Abstract
Since the identification of nicotinic acid adenine dinucleotide phosphate (NAADP) and its putative target, the two-pore channel (TPC), the NAADP/TPC/Ca2+ signaling pathway has been reported to play a role in a diverse range of functions in a variety of different cell types. TPCs have also been associated with a number of diseases, which arise when their activity is perturbed. In addition, TPCs have been shown to play key roles in various embryological processes and during the differentiation of a variety of cell types. Here, we review the role of NAADP/TPC/Ca2+ signaling during early embryonic development and cellular differentiation. We pay particular attention to the role of TPC2 in the development and maturation of early neuromuscular activity in zebrafish, and during the differentiation of isolated osteoclasts, endothelial cells, and keratinocytes. Our aim is to emphasize the conserved features of TPC-mediated Ca2+ signaling in a number of selected examples.
It was the mid-1990s when nicotinic acid adenine dinucleotide phosphate (NAADP) was first demonstrated to activate Ca2+ stores that were distinct from those sensitive to inositol 1,4,5-trisphosphate (IP3) or cyclic adenosine diphosphoribose ([cADPR]; Lee and Aarhus 1995). NAADP was reported to be highly potent, working at concentrations as low as ∼10–20 nm to stimulate the release of Ca2+ from microsomes prepared from the eggs of the sea urchin, Strongylocentrotus purpuratus. This was in comparison to concentrations of ∼75 nm cADPR and ∼0.4 µm IP3 required to elicit a similar Ca2+ response in the same cell preparation (Lee and Aarhus 1995). Furthermore, NAADP was also shown to induce rapid changes in intracellular Ca2+ in the intact eggs of another sea urchin species, Lytechinus pictus (Lee and Aarhus 1995; Aarhus et al. 1996). Following these early reports using sea urchins, NAADP was shown to mobilize Ca2+ in the oocytes of a number of other marine eggs, including those of the starfish, Asterina pectinifera, and the ascidian Phallusia mammillata (Albrieux et al. 1998; Santella et al. 2000; Lim et al. 2001; Moccia et al. 2006). In addition, NAADP was demonstrated to mobilize Ca2+ in a number of mammalian cell types, such as in mouse pancreatic acinar cells, rat astrocytes, human Jurkat T-lymphocytes, and guinea pig atrial myocytes (Cancela et al. 1999; Berg et al. 2000; Singaravelu and Deitmer 2006; Collins et al. 2011). Although the list of species and cell types reported to mobilize Ca2+ in response to NAADP is not exhaustive, the diversity of the selection that do (i.e., from evolutionary ancient echinoderms [Zamora and Rahman 2014] to the relatively more modern mammals [Lee and Beck 2015]), indicate the possible ubiquitous nature of this intracellular messenger. With the identification of more cell types that were responsive to NAADP, the race was on to identify the intracellular stores involved in generating the Ca2+ signals as well as the molecular identity of the NAADP receptor. With regard to the former, lysosome-related acidic organelles were identified as being the source of the Ca2+ that was mobilized by NAADP (Churchill et al. 2002; Kinnear et al. 2004). In addition, in reports where Ca2+ imaging data were presented, NAADP was described as stimulating a “local elevation of Ca2+” or a “spatially restricted Ca2+ wave” in the eggs and cell types under investigation (Churchill et al. 2002; Kinnear et al. 2004; Calcraft et al. 2009). Furthermore, it was suggested that such localized Ca2+ signals from the lysosomes might stimulate or “trigger” longer-range Ca2+ signaling by Ca2+-induced Ca2+ release (CICR) via ryanodine receptors (RyRs) or IP3 receptors (IP3Rs) located in the membrane of the endo-/sarcoplasmic reticulum ([ER/SR]; Cancela et al. 1999; Kinnear et al. 2004). In addition, although there remains some controversy with regard to NAADP metabolism, target receptors/ion channels, and organelles involved (Guse and Diercks 2018), there is evidence to suggest that in certain cell types, NAADP stimulates the release of Ca2+ from the acidic organelles via two-pore channels (TPCs) (Calcraft et al. 2009; Galione et al. 2009; Brailoiu et al. 2010; Zhu et al. 2010; Ruas et al. 2015).
In animals, three members of this cation-selective ion channel family have been identified, namely, TPC1, TPC2, and TPC3. TPC1 and TPC3 are localized on endosomes, whereas TPC2 is localized on lysosomes (Calcraft et al. 2009). There is some debate with regard to the identity of the endogenous agonists of these channels, and whether they conduct Ca2+ or Na+ or indeed both (Guse 2009, 2012; Wang et al. 2012; Cang et al. 2013). TPC1 is generally understood to be a voltage-regulated channel; however, there are diverging views about whether it is an NAADP-activated Ca2+ channel (Rybalchenko et al. 2012; Ogunbayo et al. 2015; Patel et al. 2017), or a phosphatidylinositol-3-5-bisphosphate (PI(3,5)P2)-activated Na+ channel (She et al. 2018). TPC2 is voltage insensitive and is reported to either conduct Ca2+ in response to NAADP, PI(3,5)P2, Mg2+ and protein kinases such as P38 and JNK (Zhang et al. 2013; Jha et al. 2014; Capel et al. 2015) or, like TPC1, conduct Na+ in response to PI(3,5)P2. Similarly, TPC3 has been identified as a voltage-activated Na+ channel (Cang et al. 2014; Kintzer and Stroud 2018), but it might also conduct Ca2+ in response to NAADP (Ogunbayo et al. 2015).
The genes of all three TPCs (tpcns) are reported to be expressed in most vertebrates although tpcn3 is not expressed in primates (specifically humans and chimps) or rodents (Calcraft et al. 2009). In addition, tpcns are found to a greater or lesser degree in many invertebrates, including echinoderms, ascidians, and some insects (Calcraft et al. 2009). Because of the diversity of species expressing tpcns, it has been suggested that they might be part of an ancient gene family, and thus play a crucial role in regulating a diverse variety of cell functions (Calcraft et al. 2009). Indeed, it has been shown that TPCs play a role in modulating the excitability and stimulus-secretion coupling in pancreatic β cells (Arredouani et al. 2015); they are important for normal platelet function (Ambrosio et al. 2015), they regulate cell pigmentation (Ambrosio et al. 2016), are involved in β-adrenoceptor signaling in the heart (Capel et al. 2015), and play a role in the contraction of smooth muscle (Tugba Durlu-Kandilci et al. 2010).
Perhaps not surprisingly, evidence is accumulating to suggest that TPCs also play a role during embryonic development and cellular differentiation. We are especially interested in the role of TPC2-mediated Ca2+ signaling in the development and innervation of the skeletal muscle in zebrafish embryos (Kelu et al. 2015, 2017, 2018). This is because we had previously demonstrated that a series of distinct Ca2+ signals are generated in the developing slow skeletal muscle cells of intact zebrafish embryos, and that both IP3Rs and RyRs play a role in generating these signals (Cheung et al. 2011). In the same year, it was reported that TPC2 is expressed in skeletal muscle tissue in mouse embryos and neonates, and that the differentiation of C2C12 myoblasts is stimulated by NAADP-induced Ca2+ release, and inhibited by the down-regulation of TPC2 (and TPC1; Aley et al. 2010a). In addition, it had previously been reported that NAADP-regulated localized Ca2+ signals from lysosomes initiates the global release of Ca2+ from RyRs in the SR, which stimulated the contraction of myocytes (Kinnear et al. 2004). These reports together with our own observations suggested that it would be important to investigate whether TPCs, and especially TPC2, might play some role in generating the Ca2+ signals we observed during the differentiation of slow skeletal muscle cells in zebrafish embryos.
In addition to skeletal muscle development, NAADP/TPC/Ca2+ signaling is also reported to play a role in other aspects of development, including the acrosome reaction (and hence activation) of mouse spermatozoa (Arndt et al. 2014) and fertilization in sea star oocytes (Ramos et al. 2014), as well as in the differentiation of various cell types, including osteoclasts, keratinocytes, and endothelial cells (Notomi et al. 2012; Favia et al. 2014; Park et al. 2015). Thus, here we review the current knowledge regarding NAADP/TPC/Ca2+ signaling during embryogenesis and tissue differentiation, and in doing so we hope to highlight the conserved features as well as the differences observed during the development of these various embryos and cell types.
TPC-MEDIATED Ca2+ SIGNALING DURING EARLY DEVELOPMENTAL AND DIFFERENTIATION EVENTS
As is quite often the case, new and important discoveries are made while working on a relatively simple nonmammalian species, and these are subsequently found to play a crucial role in the development and function of higher organisms: such was the case with the search for, and identification of, TPCs. This followed the pioneering work by Lee and colleagues who showed that NAADP could mobilize Ca2+ from an independent intracellular store other than the ER (i.e., from microsomes prepared from extracts derived from the sea urchins S. purpuratus [Lee and Aarhus 1995] and L. pictus [Lee and Aarhus 1995; Aarhus et al. 1996]). Similar results were then reported from ascidian and starfish oocytes (Albrieux et al. 1998; Santella et al. 2000), from plants (Navazio et al. 2000), and from higher eukaryotic calls (Cancela et al. 1999; Gambara et al. 2008; Aley et al. 2010a,b; Espositio et al. 2011), suggesting a highly conserved feature of this messenger molecule (Guse and Lee 2008), and presumably its endogenous receptor(s).
NAADP has been demonstrated to have distinct functions in a number of early developmental events, including during the acrosome reaction of sea urchin and mammalian sperm (Vasudevan et al. 2010; Arndt et al. 2014) and during fertilization in ascidian and starfish oocytes (Albrieux et al. 1998; Lim et al. 2001; Moccia et al. 2004). Thus, it is perhaps not surprising that TPCs have also been reported to play key roles in a variety of early developmental processes across a large number of cell types from a diverse range of organisms (e.g., from protozoans [Suárez-Cortés et al. 2017] to humans [Hockey et al. 2015; Ogunbayo et al. 2018]). These include the Ca2+ mobilization events that occur at fertilization of oocytes of the sea star Patiria miniata. Normally on fertilization in these oocytes, there is a rapid elevation of Ca2+ around the cortex (outermost region), called a “cortical flash,” which lasts for just a few seconds. This is followed by a longer-duration propagating wave of Ca2+ that crosses the oocyte starting at the location where the sperm makes contact (Ramos et al. 2014). When the expression of TPC1, TPC2, and TPC3 were individually perturbed via the introduction of morpholino oligonucleotides (MOs), there was little effect on the cortical flash or the subsequent propagating wave of Ca2+. However, when they were concomitantly perturbed, these normally well-defined Ca2+ signals were altered; in some oocytes, the cortical flash occurred after the Ca2+ wave, whereas in others, Ca2+ waves were initiated from two locations. It was suggested that in these oocytes there is a cooperative activity among the three TPC isoforms (Ramos et al. 2014).
The pharmacological inhibition of TPCs with trans-ned-19 (Naylor et al. 2009) has been shown to prevent the migration of invasive cancer cells and reduce lung metastasis of mammary mouse cancer cells (Nguyen et al. 2017). Here, the disruption of TPC1 and TPC2 function was reported to inhibit trafficking of β1 integrin, resulting in its accumulation in early endosomes. As a result, invasive cancer cells were no longer able to form leading edges that are required for migration. As TPCs are expressed in the embryos of a number of different species (Brailoiu et al. 2009; Zong et al. 2009; Ramos et al. 2014), and key early developmental events, especially those associated with gastrulation, rely on cell migration events (Keller 2005), it is possible that TPCs might regulate early embryological processes such as these as well.
TPCs have also been shown to play an essential role in the development and completion of the complex life cycle of the malaria-carrying protozoan, Plasmodium falciparum (Suárez-Cortés et al. 2017). Treatment with trans-ned-19 prevented the progression of the asexual life cycle of this parasite by inhibiting the normal spontaneous Ca2+ oscillations that are generated, as well as blocking the transition of the parasite from the early to the late trophozoite stage, and the ability of the late trophozoite to develop to the multinucleated schizont stage (Suárez-Cortés et al. 2017).
TPC2-MEDIATED Ca2+ SIGNALING DURING SKELETAL MUSCLE DEVELOPMENT
The essential role of Ca2+ during mature muscle contraction has long been recognized (Ringer 1882). More recently, evidence has accumulated to suggest that intracellular Ca2+ regulation also plays a critical role in muscle differentiation and development (see reviews by Webb and Miller 2011; Tu et al. 2016). The initial research focused on the study and characterization of the role of ER/SR-resident Ca2+ channels (i.e., RyRs and IP3Rs) during myogenesis. For example, Ferrari et al. (1996) demonstrated that cultured Xenopus myocytes exhibit spontaneous RyR-mediated Ca2+ transients during early differentiation, and when these signals are inhibited, then myofibrillogenesis is disrupted (Ferrari et al. 1998). In addition, Powell et al. (2003) reported the presence of IP3R-mediated nuclear Ca2+ transients in rodent myotubes, and demonstrated that these Ca2+ signals are involved in the regulation of gene transcription in muscle cells. Using live zebrafish embryos, Brennan et al. (2005) and Cheung et al. (2011) visualized and characterized a distinct pattern of Ca2+ signaling in the developing myotome. They suggested that these Ca2+ signals are mediated by RyRs and acetylcholine (Brennan et al. 2005) and/or by RyRs and IP3Rs (Cheung et al. 2011), and are crucial for myotomal patterning and myofibrillogenesis (Brennan et al. 2005; Cheung et al. 2011).
It was only relatively recently that lysosomal Ca2+ release was implicated in the regulation of myogenesis. The requirement of TPC2/Ca2+ signaling during skeletal muscle differentiation was first demonstrated in vitro using the C2C12 mouse myoblast cell line and primary murine myoblasts (Aley et al. 2010a). It was shown that in undifferentiated C2C12 cells, Ca2+ was released upon stimulation by NAADP-AM (a cell-permeant form of NAADP, an agonist of TPCs) and ATP (an agonist of IP3Rs), but not ryanodine (an agonist of RyRs when used at a low, stimulatory concentration; Aley et al. 2010a). This demonstrated the existence of the machinery required for NAADP signaling in the skeletal muscle precursor cells. It was also shown that the differentiation of C2C12 cells was promoted by incubation with NAADP-AM, as indicated by the increase in both myogenin (a myogenic regulatory factor) and skNAC (a skeletal and heart muscle-specific transcription factor) transcripts, as well as an increase in the number of nuclei present in cells expressing the myosin heavy chain (a late terminal myogenic differentiation marker; Aley et al. 2010a). In addition, treatment with bafilomycin A1 (an inhibitor of lysosomal H+-ATPase, which depletes acidic Ca2+ stores) and trans-ned-19 both repressed myogenin and skNAC expression in C2C12 cells and primary murine myoblasts (Aley et al. 2010a). These treatments also decreased the number of nuclei present in myosin heavy chain-positive cells (Aley et al. 2010a). It is important to note that treatment with xestospongin C (an IP3R inhibitor), or dantrolene (a RyR inhibitor), did not affect the expression of myogenin and skNAC or the number of nuclei in myosin heavy chain-positive cells (Aley et al. 2010a), implicating the specific requirement of NAADP signaling during myogenesis in vitro.
The expression profile of the transcripts of various Ca2+ channels (i.e., ip3r1-3, ryr1-3, and tpcn1-2) was also established in C2C12 cells and primary murine myoblasts (Aley et al. 2010a). It should be noted here, that somewhat similar to TPCs, there are multiple isoforms of both the IP3R and RyR. These are called IP3R1 to 3, and (in mammals) RyR1 to 3, and they are encoded by the ip3r1-3 and ryr1-3 genes, respectively. These ER-based Ca2+ channels were first described in the mid-1980s (Berridge and Irvine 1984; Fleischer et al. 1985; Berridge 1987), and recent advances in our understanding of both are reviewed by Santulli et al. (2017). Using RT-PCR, ip3r2, and ryr3 were shown to be expressed in undifferentiated C2C12 cells, whereas ip3r1-3, ryr1, and ryr3 were shown to be expressed in these cells only once they had undergone differentiation (Aley et al. 2010a). In addition, using northern blotting the relative level of expression of tpcn1-2 was characterized during the differentiation of C2C12 cells. The results showed that the expression of tpcn2 preceded the induction of differentiation, but it decreased gradually after the initiation of differentiation. In contrast, tpcn1 was expressed at a constant level before, during, and after the differentiation process (Aley et al. 2010a). Importantly, this same temporal pattern of expression of tpcn1 and tpcn2 was recapitulated in primary murine myoblasts that were obtained during embryonic and postnatal development. Thus, tpcn2 was strongly down-regulated during development, whereas tpcn1 was expressed constantly throughout all of the developmental stages examined (Aley et al. 2010a). It was suggested that TPC2 signaling might be required mainly for differentiation of the skeletal muscle cells rather than for their function once differentiation is complete (Aley et al. 2010a). To test the requirement for the various TPC isoforms in the differentiation process, the expression of tpcn1 and tpcn2 was knocked down by transfecting C2C12 cells with siRNAs against each isoform (Aley et al. 2010a). The results showed that both differentiation and the subsequent cell–cell fusion events were repressed after TPC2-knockdown, whereas the differentiation process alone was inhibited after TPC1-knockdown (Aley et al. 2010a). Together, these data support the suggestion for the requirement of NAADP/TPC/Ca2+ signaling in skeletal muscle differentiation, and they suggest that TPC1 and TPC2 have different roles in skeletal muscle differentiation and function, such that TPC2 (but not TPC1) has a specific role in the cell fusion events that occur during differentiation, whereas TPC1 (but not TPC2) is expressed in mature muscle where it might act as a trigger to release Ca2+ from the SR via RyR1 during contraction (Aley et al. 2010a).
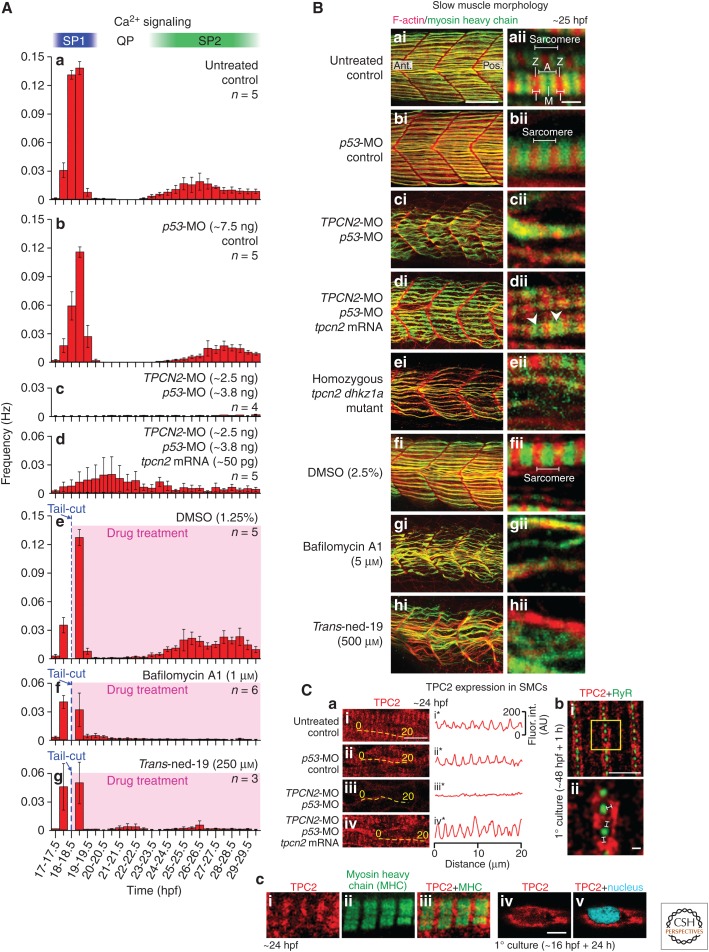
More recently, the role of TPC2 in mediating myogensis in vivo was demonstrated (Kelu et al. 2015, 2017). Earlier work characterized the endogenous pattern of Ca2+ signaling in the developing myotome in zebrafish embryos (Brennan et al. 2005; Cheung et al. 2011). Using a transgenic line of zebrafish that expresses the bioluminescent Ca2+ reporter, aequorin, specifically in skeletal muscle cells, the muscle-generated Ca2+ signals were visualized (Cheung et al. 2011). Two distinct phases of Ca2+ signaling were discovered during the development of the slow muscle cells (the first myofibers to develop; Devoto et al. 1996). These were called signaling period 1 (SP1), which occurs from ∼17.5 to ∼19.5 hours post-fertilization (hpf), and signaling period 2 (SP2), which occurs after ∼23.5 hpf (Fig. 1Aa; Cheung et al. 2011). Further pharmacological interventions suggested that the SP1 Ca2+ signals were mainly regulated by IP3R (as they were attenuated by 2-APB: an IP3R antagonist), whereas the SP2 Ca2+ signals were mainly regulated by RyR (as they were attenuated by an inhibitory concentration of ryanodine; Cheung et al. 2011). To investigate the role of TPC2-mediated Ca2+ signaling during slow muscle cell development in zebrafish embryos, Kelu et al. (2015) utilized antisense MO technology (see reviews by Blum et al. 2015; Stainier et al. 2017) to knock down the expression of TPC2. Using the same aequorin transgenic line of zebrafish described by Cheung et al. (2011), Kelu et al. (2015) showed that the usual pattern of Ca2+ signaling in the developing myotome (between ∼17 and ∼30 hpf) was significantly attenuated after TPCN2-MO injection (Fig. 1Ac). In addition, when a tpcn2 messenger RNA (mRNA) (that was not recognized by the TPCN2-MO) was injected into the morphants (MO-treated embryos), they demonstrated a partial rescue of the Ca2+ signaling signature (Fig. 1Ad); this supports the specificity of action of the TPCN2-MO (Kelu et al. 2015). In these experiments, a p53-MO was always coinjected with the TPCN2-MO (Fig. 1Ab; Kelu et al. 2015). The coinjection of p53-MO with the desired MO has been suggested to alleviate potential MO off-targeting effects that have previously been reported (Robu et al. 2007; Bedell et al. 2011). In a subsequent study, a negligible level of apoptosis was seen in the neural and somite regions (using the TUNEL assay) after the coinjection of the TPCN2-MO with p53-MO (Kelu et al. 2017); this helped to confirm the efficacy of p53 attenuation in the TPC2 morphants. As a complementary approach, embryos were treated with the lysosome/TPC inhibitors (i.e., bafilomycin A1 or trans-ned-19), and both were shown to result in a similar disruption to the muscle-generated Ca2+ signaling (Fig. 1Ae–Ag; Kelu et al. 2015). Together, the molecular knockdown and pharmacological inhibition approaches provided strong evidence for the involvement of lysosome/TPC2/Ca2+ signaling during slow muscle cell development in vivo.
Figure 1.
Role of TPC2 in the differentiation of the slow muscle cells in zebrafish embryos. (A) Effect of morpholino oligonucleotide (MO)-based knockdown (without and with messenger RNA [mRNA] rescue) or pharmacological inhibition of TPC2 on the muscle-generated Ca2+ signals from ∼17 to ∼30 hours post-fertilization (hpf). These histograms show the mean ± SEM frequency of Ca2+ signals generated every 30 min in the trunk musculature in α-actin-aeq transgenic embryos that were (Aa) untreated, (Ab–Ad) injected at the 1- to 4-cell stage with (Ab) p53-MO, (Ac) TPCN2-MO plus p53-MO, or (Ad) TPCN2-MO plus p53-MO and tpcn2 mRNA at the amounts shown; or (Ae–Ag) treated after the SP1 Ca2+ signals were first observed with (Ae) dimethylsulfoxide (DMSO), (Af) bafilomycin A1, or (Ag) trans-ned-19 (at the concentrations shown). Calcium signaling periods 1 and 2 (SP1 and SP2), and the signaling quiet period ([QP]; Cheung et al. 2011) are shown. (B) Effect of MO-based knockdown (± mRNA rescue), CRISPR/Cas9-knockout, or pharmacological inhibition of TPC2 on the organization of the trunk musculature and the formation of sarcomeres. Embryos were (Ba) untreated or (Bb–Bd) injected with (Bb) p53-MO, (Bc) TPCN2-MO plus p53-MO, or (Bd) TPCN2-MO plus p53-MO, and tpcn2 mRNA at the amounts shown in (A). In addition, (Be) shows a representative homozygous tpcn2 dhkz1a mutant, and (Bf–Bh) in some experiments embryos were treated with (Bf) DMSO, (Bg) bafilomycin A1, or (Bh) trans-ned-19 at the concentrations shown. All embryos were fixed at ∼25 hpf and dual-labeled with phalloidin and the F59 antibody to visualize F-actin (in red) and myosin heavy chain (in green) in the trunk musculature, respectively. The panels show a series of optical sections projected as single images at (Bai–Bhi) low and (Baii–Bhii) higher magnification when the red and green channels are merged; overlapping regions are shown in yellow. The higher magnification images of the slow muscle cell myofibers reveal the presence (Baii, Bbii, Beii, Bfii) or absence (Bcii, Bgii, Bhii) of the sarcomeric banding pattern of the F-actin and myosin heavy chain. Morphants that were coinjected with tpcn2 mRNA (Bdii) showed the appearance of some banding (white arrowheads) but not clear sarcomeres. Ant. and Pos. in (Bai) are anterior and posterior, respectively. (C) Localization of TPC2 in the slow muscle cells of (Cai) untreated embryos and in those injected with (Caii) p53-MO, (Caiii) TPCN2-MO plus p53-MO, or (Caiv) TPCN2-MO plus p53-MO, and tpcn2 mRNA (at the amounts shown in (A)) between the 1–4-cell stage and then fixed at 24 hpf prior to immunolabeling. The yellow dashed lines indicate the location of line scan analyses, which were performed along individual myofibers, after which (Cai*–Caiv*) line graphs were plotted to show the change in the pattern of TPC2 expression with the different treatments. (Cb) Visualization of TPC2 and RyR by dual-immunolabeling and dual-color stimulated emission depletion microscopy (STED) superresolution imaging. This is a representative muscle cell that was dissociated from the trunk of a zebrafish embryo at ∼48 hpf, plated onto a coverslip for 1 h, and then fixed and dual-immunolabeled with the 2137A anti-TPC2 and 34C anti-RyR primary antibodies. The region bounded by the yellow square in (Cbi) is shown at higher magnification in (Cbii). The white lines in (Cbii) indicate the presence of distinct gaps between the RyR and TPC2 clusters when observed via STED imaging. (Cc) Expression of TPC2 in a slow muscle cell at ∼24 hpf in relation to (Cci–Cciii) that of the myosin heavy chain, and (Cciv–Ccv) the nucleus. The former images were acquired from intact embryos fixed at ∼24 hpf, whereas the latter images were acquired from primary cultured cells prepared from embryos at ∼16 hpf and then cultured for ∼24 h. Scale bars, 50 µm (Bai–Bhi); 2 µm (Baii–Bhii); 10 µm (Cai–Caiv); 2 µm (Cbi); 200 nm (Cbii); 5 µm (Cc). (A, Ca, and Cc from Kelu et al. 2015; adapted, with permission, from UPV/EHU Press; B and Cb from Kelu et al. 2017; adapted, with permission, from Elsevier.)
To further characterize the role of TPC2 during slow muscle myogenesis, two major muscle proteins, F-actin and the myosin heavy chain, were labeled after the molecular knockdown (using TPCN2-MOs), pharmacological inhibition (using bafilomycin A1 and trans-ned-19), or genetic knockout (using CRISPR/Cas9-mediated mutagenesis) of TPC2 (Fig. 1B; Kelu et al. 2017). Strikingly, a similar pattern of muscle deformity was induced using the three different approaches, such that the slow muscle fibers became more flexuous, the somites became U-shaped, and the sarcomeric banding became disrupted (Kelu et al. 2017). Importantly, the number of slow muscle cells that were formed also decreased significantly after TPC2 intervention (Kelu et al. 2017). Together, these data suggest that in zebrafish, TPC2-mediated Ca2+ release from the lysosome is essential for the formation and differentiation of slow muscle cells, the overall patterning of the skeletal myotome, and the organization of the sarcomeres with respect to their essential contractile microfilaments.
To study the expression and localization of TPC2 during slow muscle development, a zebrafish-specific TPC2 antibody was custom made and used to label endogenous TPC2 at 2-hour intervals from ∼16 to ∼24 hpf via whole mount immunohistochemistry (Kelu et al. 2015). These stages were chosen because of the robust development of the slow muscle cells reported to occur within this time window (Devoto et al. 1996). To examine the expression of TPC2 in the slow muscle cells, in this series of experiments the slow muscle cells were dual-immunolabeled with the TPC2 antibody and a myosin heavy chain antibody (Kelu et al. 2015). At ∼16 hpf, TPC2 expression in the slow muscle cells was almost undetectable; at ∼18 hpf, TPC2 expression increased but remained relatively homogenous along the length of the slow muscle cells; at ∼20 hpf, TPC2 expression increased further, and started to assume a banding pattern as visualized by line-scan analysis. By ∼22 and ∼24 hpf, a robust and clear TPC2 banding was observed in the slow myofibrils; this observation was supported by the appearance of clear peaks of TPC2 labeling in the line-scan analysis (Fig. 1Ca; Kelu et al. 2015). The specificity of the custom-made zebrafish TPC2 antibody was also validated using the TPC2 morphants, where a clear attenuation of the TPC2 labeling was seen in the slow muscle cells. In addition, the normal localization pattern was rescued to some extent when the embryos were coinjected with the TPCN2-MO and tpcn2 mRNA (Fig. 1Ca; Kelu et al. 2015). Together, these data suggested that TPC2 is expressed in the right place (i.e., in the slow muscle cells) and at the right time (i.e., from ∼16 to 24 hpf) to generate the essential Ca2+ signaling that is required for regulating the development of the slow muscle cells.
It has been suggested that Ca2+ released from the lysosome is highly localized, and as such, it might be able to trigger further Ca2+ release from the ER/SR via CICR when the acidic stores and ER/SR are in close proximity (Galione et al. 2009; Galione 2015). Using STED superresolution microscopy, Kelu et al. (2017) showed that in immunolabeled primary cultured muscle cells, TPC2 was in close proximity to the RyR and that labeling for both was localized in the sarcomeric I-band region (Fig. 1Cb) as confirmed when TPC2 was colabeled with the myosin heavy chain (Fig. 1Cc). As subsequent quantification indicated that the separation between TPC2 and the RyR was between 57 and 82 nm (Kelu et al. 2017), it was suggested that such a nanometer distance lies within a finite range for functional interactions between the two Ca2+ release channels to be possible (Morgan et al. 2013; Fameli et al. 2014; Penny et al. 2015). Unfortunately, as the currently available TPC2 and IP3R antibodies are both raised in the same host species (i.e., rabbit), dual-immunolabeling of the TPC2 and IP3R was not conducted. Nonetheless, the results from the dual-immunolabeling and superresolution imaging of the RyR and IP3R suggest that all three of the Ca2+ release channels are localized to the sarcomeric I-bands, and that TPC2 might be even more closely opposed to the IP3R than it is to the RyR (Kelu et al. 2017). The juxapositioning of TPC2 with the IP3R and RyR therefore supports the proposed trigger hypothesis of TPC2 function (Galione et al. 2009; Galione 2015), and indicates that the subsequent recruitment of the SR to generate globalized Ca2+ signaling via CICR might be essential for myogenesis. This hypothesis was further tested by Kelu et al. (2017), who demonstrated that in the TPC2 morphants, stimulation of the IP3R or RyR could partially rescue the development of (and the generation of Ca2+ signals in) the slow muscle cells, with stimulation of the IP3R leading to a greater extent of rescue than stimulation of the RyR (Kelu et al. 2017). This partial rescue observed might be explained if there is a pool of TPC2 that works independently of IP3R or RyR in these cells. Indeed, it has been shown that in addition to being associated with the sarcomeric I-bands, TPC2 (and LAMP1; a lysosomal marker) is also localized in the perinuclear region of slow muscle cells (Fig. 1Cc; Kelu et al. 2015). IP3R and RyR are also expressed in the perinuclear and nuclear regions of these cells but the expression pattern of all these channels (TPC2, IP3R, and RyR) is more diffuse than it is in the vicinity of the myofibrils, so whether they are coupled or work independently is not yet known. However, it has been reported that TPCs can function independently of the ER/SR Ca2+ channels (Masgrau et al. 2003), and it has been suggested that the elementary Ca2+ signals generated by these channels might directly gate Ca2+-activated cation channels on the plasma membrane (Calcraft et al. 2009). This concept of distinct pools of TPC2 in cells (where one pool is coupled to the ER, while the other functions independently), has also been indicated in rat cortical neurons as the simultaneous inhibition of IP3R and RyR reduced, but did not completely block, the generation of NAADP-mediated Ca2+ signals (Brailoiu et al. 2005).
In zebrafish, it was previously proposed that the SP1 Ca2+ signals are required for slow muscle cell differentiation by mediating excitation–transcription (ET) coupling, whereas the SP2 Ca2+ signals are required for slow muscle cell function by mediating excitation–contraction (EC) coupling (Cheung et al. 2011). As TPC2 and LAMP1 have a perinuclear localization in primary cultured muscle cells (Fig. 1Cc), it was suggested that the activation of TPC2 around the nucleus during SP1 might stimulate IP3Rs and RyRs (which are also localized in the perinuclear/nuclear regions; Kelu et al. 2015) to modulate Ca2+-dependent transcription of muscle genes during ET coupling (Avila et al. 2001; Powell et al. 2001; Jaimovich and Carrasco 2002; Carrasco et al. 2003; Cárdenas et al. 2005; Stiber et al. 2005; Valdés et al. 2007). Indeed, the visualization of Ca2+ signals in the nucleus in zebrafish slow muscle cells was previously reported during the SP1 period (Cheung et al. 2011), where the duration of the elevated nuclear signal (>7.5 sec) far exceeded that of the cytoplasmic signal (∼0.58 sec), thus placing it in the time window for stimulating gene expression suggested by Berridge et al. (2003).
Even though the NAADP/TPC2 pathway has been implicated in agonist-evoked contractions and/ or EC coupling in both smooth muscle (Tugba Durlu-Kandilci et al. 2010; Aley et al. 2013) and cardiac muscle (Collins et al. 2011; Capel et al. 2015), relatively little is known about the its role in mediating skeletal muscle cell contraction. It was shown that the spontaneous coiling behavior of zebrafish embryos (Saint-Amant and Drapeau 1998) after TPC2-knockdown was severely retarded from ∼17 to ∼28 hpf, but motility was partially rescued following injection of tpcn2 mRNA (Kelu et al. 2017). In addition, when determining the touch-evoked response of embryos (Saint-Amant and Drapeau 1998), only ∼3% of TPC2 morphants responded to touch, when compared with 100% of the untreated control embryos. Again, a partial rescue was induced by the tpcn2 mRNA such that ∼30% of the embryos responded to touch (Kelu et al. 2017). Nonetheless, further investigation is required to confirm whether the effects on the muscle function are secondary to the effects on the muscle development and motor/sensory neuron activity after TPC2 knockdown.
In addition to the in vitro (Aley et al. 2010a) and in vivo (Kelu et al. 2015, 2017) evidence that is accumulating to indicate the essential requirement of NAADP/TPC2/Ca2+ signaling in skeletal muscle development and function, the role of lysosomal Ca2+ release during muscle autophagy and aging, as well as during myopathy and muscle repair have recently been described (Cheng et al. 2014, 2015; Lin et al. 2015). Thus, more in-depth investigations into the role of the TPC2/Ca2+ signaling pathway in muscle development and function might help to resolve the pathophysiology of skeletal muscle disorders.
TPC2-MEDIATED Ca2+ SIGNALING DURING NEURONAL DIFFERENTIATION
Ca2+ has long been known to regulate various aspects of neuronal development and function (reviewed by Simons 1988; Berridge 1998; Gruol et al. 2012; Kawamoto et al. 2012; Brini et al. 2014). Ca2+ entering the cell either via voltage-gated channels or via receptor-operated channels, which are regulated by ionotropic neurotransmitters, acts as a second messenger of various signal transduction pathways that regulate a diverse range of cellular processes, including neuronal excitability (Berridge 1998; Roussel et al. 2006; Lu et al. 2010), neuronal gene expression (Ghosh et al. 1994; West et al. 2001), neurotransmitter release (Harvey and MacIntosh 1940; Dodge and Rahamimoff 1967; Augustine et al. 1987; Südhof 2012), and synaptic plasticity (Lamont and Weber 2012), as well as many other events that contribute to the processing and storage of information that underlie learning and memory (Gibbs et al. 1979; Baker et al. 2013). Ca2+ signaling is also involved in various aspects of the development of the nervous system (Rosenberg and Spitzer 2011), playing a role in neuronal migration (Komuro and Rakic 1992; Tam et al. 2000), growth cone motility and guidance (Henley and Poo 2004; Bolsover 2005; Gasperini et al. 2017), neurite outgrowth and branching (Lankford and Letourneau 1989; Rønn et al. 2002), and synaptogenesis (Basarsky et al. 1994; Feng et al. 2002). Ca2+ influx from the extracellular milieu via voltage-gated Ca2+ channels, and/or the mobilization of Ca2+ from the ER via activation of IP3Rs or RyRs have long been implicated in the Ca2+-regulated aspects of neuronal development and function (Bandtlow et al. 1993; Takei et al. 1998; Tam et al. 2000; Numakawa et al. 2003; Hertle and Yeckel 2007; Gasperini et al. 2017). It is not surprising, therefore, that NAADP-mediated release of cytosolic Ca2+ from acidic stores has also been demonstrated to play a role in the extension of neurites in neurons isolated from newborn rat cerebral cortex (Brailoiu et al. 2005), and induce neuronal differentiation of PC12 cells (Brailoiu et al. 2006). More recently, Zhang et al. (2013) demonstrated that TPC2 has opposing effects on different stages of neural differentiation of mouse embryonic stem cells (ESCs), such that it inhibits the early differentiation of ESCs to neural progenitors, but it is required for the later stages of neuronal differentiation.
Most recently, NAADP/TPC2-mediated Ca2+ signaling was demonstrated to play a role in the establishment of synchronized activity in the primary motor neurons (PMNs) of intact, normally developing zebrafish embryos (Kelu et al. 2018). The SAIGFF213A:GCaMP7a double transgenic line of fish was used in which the fluorescent Ca2+ indicator, GCaMP7a is expressed in the caudal PMNs (CaPs) (Muto and Kawakami 2011; Muto et al. 2011). In normally developing fish at 18 hpf, low frequency and long duration Ca2+ signals are generated, which appear to arise in an almost stochastic manner (Kelu et al. 2018). By ∼24 hpf, however, the CaP-generated Ca2+ signals are ipsilaterally (i.e., along the same side of the trunk) correlated and contralaterally (i.e., on opposite sides of the trunk) anticorrelated (Muto and Kawakami 2011; Muto et al. 2011; Kelu et al. 2018); thus, Ca2+ signals are normally generated synchronously first along one side of the trunk and then along the other side (Fig. 2Aa, Ad). However, Kelu et al. (2018) showed that the MO-mediated knockdown (Fig. 2Ab), genetic knockout (via CRISPR/Cas9; Fig. 2Ae), or pharmacological inhibition (with bafilomycin A1 or trans-ned-19; Fig. 2Af–Ah) of TPC2 resulted in a loss of this synchronized (i.e., both the correlation and anticorrelation) Ca2+ signaling in the CaPs. TPC2 knockdown, knockout, or inhibition also resulted in a decrease in the frequency and amplitude of the CaP-generated Ca2+ signals and an increase in their duration. However, coinjection of embryos with TPCN2-MO and tpcn2 mRNA resulted in the ipsilateral and contralateral pattern of Ca2+ signaling as well as the frequency, amplitude, and duration of the signals being (at least partially) rescued (Fig. 2Ac; Kelu et al. 2018). TPC2 and LAMP1 (lysosomal-associated membrane protein 1) were shown to be expressed by the CaPs both in the cell body and in clusters along the axon (Fig. 2Ba, Bb). In addition, IP3Rs type I and II (but not IP3R type III or any of the RyR subtypes) were shown to be localized in the CaPs (Fig. 2Bc–Bf). Following MO-mediated TPC2 knockdown, treatment of embryos with the IP3R agonist, IP3/BM (but not the RyR agonists, caffeine, or ryanodine at a low agonistic concentration), partially rescued the CaP-mediated Ca2+ signals (Kelu et al. 2018). It was therefore suggested that these data support the trigger hypothesis, where the localized release of Ca2+ via TPCs in acidic stores stimulates larger-scale Ca2+ release via Ca2+ channels in the ER (IP3R or RyR) via CICR (Fig. 3; Kinnear et al. 2004; Galione et al. 2009). Indeed, the acidic organelles and ER have previously been shown to be closely apposed in primary rat medullary neurons (Brailoiu et al. 2009), and NAADP-induced Ca2+ release has been demonstrated to be linked to the activation of the IP3R in astrocytes isolated from newborn mice (Heidemann et al. 2005), as well as to the activation of IP3R or RyR in isolated nuclei from the ganglia of adult Aplysia californica and Arbacia punctata (Bezin et al. 2008). The work by Kelu et al. (2018) provides the first evidence in an intact animal model that TPC2 is a key molecular component of the Ca2+ signaling pathway that coordinates the establishment of the motor neuronal circuitry during development.
Figure 2.
Role of two-pore channel 2 (TPC2) in the differentiation of the primary motor neurons in zebrafish embryos. (A) Effect of morpholino oligonucleotide (MO)-based knockdown (without and with messenger RNA [mRNA] rescue), CRISPR/Cas9 heterozygous knockout, or pharmacological inhibition of TPC2 on the spontaneous Ca2+ activity of the caudal primary motor neurons (CaPs) in SAIGFF213A;UAS:GCaMP7a embryos at ∼24 hours post-fertilization (hpf). SAIGFF213A;UAS:GCaMP7a embryos were injected with (Aa) p53-MO; (Ab) TPCN2-MO plus p53-MO; or (Ac) TPCN2-MO plus p53-MO and the tpcn2 mRNA at the amounts shown in Figure 1A. In addition (Ad), untreated SAIGFF213A;UAS:GCaMP7a embryos (termed wild-type tpcn2 controls) were imaged and the adults were crossed with homozygous tpcn2 dhkz1a mutants to generate (Ae) double-transgenic heterozygous tpcn2 dhkz1a mutants. Furthermore (Af–Ah), some embryos were treated at ∼17 hpf with (Af) DMSO; (Ag) bafilomycin A1; or (Ah) trans-ned-19 (at the concentrations shown). (A*a) All embryos were imaged in a dorsal orientation and (A*b, A*c) time-lapse fluorescence images were acquired to show the changes in GCaMP7a fluorescence in the CaPs at different time intervals in the various treatment groups. Regions of interest (ROIs) were placed on two selected CaP cell bodies on the left (L1 and L2) and right (R1 and R2) sides of the spinal cord. Ant. and Pos. are anterior and posterior, respectively. Scale bar, 50 µm. (Aa–Ah) Line graphs showing the ΔF/F0 against time (over a period of ∼300 sec) in the ROIs selected for each embryo. (B) Expression and localization of TPC2, lysosomal-associated membrane protein 1 (LAMP1), IP3Rs, and RyRs in presumptive CaPs isolated from SAIGFF213A;UAS:GFP embryos and cultured for 24 h. The trunk of these embryos, which express green fluorescent protein (GFP) in the CaPs, was dissected at ∼18 hpf. The cells were dissociated and cultured for 24 h and then they were immunolabeled with antibodies for (Ba) TPC2, (Bb) LAMP1, (Bc–Be) IP3R types I–III, respectively, or (Bf) RyR (all subtypes). (Bg) Secondary antibody control. The cells were costained with 4′,6-diamidino-2-phenylindole (DAPI) to label the nuclei. Each panel is a series of optical sections that have been projected as a single confocal image, and they show the red (individual antibody) and blue (nuclei) channels when merged. CB and AX are the cell body and axon, respectively. The yellow, white, and blue arrowheads indicate TPC2, LAMP1, and IP3R type I clusters, respectively, localized along the axons. Scale bars, 10 µm. (From Kelu et al. 2018; adapted, with permission, from Elsevier.)
Figure 3.
Proposed model of two-pore channel 2 (TPC2)/Ca2+ signaling during early (∼17 hours post-fertilization [hpf]) and later (∼24 hpf) slow muscle cell and primary motor neuron differentiation in zebrafish embryos. This summarizes the accumulating evidence, which suggests an essential role for TPC2/Ca2+ signaling in the events involved in (A) excitation–transcription (ET) coupling during muscle differentiation and (B) excitation–contraction (EC) coupling during muscle contraction. TPC2/Ca2+ signaling appears to play a role in various aspects of neuromuscular development, including development of the spinal circuitry, outgrowth of the motor axons, formation of neuromuscular junctions, differentiation of slow muscle cells, myotomal patterning, and sarcomere formation, as well as the initiation of early motor behavior. (C) Evidence also indicates that the robustness of TPC2/Ca2+ signaling is maintained by the coordinated activation of the endo/sarcoplasmic reticulum (ER/SR) Ca2+ stores and subsequent amplification of the Ca2+ signals via Ca2+-induced Ca2+ release (CICR) within the lysosome-ER/SR junctions. (C is from Figure 2b in Galione et al. 2009; adapted, with permission.) TPC2, two-pore channel 2; IP3R, inositol 1,4,5-trisphosphate receptor; RyR, ryanodine receptor; DHPR, dihydropyridine receptor; AChR, acetylcholine receptor; ARC, ADP ribosyl cyclase; NMJ, neuromuscular junction.
TPC2-MEDIATED Ca2+ SIGNALING DURING OSTEOCLASTOGENESIS
Osteoclasts are giant multinucleated cells of the monocyte/macrophage family, which degrade and resorb bone (Udagawa et al. 1990). Working together with osteoblasts (which are responsible for synthesizing bone), osteoclasts regulate the overall mass and structure of the skeleton. The major players and main mechanisms that control osteoclast differentiation and bone resorption have been described previously and are the subject of a number of excellent reviews (Chambers 2000; Teitelbaum 2000; Boyle et al. 2003; Asagiri and Takayanagi 2007; Teitelbaum 2007; Yavropoulou and Yovos 2008; Nakashima et al. 2012). In brief, it is known that osteoclasts differentiate under the control of two main cytokines, receptor activator of nuclear factor κB ligand (RANKL) (Hsu et al. 1999; Wada et al. 2006), and macrophage colony-stimulating factor (Yoshida et al. 1990). Ca2+ signaling is known to play a key role in the differentiation of osteoclasts. Indeed, it has been shown that RANKL stimulates the generation of Ca2+ oscillations, which result in the calcineurin-mediated activation and nuclear localization of nuclear factor–activated T cells c1 (NFATc1), which in turn activates the gene transcription that regulates the terminal differentiation of these cells (Takayanagi et al. 2002). Following the Ca2+ oscillations, there is also an influx of Ca2+ on the basolateral side of osteoclasts, which is mediated by TRPV4, a Ca2+-permeable channel of the transient receptor potential family. It has been suggested that the oscillations and influx of Ca2+ together help to maintain the intracellular Ca2+ levels to ensure that NFATc1-regulated gene transcription (and hence osteoclast differentiation) is maintained (Masuyama et al. 2008).
It was also recently reported that TPC2 plays a key role in the differentiation of osteoclasts (Notomi et al. 2012, 2017). TPC2 was shown to be expressed by mouse bone marrow cells and the mouse osteoclast precursor cell line, RAW 264.7. In TPC2-knockdown cells, RANKL-regulated processes were reported to be inhibited, including the generation of the Ca2+ signals, and the nuclear localization of NFATc1 (Notomi et al. 2012). Furthermore, the TPC2 function was reported to be dependent on the amount of Mg2+, which (along with Ca2+) is stored in bone (Swaminathan 2003). Thus, whereas in normal Mg2+ conditions, TPC2 promoted osteoclast differentiation; when the level of Mg2+ was low, TPC2 inhibited this process. In addition, TPC2 was demonstrated to affect these changes by distinct mechanisms, such that it promoted and inhibited osteoclastogenesis via a Ca2+-dependent mechanism and via PI(3,5)P2-dependent changes in Na+ (rather than in Ca2+), respectively (Notomi et al. 2017). This might be the first example of the dual action of TPC2 (i.e., demonstrating its ability to transport both Ca2+ and Na+) in the same cell type (i.e., in RAW 264.7 cells) when exposed to different extracellular environments.
TPC-MEDIATED Ca2+ SIGNALING DURING THE DIFFERENTIATION OF ENDOTHELIAL AND EPITHELIAL CELLS
The formation of new blood vessels from pre-existing vessels (angiogenesis) is a key process during embryogenesis, and during the reproductive cycle of female mammals. Angiogenesis is also important during the repair and regeneration of damaged tissue, as well as in various diseases (Gupta and Zhang 2005). This process involves the proliferation, migration, and remodeling of endothelial cells from pre-existing blood vessels (Folkman and Shing 1992; Risau 1995), and it is regulated by vascular endothelial growth factor (VEGF) and its receptors, VEGFR1 and VEGFR2 (Michiels 2003). It has recently been shown both in vitro and in vivo that a VEGF/NAADP/TPC2/Ca2+ signaling cascade plays a key role in the formation of blood vessels (Favia et al. 2014). For example, treatment of human umbilical vein endothelial cells with trans-ned-19 or tpcn2 shRNA inhibited the usual VEGF-stimulated release of Ca2+ and formation of capillary-like tubes. Furthermore, in intact mice, the VEGF-induced formation of blood vessels was inhibited by trans-ned-19 in C57BL/6 (wild-type) animals and it failed to occur at all in Tpcn2−/− mutants (Favia et al. 2014).
Although there is currently no direct evidence that TPCs play a role in the differentiation of epithelial cells, NAADP has been shown to stimulate an increase in Ca2+ and promote the differentiation of human epidermal keratinocyte cells (Park et al. 2015). The latter was demonstrated by the effect of NAADP treatment on the level of expression of several protein markers for early and late keratinocyte differentiation, and the increase in activity of transglutaminases (Park et al. 2015), which are required for cross-linking proteins during terminal keratinocyte differentiation (Tharakan et al. 2010). The NAADP-stimulated Ca2+ signaling in these cells was shown to be inhibited by treatment with trans-ned-19, dipyridamole or bafilomycin, but was not affected by treatment with 8-bromo-cADPR (a cADPR inhibitor), xestospongin C (an IP3R inhibitor), or ryanodine at a RyR inhibitory concentration (Park et al. 2015). These data therefore indicate that TPCs might also play a key role in the differentiation of this specialized epithelial cell type.
CONCLUDING REMARKS
Here, we have reviewed what has been described so far about the role of NAADP/TPC/Ca2+ signaling during development and cellular differentiation. Tracking down the exact target or targets of NAADP, and identifying whether NAADP is the sole agonist of TPCs or not, as well as discovering whether TPCs conduct Na+ rather than Ca2+ is a matter of great interest for researchers in the Ca2+ signaling field. It is perhaps not surprising that a cation channel family with an evolutionary lineage such as the TPCs might have more than one agonist as well as a number of agonist-binding strategies (Lin-Moshier et al. 2014; Rahman et al. 2015; Feijóo-Bandin et al. 2017). The fact that TPCs have a somewhat promiscuous relationship with regard to the agonists that induce their opening, and the cations that they subsequently gate, in addition to their ability to act as a Ca2+ release trigger (Galione et al. 2009; Patel et al. 2010; Davis et al. 2012; Morgan et al. 2013), makes them a very interesting and challenging component of the Ca2+ signaling toolkit (Berridge et al. 2000) to study and understand, especially when it comes to the regulation of developmental and cellular differentiation. Furthermore, when considering the size and relative mobility of the acidic organelles that present TPCs to the cytosolic environment, this provides additional tantalizing evidence to suggest that they might be major players in intracellular Ca2+ signaling pathways. Indeed, endosomes and lysosomes along with closely associated ER/SR have been described as being a regulatory hub at the intersection of intracellular Ca2+ signaling (Marchant and Patel 2015; Perera and Zoncu 2016; Kilpatrick et al. 2017). In addition, while it is becoming clear that in some circumstances NAADP does appear to play a significant role in the Ca2+-mediated signaling pathways that orchestrate embryogenesis and differentiation, whether it does so via a TPC family member or some other target is (in many cases) still to be determined. Resolving these issues is essential for the role played by this pleiotropic channel family in key developmental events to be fully appreciated.
ACKNOWLEDGMENTS
Our work is supported by the HK RGC General Research Fund awards No. 16101714 and No. 16100115. We also acknowledge funding from the Hong Kong Innovation and Technology Commission (ITCPD/17-9).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Aarhus R, Dickey DM, Graeff RM, Gee KR, Walseth TF, Lee HC. 1996. Activation and inactivation of Ca2+ release by NAADP+. J Biol Chem 271: 8513–8516. 10.1074/jbc.271.15.8513 [DOI] [PubMed] [Google Scholar]
- Albrieux M, Lee HC, Villaz M. 1998. Calcium signalling by cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J Biol Chem 273: 14566–14574. 10.1074/jbc.273.23.14566 [DOI] [PubMed] [Google Scholar]
- Aley PK, Mikolajczyk AM, Munz B, Churchill GC, Galione A, Berger F. 2010a. Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels. Proc Natl Acad Sci 107: 19927–19932. 10.1073/pnas.1007381107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley PK, Noh HJ, Gao X, Tica AA, Brailoiu E, Churchill GC. 2010b. A functional role for nicotinic acid adenine dinucleotide phosphate in oxytocin-mediated contraction of uterine smooth muscle from rat. J Pharmacol Exp Ther 333: 726–735. 10.1124/jpet.110.165837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley PK, Singh N, Brailoiu GC, Brailoiu E, Churchill GC. 2013. Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger in muscarinic receptor-induced contraction of guinea pig trachea. J Biol Chem 288: 10986–10993. 10.1074/jbc.M113.458620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA, Di Pietro SM. 2015. TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release. Mol Biol Cell 26: 3263–3274. 10.1091/mbc.e15-01-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA, Aradi Al E, Christian KA, Di Pietro SM. 2016. TPC2 controls pigmentation by regulating melanosome pH and size. Proc Natl Acad Sci 113: 5622–5627. 10.1073/pnas.1600108113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt L, Castonguay J, Arlt E, Meyer D, Hassan S, Borth H, Zierler S, Wennemuth G, Breit A, Biel M, et al. 2014. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Mol Biol Cell 25: 948–964. 10.1091/mbc.E13-09-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani A, Ruas M, Collins SC, Parkesh R, Clough F, Pillinger T, Coltart G, Rietdorf K, Royle A, Johnson P, et al. 2015. Nicotinic acid adenine dinucleotide phosphate (NAADP) and endolysosomal two-pore channels modulate membrane excitability and stimulus-secretion coupling in mouse pancreatic β cells. J Biol Chem 290: 21376–21392. 10.1074/jbc.M115.671248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagiri M, Takayanagi H. 2007. The molecular understanding of osteoclast differentiation. Bone 40: 251–264. 10.1016/j.bone.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. 1987. Calcium action in synaptic transmitter release. Ann Rev Neurosci 10: 633–693. 10.1146/annurev.ne.10.030187.003221 [DOI] [PubMed] [Google Scholar]
- Avila G, O'Connell KMS, Groom LA, Dirksen RT. 2001. Ca2+ release through ryanodine receptors regulates skeletal muscle L-type Ca2+ channel expression. J Biol Chem 276: 17732–17738. 10.1074/jbc.M009685200 [DOI] [PubMed] [Google Scholar]
- Baker KD, Edwards TM, Rickard NS. 2013. The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev 37: 1211–1239. 10.1016/j.neubiorev.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Schmidt MF, Hassinger TD, Schwab ME, Kater SB. 1993. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science 259: 80–83. 10.1126/science.8418499 [DOI] [PubMed] [Google Scholar]
- Basarsky TA, Parpura V, Haydon PG. 1994. Hippocampal synaptogenesis in cell culture: Developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci 14: 6402–6411. 10.1523/jneurosci.14-11-06402.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Westcot SE, Ekker SC. 2011. Lessons from morpholino-based screening in zebrafish. Brief Funct Genomics 10: 181–188. 10.1093/bfgp/elr021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg I, Potter BVL, Mayr GW, Guse AH. 2000. Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+ signalling. J Cell Biol 150: 581–588. 10.1083/jcb.150.3.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 1987. Inositol trisphosphate and diacylglycerol: Two interacting second messengers. Ann Rev Biochem 56: 159–193. 10.1146/annurev.bi.56.070187.001111 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1998. Neuronal calcium signalling. Neuron 21: 13–26. 10.1016/S0896-6273(00)80510-3 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. 1984. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321. 10.1038/312315a0 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol 1: 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bezin S, Charpentier G, Lee HC, Baux G, Fossier P, Cancela JM. 2008. Regulation of nuclear Ca2+ signalling by translocation of the Ca2+ messenger synthesizing enzyme ADP-ribosyl cyclase during neuronal depolarization. J Biol Chem 283: 27859–27870. 10.1074/jbc.M804701200 [DOI] [PubMed] [Google Scholar]
- Blum M, De Robertis EM, Wallingford JB, Niehrs C. 2015. Morpholinos: Antisense and sensibility. Dev Cell 35: 145–149. 10.1016/j.devcel.2015.09.017 [DOI] [PubMed] [Google Scholar]
- Bolsover SR. 2005. Calcium signalling in growth cone migration. Cell Calcium 37: 395–402. 10.1016/j.ceca.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. 2003. Osteoclast differentiation and activation. Nature 423: 337–342. 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Hoard JL, Filipeanu CM, Brailoiu GC, Dun SL, Patel S, Dun NJ. 2005. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J Biol Chem 280: 5646–5650. 10.1074/jbc.M408746200 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, Dun NJ. 2006. Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J Biol Chem 281: 15923–15928. 10.1074/jbc.M602249200 [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Brailoiu E, Parkesh R, Galione A, Churchill GC, Patel S, Dun NJ. 2009. NAADP-mediated channel “chatter” in neurons of the rat medulla oblongata. Biochem J 419: 91–99. 10.1042/BJ20081138 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. 2010. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem 285: 38511–38516. 10.1074/jbc.M110.162073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C, Mangoli M, Dyer CE, Ashworth R. 2005. Acetylcholine and calcium signalling regulates muscle fibre formation in the zebrafish embryo. J Cell Sci 118: 5181–5190. 10.1242/jcs.02625 [DOI] [PubMed] [Google Scholar]
- Brini M, Calì T, Ottolini D, Carafoli E. 2014. Neuronal calcium signalling: function and dysfunction. Cell Mol Life Sci 71: 2787–2814. 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Arredouani A, Ruas M, Pan Z, Cheng X, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459: 596–600. 10.1038/nature08030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. 1999. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature 398: 74–76. 10.1038/18032 [DOI] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo Y, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. 2013. mTOR regulates lysosomal ATP-sensitive two-pore Na+ channels to adapt to metabolic state. Cell 152: 778–790. 10.1016/j.cell.2013.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Aranda K, Ren D. 2014. A non-inactivating high-voltage-activated two-pore Na+ channel that supports ultra-long action potentials and membrane bistability. Nat Commun 5: 5015 10.1038/ncomms6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel RA, Bolton EL, Lin WK, Aston D, Wang Y, Liu W, Wang X, Burton RA, Bloor-Young D, Shade KT, et al. 2015. Two-pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic β-adrenoceptor signaling in the heart. J Biol Chem 290: 30087–30098. 10.1074/jbc.M115.684076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C, Liberona JL, Molgó J, Colasante C, Mignery GA, Jaimovich E. 2005. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci 118: 3131–3140. 10.1242/jcs.02446 [DOI] [PubMed] [Google Scholar]
- Carrasco MA, Riveros N, Ríos J, Müller M, Torres F, Pineda J, Lantadilla S, Jaimovich E. 2003. Depolarization-induced slow calcium transients activate early genes in skeletal muscle cells. Am J Physiol Cell Physiol 284: C1438–C1447. 10.1152/ajpcell.00117.2002 [DOI] [PubMed] [Google Scholar]
- Chambers TJ. 2000. Regulation of the differentiation and function of osteoclasts. J Pathol 192: 4–13. 10.1002/1096-9896(2000) [DOI] [PubMed] [Google Scholar]
- Cheng X, Zhang X, Gao Q, Samie MA, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y, et al. 2014. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med 20: 1187–1192. 10.1038/nm.3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Zhang X, Yu L, Xu H. 2015. Calcium signaling in membrane repair. Semin Cell Dev Biol 45: 24–31. 10.1016/j.semcdb.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, Webb SE, Love DR, Miller AL. 2011. Visualization, characterization and modulation of calcium signaling during the development of slow muscle cells in intact zebrafish embryos. Int J Dev Biol 55: 153–174. 10.1387/ijdb.103160cc [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. 2002. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111: 703–708. 10.1016/S0092-8674(02)01082-6 [DOI] [PubMed] [Google Scholar]
- Collins TP, Bayliss R, Churchill GC, Galione A, Terrar DA. 2011. NAADP influences excitation–contraction coupling by releasing calcium from lysosomes in atrial myocytes. Cell Calcium 50: 449–458. 10.1016/j.ceca.2011.07.007 [DOI] [PubMed] [Google Scholar]
- Davis LC, Morgan AJ, Chen JL, Sneed CM, Bloor-Young D, Shenderov E, Stanton-Humphreys MN, Conway SJ, Churchill GC, Parrington J, et al. 2012. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol 22: 2331–2337. 10.1016/j.cub.2012.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto SH, Melançon E, Eisen JS, Westerfield M. 1996. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 122: 3371–3380. [DOI] [PubMed] [Google Scholar]
- Dodge FA Jr, Rahamimoff R. 1967. Co-operative action of calcium ions in transmitter release at the neuromuscular junction. J Physiol 193: 419-432. 10.1113/jphysiol.1967.sp008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espositio B, Gambara G, Lewis AM, Palombi F, D'Alessio A, Taylor LX, Genazzani AA, Ziparo E, Galione A, Churchill GC, et al. 2011. NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood 117: 4968–4977. 10.1182/blood-2010-02-266338 [DOI] [PubMed] [Google Scholar]
- Fameli N, Ogunbayo OA, van Breemen C, Evans AM. 2014. Cytoplasmic nanojunctions between lysosomes and sarcoplasmic reticulum are required for specific calcium signaling. F1000Res 3: 93 10.12688/f1000research.3720.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia A, Desideri M, Gambara G, D'Alessio A, Ruas M, Esposito B, Del Bufalo D, Parrington J, Ziparo E, Palombi F, et al. 2014. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signalling. Proc Natl Acad Sci 111: E4706–E4715. 10.1073/pnas.1406029111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijóo-Bandin S, García-Vence M, García-Rúa V, Roselló E, Portoles B, Rivera M, González-Juanatey JR, Lago F. 2017. Two-pore channels (TPCs): Novel voltage-gated ion channels with pleiotropic functions. Channels 11: 20–33. 10.1080/19336950.2016.1213929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZP, Grigoriev N, Munno D, Lukowiak K, MacVicar BA, Goldberg JI, Syed N. 2002. Development of Ca2+ hotspots between Lymnaea neurons during synaptogenesis. J Physiol 539: 53–65. 10.1113/jphysiol.2001.013125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MB, Rohrbough J, Spitzer NC. 1996. Spontaneous calcium transients regulate myofibrillogenesis in embryonic Xenopus myocytes. Dev Biol 178: 484–497. 10.1006/dbio.1996.0233 [DOI] [PubMed] [Google Scholar]
- Ferrari MB, Ribbeck K, Hagler DJ, Spitzer NC. 1998. A calcium signaling cascade essential for myosin thick filament assembly in Xenopus myocytes. J Cell Biol 141: 1349–1356. 10.1083/jcb.141.6.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Ogunbunmi EM, Dixon MC, Fleer EAM. 1985. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci 82: 7256–7259. 10.1073/pnas.82.21.7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Shing Y. 1992. Angiogenesis. J Biol Chem 267: 10931–10934. [PubMed] [Google Scholar]
- Galione A. 2015. A primer of NAADP-mediated Ca2+ signalling: From sea urchin eggs to mammalian cells. Cell Calcium 58: 27–47. 10.1016/j.ceca.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. 2009. The acid test: The discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2+ release channels. Pflugers Arch Eur J Physiol 458: 869–876. 10.1007/s00424-009-0682-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambara G, Billington RA, Debidda M, D'Alessio A, Palombi F, Ziparo E, Genazzani AA, Filippini A. 2008. NAADP-induced Ca2+ signalling in response to endothelin is via the receptor subtype B and requires the integrity of lipid rafts/caveolae. J Cell Physiol 216: 396–404. 10.1002/jcp.21407 [DOI] [PubMed] [Google Scholar]
- Gasperini RJ, Pavez M, Thompson AC, Mitchell CB, Hardy H, Young KM, Chilton JK, Foa L. 2017. How does calcium interact with the cytoskeleton to regulate growth cone motility during axon pathfinding? Mol Cell Neurosci 84: 29–35. 10.1016/j.mcn.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Ginty DD, Bading H, Greenberg ME. 1994. Calcium regulation of gene expression in neuronal cells. J Neurobiol 25: 294–303. 10.1002/neu.480250309 [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Gibbs CL, Ng KT. 1979. The influence of calcium on short-term memory. Neurosci Lett 14: 355–360. 10.1016/0304-3940(79)96174-3 [DOI] [PubMed] [Google Scholar]
- Gruol D, Manto M, Haines D. 2012. Ca2+ signalling in cerebellar Purkinje neurons—Editorial. Cerebellum 11: 605–608. 10.1007/s12311-012-0404-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Zhang J. 2005. Angiogenesis: A curse or cure? Postgrad Med J 81: 236–242. 10.1136/pgmj.2004.023309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse AH. 2009. Second messenger signaling: Multiple receptors for NAADP. Curr Biol 19: R522. [DOI] [PubMed] [Google Scholar]
- Guse AH. 2012. Linking NAADP to ion channel activity: A unifying hypothesis. Sci Signal 5: pe18 10.1126/scisignal.2002890 [DOI] [PubMed] [Google Scholar]
- Guse AH, Diercks BP. 2018. Integration of nicotinic acid adenine dinucleotide phosphate (NAADP)-dependent calcium signaling. J Physiol 596: 2735–2743. 10.1113/JP275974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse A, Lee HC. 2008. NAADP: A universal Ca2+ trigger. Sci Signal 1: re10. 10.1126/scisignal.144re10 [DOI] [PubMed] [Google Scholar]
- Harvey AM, MacIntosh FC. 1940. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol 97: 408–416. 10.1113/jphysiol.1940.sp003818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann AC, Schipke CG, Kettenmann H. 2005. Extracellular application of nicotinic acid adenine dinucleotide phosphate induces Ca2+ signalling in astrocytes in situ. J Biol Chem 280: 35630–35640. 10.1074/jbc.M507338200 [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. 2004. Guiding neuronal growth cones by Ca2+ signals: During axon pathfinding in the developing nervous system, spatiotemporal patterns of Ca2+ signals can govern growth cone extension and steering—By symmetric versus asymmetric regulation of cytoskeletal and membrane dynamics. Trends Cell Biol 14: 320–330. 10.1016/j.tcb.2004.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle DN, Yeckel MF. 2007. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neurosci 150: 625–638. 10.1016/j.neuroscience.2007.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. 2015. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci 128: 232–238. 10.1242/jcs.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et al. 1999. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci 96: 3540–3545. 10.1073/pnas.96.7.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E, Carrasco MA. 2002. IP3 dependent Ca2+ signals in muscle cells are involved in regulation of gene expression. Biol Res 35: 195–202. 10.4067/S0716-97602002000200010 [DOI] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. 2014. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J 33: 501–511. 10.1002/embj.201387035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto EM, Vivar C, Camandola S. 2012. Physiology and pathology of calcium signalling in the brain. Front Pharmacol 3: 61 10.3389/fphar.2012.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. 2005. Cell migration during gastrulation. Curr Opin Cell Biol 17: 533–541. 10.1016/j.ceb.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Kelu JJ, Chan HL, Webb SE, Cheng AH, Ruas M, Parrington J, Galione A, Miller AL. 2015. Two-pore channel 2 activity is required for slow muscle cell-generated Ca2+ signaling during myogenesis in intact zebrafish. Int J Dev Biol 59: 313–325. 10.1387/ijdb.150206am [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelu JJ, Webb SE, Parrington J, Galione A, Miller AL. 2017. Ca2+ release via two-pore channel type 2 (TPC2) is required for slow muscle cell myofibrillogenesis and myotomal patterning in intact zebrafish embryos. Dev Biol 425: 109–129. 10.1016/j.ydbio.2017.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelu JJ, Webb SE, Galione A, Miller AL. 2018. TPC2-mediated Ca2+ signaling is required for the establishment of synchronized activity in developing zebrafish primary motor neurons. Dev Biol 438: 57–68. 10.1016/j.ydbio.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Hockey LN, Yates E, Futter CE, Patel S. 2017. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep 18: 1636–1645. 10.1016/j.celrep.2017.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. 2004. Lysosome-sarcoplasmic reticulum junctions: A trigger zone for calcium signalling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem 279: 54319–54326. 10.1074/jbc.M406132200 [DOI] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM. 2018. On the structure and mechanism of two-pore channels. FEBS J 285: 233–243. 10.1111/febs.14154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. 1992. Selective role of N-type calcium channels in neuronal migration. Science 257: 806–809. 10.1126/science.1323145 [DOI] [PubMed] [Google Scholar]
- Lamont MG, Weber JT. 2012. The role of calcium in synaptic plasticity and motor learning in the cerebellar cortex. Neurosci Biobehav Rev 36: 1153–1162. 10.1016/j.neubiorev.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Lankford KL, Letourneau PC. 1989. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J Cell Biol 109: 1229–1243. 10.1083/jcb.109.3.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. 1995. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 270: 2152–2157. 10.1074/jbc.270.5.2152 [DOI] [PubMed] [Google Scholar]
- Lee MSY, Beck RMD. 2015. Mammalian evolution: A Jurassic spark. Current Biol 25: R759–R761. 10.1016/j.cub.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Lim D, Kyozuka K, Gragnaniello G, Carafoli E, Santella L. 2001. NAADP+ initiates the Ca2+ response during fertilization of starfish oocytes. FASEB J 15: 2257–2267. 10.1096/fj.01-0157com [DOI] [PubMed] [Google Scholar]
- Lin PH, Duann P, Komazaki S, Park KH, Li H, Sun M, Sermersheim M, Gumpper K, Parrington J, Galione A, et al. 2015. Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling. J Biol Chem 290: 3377–3389. 10.1074/jbc.M114.608471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, et al. 2014. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci USA 111: 13087–13092. 10.1073/pnas.1407004111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Zhang Q, Wang H, Wang Y, Nakayama M, Ren D. 2010. Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron 68: 488–499. 10.1016/j.neuron.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Patel S. 2015. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem Soc Trans 43: 434–441. 10.1042/BST20140303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJH, Galione A. 2003. NAADP: A new second messenger for glucose-induced Ca2+ responses in clonal pancreatic β cells. Curr Biol 13: 247–251. 10.1016/S0960-9822(03)00041-1 [DOI] [PubMed] [Google Scholar]
- Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, Lieben L, Torrekens S, Moermans K, Vanden Bosch A, et al. 2008. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 8: 257–265. 10.1016/j.cmet.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Michiels C. 2003. Endothelial cell functions. J Cell Physiol 196: 430–443. 10.1002/jcp.10333 [DOI] [PubMed] [Google Scholar]
- Moccia F, Lim D, Kyozuka K, Santella L. 2004. NAADP triggers the fertilization potential in starfish oocytes. Cell Calcium 36: 515–524. 10.1016/j.ceca.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Moccia F, Billington RA, Santella L. 2006. Pharmacological characterization of NAADP-induced Ca2+ signals in starfish oocytes. Biochem Biophys Res Commun 348: 329–336. 10.1016/j.bbrc.2006.05.157 [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Wagner SK, Lewis AM, Parrington J, Churchill GC, Galione A. 2013. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J Cell Biol 200: 789–805. 10.1083/jcb.201204078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Kawakami K. 2011. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun Integr Biol 4: 566–568. 10.4161/cib.15848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Ohkura M, Kotani T, Higashijima SI, Nakai J, Kawakami K. 2011. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proc Natl Acad Sci 108: 5425–5430. 10.1073/pnas.1000887108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Takayanagi H. 2012. New insights into osteoclastogenic signalling mechanisms. Trends Endocrinol Metab 23: 582–590. 10.1016/j.tem.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Navazio I, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D. 2000. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci 97: 8693–8698. 10.1073/pnas.140217897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor EA, Arredouani SR, Vasudevan AM, Lewis R, Parkesh A, Mizote D, Rosen JM, Thomas M, Izumi A, Ganesan A, et al. 2009. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol 5: 220–226. 10.1038/nchembio.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ONP, Grimm C, Schneider LS, Chao YK, Atzberger C, Bartel K, Watermann A, Ulrich M, Mayr D, Wahl-Schott C, et al. 2017. Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res 77: 1427–1438. 10.1158/0008-5472.CAN-16-0852 [DOI] [PubMed] [Google Scholar]
- Notomi T, Ezura Y, Noda M. 2012. Identification of two-pore channel 2 as a novel regulator of osteoclastogenesis. J Biol Chem 287: 35057–35064. 10.1074/jbc.M111.328930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Kuno M, Hiyama A, Nozaki T, Ohura K, Ezura Y, Noda M. 2017. Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J Biol Chem 292: 20998–21010. 10.1074/jbc.M117.780072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Nakayama H, Suzuki S, Kubo T, Nara F, Numakawa Y, Yokomaku D, Araki T, Ishimoto T, Ogura A, et al. 2003. Nerve growth factor-induced glutamate release is via p75 receptor, ceramide, and Ca2+ from ryanodine receptor in developing cerebellar neurons. J Biol Chem 278: 41259–41269. 10.1074/jbc.M304409200 [DOI] [PubMed] [Google Scholar]
- Ogunbayo OA, Zhu Y, Shen B, Agbani E, Li J, Ma J, Zhu MX, Evans AM. 2015. Organelle-specific subunit interactions of the vertebrate two-pore channel family. J Biol Chem 290: 1086–1095. 10.1074/jbc.M114.610493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunbayo OA, Duan J, Xiong J, Wang Q, Feng X, Ma J, Zhu MX, Evans AM. 2018. mTORC1 controls lysosomal Ca2+ release through the two-pore channel TPC2. Sci Signal 11: eaao5775 10.1126/scisignal.aao5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Kim KN, Park DR, Jang KY, Kim UH. 2015. Role of nicotinic acid adenine dinucleotide phosphate (NAADP) in keratinocyte differentiation. J Invest Dermatol 135: 1692–1694. 10.1038/jid.2015.43 [DOI] [PubMed] [Google Scholar]
- Patel S, Marchant J, Brailoiu E. 2010. Two-pore channels: Regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47: 480–490. 10.1016/j.ceca.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Churamani D, Brailoiu E. 2017. NAADP-evoked Ca2+ signals through two-pore channel-1 require arginine residues in the first S4-S5 linker. Cell Calcium 68: 1–4. 10.1016/j.ceca.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Eden ER, Patel S. 2015. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium 58: 387–396. 10.1016/j.ceca.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Perera RM, Zoncu R. 2016. The lysosome as a regulatory hub. Annu Rev Cell Dev Biol 32: 223–253. 10.1146/annurev-cellbio-111315-125125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JA, Carrasco MA, Adams DS, Drouet B, Rios J, Müller M, Estrada M, Jaimovich E. 2001. IP3 receptor function and localization in myotubes: An unexplored Ca2+ signaling pathway in skeletal muscle. J Cell Sci 114: 3673–3683. [DOI] [PubMed] [Google Scholar]
- Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. 2015. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal 7: ra109 10.1126/scisignal.2005450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos I, Reich A, Wessel GM. 2014. Two-pore channels function in calcium regulation in sea star oocytes and embryos. Development 141: 4598–4609. 10.1242/dev.113563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S. 1882. Concerning the influence exerted by each of the constituents of the blood on the contraction of the ventricle. J Physiol (Lond) 3: 380–393. 10.1113/jphysiol.1882.sp000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. 1995. Differentiation of endothelium. FASEB J 9: 926–933. 10.1096/fasebj.9.10.7615161 [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. 2007. p53 activation by knockdown technologies. PLoS Genet 3: e78 10.1371/journal.pgen.0030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønn LCB, Dissing S, Holm A, Berezin V, Bock E. 2002. Increased intracellular calcium is required for neurite outgrowth induced by a synthetic peptide ligand of NCAM. FEBS Lett 518: 60–66. 10.1016/S0014-5793(02)02644-3 [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Spitzer NC. 2011. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol 3: a004259 10.1101/cshperspect.a004259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel C, Erneux T, Schiffmann SN, Gall D. 2006. Modulation of neuronal excitability by intracellular calcium buffering: From spiking to bursting. Cell Calcium 39: 455–466. 10.1016/j.ceca.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, et al. 2015. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J 34: 1743–1758. 10.15252/embj.201490009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. 2012. Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J Biol Chem 287: 20407–20416. 10.1074/jbc.M112.359612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. 1998. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol 37: 622–632. [DOI] [PubMed] [Google Scholar]
- Santella L, Kyozuka K, Genazzani AA, De Riso L, Carafoli E. 2000. Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release: Interactions among distinct Ca2+ mobilizing mechanisms in starfish oocytes. J Biol Chem 275: 8301–8306. 10.1074/jbc.275.12.8301 [DOI] [PubMed] [Google Scholar]
- Santulli G, Nakashima R, Yuan Q, Marks AR. 2017. Intracellular calcium release channels: an update. J Physiol 595: 3041–3051. 10.1113/JP272781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Guo J, Chen Q, Zeng W, Jiang Y, Bai X. 2018. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 556: 130–134. 10.1038/nature26139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons TJB. 1988. Calcium and neuronal function. Neurosurg Rev 11: 119–129. 10.1007/BF01794675 [DOI] [PubMed] [Google Scholar]
- Singaravelu K, Deitmer JW. 2006. Calcium mobilization by nicotinic acid adenine dinucleotide phosphate (NAADP) in rat astrocytes. Cell Calcium 39: 143–153. 10.1016/j.ceca.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Stainier DY, Raz E, Lawson ND, Ekker SC, Burdine RD, Eisen JS, Ingham PW, Schulte-Merker S, Yelon D, Weinstein BM, et al. 2017. Guidelines for morpholino use in zebrafish. PLoS Genet 13: e1007000 10.1371/journal.pgen.1007000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiber JA, Tabatabaei N, Hawkins AF, Hawke T, Worley PF, Williams RS, Rosenberg P. 2005. Homer modulates NFAT-dependent signaling during muscle differentiation. Dev Biol 287: 213–224. 10.1016/j.ydbio.2005.06.030 [DOI] [PubMed] [Google Scholar]
- Suárez-Cortés P, Gambara G, Favia A, Palombi F, Alano P, Filippini A. 2017. Ned-19 inhibition of parasite growth and multiplication suggests a role for NAADP mediated signalling in the asexual development of Plasmodium falciparum. Malar J 16: 366 10.1186/s12936-017-2013-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. 2012. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol 4: a011353 10.1101/cshperspect.a011353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan R. 2003. Magnesium metabolism and its disorders. Clin Biochem Rev 24: 47–66. [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901. 10.1016/S1534-5807(02)00369-6 [DOI] [PubMed] [Google Scholar]
- Takei K, Shin RM, Inoue T, Kato K, Mikoshiba K. 1998. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science 282: 1705–1708. 10.1126/science.282.5394.1705 [DOI] [PubMed] [Google Scholar]
- Tam T, Mathews E, Snutch TP, Schafer WR. 2000. Voltage-gated calcium channels direct neuronal migration in Caenorhabditis elegans. Dev Biol 226: 104–117. 10.1006/dbio.2000.9854 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. 2000. Bone resorption by osteoclasts. Science 289: 1504–1508. 10.1126/science.289.5484.1504 [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. 2007. Osteoclasts: What do they do and how do they do it? Am J Pathol 170: 427–435. 10.2353/ajpath.2007.060834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharakan S, Pontiggia L, Biedermann T, Böttcher-Haberzeth S, Schiestl C, Reichmann E, Meuli M. 2010. Transglutaminases, involucrin, and loricrin as markers of epidermal differentiation in skin substitutes derived from human sweat gland cells. Pediatr Surg Int 26: 71–77. 10.1007/s00383-009-2517-5 [DOI] [PubMed] [Google Scholar]
- Tu MK, Levin JB, Hamilton AM, Borodinsky LN. 2016. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 59: 91–97. 10.1016/j.ceca.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugba Durlu-Kandilci N, Ruas M, Chuang KT, Brading A, Parrington J, Galione A. 2010. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J Biol Chem 285: 24925–24932. 10.1074/jbc.M110.129833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin J, Suda T. 1990. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci 87: 7260–7264. 10.1073/pnas.87.18.7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés JA, Hidalgo J, Galaz JL, Puentes N, Silva M, Jaimovich E, Carrasco MA. 2007. NF-κB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am J Physiol Cell Physiol 292: C1960–C1970. 10.1152/ajpcell.00320.2006 [DOI] [PubMed] [Google Scholar]
- Vasudevan SR, Lewis AM, Chan JW, Machin CL, Sinha D, Galione A, Churchill GC. 2010. The calcium-mobilizing messenger nicotinic acid adenine dinucleotide phosphate participates in sperm activation by mediating the acrosome reaction. J Biol Chem 285: 18262–18269. 10.1074/jbc.M109.087858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Nakashima T, Hiroshi N, Penninger JM. 2006. RANKL-RANK signalling in osteoclastogenesis and bone disease. Trends Mol Med 12: 17–25. 10.1016/j.molmed.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong X, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. 2012. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151: 372–383. 10.1016/j.cell.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SE, Miller AL. 2011. Visualization of Ca2+ signaling during embryonic skeletal muscle formation in vertebrates. Cold Spring Harb Perspect Biol 3: a004325 10.1101/cshperspect.a004325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. 2001. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci 98: 11024–11031. 10.1073/pnas.191352298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavropoulou MP, Yovos JG. 2008. Osteoclastogenesis—Current knowledge and future perspectives. J Musculoskelet Neuronal Interact 8: 204–216. [PubMed] [Google Scholar]
- Yoshida H, Hayashi SI, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa SI. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345: 442–444. 10.1038/345442a0 [DOI] [PubMed] [Google Scholar]
- Zamora S, Rahman IA. 2014. Deciphering the early evolution of echinoderms with Cambrian fossils. Paleontol 57: 1105–1119. 10.1111/pala.12138 [DOI] [Google Scholar]
- Zhang ZH, Lu YY, Yue J. 2013. Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells. PLoS ONE 8: e66077 10.1371/journal.pone.0066077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans M. 2010. Calcium signaling via two-pore channels: Local or global, that is the question. Am J Physiol Cell Physiol 298: C430–C441. 10.1152/ajpcell.00475.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. 2009. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pfligers Arch 458: 891–899. 10.1007/s00424-009-0690-y [DOI] [PMC free article] [PubMed] [Google Scholar]