Abstract
Introduction
Illicitly manufactured fentanyl (IMF) is responsible for a growing number of deaths. Some case series have suggested that IMF overdoses require significantly higher naloxone doses than heroin overdoses. Our objective was to determine if the naloxone dose required to treat an opioid overdose is associated with the finding of fentanyl, opiates, or both on urine drug screen (UDS).
Methods
A retrospective chart review was conducted at a single emergency department and its affiliated emergency medical services (EMS) agency. The charts of all patients who received naloxone through this EMS from 1/1/2017 to 6/15/2018 were reviewed. The study included patients diagnosed with a non-suicidal opioid overdose whose UDS was positive for opiates, fentanyl, or both. Data collected included demographics, vital signs, initial GCS, EMS and ED naloxone administrations, response to treatment, laboratory findings, and ED disposition. The fentanyl-only and fentanyl + opiate groups were compared to the opiate-only group using the stratified (by ED provider) variant of the Mann-Whitney U test.
Results
Eight hundred and thirty-seven charts were reviewed, and 121 subjects were included in the final analysis. The median age of included subjects was 38 years and 75% were male. In the naloxone dose analysis, neither the fentanyl-only (median 0.8 mg, IQR 0.4–1.6; p = 0.68) nor the fentanyl + opiate (median 0.8 mg, IQR 0.4–1.2; p = 0.56) groups differed from the opiate-only group (median 0.58 mg, IQR 0.4–1.6).
Conclusion
Our findings refute the notion that high potency synthetic opioids like illicitly manufactured fentanyl require increased doses of naloxone to successfully treat an overdose. There were no significant differences in the dose of naloxone required to treat opioid overdose patients with UDS evidence of exposure to fentanyl, opiates, or both. Further evaluation of naloxone stocking and dosing protocols is needed.
Electronic supplementary material
The online version of this article (10.1007/s13181-019-00735-w) contains supplementary material, which is available to authorized users.
Keywords: Fentanyl, Opiates, Naloxone, Drug overdose, Emergency medical services
Background
Deaths from opioid use in the USA are at record levels. In 2017, more than 63,000 Americans died due to a drug overdose involving an opioid, directly contributing to a decline in the nation’s life expectancy [1, 2]. In recent years, the opioid supply composition has changed, specifically with the entry of synthetic opioids into the illicit drug market [3, 4]. For example, a large surge in opioid related deaths from 2014 to 2015 was largely driven by a 72.2% increase in deaths due to synthetic opioids other than methadone [5, 6]. During that same time period, the number of illicit opioid samples testing positive for fentanyl increased as much as 10-fold; however, prescribing rates did not significantly change [3]. By 2017, fentanyl and fentanyl analogs were thought to be responsible for 31% of all national drug-related deaths [1].
Fentanyl and its derivatives may be hundreds of times more potent than morphine or heroin, and are often sold on the street as heroin or mixed in with other drugs such as cocaine [4]. Opioid users are often unaware of the presence of fentanyl and its increased potency, leading to accidental overdose and increased risk of death [7]. As the prevalence of illicitly manufactured fentanyl (IMF) has increased, some observational case series have noted that the quantity of naloxone needed to treat an opioid overdose may have increased relative to historic norms [8, 9]. One case series observed an average naloxone dose of 3.36 mg [10], while another noted that the majority of patients received two or more doses of intranasal naloxone (2 mg/2 mL) [11]. Directly contradicting these findings is a study evaluating the safety of a brief observation period after presumed fentanyl overdose, which noted a median naloxone dose of 0.4 mg [12]. However, this study was limited by a lack of objective data to confirm fentanyl exposure. If an IMF overdose does indeed require significantly more naloxone than a heroin overdose, prehospital provider and bystander treatment guideline changes may be warranted. For example, Emergency Medical Services (EMS) providers carry a limited supply of naloxone and could exhaust their entire supply on one patient, rendering them unable to effectively respond to the next call without restocking. Similarly, the dose of naloxone in “take home” kits distributed to laypersons would need to be increased.
Currently, convincing data do not exist to define how much naloxone is required to treat an overdose of IMF or its analogs. This relates to the lack of IMF manufacturing standardization and its control, especially given the global scope of the market. The purpose of this study was to determine if patients suffering an overdose of fentanyl or a fentanyl analog require higher doses of naloxone than patients suffering an overdose of a non-synthetic opiate, such as morphine or heroin.
Methods
Study Design
This is a single-center-based retrospective chart review conducted at a large, urban public trauma and safety net center and its affiliated EMS agency, both of which serve more than 125,000 patients per year. The EMS service processes, dispatches, and responds to all medical 9-1-1 calls for the City of Atlanta. EMS providers in this agency follow a clinical care guideline for suspected opioid overdose patients. The guideline states that patients with a respiratory rate less than 10 should receive naloxone 0.4 mg IV/IM or 2 mg IN, repeated as needed up to 10 mg. Those patients a respiratory rate greater than or equal to 10 and/or ongoing altered mental status may receive naloxone 0.4 mg IV/IM/IN, which may be repeated if there is no response. In both cases, dosing frequency is at the provider’s discretion. Chart review and data abstraction were performed by Medical Toxicology fellows who are board-certified in Emergency Medicine. Abstractors used a standardized abstraction form with specific instructions and a data dictionary (Supplemental material). They were not blinded to the study hypothesis. Most charts were reviewed by one abstractor, with the exception of a random selection used to determine interrater reliability, detailed as follows. This study was reviewed and approved by both the Emory University Institutional Review Board (IRB) and Grady Health System Research Oversight Committee.
The EMS electronic medical record (EMR) was queried for all patients, age 18 years or older, who had received prehospital naloxone between January 1, 2017, and June 15, 2018. Using this initial data set, a manual chart review of the hospital’s EMR was performed to determine the final diagnosis and group category for each case. Subjects were included if ED documentation indicated a diagnosis of opioid overdose, abuse, or toxicity, and if they had a urine drug screen (UDS) performed during their hospitalization that was positive for opiates, fentanyl, or both. Potential subjects were excluded from the study if they were suspected or confirmed to have used an opioid with suicidal intent, or if their primary diagnosis was unrelated to opioid use (e.g., alcohol intoxication or cerebrovascular accident). Data abstractors were instructed to record whether “based on your review of the ED chart, you believe that naloxone improved the patient’s clinical status.” Abstractors were not asked to specify whether this implied resolution of respiratory or central nervous system (CNS) depression because our retrospective chart review study design made it difficult to determine individual providers’ clinical endpoints for naloxone dosing, and because documentation of the respiratory rate is often unreliable [13]. It was felt that any error introduced by including patients that improved beyond just resolution of respiratory depression would lead to an over estimation of the naloxone dose required to successfully treat an overdose, which would be safer than an underestimation of this figure. Subjects who did not, in the opinion of the reviewer, exhibit a clinical response to naloxone were excluded from the primary analysis since we could not definitively determine the naloxone dose at which they might have responded, or if their mental status change was truly due to opioid intoxication.
Data Acquisition
Data were abstracted for all included subjects from both the EMS and hospital EMRs, including the subject’s location at the time of overdose (residence, workplace, street or public park, inside a motor vehicle, or other public place such as a restaurant), reported substance(s) used, route(s) of use (ingestion, insufflation, intravenous, intramuscular), time of most recent use, initial vital signs, initial Glascow Coma Scale (GCS), prehospital naloxone provider (bystander, police or basic life support provider, EMS), naloxone dose(s) and route(s), response to naloxone, prehospital CPR, qualitative UDS results, serum ethanol concentration, ED disposition, and ED provider(s) (Supplemental material). We recorded the length of ED stay for subjects who were discharged from the ED, and the indication for admission for subjects that were admitted to the hospital.
To calculate the total dose of naloxone used during each subject’s initial resuscitation, we added prehospital naloxone to in-hospital naloxone given within 1 h of ED arrival. Since naloxone’s duration of action is approximately 1 h [14], it was assumed that naloxone given more than 1 h after ED arrival reflected recurrent toxicity due to naloxone metabolism and elimination. One hundred percent bioavailability was assumed for intravenous (IV), intraosseous (IO), and intramuscular (IM) naloxone [15], and 45% bioavailability was assumed for the intranasal (IN) route [16]. Statistical analysis was performed and results presented using IV dose equivalents.
Data Analysis
Subjects’ qualitative UDS results were utilized as a surrogate marker for fentanyl and opiate exposure. The study institution’s routine UDS includes a commercially available fentanyl immunoassay (Immunalysis Corporation, Pomona, CA). The assay uses a cutoff of 2 ng fentanyl per mL of urine for positivity, and according to data provided by the manufacturer is cross-reactive with the analogs carfentanil, acetyl fentanyl, butyryl fentanyl, 4-methoxy butyryl fentanyl, isobutyryl fentanyl, and furanyl fentanyl [17]. The opiate immunoassay used is the Emit II plus (Beckman Coulter, Brea, CA) with a cutoff of 2000 ng/mL. Screening for specific synthetic opioids other than fentanyl is not routinely performed, and no hospital protocol exists at the study institution that indicates the cases in which ED providers should or should not obtain a UDS.
Subjects were divided into three groups based upon the results of their UDS: opiate positive, fentanyl positive, and both opiate and fentanyl positive. The primary aim of this study was to detect a difference in naloxone dosing of at least 2 mg between groups, which was determined to be a clinically significant increase in dosage based upon expert recommendations regarding administration of naloxone in heroin overdoses [18, 19]. Additionally, because this study design involved three hypothesis tests, a p value cutoff of .05/3 = .0167 was adopted. Using this information, the sample size necessary to achieve 80% power was calculated to be 79 subjects per group.
Continuous variables were described using medians and interquartile ranges (IQR). Categorical variables were described using frequencies and percentages. Naloxone dosages were compared using the stratified variant of the Mann-Whitney U test in order to account for variation between providers. Secondary outcomes included the incidence of precipitated withdrawal, the need for additional naloxone more than 1 h after ED arrival, ED disposition, and ED observation time (if discharged). To determine interrater reliability of abstracted data, the data abstractors all reviewed 20 (15% of all included subjects) randomly selected charts. Intraclass correlation coefficients were used for continuous variables and Cohen’s κ was used for categorical variables. Interrater reliability coefficients ranged between 0.7 and 1.0. In the case of disagreement between abstractors, the primary author re-reviewed the chart and made the final decision regarding what data to include. All statistical analyses were performed using R (v 3.5.1).
Results
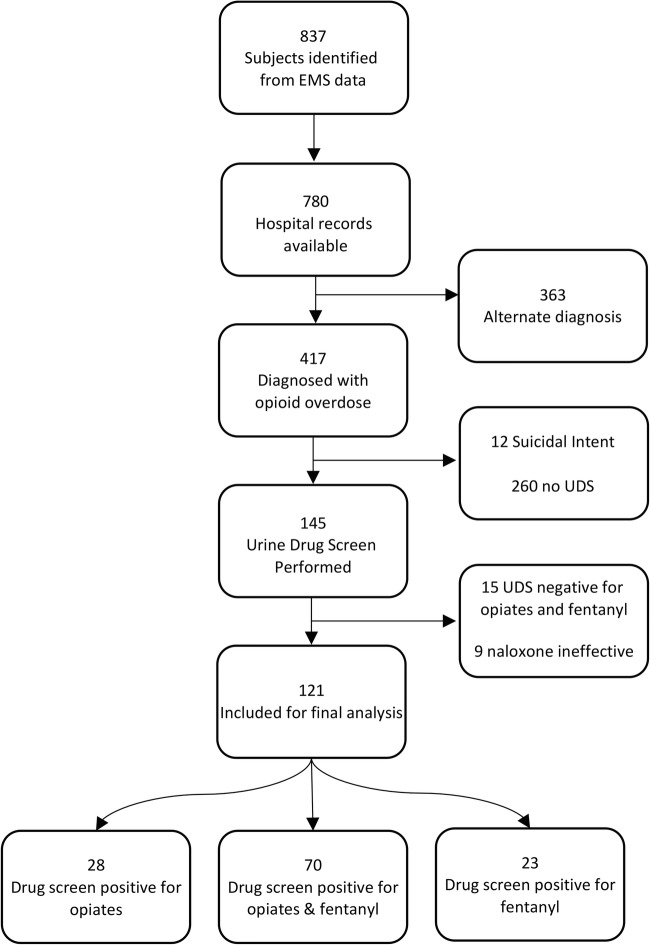
Eight hundred and thirty-seven potential subjects were identified from the initial EMS data query. Of these, 780 hospital charts were available for manual review and 417 were diagnosed with an opioid overdose according to documentation by the treating ED provider. The most common diagnoses other than opioid overdose were alcohol intoxication (n = 63, 8.1% of hospital charts reviewed), overdose of a non-opioid medication (n = 38, 4.9%), and use/abuse of a non-opioid illicit drug (n = 32, 4.1%). Twelve potential subjects were excluded because they were suspected or known to have overdosed with suicidal intent. Of the potential subjects evaluated for a non-suicidal opioid overdose, 145 had a UDS sent, 15 were excluded because their UDS was negative for both opiates and fentanyl, and 9 were analyzed separately from the primary analysis because they did not exhibit a clinical response to naloxone. One hundred and twenty-one subjects were included in the primary analysis: 28 subjects had a UDS result positive for opiates, 23 for fentanyl, and 70 for both opiates and fentanyl (Fig. 1).
Fig. 1.
Patient enrollment flowchart
The median age of included subjects was 38 years (range 18–77 years) and 75% of subjects were male (Table 1). The initial GCS and respiratory rate were documented for 106 subjects. The median initial GCS was 3 (IQR 3–8) and median initial respiratory rate was 6 breaths per minute (IQR 4–10). At the time of their overdose, subjects were most commonly located in the street or a public park (n = 36, 30%), at home (n = 33, 27%), or in a motor vehicle (n = 23, 19%). EMS providers administered the first dose of naloxone to 106 (88%) subjects and other first responders (fire department, police) administered the first dose to 12 (10%) subjects. IV was the most common route of prehospital naloxone and was administered to seventy-five (62%) subjects, followed by IN (46 subjects, 38%), IM (30 subjects, 25%), and lastly IO (1 subject, 1%, Table 2). Although only three subjects (2%) received naloxone from a bystander, 11 (9%) received bystander CPR.
Table 1.
Subject characteristics. UDS urine drug screen, IQR interquartile range, CPR cardiopulmonary resuscitation
| Any UDS (n = 121) | Fentanyl only (n = 23) | Opiates only (n = 28) | Fentanyl + Opiates (n = 70) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), year | 38 (28–54) | 37 (26–51) | 39 (27–57) | 38 (29–54) |
| Male gender, n (%) | 91 (75) | 19 (83) | 17 (61) | 55 (79) |
| Bystander CPR, n (%) | 11 (9) | 3 (13) | 2 (7) | 6 (9) |
| EMS vital signs, median (IQR) | ||||
| Glasgow Coma Scale | 3 (3–8) | 3 (3–5) | 4 (3–8) | 3 (3–9) |
| Respiratory rate | 6 (4–10) | 0 (0–0) | 8 (4–12) | 6 (4–10) |
| UDS findings, n (%) | ||||
| Amphetamines | 29 (24) | 4 (17) | 9 (32) | 16 (23) |
| Benzodiazepines | 45 (37) | 7 (30) | 11 (39) | 27 (39) |
| Cannabinoids | 48 (40) | 11 (48) | 9 (32) | 28 (40) |
| Cocaine | 67 (55) | 13 (57) | 14 (50) | 40 (57) |
Table 2.
Therapies and ED disposition. UDS urine drug screen, IQR interquartile range
| Any UDS (n = 121) | Fentanyl only (n = 23) | Opiates only (n = 28) | Fentanyl + opiates (n = 70) | |
|---|---|---|---|---|
| Prehospital naloxone route, n (%)a | ||||
| Intranasal | 46 (38) | 5 (22) | 12 (43) | 29 (41) |
| Intramuscular | 30 (25) | 3 (13) | 8 (29) | 19 (27) |
| Intraosseus | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| Intravenous (IV) | 75 (62) | 19 (83) | 14 (50) | 42 (60) |
| Prehospital naloxone dose, median (IQR, range), mgb | ||||
| Intranasal | 0 (0–0.40, 0–4.80) | 0 (0–0, 0–4.80) | 0 (0–0.40, 0–4.00) | 0 (0–0.40, 0–2.00) |
| Intramuscular | 0 (0–0, 0–2.00) | 0 (0–0, 0–0.80) | 0 (0–0.40, 0–2.00) | 0 (0–0.40, 0–2.00) |
| Intraosseus | 0 (0–0, 0–2.00) | 0 (0–0, 0–0) | 0 (0–0, 0–0) | 0 (0–0, 0–2.00) |
| Intravenous | 0.40 (0–0.80, 0–4.0) | 0.40 (0.40–1.20, 0–4.00) | 0.02 (0–0.40, 0–2.00) | 0.40 (0–0.70, 0–2.50) |
| Total prehospital naloxone, median (IQR), mg IV equivalent | 0.58 (0.40–1.16) | 0.80 (0.40–1.29) | 0.40 (0.36–0.77) | 0.72 (0.40–1.00) |
| ED therapies, n (%) | ||||
| Naloxone < 1 h after arrival | 28 (23) | 5 (22) | 10 (36) | 13 (19) |
| Naloxone > 1 h after arrival | 10 (8) | 0 (0) | 4 (14) | 6 (9) |
| Naloxone infusion | 5 (4) | 1 (4) | 2 (7) | 2 (3) |
| Intubation | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ED IV naloxone < 1 h after arrival, median (IQR, range), mg | 0 (0–0, 0–2.00) | 0 (0–0, 0–2.00) | 0 (0–0.40, 0–1.20) | 0 (0–0, 0–2.00) |
| Total naloxone dose, EMS + ED resuscitation, median (IQR, range), mg IV equivalent | 0.80 (0.40–1.38, 0.18–5.20) | 0.80 (0.40–1.60, 0.18–5.20) | 0.58 (0.40–1.25, 0.18–2.00) | 0.80 (0.40–1.38, 0.18–3.60) |
| ED disposition, n (%) | ||||
| Against medical advice | 5 (4) | 1 (4) | 0 (0) | 4 (6) |
| Home/self | 74 (61) | 13 (57) | 14 (50) | 47 (67) |
| Observation | 3 (2) | 1 (4) | 0 (0) | 2 (3) |
| Admitted to ward | 33 (27) | 7 (30) | 14 (50) | 12 (17) |
| Admitted to critical care | 6 (5) | 1 (4) | 0 (0) | 5 (7) |
aSome patients received prehospital naloxone via multiple routes. bDose provided in mg as administered, not IV dose equivalent
In the ED, all naloxone was administered IV. Twenty-eight subjects received at least one additional bolus dose of naloxone within 1 h of arrival, and five were started on a naloxone infusion (Table 2). One of these subjects, whose UDS was positive for fentanyl, received 5.20 mg IV equivalent bolus-dose naloxone before the infusion was initiated, higher than any other subject in the study. The other subjects placed on a naloxone infusion received 1.38, 0.94 mg (UDS positive for opiates), 2, and 3.6 mg (UDS positive for fentanyl + opiates) IV equivalent bolus-dose naloxone before the infusion. Ten subjects had recurrent toxicity requiring additional naloxone more than 1 h after ED arrival, four in the opiate-only group and six in the fentanyl + opiate group. No subjects in the fentanyl-only group received bolus-dose naloxone more than 1 h after ED arrival. Chart reviewers deemed that naloxone was effective at reversing overdose symptoms in 121 (93%) cases, and precipitated withdrawal was noted in 11 (8%) cases. In cases with precipitated withdrawal, the median naloxone dose was 0.60 mg (IQR 0.38–0.89, range 0.18–1.60), four (36%) were admitted to a ward bed, and three (27%) were admitted to a critical care bed. The chart reviewers concluded that naloxone did not positively improve the clinical status of nine patients (7%), three of whom had a documented cardiac arrest and six of whom were intubated. Three of these patients’ UDS were positive for opiates, one for fentanyl, and five for both fentanyl + opiates. Of the three cardiac arrest patients in this group, one had a UDS positive for opiates, and two had a UDS positive for fentanyl + opiates. The median dose of naloxone administered to this group during the initial resuscitation was 2 mg (IQR 0.58–4.00, range 0.40–5.34).
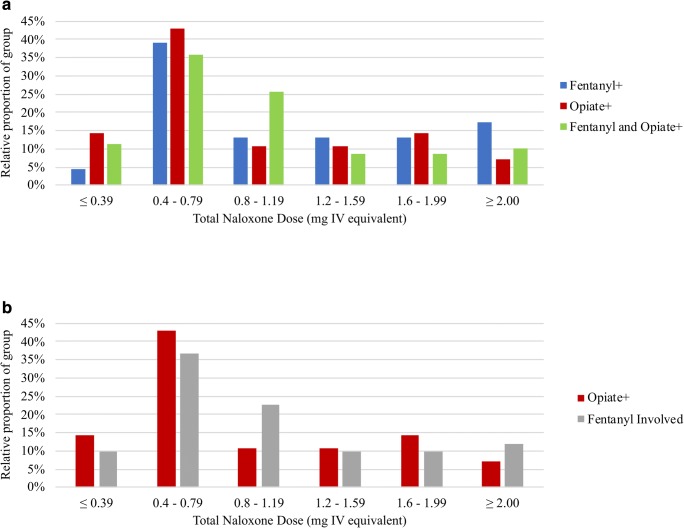
For all cases in which naloxone was deemed effective, the median intravenous equivalent dose of naloxone administered during the initial resuscitation was 0.8 mg (IQR 0.40–1.38, Fig. 2). In the naloxone dose analysis, neither the fentanyl-only (median 0.8 mg, IQR 0.40–1.38; p = 0.68) nor the fentanyl + opiate (median 0.8 mg, IQR 0.40–1.38; p = 0.56) groups differed from the opiate-only group (median 0.58 mg, IQR 0.40–1.25). A separate analysis was also performed comparing all fentanyl-involved cases (fentanyl-only and fentanyl + opiate) to opiate-only cases, and these did not differ (Fig. 2b, p = 0.79). Additionally, the fentanyl-only and fentanyl + opiate groups did not differ from each other (p = 0.36).
Fig. 2.
Distribution of naloxone dosing by UDS results. a Comparing fentanyl+, opiate+ and fentanyl and opiate+ cases. b Fentanyl+ and fentanyl and opiate+ groups are combined to form the fentanyl-involved group
At the conclusion of their ED care, 80 subjects (62%) were discharged home or left against medical advice (AMA), 49 (38%) were admitted to the hospital for reasons related to their overdose (e.g., hypoxic respiratory failure, recurrent respiratory depression requiring additional naloxone, or prolonged CNS depression), and one (1%) was admitted for gastrointestinal bleeding unrelated to the overdose. Among discharged patients for whom naloxone was deemed effective, the median observation time in the ED was 6 h (range 2–20). No patients included in the naloxone dose analysis died as a direct result of their overdose.
Discussion
To our knowledge, this is the first large case series designed to analyze the dose of naloxone required to treat an overdose effectively since the introduction of IMF into the US drug supply. In our analysis, no significant difference was discovered in the naloxone dose administered to patients whose UDS was positive for fentanyl, opiates, or for both agents. Overall, the median and average naloxone doses administered to our subjects were substantially lower than those reported in an earlier series of overdoses related to IMF [10], and are similar to reports from years before IMF was prevalent [8, 9]. Several potential explanations should be considered for the discrepancy between our experience and that observed in Chicago in 2006 [10], including the possibility that drug suppliers and/or dealers have decreased the relative amount of fentanyl in their products, or have moved to less potent analogs in response to increasing fatalities. However, this seems unlikely given that deaths from IMF were rapidly increasing throughout the study period [6]. Regional and temporal variations in the makeup and potency of products sold as heroin are common, due to their illicit unregulated manufacture, and this may partially explain these findings [20, 21]. Another possibility is that opioid users, now aware of the dangers of IMF, are using small “test” doses to evaluate potency, prior to using a full “hit” [20, 21].
Currently, two FDA-approved naloxone products are marketed for use by untrained lay responders: an autoinjector containing one 2 mg dose for intramuscular administration, and a concentrated nasal spray containing one 4 mg dose [22]. Each of these would be sufficient to successfully treat more than 90% of the naloxone responders in our study. However, our results imply that inadequate dosing would occur if many improvised take-home naloxone kits that contain only two 0.4 mg doses for IM injection were solely deployed [23], since nearly 40% of our subjects received a higher total dose. Given the limited data on patient condition at treatment termination obtained in this retrospective study, we cannot conclude whether individual providers dosed naloxone to reverse respiratory or CNS depression, and therefore, cannot definitively conclude that existing take-home naloxone kits are insufficient to improve respiratory status until EMS personnel arrive.
Although further study is needed to confirm or refute our findings, these results strongly imply that organizations utilizing these improvised kits need to consider increasing the amount of naloxone provided. Based on our results, neither changes to EMS naloxone-stocking nor dosing protocols, nor departures from current in-hospital practices, are warranted. At only 2%, a low rate of bystander naloxone administration was revealed, contrasting with rates approaching 50% in another study [12]. This was unexpected: in one survey, 77% of opiate users reported a willingness to administer naloxone in an overdose situation [24]. Observation of this low rate may be reflective of bystander naloxone administration without calling 9-1-1, low naloxone distribution rates in the community, or an urban environment where EMS typically arrives within minutes of a 9-1-1 phone call.
Limitations
This retrospective study has several limitations, most notably the use of qualitative urine immunoassays as a surrogate marker of opiate and fentanyl exposure. Without a more specific quantitative assay, we are unable to conclude if a substance found on the UDS was truly responsible for a subject’s toxidrome, an incidental finding due to a prior or clinically insignificant exposure, or a false positive result [25, 26]. For those subjects who tested positive for both fentanyl and opiates, it is not possible to determine whether the exposures were simultaneous or separated by a period of hours to days. An inability to differentiate among fentanyl analogs, some of which are far more potent than others, limits study specificity. In addition, access to testing for synthetic opioids other than fentanyl was not available, meaning that some related agents could have been missed. The potential for confounding, based upon the presence of co-exposures such as cocaine and benzodiazepines, existed; however, the prevalence of these was similar in each of our subject groups.
In addition, abstractors were not blinded to the study hypothesis, which could be a source for bias. Most importantly, this could have affected the response to “based on your review of the ED chart, you believe that naloxone improved the patient’s clinical status,” as this is a subjective question. We are also unable to determine each provider’s clinical endpoint for naloxone therapy. It is possible that some providers administer naloxone with the intent to resolve respiratory depression, while others intend to normalize the mental status. Providers may not fully understand the pharmacokinetics of naloxone, specifically the longer time to peak concentration with intranasal and intramuscular versus intravenous administration [15], causing some to administer additional naloxone before the previous dose(s) reaches peak activity. Therefore, our results may represent an overestimation of the naloxone dose needed to reverse only respiratory depression. For those subjects that received naloxone via routes other than IV, we employed literature-based assumptions about bioavailability [15, 16] to calculate an IV dose equivalent; however, the bioavailability of naloxone has not been rigorously studied in patients with impaired ventilation and/or perfusion.
Furthermore, the reasons why a UDS was obtained are not known. At the time of the study, no standardized protocol was operational at the study institution to dictate the time frame and rationale for obtaining a UDS. This decision could reflect the provider’s standard practice, but also could have been because the patient’s presentation or clinical course deviated from a perceived norm. If the included subjects with a UDS obtained did differ from those excluded because no UDS was obtained, this could be a potential source for bias. Our subjects also had an AMA rate of only 4%, contrasting with another study of opioid overdoses in which 10.4% of patients left immediately, without being seen and an additional 14.3% later left AMA [12]. This discrepancy is likely because our subjects’ clinical conditions were compromised to an extent that their ED stay duration allowed sufficient time for a UDS to be obtained, and may have biased our results towards more severely poisoned patients, leading to an overestimation of the naloxone dose required. However, we did not collect data on naloxone dosing for patients that did not have a UDS and cannot confirm this theory. Finally, our enrollment goal of 79 subjects per group was not achieved. We were unable to obtain EMS data from the EMR prior to January 1, 2017, and estimated that it would take more than three additional years to reach this enrollment goal. Therefore, we chose not to extend the study period forward. Using our sample size calculations, we estimate that our study was sufficiently powered (80%) to detect a difference of 2.8 mg for the opiate-only vs fentanyl + opiate comparison and 3.6 mg for the opiate-only vs fentanyl-only comparison, rather than the target difference of 2 mg. Nevertheless, our results reflect a significant representation of adequate naloxone dosing in the era of IMF.
Conclusions
This study provides new information that refutes the notion that high potency synthetic opioids, specifically illicitly manufactured fentanyl (IMF), require increased doses of naloxone to successfully treat an overdose. No significant difference was discovered among the naloxone doses required to treat opioid overdose patients with evidence of exposure to fentanyl, opiates, or both. Subjects in our study received adequate naloxone doses which were similar to those administered to patients in the pre-IMF era. Our results provide support for maintaining the existing marketed naloxone autoinjector and nasal spray, as well as current EMS and in-hospital protocols, which do not require substantial changes due to IMF intoxication.
Electronic Supplementary Material
(PDF 62 kb)
(PDF 15 kb)
Funding Information
This work was unfunded.
Compliance with Ethical Standards
Conflicts of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmad F, Rossen L, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics; 2018.
- 2.Kochanek K, Muprhy S, Xu J, Arias E. Mortality in the United States, 2016 National Center for Health Statistics; 2017.
- 3.Peterson AB, Gladden RM, Delcher C, Spies E, Garcia-Williams A, Wang Y, Halpin J, Zibbell J, McCarty CL, DeFiore-Hyrmer J, DiOrio M, Goldberger BA. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013-2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):844–849. doi: 10.15585/mmwr.mm6533a3. [DOI] [PubMed] [Google Scholar]
- 4.CDC Health Alert Network. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. 2015. https://emergency.cdc.gov/han/han00384.asp. Accessed 10 Apr 2019.
- 5.Rudd R, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 6.Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2017: National Center for Health Statistics; 2018.
- 7.Stogner JM. The potential threat of acetyl fentanyl: legal issues, contaminated heroin, and acetyl fentanyl disguised as other opioids. Ann Emerg Med. 2014;64(6):637–639. doi: 10.1016/j.annemergmed.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Wanger K, Brough L, Macmillan I, Goulding J, MacPhail I, Christenson JM. Intravenous vs subcutaneous naloxone for out-of-hospital management of presumed opioid overdose. Acad Emerg Med. 1998;5(4):293–299. doi: 10.1111/j.1553-2712.1998.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 9.Robertson TM, Hendey GW, Stroh G, Shalit M. Intranasal naloxone is a viable alternative to intravenous naloxone for prehospital narcotic overdose. Prehosp Emerg Care. 2009;13(4):512–515. doi: 10.1080/10903120903144866. [DOI] [PubMed] [Google Scholar]
- 10.Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol. 2008;46(6):501–506. doi: 10.1080/15563650701877374. [DOI] [PubMed] [Google Scholar]
- 11.Somerville NJ, O'Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, et al. Characteristics of fentanyl overdose—Massachusetts, 2014-2016. MMWR Morb Mortal Wkly Rep. 2017;66(14):382–386. doi: 10.15585/mmwr.mm6614a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheuermeyer FX, DeWitt C, Christenson J, Grunau B, Kestler A, Grafstein E, et al. Safety of a brief emergency department observation protocol for patients with presumed fentanyl overdose. Ann Emerg Med. 2018;72:1):1–8. e1. doi: 10.1016/j.annemergmed.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust. 2008;188(11):657–659. doi: 10.5694/j.1326-5377.2008.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 14.Evans J, Hogg M, Lunn J, Rosen M. Degree and duration of reversal by naloxone of effects of morphine in conscious subjects. Br Med J. 1974;2(5919):589–591. doi: 10.1136/bmj.2.5919.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald R, Lorch U, Woodward J, Bosse B, Dooner H, Mundin G, Smith K, Strang J. Pharmacokinetics of concentrated naloxone nasal spray for opioid overdose reversal: phase I healthy volunteer study. Addiction. 2018;113(3):484–493. doi: 10.1111/add.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Prescribing information for naloxone nasal spray. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208411lbl.pdf. Accessed 10 Apr 2019.
- 17.Immunalysis Corporation. Fentanyl urine HEIA drug screening kit. https://immunalysis.com/wp-content/uploads/2016/10/Fentanyl_HEIA_MKT50368_2.pdf. Accessed 10 Apr 2019.
- 18.Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146–155. doi: 10.1056/NEJMra1202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osterwalder JJ. Naloxone—for intoxications with intravenous heroin and heroin mixtures-harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34(4):409–416. doi: 10.3109/15563659609013811. [DOI] [PubMed] [Google Scholar]
- 20.Carroll JJ, Marshall BD, Rich JD, Green TC. Exposure to fentanyl-contaminated heroin and overdose risk among illicit opioid users in Rhode Island: a mixed methods study. Int J Drug Policy. 2017;46:136–145. doi: 10.1016/j.drugpo.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccarone D, Ondocsin J, Mars SG. Heroin uncertainties: exploring users’ perceptions of fentanyl-adulterated and-substituted ‘heroin’. Int J Drug Policy. 2017;46:146–155. doi: 10.1016/j.drugpo.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute on Drug Abuse. Opioid overdose reversal with naloxone (Narcan, Evzio). https://www.drugabuse.gov/related-topics/opioid-overdose-reversal-naloxone-narcan-evzio. Accessed 10 Apr 2019.
- 23.Harm Reduction Coalition. Guide to developing and managing overdose prevention and take-home naloxone projects. 2012.
- 24.Strang J, Manning V, Mayet S, Best D, Titherington E, Santana L, Offor E, Semmler C. Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction. 2008;103(10):1648–1657. doi: 10.1111/j.1360-0443.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- 25.Silverstein JH, Rieders MF, McMullin M, Schulman S, Zahl K. An analysis of the duration of fentanyl and its metabolites in urine and saliva. Anesth Analg. 1993;76(3):618-21. doi: 10.1213/00000539-199303000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Vandevenne M, Vandenbussche H, Verstraete A. Detection time of drugs of abuse in urine. Acta Clin Belg. 2000;55(6):323–333. doi: 10.1080/17843286.2000.11754319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 62 kb)
(PDF 15 kb)