley Interdiscip Rev Dev Biol. 2012;1:3–15. doi: 10.1002/wdev.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 6.Ingham PW, et al. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 7.Torroja C, et al. Mechanisms of Hedgehog gradient formation and interpretation. J Neurobiol. 2005;64:334–356. doi: 10.1002/neu.20168. [DOI] [PubMed] [Google Scholar]
- 8.Swarup S, Verheyen EM. Wnt/Wingless signaling in Drosophila. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007930. a007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briscoe J, Small S. Morphogen rules: design principles of gradient-mediated embryo patterning. Development. 2015;142:3996–4009. doi: 10.1242/dev.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuniga A. Next generation limb development and evolution: old questions, new perspectives. Development. 2015;142:3810–3820. doi: 10.1242/dev.125757. [DOI] [PubMed] [Google Scholar]
- 11.Kurosaka H, et al. Disrupting Hedgehog and WNT signaling interactions promotes cleft lip pathogenesis. J Clin Invest. 2014;124:1660–1671. doi: 10.1172/JCI72688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu D, Helms JA. The role of Sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- 13.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lien W-H, Fuchs E. Wnt some lose some: transcriptional governance of stem cells by Wnt/β-catenin signaling. Genes Dev. 2014;28:1517–1532. doi: 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445–3457. doi: 10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrasekaran A, et al. Astrocyte differentiation of human pluripotent stem cells: new tools for neurological disorder research. Front Cell Neurosci. 2016;10:215. doi: 10.3389/fncel.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teperino R, et al. Canonical and non-canonical Hedgehog signalling and the control of metabolism. Semin Cell Dev Biol. 2014;33:81–92. doi: 10.1016/j.semcdb.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beachy PA, et al. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 20.Pepinsky RB, et al. Identification of a palmitic acidmodified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 21.Porter JA, et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21–34. doi: 10.1016/s0092-8674(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 22.Porter JA, et al. The product of Hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- 23.Janda CY, et al. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 26.Matusek T, et al. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014;516:99–103. doi: 10.1038/nature13847. [DOI] [PubMed] [Google Scholar]
- 27.Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross JC, et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 29.Beckett K, et al. Drosophila S2 cells secrete Wingless on exosome-like vesicles but the Wingless gradient forms independently of exosomes. Traffic. 2013;14:82–96. doi: 10.1111/tra.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vyas N, et al. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep. 2014;4 doi: 10.1038/srep07357. 7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gradilla A-C, et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun. 2014;5 doi: 10.1038/ncomms6649. 5649. [DOI] [PubMed] [Google Scholar]
- 32.Parchure A, et al. Oligomerization and endocytosis of Hedgehog is necessary for its efficient exovesicular secretion. Mol Biol Cell. 2015;26:4700–4717. doi: 10.1091/mbc.E15-09-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann S, et al. Mammalian Wnt3a is released on lipoprotein particles. Traffic. 2009;10:334–343. doi: 10.1111/j.1600-0854.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 34.Panakova D, et al. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff M, et al. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15:1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders TA, et al. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanganello E, et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;6 doi: 10.1038/ncomms6846. 5846. [DOI] [PubMed] [Google Scholar]
- 38.Sagar, et al. Communication between distant epithelial cells by filopodia-like protrusions during embryonic development. Development. 2015;142:665–671. doi: 10.1242/dev.115964. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Kornberg TB. Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta–Notch signaling to the air sac primordium. Elife. 2015;4:e06114. doi: 10.7554/eLife.06114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dierker T, et al. Heparan sulfate-modulated, metalloprotease-mediated Sonic hedgehog release from producing cells. J Biol Chem. 2009;284:8013–8022. doi: 10.1074/jbc.M806838200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ching W, et al. Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callejo A, et al. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci U S A. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tukachinsky H, et al. Dispatched and Scube mediate the efficient secretion of the cholesterol-modified Hedgehog ligand. Cell Rep. 2012;2:308–320. doi: 10.1016/j.celrep.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katanaev VL, et al. Reggie-1/Flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J. 2008;27:509–521. doi: 10.1038/sj.emboj.7601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulligan KA, et al. Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility. Proc Natl Acad Sci U S A. 2012;109:370–377. doi: 10.1073/pnas.1119197109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mihara E, et al. Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein Afamin/α-albumin. Elife. 2016;5:e11621. doi: 10.7554/eLife.11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 48.Colombo M, et al. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 49.Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol. 2011;23:452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koles K, et al. Mechanism of Evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 52.Vyas N, Dhawan J. Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries. Cell Mol Life Sci. 2017;74:1567–1576. doi: 10.1007/s00018-016-2413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liegeois S, et al. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol. 2006;173:949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka Y, et al. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 55.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 56.Henne WM, et al. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a016766. a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34:2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sriram V, et al. Deep-orange and Carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J Cell Biol. 2003;161:593–607. doi: 10.1083/jcb.200210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Angelo G, et al. Endocytosis of Hedgehog through dispatched regulates long-range signaling. Dev Cell. 2015;32:290–303. doi: 10.1016/j.devcel.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Vyas N, et al. Nanoscale organization of Hedgehog is essential for long-range signaling. Cell. 2008;133:1214–1227. doi: 10.1016/j.cell.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 61.Dunst S, et al. Endogenously tagged rab proteins: a resource to study membrane trafficking in Drosophila. Dev Cell. 2015;33:351–365. doi: 10.1016/j.devcel.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greco V, et al. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- 63.Bartscherer K, et al. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Port F, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 65.Ohlig S, et al. An emerging role of Sonic hedgehog shedding as a modulator of heparan sulfate interactions. J Biol Chem. 2012;287:43708–43719. doi: 10.1074/jbc.M112.356667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palm W, et al. Secretion and signaling activities of lipoprotein-associated Hedgehog and non-sterol-modified Hedgehog in flies and mammals. PLoS Biol. 2013;11:e1001505. doi: 10.1371/journal.pbio.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gallet A, et al. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development. 2006;133:407–418. doi: 10.1242/dev.02212. [DOI] [PubMed] [Google Scholar]
- 68.Callejo A, et al. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development. 2006;133:471–483. doi: 10.1242/dev.02217. [DOI] [PubMed] [Google Scholar]
- 69.Chen MH, et al. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18:641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng X, et al. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 71.Ayers KL, et al. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev Cell. 2010;18:605–620. doi: 10.1016/j.devcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Rodenfels J, et al. Production of systemically circulating Hedgehog by the intestine couples nutrition to growth and development. Genes Dev. 2014;28:2636–2651. doi: 10.1101/gad.249763.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 74.Roy S, et al. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bilioni A, et al. Balancing Hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and Shifted/DmWif. Dev Biol. 2013;376:198–212. doi: 10.1016/j.ydbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 76.Stanganello E, et al. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;6 doi: 10.1038/ncomms6846. 5846. [DOI] [PubMed] [Google Scholar]
- 77.Phuyal S, et al. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 78.Vuong TA, et al. A Sonic hedgehog coreceptor, BOC regulates neuronal differentiation and neurite outgrowth via interaction with ABL and JNK activation. Cell Signal. 2017;30:30–40. doi: 10.1016/j.cellsig.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Luchetti G, et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote morphogenetic signaling. Elife. 2016;5:e20304. doi: 10.7554/eLife.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang P, et al. Cellular cholesterol directly activates Smoothened in Hedgehog signaling. Cell. 2016;166:1176–1187.e14. doi: 10.1016/j.cell.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Byrne EF, et al. Structural basis of Smoothened regulation by its extracellular domains. Nature. 2016;535:517–522. doi: 10.1038/nature18934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta S, et al. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237–250. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blagodatski A, et al. Targeting the Wnt pathways for therapies. Mol Cell Ther. 2014;2:28. doi: 10.1186/2052-8426-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 86.Budnik V, et al. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schiera G, et al. Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J Cell Mol Med. 2007;11:1384–1394. doi: 10.1111/j.1582-4934.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raiborg C, et al. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci. 2001;114:2255–2263. doi: 10.1242/jcs.114.12.2255. [DOI] [PubMed] [Google Scholar]
- 89.Raiborg C, et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 90.Mizuno E, et al. STAM proteins bind ubiquitinated proteins on the early endosome via the VHS domain and ubiquitin-interacting motif. Mol Biol Cell. 2003;14:3675–3689. doi: 10.1091/mbc.E02-12-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hanson PI, et al. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adell M, Alonso Y, Teis D. Assembly and disassembly of the ESCRT-III membrane scission complex. FEBS Lett. 2011;585:3191–3196. doi: 10.1016/j.febslet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in wonderland. Trends Cell Biol. 2014;24:19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 95.Chairoungdua A, et al. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Niel G, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 98.Perez-Hernandez D, et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roucourt B, et al. Heparanase activates the syndecan–syntenin–ALIX exosome pathway. Cell Res. 2015;25:412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baietti MF, et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]