Abstract
Background
The cognitive consequences and risk factors based long-term outcome of very-low-birth-weight (VLBW; < 1,500 g) infants in Korea has not been studied. The aim of this study was to determine the influence of perinatal and neonatal risk factors on the cognitive performance of VLBW children at 3 to 5 years of age.
Methods
We enrolled 88 VLBW infants without cystic periventricular leukomalacia for the assessment of their demographic data, cognitive performance, and development of cerebral palsy (CP) at 3 to 5 years of age. Cognitive performance was assessed using the Korean version of the Wechsler Preschool and Primary Scale of Intelligence IV. Growth data were assessed with measurements of weight, height, and head circumference (HC) at the corrected ages of 6, 12, and 18 months, and 3 to 5 years of age.
Results
In the VLBW group, the full-scale intelligence quotient (FSIQ) was 96.1 ± 15.2 at the mean age of 4.5 years. The incidence rate of CP was 3.4%. Overall, 17% (15/88) of the VLBW children had a below-average FSIQ (< 85). We divided the VLBW children into the abnormal FSIQ group (< 85, n = 15) and the normal FSIQ group (≥ 85, n = 73). VLBW children with intrauterine growth retardation (IUGR) was associated with a below-average FSIQ at the mean age of 4.5 years (< 85, 8/15, 53.3% vs. ≥ 85, 5/73, 6.8%; P < 0.001). After controlling for associated clinical factors, IUGR in the VLBW children was found to be associated with an abnormal FSIQ at the mean age of 4.5 years (P = 0.025). The weight, height, and HC obtained for both groups showed that normal growth was maintained at the mean age of 4.5 years with no significant difference between abnormal and normal FSIQ groups.
Conclusion
Fifteen of 88 (17%) of the VLBW children had a below-average FSIQ (< 85). VLBW with IUGR is associated with poor cognitive outcomes at the mean age of 4.5 years.
Keywords: Very-Low-Birth-Weight Infants, Cognitive Outcome, Intrauterine Growth Retardation
Graphical Abstract
INTRODUCTION
Advances in medical technologies and innovations in the management of very-low-birth-weight (VLBW; < 1.5 kg of birth weight) infants decreased neonatal mortality and morbidity through the late 1990s.1 However, compared to full-term infants, VLBW infants are prone to a range of long-term complications, such as learning disabilities, attention-deficit hyperactivity disorder, borderline mental retardation, and behavioral disorders.2 While the incidence of severe cerebral palsy (CP), blindness, and hearing impairment have decreased over time, cognitive impairments have become more prevalent sequelae in VLBW children.3 Preterm infants born at < 27 weeks of gestation have been reported to show a mean full-scale intelligence quotient (FSIQ) 14.2 points lower than controls at the age of 6.5 years.4 The greater the immaturity and the lower the birth weight, the greater the likelihood of cognitive disability in the VLBW population. Notably, a recent study reported mild cognitive disability in 30.4% of extremely preterm children, moderate cognitive disability in 18.8%, and severe cognitive disability in 11.1% of preterm children born at < 27 weeks of gestational age (GA) at the age of 6.5 years.4 Prematurity, perinatal risk factors, and environmental factors are known risk factors for neurodevelopmental impairments, especially with respect to cognitive and executive skills.5 It was suggested that the influence of extreme prematurity on cognitive disability increases over time after the age of 2.5 years, reflecting a complex relationship between perinatal and environmental factors and cognitive function.4
A recent report from the Korean Neonatal Network investigated 2 years' outcomes of Korean VLBW infants using the Bayley Scales of Infant Developmental Outcomes or the Korean Developmental Screening Test for Infants & Children from various institutions.6 While the outcome report with a large cohort size provided national initiative for VLBW's healthcare quality, the low follow-up rate for developmental assessment and heterogeneous data collection from various institutions precluded the authors from assessing the cognitive long-term outcomes adequately for VLBW infants. They recommended a further study of longer follow-up outcomes on the poor cognitive function in VLBW children. Previous studies7,8 in Korea have evaluated the neurodevelopmental outcomes of preterm preschoolers using the Korean version of the Wechsler Preschool and Primary Scale of Intelligence IV (WPPSI-IV). However, whether prematurity-related morbidity affects the poor cognitive function remains poorly understood in VLBW children at the age of 3 to 5 years.
Although premature birth in itself may adversely affect later development, insight into factors influencing cognitive outcomes is key to improving such outcomes after extremely preterm birth. The aim of this study was to determine the developmental outcomes of VLBW infants at the age of 3 to 5 years as measured by the Korean version of the WPPSI-IV and the factors associated with poor outcomes.
METHODS
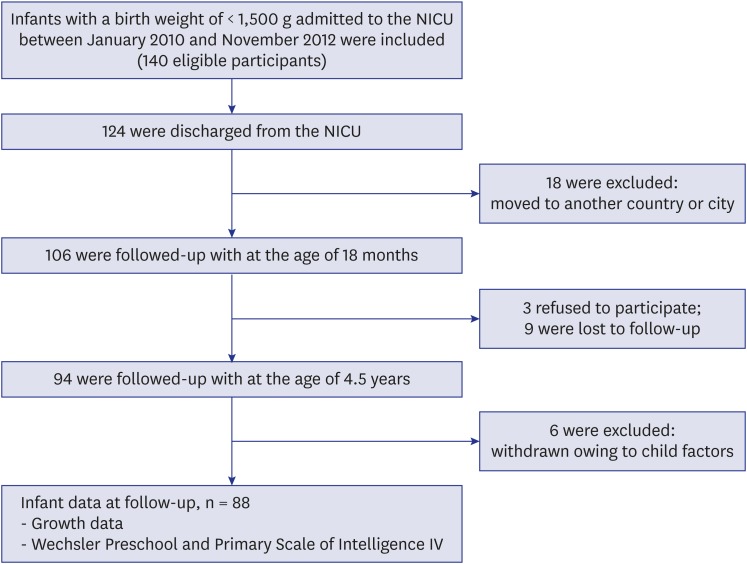
This was a prospective cohort study of VLBW infants involving a follow-up program at the Hanyang Inclusive Clinic for Developmental Disorders of the Hanyang University College of Medicine. A total of 140 infants were born and admitted to the level 3 Neonatal Intensive Care Unit at Seoul Hanyang University Hospital between November 1, 2011 and January 1, 2014. The infants received routine follow-up care at the Hanyang Inclusive Clinic for developmental checkups at 3, 6, 12, and 18 months of corrected age, and 3 to 5 years of age. The major exclusion criteria were genetic syndromes, congenital malformations, chromosomal anomalies and cystic periventricular leukomalacia, because of the increased risk of developing extensive neurological comorbidities with worse outcome even in early life. Of the infants, 124 were discharged, and eighteen of these children were living in other countries or cities. The remaining 106 patients were assessed at the follow-up clinic, at 6, 12, and 18 months of corrected age by a pediatrician who performed a detailed neurological examination and developmental checkups; all had survived to the age of 5 years. The dropout rate was 17% (18/106); the families of 3 infants refused to participate, 9 dropped out from the follow-up, and 6 were withdrawn from the study because of a factor causing the child to be unable to cooperate with testing. The remaining 83% (88/106) of subjects participated in this study at the mean age of 4.5 years (range, 3 years and 8 months to 5 years and 11 months) (Fig. 1).
Fig. 1. Study flow diagram.
NICU = neonatal intensive care unit.
At 6 and 12 months of corrected age, gross and fine motor skills, posture, tone, reflexes, ankle clonus, and spasticity were assessed by trained neonatologists and a rehabilitationist, focusing on neuromotor function. Children with abnormal muscle tone and neurological examination results were reassessed by a pediatric neurologist. Even if there were no identifiable motor delay and cognitive impairments and at the corrected age of 18 months, neurocognitive assessments including assessment of speech development were recommended for all children at 3 to 5 years of age to assess cognition, language and social functioning. Certified psychologists in the Hanyang Inclusive Clinic for Developmental Disorders assessed cognitive development for VLBW children.
Perinatal data, including maternal age, maternal education, GA, birth weight, sex, Apgar score, type of delivery, antenatal steroid use, and histological chorioamnionitis, were recorded. Intrauterine growth retardation (IUGR) was defined as any fetal growth restriction estimated below the 10th percentile of fetal weight that was either observed from serial maternal medical records or from having a birth weight below the 10th percentile based on the growth curve of Olsen et al.,9 together with an abnormal umbilical artery, such as having absent or reversed umbilical artery end-diastolic flow as assessed by fetal Doppler studies. The 3 infants with symmetric IUGR were screened for malformations and chromosomal abnormalities related to chromosomal disorders and clinical findings related to toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) infections such as hepatosplenomegaly, cardiac lesions, microcephaly, and intracranial calcifications related to IUGR. Clinical abnormalities related to TORCH such as prematurity, hearing impairment, patent ductus arteriosus, thrombocytopenia found during the neonatal period were recorded, as were neonatal sonographic findings and chromosome study results. Placental biopsy findings were gathered for all infants to identify possible causes for placental insufficiency such as placental abruption, extensive infarction, and chorioamnionitis.
We evaluated adverse clinical factors, such as respiratory distress syndrome, patent ductus arteriosus, sepsis, necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), chronic lung disease (defined as oxygen need at 36 weeks postmenstrual age), and intraventricular hemorrhage (IVH). The diagnosis of NEC was determined as modified Bell's staging criteria.10 ROP was classified in accordance with the guidelines of the Committee for Classification of ROP.11 The use of postnatal steroid was prescribed to treat or prevent bronchopulmonary dysplasia (BPD) after the first week of life. The dexamethasone given for treatment of BPD was considered as the postnatal steroid use. IVH was graded on the basis of cerebral ultrasound findings according to Volpe.12 Growth parameters (weight, length, and head circumference [HC]) were recorded at corrected age of 6, 12, and 18 months and 3 to 5 years of age. CP was defined in accordance with the definition used by Bax et al.13 and the classification proposed by the Surveillance of CP in Europe Collaborative Group. The gross motor function of the children with CP was evaluated using the Gross Motor Function Classification System (GMFCS), which is a 5-level classification system (mild CP, GMFCS level 1; moderate CP, GMFCS levels 2 to 3; and severe CP, GMFCS levels 4 to 5).14,15,16 Visual function was assessed by pediatric ophthalmologists and classified in accordance with the modified World Health Organization criteria.17 Hearing assessment findings were classified as normal, mild hearing loss without audiological intervention, and dependence on hearing aids.18
Cognitive development was assessed at the age of 3 to 5 years using the WPPSI-IV. The vocabulary comprehension intelligence quotient (IQ), visual-spatial ability IQ, and working memory IQ were calculated from the subscales. The reference mean (standard deviation [SD]) for the IQs was 100. Mild cognitive disability was defined as an FSIQ of 1 to 2 SDs below the reference mean value of 100 (i.e., 75–84 on the WPPSI-IV); moderate cognitive disability, an FSIQ of 2 to 3 SDs below the mean (i.e., 55–74); and severe cognitive disability, an FSIQ of 3 SDs below the mean (i.e., < 55). No intellectual disability was defined at an IQ of ≥ 85.19 We divided the VLBW infants into the abnormal IQ group (FSIQ of < 85, n = 15) and the normal IQ group (FSIQ of ≥ 85, n = 73), according to an FSIQ of > 1 SD below the mean. For the analyses of the clinical risk factors influencing cognitive performance, we used an IQ cutoff of < 85 to define intellectual impairment in the study infants.
Statistical analysis
All analyses were conducted using SPSS version 22 (IBM Corp., Armonk, NY, USA). Numerical data were analyzed for distribution normality using the Kolmogorov-Smirnov test and are presented as the mean ± SD. Statistical data were calculated using the Mann-Whitney U-test and Fisher's exact test for means and frequencies, respectively. The level of statistical significance was set at P < 0.05. Logistic regression analyses were performed to analyze the cognitive outcomes, accounting for GA, sex, IUGR, IVH, and maternal education. Differences in WPPSI measures among 3 subscales (vocabulary comprehension IQ, visual-spatial IQ, and working memory IQ) in abnormal IQ group were analyzed in a paired t-test and Bonferroni correction of 0.05/3 = 0.016 for multiple comparison.
Ethics statement
The study was approved by the Hanyang University Hospital Institutional Review Board (No. 201501011), and informed consent was obtained from the parents of all enrolled children to participate in the research study.
RESULTS
A total of 88 VLBW infants were assessed at a median age of 4.5 years. The mean GA and birth weight were 28.3 ± 2.8 weeks and 1,113 ± 257 g, respectively. The mean FSIQ was 96.1 ± 15.2 (range, 44–132). Overall, 15/88 (17%) of the VLBW children had a below-average FSIQ (85). Of the 88 children, 3 (3.4%: 2 moderate and 1 mild case) had CP, and 1 out of 3 children displayed impaired hearing with dependence on hearing aids in the abnormal IQ group. There were no cases of bilateral or unilateral blindness; however, 2 children demonstrated (2.3%) strabismus. The cognitive outcomes according to GA were evaluated using the FSIQ thresholds of < 55, < 75, and < 85 to define cognitive impairment. Cognitive impairment was classified as severe when the IQ was < 55, moderate at 55 to 74, and mild at 75 to 84. The number of cases of mild, moderate, and severe cognitive disabilities in the abnormal IQ group was 10, 3, and 2, respectively. The numbers of infants with cognitive impairment and its components distributed by GA are shown in Table 1.
Table 1. Neurodevelopment of very-low-birth-weight children at 4.5 years of age according to GA.
| GA, wk | No. Infants | FSIQ < 55 | FSIQ < 75 | FSIQ < 85 | CP | Hearing impaired | Bilateral blindness |
|---|---|---|---|---|---|---|---|
| 24 | 6 | 0 (0) | 1 (1.1) | 2 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| 25 | 13 | 0 (0) | 0 (0) | 2 (2.3) | 2 (2.3) | 0 (0) | 0 (0) |
| 26 | 5 | 0 (0) | 1 (1.1) | 2 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| 27 | 8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 28 | 16 | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 0 (0) |
| ≥ 29 | 40 | 1 (1.1) | 2 (2.3) | 8 (9.1) | 0 (0) | 0 (0) | 0 (0) |
| Total | 88 | 2 (2.3) | 5 (7.9) | 15 (17)) | 3 (3.4) | 1 (1.1) | 0 (0) |
Data are presented as number (%).
GA = gestational age, FSIQ = full scale intelligence quotient, CP = cerebral palsy.
The characteristics of the sociodemographic and perinatal data, along with IQ group in the VLBW infants, are shown in Table 2. In the abnormal IQ group, the mean vocabulary comprehension IQ was 81.1 ± 14.5, the visual-spatial ability IQ was 73.8 ± 15.4, the working memory IQ was 84.9 ± 21.9, and the FSIQ was 74.5 ± 13.1. Notably, the visual-spatial IQ in WPPSI was lower than vocabulary comprehension IQ (73.8 ± 15.4 vs. 81.1 ± 14.5; P = 0.134) or working memory IQ (73.8 ± 15.4 vs. 84.9 ± 21.9; P = 0.026) in the abnormal IQ group. However, difference of visual-spatial IQ and working memory IQ remained non-significant after Bonferroni correction for multiple comparisons. In the normal IQ group, the mean vocabulary comprehension IQ was 96.7 ± 11.9, the visual-spatial ability IQ was 105.6 ± 13.6, the working memory IQ was 102.0 ± 12.6, and the FSIQ was 100.6 ± 11.4. GA, sex, prenatal steroid and maternal education did not differ between the normal IQ group and the abnormal IQ group. There were significant differences in IUGR between the groups. IUGR at birth was associated with a below-average FSIQ at the mean age of 4.5 years (< 85: 8/15 [53.3%] vs. ≥ 85: 5/62 [6.8%]; P < 0.001). Ten out of the 13 IUGR cases included in the study group demonstrated the asymmetrical type of IUGR.
Table 2. Sociodemographic and perinatal characteristics.
| Variables | FSIQ < 85 (n = 15) | FSIQ ≥ 85 (n = 73) | P value | ||
|---|---|---|---|---|---|
| Social factors | |||||
| Maternal age, wk | 33.0 ± 4.1 | 33.0 ± 3.3 | 0.978 | ||
| Paternal age, wk | 37.4 ± 4.6 | 35.1 ± 3.3 | 0.142 | ||
| Maternal smoking | 0 (0.0) | 2/68 (2.9) | 1.000 | ||
| Mother's education level | 0.136 | ||||
| Middle school | 1/14 (7.1) | 2/68 (2.9) | |||
| High school | 9/14 (64.3) | 27/68 (39.7) | |||
| College degree | 4/14 (28.6) | 39/68 (57.4) | |||
| Perinatal factors | |||||
| GA, wk | 29.5 ± 4.2 | 28.2 ± 3.4 | 0.249 | ||
| Birth weight, g | 1,029.3 ± 333.4 | 1,126.0 ± 282.5 | 0.370 | ||
| Height, cm | 49.8 ± 3.2 | 50.1 ± 2.9 | 0.871 | ||
| Head circumference, cm | 34.4 ± 1.8 | 34.6 ± 1.4 | 0.847 | ||
| Male | 8 (53.3) | 36 (49.3) | 1.000 | ||
| Apgar score, 1 min | 2.7 ± 2.1 | 2.7 ± 1.8 | 0.568 | ||
| Apgar score, 5 min | 5.2 ± 2.4 | 4.8 ± 1.9 | 0.514 | ||
| PIH | 2 (13.3) | 7 (9.6) | 0.647 | ||
| GDM | 0 (0.0) | 4 (5.5) | 1.000 | ||
| C-sec | 10 (66.7) | 48 (65.8) | 1.000 | ||
| Histological chorioamnionitis | 6 (40) | 21 (28.8) | 0.539 | ||
| Prenatal steroid | 10 (66.7) | 38 (52.1) | 0.397 | ||
| IUGR | 8 (53.3) | 5 (6.8) | < 0.001 | ||
| WPPSI-IV | |||||
| Vocabulary comprehension IQ | 81.1 ± 14.5 | 96.7 ± 11.9 | < 0.001 | ||
| Visual spatial IQ | 73.8 ± 15.4 | 105.6 ± 13.6 | < 0.001 | ||
| Working memory IQ | 84.9 ± 21.9 | 102.0 ± 12.6 | 0.010 | ||
| FSIQ | 74.5 ± 13.1 | 100.6 ± 11.4 | < 0.001 | ||
| Cerebral palsy | 2 (13.3) | 1 (1.4) | 0.736 | ||
Data are presented as mean ± standard deviation or number (%).
FSIQ = full scale intelligence quotient, GA = gestational age, PIH = pregnancy induced hypertension, GDM = gestational diabetes mellitus, IUGR = intrauterine growth retardation, WPPSI-IV = Wechsler Preschool and Primary Scale of Intelligence-IV, IQ = intelligence quotient.
Table 3 shows the neonatal parameters of both groups and, although no significant differences were found, the abnormal IQ group tended towards a higher incidence of sepsis, NEC, BPD and IVH than the normal IQ group.
Table 3. Neonatal conditions.
| Conditions | FSIQ < 85 (n = 15) | FSIQ ≥ 85 (n = 73) | P value |
|---|---|---|---|
| PDA ligation | 2 (13.3) | 12 (16.4) | 1.000 |
| PDA | 9 (60) | 43 (58.9) | 1.000 |
| Sepsis | 6 (40) | 19 (26) | 0.347 |
| RDS | 11 (73.3) | 59 (80.8) | 0.498 |
| Any NEC | 5 (33.3) | 10 (13.7) | 0.123 |
| Any ROP | 4 (26.7) | 20 (27.4) | 1.000 |
| Any BPD | 9 (60) | 31 (42.5) | 0.261 |
| Ventilator, day | 14.5 ± 23.9 | 9.4 ± 12.3 | 0.670 |
| Postnatal steroid | 2 (13.3) | 6 (8.2) | 0.620 |
| IVH (grade III–IV) | 2 (13.3) | 5 (6.8) | 0.117 |
| Hearing impairment | 1 (6.7) | 0 | 0.895 |
Data are presented as mean ± standard deviation or number (%).
FSIQ = full scale intelligence quotient, PDA = patent ductus arteriosus, RDS = respiratory distress syndrome, NEC = necrotizing enterocolitis, ROP = retinopathy of prematurity, BPD = bronchopulmonary dysplasia, IVH = Intraventricular hemorrhage.
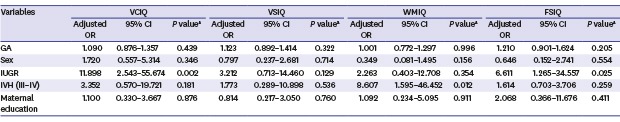
The neonatal and perinatal data that might be related to poor cognitive outcomes were analyzed (Table 4). Multiple regression analysis revealed no significant association between GA and cognitive outcomes in the VLBW children. However, after controlling for GA, sex, IVH, and maternal education, only IUGR in the VLBW children affected the cognitive outcomes, with an abnormal vocabulary comprehension IQ (95% confidence interval [CI], 2.543–55.674; P = 0.002) and FSIQ (95% CI, 1.265–34.557; P = 0.025) at the mean age of 4.5 years. Table 5 shows the details of individual clinical presentation in study infants with an abnormal FSIQ group. Table 6 shows the growth data, including the weight, height, and HC at the corrected ages of 6, 12, and 18 months, and 4.5 years of age. All average values of weight (6.6 ± 1.6 vs. 7.2 ± 1.2; P = 0.069), height (63.4 ± 5.5 vs. 63.9 ± 3.9; P = 0.751), and HC (40.4 ± 2.9 vs. 41.4 ± 2.2; P = 0.199) at 6 months in the abnormal FSIQ group were lower than those in the normal FSIQ group, although the differences were not statistically significant. Serial measurements showed that the abnormal FSIQ group reached normal weight, height, and HC by 12 months of corrected age. The growth data in 4/15 (26%) infants of the abnormal and 20/73 (27%) infants of the normal FSIQ groups had dropped at the age of 18 months, when the weight in the abnormal FSIQ group was significantly lower than those in the normal FSIQ at the age of 18 months (10.5 ± 1.4 vs. 12.3 ± 2.8; P = 0.041). However, all measurements of weight, height, and HC obtained for both groups at the mean age of 4.5 years of cognitive assessment indicated that normal growth was maintained until the mean age of 4.5 years with no significant difference between abnormal and normal FSIQ groups.
Table 4. The multivariate linear regression analysis for risk factors on FSIQ < 85.
| Variables | VCIQ | VSIQ | WMIQ | FSIQ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | P valuea | Adjusted OR | 95% CI | P valuea | Adjusted OR | 95% CI | P valuea | Adjusted OR | 95% CI | P valuea | |
| GA | 1.090 | 0.876–1.357 | 0.439 | 1.123 | 0.892–1.414 | 0.322 | 1.001 | 0.772–1.297 | 0.996 | 1.210 | 0.901–1.624 | 0.205 |
| Sex | 1.720 | 0.557–5.314 | 0.346 | 0.797 | 0.237–2.681 | 0.714 | 0.349 | 0.081–1.495 | 0.156 | 0.646 | 0.152–2.741 | 0.554 |
| IUGR | 11.898 | 2.543–55.674 | 0.002 | 3.212 | 0.713–14.460 | 0.129 | 2.263 | 0.403–12.708 | 0.354 | 6.611 | 1.265–34.557 | 0.025 |
| IVH (III–IV) | 3.352 | 0.570–19.721 | 0.181 | 1.773 | 0.289–10.898 | 0.536 | 8.607 | 1.595–46.452 | 0.012 | 1.614 | 0.703–3.706 | 0.259 |
| Maternal education | 1.100 | 0.330–3.667 | 0.876 | 0.814 | 0.217–3.050 | 0.760 | 1.092 | 0.234–5.095 | 0.911 | 2.068 | 0.366–11.676 | 0.411 |
OR = odds ratio, CI = confidence interval, GA = gestational age, VCIQ = vocabulary comprehension intelligence quotient, VSIQ = visual spatial intelligence quotient, WMIQ = working memory intelligence quotient, FSIQ = full scale intelligence quotient, IUGR = intrauterine growth retardation, IVH = intraventricular hemorrhage.
aControlling for GA, sex, IVH, and maternal education.
Table 5. Details of individual clinical presentations of infants with FSIQ < 85.
| Patients | GA, wk | Sex | BW, g | A/S | IUGR | CA | PIH | Sepsis | NEC | BPD | IVH, grade | ROP | CP | Hearing impaired | FSIQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27+2 | M | 660 | 1–3 | + | + | − | + | + | + | 2 | + | − | − | 81 |
| 2 | 30+3 | M | 910 | 1–2 | + | − | − | + | − | + | 1 | − | − | − | 84 |
| 3 | 30+1 | F | 680 | 1–3 | + | + | − | − | − | + | 1 | − | − | − | 73 |
| 4 | 28+5 | M | 640 | NA | + | − | − | + | + | + | 2 | + | − | − | 73 |
| 5 | 33+0 | M | 1,330 | 2–6 | − | − | + | − | − | − | − | − | − | − | 84 |
| 6 | 32+0 | M | 840 | 2–5 | + | + | + | + | + | − | − | + | − | − | 60 |
| 7 | 32+1 | F | 1,230 | 4–7 | + | − | − | − | + | + | 4 | − | − | − | 82 |
| 8 | 32+4 | M | 1,380 | 3–8 | − | + | − | − | − | + | − | − | − | − | 75 |
| 9 | 32+6 | M | 1,420 | 6–9 | − | − | − | − | − | − | − | − | − | − | 84 |
| 10 | 29+0 | F | 980 | 1–4 | − | + | − | − | + | + | 1 | − | − | − | 82 |
| 11 | 24+3 | F | 620 | 1–2 | − | + | − | + | − | + | 2 | + | − | − | 82 |
| 12 | 30+0 | M | 980 | 3–5 | − | − | − | − | − | − | 4 | − | + | + | 44 |
| 13 | 32+6 | F | 1,480 | 7–8 | − | − | − | − | − | − | − | − | − | − | 80 |
| 14 | 31+6 | F | 1,290 | 6–8 | + | − | − | − | − | − | − | − | − | − | 84 |
| 15 | 32+5 | F | 940 | 1–3 | + | − | − | + | − | + | − | − | − | − | 49 |
GA = gestational age, BW = birth weight, M = male, F = female, NA = not available, A/S = Apgar score, IUGR = intrauterine growth retardation, CA = histologic chorioamnionitis, PIH = pregnancy induced hypertension, NEC = necrotizing enterocolitis, BPD = bronchopulmonary dysplasia, IVH = Intraventricular hemorrhage, ROP = retinopathy of prematurity, CP = cerebral palsy, FSIQ = full scale intelligence quotient.
Table 6. Growth data for very low birth weight infant.
| Variables | 6 mon | 12 mon | 18 mon | 4.5 yr | |
|---|---|---|---|---|---|
| FSIQ < 85 | |||||
| No. | 15 | 15 | 11 | 15 | |
| Weight, kg | 6.6 ± 1.6 | 9.1 ± 1.5 | 10.5 ± 1.4a | 17.9 ± 3.4 | |
| Height, cm | 63.4 ± 5.5 | 74.9 ± 3.5 | 80.4 ± 2.8 | 108.2 ± 4.7 | |
| HC, cm | 40.4 ± 2.9 | 43.8 ± 2.1 | 45.3 ± 2.4 | 51.7 ± 1.5 | |
| FSIQ ≥ 85 | |||||
| No. | 73 | 71 | 52 | 73 | |
| Weight, kg | 7.2 ± 1.2 | 9.3 ± 1.2 | 12.3 ± 2.8 | 18.9 ± 6.8 | |
| Height, cm | 63.9 ± 3.9 | 73.2 ± 3.3 | 80.8 ± 3.8 | 110.7 ± 3.9 | |
| HC, cm | 41.4 ± 2.2 | 44.3 ± 4.7 | 46.5 ± 1.6 | 51.5 ± 3.4 | |
Data are presented as mean ± standard deviation.
FSIQ = full scale intelligence quotient, HC = head circumference.
aP < 0.05 compared with weight of FSIQ ≥ 85 group.
DISCUSSION
We analyzed data from 88 VLBW infants to investigate the cognitive outcomes and associated risk factors for poor cognitive performance at the mean age of 4.5 years. Seventeen percent (15/88) of the VLBW children had cognitive disability, with an FSIQ of 1 SD below the reference mean value. Of the 88 VLBW children, 73 (83%) had no disability, 10 had mild cognitive disability, and 5 had either moderate or severe cognitive disability at the age of 4.5 years. Except the IUGR, the sociodemographic, perinatal data and growth data did not differ between the normal IQ group and the abnormal IQ group. Our results show a strong association between IUGR and reduced cognitive performance at the mean age of 4.5 years in VLBW infants, after accounting for perinatal and environmental risk factors.
Despite the apparent progress of neonatal clinical practices, VLBW infants born in the prenatal corticosteroid and surfactant era remain at a high risk for cognitive deficits, reduced ability for sustained attention, and poor spatial working memory and school performance with the lack of clear brain injury.20,21 Serenius et al.4 showed that the FSIQ of children born in Sweden at a GA of < 27 weeks from 2004 to 2007 was 14.2 points lower than that of term infants when measured using the WPPSI-IV. They emphasized that the cognitive disability rate is better recognized and more easily detectable in older children from 2.5 to 6.5 years than in those under 2.5 years old, reflecting higher cognitive impairment rates with advancing age. Ballot et al.2 found that approximately one-third of infants were identified as being at risk for neurodevelopmental delay, however, neither birth weight nor GA predicted the neurodevelopmental outcomes. Twilhaar et al.5 emphasized that early recognition and identification of risk factors for cognitive outcomes may contribute to long-term outcomes after very preterm birth.
Previous studies have shown an association between IUGR and cognitive disability among children.22,23 IUGR, often defined as a disorder of growth and development due to decreased nutrients and oxygen supplied to the fetus, is caused by placental insufficiency during either the prenatal or perinatal period.24 In even term-born infants, the IUGR accompanied by an abnormal fetal doppler ultrasound and small GA may have a profound impact on adverse cognitive outcome. Previous study reported that 15% of children born at term with IUGR presented an abnormal cognitive performance, with an FSIQ less than 85 at the age of 9 to 10 years.22 Geva et al.23 demonstrated that term-born children with IUGR had lower IQs and marked problems including language and neuropsychologic profiles at the age of 9 years. These findings are consistent with those of previous studies showing that higher hyperactivity and conduct problems at the school-going age are associated with IUGR.25 Meta-analysis and systematic review have already shown the associations between IUGR in children born at term and neurodevelopmental outcomes in school-age children.26
A previous study on small-for-GA (SGA) infants born at a GA of < 27 weeks also showed a strong association with adverse cognitive disability on the Bayley Scales of Infant and Toddler Development-Third Edition (BSID-III) compared to the non-SGA group.27 However, it is important to distinguish between infants who are constitutionally SGA in birth weight and those who have growth restrictions due to pathological conditions, such as congenital, genetic, inflammatory, and infectious diseases.24 A systematic review of a total of 16 studies has reported that preterm birth with IUGR is associated with significant neonatal morbidity and mortality and poor neurodevelopmental outcomes within 6 months to 3 years compared with appropriate-for-GA (AGA) birth according to the BSID-III and WPPSI-IV.24 However, these studies on preterm infants with IUGR were limited by their small sample size and have shown conflicting results regarding long-term neurodevelopmental outcomes.27,28
The present study demonstrates the adverse effects on cognitive function of IUGR in VLBW children at the mean age of 4.5 years. IUGR birth in VLBW infants was associated with lower cognitive scores at a mean age of 4.5 years, reflecting the developmental vulnerability with the combined effects of IUGR and extreme prematurity. These findings are consistent with Sung et al.29 who showed that FSIQ of VLBW infants with IUGR was lower than that of VLBW infants without IUGR. Furthermore, preterm infants with lower GA may be more vulnerable to long-term outcomes with regard to cognitive development than preterm infants with higher GA. Compared with term-born infants with IUGR, preterm infants with IUGR are at higher risk of perinatal and postnatal complications, such as premature birth, IVH, lung disease and sepsis. Previous studies reported that VLBW infants with IUGR carry a significantly higher risk of long-term cognitive sequelae compared with term infants with IUGR, after adjustment for perinatal and neonatal morbidities.30,31 The primary mechanisms underlying the association between IUGR and subsequent cognitive outcomes may depend on additional risks to morbidity, such as respiratory distress syndrome, sepsis, and chronic lung disease, but these require further investigation.5 Another factor implicated in the pathogenesis of both IUGR and cognitive dysfunction in VLBW infants is hypoxia caused by placental insufficiency, which affects the gray matter despite the “brain-sparing” effect.32 IUGR has consequences on the developing brain associated with long-term impairments in both functional and structural changes, affecting white matter microstructure alterations and gray matter differences.33,34 Tolsa et al.35 described a significant reduction in intracranial volume and cerebral cortical gray matter in preterm newborns with IUGR compared with AGA preterm newborns.
In summary, our study highlights long-term neurodevelopmental outcomes and the potential influence of IUGR in VLBW infants on their cognitive outcomes at the age of 4.5 years. IUGR at birth is a potential risk factor for lower cognitive performance in VLBW children at the age of 4.5 years. This finding emphasizes the need to investigate long-term cognitive delay related to IUGR in VLBW children for early interventions targeting efficient long-term cognitive performance.
ACKNOWLEDGMENTS
We greatly appreciate the secretarial assistance of Mrs. Bo-gyung Kim and the assistance of all of our colleagues at the Hanyang Inclusive Clinic for Developmental Disorders at the Hanyang University College of Medicine. We gratefully acknowledge Chairman Il-Kewon Kim of the Korea Special Therapeutic Education Center of Anyang, Republic of Korea.
Footnotes
Funding: This research was supported by the Research Program 2018 funded by the Seoul National University College of Medicine Research Foundation and the research fund of Hanyang University (HY-2015).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee HJ, Kim EK.
- Data curation: Ahn DH, Kim MJ, Park HK, Kim HS.
- Formal analysis: Lee HJ, Kim HS.
- Investigation: Lee HJ, Park HK.
- Methodology: Lee HJ.
- Software: Kim HS.
- Validation: Lee HJ, Kim EK.
- Writing - original draft: Lee HJ, Kim HS.
- Writing - review & editing: Lee HJ, Ahn DH, Kim MJ.
References
- 1.Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–1351. doi: 10.1213/ANE.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballot DE, Potterton J, Chirwa T, Hilburn N, Cooper PA. Developmental outcome of very low birth weight infants in a developing country. BMC Pediatr. 2012;12(1):11. doi: 10.1186/1471-2431-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169(12):1162–1172. doi: 10.1001/jamapediatrics.2015.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serenius F, Ewald U, Farooqi A, Fellman V, Hafström M, Hellgren K, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. 2016;170(10):954–963. doi: 10.1001/jamapediatrics.2016.1210. [DOI] [PubMed] [Google Scholar]
- 5.Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361–367. doi: 10.1001/jamapediatrics.2017.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youn Y, Lee SM, Hwang JH, Cho SJ, Kim EK, Kim EA, et al. National registry data from Korean neonatal network: two-year outcomes of Korean very low birth weight infants born in 2013–2014. J Korean Med Sci. 2018;33(48):e309. doi: 10.3346/jkms.2018.33.e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun J, Kim EK, Shin SH, Kim HS, Lee JA, Kim ES, et al. The Bayley-III adaptive behavior and social-emotional scales as important predictors of later school-age outcomes of children born preterm. Neonatal Med. 2018;25(4):178–185. [Google Scholar]
- 8.Lee SY, Min A, Lee HJ, Park H, Oh MY, Cho JH, et al. The effect of low birth weight and age on the cognitive performance of preterm preschoolers. J Korean Acad Child Adolesc Psychiatry. 2017;28(2):141–148. [Google Scholar]
- 9.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 10.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102(8):1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 12.Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7(1):56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47(8):571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 14.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 15.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–428. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42(12):816–824. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 17.Robaei D, Rose K, Ojaimi E, Kifley A, Huynh S, Mitchell P. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112(7):1275–1282. doi: 10.1016/j.ophtha.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Leversen KT, Sommerfelt K, Rønnestad A, Kaaresen PI, Farstad T, Skranes J, et al. Prediction of neurodevelopmental and sensory outcome at 5 years in Norwegian children born extremely preterm. Pediatrics. 2011;127(3):e630–8. doi: 10.1542/peds.2010-1001. [DOI] [PubMed] [Google Scholar]
- 19.Marlow N, Wolke D, Bracewell MA, Samara M EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 20.Vicari S, Caravale B, Carlesimo GA, Casadei AM, Allemand F. Spatial working memory deficits in children at ages 3–4 who were low birth weight, preterm infants. Neuropsychology. 2004;18(4):673–678. doi: 10.1037/0894-4105.18.4.673. [DOI] [PubMed] [Google Scholar]
- 21.Ni TL, Huang CC, Guo NW. Executive function deficit in preschool children born very low birth weight with normal early development. Early Hum Dev. 2011;87(2):137–141. doi: 10.1016/j.earlhumdev.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Leitner Y, Fattal-Valevski A, Geva R, Eshel R, Toledano-Alhadef H, Rotstein M, et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol. 2007;22(5):580–587. doi: 10.1177/0883073807302605. [DOI] [PubMed] [Google Scholar]
- 23.Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118(1):91–100. doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- 24.Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni R, Foran A, Alderdice FA. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015;135(1):126–141. doi: 10.1542/peds.2014-1143. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Moreno E, Fischi-Gomez E, Batalle D, Borradori-Tolsa C, Eixarch E, Thiran JP, et al. Structural brain network reorganization and social cognition related to adverse perinatal condition from infancy to early adolescence. Front Neurosci. 2016;10:560. doi: 10.3389/fnins.2016.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Chen P, Bo T, Luo K. Cognitive and behavioral outcomes of intrauterine growth restriction school-age children. Pediatrics. 2016;137(4):e20153868. doi: 10.1542/peds.2015-3868. [DOI] [PubMed] [Google Scholar]
- 27.De Jesus LC, Pappas A, Shankaran S, Li L, Das A, Bell EF, et al. Outcomes of small for gestational age infants born at <27 weeks' gestation. J Pediatr. 2013;163(1):55–60.e1-3. doi: 10.1016/j.jpeds.2012.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunavaara V, Kallankari H, Parkkola R, Haataja L, Olsén P, Hallman M, et al. Very preterm children with fetal growth restriction demonstrated altered white matter maturation at nine years of age. Acta Paediatr. 2017;106(10):1600–1607. doi: 10.1111/apa.13954. [DOI] [PubMed] [Google Scholar]
- 29.Sung IK, Vohr B, Oh W. Growth and neurodevelopmental outcome of very low birth weight infants with intrauterine growth retardation: comparison with control subjects matched by birth weight and gestational age. J Pediatr. 1993;123(4):618–624. doi: 10.1016/s0022-3476(05)80965-5. [DOI] [PubMed] [Google Scholar]
- 30.Morsing E, Asard M, Ley D, Stjernqvist K, Marsál K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127(4):e874–e882. doi: 10.1542/peds.2010-1821. [DOI] [PubMed] [Google Scholar]
- 31.Hartkopf J, Schleger F, Keune J, Wiechers C, Pauluschke-Froehlich J, Weiss M, et al. Impact of intrauterine growth restriction on cognitive and motor development at 2 years of age. Front Physiol. 2018;9:1278. doi: 10.3389/fphys.2018.01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guellec I, Lapillonne A, Renolleau S, Charlaluk ML, Roze JC, Marret S, et al. Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics. 2011;127(4):e883–e891. doi: 10.1542/peds.2010-2442. [DOI] [PubMed] [Google Scholar]
- 33.Padilla N, Falcón C, Sanz-Cortés M, Figueras F, Bargallo N, Crispi F, et al. Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res. 2011;1382:98–108. doi: 10.1016/j.brainres.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26(1):3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004;56(1):132–138. doi: 10.1203/01.PDR.0000128983.54614.7E. [DOI] [PubMed] [Google Scholar]