Abstract
Purpose
Streptococcus suis (S. suis) is an emerging zoonotic disease mainly in pigs, causing serious infections in humans with high prevalence in Southeast Asia. Despite a relatively high mortality rate, there are limited data regarding the risk factors of this life-threatening infection. Therefore, a 13-year retrospective cohort study in Chiang Mai, Thailand during 2005–2018 was conducted to explore risk factors associated with S. suis mortality and to update the outcomes of the disease.
Patients and methods
S. suis positive cases were derived from those with positive S. suis isolates from microbiological culture results and Matrix-Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF). Potential risk factors of mortality were identified using univariate and multivariate logistic regression.
Results
Of 133 patients with culture-proven S. suis infection identified, there were 92 males and 41 females. The mean age was 56.47 years. Septicemia (55.64%) was the most common clinical manifestation followed by meningitis (37.59%) and infective endocarditis (25.56%). Alcohol drinking and raw pork consumption were documented in 66 (49.62%) and 49 (36.84%) cases respectively. The overall mortality rate was 12.03% (n=16). According to the multivariate analysis, the independent risk factors for mortality were prolonged bacteremia ≥ 6 days (OR = 43.57, 95% CI = 2.46–772.80, P =0.010), septic shock (OR = 13.34, 95% CI = 1.63–109.03, P =0.016), and direct bilirubin > 1.5 mg/dL (OR = 12.86, 95% CI = 1.91–86.59, P =0.009).
Conclusion
S. suis is not infrequent in Northern Thailand, where the cultural food habit of raw pork eating is still practiced. To the best of our knowledge, this is the largest series focusing on risk factors of S. suis mortality which has been conducted in Thailand. Prolonged bacteremia ≥ 6 days, septic shock, and direct bilirubin > 1.5 mg/dL were strong predictors associated with S. suis mortality. The mortality risk factors identified may be further utilized in clinical practice and future research to improve patient outcomes.
Keywords: Streptococcus suis, S. suis, risk factor, S. suis infection, mortality, Thailand
Introduction
Streptococcus suis (S. suis) is a gram positive diplococci alpha-hemolytic bacteria mainly found in pigs. The pathogen has been classified into 29 serotypes1 of which serotype 2 is the most prominent cause of infection in humans2 contributing to meningitis, septicemia, infective endocarditis and other serious complications. The disease is considered to be an occupational hazard via percutaneous exposure in Western countries3,4 whereas oral route transmission through ingestion of raw/undercooked pork including fresh pig’s blood, intestines, and other internal organs have been noted in the Asia region.5–8 A recent systematic review and meta-analysis demonstrated the main risk factors in acquiring S. suis infection including eating raw/undercooked pork, exposure to pigs or raw pork, male sex, and pig-related occupation.9
There is a high prevalence rate in Southeast Asia, particularly in Thailand and Vietnam.10 In Thailand, S. suis infection is an important health issue and one of the most common causes of bacterial meningitis with a high prevalence rate in the northern region due to cultural food habits involving raw pork consumption. To date, much uncertainty remains regarding the understanding of S. suis clinical manifestations and treatment responses as well as gaps between different geographical settings. The disease epidemiology in Thailand is not fully understood and epidemiological study to identify predicting factors for mortality is still limited. Although there have been a few studies on S. suis mortality in this region,11,12 their statistical significances could not be confirmed by multivariate analyses.
The highest number of investigational reports of S. suis infection in Thailand was from Chiang Mai,13 the provincial capital and the largest province in northern Thailand. Since the previous two-year series of 41 S. suis patients conducted in 2000 at Chiang Mai University Hospital (CMUH),11 there has not been any further study on this life-threatening infection in this setting. Here, we conducted a single-center retrospective cohort study during a 13-year period to determine potential risk factors of S. suis mortality and to update the evidence concerning clinical manifestations, outcomes, and treatment of the disease.
Materials and Methods
Study Design and Setting
A retrospective review, during a 13-year period, of records of S. suis infected patients admitted at CMUH, a 1400-bed tertiary teaching hospital from May 2005 to December 2018, was conducted. CMUH is the fourth largest hospital in the country and the largest hospital in northern Thailand where most of cases in northern region from 17 provinces are referred to for tertiary care (Table S1).
Study Participants
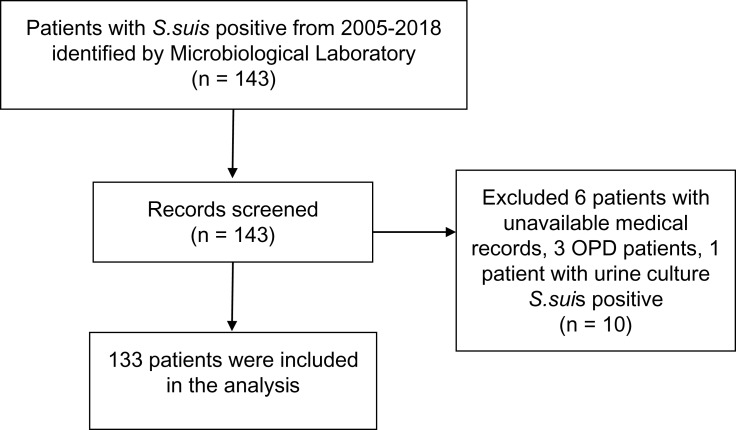
S. suis positive cases were identified from the hospital microbiology laboratory data which provided the list of patients with positive S. suis culture and their hospital numbers (HNs). S. suis isolates were cultured on sheep blood agar at 37°C and were subsequently confirmed by biochemical method (Figure S1) and Matrix-Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) Mass Spectrometry. All confirmed S. suis cases with S. suis positive culture either from blood or cerebrospinal fluid (CSF) and compatible clinical presentations admitted at CMUH with available medical records were collected and reviewed through the hospital databases (Figure 1).
Figure 1.
Patients' identification and selection.
Abbreviation: OPD, Outpatient department.
Clinical Data and Definitions
S. suis case was defined as those with S. suis positive culture from sterile site (CSF or hemoculture) with compatible clinical presentations. Time to microbiological cure is an objective measure which was defined as the duration from the date with positive cultures obtained until the date of negative cultures from the same site of S. suis isolate. In case the repeated culture was not done to show microbiological success, the date of discharge or resolution of clinical symptoms or discontinuation of antibiotics was used. The same principle was applied for dead cases with available repeated culture results, otherwise this was regarded as treatment failure. E-test method was employed to identify the minimum inhibitory concentration (MIC) of penicillin and ceftriaxone.
Echocardiography was used in the assessment of endocarditis and detection of vegetation. An audiogram was used to diagnose and monitor the degree of hearing ability.
Data Collection
Study data were collected and managed using REDCap electronic data capture tools hosted at Research Electronic Data Capture, Monash University Malaysia, a secure, web-based application designed to support data capture for research studies.14 Data including patient characteristics, pigs/pork exposure, medical history, clinical presentations, outcomes, laboratory results, treatment and antimicrobial susceptibility were collected and analyzed. Any clinical discrepancy was reviewed and discussed within the team to reach the consensus.
The study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (IRB no.010/2018) and Monash University Human Research Ethics Committee (MUHREC) (Project no.12225). Patient names, personal and other traceable information that could be used to identify the person were omitted and treated as confidential in all processes of data collection and management.
Data Analysis
Statistical analysis was done by STATA 14.2 (College Station, Texas, USA). Demographic and clinical data were initially analyzed descriptively. Variables of interest included age, gender, Glasgow coma scale (GCS) score < 8,15 underlying diseases (alcoholic liver disease, valvular heart disease (VHD), diabetes mellitus (DM)), meningitis, septicemia, septic shock, infective endocarditis, receiving corticosteroids, elevated liver functions, and hypoalbuminemia were explored for their associations with S. suis mortality. Parameters' selection was based on authors’ clinical viewpoints and previous studies on S. suis mortality.11,12 The univariate logistic regression was performed for clinical characteristics and laboratory data among dead and survivors to estimate odds ratios (OR) for potential risk factors associated with S. suis mortality. A two-tail, with p-value less than 0.05 was considered statistically significant. Any variables with P < 0.05 in the univariate analysis were carried forward in multivariate logistic regression to analyze the prognostic indicators for S. suis mortality. Potential collinearity was also checked before building the predictive model.
Results
Demographics and Clinical Characteristics
One hundred and thirty-three patients with culture-proven S. suis infection were identified. There were 92 males and 41 females. All patients were from different provinces in Northern Thailand. Majority of patients were from Chiang Mai (n=93, 70%), followed by Lumphun (n=18, 13.53%), and Chiang Rai (n=4, 3%). There were three and two patients each from Lampang, Maehongson, Prae, and Phayao, Sukhothai respectively. There was one patient each from Uttradit and Phetchabun, while there were three patients whose home address information was unavailable.
The mean age was 56.47 years ranging between 9–90 years. Most patients were middle-aged men. There was only one pediatric case which was a 9-year old boy with a medical history of congenital rheumatic heart disease (RHD) who previously underwent surgery for aortic coarctation, patent ductus arteriosus (PDA) division and ventricular septal defect (VSD) closure. All cases generally had good health status (SAPS II 27.05± 13.89) and consciousness (GCS 12.65± 3.15) during admission. Alcohol drinking and raw pork consumption were documented in 66 (49.62%) and 49 (36.84%) of included patients respectively. There were five cases (3.76%) which reported recent pig or pork exposure, 3 patients (2.26%) had occupation related to pigs, and 2 patients (1.50%) had history of skin injury. The information on patient demographics and clinical characteristics were summarized in Table 1.
Table 1.
Patient Characteristics
| Characteristics | Total (n=133) |
|---|---|
| Age (years) (mean±SD) | 56.47 ± 13.68 |
| Male | 92 (69.17%) |
| Female | 41 (30.83%) |
| GCS | 12.65 ± 3.15 |
| SAPS II | 27.05 ± 13.89 |
| Relevant social behavior | |
|
49 (36.84%) |
|
5 (3.76%) |
|
3 (2.26%) |
|
2 (1.50%) |
|
66 (49.62%) |
|
24 (18.05%) |
| Underlying diseases | |
|
26 (19.55%) |
|
16 (12.03%) |
|
2 (1.50%) |
|
44 (33.08%) |
|
2 (1.50%) |
|
3 (2.26%) |
|
2 (1.50%) |
|
27 (20.30%) |
|
21 (15.79%) |
| Exposure to onset (days) (mean±SD)† | 7.67 ± 11.66 |
| Time from exposure to admission (days) (mean±SD)‡ | 13.55 ± 19.21 |
Notes: †Available data from 37 patients; ‡available data from 40 patients.
Abbreviations: GCS, Glasgow coma scale; SAPS II, The Simplified Acute Physiology Score; DM, Diabetes Mellitus; ALD, Alcoholic liver disease; HIV, Human immunodeficiency virus infection; AIDS, Acquired immune deficiency syndrome; SLE, Systemic lupus erythematosus; VHD, Valvular heart disease.
About one third of patients had underlying valvular heart disease (VHD) (n=44, 33.08%). There were 27 patients (20.30%) with medical history of spondylodiscites, 26 (19.55%) with diabetes mellitus (DM), 21 (15.79%) with systemic lupus erythematosus (SLE), and 16 (12.03%) reported alcoholic liver disease (ALD). Two patients each (1.50%) were noted to have history of cancer and splenectomy, and human immunodeficiency virus (HIV) infection. The mean duration from exposure to onset and admission were about 8 days (7.67± 11.66 days) and 14 days (13.55± 19.21 days) respectively (Table 1).
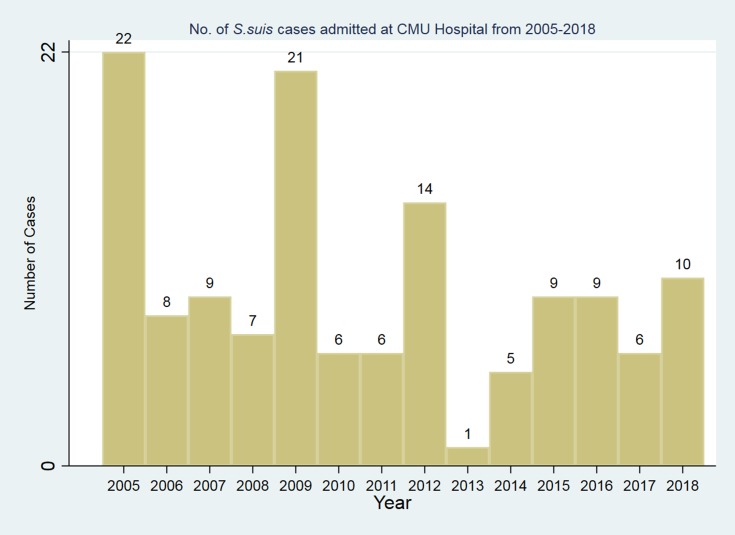
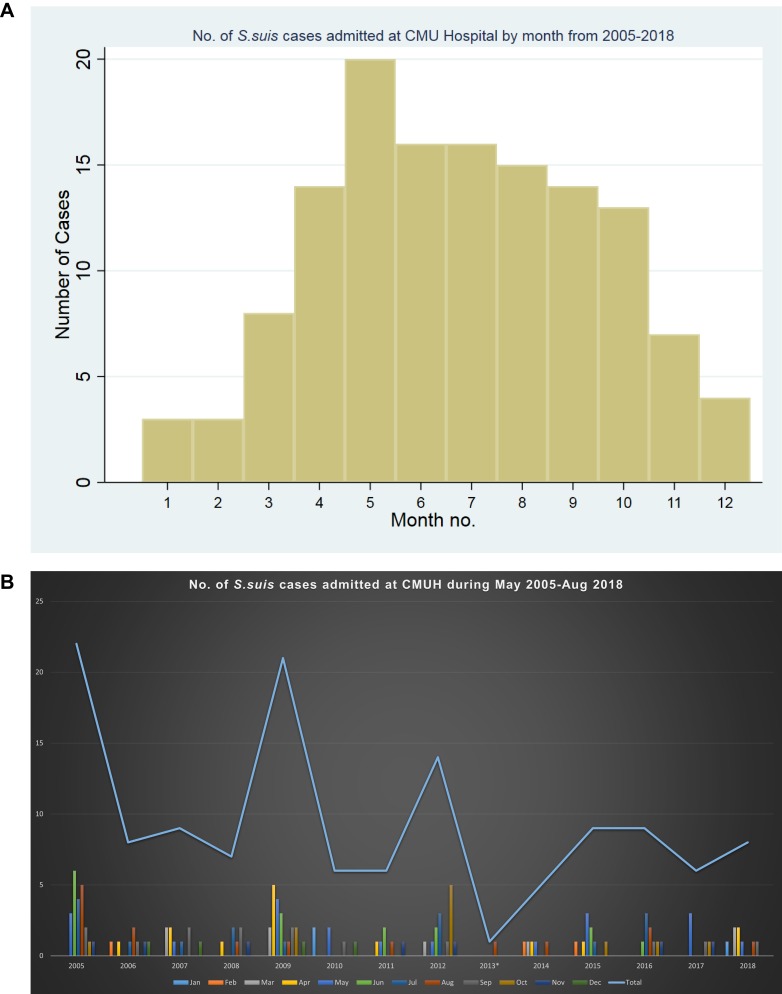
The highest number of patients admitted was in the year 2005 (n=22), 2009 (n=21), and 2012 (n=14) (Figure 2). The number of S. suis cases admitted was quite high during summer and rainy season from April to June, and in August and October respectively (Figures 3A and B).
Figure 2.
Number of S. suis cases admitted at CMUH from 2005–2018.
Figure 3.
(A and B) Number of S. suis cases admitted at CMUH by month from 2005–2018.
Clinical Manifestations and Outcomes
Septicemia (n=74, 55.64%) was the most common clinical manifestation found followed by meningitis (n=50, 37.59%) in which neck stiffness was recognized among the majority of cases. Infective endocarditis (IE) ranked third, involving 34 patients (25.56%) of which 31 had underlying VHD and 20 patients had undergone valve replacement. The most common vegetation site was aortic valve (n=26, 19.55%), followed by mitral valve (n=19, 14.29%) and tricuspid valve (n=4, 3.01%). There were 1 and 4 patients with three and two vegetation sites detected respectively (1 patient with aortic, tricuspid and mitral valve, 3 patients with aortic and mitral valve and 1 patient with tricuspid and mitral valve). Thirty-seven patients (27.82%) experienced diarrhea and twenty-seven patients (20.03%) had vomiting while only 10 patients (7.52%) presented with vertigo. Septic shock and infective spondylodiscites were recognized in 20 (15.04%) and 12 patients (9.02%) respectively whereby 8 out of 12 had underlying disease of spondylodiscites. The key clinical manifestations and outcomes were summarized in Table 2.
Table 2.
Clinical Manifestations and Outcomes
| Characteristics | Total (n=133) |
|---|---|
| Signs and symptoms | |
|
37 (27.82%) |
|
27 (20.03%) |
|
10 (7.52%) |
|
47 (35.34%) |
| Major clinical manifestation | |
|
50 (37.59%) |
|
74 (55.64%) |
|
20 (15.04%) |
|
34 (25.56%) |
| ● Aortic valve | 26 (19.55%) |
| ● Mitral valve | 19 (14.29%) |
| ● Tricuspid valve | 4 (3.01%) |
| ● Pulmonary valve | 2 (1.50%) |
| ● Aortic, mitral | 3 (2.26%) |
| ● Tricuspid, mitral | 1 (0.75%) |
| ● Aortic, tricuspid, mitral | 1 (0.75%) |
|
9 (6.77%) |
|
12 (9.02%) |
| Complications | |
|
42 (31.58%) |
|
13 (9.77%) |
|
7 (5.26%) |
|
4 (3.01%) |
|
12 (9.02%) |
|
20 (15.04%) |
|
30 (22.56%) |
|
2 (1.50%) |
| Outcomes | |
|
70 (52.73%) |
|
44 (33.08%) |
|
1 (0.75%) |
|
16 (12.03%) |
| Mean duration of admission (days) | 18.18 ± 17.09 |
| Main antibiotic regimen use | |
|
47 (36%) |
|
49 (37%) |
|
10 (7%) |
|
5 (4%) |
|
21 (16%) |
| Received dexamethasone | 27 (20.30%) |
|
8.82 ± 14.04 |
Note: Treatment and outcomes were unavailable in 1 patient.
Abbreviations: IE, Infective endocarditis; SNHL, Sensorineural hearing loss; PGS, Penicillin G, Others include vancomycin in combination with gentamicin and levofloxacin, cefotaxime in combination with vancomycin or metronidazole, ciprofloxacin, clindamycin, and doxycycline.
The overall case fatality rate was 12.03% (n=16). The main causes of death were septic shock (9/16, 56.25%) and metabolic acidosis (7/16, 43.75%). Of 16 deaths, 7 developed metabolic acidosis in which 2 had multi-organ failure and 1 patient each had HIV positive, and hepatic encephalopathy. Of 5 patient deaths with underlying alcoholic liver disease; 1 developed liver cirrhosis with diffused hematoma and suspected portal vein thrombosis; 2 had hepatic encephalopathy; 1 had underlying coronary artery disease from steroid abuse and developed chronic subdural hematoma septicemia and end-stage renal disease (ESRD); 1 had medical history of hepatocellular carcinoma (HCC) and spondylodiscites. There were 2 HIV infected patients. The first one was a 52-year old man who died within a day of admission from severe metabolic acidosis. The other one was a 25-year old female who was admitted with diarrhea and shock. She later presented with septicemia, pharyngeal candidiasis, herpes zoster infection at right side of face and expired due to acute renal failure and electrolyte imbalance. One patient died from rupture of infected abdominal aortic aneurysm, and cardiac arrest. One patient with history of post ST-Elevation Myocardial Infarction (STEMI) had infective endocarditis and died from pulmonary edema with respiratory failure. Among 2 patient deaths with no relevant medical history nor risk factors; one patient was a carpenter and admitted with shortness of breath in left side of chest, then developed massive hemoptysis and died shortly on day 3; the other presented with septicemia and mottled skin.
Approximately half of patients, including the 9-year-old boy with congenital rheumatic heart disease, recovered from infection (52.73%), whereas 44 patients (33.08%) had sequelae. Hearing loss was the most prominent complication found followed by vestibular dysfunction. Out of 42 (31.58%) affected with hearing loss, 13 had mild sensory neural hearing loss (SNHL); 7 had moderate SNHL; 4 had severe SNHL; 12 had profound SNHL in which one patient also developed Bell’s palsy while the others had no information specified concerning the degree of hearing impairment. There were 30 (22.56%) and 2 (1.5%) patients affected by vestibular dysfunction and endophthalmitis respectively. One patient who experienced both meningitis and septicemia also developed Bell’s palsy on the left side. The mean duration of admission was about 18 days.
Treatments
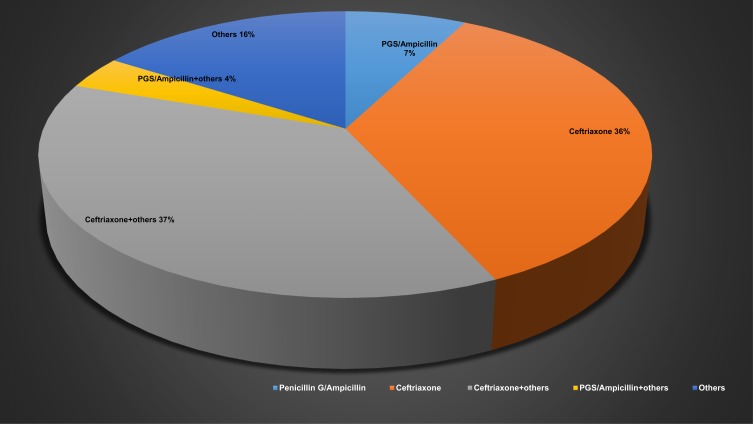
The empirical antibiotics prescribed varied, including gentamicin, ciprofloxacin, ceftriaxone, and penicillin antibiotics. The main antibiotic regimens prescribed were ceftriaxone, followed by ceftriaxone in combination with ampicillin. Other regimens included vancomycin in combination with gentamicin and levofloxacin, cefotaxime in combination with vancomycin or metronidazole, ciprofloxacin, clindamycin, and doxycycline. Ceftriaxone as adjunct with dexamethasone was used in 12 patients (9.4%), whereas Penicillin G (PGS) or ampicillin was administered in 10 patients (7%). Only 27 (20.3%) patients received adjunctive dexamethasone (Table 2 and Figure 4). The average time to microbiological cure was around 9 days. The minimum inhibitory concentration (MIC) of penicillin and ceftriaxone was carried out in 57 and 53 patients respectively. S. suis isolates were generally susceptible to penicillin and ceftriaxone. The mean MIC for penicillin and ceftriaxone were 0.16 µg/mL and 0.24 µg/mL respectively (Table 3).
Figure 4.
Main antibiotic therapy use.
Table 3.
Laboratory Investigations
| Characteristics | Total |
|---|---|
| Total bilirubin (0.00–1.20 mg/dL) (n=113) | 1.78 ± 3.33 |
| Direct bilirubin (>,=0.30mg/dL) (n=113) | 0.89 ± 2.01 |
| WBCs count (cells/cu.mm) (5000–10,000) (n=129) | 19,897.21 ± 37,555.65 |
| Total protein (6.6–8.7g/dL) (n=113) | 6.65 ± 0.94 |
| Platelet count (cells/cu.mm) (n=130) | 221,983 ± 143,810 |
| Albumin (3.2–5.2 g/dL) (n=113) | 3.12 ± 0.59 |
| Globulin (3.1–3.5 g/dL) (n=113) | 3.53 ± 0.80 |
| BUN (6–20 mg/dL) (n=129) | 25.64 ± 22.71 |
| AST (0–40 U/L) (n=113) | 100.31 ± 161.64 |
| ALT (0–41 U/L) (n=113) | 62.23 ± 66.93 |
| Hb (g/dL) (10–15) (n=130) | 11.58 ± 2.58 |
| Hct (%) (40–50) (n=130) | 35.20 ± 7.876 |
| Microbiological results: | |
| ● Mean MIC to penicillin (µg/mL) ±SD | 0.16 ± 0.16 |
| ● Mean MIC to ceftriaxone (µg/mL) ±SD | 0.24 ± 0.27 |
Abbreviations: WBC, white blood cells; BUN, Blood Urea Nitrogen; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; Hb, Hemoglobin; Hct, Hematocrit; MIC, Minimal Inhibitory Concentration.
Potential Risk Factors for Mortality
The estimated odds ratios (ORs) for potential risk factors of S. suis fatality among deaths vs survivors were presented in Table 5. From the univariate analysis, independent risk factors of mortality included GCS score < 8, SAPS II score > 50, time to microbiological cure ≥ 6 days, ALD, presence of acute meningitis, septic shock, total bilirubin > 2.5 mg/dL, direct bilirubin > 1.5 mg/dL, BUN > 28 mg/dL, creatinine > 1.8 mg/dL, bicarbonate < 18 mmol/, albumin < 3.5 g/dL (Table 4).
Table 5.
Significant Predictors of S. Suis Mortality
| Predictors | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value | |
| GCS <8 | 9.42 | 2.08–42.55 | 0.0058 | 1.71 | 0.10–28.50 | 0.709 |
| Time to microbiological cure ≥ 6 days | 6.21 | 1.35–28.55 | 0.0052 | 43.57 | 2.46–772.80 | 0.010 |
| ALD | 4.38 | 1.29–14.93 | 0.0262 | 2.24 | 0.32–15.84 | 0.417 |
| Acute meningitis | 0.21 | 0.045–0.95 | 0.0176 | 0.24 | 0.03–2.33 | 0.236 |
| Septic shock | 12.39 | 3.86–39.79 | <0.001 | 13.34 | 1.63–109.03 | 0.016 |
| Direct bilirubin >1.5 mg/dL | 5.30 | 1.77–15.88 | 0.0024 | 12.86 | 1.91–86.59 | 0.009 |
| Creatinine >1.8 mg/dL | 4.73 | 1.60–13.96 | 0.0050 | 0.41 | 0.01–6.49 | 0.414 |
| Bicarbonate <18 mmol/L | 4.91 | 1.60–15.04 | 0.0073 | 3.00 | 0.12–73.62 | 0.500 |
| Albumin <3.5 g/dL | 5.04 | 1.10–23.21 | 0.0149 | 10.97 | 0.96–125.81 | 0.054 |
Note: All significant predictors, their 95% confidence intervals and p-value are indicated in bold.
Table 4.
Clinical Characteristics of S. Suis Infected Patients for Mortality
| Characteristics | Total | Died (n=16, 12.03%) | Survived (n=117, 87.97%) | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Age (years) (mean±SD) | 56.47± 13.68 | 58.25±15.61 | NA | 56.23±13.46 | NA | 1.01 | 0.97–1.05 | 0.578 |
| Male sex | 92 (69.17%) | 11 | 68.75 | 81 | 69.23 | 0.98 | 0.32–3.02 | 0.969 |
| GCS < 8 | NA | 9.92± 3.95 | NA | 13.06± 2.83 | NA | 9.42 | 2.08–42.55 | 0.006 |
| SAPS II > 50 | NA | 46.56± 17.64 | NA | 24.34±10.87 | NA | 21.97 | 5.40–89.42 | <0.001 |
| Time to microbiological cure ≥ 6 days | NA | 6.33± 3.44 | NA | 8.96± 14.39 | NA | 6.21 | 1.35–28.55 | 0.005 |
| Underlying disease | ||||||||
|
44 (33.08%) | 5 | 11.36 | 39 | 88.64 | 0.91 | 0.30–2.80 | 0.868 |
|
16 (12.03%) | 5 | 31.25 | 11 | 68.75 | 4.38 | 1.29–14.93 | 0.026 |
|
26 (19.55%) | 4 | 15.38 | 22 | 84.62 | 1.43 | 0.42–4.89 | 0.568 |
| Major clinical manifestations | ||||||||
|
50 (37.59%) | 2 | 4 | 48 | 96 | 0.21 | 0.04–0.95 | 0.018 |
|
74 (55.64%) | 12 | 16.22 | 62 | 83.78 | 2.66 | 0.81–8.73 | 0.088 |
|
20 (15.27%) | 9 | 45.00 | 11 | 55.00 | 12.39 | 3.86–39.79 | <0.001 |
|
34 (25.56%) | 3 | 8.82 | 31 | 91.18 | 0.64 | 0.17–2.40 | 0.493 |
| Received dexamethasone | 27 (20.30%) | 1 | 3.740 | 26 | 96.30 | 0.23 | 0.03–1.85 | 0.096 |
| Total bilirubin >2.5 mg/dL | 1.78±3.33 | 4.90±8.19 | NA | 1.30±1.17 | NA | 3.90 | 1.33–11.41 | 0.013 |
| Direct bilirubin >1.5mg/dL | 0.89±2.01 | 2.74±4.89 | NA | 0.60±0.76 | NA | 5.30 | 1.77–15.88 | 0.002 |
| WBC count (cells/cu.mm) | 19,897±37,555 | 15,117± 8512.37 | NA | 20,574±39,981 | NA | 1.0 | 1.00–1.00 | 0.472 |
| AST >300 U/L | 100.31± 161.64 | 179.53±252.09 | NA | 88.18±140.98 | NA | 2.0 | 0.67–6.01 | 0.228 |
| ALT >300 U/L | 62.23± 66.93 | 84.33± 110 | NA | 58.84±57.78 | NA | 1.44 | 0.42–4.89 | 0.568 |
| BUN >28 mg/dL | 25.64± 22.71 | 38.25± 42.83 | NA | 23.86±17.83 | NA | 4.24 | 1.43–12.61 | 0.008 |
| Creatinine > 1.8 mg/dL | 1.80± 2.50 | 3.01± 4.97 | NA | 1.63± 1.90 | NA | 4.73 | 1.60–13.96 | 0.005 |
| Bicarbonate < 18 mmol/L | 21.44± 4.39 | 18.81± 5.86 | NA | 21.82±4.03 | NA | 4.91 | 1.60–15.04 | 0.007 |
| Phosphorus > 4.5 mg/dL | 3.75± 1.90 | 5.27± 3.00 | NA | 3.48± 1.50 | NA | 1.91 | 0.67–5.50 | 0.225 |
| Albumin < 3.5 g/dL | 3.12± 0.59 | 2.75± 0.53 | NA | 3.17± 0.58 | NA | 5.04 | 1.10–23.21 | 0.015 |
| Globulin > 4 g/dL | 3.53± 0.80 | 3.99± 0.87 | NA | 3.46± 0.76 | NA | 2.48 | 0.86–7.14 | 0.092 |
In the multivariate analysis, the remaining risk factors for mortality were time to microbiological cure ≥ 6 days, septic shock, and direct bilirubin > 1.5 mg/dL (Table 5). Forward and backward stepwise multivariate logistic regression was also performed. According to the analyses, significant risk factors associated with S. suis mortality identified were similar, with low albumin level below 3.5 g/dL as addition. (Table S2 shows significant predictors of S. suis mortality).
Discussion
In this 13-year retrospective cohort study, we identified a number of potential risk factors for S. suis mortality. The remaining prognostic indicators from multivariate analysis were time to microbiological cure ≥ 6 days, septic shock, and direct bilirubin > 1.5 mg/dL. The risk factors identified are straight-forward in terms of clinical explanations.
Mortality from 72 hrs to first ten days could be a reliable indicator from antimicrobial treatment failure.16 Observations of patients whether they received effective antibiotic treatment have shown relatively lower mortality during that interval.17
Hyperbilirubinemia reflects impact from liver damage which could be potentially caused by systemic infection.18,19 This is consistent with other clinical symptoms including febrile illnesses, elevated liver enzymes, and low albumin levels caused by septicemia.
Hypoalbuminemia could potentially be a predictor of S. suis mortality. Although the association was not statistically significant in the full model analysis, the p-value was very near significant level (0.054), which may be due to limited sample size. Nevertheless, in forward and backward stepwise multivariate logistic regression, low serum albumin level <3.5 g/dL was significantly associated with S. suis mortality.
The findings were generally consistent with the previous studies.5,11,12 Septic shock was a major risk factor of mortality in both our study and two previous series.5,12 This clinical event could result in liver dysfunction through hemodynamic changes contributing to elevated serum creatinine, bilirubin and liver enzymes.20 Consistently, this variable was also a significant predictor of S. suis mortality in two previous studies.11,12 Early diagnosis, prompt antibiotic therapy, close patient monitoring regarding these risk parameters, and supportive fluid resuscitation are essential to improve clinical outcomes. Daily physical examination and serial microbiological work-up may be necessary in non-responsive cases.
A history of raw pork consumption was recognized in more than one third of included patients despite the fact of retrospective nature, which is in contrast with the previous study in the same setting whereby this correlation could not be demonstrated.11 Interestingly, a history of alcohol drinking was documented in nearly half of all S. suis cases. This reflects the northern Thai culture involving “Larb Lu” a famous northern raw pork dish flecked with herb and chili consumption together with alcohol drinking during social events/gatherings. Although alcohol drinking is not a risk factor, heavy drinking and alcoholic liver disease could potentially result in immunocompromised condition which makes patients more susceptible to infection. Skin injury, pig-related occupation, recent exposure to pigs/raw pork were documented only in small percentages of patients. This is different from the reports from Western countries that showed S. suis is a disease among occupations involving swine contact.3,4,21
Similar to previous studies,2,3,5,7,8,11,12,22–34 most S. suis patients were middle-aged men. The rarity of the disease in children was probably due to the lack of exposure to risk factors in acquiring the infection in pediatric population. In our study, there was only one pediatric case with medical history of congenital rheumatic heart disease without any predisposing risk factors. The underlying disease would probably make the patient more susceptible to infection. The frequent occurrence of the disease in summer and rainy season is consistent with previous findings2,5,6,8,11,23,35–37 that these weather conditions precipitate the pathogen's infectivity and proliferation.35 A relatively high number of cases in April may be related to Songkran’s festival or Thai New Year which is one of the biggest social events among locals. The overall mortality rate (12%) was comparatively lower than findings from previous studies (19%).11,12 The relatively low mortality rate in this study might be explained by the generally good health status (low SAPS II score) of included patients during admission.
Discrepancies regarding clinical manifestations were noted among studies despite the fact that they were conducted within the same region. In the study by Wangkaew et al (2006),11 IE was the most common clinical manifestation while only 8% of IE cases were found in another hospital setting in the lower northern region of Thailand.12 According to our results, the most common clinical presentations found were septicemia, meningitis, diarrhea, and IE. The observation is still similar to previous reports in terms of spectrum of presentations in which meningitis and septicemia were still among the most prominent. Septicemia was usually found concomitantly with either meningitis or IE and one of the major causes of complications including septic shock, multi-organ failure and DIC (Disseminated Intravascular Coagulation). This indicates that supportive treatment is also important apart from timely and adequate antibiotic treatment. The relatively high proportion of patients with diarrhea symptoms suggests an oral route transmission which is consistent with the considerably high number of raw pork and alcohol consumption.
The most common underlying diseases were VHD, spondylodiscites, DM, SLE, and ALD in which ALD was also a significant risk factor of disease mortality in the univariate analysis. These underlying medical conditions could lead to immunocompromised conditions and make patients more susceptible to infection. The fact that nearly all patients with IE were found with history of VHD may suggest the association of IE with this underlying condition.
To the best of our knowledge, this 13-year retrospective cohort study was probably the largest series focusing on risk factors of S. suis mortality and clinical outcomes conducted in Thailand. The longer duration and higher number of included cases enabled us to demonstrate several significant factors of the disease fatality and to see a better clinical picture of this neglected life-threatening infection. The inclusion of all S. suis culture-proven positive patients regardless of their ages, unlike previous studies which usually included only adult population,11,12,22 may provide more insights on the burden of the disease. In addition, some significant risk factors of mortality were also confirmed in the multivariate analyses which could not be demonstrated in the previous studies due to small sample size. Nonetheless, there are some limitations to be noted. The number of cases included may not represent the true incidence within the region due to the retrospective nature which could potentially lead to missing or incomplete data. As only admitted patients with available medical records were included, outpatients or few patients whose medical data could not be located or had been destroyed were not accounted for in the findings. However, this number was quite small and should not have affected the results. S. suis is a generally serious and life-threatening infection which requires hospitalization. Therefore, majority of S. suis cases should have been captured in our study. The unavailability of S. suis serotyping at the hospital made it impossible to gather the genotypic profile, yet it could be assumed that most infections should be from serotype 2 based on the previous genotyping study.37,38 In addition, the selection of only positive hemoculture or CSF cases may have led to us missing some clinical presentations such as ecchymosis, petechiae and other skin abnormalities. However, we believe that this selection bias would indicate positive cases from true infection. Finally, the uniqueness of local traditional culture of raw pork consumption in northern Thailand may have affected the findings regarding mortality and outcomes of S. suis infection in this setting. Therefore, the findings may not be applicable in the settings where there is no existence of this eating behavior.
Whereas S. suis is considered to be uncommon and an occupational hazard in the Western region, this disease is considerably common in Asian countries, especially in Southeast Asia. This warrants the need for continuous educational or public health awareness programs for sustainable results. The incidence is largely under-reported and serotyping detection is usually not routinely available in hospital settings. Development of surveillance and strengthening of laboratory networks are essential to monitor the extent of the current disease situation. The clinical risk factors identified from this study may be further utilized in future research, including a mortality risk score development to improve patient outcomes.
Acknowledgment
We would like to thank the staff at Chiang Mai University Hospital, Boonchira Kitisith, Ekkachai Jongkire, Areerat Kittikunakorn of the Office of Medical Records and Statistics, Wilai Baosoung, Paduangkiat Kamnoi of CMUH Microbiology Laboratory for their kind support in data access and facilitating our 6-month data collection, Donsuk Pongnikorn and Weerawat Chanla of Lampang Cancer Hospital for the introduction and guidance regarding case record form (CRF) use and design, Shaun Lee of School of Pharmacy, Monash University Malaysia for his advice on statistical analysis. The work was financially supported by Monash Global Asia in the 21st Century (GA21) research grant (GA-HW-19-L01 & GA-HW-19-S01) and Fundamental Research Grant Scheme (FRGS/1/2019/WAB09/MUSM/02/1& FRGS/1/2019/SKK08/MUSM/02/7).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflict of interest in this work.
References
- 1.Athey TBT, Teatero S, Lacouture S, Takamatsu D, Gottschalk M, Fittipaldi N. Determining Streptococcus suis serotype from short-read whole-genome sequencing data. BMC Microbiol. 2016;16(1):162. doi: 10.1186/s12866-016-0782-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim HF, Nguyen HN, Taylor W, et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One. 2009;4(6):e5973. doi: 10.1371/journal.pone.0005973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10(1):131–137. doi: 10.1093/clinids/10.1.131 [DOI] [PubMed] [Google Scholar]
- 4.Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3:e45. doi: 10.1038/emi.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fongcom A, Pruksakorn S, Netsirisawan P, Pongprasert R, Onsibud P. Streptococcus suis infection: a prospective study in northern Thailand. Southeast Asian J Trop Med Public Health. 2009;40(3):511–517. [PubMed] [Google Scholar]
- 6.Navacharoen N, Chantharochavong V, Hanprasertpong C, Kangsanarak J, Lekagul S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J Laryngol Otol. 2009;123(8):857–862. doi: 10.1017/S0022215109004939 [DOI] [PubMed] [Google Scholar]
- 7.Huong VTL, Thanh LV, Phu VD, et al. Temporal and spatial association of Streptococcus suis infection in humans and porcine reproductive and respiratory syndrome outbreaks in pigs in northern Vietnam. Epiemiol Infect. 2016;144(1):35–44. doi: 10.1017/S0950268815000990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM. 1995;88(1):39–47. [PubMed] [Google Scholar]
- 9.Rayanakorn A, Goh B-H, Lee L-H, Khan TM, Saokaew S. Risk factors for Streptococcus suis infection: a systematic review and meta-analysis. Sci Rep. 2018;8(1):13358. doi: 10.1038/s41598-018-31598-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huong VTL, Ha N, Huy NT, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014;20(7):1105–1114. doi: 10.3201/eid2007.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wangkaew S, Chaiwarith R, Tharavichitkul P, Supparatpinyo K. Streptococcus suis infection: a series of 41 cases from Chiang Mai University Hospital. J Infect. 2006;52(6):455–460. doi: 10.1016/j.jinf.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 12.Wangsomboonsiri W, Luksananun T, Saksornchai S, Ketwong K, Sungkanuparph S. Streptococcus suis infection and risk factors for mortality. J Infect. 2008;57(5):392–396. doi: 10.1016/j.jinf.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 13.Wongkumma A, Hinjoy S, Choomkhasian P A surveillance report of Streptococcus suis infection in humans, Thailand, 2011–2013. Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health: Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health; June 6, 2014. [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rucker JCMD. Neurology and clinical neuroscience. J Neuro Ophthalmol. 2009;29(3):253–254. doi: 10.1097/01.wno.0000360540.92648.4d [DOI] [Google Scholar]
- 16.Musher D. Clinical and microbiological end points in the treatment of pneumonia. Clin Inf Dis. 2008;47:S207. doi: 10.1086/596035 [DOI] [PubMed] [Google Scholar]
- 17.Musher MD, Alexandraki AI, Graviss AE, et al. Bacteremic and nonbacteremic pneumococcal pneumonia a prospective study. Medicine. 2000;79(4):210–221. doi: 10.1097/00005792-200007000-00002 [DOI] [PubMed] [Google Scholar]
- 18.Szabo G, Romics L Jr., Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis. 2002;6(4):1045–1066, x. doi: 10.1016/S1089-3261(02)00058-2 [DOI] [PubMed] [Google Scholar]
- 19.Subbiah V, West H. Jaundice (Hyperbilirubinemia) in cancer. JAMA Oncol. 2016;2(8):1103. doi: 10.1001/jamaoncol.2016.1236 [DOI] [PubMed] [Google Scholar]
- 20.Nesseler N, Launey Y, Aninat C, Morel F, Mallédant Y, Seguin P. Clinical review: the liver in sepsis. Crit Care. 2012;16(5):235. doi: 10.1186/cc11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottschalk M, Xu J, Lecours M, Grenier D, Fittipaldi N, Segura M. Streptococcus suis infections in humans: what is the prognosis for Western countries? (Part II). Clin Microbiol Newsl. 2010;32(13):97–102. doi: 10.1016/j.clinmicnews.2010.06.001 [DOI] [Google Scholar]
- 22.Mai NT, Hoa NT, Nga TV, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008;46(5):659–667. doi: 10.1086/527385 [DOI] [PubMed] [Google Scholar]
- 23.Khin Thi OO, Chan J. The epidemic of group - R streptococcal (Streptococcus suis) meningitis and septicaemia in Hong Kong. Hong Kong Med J. 1985;37(3):134–136. [Google Scholar]
- 24.Ho DTN, Wolbers M, Cao QT, et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One. 2011;6(3):e17604. doi: 10.1371/journal.pone.0017604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu HJ, Liu XC, Wang SW, et al. Matched case-control study for risk factors of human Streptococcus suis infection in Sichuan Province, China. [Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(9):636–639. [PubMed] [Google Scholar]
- 26.Kong D, Zhang X, Mei S. Epidemiological analysis of four human cases of Streptococcus suis infection in Shenzhen [Chinese]. J Trop Med. 2009;9(3):320–321, 340. [Google Scholar]
- 27.Takeuchi D, Kerdsin A, Akeda Y, et al. Impact of a Food safety campaign on Streptococcus suis infection in humans in Thailand. Am J Trop Med Hyg. 2017;96(6):1370–1377. doi: 10.4269/ajtmh.16-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tall H, Njanpop-Lafourcade B-M, Mounkoro D, et al. Identification of Streptococcus suis meningitis through population-based surveillance, Togo, 2010–2014. Emerg Infect Dis. 2016;22(7):1262–1264. doi: 10.3201/eid2207.151511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh B, Williams AE, Satsangi J. Streptococcus suis type 2: pathogenesis and clinical disease. Rev Med Microbiol. 1992;3(2):65–71. [Google Scholar]
- 30.Donsakul K, Dejthevaporn C, Witoonpanich R. Streptococcus suis infection: clinical features and diagnostic pitfalls. Southeast Asian J Trop Med Public Health. 2003;34(1):154–158. [PubMed] [Google Scholar]
- 31.Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health. 2004;35(4):868–876. [PubMed] [Google Scholar]
- 32.Beek D, Spanjaard L, Gans J. Streptococcus suis meningitis in the Netherlands. J Infect. 2008;57(2):158–161. doi: 10.1016/j.jinf.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 33.Ma E, Chung PH, So T, et al. Streptococcus suis infection in Hong Kong: an emerging infectious disease? Epidemiol Inf. 2008;136(12):1691–1697. doi: 10.1017/S0950268808000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rusmeechan S, Sribusara P. Streptococcus suis meningitis: the newest serious infectious disease. J Med Assoc Thai. 2008;91(5):654–658. [PubMed] [Google Scholar]
- 35.Chau PY, Huang CY, Kay R. Streptococcus suis meningitis. An important underdiagnosed disease in Hong Kong. Med J Aust. 1983;1(9):414–416, 417. doi: 10.5694/j.1326-5377.1983.tb136138.x [DOI] [PubMed] [Google Scholar]
- 36.Yu H, Jing H, Chen Z, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12(6):914–920. doi: 10.3201/eid1206.051194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerdsin A, Dejsirilert S, Puangpatra P, et al. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg Infect Dis. 2011;17(5):835–842. doi: 10.3201/eid1705.100754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerdsin A, Akeda Y, Takeuchi D, Dejsirilert S, Gottschalk M, Oishi K. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur J Clin Microbiol Infect Dis. 2018;37(5):917–925. doi: 10.1007/s10096-018-3208-8 [DOI] [PubMed] [Google Scholar]