In this study, Park et al. set out to elucidate the mechanism by which adipose-resident invariant natural killer T cells (iNKT) cells impact adipose tissue remodeling in obesity. Using in vitro and ex vivo approaches, the authors found that, in obesity, adipose iNKT cells can kill hypertrophic and pro-inflammatory adipocytes via FasL-Fas-dependent apoptosis, thus providing new insight into the role adipose iNKT cells play in promoting healthy adipose tissue remodeling.
Keywords: adipocyte death, adipocyte turnover, adipogenesis, adipose tissue remodeling, iNKT cell
Abstract
In obesity, adipose tissue undergoes dynamic remodeling processes such as adipocyte hypertrophy, hypoxia, immune responses, and adipocyte death. However, whether and how invariant natural killer T (iNKT) cells contribute to adipose tissue remodeling are elusive. In this study, we demonstrate that iNKT cells remove unhealthy adipocytes and stimulate the differentiation of healthy adipocytes. In obese adipose tissue, iNKT cells were abundantly found nearby dead adipocytes. FasL-positive adipose iNKT cells exerted cytotoxic effects to eliminate hypertrophic and pro-inflammatory Fas-positive adipocytes. Furthermore, in vivo adipocyte-lineage tracing mice model showed that activation of iNKT cells by alpha-galactosylceramide promoted adipocyte turnover, eventually leading to potentiation of the insulin-dependent glucose uptake ability in adipose tissue. Collectively, our data propose a novel role of adipose iNKT cells in the regulation of adipocyte turnover in obesity.
Adipose tissue is a dynamic organ that flexibly expands and actively remodels in the process of storing excess nutrients (Sun et al. 2011; Choe et al. 2016). Accumulating evidence indicates that unhealthy adipose tissue remodeling processes, including adipocyte hypertrophy, hypoxia, and immune cell infiltration, are closely linked to adipocyte dysfunction, resulting in insulin resistance and metabolic complications (Ham et al. 2013; Choe et al. 2014; Rosen and Spiegelman 2014; DiSpirito and Mathis 2015; Sohn et al. 2018). Adipose tissue contains various innate and acquired immune cells that actively communicate with adipocytes (Huh et al. 2014; Li et al. 2018). For instance, deficiency of certain immune cells, such as eosinophils and iNKT cells, aggravates obesity and promotes unhealthy adipose tissue remodeling (Wu et al. 2011; Lynch et al. 2012; Huh et al. 2013). However, whether adipose immune cells would directly affect obesity-induced adipocyte hypertrophy, which is a key factor for morbidity of metabolic complications, remains largely unknown.
NKT cells are innate-like T lymphocytes that participate in rapid activation compared with adaptive immune cells. NKT cells are subdivided into three clusters; iNKT cells (type I), diverse NKT cells (type II), and NKT-like cells (Park et al. 2018). The TCR in iNKT cells has a semi-invariant TCRα chain and a restricted β-chain—Vα14Jα18 in the mouse and Vα24Jα18 in humans (Bendelac et al. 1997; Benlagha et al. 2000; Matsuda et al. 2000). Unlike conventional T cells, which are activated by peptides, iNKT cells recognize lipid antigens loaded on major histocompatibility complex class I (MHC I)-related protein CD1d through TCR (Beckman et al. 1994; Huh et al. 2018). iNKT cells have been implicated in autoimmunity, microbial infection, cancer, and obesity (Bessoles et al. 2009; Lynch et al. 2009; Gaya et al. 2018). Depending on stimuli from antigen-presenting cell types, lipid antigens, and microenvironments, iNKT cells are able to exert cytotoxic functions through FasL (CD95L) and perforin/granzyme or exhibit immunoregulatory roles through secreting Th1- or Th2-type cytokines, such as interferon gamma, interleukin (IL)-2, IL-4, IL-10, and IL-13 (Bendelac et al. 2007; Berzins et al. 2011; Brennan et al. 2013; Sag et al. 2014). Marine sponge-derived α-galactosylceramide (α-GC) is a specific glycolipid antigen that potently activates iNKT cells and is an effective adjuvant against infections, tumors, and obesity-induced inflammation (Kawano et al. 1997). Numerical and functional reductions in iNKT cells in adipose tissue are closely associated with increased inflammatory responses, obesity, adipocyte hypertrophy, and insulin resistance in obesity (Lynch et al. 2009; Ji et al. 2012; Schipper et al. 2012; Huh et al. 2017).
Yearly, 10% of human adipocytes die and are replaced (Spalding et al. 2008). Patients with cachexia, human immunodeficiency virus, or lipodystrophy show loss of adipocytes (Prins et al. 1994; Domingo et al. 1999; Fischer-Posovszky et al. 2006; Hussain and Garg 2016). In mice, as the morbidity of obesity becomes worse, dead adipocytes are frequently found in epididymal adipose tissue (EAT). It has been suggested that several mechanisms, including apoptosis, necrosis, and pyroptosis, mediate adipocyte death in obesity (Cinti et al. 2005; Alkhouri et al. 2010; Giordano et al. 2013). It seems that different types of adipocyte death mediate different effects on inflammatory responses and metabolic diseases. For instance, targeted induction of apoptosis in adipocytes recruits anti-inflammatory macrophages into adipose tissue, leading to the alleviation of inflammatory responses (Pajvani et al. 2005; Fischer-Posovszky et al. 2011b). Fas (CD95), a member of the tumor necrosis factor (TNF) receptor family, plays a key role in programmed cell death (apoptosis) (Fischer-Posovszky et al. 2011a). When FasL binds to Fas, a death-inducing signaling complex is assembled and causes apoptosis in a caspase-8- and caspase-3-dependent manner, without significant induction of inflammatory responses (Rock and Kono 2008; Nagata and Tanaka 2017). However, necrosis has been characterized as accidental cell death with release of intracellular components, accompanied with pro-inflammatory responses and insulin resistance (Cinti et al. 2005; Fink and Cookson 2005; Alkhouri et al. 2010).
In obesity, adipose tissue expands through mainly two processes; enlargement of pre-existing adipocytes (adipocyte hypertrophy) and induction of new adipocyte differentiation from adipocyte progenitor cells (adipocyte hyperplasia) (Rosen and Spiegelman 2014). Adipocyte hypertrophy is closely associated with “unhealthy obesity” features, including fibrosis, inflammation, adipocyte death, and insulin resistance. In contrast, adipocyte hyperplasia is a relatively low-risk factor of metabolic diseases (Gustafson et al. 2009; Sun et al. 2011; Rydén et al. 2014). Genetic and pharmacologic stimulation of adipocyte hyperplasia could suppress unhealthy adipose tissue remodeling and improve metabolic phenotypes (Shepherd et al. 1993; Berg et al. 2001; Kim et al. 2007; Kusminski et al. 2012; Shao et al. 2018). Although surface markers of adipocyte progenitor cells have not been fully identified, platelet-derived growth factor receptor (PDGFR) α- or PDGFR β-expressing cells reportedly contribute to adipocyte hyperplasia upon high-fat diet (HFD), protecting against unhealthy adipose tissue expansion (Lee et al. 2012; Vishvanath et al. 2016; Shao et al. 2018). However, the regulatory mechanisms underlying the balance between adipocyte hypertrophy and hyperplasia in obese adipose tissue are largely unknown.
Previously, we have shown that HFD-fed iNKT cell-deficient mice (Jα18 KO) are prone to unhealthy adipose tissue expansion, including adipocyte hypertrophy and insulin resistance, as well as elevated pro-inflammatory responses in obesity (Huh et al. 2013). Although we and other groups have investigated the relationship between iNKT cells and adipose tissue inflammation in obesity (Ji et al. 2012; Lynch et al. 2012; Schipper et al. 2012; Huh et al. 2013; Satoh et al. 2016), it is not thoroughly understood how iNKT cells can prevent unhealthy adipose tissue expansion in obesity. In this study, we investigated the roles of adipose iNKT cells in the regulation of adipocyte death in obese adipose tissue. Moreover, by using specific lipid antigen and adipocyte lineage-tracing mouse model, we examined whether activated iNKT cells can modulate adipose tissue remodeling. Collectively, our findings suggest that adipose iNKT cell can drive healthy adipose tissue remodeling by modulating adipocyte death and birth in obesity.
Results
In obese adipose tissue, cytotoxic potential of iNKT cells is potentiated
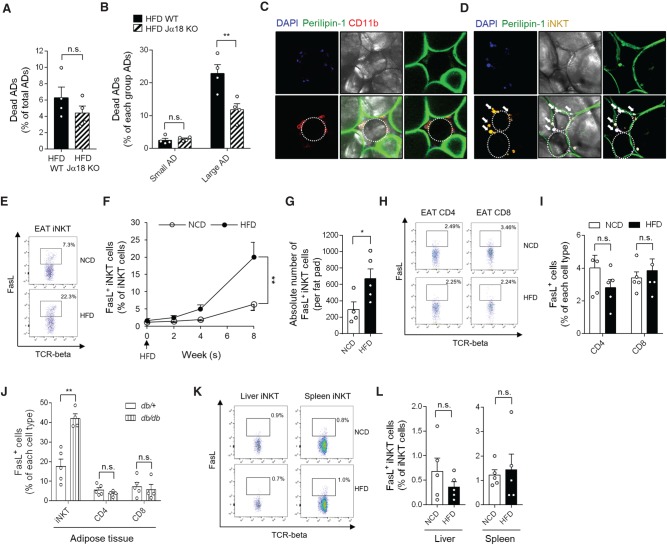
Consistent with previous reports (Lynch et al. 2012; Huh et al. 2013), Jα18 KO mice gained more body weight and EAT mass, and increased adipocyte size than did wild-type (WT) mice upon HFD (Supplemental Fig. S1A–D). Although iNKT cells reportedly have cytotoxic ability, it is unclear whether adipose iNKT cells would kill adipocytes or remove damaged adipocytes. To address this, we investigated the survival rate of adipocytes in HFD-fed WT mice and Jα18 KO mice. To assess the frequency of dead adipocytes from WT and Jα18 KO mice, we used a BioSorter instrument (Supplemental Fig. S1E,F), which enables quantitative analysis of large adipocytes. As shown in Figure 1A, total dead adipocytes displayed a decreased trend in obese Jα18 KO mice than in obese WT control littermates. When we examined the frequency of dead adipocytes in the small (≤60 μm) and large adipocyte (>60 μm) populations, the fraction of dead cells was significantly lower in the large adipocyte population isolated from HFD-fed Jα18 KO mice than in that of HFD-fed WT littermates (Fig. 1B; Supplemental Fig. S1G), suggesting that iNKT cells probably participate in large adipocyte death in diet-induced obesity (DIO). In this study, large adipocytes were defined based on a diameter >60 µm, because the adipocyte population meeting this criterion was increased by HFD (Supplemental Fig. S1H). In addition, we found that iNKT cells were abundantly present nearby dead adipocytes that were identified as perilipin-negative cells (Cinti et al. 2005; Strissel et al. 2007) and surrounded by adipose tissue macrophages (Fig. 1C,D). Taken together, these results suggest that iNKT cells might be involved in the death of hypertrophic adipocytes in obesity.
Figure 1.
In DIO, cytotoxic FasL-positive iNKT cells are increased. (A) Dead adipocytes were counted using a BioSorter, after PI staining. About 150,000–200,000 adipocytes were analyzed. (B) Fractions of dead adipocytes per size category. (C,D) Localization of macrophages (C) and iNKT cells (D) around dead adipocytes; DAPI (blue), perilipin-1 (green), CD11b (red), and α-GC/CD1d tetramer (yellow) were used as a marker for the nucleus, adipocytes, macrophages, and iNKT cells, respectively. Dotted circles and arrows indicate perilipin-1-negative dead adipocytes and iNKT cells, respectively. (E) Dot graph of iNKT cells from EAT. (F) FasL-positive iNKT cell fraction in EATs over 8 wk of HFD feeding. (G) Mean number of FasL-positive iNKT cells per EAT fat pad. (H) Dot graphs of CD8 T and CD4 T cells. (I) Percentages of FasL-positive cells in CD4 T cells and CD8 T cells in EAT. (J) Percentages of FasL-positive cells in iNKT cells, CD4 T cells, and CD8 T cells, respectively, in EAT from db/db mice. (K) Dot graph of liver and spleen iNKT cells. (L) Fractions of FasL-positive iNKT cells among total hepatic (left) and splenic (right) iNKT cells. (*) P < 0.05 and (**) P < 0.01 (t-test, RM-ANOVA, or two-way ANOVA with Bonferroni post-hoc test).
To evaluate the cytotoxic potential of adipose iNKT cells, the population of FasL-positive iNKT cells was quantified in EAT from normal chow diet (NCD)- and HFD-fed mice. The fraction of FasL-positive iNKT cells (Fig. 1E,F) and absolute number of FasL-positive iNKT cells per fat pad were increased in obese mice (Fig. 1G). Given that CD4 and CD8 T cells also exert cytotoxic effects by expressing FasL (Kagi et al. 1994; Kojima et al. 1994; Lowin et al. 1994), we investigated the populations of FasL-positive CD4 and CD8 T cells in EAT from DIO or genetically obese db/db mice compared with NCD-fed lean or db/+ heterozygote mice, respectively. Unlike adipose iNKT cells, the levels of FasL in CD4 and CD8 T cells did not change in DIO (Fig. 1H,I) nor db/db mice (Fig. 1J). Furthermore, in hepatic and splenic iNKT cells, there were no significant differences in the fractions of FasL-positive iNKT cells (Fig. 1K,L). Thus, these data imply that iNKT cells are a major cell type exhibiting increased FasL expression in obese adipose tissue.
Hypertrophic adipocytes express high level of Fas, accompanied with their mortality
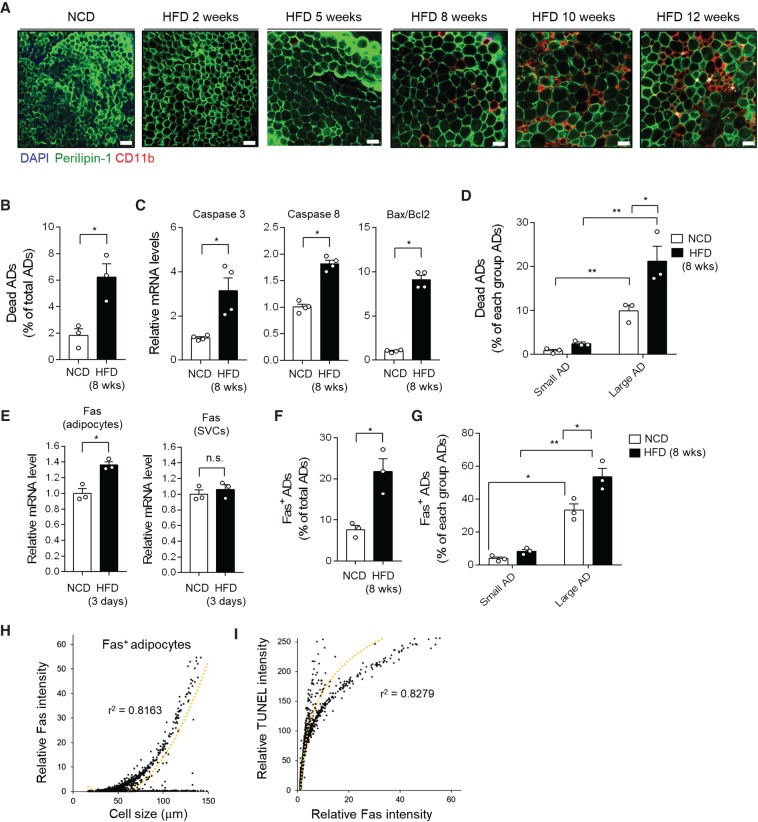
Dead adipocytes were frequently observed in obese adipose tissue over HFD period (Fig. 2A; Supplemental Fig. S2A; Cinti et al. 2005; Strissel et al. 2007). To investigate the characters of dead/dying adipocytes, the frequency of dead adipocytes was analyzed at a single-cell level. As shown in Figure 2B, the fraction of dead adipocytes was ∼1.8% or 6.2% of total adipocytes upon NCD or HFD, respectively. The mRNA levels of the pro-apoptotic genes such as Caspase-3 and 8, and the Bax/Bcl2 ratio were also elevated in EAT (Fig. 2C; Supplemental Fig. S2B). Given that adipocytes have different characters depending on their sizes, we explored the relationship between adipocyte size and dead adipocyte frequency. The frequency of dead adipocytes was higher in the large adipocyte population (>60 µm) than in the small one (≤60 µm) in NCD- and HFD-fed WT and Jα18 KO mice (Fig. 2D; Supplemental Fig. S2C,D). As shown in Figure 2D, the frequency of large adipocyte death was up-regulated in HFD-fed WT mice compared with that of large adipocyte death in NCD-fed WT mice. On the contrary, in HFD-fed Jα18 KO mice, the frequency of dead adipocytes in large adipocyte population was comparable to NCD-fed mice (Supplemental Fig. S2D), implying that the process of hypertrophic adipocyte death might be alleviated in HFD-fed Jα18 KO mice.
Figure 2.
Hypertrophic adipocytes strongly express Fas, accompanied with increased mortality. (A) Histological analysis of dead adipocytes in EAT from HFD-fed WT mice. (B) Dead adipocytes were analyzed using a BioSorter, after PI staining. (C) mRNA levels of apoptosis-related genes in adipocyte fractions. (D,F–I) About 20,000 to 30,000 adipocytes were analyzed. (D) Percentage of dead adipocytes per size category (small adipocytes, <60 μm; large adipocytes, >60 μm). (E) mRNA level of Fas in adipocyte fraction and stromal vascular cells (SVCs) from mice fed HFD for 3 d. (F) Percentage of total Fas-positive adipocytes from NCD- and HFD-fed mice. (G) Abundance of Fas-positive adipocytes in small and large adipocytes from NCD- and HFD-fed mice. (H) Correlation between adipocyte size and Fas protein expression. (I) Correlation between Fas protein expression and TUNEL intensity. Dotted lines are trend lines. r2 is indicated on the graph. All data represent mean ± SEM. (*) P < 0.05 (t-test or two-way ANOVA with Bonferroni post-hoc test).
As adipose iNKT cells expressed the cytotoxicity mediator FasL, we examined the expression of Fas as a binding partner for FasL in iNKT cells. The mRNA level of Fas was tended to be up-regulated in adipocyte fractions after 3 d of HFD feeding, but not stromal vascular cells (Fig. 2E; Supplemental Fig. S2E). The fraction of Fas-positive adipocytes was higher in HFD-fed than in NCD-fed mice (Fig. 2F; Supplemental Fig. S2F). Next, we analyzed the relationship between adipocyte size and adipocyte Fas expression. The fraction of Fas-positive adipocytes was significantly increased in large adipocytes in both NCD and HFD (Fig. 2G). Moreover, the intensity of adipocyte Fas in HFD-fed mice markedly increased with adipocyte size (Fig. 2H). To determine the relevance of adipocyte Fas expression to the frequency of dead adipocytes, adipocytes isolated from HFD-fed mice were subjected to Fas and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stainings. As shown in Figure 2I, TUNEL-staining intensity was positively correlated with Fas expression in adipocytes, indicating that adipocyte Fas expression might be one of indicators of adipocyte death in obesity.
Fas/FasL signaling is a key component in iNKT cell-induced adipocyte death
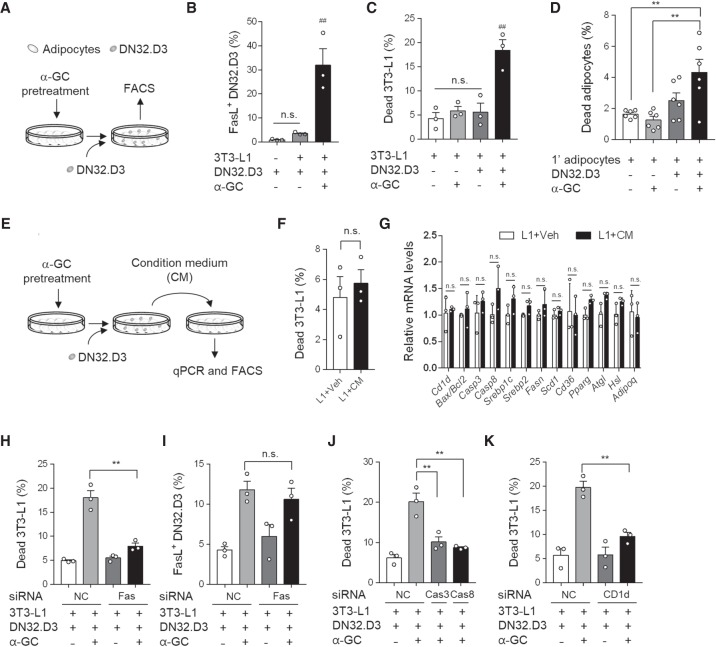
To gain further insights into the mechanism by which iNKT cells induce adipocyte death, we conducted in vitro coculture experiments with differentiated 3T3-L1 adipocytes and DN32.D3 (iNKT hybridoma) cells. Differentiated adipocytes were directly cocultured with DN32.D3 cells in the absence or presence of α-GC, a potent iNKT cell lipid antigen (Fig. 3A). Upon exposure to α-GC, the population of FasL-positive DN32.D3 cells was elevated (Fig. 3B), concurrently with an increase in the frequency of dead 3T3-L1 adipocytes and dead primary adipocytes (Fig. 3C,D; Supplemental Fig. S3A), indicating that FasL might be a potent mediator of iNKT cell-mediated adipocyte death. However, iNKT cells could also exert cytotoxic functions via secretion of cytotoxic molecules such as perforin/granzyme. To test the possibility that iNKT cells might induce adipocyte death through cytotoxic molecules secretion, condition media collected from co-culture between DN32.D3 cells and 3T3-L1 adipocytes were incubated with differentiated 3T3-L1 adipocytes (Fig. 3E). Treatment of condition media did not affect dead adipocyte frequency and mRNA expressions related with adipocyte function (Fig. 3F,G). These data propose that physical contact between adipocytes and iNKT cells would be important to provoke iNKT cell-mediated adipocyte death.
Figure 3.
The Fas/FasL pathway is essential for iNKT cells to remove adipocytes. (A) Schematic illustration of experimental set-up. Differentiated adipocytes were challenged with α-GC and then cocultured with DN32.D3 cells for 48 h. (B) Percentage of FasL-positive DN32.D3 cells among total DN32.D3 cells. (C) Percentage of dead 3T3-L1 adipocytes. About 30,000 to 50,000 adipocytes were analyzed. (D) Percentage of dead primary adipocytes isolated from NCD-fed mice. (E) Schematic illustration of experimental set-up. Differentiated 3T3-L1 adipocytes were challenged with α-GC and then cocultured with DN32.D3. Condition medium (CM) was collected and treated differentiated 3T3-L1 adipocytes. (F) Percentages of dead 3T3-L1 adipocytes. About 30,000 to 50,000 adipocytes were analyzed. (G) mRNA levels of condition medium-treated 3T3-L1 adipocytes. (H–K) Percentages of dead 3T3-L1 adipocytes (H,J,K) and FasL-positive DN32. D3 cells (I). Fas (H,I), the Fas downstream genes caspase 3 (Casp3), and caspase 8 (Casp8) (J), and MHC I-related protein CD1d (K) were knocked down via siRNA in differentiated 3T3-L1 adipocytes. All data represent mean ± SEM. (**) P < 0.01 and (##) P < 0.01 versus all other groups (t-test, one-way ANOVA and two-way ANOVA with Bonferroni post-hoc test).
To investigate whether the FasL/Fas pathway is involved in adipocyte death, Fas and Fas downstream genes were suppressed in differentiated adipocytes using siRNAs (Supplemental Fig. S3B–D). As shown in Figure 3H, knockdown of Fas in differentiated 3T3-L1 adipocytes significantly reduced adipocyte death induced by iNKT cells activated with α-GC, although there was no difference in the fractions of FasL-positive DN32.D3 cells between siNC- and siFas-transfected groups (Fig. 3I). Similarly, when Caspase-3 or Caspase-8 was suppressed in differentiated adipocytes, activated iNKT cells failed to promote adipocyte death (Fig. 3J). Given that CD1d is an antigen-presenting molecule that is essential for iNKT cell activation, the effect of adipocyte CD1d on iNKT cell-mediated adipocyte death was investigated. As indicated in Figure 3K and Supplemental Figure S3E, knockdown of CD1d in differentiated 3T3-L1 adipocytes blunted iNKT cell-induced adipocyte death, indicating that antigen presentation by adipocyte CD1d is an essential step in the mediation of adipocyte death by iNKT cells. Taken together, these data suggest that both the recognition of antigens loaded on adipocyte CD1d by iNKT cells and the interaction between FasL and Fas are crucial to provoke iNKT cell-mediated adipocyte death.
Hypertrophic adipocytes with pro-inflammatory characters are vulnerable to iNKT cell-mediated adipocyte death
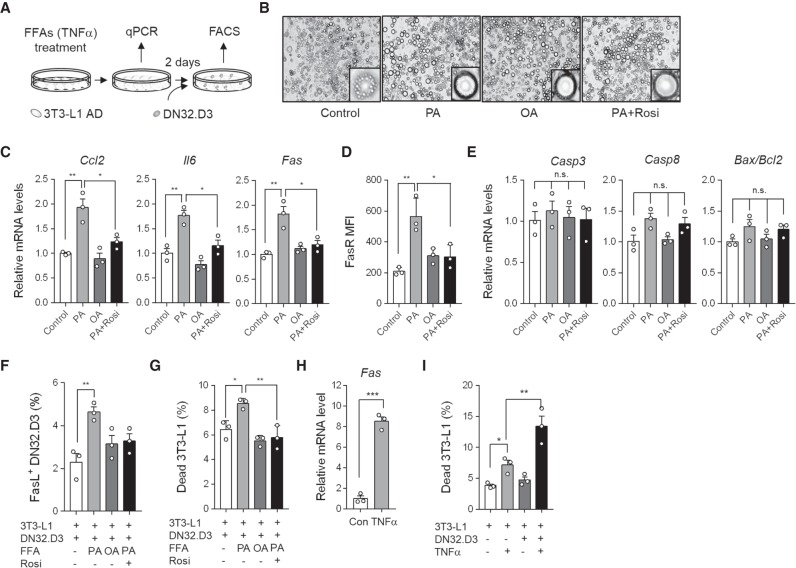
In obesity, Fas expression in large adipocytes was elevated, which was positively correlated with the frequency of dead adipocytes (Fig. 2H,I). To explore mechanistic understanding whether hypertrophic adipocytes would be vulnerable to iNKT cell-induced death, we decided to test lipid-overloaded large adipocytes treated with free fatty acids (FFAs). When differentiated adipocytes were challenged with saturated fatty acids (palmitic acid, PA) or mono-unsaturated fatty acids (oleic acid, OA) (Fig. 4A), adipocytes became enlarged with an unilocular lipid droplet (Fig. 4B). Moreover, the mRNA levels of pro-inflammatory genes such as chemokine (C-C motif) ligand 2 (Ccl2), Il-6, and mRNA and protein levels of Fas were markedly up-regulated in large adipocytes induced by PA, but not OA (Fig. 4C,D). However, FFA treatment did not induce apoptosis-related genes such as Caspase-3, Caspase-8, and Bax/Bcl2 ratio (Fig. 4E). After FFA treatment, DN32.D3 cells were directly cocultured with enlarged 3T3-L1 adipocytes to investigate whether enlarged adipocytes might be susceptible to death by activated iNKT cells. The fractions of FasL-positive DN32.D3 cells and dead 3T3-L1 adipocytes were increased in PA-induced, but not OA-induced hypertrophic adipocytes (Fig. 4F,G). Given that saturated fatty acids such as PA potently stimulate pro-inflammatory responses in adipocytes, we asked whether suppression of pro-inflammatory responses in PA-induced hypertrophic adipocytes would attenuate the induction of adipocyte death by iNKT cells. To address this, lipid-overloaded adipocytes were treated with the anti-inflammatory agent rosiglitazone. As shown in Figure 4C–G, rosiglitazone repressed pro-inflammatory responses and adipocyte death provoked by DN32.D3 cells, indicating that hypertrophic adipocytes with pro-inflammatory characters would be preferentially removed by activated iNKT cells. To acquire further insight into the relationship between inflammatory stimuli and adipocyte death, differentiated adipocytes were treated with TNFα. TNFα up-regulated the Fas mRNA level in differentiated 3T3-L1 adipocytes (Fig. 4H) and further promoted the cytotoxic effects of DN32.D3 cells against adipocytes (Fig. 4I). Together, these data suggest that pro-inflammatory characters caused by excess nutrients and/or pro-inflammatory stimuli render unhealthy adipocytes susceptible to iNKT cell-mediated apoptosis.
Figure 4.
Hypertrophic adipocytes with pro-inflammatory characters are preferentially removed by iNKT cells. (A) Schematic illustration of experimental setup. Differentiated 3T3-L1 adipocytes were challenged with FFAs (500 μM, palmitic acid; PA and oleic acid; OA) or TNFα (10 nM) for 2 wk or 1 d, respectively, and then cocultured with DN32.D3 cells for 48 h. Palmitic acid-treated 3T3-L1 adipocytes were challenged with rosiglitazone (Rosi, 10 nM). (B) Microscopic images of 3T3-L1 adipocytes. (C) mRNA levels of Ccl2, Il-6, and Fas. (D) FasR mean fluorescence intensity (MFI). (E) mRNA levels of apoptosis-related genes, including Casp3, Casp8, and Bax/Bcl2 ratio. (F,G) Percentages of FasL-positive DN32.D3 cells (F) and dead 3T3-L1 adipocytes (G). (H) mRNA level of Fas in TNFα-treated 3T3-L1 adipocytes. (I) Percentage of dead 3T3-L1 adipocytes. (#) P < 0.05 versus all other groups and (*) P < 0.05 (t-test or one-way ANOVA with Bonferroni post-hoc test). All data represent mean ± SEM.
In obesity, iNKT cell activation promotes adipocyte death
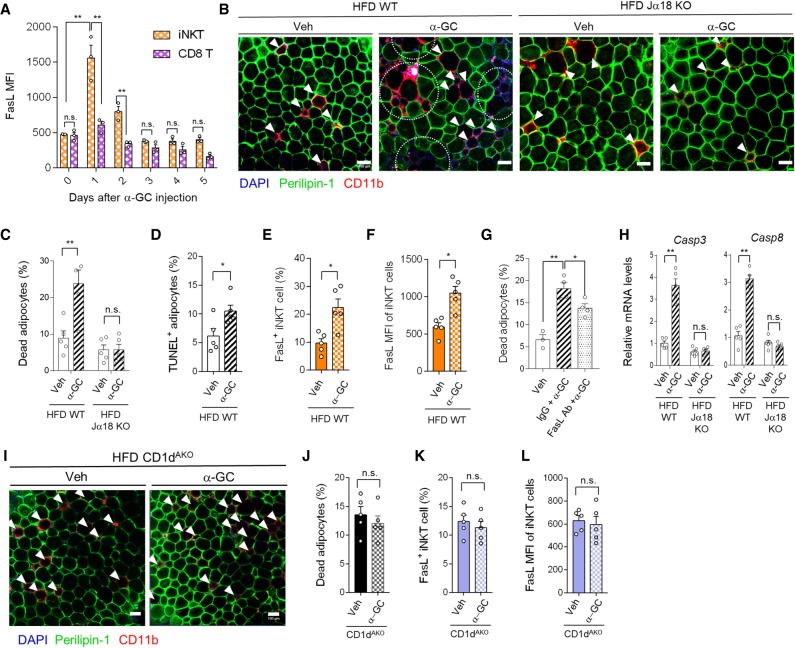
To explore physiological roles of iNKT cell-induced adipocyte death in vivo, WT and Jα18 KO mice fed NCD or HFD were administered α-GC and the frequency of adipocyte death was assessed. Administration of α-GC did not significantly affect total body weight and EAT mass upon NCD and HFD (Supplemental Fig. S4A,B). In adipose tissue, α-GC increased FasL expression in iNKT cells, but not CD8 T cells, which was persisted for 2 d (Fig. 5A). Since the effect of α-GC on FasL expression in iNKT cells was remarkable at day 1, we decided to analyze adipocyte death 1 d after α-GC injection in following experiments. Upon NCD, the effect of α-GC on adipocyte death was not detected (Supplemental Fig. S4C), even though FasL expression was increased in iNKT cells (Supplemental Fig. S4D). On the contrary, in HFD-fed mice, α-GC markedly increased the fractions of perilipin-negative dead adipocytes and TUNEL-positive adipocytes (Fig. 5B–D) as well as the up-regulated portion of FasL-positive iNKT cells and FasL mean fluorescence intensity (MFI) in adipose iNKT cells (Fig. 5E,F). However, in HFD-fed Jα18 KO mice, α-GC did not affect adipocyte death (Fig. 5B,C) as well as total body weight and EAT mass (Supplemental Fig. S4E,F). Moreover, the extent of adipocyte death induced by α-GC was diminished in FasL-neutralized WT mice (Fig. 5G), implying that α-GC-induced adipocyte death is mediated by FasL-positive iNKT cells. Concurrently, Caspase-3 and Caspase-8 mRNA levels were higher in adipocytes from α-GC-injected WT mice than in those from α-GC-injected Jα18 KO mice (Fig. 5H). These data imply that adipocyte dysregulation by HFD might be required for iNKT cell-mediated adipocyte death.
Figure 5.
α-GC promotes iNKT cell-mediated adipocyte death. (A) FasL MFI of iNKT, and CD8 T cells after α-GC (1 μg/mouse) injection into NCD-fed mice. (B–L) α-GC was intraperitoneally injected into mice fed HFD for 8 wk. EATs were analyzed 1 d after α-GC administration. (B) Histological analysis of dead adipocytes in EAT from HFD-fed WT and Ja18 KO mice 1 d after vehicle (Veh) or α-GC injection. (C) Quantification of dead adipocytes as perilipin-negative cells. (D) TUNEL assay in EAT from HFD-fed Veh or α-GC injected mice. (E) Percentage of FasL-positive iNKT cells. (F) FasL MFI of iNKT cells in EAT from Veh and α-GC-injected mice. (G) Percentage of dead adipocytes in EAT from Veh, α-GC, or FasL neutralizing antibody (Ab) injected mice. (H) mRNA levels of Casp3 and Casp8. (I) Histological analysis of dead adipocytes in CD1dAKO 1 d after Veh and α-GC administration. (J) Quantification of dead adipocytes as perilipin-negative cells in Veh- and α-GC-injected HFD-fed CD1dAKO mice. (K) Percentage of FasL-positive iNKT cells in Veh- and α-GC-injected HFD-fed CD1dAKO mice. (L) FasL MFI of iNKT cells in EAT from Veh- and α-GC-injected mice. Arrows and dotted (semi)circles indicate dead adipocytes. All data represent mean ± SEM. (*) P < 0.05 and (**) P < 0.01 (t-test or two-way ANOVA with Tukey post-hoc test).
We have previously demonstrated that adipocyte CD1d is crucial for the regulation of adipose iNKT cell activity (Huh et al. 2017). To examine whether adipocyte CD1d is required for iNKT cell-induced adipocyte death in obesity, HFD-fed adipocyte-specific CD1d KO (CD1dAKO) mice were treated with α-GC. Similar to HFD-fed Jα18 KO mice (Fig. 5B,C), there was no significant difference in the fraction of dead adipocytes in CD1dAKO mice with or without α-GC (Fig. 5I,J). Moreover, the levels of FasL in adipose iNKT cells were not different between vehicle (Veh)- and α-GC-treated CD1dAKO mice. (Fig. 5K,L). These data suggest that antigen recognition of iNKT cells via adipocyte CD1d is an indispensable step in iNKT cell-mediated adipocyte death in DIO.
Activation of iNKT cells causes healthy adipose tissue remodeling by inducing adipogenesis
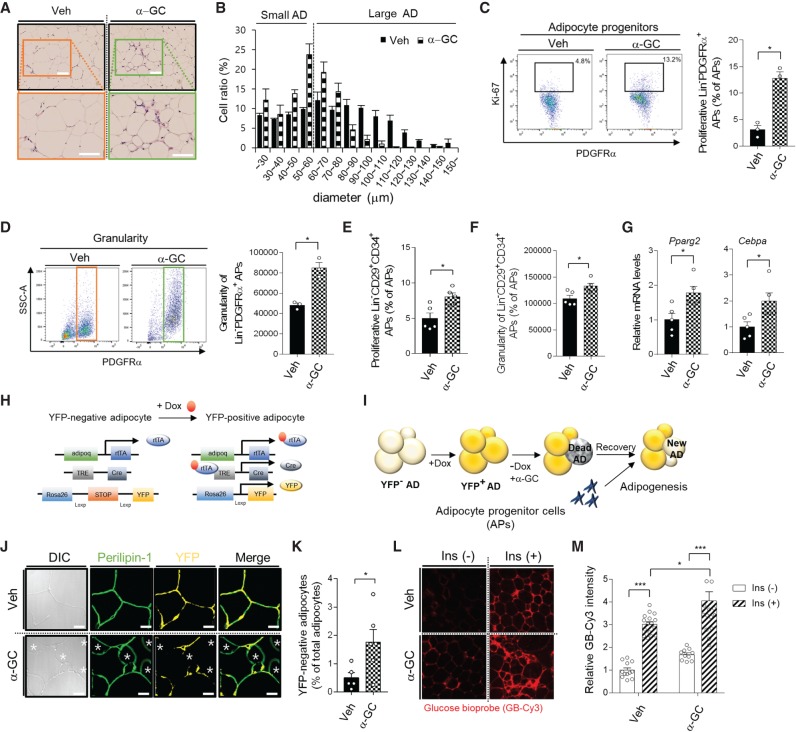
In adulthood, the ratio of adipocyte birth to adipocyte death is quite constant, which contributes to the maintenance of adipose tissue homeostasis (Salans et al. 1973; Jo et al. 2009). To examine the dynamics of adipocyte turnover by iNKT cell activation, we evaluated adipose tissue 1 wk after α-GC treatment of HFD-fed mice. Histological analysis revealed that small adipocytes were increased in α-GC-treated obese adipose tissue (Fig. 6A,B). When adipocyte differentiation is initiated in adipocyte progenitor cells, it seems that they actively proliferate to secure the number of progenitor cells, which is required for both adipocyte differentiation and maintenance of progenitor cells (Rodeheffer et al. 2008; Lee et al. 2012; Jeffery et al. 2015). Further, adipocyte progenitor cells start to accumulate lipid metabolites during adipogenesis (Lee et al. 2004). The proliferation rate and granularity of adipocyte progenitor cells were analyzed by FACS from two populations such as CD31−CD45−PDGFRα+ cells and CD31−CD45− CD29+CD34+ cells (Jeffery et al. 2015). Upon α-GC treatment, the proliferation rate and granularity of adipocyte progenitor cells (CD31−CD45−PDGFRα+Ki67+) was increased 4.2-fold (Fig. 6C) and 1.8-fold (Fig. 6D), respectively, in WT mice, but not in Jα18 KO mice (Supplemental Fig. S5A,B). Similarly, the proliferation rate and granularity of another type of adipocyte progenitor cells (CD31−CD45−CD29+CD34+) was increased upon α-GC treatment (Fig. 6E,F). Next, after adipocyte progenitor cells (CD31−CD45−PDGFRα+) were sorted by FACS, mRNA expression was analyzed. As shown in Figure 6G, the mRNA level of Pparg2 and Cebpa were up-regulated 1.7-fold and 2.0-fold, implying that adipocyte differentiation might be potentiated after activation of iNKT cells to replace dead adipocytes in DIO.
Figure 6.
Activated iNKT cells stimulate new and healthy adipocyte generation. α-GC (1 μg/mouse) was intraperitoneally administrated into mice fed the HFD for 8 wk. All analyses were conducted 1 wk after α-GC injection. (A) Histological analysis of EATs from Veh and α-GC injected mice. Scale bars, 100 μm. (B) Adipocyte size distribution. (C) Dot graph (left) and percentage of proliferative adipocyte progenitor cells (CD31–CD45–PDGFRα+Ki-67+). CD31–CD45–PDGFRα+ and Ki67 were used as a marker of adipocyte progenitor cells and proliferation, respectively. (D) Dot graphs (left) and average value (right) of granularity of adipocyte progenitor cells. (E) Proliferation rate of another type of adipocyte progenitor cells (CD31−CD45−CD29+CD34+). (F) Granularity of adipocyte progenitor cells (CD31−CD45−CD29+CD34+). (G) mRNA levels of Pparg2 and Cebpa in adipocyte progenitor cells from Veh and α-GC injected mice. (H,I) Illustration of in vivo experiments using an adipocyte lineage-tracing mouse model (H). Adipocyte lineage-tracing mice were fed the HFD for 6 wk, followed by a Dox-containing HFD for 2 wk. After withdrawing Dox, α-GC (1 μg/mouse) was intraperitoneally administrated into mice fed the HFD for 8 wk (I). (J) Immunohistochemistry of YFP-positive and YFP-negative adipocytes; differential interference contrast (DIC), perilipin-1 (green), YFP (yellow). (K) Quantification of new adipocytes as YFP-negative and perilipin-1-positive cells in Veh- or α-GC-injected HFD-fed mice. (L) Glucose uptake assay in EAT from α-GC injected adipocyte lineage-tracing mice. (M) Quantification of glucose uptake in Veh- or α-GC-injected HFD-fed mice. (*) P < 0.05 (t-test or two-way ANOVA with a Bonferroni post-hoc test). All data represent mean ± SEM.
To investigate the origin of small adipocytes induced by α-GC treatment, we decided to utilize an adipocyte lineage-tracing mouse model (AdiponectinrtTA; TRE-Cre; Rosa26-loxp-stop-loxp-YFP) (Wang et al. 2013) in which adiponectin-expressing adipocytes express YFP in a doxycycline (Dox)-inducible manner (Fig. 6H). The mice were fed HFD for 6 wk, followed by Dox-containing HFD for 2 wk to label mature adipocytes with YFP (Supplemental Fig. S5C-E). After withdrawing Dox, α-GC was administered. One week after α-GC administration, newly differentiated (perilipin-positive and YFP-negative) adipocytes were observed by microscopy (Fig. 6I). As shown in Figure 6J,K, newly differentiated small adipocytes were evidently observed in α-GC-treated, but not Veh-injected mice. To determine whether newly differentiated small adipocytes induced by iNKT cell activation would contribute to insulin sensitivity in adipose tissue, we performed an insulin-dependent glucose uptake assay using a Cy3-tagged glucose bioprobe. In α-GC-treated obese mice, small adipocytes exhibited elevated insulin-dependent glucose uptake ability compared with large adipocytes (Fig. 6L,M), which might contribute to improved insulin resistance in EAT and whole body of DIO mice (Supplemental Fig. S5F,G). Overall, these data suggest that iNKT cells can trigger healthy adipose tissue remodeling in obese mice by modulating adipogenesis.
Discussion
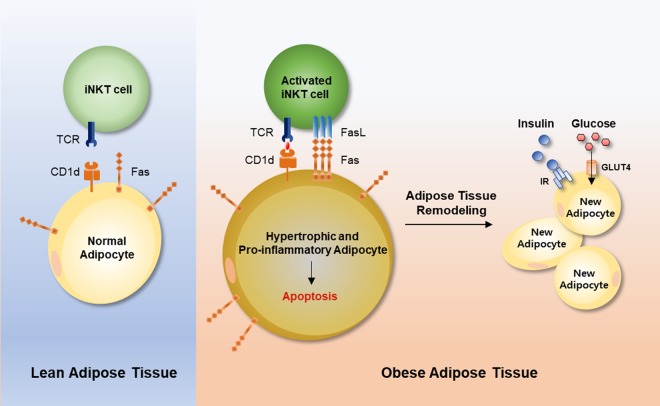
Adipocyte death is frequently observed in obesity (Cinti et al. 2005; Strissel et al. 2007; Spalding et al. 2008). However, the causes and physiological implications of adipocyte death have not been fully understood. There are significant technical limitations to in vivo analysis of adipocyte death, because adipocytes are floating, physically fragile, and too large to be analyzed by conventional microscopes and FACS. Here, we tried to overcome these issues by adopting new approaches to the analysis of large adipocytes and we investigated the physiological functions of adipocyte death in obesity. Our findings demonstrated that adipose iNKT cells stimulated death of hypertrophic adipocytes with pro-inflammatory characters. In obese adipose tissue, FasL-positive iNKT cells were increased and removed hypertrophic Fas-positive adipocytes. Interestingly, activation of iNKT cells not only induced adipocyte death, but also involved in stimulation of de novo adipogenesis in obesity, which eventually led to improved insulin-dependent glucose uptake ability in adipose tissue (Fig. 7). Taken together, these data suggest that adipose iNKT cells play an important role in the regulation of adipocyte turnover, contributing to maintenance of adipose tissue homeostasis in obesity.
Figure 7.
Working model. In obesity, excess energy renders adipocytes hypertrophic and pro-inflammatory, with up-regulated Fas expression. These adipocytes activate iNKT cells through CD1d and augment FasL in iNKT cells. Interaction between FasL-positive iNKT cells and Fas-positive adipocytes provokes apoptosis in hypertrophic and pro-inflammatory adipocytes, followed by the generation of new and healthy adipocytes.
iNKT cells exert effector functions through FasL expression. Also, iNKT cells mediate effector functions via activation of NK cells that secrete perforin/granzyme (Wilson and Delovitch 2003). However, given that perforin KO mice do not show any change in dead adipocytes in obesity (Revelo et al. 2015), it seems that the perforin/granzyme pathway is not crucial in obesity-induced adipocyte death. Here, we found that iNKT cells were abundantly localized around dead adipocytes and FasL-positive iNKT cells augmented adipocyte death. Given that absolute number of cytotoxic iNKT cells was increased in obese adipose tissue, it is likely that adipose iNKT cells would elevate cytotoxic capacity. To entail iNKT cell-mediated adipocyte death, it seems that iNKT cells have to be activated via adipocyte CD1d and that FasL of activated iNKT cells has to bind to Fas in adipocytes. Accordingly, our data revealed that hypertrophic adipocytes with pro-inflammatory characters activated iNKT cells and up-regulated FasL in iNKT cells. Nonetheless, it remains elusive which kinds of endogenous lipid antigens might be presented by adipocyte CD1d in obesity. Identification of endogenous lipid antigens would improve our understanding of the roles of iNKT cells in the regulation of adipocyte turnover.
We found that death of hypertrophic Fas-positive adipocytes was induced by FasL-positive iNKT cells in obese adipose tissue. In iNKT cell-depleted Jα18 KO mice, hypertrophic adipocytes were retained in obese adipose tissue, resulting in unhealthy adipose tissue expansion upon obesity. Hypertrophic Fas-positive adipocytes induced by saturated fatty acids exhibited pro-inflammatory characters and they were removed by activated iNKT cells. It has been demonstrated that hypertrophic or Fas-positive adipocytes per se secrete pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6, and CCL2, aggravating adipose tissue inflammation, insulin resistance, and type 2 diabetes (Salans et al. 1968; Brook and Lloyd 1973; Schaub et al. 2003; Farley et al. 2008; Wueest et al. 2010; Blüher et al. 2014; Cotillard et al. 2014). Clearance of Fas-positive cells by FasL is one of the major mechanisms inducing apoptosis without eliciting improper immune responses (Nagata and Tanaka 2017). It has been demonstrated that targeted apoptosis in adipocytes through activation of Caspase-8, a key downstream component of Fas signaling, attenuates adipose tissue inflammation by recruiting anti-inflammatory macrophages (Pajvani et al. 2005; Fischer-Posovszky et al. 2011b). In addition, macrophages that phagocytose apoptotic cells are able to secrete anti-inflammatory cytokines, such as IL-10, transforming growth factor-β, platelet activating factor, and prostaglandin E2, to down-regulate pro-inflammatory responses (Fadok et al. 1998; Gao et al. 1998; Xu et al. 2006; Szondy et al. 2017). In EAT of HFD-fed mice, α-GC administration altered the abundance of pro-inflammatory macrophages (CD11b+F4/80+CD11c+) and anti-inflammatory macrophages (CD11b+F4/80+CD206+) (Supplemental Fig. S5H,I). It seems that the relative ratio of anti-inflammatory to pro-inflammaotry macrophages might be increased from 4 d after iNKT cell activation. Thus, it is feasible to speculate that apoptotic removal of hypertrophic and pro-inflammatory Fas-positive adipocytes by FasL-positive iNKT cells would contribute to the suppression of pro-inflammatory responses in obese adipose tissue.
Emerging evidence suggests that adipocyte hypertrophy is an indicator of adipose tissue dysfunction and leads to insulin resistance and dyslipidemia in obesity, whereas adipocyte hyperplasia plays relatively beneficial roles in the relief of metabolic complications (Salans et al. 1968; Stern et al. 1972; Cotillard et al. 2014; An et al. 2017). However, the factors that modulate the fate of adipose tissue expansion into hypertrophy or hyperplasia have not been fully elucidated. Our data suggest that adipose iNKT cells would suppress unhealthy adipose tissue expansion by removing hypertrophic adipocytes and promoting the generation of small adipocytes. The new and small adipocytes exhibited a stronger glucose uptake capacity than old and hypertrophic adipocytes, probably leading to improvement of insulin resistance. Although it remains to be clarified how adipogenesis is promoted by activated iNKT cells in obesity, macrophages reportedly can cause proliferation and differentiation of PDGFRα-positive adipocyte progenitor cells (Lee et al. 2013; Hill et al. 2018), and iNKT cells interact with macrophages around dead adipocytes (Lynch et al. 2015). Moreover, it has been suggested that FasL might promote proliferation and differentiation of adipocyte progenitor cells (Solodeev et al. 2018). Thus, elucidation of ternary relations between adipocytes, iNKT cells, and macrophages in adipocyte turnover will be helpful to decipher the dynamic regulation of adipose tissue remodeling.
Numerous studies have highlighted T cell cytotoxicity as an immunotherapeutic approach to remove harmful or dysregulated cells (Fesnak et al. 2016; June et al. 2018). However, the application of such immunotherapy for the treatment of metabolic disorders such as obesity and type 2 diabetes has not been explored. It is feasible to speculate that iNKT cells would be able to sense and respond to imbalanced lipid metabolites in obese adipose tissue because they are activated by recognizing lipid antigens loaded onto CD1d. Among several tissues, such as adipose tissue, liver, and spleen, where iNKT cells are abundant, adipose iNKT cells prominently expressed FasL in DIO and genetically obese mice. Both Fas and FasL are up-regulated in obese and diabetic patients (Fischer-Posovszky et al. 2004; Blüher et al. 2014) and Fas-mediated apoptosis provokes adipocyte loss in patients with acquired lipodystrophy disorders (Hussain and Garg 2016). Nevertheless, the sources of FasL have not been yet elucidated. Further, even though iNKT cells are abundant in human adipose tissue (Lynch et al. 2012), their roles in adipocyte pathophysiology have not been fully defined. Based on our findings, we believe that the cytotoxic roles of iNKT cells in human adipose tissue are worth being considered in the fight against metabolic dysfunction.
In conclusion, we have elucidated a novel role of adipose iNKT cells in the regulation of adipocyte turnover, potentially leading to healthy adipose tissue expansion. In obesity, activated adipose iNKT cells participate in adipose tissue remodeling through the stimulation of damaged adipocyte death as well as the induction of adipogenesis (Fig. 7). Collectively, our data suggest that adipocyte turnover by iNKT cells is a key process to maintain adipose tissue homeostasis.
Materials and methods
Animals and treatments
C57BL6/J mice were obtained from Central Lab Animal Incorporation. After 1 wk of acclimatization, 8-wk-old mice were fed a NCD or a 60% HFD purchased from Research Diets Incorporation for 8 wk. db/db mice were purchased from Central Lab Animal Incorporation and sacrificed at 12 wk of age. Jα18 heterozygous mice were bred to generate WT mice and knock-out (KO) littermates. Adipocyte-specific CD1d KO (CD1dAKO) mice were generated by crossing adiponectin Cre mice with CD1dflox mice (C57BL/6-CD1d1tm1.Aben/J; The Jackson Laboratory). AdipoChaser mice (AdiponectinrtTA; TRE-Cre; Rosa26-loxp-stop-loxp-YFP) were kindly provided by Dr. Philipp Scherer (UT Southwestern). AdipoChaser mice were kept on a HFD for 6 wk and were then fed a Dox (600 mg/kg)-containing HFD for 2 wk. To activate iNKT cells, α-GC (1 μg/mouse; Adipogen) was intraperitoneally injected. Glucose tolerance test was performed using intraperitoneal glucose injection (1 g/kg body weight) after overnight fasting. All experiments with mice were approved by the Seoul National University Institutional Animal Care and Use Committee (SNUIACUC). Neutralizing anti-FasL monoclonal IgG (BioLegend) was previously described (Hattori et al. 1997). HFD-fed WT mice were injected intraperitoneally with 300 μg of anti-FasL antibody, followed by α-GC injection. Age-matched control mice were treated similarly with control IgG. Mice were monitored 1 d after α-GC injection to investigate whether neutralization of FasL affects iNKT cell-induced adipocyte death.
Primary adipocyte generation and analysis
Adipocyte fractionation from whole epididymal adipose tissue was conducted as previously described (Lee et al. 2011). Briefly, adipose tissues were digested with type I collagenase for 30 min in a water bath at 37°C with shaking. After centrifugation at 1000 rpm and at room temperature (RT) for 5 min, floating adipocytes were collected. Primary adipocytes were washed several times with phosphate-buffered saline (PBS). Primary adipocytes were incubated with fluorescence-labeled Fas mAb (eBioscience) at RT for 1 h to detect Fas-positive adipocytes. To distinguish dead/dying adipocytes from live adipocytes, primary adipocytes were stained with propidium iodide (PI, eBioscience) or TUNEL assay (Roche) at RT for 1 h. After staining, the primary adipocytes were analyzed using a BioSorter instrument (Union Biometrica) following the manufacturer's instruction.
Whole-mount immunohistochemistry
Whole-mount immunohistochemistry was performed as previously described (Ham et al. 2016). Whole epididymal adipose tissues were removed and fixed with 4% paraformaldehyde for 1 h. The samples were blocked with 1% horse serum for 1 h and then stained with primary antibody at 4°C overnight. Monoclonal antibodies (mAbs) against PERILIPIN-1 (Fitzgerald), CD11b (eBioscience), phycoerythrin-conjugated PBS57-loaded CD1d-tetramer (National Institutes of Health Tetramer Core Facility), and GFP (Santa Cruz Biotechnology) were used to detect adipocytes, macrophages, iNKT cells, and YFP-positive adipocytes, respectively. After a 1-h wash, the samples were incubated with fluorescence-labeled secondary antibody at RT for 4 h. After incubation with mounting solution containing 4′,6-diamidino-2-phenylindole (DAPI, Vector Lab Incorporation), and the samples were observed using a Zeiss LSM 700 confocal microscope (Carl Zeiss).
Flow cytometry and fluorescence-assisted cell sorting (FACS)
Flow-cytometric iNKT cell analysis was carried out as previously described (Shin et al. 2017). The levels of FasL in iNKT cells and DN32.D3 cells, an iNKT cell hybridoma cell line, were assessed using FasL mAb (eBioscience). Fas expression and the frequency of dead 3T3-L1 adipocytes were investigated using Fas mAb (eBioscience) and PI, respectively. The proliferation rate was analyzed using Ki67 mAb (eBioscience). Macrophages were analyzed using CD11b, F4/80, CD11c, and CD206 mAb (eBioscience). Cells were analyzed using a FACS Canto II instrument (BD Biosciences). To isolate adipocyte progenitor cells, epididymal adipose tissues were fractionated into adipocytes and stromal vascular cells (SVCs) and SVCs were stained using CD29 (BioLegend), CD34, CD31, CD45, and PDGFRα (BD Biosciences) mAbs. After SVCs were stained with mAbs against, CD31−CD45−PDGFRα+, adipocyte progenitor cells were sorted using a FACS Aria II (BD Biosciences) at the National Center for Inter-university Research Facilities (NCIRF) at Seoul National University.
Coculture
3T3-L1 pre-adipocytes were grown to confluence and then differentiated in 12-well culture plates. Differentiated 3T3-L1 adipocytes were pretreated with α-GC (100 ng/mL) for 4 h and then washed with PBS. DN32.D3 cells (1 × 105) were incubated with differentiated 3T3-L1 adipocytes for 48 h. FFA treatment (500 μm) was conducted as previously described (Kim et al. 2015). Briefly, FFAs were dissolved in ethanol and diluted in DMEM containing 1% fetal bovine serum and 2% bovine serum albumin (BSA) at 55°C for 10 min. BSA-conjugated FFA-containing media were used for challenging differentiated 3T3-L1 adipocytes for 2 wk at 2-d intervals. Then, the cells were challenged with TNFα (10 nM) for 1 d. Dead adipocytes and FasL-positive DN32.D3 cells were analyzed by FACS.
Apoptosis assay
The frequency of apoptotic adipocytes was determined using an in situ cell death detection kit (Roche) according to the manufacturer's protocol. TUNEL-positive cells were analyzed under a microscope, and statistical analysis was performed using LSM510 software (Carl Zeiss). For the detection of annexin V-positive cells, we used the annexin V staining kit purchased from BD Biosciences. According to the manufacturer's protocol, the frequency of annexin V-positive adipocytes was analyzed using FACS.
Ex vivo glucose bioprobe uptake assay
Ex vivo glucose bioprobe uptake was assayed as previously described (Kim et al. 2015).
Western blot analysis
Western blotting was performed as previously described (Sohn et al. 2018).
RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was carried out as previously described (Lee et al. 2017). The mRNA level of cyclophilin was used for normalization. Sequence information for the primers used is provided in Supplemental Table S1.
Transfection with small interfering RNA (siRNA)
siRNA duplexes for Cd1d1, caspase-3, caspase-8, and Fas were synthesized at Bioneer Incorporation. siRNAs were delivered into 3T3-L1 cells by electroporation. As a negative control, a scrambled siRNA (siNC) was used.
Statistical analysis
Data are reported as the mean ± standard error of the mean (SEM). Means were compared by two-tailed Student's t-tests or two-way ANOVA with a Bonferroni post-hoc test. Statistical analyses were performed using Prism (GraphPad Software), and differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We would like to thank Dr. Philipp Scherer for providing the adipocyte lineage-tracing mouse model. The authors thank the National Institutes of Health Tetramer Core Facility for the generous gift of CD1d tetramers. This study was supported by the National Research Foundation (NRF) grant funded by the Korea government (MIST) (2011-0018312, J.B.K., 2017M3A9C4065956, J.P.). J.P. (Seoul National University), J.I.K., S.M.H., K.C.S., and Y.G.J. were supported by the BK21 plus program.
Author contributions: J.P. (Seoul National University) designed and conducted the study and wrote the paper. J.Y.H., J.O., J.I.K., S.M.H., K.C.S., Y.G.J., S.S.C., and J.P. (UNIST) performed the experiments and analyzed the data. J.B.K. supervised the study and manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.329557.119.
References
- Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. 2010. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 285: 3428–3438. 10.1074/jbc.M109.074252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An YA, Sun K, Joffin N, Zhang F, Deng Y, Donzé O, Kusminski CM, Scherer PE. 2017. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. Elife 6: e24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. 1994. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372: 691–694. 10.1038/372691a0 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Rivera MN, Park SH, Roark JH. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol 15: 535–562. 10.1146/annurev.immunol.15.1.535 [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. 2007. The biology of NKT cells. Annu Rev Immunol 25: 297–336. 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med 191: 1895–1904. 10.1084/jem.191.11.1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953. 10.1038/90992 [DOI] [PubMed] [Google Scholar]
- Berzins SP, Smyth MJ, Baxter AG. 2011. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol 11: 131–142. 10.1038/nri2904 [DOI] [PubMed] [Google Scholar]
- Bessoles S, Dudal S, Besra GS, Sanchez F, Lafont V. 2009. Human CD4+ invariant NKT cells are involved in antibacterial immunity against Brucella suis through CD1d-dependent but CD4-independent mechanisms. Eur J Immunol 39: 1025–1035. 10.1002/eji.200838929 [DOI] [PubMed] [Google Scholar]
- Blüher M, Klöting N, Wueest S, Schoenle EJ, Schön MR, Dietrich A, Fasshauer M, Stumvoll M, Konrad D. 2014. Fas and FasL expression in human adipose tissue is related to obesity, insulin resistance, and type 2 diabetes. J Clin Endocrinol Metab 99: E36–E44. 10.1210/jc.2013-2488 [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. 2013. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13: 101–117. 10.1038/nri3369 [DOI] [PubMed] [Google Scholar]
- Brook CG, Lloyd JK. 1973. Adipose cell size and glucose tolerance in obese children and effects of diet. Arch Dis Child 48: 301–304. 10.1136/adc.48.4.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB. 2014. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63: 3359–3371. 10.2337/db13-1965 [DOI] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. 2016. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. 2005. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355. 10.1194/jlr.M500294-JLR200 [DOI] [PubMed] [Google Scholar]
- Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Klöting N, Grégoire C, Lolmede K, Blüher M, Clément K. 2014. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab 99: E1466–E1470. 10.1210/jc.2014-1074 [DOI] [PubMed] [Google Scholar]
- DiSpirito JR, Mathis D. 2015. Immunological contributions to adipose tissue homeostasis. Semin Immunol 27: 315–321. 10.1016/j.smim.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo P, Matias-Guiu X, Pujol RM, Francia E, Lagarda E, Sambeat MA, Vázquez G. 1999. Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. AIDS 13: 2261–2267. 10.1097/00002030-199911120-00008 [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898. 10.1172/JCI1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley SM, Purdy DE, Ryabinina OP, Schneider P, Magun BE, Iordanov MS. 2008. Fas ligand-induced proinflammatory transcriptional responses in reconstructed human epidermis. Recruitment of the epidermal growth factor receptor and activation of MAP kinases. J Biol Chem 283: 919–928. 10.1074/jbc.M705852200 [DOI] [PubMed] [Google Scholar]
- Fesnak AD, June CH, Levine BL. 2016. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16: 566–581. 10.1038/nrc.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916. 10.1128/IAI.73.4.1907-1916.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Tornqvist H, Debatin KM, Wabitsch M. 2004. Inhibition of death-receptor mediated apoptosis in human adipocytes by the insulin-like growth factor I (IGF-I)/IGF-I receptor autocrine circuit. Endocrinology 145: 1849–1859. 10.1210/en.2003-0985 [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Hebestreit H, Hofmann AK, Strauss G, Möller P, Debatin KM, Wabitsch M. 2006. Role of CD95-mediated adipocyte loss in autoimmune lipodystrophy. J Clin Endocrinol Metab 91: 1129–1135. 10.1210/jc.2005-0737 [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Keuper M, Nagel S, Hesse D, Schürmann A, Debatin KM, Strauss G, Wabitsch M. 2011a. Downregulation of FLIP by cycloheximide sensitizes human fat cells to CD95-induced apoptosis. Exp Cell Res 317: 2200–2209. 10.1016/j.yexcr.2011.06.016 [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE. 2011b. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 152: 3074–3081. 10.1210/en.2011-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. 1998. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med 188: 887–896. 10.1084/jem.188.5.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaya M, Barral P, Burbage M, Aggarwal S, Montaner B, Warren Navia A, Aid M, Tsui C, Maldonado P, Nair U, et al. 2018. Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 172: 517–533.e20. 10.1016/j.cell.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, Barazzoni R, Scherer PE, Cinti S. 2013. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res 54: 2423–2436. 10.1194/jlr.M038638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. 2009. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab 297: E999–E1003. 10.1152/ajpendo.00377.2009 [DOI] [PubMed] [Google Scholar]
- Ham M, Lee JW, Choi AH, Jang H, Choi G, Park J, Kozuka C, Sears DD, Masuzaki H, Kim JB. 2013. Macrophage glucose-6-phosphate dehydrogenase stimulates proinflammatory responses with oxidative stress. Mol Cell Biol 33: 2425–2435. 10.1128/MCB.01260-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham M, Choe SS, Shin KC, Choi G, Kim JW, Noh JR, Kim YH, Ryu JW, Yoon KH, Lee CH, et al. 2016. Glucose-6-Phosphate dehydrogenase deficiency improves insulin resistance with reduced adipose tissue inflammation in obesity. Diabetes 65: 2624–2638. 10.2337/db16-0060 [DOI] [PubMed] [Google Scholar]
- Hattori K, Hirano T, Ushiyama C, Miyajima H, Yamakawa N, Ebata T, Wada Y, Ikeda S, Yoshino K, Tateno M, et al. 1997. A metalloproteinase inhibitor prevents lethal acute graft-versus-host disease in mice. Blood 90: 542–548. 10.1182/blood.V90.2.542 [DOI] [PubMed] [Google Scholar]
- Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, et al. 2018. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci 115: E5096–E5105. 10.1073/pnas.1802611115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, Lee SK, Alfadda AA, Kim SS, Choi SH, et al. 2013. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol 33: 328–339. 10.1128/MCB.00552-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Park YJ, Ham M, Kim JB. 2014. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 37: 365–371. 10.14348/molcells.2014.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Park J, Kim JI, Park YJ, Lee YK, Kim JB. 2017. Deletion of CD1d in adipocytes aggravates adipose tissue inflammation and insulin resistance in obesity. Diabetes 66: 835–847. 10.2337/db16-1122 [DOI] [PubMed] [Google Scholar]
- Huh JY, Park YJ, Kim JB. 2018. Adipocyte CD1d determines adipose inflammation and insulin resistance in obesity. Adipocyte 7: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Garg A. 2016. Lipodystrophy syndromes. Endocrinol Metab Clin North Am 45: 783–797. 10.1016/j.ecl.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. 2015. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 17: 376–385. 10.1038/ncb3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. 2012. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem 287: 13561–13571. 10.1074/jbc.M112.350066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. 2009. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5: e1000324 10.1371/journal.pcbi.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. 2018. CAR T cell immunotherapy for human cancer. Science 359: 1361–1365. 10.1126/science.aar6711 [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. 1994. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265: 528–530. 10.1126/science.7518614 [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278: 1626–1629. 10.1126/science.278.5343.1626 [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637. 10.1172/JCI31021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. 2015. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol 35: 1686–1699. 10.1128/MCB.01321-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. 1994. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity 1: 357–364. 10.1016/1074-7613(94)90066-3 [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, et al. 2012. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 18: 1539–1549. 10.1038/nm.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Chen SY, Wiesner RJ, Huang YF. 2004. Simple flow cytometric method used to assess lipid accumulation in fat cells. J Lipid Res 45: 1162–1167. 10.1194/jlr.D300028-JLR200 [DOI] [PubMed] [Google Scholar]
- Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, et al. 2011. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483. 10.2337/db11-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. 2012. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab 15: 480–491. 10.1016/j.cmet.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Granneman JG. 2013. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 18: 355–367. 10.1016/j.cmet.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jeon YG, Lee KH, Lee HW, Park J, Jang H, Kang M, Lee HS, Cho HJ, Nam DH, et al. 2017. RNF20 suppresses tumorigenesis by inhibiting the SREBP1c-PTTG1 axis in kidney cancer. Mol Cell Biol 37: e00265-17 10.1128/MCB.00265-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, Mathis D. 2018. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-Treg phenotype. Cell 174: 285–299.e12. 10.1016/j.cell.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin B, Hahne M, Mattmann C, Tschopp J. 1994. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370: 650–652. 10.1038/370650a0 [DOI] [PubMed] [Google Scholar]
- Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG, O'Farrelly C. 2009. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol 39: 1893–1901. 10.1002/eji.200939349 [DOI] [PubMed] [Google Scholar]
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. 2012. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37: 574–587. 10.1016/j.immuni.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, et al. 2015. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat Immunol 16: 85–95. 10.1038/ni.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med 192: 741–754. 10.1084/jem.192.5.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Tanaka M. 2017. Programmed cell death and the immune system. Nat Rev Immunol 17: 333–340. 10.1038/nri.2016.153 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. 2005. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 11: 797–803. 10.1038/nm1262 [DOI] [PubMed] [Google Scholar]
- Park YJ, Park J, Huh JY, Hwang I, Choe SS, Kim JB. 2018. Regulatory roles of invariant natural killer T cells in adipose tissue inflammation: defenders against obesity-induced metabolic complications. Front Immunol 9: 1311 10.3389/fimmu.2018.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JB, Walker NI, Winterford CM, Cameron DP. 1994. Human adipocyte apoptosis occurs in malignancy. Biochem Biophys Res Commun 205: 625–630. 10.1006/bbrc.1994.2711 [DOI] [PubMed] [Google Scholar]
- Revelo XS, Tsai S, Lei H, Luck H, Ghazarian M, Tsui H, Shi SY, Schroer S, Luk CT, Lin GH, et al. 2015. Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes 64: 90–103. 10.2337/db13-1524 [DOI] [PubMed] [Google Scholar]
- Rock KL, Kono H. 2008. The inflammatory response to cell death. Annu Rev Pathol 3: 99–126. 10.1146/annurev.pathmechdis.3.121806.151456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. 2008. Identification of white adipocyte progenitor cells in vivo. Cell 135: 240–249. 10.1016/j.cell.2008.09.036 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. 2014. What we talk about when we talk about fat. Cell 156: 20–44. 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén M, Andersson DP, Bergström IB, Arner P. 2014. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab 99: E1870–1876. 10.1210/jc.2014-1526 [DOI] [PubMed] [Google Scholar]
- Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. 2014. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest 124: 3725–3740. 10.1172/JCI72308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans LB, Knittle JL, Hirsch J. 1968. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest 47: 153–165. 10.1172/JCI105705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans LB, Cushman SW, Weismann RE. 1973. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest 52: 929–941. 10.1172/JCI107258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Hoshino M, Fujita K, Iizuka M, Fujii S, Clingan CS, Van Kaer L, Iwabuchi K. 2016. Adipocyte-specific CD1d-deficiency mitigates diet-induced obesity and insulin resistance in mice. Sci Rep 6: 28473 10.1038/srep28473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub FJ, Liles WC, Ferri N, Sayson K, Seifert RA, Bowen-Pope DF. 2003. Fas and Fas-associated death domain protein regulate monocyte chemoattractant protein-1 expression by human smooth muscle cells through caspase- and calpain-dependent release of interleukin-1α. Circ Res 93: 515–522. 10.1161/01.RES.0000093205.42313.7C [DOI] [PubMed] [Google Scholar]
- Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, et al. 2012. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest 122: 3343–3354. 10.1172/JCI62739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, Chen S, Yu X, An YA, Zhu Y, et al. 2018. De novo adipocyte differentiation from Pdgfrβ+ preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun 9: 890 10.1038/s41467-018-03196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. 1993. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 268: 22243–22246. [PubMed] [Google Scholar]
- Shin KC, Hwang I, Choe SS, Park J, Ji Y, Kim JI, Lee GY, Choi SH, Ching J, Kovalik JP, et al. 2017. Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat Commun 8: 1087 10.1038/s41467-017-01232-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JH, Lee YK, Han JS, Jeon YG, Kim JI, Choe SS, Kim SJ, Yoo HJ, Kim JB. 2018. Perilipin 1 (Plin1) deficiency promotes inflammatory responses in lean adipose tissue through lipid dysregulation. J Biol Chem 293: 13974–13988. 10.1074/jbc.RA118.003541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodeev I, Meilik B, Volovitz I, Sela M, Manheim S, Yarkoni S, Zipori D, Gur E, Shani N. 2018. Fas-L promotes the stem cell potency of adipose-derived mesenchymal cells. Cell Death Dis 9: 695 10.1038/s41419-018-0702-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al. 2008. Dynamics of fat cell turnover in humans. Nature 453: 783–787. 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- Stern JS, Hollander N, Batchelor BR, Cohn CK, Hirsch J. 1972. Adipose-cell size and immunoreactive insulin levels in obese and normal-weight adults. Lancet 300: 948–951. 10.1016/S0140-6736(72)92474-9 [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. 2007. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56: 2910–2918. 10.2337/db07-0767 [DOI] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. 2011. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101. 10.1172/JCI45887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szondy Z, Sarang Z, Kiss B, Garabuczi E, Köröskényi K. 2017. Anti-inflammatory mechanisms triggered by apoptotic cells during their clearance. Front Immunol 8: 909 10.3389/fimmu.2017.00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK. 2016. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 23: 350–359. 10.1016/j.cmet.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. 2013. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19: 1338–1344. 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SB, Delovitch TL. 2003. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol 3: 211–222. 10.1038/nri1028 [DOI] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332: 243–247. 10.1126/science.1201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, Chervonsky AV, Rudich A, Schoenle EJ, Donath MY, et al. 2010. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest 120: 191–202. 10.1172/JCI38388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. 2006. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood 107: 4930–4937. 10.1182/blood-2005-10-4144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.