Abstract
Purpose of Review
Over the past decade, our understanding of the biomechanics of the reverse total shoulder arthroplasty (RTSA) has advanced, resulting in design adjustments, improved outcomes, and expanding indications. The purpose of this review is to summarize recent literature regarding the biomechanics of RTSA and the evolving indications for its use.
Recent Findings
While Grammont’s principles of RTSA biomechanics remain pillars of contemporary designs, a number of modifications have been proposed and trialed in later generations to address complications such as impingement and glenoid failure. Clinical and biomechanical literature suggest that less medialized, more inferior glenospheres result in less impingement and notching. On the humerus, a more vertical neck cut is associated with less impingement. Indications for RTSA continue to expand beyond the classic indication of cuff tear arthropathy (CTA). Patients without a functional cuff but no arthritis now have a reliable option in the RTSA. RTSA has also replaced hemiarthroplasty as the implant of choice for displaced three- and four-part proximal humerus fractures in the elderly. Finally, updated design options and modular components now allow for treatment of glenoid bone loss, failed arthroplasty, and proximal humerus tumors with RTSA implants.
Summary
Reverse total shoulder arthroplasty design has been modernized on both the glenoid and humerus to address biomechanical challenges of early implants. As outcomes improve with these modifications, RTSA indications are growing to address complex bony pathologies such as tumor and bone loss. Longitudinal follow-up of patients with updated designs and novel indications is essential to judicious application of RTSA technology.
Keywords: Reverse total shoulder arthroplasty, Rotator cuff, Biomechanics, Scapular notching, Proximal humerus fracture
Introduction
Following its Food and Drug Administration (FDA) approval in 2003, reverse total shoulder arthroplasty (RTSA) has become increasingly popular in the USA. RTSAs comprised one-third of shoulder arthroplasties in the USA performed in 2011, and 46% in 2014 [1, 2]. The popularity of the RTSA stemmed initially from its success in pseudoparalytic shoulders secondary to cuff tear arthropathy (CTA), a condition for which the anatomic total shoulder arthroplasty (TSA) performed poorly [3, 4]. While initial reverse-polarity arthroplasties faced similar issues as the anatomic TSA, the introduction of the Grammont RTSA in 1985 provided a solution by re-tensioning the deltoid and medializing the center of rotation [3, 5].
The initial description of the basic biomechanics of the RTSA provided insight into how and why this prosthesis works, but since its introduction there has been an expanding body of literature on implications of this shoulder arthroplasty design [3, 6–10]. Updated understandings of the biomechanics and associated complications, such as scapular notching, have allowed for refinements in component positioning and implant design to improve range of motion (ROM), maintain the deltoid lever arm, and minimize joint reactive forces [8, 11–16]. The importance of understanding the biomechanics of reverse shoulder anatomy is crucial to produce optimal outcomes after RTSA. The indications for RTSA continue to expand from its FDA-approved use for CTA to indications ranging from massive rotator cuff tears, proximal humerus fractures, primary osteoarthritis, failed anatomic TSA, and orthopedic oncologic conditions. This review will focus on the basic biomechanics, contemporary updates to prosthesis design, and the expanding indications of RTSA.
Biomechanics
Prior to the development of the RTSA, addressing arthritis in cuff-deficient shoulders was challenging. Available implants failed to address the inherent instability of the shoulder girdle occurring with loss of dynamic compression from the rotator cuff [17, 18]. As a result, early prosthesis failure through superior humeral head migration and glenoid loosening from eccentric loading occurred frequently [5, 19].
The revolutionary Grammont reverse prosthesis was available in Europe in 1985, and was based on four key principles that altered the biomechanics of the prosthesis to mitigate shortcomings of its predecessors in the cuff-deficient shoulder [4]. These principles included (1) medialization of the center of rotation, (2) re-tensioning of the deltoid by distalizing the humerus, (3) a constant center of rotation leading to an inherently stable implant, and (4) a semi-constrained prosthesis with a larger arc of motion [3, 6]. These four principles have remained a staple in the understanding of RTSA biomechanics.
Medialized Center of Rotation
With the center of rotation (COR) medialized compared with an anatomic shoulder (Fig. 1a and b), but lateralized to or flush with the glenoid, the RTSA confers stability at the bone-implant interface. Movements around the fixed COR convert the compressive and shear forces into a largely compressive vector (Fig. 1b) [6, 9]. Forces across the shoulder joint are altered due to these altered biomechanics of the RTSA. In a native shoulder, at 90° of abduction there is a 90% body weight joint reactive force, and up to 42% body weight shearing force is seen at 60° abduction [20]. Peak forces are reduced in both compression and shear across the shoulder joint throughout ROM in reverse total shoulders [21, 22]. In one cadaveric study, Ackland and colleagues suggested that that glenohumeral joint force in abduction decreases by 41.5%BW [22]. In a shoulder lacking the compressive force of the cuff, minimization of the ratio of shear to compressive forces at the joint leads to an inherently stable prosthesis.
Fig. 1.
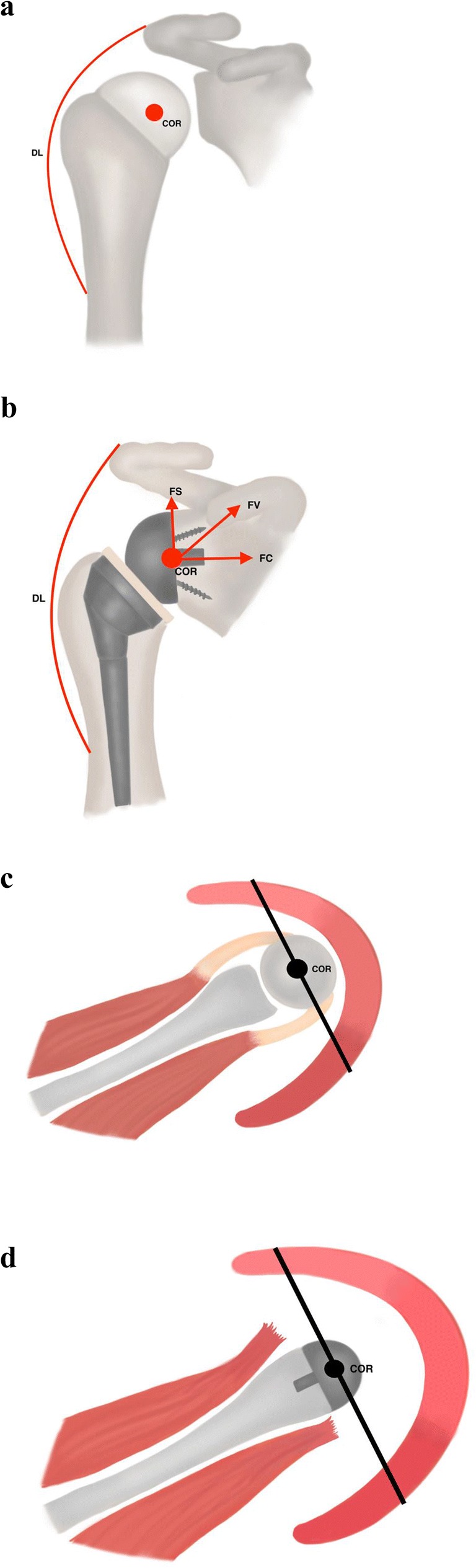
(a–d) Biomechanical improvements of the reverse total shoulder implants. a The natural center of rotation (COR) and deltoid lever arm (DL) in a native shoulder. (b) Starting with the Grammont implant, more modern reverse total shoulder arthroplasty implants medialize and distalize the center of rotation, which minimizes shear forces (FS), and increases compressive forces (FC), to create an overall favorable force vector (FV) at the bone-glenoid interface, as well as re-tensions the deltoid to provide a mechanical advantage. (c) In a native shoulder, the middle deltoid (red) and part of the anterior deltoid (light red) provide an abduction force. The posterior deltoid in dark red provides extension force. (d) With medialization of the center of rotation in a reverse total shoulder arthroplasty, a larger part of the anterior deltoid and posterior deltoid are recruited and contribute to the active abduction force. (Adapted from Berliner et al, JSES 2015 and Boileau et al, JSES 2005)
Re-tensioning of the Deltoid
With the deltoid as the primary workhorse for motion in the cuff-deficient shoulder, the RTSA design has maximized deltoid efficiency by COR medialization and deltoid lengthening. Several changes occur regarding the deltoid biomechanics in RTSA. The orientation of the muscle fibers becomes more vertical, and muscle recruitment changes such that all three sub-regions of the deltoid become primary shoulder abductors (Fig. 1c and d) [22, 23]. One cadaver study demonstrated increased moment arms of the anterior and middle deltoid by 10 and 15 mm respectively [23]. Other studies have noted a 20–42% increase in the deltoid moment arm, with a peak in the middle deltoid moment arm at 90° [9, 21], as well as improved deltoid abduction efficiency by 30% compared with native shoulders [21, 24]. Clinically, these changes have translated to improved range of motion in multiple studies [25, 26].
These changes in muscle recruitment for abduction are not without cost. As the posterior deltoid is recruited to become an abductor, its external rotation moment arm is lost, contributing to the common external rotation deficit seen following RTSA [16, 27]. Other directional moment arms are affected following RTSA as well, and, more recently, subscapularis repair after RTSA has been investigated. A sonographic study correlated the integrity of a subscapularis repair with post-operative PROs and determined that there was no significant difference between intact, attenuated, or absent subscapularis muscles, though internal rotation was improved in patients with intact tendons [28].
An Inherently Stable Shoulder
The minimization of shear forces conferred by a constant, medial COR leads to an inherently stable prosthesis. Furthermore, the radius of curvature in the glenoid and humeral components are congruent in an RTSA, allowing it to tolerate a greater joint reaction force vector up to 45° [6]. RTSA stability has been found to be two to three times higher than an anatomic TSA, and up to five times more stable than a native shoulder joint at 90° abduction [29]. Furthermore, likely due to the larger muscle forces acting throughout abduction, increasing angles of abduction confer greater forces required to dislocate the RTSA [30].
A Semi-constrained Prosthesis
A semi-constrained prosthesis is achieved by utilizing a relatively larger glenosphere relative to the humeral cup component. Early models consisted of a ball on socket design, similar to that of a total hip arthroplasty, but these highly constrained implants failed frequently due to elevated torque at the bone-implant interface leading to loosening. Furthermore, functional outcomes were poor due to low ROM prior to impingement. The Grammont RTSA offered a semi-constrained design, with a smaller humeral cup to provide larger ROM prior to impingement.
Modern RTSA implants have strove to balance the amount of constraint—with a humeral component deep enough to allow inherent stability in a cuff-deficient shoulder, but shallow enough to minimize impingement and shear forces generated in extremes of motion. More constraint increases the force required to dislocate the prosthesis, though this depends on the direction of force [30]. When compared with other variables possibly affecting ROM prior to impingement, the largest impact was shown to be humeral cup depth—a more retentive humeral cup reduced the ROM by 26° compared with the standard semi-constrained cup [31]. Furthermore, although highly constrained RTSA implants had elevated forces at the bone-implant interface, a more recent study demonstrated that less constrained implants also have increased contact stresses, particularly at the inferior edge of the humeral component [32]. These results provide support for a semi-constrained prosthesis, rather than one at the extremes.
Design Changes
Since the introduction of Grammont’s principles, there have been multiple proposed changes to the design and component placement of RTSAs with goals of improve ROM and outcomes while reducing impingement. These adjustments include glenoid baseplate placement modifications as well as humeral-sided design changes.
Glenoid Position
Medialization of the COR decreases shear forces across the glenoid component and creates compressive forces at the bone-implant interface. Medialization results in less glenoid baseplate motion [9, 33, 34] and lower force generation by the deltoid to initiate motion compared with lateralized components [35, 36]. However, medialization may lead to increased scapular notching and reduced shoulder ROM due to impingement [36–38]. Clinically, in a study of 146 consecutive RTSAs, patients with increased medialization had decreased external rotation, but improved pain scores. Those with increased glenoid lateralization had less radiographic notching [39]. Other biomechanical and clinical studies have also demonstrated improved ROM with glenoid lateralization [11, 40, 41•, 42]. Solutions to address these contrasting benefits have included improving glenoid baseplate fixation, moving from a highly constrained to a semi-constrained joint, and more inferior placement and inferior tilt of the glenosphere to avoid notching [6, 43].
The superior-to-inferior position and tilt of the glenosphere has also been studied with regard to reducing impingement. Initially, a computer-based model predicted less impingement in inferiorly placed and inferiorly tilted implants [8]. In a CT modeling study, inferior tilt of the glenosphere and inferior eccentric placement of the glenosphere both improved predicted ROM compared with a standard concentric glenosphere [40]. However, other studies have raised concerns about glenoid fixation with a tilted configuration [44, 45]. Currently, inferiorly tilted and eccentric designs are available to allow for inferior positioning of the glenosphere. While short-term results have been promising [13, 46, 47], long-term results with these modern implants are not yet available.
Humeral Component Design
Humeral component design and position have also been modified to diminish impingement and improve ROM since Grammont’s initial designs. The Delta prosthesis humeral component neck-shaft angle was in 155° of valgus, providing superior stability in a cuff-deficient shoulder. However, this non-anatomic, nearly-horizontal humeral component is more likely to impinge on the lateral pillar of the scapula. More contemporary designs offer a neck-shaft angle closer to normal anatomy, with options between 135 and 145°. Biomechanical studies have demonstrated reduced impingement and improved ROM [8, 48], findings further supported in clinical studies demonstrating reduced notching in patients receiving implants with a lower neck-shaft angle [49, 50].
Humerus preparation has also been modified since Grammont’s inlay prosthesis. Grammont’s initial stem was straight with a horizontal inlay-type humeral tray. A theoretical advantage of the inlay stem is increased bony contact between the proximal component and bone. However, the inlay design involves reaming more metaphyseal bone and preparation may risk greater tuberosity fracture [51]. Curved-stem designs with an onlay proximal interface have been utilized as well. The onlay design preserves proximal bone, and is generally has a more varus cut, preserving greater tuberosity bone and minimizing damage to the remaining rotator cuff. Furthermore, these prostheses may be convertible to or from hemiarthroplasty and anatomic TSA. Intrinsic to the more vertical inclination is increased humeral offset. Modular onlay humeral stems allow for the humeral tray to be rotated on the stem, with intraoperative adjustment to low/high offset configurations [52]. A 3D modeling study comparing Grammont inlay stems with short onlay stems of different inclinations demonstrated improved ROM in adduction, extension, and external rotation with onlay stems [51]. A clinical study comparing 2-year outcomes of the Grammont-style inlay stem with a short, curved onlay stem found that scapular fracture was more common for the onlay stems, but radiographic notching occurred less and external rotation improvement was greater for the onlay stems [53]. Currently, both stem types are available on the market, though discrepant design features between manufacturers makes comparative studies for this particular variable difficult.
Scapular Notching
Scapular notching has been found to be present radiographically in approximately two-thirds of RTSAs at 2-year follow-up [3, 10, 54, 55]. In a prospective study predictors of notching in RTSA patients, concentric or superior glenoid placement were shown to have the largest effect on the occurrence of notching. Notching was significantly correlated with less active abduction and flexion [56]. A retrospective review demonstrated superior placement and superior tilting of the glenoid to be associated with notching, and notching has been associated with less strength and reduced active elevation [54]. Despite these findings, two studies found no observed difference in PROs in patients with and without notching [10, 54]. Other studies, however, have demonstrated differences in clinical outcomes regarding PROs, strength, and ROM [57, 58]. In addition to ROM concerns, the longevity of the glenoid component is of primary concern. Biomechanical studies demonstrate that in scapulae with a notch, glenoid fixation is impaired, with increased micromotion of the glenosphere to shear loads [34]. However, the relationship to long-term glenoid longevity remains unclear [59–62].
Multiples studies have examined glenoid component position as it relates to post-operative notching [46, 55, 57]. Eccentric inferior glenoid placement may allow for increased abduction/adduction ROM [55]. Even in other planes of motion such as internal and external rotation, an inferiorly translated glenoid improves motion [11]. A randomized trial with 50 patients found that utilization of eccentric versus concentric glenoid components demonstrated no difference in functional scores, shoulder ROM, or notching incidence. However, inferior placement with > 3.5 mm of overhang was shown to prevent notching [46]. Another series of 40 patients with inferiorly placed glenoid components had no events of notching post-operatively at 2-year minimum follow-up [14].
Indications for RTSA
Cuff Tear Arthropathy
Cuff tear arthropathy remains the only FDA-approved indication for RTSA, and outcomes after the initial learning curve in the USA are promising. A study with 5-year follow-up of RTSA in CTA demonstrated improved ROM in abduction (34° pre-operatively to 71° post-operatively) and forward flexion (55° to 110°), as well as significantly improved Constant scores at this time point [63]. The 10-year implant survival rate for CTA patients has been reported as 95% [64]. A systematic review of RTSA in CTA and massive cuff tears, however, showed a high complication rate across the studies of 17.4%, while ROM was significantly improved in all directions [65]. Aside from survivorship and functional outcomes, several reports have examined the impact of other factors, including pre-operative deltoid size and patient sex, on post-operative outcomes. Pre-operative deltoid size correlated with improved ASES scores and women overall had more pain post-operatively and inferior functional scores compared with men [66, 67].
Massive Irreparable Cuff Tears
Given its biomechanical advantage in a rotator cuff-deficient shoulder, RTSA is often used to address massive irreparable cuff tears in the absence of arthritis (Fig. 2). In a series of 112 patients with massive cuff tears, Cuff et al. reported 94% survivorship at 5 years and 90% at 10 years. Improved motion and functional scores were maintained at 10 years [68, 69]. Other studies demonstrated improvement in abduction and forward flexion by over 70° in each direction at mid-term follow-up [70]. Longer follow-up results, however, have been mixed. A longitudinal study with 15-year follow-up suggested a failure rate of 27% and almost a 60% complication rate, although constant scores and anterior elevation were improved overall at final follow-up. Constant scores for those who had complications (excluding those requiring revisions) were comparable with those without complications [71]. A systematic review showed no significant decrease in functional scores or ROM up to 20 years post-surgery [72]. Overall, a younger age at the time of surgery, prior rotator cuff repair, and higher pre-operative shoulder scores were associated with poorer outcomes in this population [73, 74].
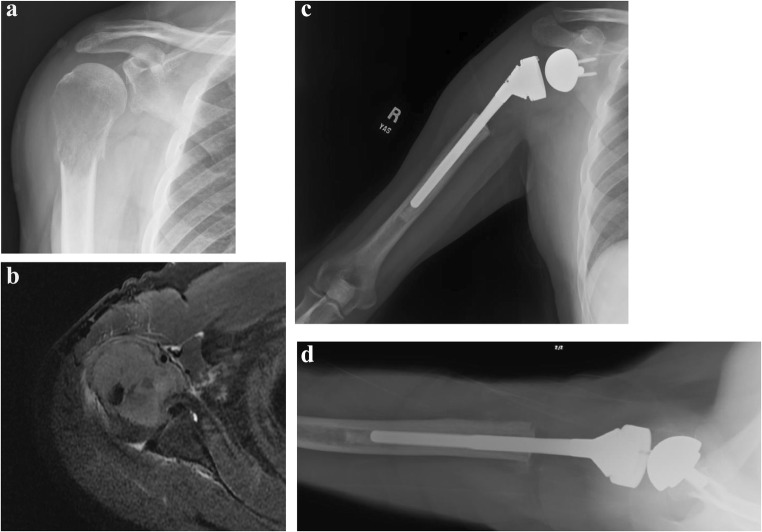
Fig. 2.
(a, b) Massive rotator cuff tear with anterosuperior escape treated with reverse total shoulder arthroplasty. (a) A weighted abducted AP view demonstrates anterosuperior escape of the humeral head, indicating a lack of functional rotator cuff. (b) Post-operative radiograph demonstrating cemented reverse total shoulder arthroplasty
Proximal Humerus Fractures
One condition for which RTSA is becoming increasingly utilized is proximal humerus fractures (PHF) (Fig. 3). From 2011 to 2013, the rates of RTSA in management of PHF increased 1.8-fold, comprising 3 to 24% of surgical management of PHF [75]. One of the challenges with either primary fixation or hemiarthroplasty for PHF is achieving tuberosity healing, which is associated with improved outcomes. Again, given the altered biomechanics of the RTSA, this implant offers the possibility of improved function regardless of tuberosity healing. The use of RTSA surpassed the use of hemiarthroplasties by 2015, representing 67.4% of arthroplasty implants used for PHF in one registry report [76]. A prospective study of RTSA in comminuted PHF resulted in 97% of patients with stable fixation, average forward flexion of 130° and average external rotation of 32° [77]. A multicenter review of 52 shoulders, mean age of 77 with 35-month follow-up, demonstrated final absolute and relative constant scores of 62 and 86, with 92% of patients rating their outcome as excellent or good. Resected or displaced greater tuberosities on imaging did correlate with inferior clinical outcomes in this cohort [78]. Ross et al. demonstrated excellent results in a retrospective review of 29 shoulders with 4.5 year average follow-up—no revisions, an average constant score of 88, and an average American Shoulder and Elbow Surgeon Score of 89 in this population with three and four-part PHF [79].
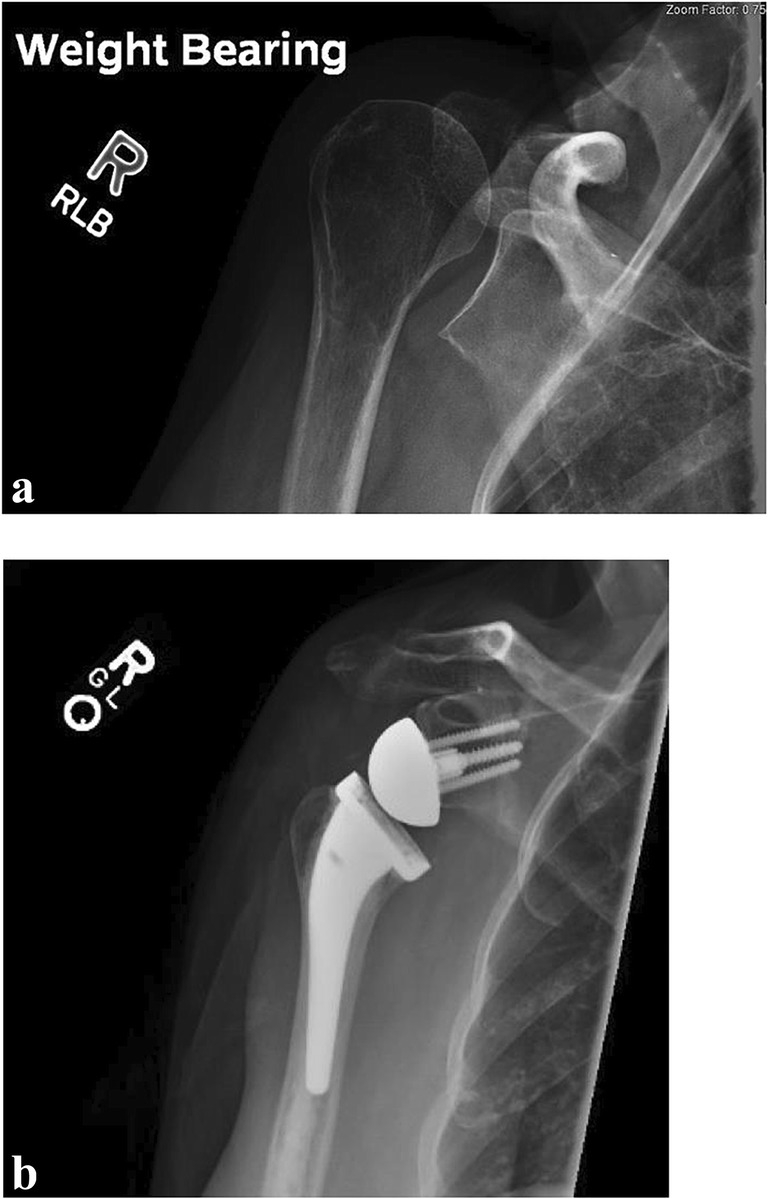
Fig. 3.
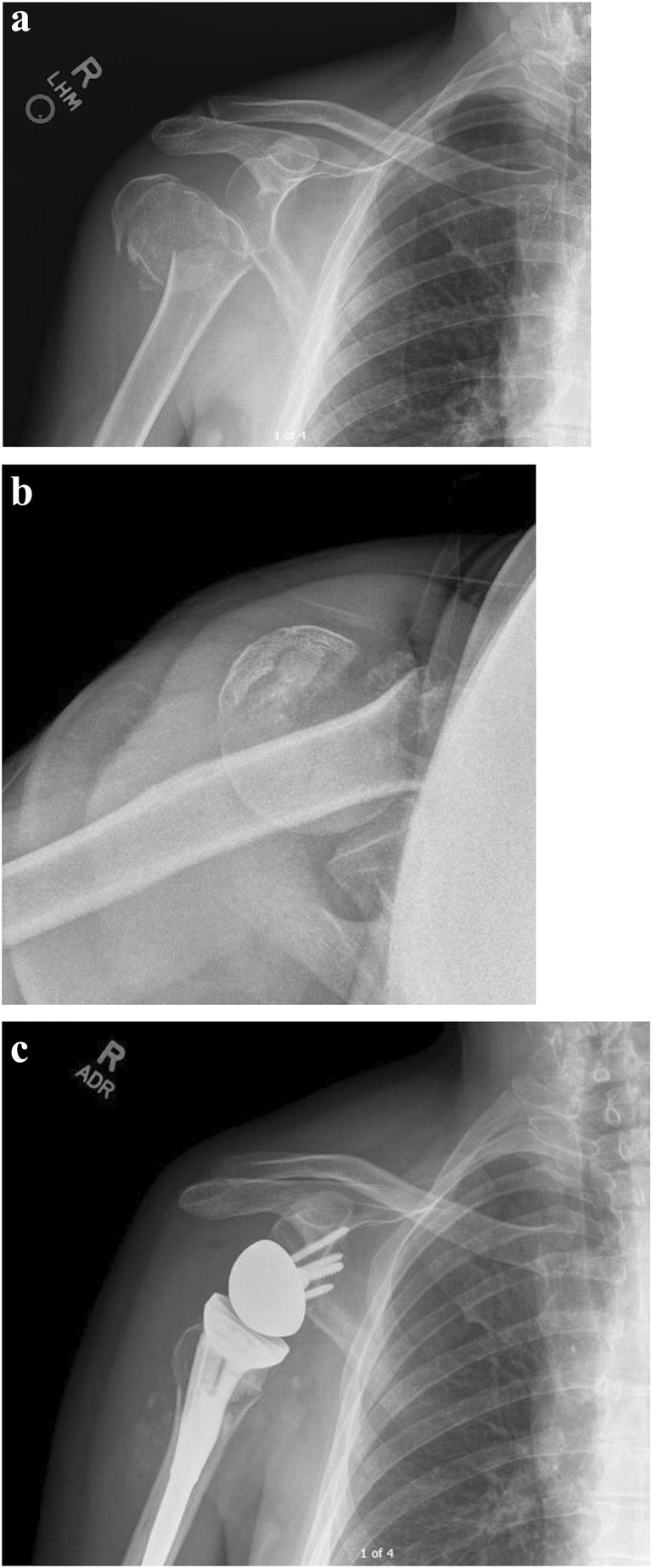
(a–c) Reverse total shoulder arthroplasty for a proximal humerus fracture. (a, b) Anterior-posterior (a) and lateral (b) radiograph of the right shoulder demonstrates a displaced four-part proximal humerus fracture. (c) Following reverse total shoulder arthroplasty with a cemented stem with suture fixation of the greater and lesser tuberosities around the humeral component
Comparisons of RTSA with hemiarthroplasty for treatment of PHF has generally favored RTSA in terms of improved functional motion, pain scores, and revision rate. A matched case-control prospective study in three- and four-part PHF demonstrated a higher proportion of subjects with forward elevation > 90° in the RTSA cohort, although there were no detectable differences in function scores between the two groups [80]. In a randomized trial of 62 patients over the age of 70 with PHF comparing hemiarthroplasty with RTSA, the RTSA group had higher UCLA and constant scores, and superior ROM in abduction and forward elevation. The healing status of the tuberosities did not affect the functional outcomes of the RTSA group, and more hemiarthroplasties required revision [81]. A meta-analysis confirmed superior outcomes regarding ROM, pain, and functional scores in RTSA compared with hemiarthroplasty [82].
Despite promising data on outcomes of this population when managed with RTSA, comparison between RTSA and non-operative management of PHF does not consistently demonstrate that superiority of RTSA. A 5-year review of 218 RTSA and 427 hemiarthroplasties showed no difference between functional outcomes or revision rate [83]. In a retrospective review of 39 patients with three- or four-part PHF managed with either RTSA or non-operatively, there was no difference in forward elevation, external rotation, or PROs at 2 years [84]. Chivot et al. reviewed 60 patients aged 70 or older with three- or four-part PHF, and although the RTSA cohort demonstrated higher constant scores than the non-operative cohort, there was no difference in other functional scores. Anterior elevation was improved in the RTSA group compared with the non-operative group (110° versus 98°); however, there were more complications in the RTSA group (7% versus 0%) [85]. Furthermore, delayed primary RTSA compared with acute primary RTSA yielded similar clinical results and reoperation rates, suggesting that perhaps in this frail population a trial of non-operative management may be prudent when appropriate [86].
Glenoid Bone Loss
Due to the potential fixation strength of the glenoid component in RTSA, RTSA may be considered for patients with severe glenoid bone loss, such as from primary osteoarthritis, tumor, inflammatory arthritis, or failed prior arthroplasty [87]. Glenoid bone loss resulting in a biconcave (B2) or severely retroverted and dysplastic (C) glenoid may be considerations for RTSA based on other patient characteristics (Fig. 4). For instance, Walch reported glenoid loosening in anatomic shoulder arthroplasties with biconcave glenoids that was present in over 20% of patients and the revision rate was 16.3% at average 77-months follow-up [88].
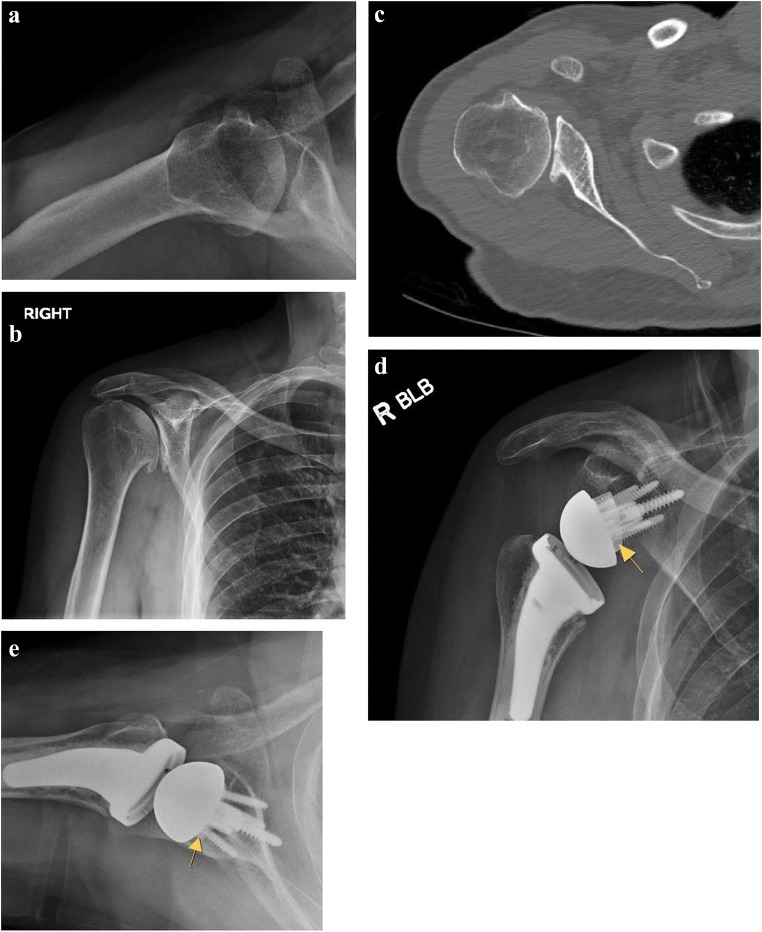
Fig. 4.
(a–e) Reverse total shoulder arthroplasty with an augmented glenoid component. Grashey (a) and axillary lateral (b) radiographs demonstrate glenohumeral arthritis with superior migration of the humeral head. (c) CT scan axial cuts demonstrate a dysplastic Walch C type glenoid. AP (d) and axillary lateral (e) radiographs following reverse total shoulder arthroplasty. The glenoid baseplate consists of a porous metal backed lateralizing augment, marked with a yellow arrow. Note the restoration of glenoid version with the posteriorly augmented component
Bone grafting of the glenoid to achieve sufficient bony fixation, to restore the glenoid version with posterior augmentation, and to lateralize the COR to avoid impingement on the coracoid and scapula is also a possible option [89–91]. Mizuno et al. reported results from a retrospective series of 27 patients with primary osteoarthritis of the shoulder with a biconcave glenoid treated with RTSA from 1998 to 2009 [92]. In this series, average retroversion was 32°; 10 patients received posterior bone grafting. There were significant improvements in PROs and ROM with complications in 15% of patients and no recurrent posterior instability at minimum 2-year follow-up. Gupta et al. described outcomes following bone grafting during RTSA in one of the largest contemporary series of 94 patients [93•]. A single-stage procedure was feasible in 92.5% of patients, and the authors recommended considering a two-stage procedure when intraoperative glenoid baseplate stability was unsatisfactory. Bone grafting was recommended if medialization occurred past the point of the coracoid.
Some companies have marketed implants or techniques specifically to address glenoid bone loss. The bony increased offset-reversed shoulder arthroplasty (BIO-RSA) is an option in which cancellous humeral head autograft is used to lateralize the COR, and in medium-term follow-up, has demonstrated excellent graft incorporation, a low rate of scapular notching, and satisfactory post-operative ROM [89]. Novel implant designs (Fig. 4) may allow for correction of retroversion or bone loss with a wedge or stepped implant. At this time there is no graft technique or implant which has comparative data demonstrating clear superiority. Future clinical studies will better define the appropriate indications for these implants and patient outcomes after implantation.
Revision Shoulder Arthroplasty
Reverse total shoulder arthroplasty may also be indicated for use in the setting of failed anatomic or hemiarthroplasty. If the rotator cuff fails in the setting of a hemiarthroplasty or anatomic TSA, instability and anterosuperior escape may manifest [94]. The reverse prosthesis is a reasonable solution as it does not rely on the rotator cuff for stability. In a study of 22 patients with failed total shoulder arthroplasty, conversion to RTSA resulted in improved subjective and functional outcomes though with higher complication rate than primary RTSA [95]. Relatedly, if there is nonunion, malunion, or resorption of the tuberosities following HA for PHF, RTSA can be used as a salvage operation [96].
Another indication for revision may be glenoid wear in hemiarthroplasty, or glenoid component failure in anatomic or reverse TSA [97]. In these settings, glenoid bone stock may not be adequately addressed by a revision anatomic TSA, even if the rotator cuff is intact. Due to the ability of the RTSA prosthesis to make up for deficient rotator cuff (which is necessary for both hemiarthroplasty and anatomic TSA) and provide glenoid resurfacing with less glenoid bone stock due to enhanced fixation options, it is becoming a more common solution to challenging revision arthroplasty cases.
Tumor
Reverse total shoulder arthroplasty is also a viable option in the setting of oncologic resection (Fig. 5) [98]. These patients may be younger and require substantial resection depending on the tumor size. If a wide oncologic resection necessitates removal of the tuberosities, then RTSA may be the only implant that allows for restoration of joint stability and preservation of shoulder function. In a study of 8 patients who underwent reversed proximal humeral endoprosthesis following tumor resection, Maclean et al. showed 100% revision-free survival at mean follow-up of 49 months with no local recurrence [99]. Forward flexion and abduction were less than 90° on average, but pain control was satisfactory. In a study of 13 patients with proximal humerus tumors necessitating resection, a two-surgeon approach with an orthopedic oncologist and a shoulder-trained surgeon yielded acceptable clinical results and no complications [100]. The authors recommended long-stemmed, modular components, optimization of stability through component positioning and soft tissue tensioning, and consideration for patients with a life expectancy of greater than 6 months.
Fig. 5.
(a–d) Reverse total shoulder reconstruction with endoprosthesis for tumor. (a) AP radiograph of the right shoulder demonstrates a lytic lesion in the right humeral head and metaphysis with pathologic fracture from metastatic hepatocellular carcinoma. (b) T2-weighted axial MRI image demonstrates a T2-bright heterogenous lesion replacing the bone of the humeral head. (c and d) Post-operative AP (c) and axillary lateral (d) radiographs of the right shoulder and humerus following proximal humerus resection and reconstruction with long-stemmed, cemented reversed polarity implant, which was performed with a shoulder arthroplasty-trained surgeon and an orthopaedic oncology surgeon
Conclusions
The reverse shoulder replacement has revolutionized the treatment of many challenging and complex shoulder pathologies. Through alterations to the native shoulder biomechanics, the RTSA provides a stable shoulder in the absence of a functioning rotator cuff. While only FDA-approved for patients with cuff tear arthropathy, emerging clinical evidence shows the efficacy of this treatment for a variety of clinical conditions. Future clinical studies will help clarify the role of further advances in implant design and surgical technique to optimize outcomes.
Compliance with Ethical Standards
Conflict of Interest
Caitlin M. Rugg, Monica J. Coughlan and Drew. A. Lansdown declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Reverse Shoulder Arthroplasty
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Caitlin M. Rugg, Email: Caitlin.rugg@ucsf.edu
Monica J. Coughlan, Email: monica.coughlan@ucsf.edu
Drew A. Lansdown, Email: drew.lansdown@ucsf.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 2.Palsis JA, Simpson KN, Matthews JH, Traven S, Eichinger JK, Friedman RJ. Current trends in the use of shoulder arthroplasty in the United States. Orthopedics. 2018;41(3):e416–ee23. doi: 10.3928/01477447-20180409-05. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Grammont PTP, Laffay JP, Deries X. Concept study and reaslization of a new total shoulder prosthesis [French] Rhumatologie. 1987;39:407–418. [Google Scholar]
- 5.Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469(9):2432–2439. doi: 10.1007/s11999-010-1733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berliner JL, Regalado-Magdos A, Ma CB, Feeley BT. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(1):150–160. doi: 10.1016/j.jse.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Bigorre N, Lancigu R, Bizot P, Hubert L. Predictive factors of scapular notching in patients with reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2014;100(7):711–714. doi: 10.1016/j.otsr.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17(4):608–615. doi: 10.1016/j.jse.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Kontaxis A., Johnson G.R. The biomechanics of reverse anatomy shoulder replacement – A modelling study. Clinical Biomechanics. 2009;24(3):254–260. doi: 10.1016/j.clinbiomech.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Levigne C, Boileau P, Favard L, Garaud P, Mole D, Sirveaux F, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(2):151–158. doi: 10.1016/j.jse.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Boileau Pascal, Gauci Marc-Olivier, Wagner Eric R., Clowez Gilles, Chaoui Jean, Chelli Mikaël, Walch Gilles. The reverse shoulder arthroplasty angle: a new measurement of glenoid inclination for reverse shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2019;28(7):1281–1290. doi: 10.1016/j.jse.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Choi CH, Kim SG, Lee JJ, Kwack BH. Comparison of clinical and radiological results according to glenosphere position in reverse total shoulder arthroplasty: a short-term follow-up study. Clin Orthop Surg. 2017;9(1):83–90. doi: 10.4055/cios.2017.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg. 2012;96(Suppl 1):S27–S34. doi: 10.1007/s12306-012-0193-4. [DOI] [PubMed] [Google Scholar]
- 15.Edwards TB, Trappey GJ, Riley C, O'Connor DP, Elkousy HA, Gartsman GM. Inferior tilt of the glenoid component does not decrease scapular notching in reverse shoulder arthroplasty: results of a prospective randomized study. J Shoulder Elbow Surg. 2012;21(5):641–646. doi: 10.1016/j.jse.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 16.Meisterhans Michel, Bouaicha Samy, Meyer Dominik C. Posterior and inferior glenosphere position in reverse total shoulder arthroplasty supports deltoid efficiency for shoulder flexion and elevation. Journal of Shoulder and Elbow Surgery. 2019;28(8):1515–1522. doi: 10.1016/j.jse.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Leung AS, Hippe DS, Ha AS. Cuff tear arthropathy shoulder hemiarthroplasty: a radiographic outcome study. Skeletal Radiology. 2017;46(7):909–918. doi: 10.1007/s00256-017-2631-8. [DOI] [PubMed] [Google Scholar]
- 18.Mahony GT, Werner BC, Chang B, Grawe BM, Taylor SA, Craig EV, et al. Risk factors for failing to achieve improvement after anatomic total shoulder arthroplasty for glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2018;27(6):968–975. doi: 10.1016/j.jse.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Neer CS, 2nd, Watson KC, Stanton FJ. Recent experiences in total shoulder replacement. J Bone Joint Surg. 1982;64:319–337. doi: 10.2106/00004623-198264030-00001. [DOI] [PubMed] [Google Scholar]
- 20.Poppen NKWP. Forces at the glenohumeral joint in abduction. Clin Orthop Relat Res. 1978;135:165–170. [PubMed] [Google Scholar]
- 21.Terrier A., Reist A., Merlini F., Farron A. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. The Journal of Bone and Joint Surgery. British volume. 2008;90-B(6):751–756. doi: 10.1302/0301-620X.90B6.19708. [DOI] [PubMed] [Google Scholar]
- 22.Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Muscle and joint-contact loading at the glenohumeral joint after reverse total shoulder arthroplasty. J Orthop Res. 2011;29(12):1850–1858. doi: 10.1002/jor.21437. [DOI] [PubMed] [Google Scholar]
- 23.Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221–1230. doi: 10.2106/JBJS.I.00001. [DOI] [PubMed] [Google Scholar]
- 24.Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483–490. doi: 10.1016/j.jse.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Jobin CM, Brown GD, Bahu MJ, Gardner TR, Bigliani LU, Levine WN, et al. Reverse total shoulder arthroplasty for cuff tear arthropathy: the clinical effect of deltoid lengthening and center of rotation medialization. J Shoulder Elbow Surg. 2012;21(10):1269–1277. doi: 10.1016/j.jse.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 26.Lädermann Alexandre, Williams Matthew D., Melis Barbara, Hoffmeyer Pierre, Walch Gilles. Objective evaluation of lengthening in reverse shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2009;18(4):588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Ackland DC, Richardson M, Pandy MG. Axial rotation moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(20):1886–1895. doi: 10.2106/JBJS.J.01861. [DOI] [PubMed] [Google Scholar]
- 28.Dedy NJ, Gouk CJ, Taylor FJ, Thomas M, Tan SLE. Sonographic assessment of the subscapularis after reverse shoulder arthroplasty: impact of tendon integrity on shoulder function. J Shoulder Elbow Surg. 2018;27(6):1051–1056. doi: 10.1016/j.jse.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(4):550–556. doi: 10.1016/j.jse.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 30.Clouthier AL, Hetzler MA, Fedorak G, Bryant JT, Deluzio KJ, Bicknell RT. Factors affecting the stability of reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2013;22(4):439–444. doi: 10.1016/j.jse.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 31.North LR, Hetzler MA, Pickell M, Bryant JT, Deluzio KJ, Bicknell RT. Effect of implant geometry on range of motion in reverse shoulder arthroplasty assessed using glenohumeral separation distance. J Shoulder Elbow Surg. 2015;24(9):1359–1366. doi: 10.1016/j.jse.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Langohr GD, Willing R, Medley JB, Athwal GS, Johnson JA. Contact mechanics of reverse total shoulder arthroplasty during abduction: the effect of neck-shaft angle, humeral cup depth, and glenosphere diameter. J Shoulder Elbow Surg. 2016;25(4):589–597. doi: 10.1016/j.jse.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Elwell J, Choi J, Willing R. Quantifying the competing relationship between adduction range of motion and baseplate micromotion with lateralization of reverse total shoulder arthroplasty. J Biomech. 2017;52:24–30. doi: 10.1016/j.jbiomech.2016.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roche CPSN, Martin BL, Steiler CA, Flurin PH, Wright TW, et al. The impact of scapular notching on reverse shoulder glenoid fixation. J Shoulder Elbow Surg. 2013;22:963–970. doi: 10.1016/j.jse.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 35.Permeswaran VN, Caceres A, Goetz JE, Anderson DD, Hettrich CM. The effect of glenoid component version and humeral polyethylene liner rotation on subluxation and impingement in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(10):1718–1725. doi: 10.1016/j.jse.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Hettrich CM, Permeswaran VN, Goetz JE, Anderson DD. Mechanical tradeoffs associated with glenosphere lateralization in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(11):1774–1781. doi: 10.1016/j.jse.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Melis B, DeFranco M, Ladermann A, Mole D, Favard L, Nerot C, et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93(9):1240–1246. doi: 10.1302/0301-620x.93b9.25926. [DOI] [PubMed] [Google Scholar]
- 38.Helmkamp JK, Bullock GS, Amilo NR, Guerrero EM, Ledbetter LS, Sell TC, et al. The clinical and radiographic impact of center of rotation lateralization in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27(11):2099–2107. doi: 10.1016/j.jse.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Rhee SM, Lee JD, Park YB, Yoo JC, Oh JH. Prognostic radiological factors affecting clinical outcomes of reverse shoulder arthroplasty in the Korean population. Clin Orthop Surg. 2019;11(1):112–119. doi: 10.4055/cios.2019.11.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner BS, Chaoui J, Walch G. Glenosphere design affects range of movement and risk of friction-type scapular impingement in reverse shoulder arthroplasty. Bone Joint J. 2018;100-b(9):1182–1186. doi: 10.1302/0301-620x.100b9.Bjj-2018-0264.R1. [DOI] [PubMed] [Google Scholar]
- 41.Boutsiadis A, Lenoir H, Denard PJ, Panisset JC, Brossard P, Delsol P, et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(7):1226–1234. doi: 10.1016/j.jse.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 42.Kim SJ, Jang SW, Jung KH, Kim YS, Lee SJ, Yoo YS. Analysis of impingement-free range of motion of the glenohumeral joint after reverse total shoulder arthroplasty using three different implant models. J Orthop Sci. 2019;24(1):87–94. doi: 10.1016/j.jos.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 43.de Wilde LF, Poncet D, Middernacht B, Ekelund A. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop. 2010;81(6):719–726. doi: 10.3109/17453674.2010.538354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Junaid S, Gregory T, Hansen U, Cheng CK. Effect of baseplate positioning on fixation of reverse total shoulder arthroplasty. Clin Biomech (Bristol, Avon) 2019;62:15–22. doi: 10.1016/j.clinbiomech.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Chae SW, Lee J, Han SH, Kim SY. Inferior tilt fixation of the glenoid component in reverse total shoulder arthroplasty: a biomechanical study. Orthop Traumatol Surg Res. 2015;101(4):421–425. doi: 10.1016/j.otsr.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Poon PC, Chou J, Young SW, Astley T. A comparison of concentric and eccentric glenospheres in reverse shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(16):e138. doi: 10.2106/jbjs.M.00941. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Dines JS, Warren RF, Craig EV, Dines DM. Inferior glenosphere placement reduces scapular notching in reverse total shoulder arthroplasty. Orthopedics. 2015;38(2):e88–e93. doi: 10.3928/01477447-20150204-54. [DOI] [PubMed] [Google Scholar]
- 48.Jeon BK, Panchal KA, Ji JH, Xin YZ, Park SR, Kim JH, et al. Combined effect of change in humeral neck-shaft angle and retroversion on shoulder range of motion in reverse total shoulder arthroplasty - a simulation study. Clin Biomech (Bristol, Avon) 2016;31:12–19. doi: 10.1016/j.clinbiomech.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Nelson R, Lowe JT, Lawler SM, Fitzgerald M, Mantell MT, Jawa A. Lateralized center of rotation and lower neck-shaft angle are associated with lower rates of scapular notching and heterotopic ossification and improved pain for reverse shoulder arthroplasty at 1 year. Orthopedics. 2018;41(4):230–236. doi: 10.3928/01477447-20180613-01. [DOI] [PubMed] [Google Scholar]
- 50.Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of prosthesis design on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):571–576. doi: 10.1016/j.jse.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 51.Ladermann A, Denard PJ, Boileau P, Farron A, Deransart P, Terrier A, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39(11):2205–2213. doi: 10.1007/s00264-015-2984-3. [DOI] [PubMed] [Google Scholar]
- 52.Berhouet J, Kontaxis A, Gulotta LV, Craig E, Warren R, Dines J, et al. Effects of the humeral tray component positioning for onlay reverse shoulder arthroplasty design: a biomechanical analysis. J Shoulder Elbow Surg. 2015;24(4):569–577. doi: 10.1016/j.jse.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 53.Merolla G, Walch G, Ascione F, Paladini P, Fabbri E, Padolino A, et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg. 2018;27(4):701–710. doi: 10.1016/j.jse.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Levigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512–2520. doi: 10.1007/s11999-010-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Simovitch RWZM, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg. 2007;89-A:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 57.Mollon B, Mahure SA, Roche CP, Zuckerman JD. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg. 2017;26(7):1253–1261. doi: 10.1016/j.jse.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 58.Sirveaux FFL, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 59.Bitzer A, Rojas J, Patten IS, Joseph J, McFarland EG. Incidence and risk factors for aseptic baseplate loosening of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(12):2145–2152. doi: 10.1016/j.jse.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 60.Katz D, Valenti P, Kany J, Elkholti K, Werthel JD. Does lateralisation of the centre of rotation in reverse shoulder arthroplasty avoid scapular notching? Clinical and radiological review of one hundred and forty cases with forty five months of follow-up. Int Orthop. 2016;40(1):99–108. doi: 10.1007/s00264-015-2976-3. [DOI] [PubMed] [Google Scholar]
- 61.Wellmann M, Struck M, Pastor MF, Gettmann A, Windhagen H, Smith T. Short and midterm results of reverse shoulder arthroplasty according to the preoperative etiology. Arch Orthop Trauma Surg. 2013;133(4):463–471. doi: 10.1007/s00402-013-1688-7. [DOI] [PubMed] [Google Scholar]
- 62.van Ochten JHM, van der Pluijm M, Pouw M, Felsch QTM, Heesterbeek P, de Vos MJ. Long-term survivorship and clinical and radiological follow - up of the primary uncemented Delta III reverse shoulder prosthesis. J Orthop. 2019;16(4):342–346. doi: 10.1016/j.jor.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014;23(11):1662–1668. doi: 10.1016/j.jse.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742–1747. doi: 10.2106/jbjs.e.00851. [DOI] [PubMed] [Google Scholar]
- 65.Petrillo S, Longo UG, Papalia R, Denaro V. Reverse shoulder arthroplasty for massive irreparable rotator cuff tears and cuff tear arthropathy: a systematic review. Musculoskelet Surg. 2017;101(2):105–112. doi: 10.1007/s12306-017-0474-z. [DOI] [PubMed] [Google Scholar]
- 66.Wiater BP, Koueiter DM, Maerz T, Moravek JE, Jr, Yonan S, Marcantonio DR, et al. Preoperative deltoid size and fatty infiltration of the deltoid and rotator cuff correlate to outcomes after reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2015;473(2):663–673. doi: 10.1007/s11999-014-4047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong SE, Pitcher AA, Ding DY, Cashman N, Zhang AL, Ma CB, et al. The effect of patient gender on outcomes after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(11):1889–1896. doi: 10.1016/j.jse.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 68.Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996–2000. doi: 10.2106/jbjs.K.01206. [DOI] [PubMed] [Google Scholar]
- 69.Cuff DJ, Pupello DR, Santoni BG, Clark RE, Frankle MA. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of 10 years, of previous reports. J Bone Joint Surg Am. 2017;99(22):1895–1899. doi: 10.2106/jbjs.17.00175. [DOI] [PubMed] [Google Scholar]
- 70.Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544–2556. doi: 10.2106/jbjs.i.00912. [DOI] [PubMed] [Google Scholar]
- 71.Gerber C, Canonica S, Catanzaro S, Ernstbrunner L. Longitudinal observational study of reverse total shoulder arthroplasty for irreparable rotator cuff dysfunction: results after 15 years. J Shoulder Elbow Surg. 2018;27(5):831–838. doi: 10.1016/j.jse.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 72.Ernstbrunner L, Andronic O, Grubhofer F, Camenzind RS, Wieser K, Gerber C. Long-term results of reverse total shoulder arthroplasty for rotator cuff dysfunction: a systematic review of longitudinal outcomes. J Shoulder Elbow Surg. 2019;28(4):774–781. doi: 10.1016/j.jse.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE, et al. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg. 2015;24(11):1698–1706. doi: 10.1016/j.jse.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Shields EJW, Koueiter DM, Maerz T, Schwark A, Wiater JM. Previous rotator cuff repair is associated with inferior clinical outcomes after reverse total shoulder arthroplasty. Orthop J Sports Med. 2017;5(10):2325967117730311. doi: 10.1177/2325967117730311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajaee SS, Yalamanchili D, Noori N, Debbi E, Mirocha J, Lin CA, et al. Increasing use of reverse total shoulder arthroplasty for proximal humerus fractures in elderly patients. Orthopedics. 2017;40(6):e982–e9e9. doi: 10.3928/01477447-20170925-01. [DOI] [PubMed] [Google Scholar]
- 76.Dillon MT, Prentice HA, Burfeind WE, Chan PH, Navarro RA. The increasing role of reverse total shoulder arthroplasty in the treatment of proximal humerus fractures. Injury. 2019;50(3):676–680. doi: 10.1016/j.injury.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 77.Wright Jon O., Ho Anthony, Kalma Jeremy, Koueiter Denise, Esterle Jason, Marcantonio David, Wiater J. Michael, Wiater Brett. Uncemented Reverse Total Shoulder Arthroplasty as Initial Treatment for Comminuted Proximal Humerus Fractures. Journal of Orthopaedic Trauma. 2019;33(7):e263–e269. doi: 10.1097/BOT.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 78.Grubhofer F, Wieser K, Meyer DC, Catanzaro S, Beeler S, Riede U, et al. Reverse total shoulder arthroplasty for acute head-splitting, 3- and 4-part fractures of the proximal humerus in the elderly. J Shoulder Elbow Surg. 2016;25(10):1690–1698. doi: 10.1016/j.jse.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 79.Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215–222. doi: 10.1016/j.jse.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 80.Chalmers PN, Slikker W, 3rd, Mall NA, Gupta AK, Rahman Z, Enriquez D, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197–204. doi: 10.1016/j.jse.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 81.Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419–1426. doi: 10.1016/j.jse.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 82.Shukla DR, McAnany S, Kim J, Overley S, Parsons BO. Hemiarthroplasty versus reverse shoulder arthroplasty for treatment of proximal humeral fractures: a meta-analysis. J Shoulder Elbow Surg. 2016;25(2):330–340. doi: 10.1016/j.jse.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 83.van der Merwe M, Boyle MJ, Frampton CMA, Ball CM. Reverse shoulder arthroplasty compared with hemiarthroplasty in the treatment of acute proximal humeral fractures. J Shoulder Elbow Surg. 2017;26(9):1539–1545. doi: 10.1016/j.jse.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Roberson TA, Granade CM, Hunt Q, Griscom JT, Adams KJ, Momaya AM, et al. Non-operative management versus reverse shoulder arthroplasty for treatment of 3- and 4-part proximal humeral fractures in older adults. J Shoulder Elbow Surg. 2017;26(6):1017–1022. doi: 10.1016/j.jse.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Chivot M, Lami D, Bizzozero P, Galland A, Argenson JN. Three- and four-part displaced proximal humeral fractures in patients older than 70 years: reverse shoulder arthroplasty or nonsurgical treatment? J Shoulder Elbow Surg. 2019;28(2):252–259. doi: 10.1016/j.jse.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 86.Torchia MT, Austin DC, Cozzolino N, Jacobowitz L, Bell J-E. Acute versus delayed reverse total shoulder arthroplasty for the treatment of proximal humeral fractures in the elderly population: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2019;28(4):765–773. doi: 10.1016/j.jse.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Hyun YS, Huri G, Garbis NG, McFarland EG. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg. 2013;5(4):243–255. doi: 10.4055/cios.2013.5.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526–1533. doi: 10.1016/j.jse.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 89.Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sears BW, Johnston PS, Ramsey ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604–613. doi: 10.5435/jaaos-20-09-604. [DOI] [PubMed] [Google Scholar]
- 91.Cofield RH. Bone grafting for glenoid bone deficiencies in shoulder arthritis: a review. J Shoulder Elbow Surg. 2007;16(5 Suppl):S273–S281. doi: 10.1016/j.jse.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297–1304. doi: 10.2106/jbjs.L.00820. [DOI] [PubMed] [Google Scholar]
- 93.Gupta A, Thussbas C, Koch M, Seebauer L. Management of glenoid bone defects with reverse shoulder arthroplasty-surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2018;27(5):853–862. doi: 10.1016/j.jse.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure–midterm results. Int Orthop. 2011;35(1):53–60. doi: 10.1007/s00264-010-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514–522. doi: 10.1016/j.jse.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. doi: 10.2106/E.01310. [DOI] [PubMed] [Google Scholar]
- 97.Holcomb JO, Cuff D, Petersen SA, Pupello DR, Frankle MA. Revision reverse shoulder arthroplasty for glenoid baseplate failure after primary reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):717–723. doi: 10.1016/j.jse.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 98.Chalmers PN, Keener JD. Expanding roles for reverse shoulder arthroplasty. Curr Rev Musculoskelet Med. 2016;9(1):40–48. doi: 10.1007/s12178-016-9316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maclean S, Malik SS, Evans S, Gregory J, Jeys L. Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg. 2017;26(11):1990–1994. doi: 10.1016/j.jse.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Grosel TW, Plummer DR, Mayerson JL, Scharschmidt TJ, Barlow JD. Oncologic reconstruction of the proximal humerus with a reverse total shoulder arthroplasty megaprosthesis. J Surg Oncol. 2018;118(6):867–872. doi: 10.1002/jso.25061. [DOI] [PubMed] [Google Scholar]