Abstract
Cystic renal lesions are a common incidental finding on routinely imaging examinations. Although a benign simple cyst is usually easy to recognize, the same is not true for complex and multifocal cystic renal lesions, whose differential diagnosis includes both neoplastic and non-neoplastic conditions. In this review, we will show a series of cases in order to provide tips to identify benign cysts and differentiate them from malignant ones.
Keywords: Bosniak, Cystic renal lesion, Cystic renal cell carcinoma, CT, MR
Key points
Cystic renal lesions are a common incidental finding on routinely imaging examinations.
Benign simple cyst is usually easy to recognize at imaging.
Differential diagnosis of complex and multifocal cystic renal lesions include both neoplastic and non-neoplastic conditions.
The most widely used system to classify cystic renal lesions was introduced by Bosniak in 1984 and revised in 1997.
Renal cysts can be divided into focal and multifocal.
Introduction
Cystic renal lesions are very commonly encountered at abdominal ultrasound, computed tomography (CT), and magnetic resonance (MR) imaging. Most lesions are asymptomatic and incidentally found, but they can rarely manifest with abdominal pain, hematuria, and signs of infection (e.g., fever). Although the majority represents simple cysts, their pathologic spectrum is broad and includes developmental, neoplastic, and inflammatory processes.
Ultrasound represents usually the first-line imaging examination of the abdomen and kidney and can easily recognize simple, fluid-filled renal cysts with the following criteria: homogeneous anechoic content, marked posterior enhancement, and well-defined borders [1, 2].
When these criteria are absent, a cystic renal lesion is classified as a complex cyst [1, 2].
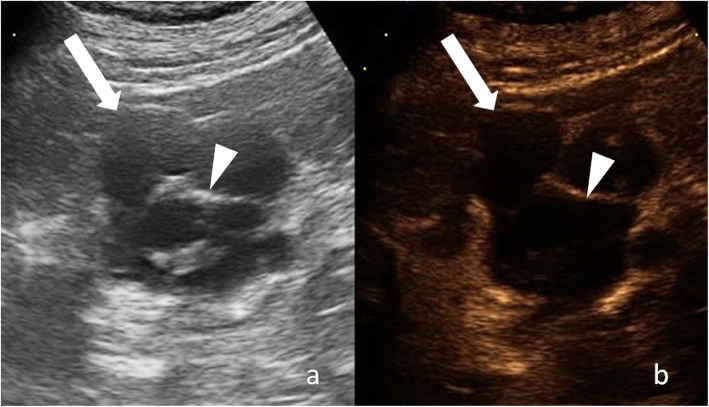
The term “complicated cyst” must be reserved to those cysts, which undergo morphological changes due to documented rupture, hemorrhage, or infection [1, 2]. Complex and complicated renal cysts cannot be accurately characterized at ultrasound and usually warrant contrast-enhanced CT or magnetic resonance (MR) imaging [1, 2]. Because of absence of ionizing radiation and low-cost contrast-enhanced US (CEUS) is emerging as a valuable alternative to contrast-enhanced CT and MR [3, 4]. A growing body of evidence suggests that CEUS is useful to evaluate the vascularity of both cystic renal and hepatic lesion in real time using microbubble-based, purely intravascular, contrast agents (Fig. 1) [3–7]. However, the use of CEUS hampered operator dependency and technical limitations due to deep lesion location, bowel interposition, patient body habitus, and patient cooperation [3, 8]. Knowledge of the imaging characteristics and understanding the pathophysiology of cystic renal lesions helps the radiologist to derive the correct diagnosis.
Fig. 1.
Cystic renal lesion in a 76-year-old-man. a Gray-scale ultrasound shows a cystic lesion (arrow) with a thin wall and thin septa (arrowhead), which contains fine calcifications. b Corresponding CEUS image shows enhancement of cyst wall and septa
A useful strategy for the evaluation of renal cysts is to divide them into focal and multifocal.
In this paper, we will expose radiologists to a series of CT and MRI cases in order to provide tips to identify benign cysts and differentiate them from malignant ones in adult patients.
CT or MRI: advantages and disadvantages
Contrast-enhanced CT is the modality of choice in evaluating cystic renal masses. Narrow detector thickness (< 1 mm) and intravenous administration of contrast agent are mandatory to detect thin septa and small enhancing nodules [9]. Also, demonstration of enhancing areas helps differentiate solid components from hemorrhage or debris [10]. MRI is used when CT is contraindicated (e.g., patients with allergy to iodinated contrast agent) or as a problem-solving modality for equivocal findings. Indeed, MRI can show some septa that are less apparent at CT and demonstrate definitive enhancement in those cysts that show only equivocal enhancement at CT [11]. As a consequence, renal cysts can be placed in a higher Bosniak category with MRI than with CT [11].
Focal renal cysts
Focal renal cysts are common in older subjects. Their prevalence, size, and number increase with age, with approximately 30% of people after the fourth decade and 40% after the fifth decade having at least one renal cyst [12, 13]. The majority is benign simple renal cysts and can be diagnosed with confidence. However, cystic renal lesions can have benign as well as malignant causes. Possible malignant causes include renal cell carcinoma (RCC) and metastasis. Since cystic RCCs, benign complicated cysts, and other cystic tumors can be radiologically indistinguishable, the goal of imaging when a renal cyst is found is to differentiate a benign “leave-alone” lesion from a lesion that requires treatment.
Bosniak classification system for renal cysts
The most widely used system to classify cystic renal lesions was introduced by Bosniak in 1984 and revised in 1997 [14, 15]. This system was originally developed on CT findings, but it can be also used at MRI [11, 16, 17].
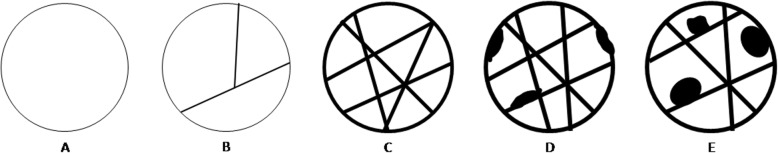
Renal cysts are divided into five categories on the basis of imaging appearance (Table 1, Fig. 2). Each Bosniak category reflects the likelihood of cystic RCC that ranges from I (simple cyst) to IV (cystic tumors). Category I, II, and, IIF cysts are nonsurgical, while categories III and IV are surgical.
Table 1.
The five categories of renal cysts, divided on the basis of imaging appearance
| Bosniak category | Wall | Septa | Calcifications | Enhancing nodularity |
|---|---|---|---|---|
| I |
Thin Non-enhancing |
- | - | - |
| II |
Thin Non-enhancing |
Few Thin |
Fine or slightly thickened | - |
| II-Fa |
Minimal thickening Perceived enhancement |
Several Minimal thickening Perceived enhancement |
Irregular or nodular | - |
| IIIb |
Irregularly thick Measurable enhancement |
Several Irregularly thick Measurable enhancement |
Variable | - |
| IV |
Irregularly thick Measurable enhancement |
Several Minimal thickening Measurable enhancement |
Variable | Wall and/or septa |
aThis category includes complicated (< 3 cm) cysts
bThis category includes complicated (> 3 cm) cysts
Fig. 2.
Imaging features of cystic renal lesions according to Bosniak classification. a Bosniak category I cyst: thin wall. b Bosniak category II cyst: thin wall; few, thin septa. c Bosniak category II-F cyst: minimally thickened wall; several, minimally thickened septa. d Bosniak category III cyst: irregularly thickened wall; several, irregularly thickened septa. e Bosniak category IV cyst: enhancing nodularity; irregularly thickened wall; several, irregularly thickened septa
Imaging findings include attenuation/signal intensity, size, presence of calcifications, septa and enhancing nodularity. Among these, enhancing nodularity is considered the most important predictor of malignancy [18]. At CT, enhancement requires an increase of attenuation of at least 15–20 HU from unenhanced to the contrast enhanced images [18]. A 10–15 HU change in attenuation can be due to incorrect placement of the region of interest, patient motion, or beam hardening from adjacent enhancing renal parenchyma (the so called “pseudoenhancement”) [19]. To overcome this problem, it has been suggested to use dual-energy CT, where true unenhanced images can be replaced by virtual unenhanced images [20]. Iodine quantification and iodine-related attenuation are used to differentiate nonenhancing cysts from enhancing solid masses [20].
In equivocal cases, another option is to use subtraction MRI to assess the presence or absence of enhancement [21].
Septa are defined as dividing wall within a renal cyst and are best appreciated at MRI than at CT. When present, they can be classified as thin, minimally thickened, or grossly thickened and irregular, and as enhancing or non-enhancing.
Calcifications are usually easy to recognize at CT but may be unapparent at MRI. Despite the importance in predicting the malignancy of solid renal masses, calcifications have limited utility in the Bosniak classification since they can be found in the wall or septa of both benign and malignant cysts [22]. Similarly, the size does not reliably predict the benignity or malignity of a renal cyst. Indeed, larger cysts can be benign and small ones can be malignant.
Category I renal cysts
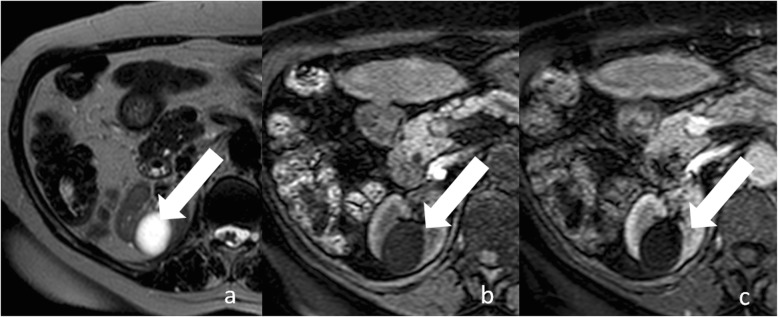
Category I cysts are simple benign cysts. The exact pathogenesis is unknown. It has been suggested that they originate from weakening of the basement membrane of distal convoluted or collecting tubules [23]. Imaging appearance is consistent with water content: 0–20 HU attenuation on unenhanced CT, strong hyperintensity on T2-weighted MRI sequences, hypointensity on T1-weighted MRI sequences (Figs. 3 and 4). The wall is thin, hair-line, and non-enhancing. Calcifications, septa, and enhancing nodularity are absent. Almost all are benign. In a study including 1700 individuals with at least one renal cyst, only two patients developed a renal neoplasm [12]. Category I renal can grow in size over time. Treatment or follow-up are not recommended.
Fig. 3.
Bosniak category I renal cyst. Axial non-enhanced (a) and contrast-enhanced (b) CT images shows a cyst (arrow) with a thin and non-enhancing wall
Fig. 4.
Bosniak category I renal cyst. a Axial T2-weighted MR image shows a lesion (arrow) with strong hyperintensity and a thin wall. Corresponding axial non-enhanced (b) and contrast-enhanced (c) T1-weighted MR images show a hypointense lesion with a thin and non-enhancing wall
Category II renal cysts
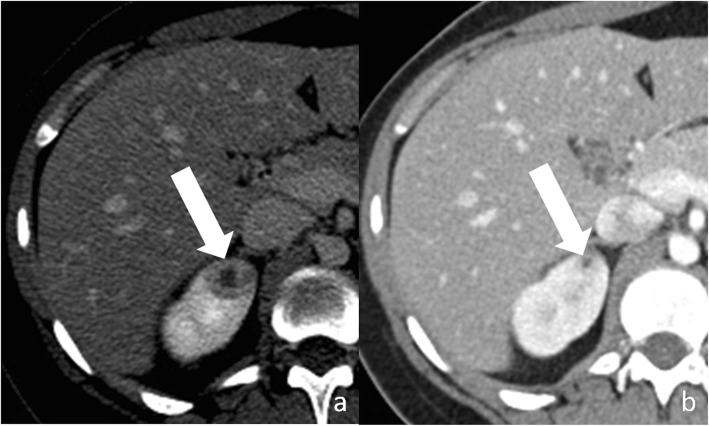
Category II renal cysts are slightly more complicated in that they show hair-line wall, and few, thin septa, which can show perceived (not measurable) enhancement (Fig. 5). Fine calcifications or a short segment of slightly thickened calcifications can be present in the wall or septa. Complicated (proteinaceous or hemorrhagic) renal cysts measuring less than 3 cm are also included in the category II. These cysts show hyperattenuation (> 20 HU) on unenhanced CT, high signal intensity on unenhanced T1-weighted MRI sequences, and no enhancement, which helps differentiate benign cyst from RCC. Lesion homogeneity and smooth borders also are highly suggestive of a benign cyst [24]. In general, proteinaceous cysts measure 20–40 HU and are anechoic at ultrasound, while hemorrhagic cysts measure over 40 50 HU and can show a complex appearance at ultrasound [25]. Category II renal cysts are benign, and do not require treatment or follow-up.
Fig. 5.
Bosniak category II renal cyst. a Axial non-enhanced CT image shows a lesion with a thin wall (arrow) and a thin septum (arrowhead), which contains fine calcifications. b Corresponding axial contrast-enhanced CT image shows enhancement of cyst wall and septum
Category IIF renal cysts
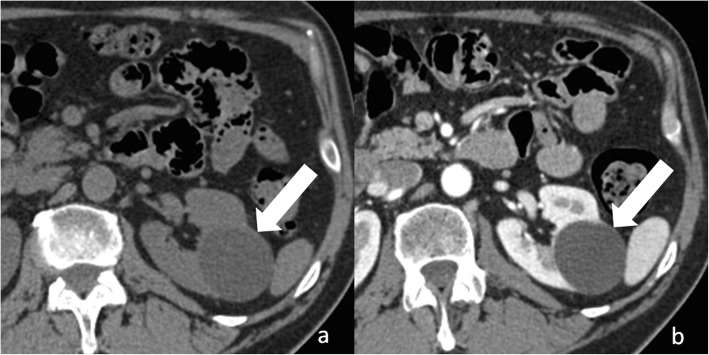
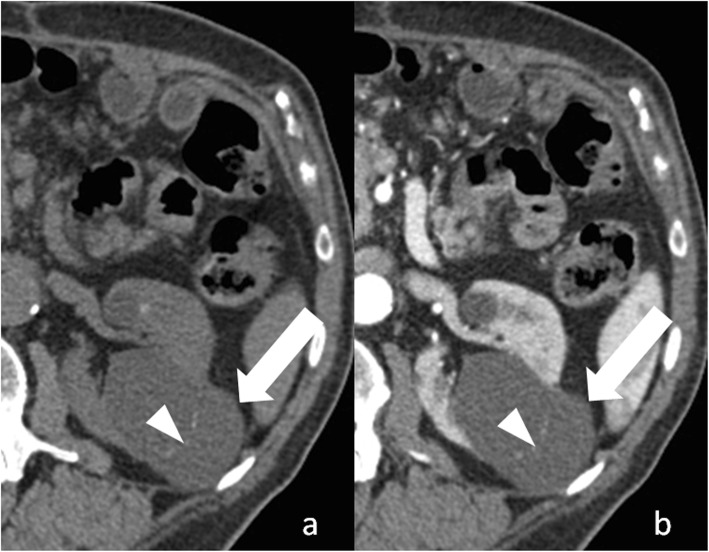
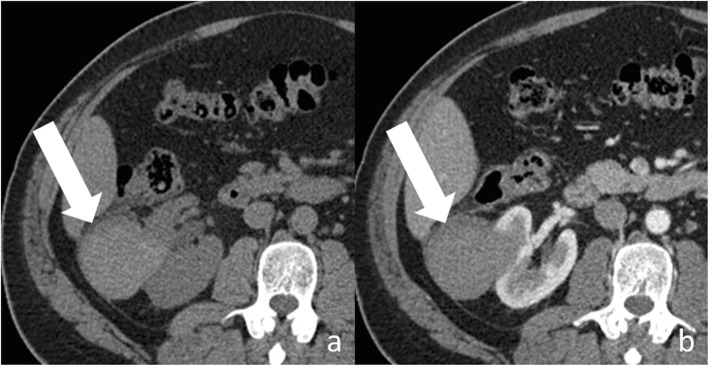
Category IIF renal cysts (“F” means follow-up) are more worrisome than category I and II [15, 26, 27]. The wall and septa can show minimal thickening and perceived (not measurable) enhancement and can contain irregular or nodular calcifications (Fig. 6). Unlike category II cysts, they can contain several septa. Complicated renal cysts measuring more than 3 cm are included in the category IIF (Fig. 7). Category IIF renal cysts are benign in 75–95% of time [28–30]. Imaging follow-up is required to exclude the malignancy by showing stability over time. However, the optimal interval time for follow-up is unclear and is influenced by cyst complexity. Bosniak had suggested that category IIF cysts with minimal complications need a 1–2-year follow-up, while more complex ones require at least a 3–4 year follow-up [31].
Fig. 6.
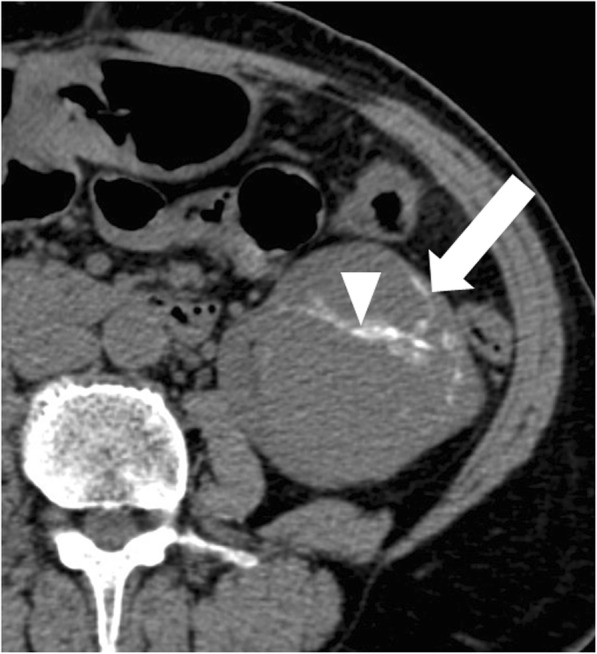
Bosniak category IIF renal cyst. Axial non-enhanced CT image shows a lesion with irregular calcifications within the wall (arrow) and septa (arrowhead)
Fig. 7.
Bosniak category IIF renal cyst. Axial non-enhanced (a) and contrast-enhanced (b) CT images shows a large (> 3 cm) lesion (arrow) with spontaneous hyperattenuation and no enhancement
Category III renal cysts
Category III renal cysts are indeterminate lesions with a reported malignancy of nearly 50% [28]. This category includes multilocular cysts, hemorrhagic and infected cysts, multilocular cystic nephroma, and cystic RCC [32]. Wall and septa are irregularly thick, show a measurable enhancement, and can contain thick nodular calcifications (Fig. 8). Septa are increased in number compared to category II cysts. Surgical removal of category III renal cysts is recommended because of their increased risk of malignancy.
Fig. 8.
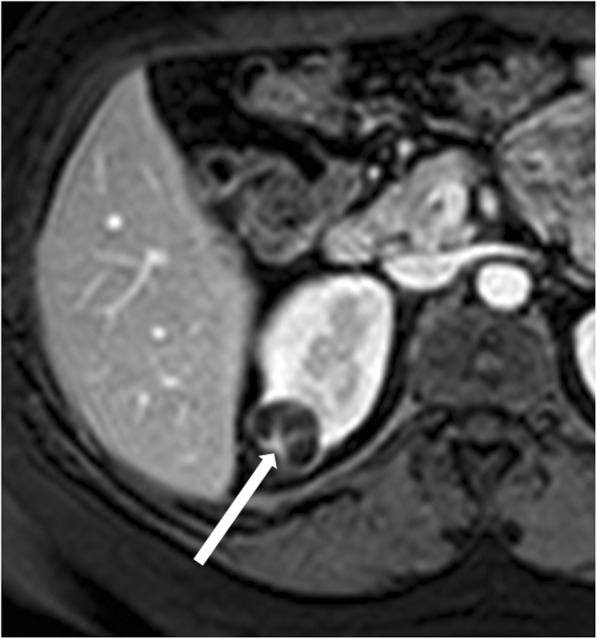
Bosniak category III renal cyst. Axial contrast-enhanced T1-weighted MR image shows a lesion with thick, enhancing wall and septa (arrow)
Category IV renal cysts
Category IV renal cysts are considered malignant lesions. Nearly all are RCCs or, more rarely, metastases [32]. However, there are few benign lesions such as mixed epithelial and stromal tumor (MEST) and cystic angiomyolipomas that can be classified as category IV renal cysts [32]. The hallmark of this category is the presence of enhancing nodularity (Fig. 9). These cysts can also contain all findings observed in category III. Surgical removal is strongly recommended.
Fig. 9.
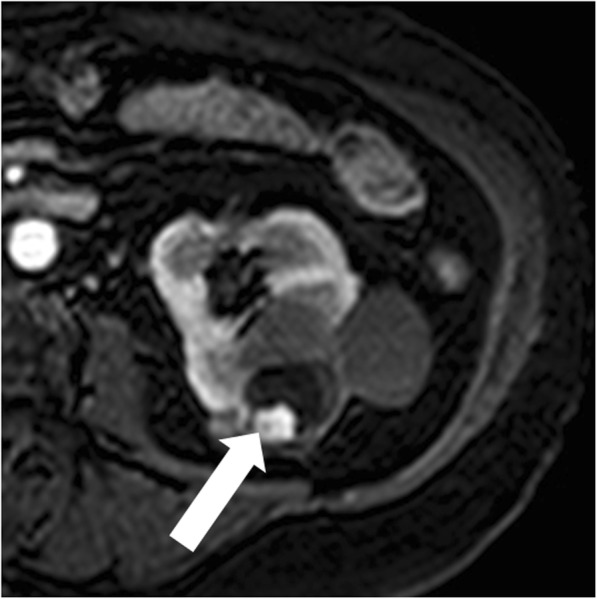
Bosniak category IV renal cyst. Axial contrast-enhanced T1-weighted MR image shows a lesion with a peripheral, enhancing, nodule (arrow)
Cystic renal cell carcinoma
Cystic RCC is relatively rare and comprises approximately 3–15 % of all cases of RCCs. It is found more commonly in younger age and in females compared with solid RCC [33]. The cystic appearance can be related to their inherent architecture or secondary to cystic degeneration and extensive necrosis [34]. Clear cell type RCC is the most common subtype, followed by papillary and chromophobe RCC. Clear cell type RCC can show a dominant cystic component or can arise in a simple cyst [35]. Multilocular cystic RCC of low malignant potential is a rare variant of clear cell type RCC with no reported recurrence or metastasis. This tumor is composed exclusively by cysts with low-grade tumor cell [36] and shows a variable imaging appearance, which ranges from category IIF to category IV renal cysts [35]. Papillary RCC can appear as a cyst with hemorrhagic or necrotic content and a thick pseudocapsule [35]. Cystic renal RCCs have a more favorable prognosis of all subtypes of RCC: they have a low Fuhrman grade, grow slowly, and rarely metastasize or recur [37].
Renal metastases
Renal metastases are not uncommon, with reported frequencies ranging from 7 to 20% at post-mortem studies [38–41]. The most common primary malignancies are the lung, breast, gastrointestinal tract, and melanoma. CT and MR imaging diagnosis is less frequent because post-mortem studies included microscopic lesions, which are beyond CT resolution [42, 43].
Renal metastases can show a solid or cystic appearance. The differentiation of renal metastasis from RCC on the basis of CT and MR findings alone may be impossible [42–44]. However, some features are likely to be distinctive: renal metastases are frequently multiple, bilateral and small [42, 43].
Mixed epithelial and stromal tumors
The MESTs area heterogeneous group of rare renal tumors occurring predominantly in perimenopausal women (female-to-male ratio, 11:1). The MEST appears as a well-marginated lesion with a variable proportion of solid and cystic components [45]. Septa and nodules can show heterogeneous and delayed enhancement [45]. MEST can show an exophytic growth or herniate into the renal pelvis [45]. Adult cystic nephroma is now classified within MEST family due to similar histologic and epidemiologic findings [36]. This tumor appears as an encapsulated lesion, with cysts of variable size, and thin, variably enhancing, septa [46]. Calcifications are peripheral and curvilinear [46]. Solid components are typically absent [46]. At MRI, the capsule and septa can show hypointensity on both T1- and T2-weighted images due to the fibrous composition. Since imaging features are non-specific, differentiation between MEST and cystic RCC requires pathologic examination.
Renal abscess
Renal abscess is an uncommon entity that usually results from a complication of untreated or inadequately treated acute pyelonephritis or ascending urinary tract infection. More rarely, it results from hematogenous spread from an extra-urinary source of infection (e.g., diverticulitis, pancreatitis). Patients may present with signs and symptoms of infection. Renal abscess can appear as a complex renal cyst with inhomogeneous areas of fluid attenuation/intensity and a thick and irregular wall that shows a little enhancement on excretory phase (Fig. 10). Because of the presence of viscous pus, the fluid component shows a characteristic strong and heterogeneous diffusion restriction on diffusion-weighted imaging, which favors the diagnosis of renal abscess over that of RCC [47]. Renal parenchyma around the abscess can show low density/intensity on early phases and delayed enhancement [48, 49]. Fat stranding is often found adjacent to the renal abscess [50]. Gas can be rarely present within the lesion and strongly suggests abscess formation. When imaging findings, clinical history and laboratory tests do not permit a confident differentiation between renal abscess and cystic RCC; biopsy/drainage should be performed to obtain the correct diagnosis.
Fig. 10.
Renal abscess. a Axial contrast-enhanced CT shows a cystic lesion (arrow) with a peripheral, thick, enhancing, wall. b Axial contrast-enhanced CT obtained 3 months after antibiotic therapy shows decrease in size of the lesion
Multifocal renal cysts
Multifocal cystic renal diseases comprise a heterogeneous spectrum of hereditary and nonhereditary diseases characterized by the presence of multiple simple kidney cysts [32]. Hereditary entities are due to mutations of genes involved in the formation and functioning of renal cilia, which result in epithelial proliferation and development of renal cysts [51]. Autosomal dominant polycystic kidney disease is the most common hereditary multifocal renal disease. Nonhereditary entities are due to obstructive, stromal-epithelial malinductive and neoplastic mechanisms [52]. Most common causes of nonhereditary multifocal cysts formation include lithium-induced nephrotoxicity, acquired cystic renal disease, and localized cystic renal disease. The location and appearance of renal cysts, presence of interposed normal renal parenchyma, size of the kidneys, patient’s age at presentation, and degree of renal function help differentiate at imaging multifocal cystic renal diseases.
Autosomal dominant polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disorder and occurs in approximately one of 500 live births [51]. Mutations in one of the two genes encoding plasma membrane—spanning polycystin 1 and polycystin 2 (PKD1 and PKD2)—are responsible of the disease. It is characterized by progressive development and growth in size of simple renal cysts, leading to symmetric enlargement of the kidneys and chronic renal failure by late middle-age [52, 53] (Fig. 11). Cysts have variable dimension (from few millimeters to several centimeters) and are diffusely distributed through the kidneys. Cyst complications include hemorrhage, pyogenic infection, and, more rarely, rupture. The risk for RCC is not increased in comparison with the general population except in patients on dialysis [54]. The added risk of malignancy in dialysis patients is probably related to the effects of coexistent acquired cystic renal disease [54].
Fig. 11.
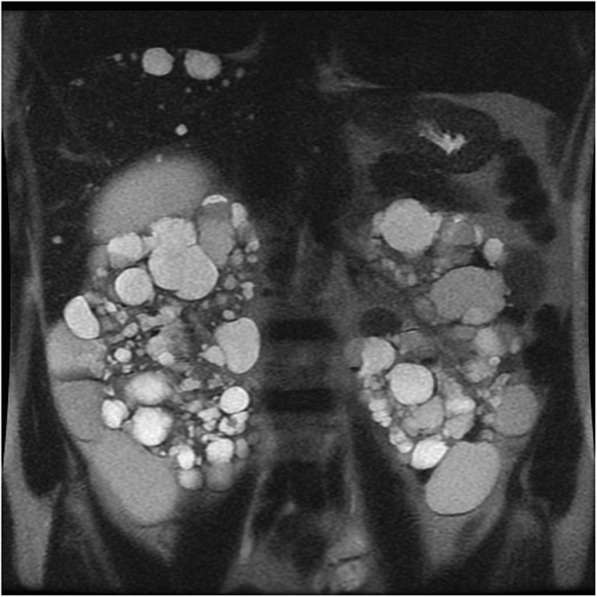
Autosomal dominant polycystic kidney disease. Coronal T2-weighted image shows symmetric enlargement of the kidneys, which contain multiple cysts with variable size
Hepatic cysts are the most common extra-renal manifestations of ADPKD and show variable number, size, location, and distribution [51, 54]. Polycystic liver disease is uncommon and leads to hepatomegaly [51, 54]. More rare hepatic complications include congenital hepatic fibrosis and segmental dilatation of biliary tract [54].
The other extra-renal manifestations of ADPKD include cysts in other organs such as pancreas and non-cystic abnormalities such as cardiac valvulopathies and intracranial aneurysms [51]. Imaging plays a crucial role in the identification of ADPKD in high-risk individuals (those with a positive family history). The diagnosis of ADPKD requires at least three renal cysts (unilateral or bilateral) in high-risk patients 15–39 years of age, at least two cysts in each kidney in high-risk patients 40–59 years of age, and several bilateral renal cysts in high-risk patients 60 years of age or older [55]. Since renal enlargement correlates with a decline of renal function, estimation of renal volume can predict the risk for renal failure [53].
Acquired cystic renal disease
Acquired cystic kidney disease (ACKD) is a consequence of sustained uremia in patient with end-stage renal disease [52]. The disease is found in 8–13% patients with end-stage renal disease and in approximately 50% patients on dialysis. The disease is multifactorial. It is the progressive destruction of renal functioning nephrons with compensatory hypertrophy of remaining renal parenchyma, obstruction of renal tubules by interstitial fibrosis or oxalate crystals, and cyst formation [52]. Kidneys are atrophic and contain multiple cysts with variable size (from few millimeters to several centimeters) and imaging appearance (Fig. 12). Since renal cysts are extremely common in the adult population, the diagnosis of ACKD requires the presence of three or more cysts in each kidney, in conjunction to end-stage renal disease, and no history of hereditable renal disease [56]. Cyst hemorrhage is a common complication and can cause hematuria, whereas cyst rupture, perinephric hematoma, and retroperitoneal hemorrhage are less frequent [52]. Development of RCC in the wall of the cyst is the most serious complication of ACKD, with a higher rate in comparison to the general population [55].
Fig. 12.
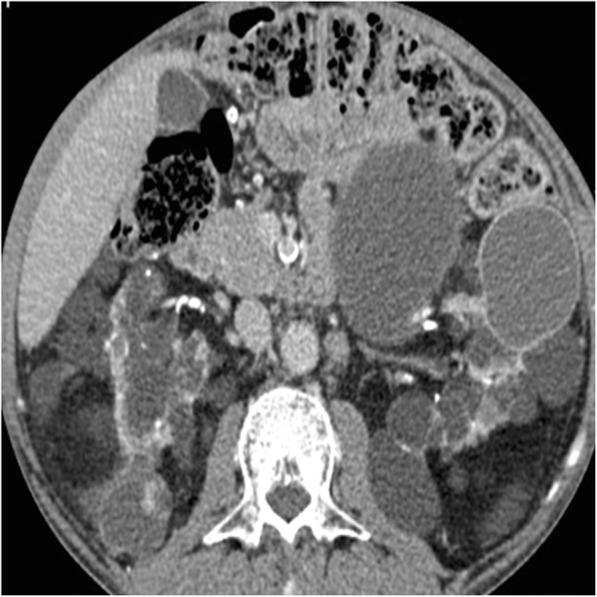
Acquired cystic renal disease. Axial contrast-enhanced CT image shows atrophic kidneys, which contain multiple cysts of variable size
The most common tumor type in patients with ACKD is acquired cystic disease (ACD)-associated RCC, followed by papillary and clear cell type RCC [36]. ACD-associated RCC has unique morphologic features and is found exclusively in patients with end-stage renal disease and ACKD [36].
Lithium-induced nephropathy
Long-term lithium therapy is a well-known cause of nephrotoxicity in the form of polyuria-polydipsia syndrome (diabetes insipidus) and chronic renal insufficiency [57]. Characteristic imaging findings include normal or slightly decreased size of kidneys with multiple, uniformly, and symmetrically distributed microcysts [58]. Microcysts measure 1–2 mm in diameter and are located in both cortex and medulla [58].
Localized cystic renal disease
Localized cystic renal disease is a rare, nonhereditary, form of cystic renal disease, which manifests as a conglomeration of multiple simple cysts of variable size [59] (Fig. 13). In contrast to ACKD and ADPKD, localized cystic renal disease is typically unilateral and not progressive. The disease usually involves only a portion of the kidney with a polar predilection [59]. Entire renal involvement is rare [58]. The contralateral kidney is normal. The presence of interposed normal renal parenchyma and the absence of a capsule help to differentiate localized cystic renal disease from cystic nephroma and multiloculated cystic RCC [58]. Cystic involvement of other organs is typically absent [58].
Fig. 13.
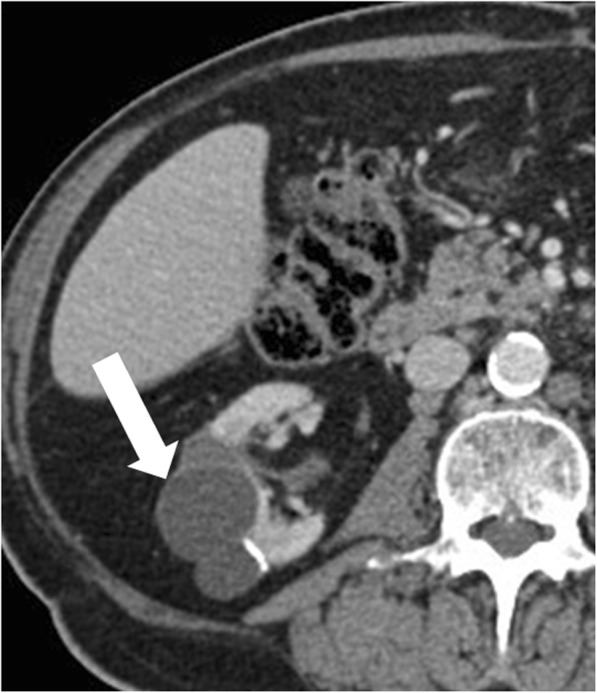
Localized cystic renal disease. Axial contrast-enhanced CT image shows a conglomeration of multiple simple cysts of variable size (arrow) in the right kidney
Conclusions
Cystic renal lesions are commonly encountered on radiologic examinations. Complex and multifocal cystic renal lesions are often a diagnostic challenge, since they can represent neoplastic and non-neoplastic conditions. The Bosniak classification system is a well-established imaging method, which helps radiologists and surgeons in daily practice in the differentiation of nonsurgical from surgical lesions. Radiologists should also recognize the imaging appearances of specific types of cystic lesions in order to better characterize them.
Abbreviations
- ACKD
Acquired cystic kidney disease
- ADPKD
Autosomal dominant polycystic kidney disease
- CEUS
Contrast-enhanced US
- CT
Computed tomography
- MESTs
Mixed epithelial and stromal tumors
- MRI
Magnetic resonance imaging
- RCC
Renal cell carcinoma
Authors’ contributions
All authors read and approved the final manuscript.
Funding
None
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hartman DS, Choyke PL, Hartman MS. From the RSNA refresher courses: a practical approach to the cystic renal mass. Radiographics. 2004;24(Suppl 1):S101–S115. doi: 10.1148/rg.24si045515. [DOI] [PubMed] [Google Scholar]
- 2.Hélénon O, Crosnier A, Verkarre V, Merran S, Méjean A, Correas JM. Simple and complex renal cysts in adults: classification system for renal cystic masses. Diagn Interv Imaging. 2018;99(4):189–218. doi: 10.1016/j.diii.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Park BK, Kim B, Kim SH, Ko K, Lee HM, Choi HY. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast-enhanced US. Eur J Radiol. 2007;61(2):310–314. doi: 10.1016/j.ejrad.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Ascenti Giorgio, Mazziotti Silvio, Zimbaro Giovanni, Settineri Nicola, Magno Carlo, Melloni Darwin, Caruso Rosario, Scribano Emanuele. Complex Cystic Renal Masses: Characterization with Contrast-enhanced US. Radiology. 2007;243(1):158–165. doi: 10.1148/radiol.2431051924. [DOI] [PubMed] [Google Scholar]
- 5.Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic performance and confidence of contrast-enhanced ultrasound in the differential diagnosis of cystic and cysticlike liver lesions. AJR Am J Roentgenol. 2017;209(3):W119–W127. doi: 10.2214/AJR.16.17062. [DOI] [PubMed] [Google Scholar]
- 6.Corvino A, Catalano O, Setola SV, Sandomenico F, Corvino F, Petrillo A. Contrast-enhanced ultrasound in the characterization of complex cystic focal liver lesions. Ultrasound Med Biol. 2015;41(5):1301–1310. doi: 10.1016/j.ultrasmedbio.2014.12.667. [DOI] [PubMed] [Google Scholar]
- 7.Corvino A, Sandomenico F, Setola SV, Corvino F, Tafuri D, Catalano O. Morphological and dynamic evaluation of complex cystic focal liver lesions by contrast-enhanced ultrasound: current state of the art. J Ultrasound. 2019;22(3):251–259. doi: 10.1007/s40477-019-00385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quaia Emilio, Bertolotto Michele, Cioffi Vincenzo, Rossi Alexia, Baratella Elisa, Pizzolato Riccardo, Cova Maria Assunta. Comparison of Contrast-Enhanced Sonography with Unenhanced Sonography and Contrast-Enhanced CT in the Diagnosis of Malignancy in Complex Cystic Renal Masses. American Journal of Roentgenology. 2008;191(4):1239–1249. doi: 10.2214/AJR.07.3546. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PT, Horton KM, Fishman EK. Optimizing detectability of renal pathology with MDCT: protocols, pearls, and pitfalls. AJR Am J Roentgenol. 2010;194(4):1001–1012. doi: 10.2214/AJR.09.3049. [DOI] [PubMed] [Google Scholar]
- 10.Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology. 2005;236(2):441–450. doi: 10.1148/radiol.2362040218. [DOI] [PubMed] [Google Scholar]
- 11.Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004;231(2):365–371. doi: 10.1148/radiol.2312031025. [DOI] [PubMed] [Google Scholar]
- 12.Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology. 2008;71(1):7–11. doi: 10.1016/j.urology.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 13.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58(8):626–629. doi: 10.1016/S0009-9260(03)00165-X. [DOI] [PubMed] [Google Scholar]
- 14.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158(1):1–10. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 15.Bosniak MA. The use of the Bosniak classification system for renal cysts and cystic tumors. J Urol. 1997;157(5):1852–1853. doi: 10.1016/S0022-5347(01)64883-3. [DOI] [PubMed] [Google Scholar]
- 16.Balci N C, Semelka R C, Patt R H, Dubois D, Freeman J A, Gomez-Caminero A, Woosley J T. Complex renal cysts: findings on MR imaging. American Journal of Roentgenology. 1999;172(6):1495–1500. doi: 10.2214/ajr.172.6.10350279. [DOI] [PubMed] [Google Scholar]
- 17.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66(3):484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Benjaminov O, Atri M, O'Malley M, Lobo K, Tomlinson G. Enhancing component on CT to predict malignancy in cystic renal masses and interobserver agreement of different CT features. AJR Am J Roentgenol. 2006;186(3):665–672. doi: 10.2214/AJR.04.0372. [DOI] [PubMed] [Google Scholar]
- 19.Galia Massimo, Albano Domenico, Bruno Alberto, Agrusa Antonino, Romano Giorgio, Di Buono Giuseppe, Agnello Francesco, Salvaggio Giuseppe, La Grutta Ludovico, Midiri Massimo, Lagalla Roberto. Imaging features of solid renal masses. The British Journal of Radiology. 2017;90(1077):20170077. doi: 10.1259/bjr.20170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mileto Achille, Allen Brian C., Pietryga Jason A., Farjat Alfredo E., Zarzour Jessica G., Bellini Davide, Ebner Lukas, Morgan Desiree E. Characterization of Incidental Renal Mass With Dual-Energy CT: Diagnostic Accuracy of Effective Atomic Number Maps for Discriminating Nonenhancing Cysts From Enhancing Masses. American Journal of Roentgenology. 2017;209(4):W221–W230. doi: 10.2214/AJR.16.17325. [DOI] [PubMed] [Google Scholar]
- 21.Fananapazir G, Lamba R, Lewis B, Corwin MT, Naderi S, Troppmann C. Utility of MRI in the characterization of indeterminate small renal lesions previously seen on screening CT scans of potential renal donor patients. AJR Am J Roentgenol. 2015;205(2):325–330. doi: 10.2214/AJR.14.13956. [DOI] [PubMed] [Google Scholar]
- 22.Israel GM, Bosniak MA. Calcification in cystic renal masses: is it important in diagnosis? Radiology. 2003;226(1):47–52. doi: 10.1148/radiol.2261011704. [DOI] [PubMed] [Google Scholar]
- 23.Baert L, Steg A. Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? J Urol. 1977;118(5):707–710. doi: 10.1016/S0022-5347(17)58167-7. [DOI] [PubMed] [Google Scholar]
- 24.Davarpanah AH, Spektor M, Mathur M, Israel GM. Homogeneous T1 Hyperintense renal lesions with smooth borders: is contrast-enhanced MR imaging needed? Radiology. 2016;281(1):326. doi: 10.1148/radiol.2016164032. [DOI] [PubMed] [Google Scholar]
- 25.Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008;249(1):16–31. doi: 10.1148/radiol.2491070783. [DOI] [PubMed] [Google Scholar]
- 26.Bosniak MA. Problems in the radiologic diagnosis of renal parenchymal tumors. Urol Clin North Am. 1993;20(2):217–230. [PubMed] [Google Scholar]
- 27.Bosniak MA. Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am J Roentgenol. 1997;169(3):819–821. doi: 10.2214/ajr.169.3.9275903. [DOI] [PubMed] [Google Scholar]
- 28.Smith Andrew D., Remer Erick M., Cox Kelly L., Lieber Michael L., Allen Brian C., Shah Shetal N., Herts Brian R. Bosniak Category IIF and III Cystic Renal Lesions: Outcomes and Associations. Radiology. 2012;262(1):152–160. doi: 10.1148/radiol.11110888. [DOI] [PubMed] [Google Scholar]
- 29.Hindman NM, Hecht EM, Bosniak MA. Follow-up for Bosniak category 2F cystic renal lesions. Radiology. 2014;272(3):757–766. doi: 10.1148/radiol.14122908. [DOI] [PubMed] [Google Scholar]
- 30.O'Malley RL, Godoy G, Hecht EM, Stifelman MD, Taneja SS. Bosniak category IIF designation and surgery for complex renal cysts. J Urol. 2009;182(3):1091–1095. doi: 10.1016/j.juro.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Bosniak MA. The Bosniak renal cyst classification: 25 years later. Radiology. 2012;262(3):781–785. doi: 10.1148/radiol.11111595. [DOI] [PubMed] [Google Scholar]
- 32.Wood Cecil G., Stromberg LeRoy J., Harmath Carla B., Horowitz Jeanne M., Feng Chun, Hammond Nancy A., Casalino David D., Goodhartz Lori A., Miller Frank H., Nikolaidis Paul. CT and MR Imaging for Evaluation of Cystic Renal Lesions and Diseases. RadioGraphics. 2015;35(1):125–141. doi: 10.1148/rg.351130016. [DOI] [PubMed] [Google Scholar]
- 33.Winters BR, Gore JL, Holt SK, Harper JD, Lin DW, Wright JL. Cystic renal cell carcinoma carries an excellent prognosis regardless of tumor size. Urol Oncol. 2015;33(12):505.e9–505.13. doi: 10.1016/j.urolonc.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartman DS, Davis CJ, Jr, Johns T, Goldman SM. Cystic renal cell carcinoma. Urology. 1986;28(2):145–153. doi: 10.1016/0090-4295(86)90109-3. [DOI] [PubMed] [Google Scholar]
- 35.Moch H. Cystic renal tumors: new entities and novel concepts. Adv Anat Pathol. 2010;17(3):209–214. doi: 10.1097/PAP.0b013e3181d98c9d. [DOI] [PubMed] [Google Scholar]
- 36.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 37.Jhaveri Kartik, Gupta Priya, Elmi Azadeh, Flor Lior, Moshonov Hadas, Evans Andrew, Jewett Michael. Cystic Renal Cell Carcinomas: Do They Grow, Metastasize, or Recur? American Journal of Roentgenology. 2013;201(2):W292–W296. doi: 10.2214/AJR.12.9414. [DOI] [PubMed] [Google Scholar]
- 38.Meilstrup JW, Mosher TJ, Dhadha RS, Hartman DS. Other renal tumors. Semin Roentgenol. 1995;30:168–184. doi: 10.1016/S0037-198X(05)80032-X. [DOI] [PubMed] [Google Scholar]
- 39.Bracken RB, Chica G, Johncon DE, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979;72:806–807. doi: 10.1097/00007611-197907000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Abrams H, Spira R, Goldstein M. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::AID-CNCR2820030111>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Klinger ME. Secondary tumors of the genitourinary tract. J Urol. 1951;65:144–153. doi: 10.1016/S0022-5347(17)68470-2. [DOI] [PubMed] [Google Scholar]
- 42.Patel U, Ramachandran N, Halls J, Parthipun A, Slide C. Synchronous renal masses in patients with a nonrenal malignancy: incidence of metastasis to the kidney versus primary renal neoplasia and differentiating features on CT. AJR Am J Roentgenol. 2011;197(4):W680–W686. doi: 10.2214/AJR.11.6518. [DOI] [PubMed] [Google Scholar]
- 43.Choyke PL, White EM, Zeman RK, Jaffe MH, Clark LR. Renal metastases: clinicopathologic and radiologic correlation. Radiology. 1987;162(2):359–363. doi: 10.1148/radiology.162.2.3797648. [DOI] [PubMed] [Google Scholar]
- 44.Fan G, Xie YU, Pei X, Lei J, Ye M, Zeng G, Li F, Xiong Y, Han W. Renal metastasis from cervical carcinoma presenting as a renal cyst: a case report. Oncol Lett. 2015;10(5):2761–2764. doi: 10.3892/ol.2015.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moslemi MK. Mixed epithelial and stromal tumor of the kidney or adult mesoblastic nephroma: an update. Urol J. 2010;7(3):141–147. [PubMed] [Google Scholar]
- 46.Lane BR, Campbell SC, Remer EM, et al. Adult cystic nephroma and mixed epithelial and stromal tumor of the kidney: clinical, radiographic, and pathologic characteristics. Urology. 2008;71:1142–1148. doi: 10.1016/j.urology.2007.11.106. [DOI] [PubMed] [Google Scholar]
- 47.Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A. Diffusion-weighted MRI in inflammatory renal lesions: all that glitters is not RCC! Eur Radiol. 2013;23(1):272–279. doi: 10.1007/s00330-012-2577-0. [DOI] [PubMed] [Google Scholar]
- 48.Kawashima A, Sandler CM, Goldman SM. Imaging in acute renal infection. BJU Int. 2000;86(Suppl 1):70–79. doi: 10.1046/j.1464-410x.2000.00578.x. [DOI] [PubMed] [Google Scholar]
- 49.Papanicolaou N, Pfister RC. Acute renal infections. Radiol Clin North Am. 1996;34(5):965–995. [PubMed] [Google Scholar]
- 50.Katabathina VS, Kota G, Dasyam AK, Shanbhogue AK, Prasad SR. Adult renal cystic disease: a genetic, biological, and developmental primer. Radiographics. 2010;30(6):1509–1523. doi: 10.1148/rg.306105513. [DOI] [PubMed] [Google Scholar]
- 51.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329(5):332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 52.Choyke PL. Acquired cystic kidney disease. Eur Radiol. 2000;10(11):1716–1721. doi: 10.1007/s003300000601. [DOI] [PubMed] [Google Scholar]
- 53.Grantham Jared J., Torres Vicente E., Chapman Arlene B., Guay-Woodford Lisa M., Bae Kyongtae T., King Bernard F., Wetzel Louis H., Baumgarten Deborah A., Kenney Phillip J., Harris Peter C., Klahr Saulo, Bennett William M., Hirschman Gladys N., Meyers Catherine M., Zhang Xiaoling, Zhu Fang, Miller John P. Volume Progression in Polycystic Kidney Disease. New England Journal of Medicine. 2006;354(20):2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 54.Chauveau D, Fakhouri F, Grünfeld JP. Liver involvement in autosomal-dominant polycystic kidney disease: therapeutic dilemma. J Am Soc Nephrol. 2000;11(9):1767–1775. doi: 10.1681/ASN.V1191767. [DOI] [PubMed] [Google Scholar]
- 55.Tickoo Satish K, dePeralta-Venturina Mariza N, Harik Lara R, Worcester Heath D, Salama Mohamed E, Young Andrew N, Moch Holger, Amin Mahul B. Spectrum of Epithelial Neoplasms in End-Stage Renal Disease. The American Journal of Surgical Pathology. 2006;30(2):141–153. doi: 10.1097/01.pas.0000185382.80844.b1. [DOI] [PubMed] [Google Scholar]
- 56.Pei York, Obaji James, Dupuis Annie, Paterson Andrew D., Magistroni Riccardo, Dicks Elizabeth, Parfrey Patrick, Cramer Benvon, Coto Eliecer, Torra Roser, San Millan Jose L., Gibson Robert, Breuning Martijn, Peters Dorien, Ravine David. Unified Criteria for Ultrasonographic Diagnosis of ADPKD. Journal of the American Society of Nephrology. 2008;20(1):205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D'Agati VD. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439–1448. doi: 10.1681/ASN.V1181439. [DOI] [PubMed] [Google Scholar]
- 58.Farres Maria Teresa, Ronco Pierre, Saadoun David, Remy Philippe, Vincent François, Khalil Antoine, Le Blanche Alain Ferdinand. Chronic Lithium Nephropathy: MR Imaging for Diagnosis. Radiology. 2003;229(2):570–574. doi: 10.1148/radiol.2292020758. [DOI] [PubMed] [Google Scholar]
- 59.Slywotzky CM, Bosniak MA. Localized cystic disease of the kidney. AJR Am J Roentgenol. 2001;176(4):843–849. doi: 10.2214/ajr.176.4.1760843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable