Abstract
Ionized calcium (Ca2+) is the most versatile cellular messenger. All cells use Ca2+ signals to regulate their activities in response to extrinsic and intrinsic stimuli. Alterations in cellular Ca2+ signaling and/or Ca2+ homeostasis can subvert physiological processes into driving pathological outcomes. Imaging of living cells over the past decades has demonstrated that Ca2+ signals encode information in their frequency, kinetics, amplitude, and spatial extent. These parameters alter depending on the type and intensity of stimulation, and cellular context. Moreover, it is evident that different cell types produce widely varying Ca2+ signals, with properties that suit their physiological functions. This primer discusses basic principles and mechanisms underlying cellular Ca2+ signaling and Ca2+ homeostasis. Consequently, we have cited some historical articles in addition to more recent findings. A brief summary of the core features of cellular Ca2+ signaling is provided, with particular focus on Ca2+ stores and Ca2+ transport across cellular membranes, as well as mechanisms by which Ca2+ signals activate downstream effector systems.
GENERAL PRINCIPLES OF CELLULAR Ca2+ SIGNALING
A key principle of Ca2+ signaling is that a change of the intracellular Ca2+ concentration provokes a cellular response (Berridge et al. 2000). In unstimulated cells, the cytosolic Ca2+ concentration is maintained at ∼100 nm (often referred to in the Ca2+ signaling literature as the “resting” or “basal” Ca2+ concentration). Extrinsic stimulation of cells can take many forms—hormonal, neurotransmitter, growth factor, antibody, mechanical, electrical, gasotransmitter, temperature, pH change, osmotic change, cytotoxic reagents, microbial invasion, and gap junction-mediated passage of cellular signals—all of which have been shown to elevate cytosolic Ca2+ concentration. Alternatively, Ca2+ signaling can occur because of intrinsic cellular cues, such as the spontaneous Ca2+ signals within cardiac myocytes (Hüser et al. 2000) and developing neurons (Ciccolini et al. 2003).
Typically, stimulation of cells leads to an acute increase in cytosolic Ca2+ concentration from the resting level of 100 nm, and at the end of stimulation the Ca2+ concentration returns back to the resting state. The level of cytosolic Ca2+ attained depends on the nature of the stimulus, as well as factors such as the concentration, intensity and duration of the stimulus, and presence of Ca2+-buffering proteins (Schwaller 2010) and respiring mitochondria (Wacquier et al. 2019). If the cytosolic Ca2+ concentration is monitored by taking an average measure across a whole cell, as is often done using Ca2+-sensitive fluorescent indicators such as Fura-2 (Bootman et al. 2013), Ca2+ signals typically reach peak levels of 0.5–1 µm (Fig. 1).
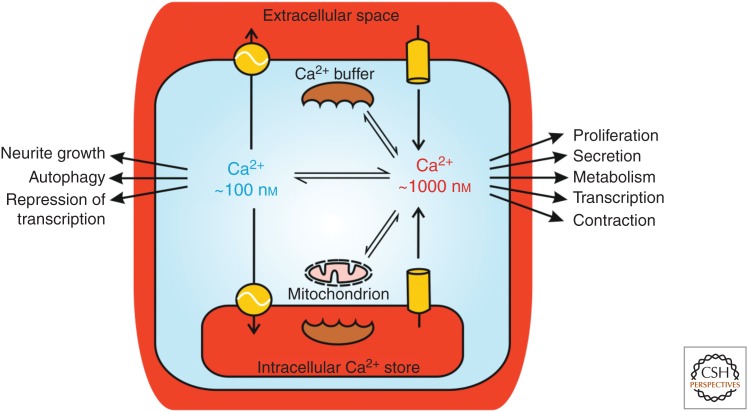
Figure 1.
Cytosolic Ca2+ signals arise via Ca2+ release from intracellular organelles and/or Ca2+ influx across the plasma membrane. During physiological Ca2+ signaling, the averaged cytosolic Ca2+ concentration typically increases from ∼100 nm to ∼1 µm, depending on the stimulus and factors such as buffering of Ca2+ by mitochondria and Ca2+-binding proteins. Cellular processes are specifically switched on or off when the cytosolic Ca2+ concentration is altered. The main intracellular Ca2+ store is the endoplasmic reticulum (ER), but also the nuclear envelope, Golgi, and acidic compartments such as lysosomes function as Ca2+ stores (see text).
Averaged cytosolic Ca2+ signals >1 µm have been recorded, but such large elevations of Ca2+ concentration require a substantial movement of Ca2+ into the cytosol to counteract the energy-dependent homeostatic Ca2+ clearance mechanisms employed by cells. Such excessive Ca2+ signals are often caused by cellular damage, and are generally considered to be in a nonphysiological range. Large elevations of cytosolic Ca2+ concentration can lead to a variety of deleterious cellular effects, particularly if they are sustained for minutes, because of the activation of Ca2+-dependent proteases, production of reactive oxygen species, acute organelle remodeling, and mitochondrial permeability transition (Orrenius et al. 2003). Examples of the disastrous cellular effects of large Ca2+ elevations include excitotoxic stimulation of neurons (Vergun et al. 1999) and death of vascular smooth muscle cells exposed to naturally occurring calcified particles (Proudfoot 2019). However, it is not only large elevations of Ca2+ concentration that are linked to poor cellular outcomes. Indeed, Ca2+ is implicated in numerous pathologies (Parys and Bultynck 2018) as well as in processes underlying natural aging (Verkhratsky 2019). In many cases, subtle alterations of Ca2+ signaling mediate functional or phenotypic changes (Berridge 2012, 2017). The discussion above alludes to the precarious position of cells with regard to Ca2+ signaling. On one hand, Ca2+ is a dynamic and versatile signal within cells, whereby the chemistry of Ca2+ makes it more suitable than other ions for this purpose (Clapham 2007). On the other hand, too much, too little, or misappropriate Ca2+ signaling will ultimately affect cell behavior and fate (Mekahli et al. 2011; Giorgi et al. 2018a).
Understanding the principles underlying Ca2+ dynamics in health and their perturbation in disease and aging also presents unique opportunities to develop strategies to restore normal cell function, which is a central theme in regenerative medicine. Therefore, researchers have turned their focus on studying Ca2+ signaling in regeneration, for example, using organisms and model systems with a powerful inherent regenerative capacity, such as planarian flatworms (Marchant 2019).
Ca2+ is often regarded as a signal when its cytosolic concentration is elevated. Indeed, Ca2+ has many dramatic effects within cells when its concentration is increased. However, it is important to remember that the absence of Ca2+ elevation (i.e., the resting Ca2+ concentration), may also have a signaling function because some processes within cells are inhibited by an elevation of cytosolic Ca2+ concentration. For example, the transcriptional repressor downstream regulatory element antagonist modulator (DREAM) binds to target genes and prevents their transcription at resting Ca2+ concentration (Hagenston et al. 2019). In addition, neurite outgrowth has been negatively correlated with Ca2+ signaling in some neuronal cell types (Mattson et al. 1988). A lack of Ca2+ signaling can trigger processes such as quiescence (a reversible growth/proliferation arrest) (Humeau et al. 2018) or autophagy, a lysosomal turnover pathway responsible for the clearance of damaged or unwanted proteins and organelles (Bootman et al. 2018). Consequently, it should be remembered that Ca2+ signals have pleiotropic actions within cells: some processes will be switched on, while others are switched off, when the cytosolic Ca2+ concentration is elevated. The cellular effect of Ca2+ signaling also depends on the context of a cell in terms of its position in the cell cycle, energetic status, and other incoming external signaling cues. All of the above paints a complex picture of cellular responses to stimuli that evoke cytosolic Ca2+ signals. Indeed, it is this complexity, coupled with a desire to understand how specific cell types generate their individualistic Ca2+ signals, which sustains the interest of the many laboratories around the world who study signal transduction by Ca2+.
Cells can access two principal sources of Ca2+ to generate signals: Ca2+ release from intracellular stores and Ca2+ influx from the extracellular space (Fig. 1). Both of these Ca2+ sources are utilized by cells, but the balance between the two can differ. For example, cytosolic Ca2+ signals mediate contraction of both skeletal and cardiac muscle by activating the engagement of actin and myosin fibers (Santulli et al. 2017). However, skeletal muscle cells can continue to contract for some time in the absence of extracellular Ca2+, whereas cardiac muscle cells will immediately cease contracting if extracellular Ca2+ is withdrawn (Eisner et al. 2017).
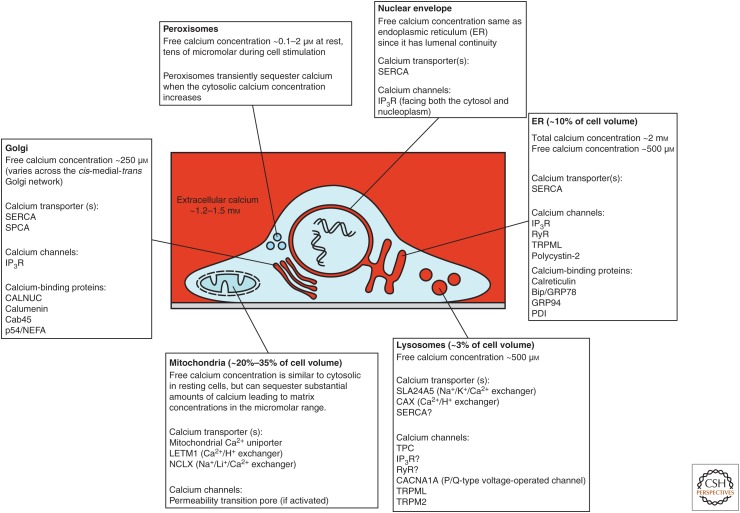
Ca2+ release from intracellular stores occurs via channels that span the membranes of organelles. Principal Ca2+ releasing channels are inositol 1,4,5-trisphosphate receptors (IP3Rs) (Prole and Taylor 2019), ryanodine receptors (RyRs) (Lanner et al. 2010), two-pore channels (TPCs) (Galione 2019; Lloyd-Evans and Waller-Evans 2019; Webb et al. 2019), and the mucolipin subfamily of transient receptor potential channels (TRPML) (Vangeel and Voets 2019). The endoplasmic reticulum (ER) in nonexcitable cells and neurons, and the sarcoplasmic reticulum (SR) in muscle cells, have long been known as major intracellular Ca2+ stores that play roles in cellular Ca2+ signaling. However, other organelles—the Golgi, nuclear envelope, lysosomes, and other acidic vesicles—also participate in cellular Ca2+ signals when cells are appropriately stimulated (Fig. 2). These Ca2+ stores contain substantial amounts of Ca2+ through the expression of various Ca2+ transporters (Vandecaetsbeek et al. 2011; Chen et al. 2019; Lloyd-Evans and Waller-Evans 2019) and a range of Ca2+-binding proteins (Wang et al. 2019). Other organelles, notably mitochondria and peroxisomes, also play roles in intracellular Ca2+ signaling, but are not substantial constitutive Ca2+ stores. Both mitochondria and peroxisomes sequester Ca2+ during cytosolic Ca2+ increases. The accumulation of Ca2+ by these organelles serves to limit the amplitude of cytosolic Ca2+ signals, just as cytosolic Ca2+-binding proteins do (Fig. 1; Schwaller 2019; Wacquier et al. 2019). However, the role of these compartments is not just to dampen cytosolic Ca2+ rises. For instance, the uptake of Ca2+ by mitochondria intimately links cellular signaling to metabolism and bioenergetics (Cárdenas et al. 2010) and cell fate (Walter and Hajnóczky 2005). In the mitochondrial matrix, Ca2+ is a cofactor for some enzymes within the tricarboxylic acid cycle, and promotes ATP production, but can also trigger release of proapoptotic factors (Giorgi et al. 2018b). Moreover, not only does Ca2+ impact mitochondrial metabolism, but mitochondrial metabolism also boosts cellular Ca2+ signaling through regulation of cytosolic Ca2+ signals and redox signaling (Booth et al. 2016; Joseph et al. 2019).
Figure 2.
Ca2+-sequestering organelles. Cellular Ca2+ signaling involves combinations of organelles depending on the tissue type and stimulus. Although there is considerable overlap in some of the organelles’ characteristics, they also have discrete properties that imbue each of the organelles with the ability to generate distinctive Ca2+ signals. Mitochondria and peroxisomes are not constitutive Ca2+ stores, but have the capacity to sequester Ca2+ during cytosolic Ca2+ increases. For more details, see Wang et al. (2019), Chen et al. (2019), Lloyd-Evans and Waller-Evans (2019), Wacquier et al. (2019), and Vangeel and Voets (2019). A significant Ca2+ store not shown in the figures is the sarcoplasmic reticulum (SR), which plays a critical Ca2+ signaling role in muscle cells. For details about SR function and Ca2+ homeostasis see Wang et al. (2019) and Gilbert et al. (2019). SERCA, sarcoendoplasmic reticulum Ca2+-ATPase; SPCA, Golgi/secretory pathway Ca2+ ATPase; TPC, two-pore channel; IP3R, inositol 1,4,5-trisphosphate receptor; RyR, ryanodine receptor.
Although organelles may contain sufficient Ca2+ to initiate and sustain signaling for minutes, they are finite Ca2+ stores and will run down if not replenished. Ultimately, Ca2+ from the extracellular space is required to renew organellar Ca2+ and prolong cytosolic Ca2+ signals. However, it should be noted that extracellular Ca2+ can also function to trigger the release of Ca2+ from intracellular stores, as happens in cardiac myocytes (Gilbert et al. 2019), and can directly activate downstream effectors, as happens in neurons (Barak and Parekh 2019; Burgoyne et al. 2019; Hagenston et al. 2019). Indeed, Ca2+ signals arising from organellar Ca2+ release or from Ca2+ influx can have discrete cellular outcomes (Barak and Parekh 2019).
A Ca2+ SIGNALING TOOLKIT
A simple paradigm that rationalizes the enormous number of components that cells express to generate Ca2+ signals, and respond to them, is that of a toolkit (Berridge et al. 2000). The “Ca2+ signaling toolkit” is essentially all the Ca2+ transporters (channels, pumps, and exchangers), Ca2+-binding proteins, and Ca2+-dependent effectors that exist in nature. From this vast toolkit, cells express the components that fit their function. For example, cardiac myocytes express a specific set of proteins—voltage-operated Ca2+ channels, RyRs, sodium-calcium exchangers, SERCA2a, and troponin C—that enable them to generate Ca2+ signals that rise and recover within tens of milliseconds, and so trigger pulsatile cellular contraction to pump blood (Bers 2008; Fearnley et al. 2011). In the average human lifetime, cardiac myocytes beat over 2 billion times, and are activated every second by an action potential arriving from the sinoatrial node, so they need to have the ability to rapidly respond and recover with great fidelity. Missing a beat is not an option! In contrast, oocytes are largely dormant cells, waiting for sperm to trigger Ca2+ signaling, cause resumption of the cell cycle, and initiate development (Wakai et al. 2019). Mammalian oocytes express different components from the Ca2+ signaling toolkit—IP3Rs and SERCA2b—and following fertilization they display a series of long-lasting Ca2+ oscillations with much slower kinetics than the rapid Ca2+ transients observed in cardiomyocytes. Elucidating the specific Ca2+ signaling toolkit components that are discretely expressed by each tissue type, and how they collectively shape Ca2+ signal generation and cellular outcomes, is the key to understanding signal transduction via Ca2+.
It is important to note that the Ca2+ signaling components expressed by cells can be remodeled because of environmental factors or genetic mutation. A well-known example occurs during hypertrophic growth of cardiac myocytes. Hypertrophic stimuli cause an increase in the amplitude of cytosolic Ca2+ signals and larger myocyte contraction (Harzheim et al. 2009), which is a necessary compensation for greater hemodynamic demand, for example, during pregnancy or athletic training. However, in some deleterious situations (e.g., hypertension) hypertrophy can progress to a maladapted state in which Ca2+ signals are reduced, and the heart becomes weaker (Roderick et al. 2007). Moreover, the altered expression or mutation of the Ca2+ signaling toolkit components can lead to oncogenesis and malignant cellular behavior, as is observed in several cancer types (Distelhorst and Bootman 2019; Roberts-Thomson et al. 2019).
IP3Rs AS AN EXAMPLE OF A CELLULAR SIGNALING HUB
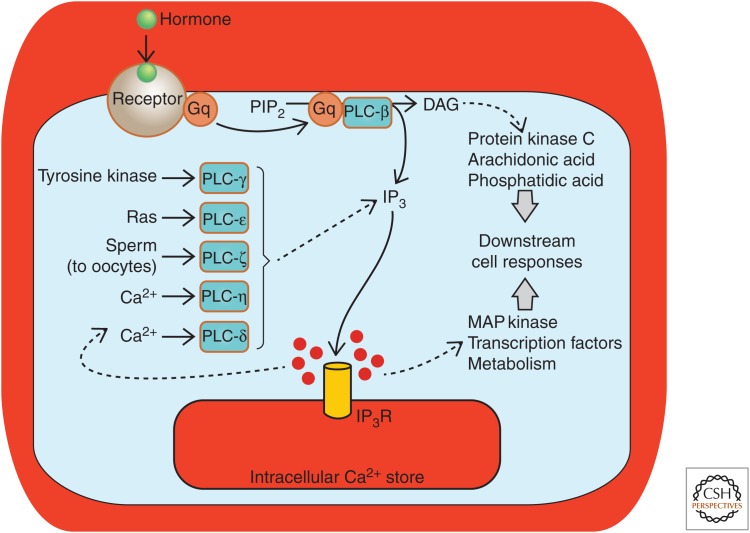
There are too many components in the Ca2+ signaling toolkit to describe in this limited primer, but some elements are so widely expressed and commonly involved in the generation of Ca2+ signals that they deserve a mention. In particular, IP3Rs, which are a principal means of releasing Ca2+ from intracellular organelles, participate in Ca2+ signaling within many excitable and nonexcitable cell types (Foskett et al. 2007; Mikoshiba 2015). IP3Rs are activated following the production of the intracellular messenger IP3 and release Ca2+ from the ER, Golgi, and nuclear envelope (Prole and Taylor 2019), as well as activating a small number of IP3Rs that are localized at the plasma membrane (Dellis et al. 2006). IP3 production within cells is triggered by a variety of extrinsic stimuli (e.g., hormones, growth factors) that bind to cell-surface receptors (e.g., G-protein-coupled receptors or receptor tyrosine kinases). Application of these stimuli typically induces Ca2+ signals inside cells within a few seconds (Berridge and Galione 1988). As with many components of the Ca2+ signaling toolkit, IP3-mediated Ca2+ release is far from simply being a discrete linear pathway. Indeed, the phosphoinositide signaling pathway (a name commonly used to denote the production of IP3 and activation of IP3Rs within cells) is at the center of a web of interactions with other signaling pathways. Phospholipase C (PLC), the enzyme that produces IP3 inside cells (via the hydrolysis of the minor membrane phospholipid phosphatidylinositol 4,5-bisphosphate [PIP2]), is expressed as several isoforms, and only some (e.g., PLC-β, PLC-γ) are activated by extrinsic stimuli. Other isoforms are activated by Ca2+ (most PLCs, but especially PLC-δ and PLC-η), the small G-protein Ras (PLC-ε), or introduced into oocytes by sperm at fertilization (PLC-ζ) (Fig. 3). Besides producing IP3, the hydrolysis of PIP2 by PLC yields diacylglycerol (DAG) that stays within the plasma membrane. DAG can activate protein kinase C (often in cooperation with Ca2+ signals) (Lipp and Reither 2011) or can be further metabolized to produce additional cellular messengers such as arachidonic acid.
Figure 3.
IP3-mediated Ca2+ release is a common outcome from a number of cellular signaling processes and evokes a range of downstream outcomes. A generic pathway leading from hormone-receptor activation, activation of a heterotrimeric G-protein (Gq) and phospholipase C β (PLC-β) is depicted centrally. However, the other PLC isoforms provide alternative mechanisms for activating Ca2+ signaling via IP3Rs. (From Bootman et al. 2009; adapted, with permission, from Company of Biologists © 2009.)
Profound insights into the activation of IP3Rs have been gained via high-resolution structural studies using cryo-electron microscopy (cryo-EM), a major tour de force given the large size of IP3R proteins (IP3Rs are tetramers of ∼270 kDa subunits, giving functional channels of ∼1100 kDa) (Fan et al. 2015, 2018; Hamada et al. 2017). Cryo-EM allows the determination of a protein's structure without requiring its crystallization, an advantage compared to X-ray crystallography or nuclear magnetic resonance spectroscopy, since crystallization is particularly challenging for large, membranous proteins. The structural organization of IP3Rs is further discussed in Ivanova et al. (2019).
IP3Rs are known to bind a range of accessory proteins, many of which convey messages to and from other signaling pathways (Prole and Taylor 2019). Some of the accessory proteins associated with IP3Rs affect channel opening and Ca2+ release, while others determine IP3R degradation, cellular location, or serve to tether additional proteins that do not directly impact on IP3R function. An emerging class of IP3R-associated proteins is the Bcl-2 family, which are critical controllers of apoptotic cell death (Vervliet et al. 2016; Ivanova et al. 2019). As the expression of Bcl-2-family members is altered in cancer, such IP3R/Bcl-2-protein complexes form a mechanistic link between Ca2+ signaling and cell death, a pathway that is often dysregulated in cancers but might be therapeutically targeted (Distelhorst and Bootman 2019; Ivanova et al. 2019).
Stimulation of cells with agonists that activate IP3 production typically leads to the generation of cytosolic Ca2+ oscillations (sometimes referred to in the Ca2+ literature as “Ca2+ spikes” or “Ca2+ transients”) (Berridge and Galione 1988; Dupont et al. 2011). The patterns of Ca2+ oscillation can vary between cell types, and even within a cell type for different stimuli, but in most cases Ca2+ oscillations are brief increases in cytosolic Ca2+ that last for a few tens of seconds. Generally, Ca2+ oscillations have a rapid rising phase, reaching a peak cytosolic Ca2+ concentration ∼500 nm, before more slowly decaying back to the resting basal Ca2+ concentration. It was demonstrated many years ago that intracellular signaling systems display oscillatory activity (Berridge and Rapp 1979), and that cellular Ca2+ signaling in particular was similarly encoded as oscillations (Woods et al. 1986). Studies have shown that both the frequency of Ca2+ oscillations displayed by cells (Rooney et al. 1989) and the downstream cellular responses (Dolmetsch et al. 1998) are proportional to the concentration of stimulus applied. Hence, Ca2+ signaling is often considered as information transmission in a frequency-encoded manner where the successive pulses of cytosolic Ca2+ that arise with each Ca2+ oscillation trigger a cumulative cellular response (Bhattacharyya et al. 2019; Roy and Cyert 2019).
Ca2+ OSCILLATIONS AS A HIGH-FIDELITY SIGNALING MECHANISM
There are a number of advantages to using oscillatory Ca2+ signals for information transfer within cells. As mentioned earlier, sustained increases in cytosolic Ca2+ are energetically costly as they need to overcome cellular Ca2+ transport processes, and if the Ca2+ elevation is too great it may cause deleterious effects. So, Ca2+ oscillations are energetically more favorable. Additionally, there is considered to be greater fidelity in frequency-encoded signaling systems than those based on graded amplitude changes (Berridge 1997). Moreover, cells have mechanisms for decoding, and responding to, pulsatile increases in Ca2+ in preference to sustained Ca2+ signals (Hajnóczky et al. 1995). An important mechanism by which Ca2+ signals are decoded involves phosphorylation-dependent control of proteins via both kinases and phosphatases that are activated by Ca2+, such as Ca2+/calmodulin-dependent kinase II and calcineurin (Bhattacharyya et al. 2019; Roy and Cyert 2019). Additionally, the Ca2+-binding protein calmodulin mediates a number of cytosolic and nuclear effects of Ca2+ signals (Hagenston et al. 2019). The mechanism through which cells generate Ca2+ oscillations is still not completely understood, particularly for situations where there is an interval of many tens of seconds, or even minutes, between successive cytosolic Ca2+ increases (Skupin et al. 2008). A common mechanism invoked to explain cytosolic Ca2+ oscillations is based on the feedback of Ca2+ itself on IP3Rs. Indeed, IP3R activation has a “bell-shaped” dependence on cytosolic Ca2+: relatively low Ca2+ concentrations (250–500 nm) promote Ca2+ release, whereas greater Ca2+ concentrations (∼1 µm) inhibit Ca2+ release (Bezprozvanny et al. 1991; Prole and Taylor 2019).
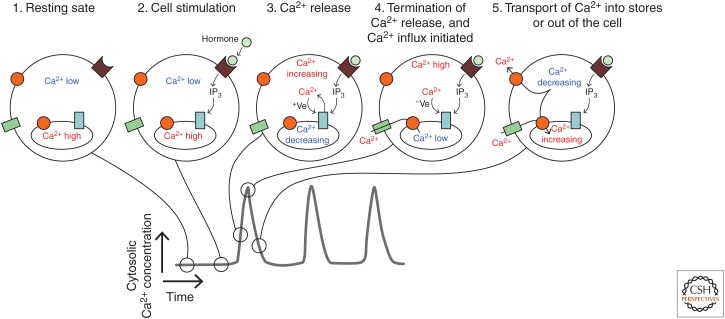
Following stimulation of cells with an agonist that activates phospholipase C, IP3 produced at the plasma membrane will diffuse within the cytosol and bind to its receptor, IP3R, on intracellular organelles. At first, the Ca2+ signal is limited, but as the cytosolic Ca2+ concentration increases it can feed back in a positive manner to stimulate further Ca2+ release, a process known as Ca2+-induced Ca2+ release (Roderick et al. 2003). However, as the cytosolic Ca2+ concentration continues to increase it will reach the range in which the feedback becomes inhibitory. At that point the IP3Rs will close and the cell can then recover back to the resting situation as Ca2+ is transported out the cell and back into the stores (Fig. 4). In this scheme, IP3Rs require IP3 binding to become activated, but are actually opened and closed by Ca2+ binding to discrete stimulatory and inhibitory sites, respectively. As discussed above, Ca2+ influx plays a part in restoring the Ca2+ content of the stores so that the cell is set for another oscillatory cycle. In fact, altering the concentration of extracellular Ca2+ has a significant effect on the frequency of cytosolic Ca2+ oscillations, suggesting that the loading of intracellular Ca2+ stores is a key aspect of a cell recovering from one Ca2+ release event to the next (Bootman et al. 1996).
Figure 4.
Generation of Ca2+ oscillations via positive and negative feedback on IP3Rs. The sequence of cartoons 1–5 depict the status of cytosolic Ca2+, IP3R activity, and Ca2+ store content during the various phases of a Ca2+ oscillation. Phase 1 represents the cell at rest, before application of an extracellular stimulus. Phase 2 shows the cell subsequent to agonist–receptor interaction, when there is IP3 production but no Ca2+ release via IP3Rs. Phase 3 indicates the initial opening of IP3Rs and an increasing cytosolic Ca2+ concentration, which exerts a positive (+Ve) feedback on the IP3R, thereby promoting further Ca2+ release. At phase 4, the cytosolic Ca2+ concentration has increased to the point where it inhibits IP3R activity via negative (−Ve) feedback and hence Ca2+ release terminates. At this point, the Ca2+ store is depleted and Ca2+ influx is activated. Phase 5 indicates the recovery of the cell as Ca2+ is transported from the cytosol toward the intracellular stores and the extracellular environment and the negative feedback from cytosolic Ca2+ is relaxed.
Hormone-evoked Ca2+ oscillations originate in the cytosol through initial activation of clusters of IP3Rs. Intriguingly, not all IP3Rs are equal in this respect and only a fraction of the IP3Rs that are expressed, specifically those within clusters, seem to be able to respond (Prole and Taylor 2019). The activation of clusters of IP3Rs has been visualized using rapid imaging techniques, and the localized cytosolic Ca2+ elevation caused by the activation of an IP3R cluster is termed a “Ca2+ puff” (Yao et al. 1995; Bootman et al. 1997). With low levels of cell stimulation, and consequently low intracellular levels of IP3, a few Ca2+ puffs may be all that occurs. However, with higher levels of cell stimulation the Ca2+ that diffuses from one cluster of IP3Rs during a Ca2+ puff is likely to encounter a neighboring cluster in which the IP3Rs are liganded with IP3. In that case, the second IP3R cluster can be triggered and a further Ca2+ puff will occur. Through successive rounds of diffusion and Ca2+-induced Ca2+ release, neighboring IP3R clusters can be recruited and Ca2+ signals can propagate throughout a cell. Saltatoric Ca2+ waves, which reflect this fire-diffuse-fire scheme for Ca2+ signal propagation (Thul et al. 2008), have been visualized in the cytosol of a number of cell types including nonexcitable cells (Bootman et al. 1997; Callamaras et al. 1998), cardiac myocytes (Kockskämper et al. 2001), and also the nanotunnels that connect the cytoplasm of adjacent cells (Smith et al. 2011). As Ca2+ waves propagate through cells, they encounter effector proteins that can become activated (Schwaller 2019), as well as other organelles that may sequester, or respond to, the oncoming Ca2+ signal. Moreover, Ca2+ waves can permeate the nucleoplasm by diffusing through nuclear pore complexes (Bootman et al. 2009), and thereby modulate gene transcription (Hagenston et al. 2019). As a result of the relative lack of Ca2+ sequestration within the nucleus, Ca2+ increases within this organelle can persist for longer than in the cytosol (Lipp et al. 1997), and indeed have discrete functions (Higazi et al. 2009; Hagenston and Bading 2011).
Although Ca2+ oscillations constitute a form a cell signaling that is used by many cell types for the reasons described above, there are numerous examples of cellular responses being controlled by the amplitude/kinetics (Dolmetsch et al. 1997), or spatial extent, of cytosolic Ca2+ signals (Mackenzie et al. 2004). Indeed, sometimes there is an interplay of different parameters in controlling cellular responses. Within the heart for example, β-adrenergic receptor stimulation causes higher amplitude Ca2+ signals that contribute to stronger contraction (positive inotropy), faster kinetics for Ca2+ signal recovery (positive lusitropy to enable the heart to relax quicker), and a faster rate of beating (positive chronotropy; although in the intact heart faster beating is caused by more frequent action potentials arising from the sinoatrial node pacemaker).
LOCAL Ca2+ SIGNALS AND MEMBRANE CONTACT SITES ENABLE DISCRETE COMMUNICATION
Earlier in this article, it was mentioned that averaged Ca2+ signals within cells are typically seen to reach peak levels of 0.5–1 µm. However, it is important to point out that at the mouth of an open Ca2+ channel (or Ca2+ channel cluster) the concentration of Ca2+ can be reach in excess of 100 µm (Thul and Falcke 2004; Demuro and Parker 2006). The concentration of Ca2+ falls dramatically with distance from an active channel, because of dissipation via diffusion and buffering (Thul and Falcke 2004). So, when a cell is stimulated, Ca2+ increases of many tens of micromolar will develop around the locations of the activated Ca2+ channels, and diffusion of Ca2+ away from these channels will yield a more widespread distribution of Ca2+ with a substantially lower Ca2+ concentration. The regions of high Ca2+ concentration around activated Ca2+ channels have been increasingly recognized as having distinct signaling functions (Barak and Parekh 2019; Wang et al. 2019) and are often referred to as “Ca2+ signaling microdomains” (or nanodomains, depending on the distance involved; sometimes the terms “Ca2+ hotspots” or “local Ca2+ signals” are used in the literature). Some Ca2+-sensitive effectors are localized close to Ca2+ channels, thereby providing a means for rapid and specific cellular outcomes (Bootman et al. 2001; Berridge 2006).
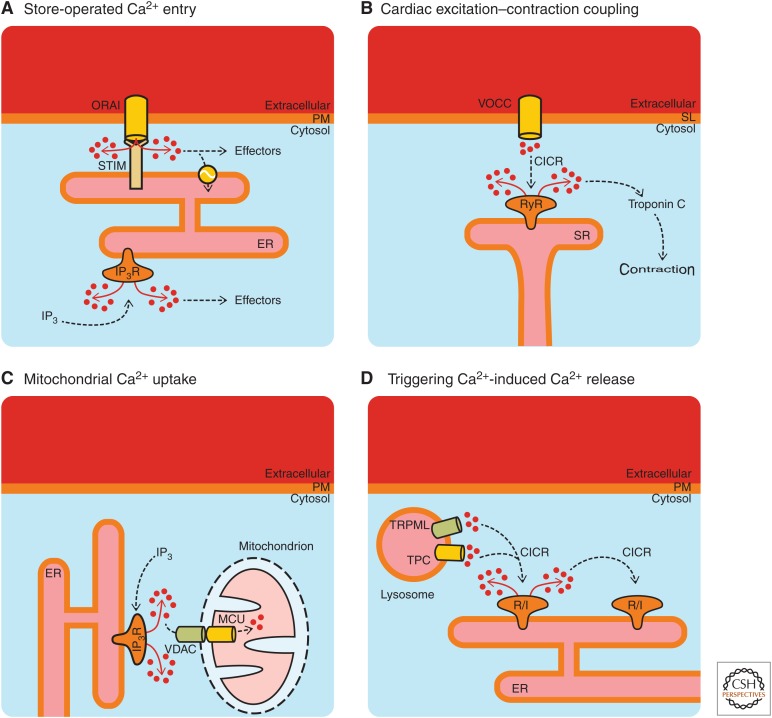
Ca2+ signaling microdomains also occur because of the close apposition of cellular organelles (Ahuja et al. 2019), for example, between the ER and mitochondria, the ER and lysosomes, or organelles with the plasma membrane (La Rovere et al. 2016; Raffaello et al. 2016; Csordás et al. 2018). Ca2+ signaling microdomains are a rapidly growing aspect of the wider topic of membrane contact sites in cell biology (Dolgin 2019; Scorrano et al. 2019). The close proximity of organelles and membranes is brought about through tethering proteins (Scorrano et al. 2019), thereby enabling Ca2+ signals originating at one membrane to activate processes at the apposed membrane. These membrane contact sites limit the diffusion of Ca2+ and provide a means for highly specific interactions between Ca2+ sources and effectors. Examples of four different Ca2+ signaling mechanisms in which membrane contact sites are essential are depicted in Figure 5. Although the four examples shown in Figure 5 are all based on the essential apposition of cellular organelles/membranes, they are different in terms of Ca2+ toolkit components and physiological outcomes.
Figure 5.
Examples of Ca2+ signaling via membrane contact sites. (A)–(D) Well-known situations where the close apposition of membranes/organelles is essential for initiation or communication of Ca2+ signals. VOCC, voltage-operated Ca2+ channel; SL, sarcolemma; VDAC, voltage-dependent anion channel; MCU, mitochondrial Ca2+ uniporter; TPC, two-pore channel; TRPML, mucolipin subfamily of transient receptor potential channels; CICR, Ca2+-induced Ca2+ release; R/I, ryanodine receptor or IP3 receptor.
Store-operated Ca2+ entry (SOCE) (Fig. 5A) was postulated many years ago to explain the observation that depletion of intracellular Ca2+ stores led to Ca2+ influx across the plasma membrane (Putney 1990; Ahuja et al. 2019; Lewis 2019). Moreover, a highly Ca2+-selective current was identified that was triggered by Ca2+ store depletion (Hoth and Penner 1992). This current was termed “Ca2+ release-activated current” (ICRAC) and it is the electrophysiological basis of SOCE. The molecular components of SOCE were established sometime later: stromal interaction molecule (STIM), which acts as a sensor of ER luminal Ca2+ loss (Liou et al. 2005; Zhang et al. 2005), and Orai, which forms the Ca2+ channel in the plasma membrane (Prakriya et al. 2006). STIM is a transmembrane protein that spans the ER and projects its carboxy terminus into the cytosol. A substantial amount of work has been focused on understanding how STIM regulates SOCE (Lewis 2019). Briefly, STIM can bind Ca2+ within the lumen of the ER, and if the Ca2+ concentration drops below a threshold level then Ca2+ dissociates from STIM. Consequently, STIM oligomerizes and triggers the opening Orai channels at the plasma membrane (Soboloff and Romanin 2019). The loss of Ca2+ from STIM causes substantial molecular rearrangements within the STIM protein such that its carboxy-terminal domain projects toward Orai channels so that they physically interact. This interaction occurs in regions where the ER and plasma membrane come within 15 nm of each other (Zhou et al. 2017).
In addition to Orai, SOCE can be mediated by members of the transient receptor potential (TRP) family, and in particular the subfamily of canonical TRP channels (TRPCs) (Ahuja et al. 2019; Ong and Ambudkar 2019; Vangeel and Voets 2019). Indeed, Orai and TRPC channels can cooperate in the activation of Ca2+ entry (Ambudkar et al. 2017). Ca2+ store depletion and STIM1–Orai interaction leads to the insertion of TRPCs into the plasma membrane. The participation of TRPCs alongside Orai prolongs SOCE and downstream signaling (Cheng et al. 2011). The interaction of STIM1, Orai, and TRPCs gives rise to a current that has been termed ISOC, which is distinct from ICRAC (Desai et al. 2015). Insights into the properties and mode of TRP activation have been propelled forward by advances in structural information (Vangeel and Voets 2019).
Excitation–contraction coupling in cardiac myocytes (Fig. 5B) also relies on the close apposition of the cell membrane with a major intracellular Ca2+ store, but in this case it is the sarcolemma (the myocyte cell membrane) and the SR (the Ca2+ store within muscle cells that is used to activate contraction). The Ca2+ channels responsible for myocyte contraction are voltage-operated Ca2+ channels (specifically, CaV1.2 or “L-type” voltage-operated Ca2+ channels) on the sarcolemma, and RyRs on the SR. RyRs are primarily activated by Ca2+, but can also respond to cellular messengers such as cyclic adenosine diphosphate ribose (Galione and Churchill 2000).
Blood pumping by the heart occurs via the coordinated contraction of the atrial and ventricular chambers in a process known as the cardiac cycle (Bers 2002). Each cardiac cycle is initiated by a group of specialized pacemaking cells in the right atrial chamber (the sinoatrial node), which spontaneously discharge electrical signals (action potentials) that propagate through the heart. Ca2+ is the cellular messenger that links propagating action potentials and cardiomyocyte contraction (Gilbert et al. 2019). When an action potential arrives at a cardiac myocyte, it triggers a brief depolarization of the sarcolemma, which consequently activates the voltage-operated Ca2+ channels, leading to the influx of Ca2+ from outside of the cells into a membrane-delimited region called the dyadic cleft (Eisner et al. 2017). The sarcolemma and SR come within 10–15 nm of each other at a dyadic cleft. This proximity is necessary so that the Ca2+ influx through voltage-operated Ca2+ channels can diffuse to the RyRs present on the SR membrane at a concentration sufficient to trigger Ca2+-induced Ca2+ release (Fearnley et al. 2011).
The activation of RyRs leads to a rapid increase of the Ca2+ concentration within the dyadic cleft. The Ca2+ signal within the dyadic cleft subsequently diffuses out into the cytoplasm and encounters troponin C (among other targets), which promotes the association of actin and myosin to trigger cell contraction. A single cardiac myocyte contains thousands of dyadic clefts that simultaneously respond during an action potential, and all contribute to the Ca2+ signal that is required for contraction. It has been suggested that around 25 voltage-operated Ca2+ channels and 100 RyRs are closely apposed within a single dyadic cleft (Bers and Guo 2005). In some cardiac diseases, the sarcolemma and SR membranes become dissociated such that there is no cross talk between voltage-operated Ca2+ channels and RyRs (Louch et al. 2004). These “orphaned RyRs” are not recruited during excitation–contraction coupling, hence myocyte Ca2+ signaling and contraction become weaker (Heinzel et al. 2011).
Mitochondrial Ca2+ uptake (Fig. 5C) relies on the close association of mitochondria to Ca2+ channels. In fact, mitochondria can accumulate Ca2+ from various sources (i.e., Ca2+ release from organelles and Ca2+ influx) (Collins et al. 2001). However, the rate of Ca2+ uptake by mitochondria depends on the proximity of these organelles to Ca2+ channels. It was demonstrated some time ago that mitochondria are relatively insensitive to the average measured cytosolic Ca2+ concentrations that occur during cell stimulation (i.e., 100–500 nm) (Kirichok et al. 2004), and that mitochondrial Ca2+ uptake relies on the microdomains of high Ca2+ concentration that occur close to activated channels (Rizzuto et al. 1993; Csordás et al. 1999). Mitochondria-associated membranes (MAMs) are a specific form of membrane contact site, and are intensely studied as sites of Ca2+ communication between the ER and mitochondria (in addition to being sites of protein and lipid transfer) (Marchi et al. 2018). This interorganellar complex enables the “quasisynaptic” transfer of Ca2+ between the ER and mitochondria (Rizzuto et al. 1998; Csordás et al. 1999).
Within MAMs, IP3Rs on the ER membrane are coupled via chaperones with voltage-dependent anion channels (VDACs) situated in the outer mitochondrial membrane (Szabadkai et al. 2006). VDAC proteins permit the passage of Ca2+ across the outer mitochondrial membrane. Ca2+ transport across the inner mitochondrial membrane is enabled by the mitochondrial Ca2+ uniporter complex (Granatiero et al. 2017), which has key roles in cell death (Penna et al. 2018), organ physiology (Mammucari et al. 2018), and disease (Mammucari et al. 2017). Changes in MAM organization have been linked to neurodegenerative diseases and cancer (Kerkhofs et al. 2017; Rossi et al. 2019). Moreover, the cellular sensitivity of cancer cells toward chemotherapeutics seems to be dependent on Ca2+ fluxes at the MAMs (Kerkhofs et al. 2018). These insights offer novel opportunities to promote cancer cell death, for example through novel peptide tools that act at the MAM interface (Kerkhofs et al. 2019).
Triggering of Ca2+-induced Ca2+ release (Fig. 5D) can occur when Ca2+ channels are in close proximity. As mentioned above, IP3Rs and RyRs are activated by an increase in the cytosolic Ca2+ concentration (Bezprozvanny et al. 1991). Both types of channel have been shown to amplify small Ca2+ signals into much larger responses via Ca2+-induced Ca2+ release. This amplification is critical for relatively small Ca2+ stores such as lysosomes to trigger substantial cellular Ca2+ signals (Galione 2019). For example, the cellular messenger nicotinamide adenine dinucleotide phosphate can cause a limited release of Ca2+ from lysosomes via TPCs (Lloyd-Evans and Waller-Evans 2019), which can then be amplified by IP3Rs and RyRs (Zhu et al. 2010). IP3Rs have been found to be strategically located within ER-lysosomal contact sites (Atakpa et al. 2018), where they serve to deliver Ca2+ from the ER to the lysosomal compartment (Patel 2019).
CONCLUDING REMARKS
Cellular Ca2+ signaling is complex, multifactorial, and dynamic, and is critical to many physiological processes. Ca2+ signals can arise from a number of sources and via a plethora of channels and other transporters. Ca2+ signaling is tightly integrated with other cellular signal transduction pathways, and there are many examples of cross talk and synergy. Cellular signaling can remodel depending on environmental conditions, and alterations to Ca2+ signals or Ca2+ homeostasis occur in disease conditions. The discussion above is a necessarily superficial tour of some of the key aspects of cellular Ca2+ signaling. For each of the topics discussed, there is a wealth of underlying publications and knowledge. The intention of this article was to stimulate appreciation of Ca2+ signaling so that the interested enquirer might continue reading. Fortunately, while the Ca2+ signaling literature is vast, there are some underpinning principles of Ca2+ signaling that apply irrespective of the cell type and its function. In particular, Ca2+ signals are highly organized in terms of kinetics and cellular location. The organization of Ca2+ signals enables cells to use Ca2+ as a means of simultaneously controlling diverse processes. It is clear that perturbations of Ca2+ signaling are a proximal factor in the pathogenesis of debilitating and fatal pathologies, but our increasing understanding of aberrant Ca2+ signals offers unprecedented opportunities for novel therapeutic strategies.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- *.Ahuja M, Young Chung W, Lin W-Y, McNally BA, Muallem S. 2019. Ca2+ signaling in exocrine cells. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar IS, de Souza LB, Ong HL. 2017. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium 63: 33–39. 10.1016/j.ceca.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atakpa P, Thillaiappan NB, Mataragka S, Prole DL, Taylor CW. 2018. IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep 25: 3180–3193.e7. 10.1016/j.celrep.2018.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Barak P, Parekh AB. 2019. Signaling through Ca2+ microdomains from store-operated CRAC channels. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 1997. The AM and FM of calcium signalling. Nature 386: 759–760. 10.1038/386759a0 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2006. Calcium microdomains: Organization and function. Cell Calcium 40: 405–412. 10.1016/j.ceca.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2012. Calcium signalling remodelling and disease. Biochem Soc Trans 40: 297–309. 10.1042/BST20110766 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 2017. Calcium signalling in health and disease. Biochem Biophys Res Commun 485: 5 10.1016/j.bbrc.2017.01.098 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Galione A. 1988. Cytosolic calcium oscillators. FASEB J 2: 3074–3082. 10.1096/fasebj.2.15.2847949 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Rapp PE. 1979. A comparative survey of the function, mechanism and control of cellular oscillators. J Exp Biol 81: 217–279. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bers DM. 2002. Cardiac excitation–contraction coupling. Nature 415: 198–205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Bers DM. 2008. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49. 10.1146/annurev.physiol.70.113006.100455 [DOI] [PubMed] [Google Scholar]
- Bers DM, Guo T. 2005. Calcium signaling in cardiac ventricular myocytes. Ann NY Acad Sci 1047: 86–98. 10.1196/annals.1341.008 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. 1991. Bell-shaped calcium-response curves of lns(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754. 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- *.Bhattacharyya M, Karandur D, Kuriyan J. 2019. Structural insights into the regulation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DM, Enyedi B, Geiszt M, Várnai P, Hajnóczky G. 2016. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol Cell 63: 240–248. 10.1016/j.molcel.2016.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Young KW, Young JM, Moreton RB, Berridge MJ. 1996. Extracellular calcium concentration controls the frequency of intracellular calcium spiking independently of inositol 1,4,5-trisphosphate production in HeLa cells. Biochem J 314: 347–354. 10.1042/bj3140347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M, Niggli E, Berridge M, Lipp P. 1997. Imaging the hierarchical Ca2+ signaling system in HeLa cells. J Physiol 499: 307–314. 10.1113/jphysiol.1997.sp021928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Lipp P, Berridge MJ. 2001. The organisation and functions of local Ca2+ signals. J Cell Sci 114: 2213–2222. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. 2009. An update on nuclear calcium signalling. J Cell Sci 122: 2337–2350. 10.1242/jcs.028100 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Rietdorf K, Collins T, Walker S, Sanderson M. 2013. Ca2+-sensitive fluorescent dyes and intracellular Ca2+ imaging. Cold Spring Harb Protoc 2013: 83–99. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Chehab T, Bultynck G, Parys JB, Rietdorf K. 2018. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium 70: 32–46. 10.1016/j.ceca.2017.08.005 [DOI] [PubMed] [Google Scholar]
- *.Burgoyne RD, Helassa N, McCue HV, Haynes LP. 2019. Calcium sensors in neuronal function and dysfunction. Cold Spring Harb Perspect Biol 11: a035154 10.1101/cshperspect.a035154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callamaras N, Marchant JS, Sun XP, Parker I. 1998. Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes. J Physiol 509: 81-91. 10.1111/j.1469-7793.1998.081bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, et al. 2010. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142: 270–283. 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chen J, Sitsel A, Benoy V, Sepúlveda MR, Vangheluwe P. 2019. Primary active Ca2+ transport systems in health and disease. Cold Spring Harb Persepct Biol 10.1101/cshperspect.a035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. 2011. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol 9: e1001025 10.1371/journal.pbio.1001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. 2003. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci 23: 103–111. 10.1523/jneurosci.23-01-00103.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. 2007. Calcium signaling. Cell 131: 1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Collins TJ, Lipp P, Berridge MJ, Bootman MD. 2001. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J Biol Chem 276: 26411–26420. 10.1074/jbc.M101101200 [DOI] [PubMed] [Google Scholar]
- Csordás G, Thomas AP, Hajnóczky G. 1999. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18: 96–108. 10.1093/emboj/18.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Weaver D, Hajnóczky G. 2018. Endoplasmic reticulum-mitochondrial contactology: Structure and signaling functions. Trends Cell Biol 28: 523–540. 10.1016/j.tcb.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. 2006. Ca2+ entry through plasma membrane IP3 receptors. Science 313: 229–233. 10.1126/science.1125203 [DOI] [PubMed] [Google Scholar]
- Demuro A, Parker I. 2006. Imaging single-channel calcium microdomains. Cell Calcium 40: 413–422. 10.1016/j.ceca.2006.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. 2015. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci Signal 8: ra74 10.1126/scisignal.aaa8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Distelhorst CW, Bootman MD. 2019. Creating a new cancer therapeutic agent by targeting the interaction between Bcl-2 and IP3 receptors. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. 2019. How secret conversations inside cells are transforming biology. Nature 567: 162–164. 10.1038/d41586-019-00792-9 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858. 10.1038/386855a0 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936. 10.1038/31960 [DOI] [PubMed] [Google Scholar]
- Dupont G, Combettes L, Bird GS, Putney JW. 2011. Calcium oscillations. Cold Spring Harb Perspect Biol 3: a004226 10.1101/cshperspect.a004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Caldwell JL, Kistamás K, Trafford AW. 2017. Calcium and excitation-contraction coupling in the heart. Circ Res 121: 181–195. 10.1161/circresaha.117.310230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II. 2015. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature 527: 336–341. 10.1038/nature15249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Baker MR, Wang Z, Seryshev AB, Ludtke SJ, Baker ML, Serysheva II. 2018. Cryo-EM reveals ligand induced allostery underlying InsP3R channel gating. Cell Res 28: 1158–1170. 10.1038/s41422-018-0108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley CJ, Roderick HL, Bootman MD. 2011. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol 3: a004242 10.1101/cshperspect.a004242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658. 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Galione A. 2019. NAADP receptors. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Churchill GC. 2000. Cyclic ADP ribose as a calcium-mobilizing messenger. Sci STKE 2000: pe1 10.1126/stke.2000.18.pe1 [DOI] [PubMed] [Google Scholar]
- *.Gilbert G, Demydenko K, Dries E, Puertas RD, Jin X Sipido K, Roderick HL. 2019. Calcium signaling in cardiomyocyte function. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Danese A, Missiroli S, Patergnani S, Pinton P. 2018a. Calcium dynamics as a machine for decoding signals. Trends Cell Biol 28: 258–273. 10.1016/j.tcb.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Giorgi C, Marchi S, Pinton P. 2018b. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 19: 713–730. 10.1038/s41580-018-0052-8 [DOI] [PubMed] [Google Scholar]
- Granatiero V, De Stefani D, Rizzuto R. 2017. Mitochondrial calcium handling in physiology and disease. Adv Exp Med Biol 982: 25–47. 10.1007/978-3-319-55330-6_2 [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Bading H. 2011. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harb Perspect Biol 3: a004564 10.1101/cshperspect.a004564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hagenston AM, Bading H, Bas-Orth C. 2019. Functional consequences of calcium-dependent synapse-to-nucleus communication: Focus on transcription-dependent metabolic plasticity. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. 1995. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424. 10.1016/0092-8674(95)90430-1 [DOI] [PubMed] [Google Scholar]
- Hamada K, Miyatake H, Terauchi A, Mikoshiba K. 2017. IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography. Proc Natl Acad Sci 114: 4661–4666. 10.1073/pnas.1701420114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D, Movassagh M, Foo RS, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL. 2009. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci 106: 11406–11411. 10.1073/pnas.0905485106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel FR, MacQuaide N, Biesmans L, Sipido K. 2011. Dyssynchrony of Ca2+ release from the sarcoplasmic reticulum as subcellular mechanism of cardiac contractile dysfunction. J Mol Cell Cardiol 50: 390–400. 10.1016/j.yjmcc.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. 2009. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell 33: 472–482. 10.1016/j.molcel.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. 1992. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355: 353–356. 10.1038/355353a0 [DOI] [PubMed] [Google Scholar]
- Humeau J, Bravo-San Pedro JM, Vitale I, Nuñez L, Villalobos C, Kroemer G, Senovilla L. 2018. Calcium signaling and cell cycle: Progression or death. Cell Calcium 70: 3–15. 10.1016/j.ceca.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Hüser J, Blatter LA, Lipsius SL. 2000. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524: 415–422. 10.1111/j.1469-7793.2000.00415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ivanova H, Vervliet T, Monaco G, Terry LE, Rosa N, Baker MR, Parys JB, Serysheva II, Yule DI, Bultynck G. 2019. Bcl-2 protein family as modulators of IP3 receptors and other organeller Ca2+ channels. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Booth DM, Young MP, Hajnóczky G. 2019. Redox regulation of ER and mitochondrial Ca2+ signaling in cell survival and death. Cell Calcium 79: 89–97. 10.1016/j.ceca.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhofs M, Giorgi C, Marchi S, Seitaj B, Parys JB, Pinton P, Bultynck G, Bittremieux M. 2017. Alterations in Ca2+ signalling via ER-mitochondria contact site remodelling in cancer. Adv Exp Med Biol 997: 225–254. 10.1007/978-981-10-4567-7_17 [DOI] [PubMed] [Google Scholar]
- Kerkhofs M, Bittremieux M, Morciano G, Giorgi C, Pinton P, Parys JB, Bultynck G. 2018. Emerging molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis 9: 334 10.1038/s41419-017-0179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhofs M, Bultynck G, Vervliet T, Monaco G. 2019. Therapeutic implications of novel peptides targeting ER–mitochondria Ca2+-flux systems. Drug Discov Today 24: 1092–1103. 10.1016/j.drudis.2019.03.020 [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364. 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Kockskämper J, Sheehan KA, Bare DJ, Lipsius SL, Mignery GA, Blatter LA. 2001. Activation and propagation of Ca2+ release during excitation–contraction coupling in atrial myocytes. Biophys J 81: 2590–2605. 10.1016/S0006-3495(01)75903-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. 2010. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere RM, Roest G, Bultynck G, Parys JB. 2016. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60: 74–87. 10.1016/j.ceca.2016.04.005 [DOI] [PubMed] [Google Scholar]
- *.Lewis RS. 2019. Store-operated calcium channels: From function to structure and back again. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrel JE Jr, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Reither G. 2011. Protein kinase C: the “masters” of calcium and lipid. Cold Spring Harb Perspect Biol 3: a004556 10.1101/cshperspect.a004556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Thomas D, Berridge MJ, Bootman MD. 1997. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J 16: 7166–7173. 10.1093/emboj/16.23.7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lloyd-Evans E, Waller-Evans H. 2019. Lysosomal Ca2+ homeostasis and signaling in health and disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. 2004. Reduced synchrony of Ca2+ release with loss of T-tubules—A comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73. 10.1016/j.cardiores.2003.12.031 [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. 2004. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci 117: 6327–6337. 10.1242/jcs.01559 [DOI] [PubMed] [Google Scholar]
- Mammucari C, Gherardi G, Rizzuto R. 2017. Structure, activity regulation, and role of the mitochondrial calcium uniporter in health and disease. Front Oncol 7: 139 10.3389/fonc.2017.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Raffaello A, Vecellio Reane D, Gherardi G, De Mario A, Rizzuto R. 2018. Mitochondrial calcium uptake in organ physiology: From molecular mechanism to animal models. Pflugers Arch 470: 1165–1179. 10.1007/s00424-018-2123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Marchant JS. 2019. Ca2+ signaling and regeneration. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P. 2018. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69: 62–72. 10.1016/j.ceca.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Taylor-Hunter A, Kater SB. 1988. Neurite outgrowth in individual neurons of a neuronal population is differentially regulated by calcium and cyclic AMP. J Neurosci 8: 1704–1711. 10.1523/jneurosci.08-05-01704.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. 2011. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3: a004317 10.1101/cshperspect.a004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. 2015. Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul 57: 217–227. 10.1016/j.jbior.2014.10.001 [DOI] [PubMed] [Google Scholar]
- *.Ong HL, Ambudkar IS. 2019. The endoplasmic reticulum–plasma membrane junction: A hub for agonist regulation of Ca2+ entry. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. 2003. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565. 10.1038/nrm1150 [DOI] [PubMed] [Google Scholar]
- Parys JB, Bultynck G. 2018. Calcium signaling in health, disease and therapy. Biochim Biophys Acta Mol Cell Res 1865: 1657–1659. 10.1016/j.bbamcr.2018.08.019 [DOI] [PubMed] [Google Scholar]
- Patel S. 2019. Getting close. Lysosome-ER contact sites tailor Ca2+ signals. Cell Calcium 80: 194–196. 10.1016/j.ceca.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Penna E, Espino J, De Stefani D, Rizzuto R. 2018. The MCU complex in cell death. Cell Calcium 69: 73–80. 10.1016/j.ceca.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233. 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- *.Prole DL, Taylor CW. 2019. Structure and function of IP3 receptors. Cold Spring Harb Perspect Biol 11: a035063 10.1101/cshperspect.a035063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Proudfoot D. 2019. Calcium signaling and tissue calcification. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr. 1990. Capacitative calcium entry revisited. Cell Calcium 11: 611–624. 10.1016/0143-4160(90)90016-N [DOI] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, Rizzuto R. 2016. Calcium at the center of cell signaling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci 41: 1035–1049. 10.1016/j.tibs.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. 1993. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747. 10.1126/science.8235595 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766. [DOI] [PubMed] [Google Scholar]
- *.Roberts-Thomson SJ, Chalmers SB, Monteith GR. 2019. The calcium signaling toolkit in cancer: Remodeling and targeting. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick HL, Berridge MJ, Bootman MD. 2003. Calcium-induced calcium release. Curr Biol 13: R425 10.1016/S0960-9822(03)00358-0 [DOI] [PubMed] [Google Scholar]
- Roderick HL, Higazi DR, Smyrnias I, Fearnley C, Harzheim D, Bootman MD. 2007. Calcium in the heart: When it's good, it's very very good, but when it's bad, it's horrid. Biochem Soc Trans 35: 957–961. 10.1042/BST0350957 [DOI] [PubMed] [Google Scholar]
- Rooney TA, Sass EJ, Thomas AP. 1989. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem 264: 17131–17141. [PubMed] [Google Scholar]
- Rossi A, Pizzo P, Filadi R. 2019. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim Biophys Acta Mol Cell Res 1866: 1068–1078. 10.1016/j.bbamcr.2018.10.016 [DOI] [PubMed] [Google Scholar]
- *.Roy J, Cyert MS. 2019. Identifying new substrates and functions for an old enzyme: Calcineurin. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G, Lewis DR, Marks AR. 2017. Physiology and pathophysiology of excitation-contraction coupling: The functional role of ryanodine receptor. J Muscle Res Cell Motil 38: 37–45. 10.1007/s10974-017-9470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B. 2010. Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol 2: a004051 10.1101/cshperspect.a004051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schwaller B. 2019. Cytosolic Ca2+ buffers are inherently Ca2+ signal modulators. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, De Matteis MA, Emr S, Giordano F, Hajnóczky G, Kornmann B, Lackner LL, Levine TP, Pellegrini L, Reinisch K, et al. 2019. Coming together to define membrane contact sites. Nat Commun 10: 1287 10.1038/s41467-019-09253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skupin A, Kettenmann H, Winkler U, Wartenberg M, Sauer H, Tovey SC, Taylor CW, Falcke M. 2008. How does intracellular Ca2+ oscillate: By chance or by the clock? Biophys J 94: 2404–2411. 10.1529/biophysj.107.119495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Shuai J, Parker I. 2011. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J 100: L37–L39. 10.1016/j.bpj.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Romanin C. 2019. STIM1 structure-function and downstream signaling pathways. Cell Calcium 80: 101–102. 10.1016/j.ceca.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. 2006. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911. 10.1083/jcb.200608073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul R, Falcke M. 2004. Release currents of IP3 receptor channel clusters and concentration profiles. Biophys J 86: 2660–2673. 10.1016/S0006-3495(04)74322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul R, Smith GD, Coombes S. 2008. A bidomain threshold model of propagating calcium waves. J Math Biol 56: 435–463. 10.1007/s00285-007-0123-5 [DOI] [PubMed] [Google Scholar]
- Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J. 2011. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 3: a004184 10.1101/cshperspect.a004184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Vangeel L, Voets T. 2019. Transient receptor potential (TRP) channels and calcium signaling. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergun O, Keelan J, Khodorov BI, Duchen MR. 1999. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol 519: 451–466. 10.1111/j.1469-7793.1999.0451m.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Verkhratsky A. 2019. Astroglial calcium signaling in aging and Alzheimer's disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet T, Parys JB, Bultynck. 2016. Bcl-2 proteins and calcium signaling: Complexity beneath the surface. Oncogene 35: 5079–5092. 10.1038/onc.2016.31 [DOI] [PubMed] [Google Scholar]
- *.Wacquier B, Combettes L, Dupont G. 2019. Cytoplasmic and mitochondrial calcium signaling: A two-way relationship. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wakai T, Mehregan A, Fissore RA. 2019. Ca2+ signaling and homeostasis in mammalian oocytes and eggs. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Hajnóczky G. 2005. Mitochondria and endoplasmic reticulum: The lethal interorganelle cross-talk. J Bioenerg Biomembr 37: 191–206. 10.1007/s10863-005-6600-x [DOI] [PubMed] [Google Scholar]
- *.Wang W-A, Agellon LB, Michalak M. 2019. Organellar calcium handling in the cellular reticular network. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a038265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Webb SE, Kelu JJ, Miller AL. 2019. Role of two-pore channels in embryonic development and cellular differentiation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a035170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NM, Cuthbertson KS, Cobbold PH. 1986. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319: 600–602. 10.1038/319600a0 [DOI] [PubMed] [Google Scholar]
- Yao Y, Choi J, Parker I. 1995. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol 482: 533–553. 10.1113/jphysiol.1995.sp020538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. 2005. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905. 10.1038/nature04147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cai X, Nwokonko RM, Loktionova NA, Wang Y, Gill DL. 2017. The STIM-Orai coupling interface and gating of the Orai1 channel. Cell Calcium 63: 8–13. 10.1016/j.ceca.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. 2010. Calcium signaling via two-pore channels: Local or global, that is the question. Am J Physiol Cell Physiol 298: C430–C441. 10.1152/ajpcell.00475.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]