Abstract
Childhood adversity, such as physical, sexual, and verbal abuse, as well as neglect and family conflict, is a risk factor for schizophrenia. Such adversity can lead to disruptions of cognitive function during development, undermining intellectual capabilities and academic achievement. Schizophrenia is a neurodevelopmental disorder that is associated with cognitive impairments that may become evident during childhood. The Australian Schizophrenia Research Bank database comprises a large community cohort (N = 1169) in which we previously identified 3 distinct cognitive groups among people with schizophrenia: (1) Compromised, current, and estimated premorbid cognitive impairment; (2) Deteriorated, substantial decline from estimated premorbid function; and (3) Preserved, performing in the normal cognitive range without decline. The compromised group displayed the worst functional and symptom outcomes. Here, we extend our previous work by assessing the relationship among these categories of cognitive abilities and reported childhood adversity in 836 patients and healthy controls. Exploratory factor analysis of the Childhood Adversity Questionnaire revealed 3 factors (lack of parental involvement; overt abuse; family breakdown and hardship). People with schizophrenia reported significantly more childhood adversity than healthy controls on all items and factors. People with schizophrenia in the compromised group reported significantly more lack of parental involvement and family breakdown and hardship and lower socioeconomic status than those in the deteriorated group. The cognitive groups were not related to family history of psychosis. These findings identify specific social and family factors that impact cognition, highlighting the important role of these factors in the development of cognitive and functional abilities in schizophrenia.
Keywords: schizophrenia, cognition, childhood adversity, functional ability, development, IQ
Introduction
Childhood adversity may be defined as adverse life events which overwhelm a child’s capacity to cope,1 and may include emotional, psychological, physical or sexual abuse,2 or neglect.3 Consequences of childhood adversity include lower IQ and cognitive impairment in adulthood.1,4,5 Further, these adverse life events can lead to the development of a wide range of mental illness, including schizophrenia.6–8 A subset of people with schizophrenia display marked cognitive impairments which may have begun in childhood.9,10 There is evidence of increased risk of schizophrenia and psychotic disorders among people who have experienced childhood adversity, including a “dose–response” effect between increased frequency of child abuse, psychosis risk,11 and “positive” symptom severity.4 Compared with healthy controls, people with a psychotic illness were 15.5 times more likely to have experienced abuse; whereas, people with other psychiatric disorders were only 6.9 times more likely to have experienced abuse.12
People who have experienced childhood adversity and people with schizophrenia display similar patterns of cognitive impairment.4,13 Lower IQ scores14 and cognitive deficits in executive function, verbal, visual, and working memory are consistently observed in both cohorts.1,9,15–17 These cohorts also display impaired inhibitory capacity on go-no-go tasks18,19 and display abnormal prefrontal-striatal circuitry,20–22 which may be related to similar underlying patterns of altered brain morphology. Volume reductions in cerebral, prefrontal, hippocampal, and corpus callosum regions have been reported in people who have experienced childhood adversity,1,19,23,24 and in people with schizophrenia.25–27 McLaughlin and colleagues28 suggest that chronic stress associated with childhood adversity can disrupt brain development through differential mechanisms depending on the nature of adversity. Adversity that threatens personal bodily integrity may lead to sensitization of the hypothalamic-pituitary-adrenal (HPA) axis4,15 and to abnormalities in dopamine regulation. Adversity in the form of deprivation, such as neglect, may lead to more widespread volumetric reductions in association cortices implicated in executive function. These would be consistent with neurodevelopmental abnormalities observed in schizophrenia29 as HPA sensitization,3,15 aberrant dopamine function,18,30 and executive function deficits17have all been reported among people with schizophrenia. However, it is important to note that not all children who experience childhood adversity go on to have cognitive impairment or schizophrenia. At least one recent study suggests that childhood trauma does not negatively influence IQ in patients with psychosis.31 Certainly, not all people with schizophrenia experience early childhood adverse events or display cognitive deficits. Previously, we found that approximately 25% of people with schizophrenia display near average intellectual ability with no clear decline from their premorbid estimates (the preserved group), while approximately 50% display a marked decline from their premorbid IQ estimate (the deteriorated group). A third group of approximately 25% display impairments on measures of both premorbid and current cognitive ability, suggesting disruptions in childhood cognitive development (the compromised group).9,10
The aim of the present work was to determine the extent to which childhood adversity influences cognitive subtypes of people with schizophrenia who differ in cognitive decline from premorbid IQ to current cognitive abilities. Here, we used similar groupings derived from the application of empirical clustering procedures to cognitive variables in the Australian Schizophrenia Research Bank (ASRB) data set, to now determine the extent to which childhood adversity predicts specific cognitive subgroups of people with schizophrenia. Childhood adversity has been reported previously in the ASRB.4 However, we chose to perform new analyses given that we had access to a larger, updated ASRB data set and we aimed to test the novel question as to whether childhood adversity predicts cognitive subgroup which may reveal additional effects. Testing the relationship between childhood adversity and cognitive subgroups of patients with schizophrenia based on the presence or absence of cognitive decline from premorbid abilities could help to explain discrepant findings pertaining to the effects of childhood adversity on cognition in schizophrenia which could not be addressed by assessing the “dose–response” relationship between adversity and IQ.4
In our previous study, we reported that approximately 25% of the people with schizophrenia displaying low premorbid and current cognitive abilities (the compromised subgroup) were more likely to have worse negative symptoms and poorer general functioning than other people with schizophrenia.10 For these people, there may be environmental factors (eg, childhood adversity) that interact with other etiological factors in schizophrenia to influence early cognitive development. First, consistent with previous work,4 we hypothesized that people with schizophrenia would be more likely to have childhood adversity than controls; however, people with schizophrenia and childhood adversity would more likely fall into the compromised (low premorbid IQ and current cognitive function) cognitive subgroup. The compromised group is the only group characterized by both premorbid and current cognitive impairment. Given the relationship between childhood adversity and lower IQ, we hypothesized that the compromised group would be most likely to have experienced childhood adversity. Second, we hypothesized that a family history of psychosis would predict schizophrenia diagnosis but not cognitive subgroup membership, given that differences in premorbid IQ may be primarily driven by a variety of childhood environmental factors.
Method
Participants
Data were acquired from the ASRB, a research data bank compiled from 5 Australian states32 for 635 healthy controls and 534 people with schizophrenia. Diagnosis of schizophrenia (n = 448) or schizoaffective disorder (n = 86) was confirmed with DSM-IV criteria from the Diagnostic Interview for Psychosis (DIP).33 Exclusion criteria were a history of: organic brain disorder; serious brain injury; an IQ <70; electroconvulsive therapy or current substance dependence within the past year; or family or personal history of psychosis or bipolar 1 disorder among healthy controls. Participants were not excluded if they had posttraumatic stress disorder. A subset of the ASRB sample (N = 836), who answered questions regarding childhood adversity, was assessed in the present study. Table 1 displays demographic characteristics of the participants. Ethics approval was obtained for data collection (HNE HREC 08/12/17/5.20, HREC/08/HNE/438, SSA/09/HNE/23) and analysis (Human Research Ethics Advisory Panel of the University of New South Wales, HREAP D UNSW HC14124). All participants provided written informed consent.
Table 1.
Demographic Characteristics of Participants
| All Participants | CAQ Participants (Current Study) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Sex (% Male) | Age (Years) | Education (Years) | CAQ (Mean) | N | Sex (% Male) | Age (Years) | Education (Years) | |
| CIQ | 138 | 73.2 | 38.4 (9.7) | 10.9 (2.4) | 6.8 (4.9) | 80 | 75 | 38.6 (9.8) | 11.3 (2.2) |
| DIQ | 239 | 66.1 | 38.1 (10.1) | 13.2 (2.4) | 5.4 (4.2) | 165 | 64.2 | 38 (10.2) | 13.6 (2.4) |
| PIQ | 157 | 61.1 | 41.5 (11.4) | 14.2 (2.8) | 6.3 (4.7) | 116 | 57.8 | 41.7 (11.9) | 14.4 (2.9) |
| Controls | 635 | 44.7 | 41.9 (13.5) | 15.1 (3.1) | 2.8 (3.2) | 475 | 41.1 | 42.6 (13.3) | 15.3 (3.2) |
Note: HC, healthy control group; CAQ, Childhood Adversity Questionnaire; CIQ, compromised group; DIQ, deteriorated group; PIQ, preserved group. Means are provided with standard deviations in parentheses.
Cognitive Function
The Wechsler Test of Adult Reading (WTAR)34–36 was used to assess premorbid IQ.34–36 The WTAR (mean = 100, standard deviation = 10) is an assay of pronunciation of English words. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)37 (index scores age adjusted) is validated among people with schizophrenia.38 The RBANS Attention and Immediate Memory indexes and the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) Letter Number Sequencing (LNS) subtest (age-adjusted scaled scores35) were used as an assay of current cognitive function.
Cognitive Groups
People with schizophrenia were classified into 3 groups (ie, compromised, deteriorated, and preserved) using z-scores (based on control means and standard deviations) of WTAR, LNS, and the RBANS Attention and Immediate Memory index scores. Hierarchical cluster analysis using complete linkage and squared Euclidean distances followed by 3-group K-means clustering was applied to the variables. See our previous work10 for characterization of the preserved (PIQ), deteriorated (DIQ), and compromised (CIQ) groups.
Childhood Adversity
The Childhood Adversity Questionnaire (CAQ) is a 21-item self-report questionnaire previously used to measure childhood adversity in Australia, however, to our knowledge, the psychometric properties for this scale have not been reported.39 Items assess lack of parental affection, not having needs met, parental emotional trouble and substance use, financial hardship, family conflict and divorce or separation, and abuse, including neglect, authoritarian parenting, physical, psychological, sexual or verbal abuse, excessive physical punishment, witnessing or other abuse. In addition to the CAQ, as part of the ASRB, participants answered a number of general questions related to childhood adversity, eg, “mother smoked during pregnancy, mother drank alcohol during pregnancy, developmental problems (delayed milestones), externalising behaviour (loud and hyperactive or other behavioural problems), living in care or with guardian;” which were included in a separate analysis.
Socioeconomic Status
Area of residency (using postal codes) was used to determine each participant’s early childhood socioeconomic status (SES) based on the Index of Relative Socio-Economic Disadvantage (IRSED) created by the Australian Bureau of Statistics (2011), with a range from 0 to 100. The IRSED is a general socioeconomic index that summarizes a range of information about the economic and social conditions of people and households within an area. A low index score reflects relatively greater disadvantage (lower SES), while a high index score reflects a relative lack of disadvantage in general (higher SES).
Family History of Psychosis
Trajectories of cognitive development and psychosis are influenced by both genetic and environmental factors. Family history of psychosis may provide a rough approximation of genetic risk. In addition, premorbid and current IQ may be adversely impacted by growing up with family members who have psychosis. Thus, we tested whether cognitive group membership was independent of family history of psychosis.
Statistical Analyses
Analyses were performed in SPSS version 22, except polychoric factor analyses, which were performed in R 3.4.1. Non-dichotomous CAQ variable (father’s affection, mother’s affection, rated 0–2) items were converted into dichotomous variables indicating presence or absence of this adversity (>0 = 1) to match the scale of the other CAQ items. Conflict and tension in the household was recoded to (>1 = 1) as the lower score of 1 was endorsed by more than half of participants. All CAQ items were summed to create a total score (range 0–21). A 1-way analysis of variance (ANOVA) was used to test for group differences among healthy controls and patient cognitive groups in total CAQ score with post hoc tests to identify significant group differences (significance level P < .05, Bonferroni adjustment P < .0125).
Factor Analysis
Given the expanded sample since the previous ASRB childhood adversity study,4 we performed new factor analyses. Dimension reduction analyses were completed in 2 steps. The sample was randomly split into 2 equal samples. An exploratory ordinary least squares factor analysis with polychoric correlations and oblimin rotation was performed on sample 1. Velicer’s MAP test and parallel analysis were used to determine the number of factors to be extracted. A 3-stage robust diagonally least squared confirmatory factor analysis with polychoric correlations was performed on sample 2. Items from the resulting latent variables were summed to generate factor scores.
Group Differences in CAQ Scores
A 4-group multivariate analysis of variance (MANOVA) was performed to test for differences among groups in overall factor scores, with sex as a factor to test for effects of sex. Follow-up least significant difference (LSD) Post hoc tests of significant main effects and interactions were used to test for differences among cognitive groups and between people with schizophrenia versus healthy controls. Bonferroni corrections were applied for 3 multiple comparisons (significance level P < .05, Bonferroni adjustment P < .017) for each CAQ factor score. Tests were 1-tailed when comparing the compromised group to the other groups as our hypotheses predicted that the compromised group would report more childhood adversity based on our previous findings where people in the compromised group reported worse cognitive function, general functioning, and symptoms. Tests were 2-tailed when comparing the preserved and deteriorated groups. Where post hoc tests indicated a significant pairwise comparison in overall CAQ factor scores, logistic regression was used to determine whether group membership was predicted by individual items. Bonferroni correction was not used for each individual item as the significant difference in factor score indicated a pattern of significance in the predicted direction across items in that factor.
Demographics, Family History, and SES
Chi-square was used to test for group differences among people with schizophrenia and between people with schizophrenia and controls for noncontinuous demographic items relating to childhood adversity, Bonferroni corrected for each variable (significance level P < .05, Bonferroni adjustment P < .01, 1-tailed). A weighted family history of psychosis variable was calculated such that parental history or sibling history = .5, uncle, aunt, or grandparent = .25. Logistic regression was conducted to test whether group membership (patient cognitive groups or people with schizophrenia compared with controls) was related to family history of psychosis. A 1-way between groups ANOVA was used to test for group differences in SES among the schizophrenia cognitive subgroups, with LSD post hoc tests to follow up significant effects (significance level P < .05, Bonferroni adjustment P < .017, 1-tailed).
Results
Demographic characteristics and total CAQ scores are presented in table 1. See our previous report10 for a detailed description of participant characteristics. Given that a subset of participants answered the CAQ, demographic analysis was run again only including those participants. Patterns of group differences in the demographic factors of age, education, and sex were the same as those described in our previous report10 (table 1).
People with schizophrenia endorsed significantly more childhood adversity items than healthy controls, t(834) = 14.46, P < .001, reporting 6.0 adverse events on average, while the mean for healthy controls was 2.8. Patients in the compromised group reported significantly more adversity overall than patients in the deteriorated group, t(243) = 3.3, P = .009. There were no other significant differences among the schizophrenia cognitive subgroups in relation to childhood adversity.
Factor Analysis
There were no significant differences between the 2 randomly split samples in age, years of education, biological sex, total CAQ score or proportions from each cognitive group (supplementary table S1).
Exploratory Factor Analysis
Velicer’s MAP test and visual inspection of the scree plot indicated 3 factors, while parallel analysis suggested 8. An oblimin rotated 3-factor solution was performed which accounted for 66% of the variance. Factor 1 (25% of the variance) included items related to overt abuse. Factor 2 (23% of the variance) included items related to lack of parental care and involvement. Factor 3 (18% of the variance) included items related to family dysfunction, breakdown, and hardship (table 2).
Table 2.
Childhood Adversity Questionnaire Exploratory and Confirmatory Factor Analyses
| Exploratory Factor Analysis | Confirmatory Factor Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAQ items | Factor Loadings | ||||||||
| 1 | 2 | 3 | B | Beta | SE | Z Score | Sig. | Factor Covariances | |
| Total CAQ | |||||||||
| Factor 1—Overt abuse | |||||||||
| I had a strict, authoritarian or regimented childhood | 0.83 | −0.06 | −0.27 | 1.00 | 0.52 | 0.06 | 8.88 | <0.010 | Abuse - neglect beta = 0.89, SE = 0.03, z = 34.73, P = .02 Abuse - breakdown beta = 0.74, SE = 0.05, z = 16.34, P = .02 |
| I was verbally abused by a parent | 0.69 | 0.24 | 0.14 | 1.79 | 0.93 | 0.02 | 42.55 | <0.011 | |
| I suffered humiliation, ridicule, bullying or mental cruelty from a parent | 0.64 | 0.29 | 0.15 | 1.82 | 0.94 | 0.02 | 41.80 | <0.012 | |
| I witnessed physical or sexual abuse of others in family | 0.68 | −0.11 | 0.48 | 1.60 | 0.83 | 0.04 | 20.78 | <0.013 | |
| I was physically abused by a parent | 0.84 | 0.08 | 0.05 | 1.69 | 0.87 | 0.03 | 28.30 | <0.014 | |
| I received too much physical punishment | 0.84 | 0.19 | −0.07 | 1.73 | 0.90 | 0.03 | 31.90 | <0.015 | |
| Factor 2—Lack of care | |||||||||
| No fatherly affectiona | 0.11 | 0.59 | 0.06 | 0.66 | 0.55 | 0.06 | 8.97 | <0.002 | Lack of care - breakdown beta = 0.85, SE = 0.04, z = 21.75, P = .02 |
| No maternal affectiona | 0.03 | 0.95 | −0.24 | 0.78 | 0.65 | 0.06 | 10.56 | <0.003 | |
| I did not have a happy childhood | 0.34 | 0.60 | 0.08 | 0.99 | 0.83 | 0.04 | 23.12 | <0.004 | |
| Mother suffered nervous, emotional trouble or depression | 0.11 | 0.45 | 0.33 | 0.75 | 0.62 | 0.06 | 11.24 | <0.007 | |
| Parents did not do their best for me | 0.33 | 0.53 | 0.11 | 1.00 | 0.83 | 0.05 | 16.56 | <0.001 | |
| I was neglected | 0.26 | 0.58 | 0.25 | 1.02 | 0.85 | 0.04 | 21.43 | <0.005 | |
| I did not have a normal upbringing | 0.26 | 0.57 | 0.28 | 1.15 | 0.96 | 0.02 | 46.02 | <0.006 | |
| I was sexually abused by a parent | 0.17 | 0.44 | 0.4 | 0.99 | 0.82 | 0.06 | 14.77 | <0.008 | |
| I experienced another type of mistreatment | 0.31 | 0.39 | 0.31 | 0.94 | 0.78 | 0.05 | 16.97 | <0.009 | |
| Factor 3—Family breakdown and hardship | |||||||||
| Father suffered nervous, emotional trouble, or depression | 0.15 | −0.02 | 0.66 | 1.00 | 0.63 | 0.06 | 10.28 | <0.016 | |
| Father had trouble with drinking or drugs | −0.28 | 0.19 | 0.78 | 0.69 | 0.43 | 0.07 | 6.14 | <0.017 | |
| Parents divorced or permanently separated in childhood | 0.23 | −0.27 | 0.77 | 0.68 | 0.43 | 0.07 | 6.07 | <0.018 | |
| Mother had trouble with drinking or drugs | −0.24 | 0.48 | 0.48 | 0.89 | 0.56 | 0.08 | 6.60 | <0.021 | |
| Conflict or tension in the householdb | 0.35 | 0.35 | 0.44 | 1.56 | 0.98 | 0.04 | 23.00 | <0.020 | |
| I grew up in poverty or financial hardship | 0.22 | 0.14 | 0.36 | 0.80 | 0.50 | 0.07 | 7.40 | <0.019 |
Note: Bold values in table 2 indicate that the given item loads on the factor displayed in the column.
aParent who was not at all affectionate or no parental figure.
bA lot of conflict or tension
Confirmatory Factor Analysis
The data fitted the model, replicating the factor structure observed in the exploratory factor analysis. The comparative fit index (CFI = 0.979), Tucker–Lewis Index (TLI = 0.976) and root mean square error or approximation (RMSEA = 0.038, CI = 0.033–0.042) were all within ranges specified to indicate good or excellent fit.40 The chi-square was large and highly significant, = 427.27, P < .001. Loadings on each latent factor are provided in table 2.
Differences Between People With Schizophrenia and Controls in Childhood Adversity
There were significant differences between people with schizophrenia and controls for all factors and for each individual variable (table 3). Additional items relating to childhood adversity indicated that relative to controls, a greater proportion of people with schizophrenia reported having a mother who drank alcohol during pregnancy, developmental problems, externalizing behavior, and living in care or with a guardian (table 4).
Table 3.
Childhood Adversity Questionnaire Differences Among Cognitive Groups and by Diagnosis
| HC | CIQ | DIQ | PIQ | All Groups | Gender | HC vs patients | CIQ vs DIQ | CIQ vs PIQ | DIQ vs PIQ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAQ Item | Statistic/Value | Sex | F | P | F | P | P | P c | P | P | ||||
| Total CAQ | Mean | M | 2.4 | 6.1 | 4.8 | 5.4 | 60 | <.001 | 24.7 | <.001 | <.001 | .004 c | .200 | .049 |
| F | 2.9 | 8.8 | 6.5 | 7.5 | ||||||||||
| Factor 1—Overt abuse | Mean | M | 0.7 | 1.5 | 1.5 | 1.5 | 32.4 | <.001 | 22.7 | <.001 | <.001 | .228c | .24 | .093 |
| F | 0.8 | 2.6 | 1.9 | 2.7 | ||||||||||
| χ2 | P | χ2 | P | |||||||||||
| I had a strict, authoritarian or regimented childhood | % | M | 24 | 43 | 38 | 41 | 31.6 | <.001 | 0.1 | .732 | <.001 | |||
| F | 28 | 48 | 48 | 58 | ||||||||||
| I was verbally abused by a parent | % | M | 16 | 31 | 39 | 34 | 62.8 | <.001 | 3.5 | .06 | <.001 | |||
| F | 13 | 40 | 35 | 46 | ||||||||||
| I suffered humiliation, ridicule, bullying or mental cruelty from a parent | % | M | 8 | 29 | 26 | 26 | 69.3 | <.001 | 0.4 | 0.521 | <.001 | |||
| F | 11 | 40 | 33 | 48 | ||||||||||
| I witnessed physical or sexual abuse of others in family | % | M | 6 | 11 | 11 | 14 | 19.8 | <.001 | 12.5 | <.001 | <.001 | |||
| F | 11 | 36 | 25 | 35 | ||||||||||
| I was physically abused by a parent | % | M | 9 | 19 | 17 | 22 | 32.3 | <.001 | 1.4 | .241 | <.001 | |||
| F | 11 | 36 | 29 | 37 | ||||||||||
| I received too much physical punishment | % | M | 9 | 20 | 15 | 18 | 38.9 | <.001 | 1.6 | .209 | <.001 | |||
| F | 9 | 36 | 25 | 44 | ||||||||||
| Factor 2—Lack of care | F | P | F | P | ||||||||||
| Mean | M | 0.7 | 3.1 | 2.3 | 2.8 | 52 | <.001 | 21.4 | <.001 | <.001 | .003 c | .421 | .035 | |
| F | 0.6 | 2.2 | 1.5 | 1.8 | ||||||||||
| χ2 | P | χ2 | P | |||||||||||
| No fatherly affectiona | % | M | 20 | 30 | 24 | 31 | 24.6 | <.001 | 3.9 | .046 | <.001 | .03 c | ||
| F | 13 | 41 | 22 | 30 | ||||||||||
| No maternal affectiona | % | M | 4 | 17 | 8 | 10 | 21 | <.001 | 4.5 | .034 | <.001 | .080c | ||
| F | 7 | 20 | 19 | 25 | ||||||||||
| I did not have a happy childhood | % | M | 8 | 32 | 26 | 27 | 73 | <.001 | <0.1 | .984 | <.001 | .040 c | ||
| F | 11 | 52 | 30 | 46 | ||||||||||
| Mother suffered nervous, emotional trouble or depression | % | M | 10 | 40 | 31 | 34 | 48.7 | <.001 | 5.1 | .024 | <.001 | .880c | ||
| F | 23 | 36 | 51 | 51 | ||||||||||
| Parents did not do their best for me | % | M | 4 | 8 | 8 | 14 | 38.7 | <.001 | 0.8 | .362 | <.001 | .890c | ||
| F | 3 | 20 | 19 | 29 | ||||||||||
| I was neglected | % | M | 5 | 25 | 11 | 14 | 56.3 | <.001 | 0.3 | .593 | <.001 | .010 c | ||
| F | 4 | 32 | 22 | 29 | ||||||||||
| I did not have a normal upbringing | % | M | 6 | 29 | 31 | 32 | 100.9 | <.001 | 0.3 | .604 | <.001 | .190c | ||
| F | 11 | 40 | 51 | 42 | ||||||||||
| I was sexually abused by a parent | % | M | 1 | 8 | 3 | 2 | 24.9 | <.001 | 5.1 | .025 | <.001 | .040 c | ||
| F | 2 | 20 | 10 | 15 | ||||||||||
| I experienced another type of mistreatment | % | M | 4 | 25 | 16 | 27 | 87.4 | <.001 | 1.3 | .264 | <.001 | .112c | ||
| F | 4 | 36 | 30 | 27 | ||||||||||
| Factor 3—Family breakdown and hardship | F | P | F | P | ||||||||||
| Mean | M | 1.2 | 2.7 | 2.1 | 2.5 | 40.2 | <.001 | 6.6 | .01 | <.001 | .002 c | .036 | .299 | |
| F | 1.6 | 3.2 | 2.6 | 2.5 | ||||||||||
| χ2 | P | χ2 | P | |||||||||||
| Father suffered nervous, emotional trouble or depression | % | M | 10 | 37 | 36 | 38 | 70.4 | <.001 | 0.2 | .694 | <.001 | .830c | ||
| F | 16 | 40 | 44 | 39 | ||||||||||
| Father had trouble with drinking or drugs | % | M | 15 | 42 | 26 | 34 | 35.9 | <.001 | <.1 | .907 | <.001 | .020 c | ||
| F | 18 | 45 | 37 | 27 | ||||||||||
| Parents divorced or permanently separated in childhood | % | M | 16 | 40 | 29 | 34 | 32.8 | <.001 | 1.7 | .192 | <.001 | .005 c | ||
| F | 20 | 56 | 27 | 17 | ||||||||||
| Mother had trouble with drinking or drugs | % | M | 3 | 13 | 10 | 11 | 18.17 | <.001 | 1.1 | .297 | <.001 | .163c | ||
| F | 7 | 28 | 18 | 14 | ||||||||||
| Conflict or tension in the householdb | % | M | 56 | 88 | 76 | 81 | 46.9 | <.001 | 0.3 | .606 | <.001 | .044 c | ||
| F | 63 | 84 | 81 | 83 | ||||||||||
| I grew up in poverty or financial hardship | % | M | 14 | 28 | 20 | 31 | 13.4 | .004 | 5.1 | .024 | .005 | .012 c | ||
| F | 23 | 48 | 22 | 29 |
Note: HC, healthy control group; CIQ, compromised group; DIQ, deteriorated group; PIQ, preserved group. Bold font indicates significant result. Factor and total scores: results of MANOVAs for CAQ total and factor scores indicated by F tests across all group comparisons (including healthy controls and the 3 cognitive groups). Healthy controls compared with all patients combined were tested by post hoc t tests. For post hoc tests, 2-tailed significance levels are shown unless otherwise noted. Only significance values passing Bonferroni correction are bolded. Individual item scores: Results of logistic regressions for binary outcomes with odds ratio (OR) shown for each item. Percentage of participants in each group (%) endorsing each item are shown. Bonferroni correction was not used for each individual item since the significant difference in factor score was already controlled for multiple comparisons.
aParent who was not at all affectionate or no parental figure.
bA lot of conflict or tension.
cOne-tailed tests.
Table 4.
Additional Childhood Adversity Items, Socioeconomic Status and Weighted Family History of Psychosis by Diagnosis and Cognitive Group
| HC | CIQ | DIQ | PIQ | HC vs Patients | CIQ vs DIQ | CIQ vs PIQ | DIQ vs PIQ | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Adversity Item | Yes/No | % | % | % | % | P | P | P | P | |
| Mother smokes during pregnancy | Yes | 17.00 | 27.00 | 23.70 | 16.90 | .109 | ||||
| No | 83.00 | 72.00 | 76.00 | 83.10 | ||||||
| Alcohol during pregnancy | Yes | 2.50 | 12.70 | 4.70 | 2.10 | .001 | .010 | .001 | .191 | |
| No | 97.50 | 87.30 | 95.30 | 97.90 | ||||||
| Developmental problems | Yes | 3.70 | 15.90 | 10.50 | 8.70 | .015 | .144 | .066 | .552 | |
| No | 96.30 | 84.10 | 89.50 | 91.30 | ||||||
| Externalizing behavior | Yes | 9.10 | 30.50 | 13.40 | 14.40 | .001 | <.001 | .001 | .790 | |
| No | 90.90 | 69.50 | 86.60 | 85.60 | ||||||
| Live in care or with guardian | Yes | 3.30 | 16.10 | 6.70 | 9.60 | .015 | .004 | .098 | .297 | |
| No | 69.70 | 83.90 | 93.30 | 90.40 | ||||||
| HC vs CIQ | .79 | |||||||||
| Socioeconomic status | Mean (SD) | 61.10 (29.98) | 63.55 (28.84) | 60.33 (28.12) | 68.34 (26.74) | HC vs DIQ | .003 | .016 | .377 | .143 |
| All groups | F | P | HC vs PIQ | .4 | ||||||
| 3.33 | .019 | |||||||||
| Weighted family history of psychosis | Mean | 0.011 | 0.174 | 0.179 | 0.175 | OR | 940.88 | 1.07 | 1.00 | 0.95 |
| P | <.001 | .876 | .986 | .886 | ||||||
| 95% CI | 208.44–3392.30 | 0.48–2.35 | 0.46–2.22 | 0.44–2.02 |
Notes: Results of logistic regressions for binary outcomes with odds ratio (OR) shown for each item. Percentage of participants in each group (%) endorsing each item are shown. Bonferroni correction was not used for each individual item since the significant difference in factor score was already controlled for multiple comparisons.
Differences in Childhood Adversity Among Cognitive Subgroups Within Schizophrenia
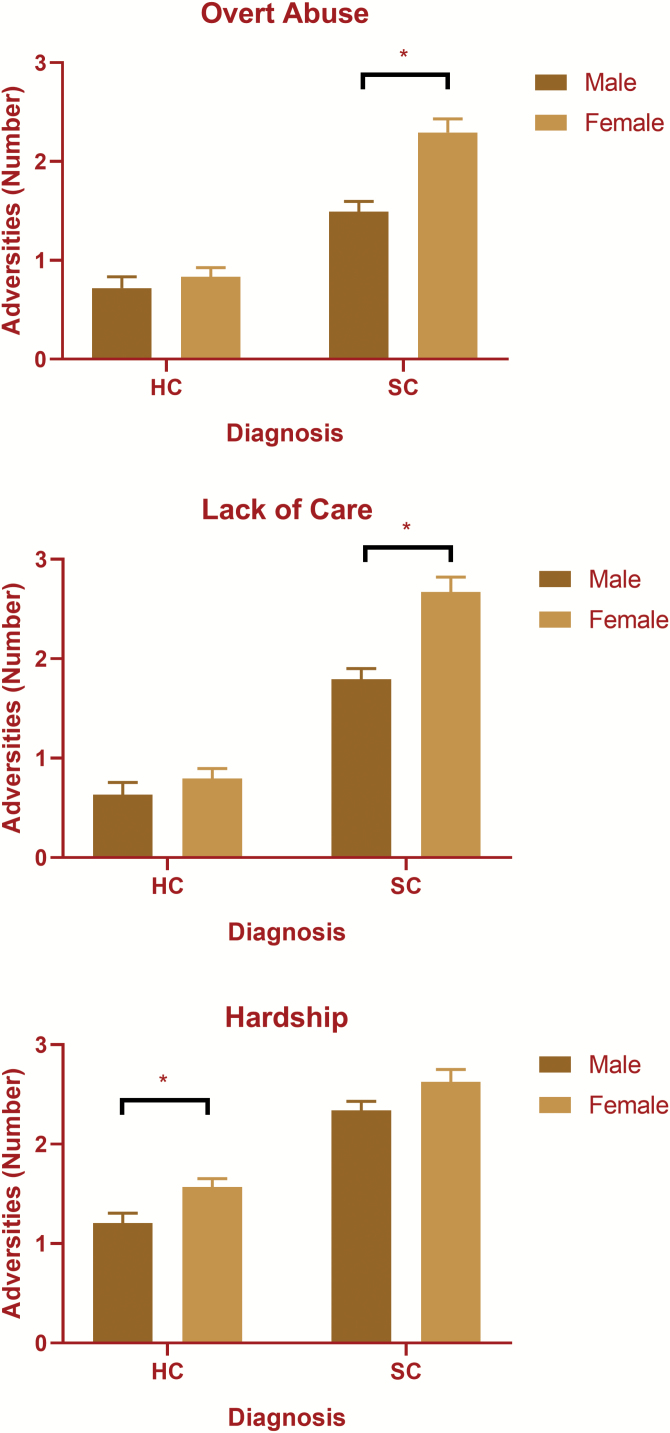
The MANOVA showed significant main effects of diagnostic/cognitive group, F(9, 2484) = 18.67, P = <.001; sex, F(3, 826) = 9.06, p = <.001; and a significant interaction between sex and diagnostic/cognitive group, F(9, 2484) = 18.67, P = .003. Females reported significantly higher CAQ scores than males on all factors (table 3 and figure 1 for means and supplementary table S2 for statistical results of sex analyses). There were significant interactions between group and sex on overt abuse, F(3, 828) = 4.184, P = .006, lack of care, F(3, 828) = 3.331, P = .019, but not family hardship, F(3, 828) = 0.725, P = .537. Follow-up interaction contrasts showed significant interactions between patients and healthy controls on all factors (P < .001 for all contrasts) with females with schizophrenia reporting significantly more childhood adversity than female healthy controls (figure 1). There were no significant interactions between sex and pairwise cognitive patient group comparisons (supplementary table S2).
Fig. 1.
Significant sex by diagnostic group interaction in the number of childhood adversities reported. *P < .05. HC, healthy controls; SC, schizophrenia; Adversities, mean CAQ score for a given factor.
The compromised group reported significantly more lack of parental care and neglect (Factor 2) than the deteriorated group but not more than the preserved group. The compromised group also reported significantly more family breakdown and hardship (Factor 3) than the deteriorated group, but not more than the preserved group. There were no significant differences among groups in overt abuse (Factor 1). Logistic regressions comparing the compromised and deteriorated groups on individual items revealed significant differences in lack of fatherly affection; not having a happy childhood; neglect; sexual abuse; father having a substance abuse problem; conflict or tension in the house; poverty and parental divorce (table 3). Experiencing parental divorce also predicted compromised group membership over the preserved group. Among other adversity items, a greater proportion of the compromised group reported having a mother who drank alcohol during pregnancy and exhibiting externalizing behavior than both the deteriorated or preserved groups (table 4). A greater proportion of the compromised group relative to the deteriorated group also reported living in care or with a guardian (table 4).
Family History of Psychosis
Participants with a greater family history of psychosis had greater odds of having a diagnosis of schizophrenia than healthy controls, OR = 940.9 (CI = 208.4–3392.3), P < .001. There were no significant differences among the 3 schizophrenia cognitive subgroups regarding family history of psychosis (table 4).
Socioeconomic Status
There was a significant overall difference in SES among the groups (table 4). Pairwise comparisons revealed that healthy controls and the compromised patient group had significantly lower SES scores than the deteriorated group. There were no other significant differences among the groups in relation to SES.
Discussion
Multiple studies have shown distinct cognitive subgroups in schizophrenia9,10,41–44 that may be related to different trajectories of cognitive development or impairment. In the present study, people with schizophrenia displaying compromised cognition based on premorbid and current estimates reported significantly greater childhood adversity than healthy controls and people with schizophrenia displaying deteriorated cognitive function. This finding is consistent with previous research highlighting the association between childhood adversity and low premorbid IQ4,14 and with evidence of detrimental long-term effects of childhood adversity on neural development,1,23 which may be mediated by other factors, such as educational disruptions and poor school attendance. In addition, low premorbid IQ is a well-established risk factor for schizophrenia.13 Participants in the preserved group did not report significantly less childhood adversity than those in other groups suggesting they may have experienced some protective factors (such as genetic factors) that prevent or limit cognitive decline, some of which were not assessed in this study (n.b., some cognitive decline may have been present in this subgroup but was not detectable given the tests available). Participants in the compromised group were also more likely to have been born in an area of lower socioeconomic status than the deteriorated group, highlighting the role of social factors in influencing both childhood cognitive development and exposure to childhood adversity. Given that the control group was also more likely to come from a low socioeconomic status background, other factors (such as genetic factor) may be influential here.
The impact observed of lack of care and neglect (Factor 2) on cognitive functioning is consistent with previous findings.29 Childhood neglect is strongly associated with cognitive impairment and detrimental psychological outcomes.5 Emotional and environmental deprivation have been consistently shown to result in altered neurodevelopment,29 as children who are neglected may rarely be given opportunities to experience the nurturing necessary to alleviate chronic stress responses. Sexual abuse may relate to neglect in that it may occur because of inadequate parental supervision.45 In addition, when inflicted by parents, sexual abuse exemplifies a lack of concern for the child’s needs.46
Consistent with other studies, reports of family breakdown and hardship (Factor 3) also defined the compromised intellect group. Family dysfunction, as evident from parental conflict and divorce can impact cognitive development47 and interrupt education.48 Financial hardship can influence cognitive development due to a lack of access to resources.49 The compromised group had lower SES than the deteriorated group, consistent with a body of research demonstrating the deleterious effect of lower SES on childhood cognitive development.50 The impacts of SES, parental mental health and substance use, family conflict and divorce on child cognitive development are complex and interconnected. What is clear, is that people with schizophrenia in the compromised group appear to have been disadvantaged at home, compared with those in the deteriorated group, including being twice as likely to be neglected and 2.3 times as likely to have been sexually abused compared with the deteriorated group.
Contrary to our hypothesis and the literature, overt abuse (Factor 1) was not related to compromised intellect in the present cohort, despite the known association between domestic violence and lower childhood IQ.14 Of note is the greatly increased occurrence of overt abuse in people with schizophrenia, who were 4.3 times as likely to experience parental cruelty and 2.3 times as likely to witness family members being abused. Overt abuse was common across all cognitive groups (eg, 33–39% for verbal abuse). While experiences of overt abuse may have impacted on the cognitive functioning of people with schizophrenia in this study, this factor did not differ among cognitive groups.
The finding that the compromised group had more experiences of living in guardianship or care, also adds to our findings of the effects of Factors 2 and 3 (ie, neglect, emotional deprivation, sexual abuse, hardship, and family dysfunction) on cognitive functioning. Inadequate or harmful parenting are likely to result in guardianship placement. Furthermore, living in guardianship or care indicates a complete breakdown of the family unit, probably leading to additional traumas due to isolation. Reports of prenatal alcohol use also accounted for compromised group membership, which is consistent with a broad range of literature on the devastating effects of prenatal alcohol exposure on cognitive development.51 Taken together with evidence that both trauma7 and impaired premorbid cognitive performance13 are risk factors for schizophrenia, programs to prevent or ameliorate these childhood adversities may positively alter trajectories of cognitive and functional outcomes for those who develop schizophrenia and may possibly prevent development of schizophrenia for others.
As predicted, greater levels of externalizing behavior were found among individuals with compromised intellect, supporting previous research and suggesting links between childhood adversity, childhood behavioral problems, and cognitive impairment52 and the later development of schizophrenia.53 Impaired emotional regulation and verbal learning capacity as a result of childhood adversity may lead children to use physical aggression more frequently when communicating their needs.5 These behavioral patterns may lead to further adversity for children who do not receive adequate support from caregivers.
This study contributes to the body of literature regarding both schizophrenia and childhood adversity which highlight the need to assess heterogeneity. The results of our factor analysis are congruent with McLaughlin and colleagues’28 conceptual framework for differential neurodevelopmental impacts of deprivation and threat-based childhood adversity. They propose that deprivation and threat constitute distinct environmental challenges leading to distinct effects on brain development. The categories they describe are broadly consistent with the factors we established in the CAQ. For example, neglect (lack of care factor) may involve high deprivation and low threat, while physical abuse (overt abuse factor) may involve low or high deprivation and high threat, and finally, poverty (family breakdown and hardship factor) may comprise a mixture of both. Based on extensive literature review, McLaughlin and colleagues predict that high threat CA will lead to hippocampal, amygdala and medial prefrontal cortex dysfunction, leading to impairments in extinction learning and heightened sensitivity to social threat cues. Social cognition was not measured in our study, however it is noteworthy that overt abuse was not different among the cognitive groups in our study. McLaughlin and colleagues further predict that lack of environmental enrichment and complexity during development will lead to widespread reductions in the volume of association cortices possibly due to synaptic pruning, leading to impairments in complex cognitive tasks requiring high cognitive control, such as those used to measure current cognitive function in our present study. Our findings indicate that exposure to different kinds of adversity can have distinct impacts on trajectories of cognitive function among people with schizophrenia. All groups reported high levels of overt abuse, while the compromised group reported higher levels of lack of care (high deprivation) and levels of hardship (mixed deprivation and threat) than the deteriorated group while also displaying widespread cognitive impairments. However, this theory would not explain why the preserved group experienced similar levels of adversity to the compromised group, but did not display these impairments. Future research could examine whether overt abuse and SES are predicitve of cognitive function, including social cognition, problem solving, and processing speed, within the cognitive trajectory patient groups.
In psychiatry, there is an emphasis on developing treatments to address the biological roots of cognitive impairment, but this may miss an important part of the broader picture. Since childhood adversity and maltreatment is common worldwide, the World Health Organization (WHO) has called for greater attention to be given to its effects and prevention.54 Many of these adversities and subsequent psychological challenges are preventable. At a time when many government services, which provide help to mothers and children seeking refuge from damaging home environments (such as women’s shelters), are not being sufficiently or consistently funded, eg, in Australia,55 these findings indicate just how damaging and costly failures to protect children from serious adversity can be to individuals and society in the long term. Of particular note is the interaction between sex and diagnosis, such that females with schizophrenia report significantly greater childhood adversity than males with schizophrenia (figure 1), indicating possible cumulative forms of marginalization (figure 1). In particular, the high rates of sexual abuse among females with schizophrenia highlight a need for a gender sensitive approach to prevention efforts. Intervention at the family level to address issues such as family conflict, can reduce emotional and behavioral problems in children exposed to adversity and may alter trajectories that could otherwise lead to serious mental health impairment.56 Given our previous findings in this cohort,10 that people in the compromised group also displayed greater symptom and functional impairments, prevention efforts could improve quality of life for people living with schizophrenia.
Limitations of our study include its cross-sectional nature. Although the WTAR is a validated measure of estimated premorbid IQ,57 an actual premorbid IQ measure may have more accurately classified people with schizophrenia. Social cognition, which was not measured in our study, may have provided further insight into the unique contributions of neglect and lack of care on social and occupational functioning. The current study also relied on subjective reporting of childhood adversities and thus, general recall bias and memory problems may have affected the accuracy of the results. The CAQ does not give information about the developmental timing or frequency of exposure, which may mediate effects on cognitive development. Corroborative information, such as school records, could provide more evidence of adversities. However, studies suggest that underreporting of childhood adversity (rather than overreporting) is more likely in retrospective studies58 and that people with schizophrenia are no more likely to make errors in reporting childhood adversity.6 Additional measurement of potential adult traumatic experiences may have assisted in disambiguating the unique role of childhood adversity. Further, the lack of significant differences in genetic liability among groups may have been due to our use of family history of psychosis to determine genetic liability rather than using specific genetic polymorphisms or performing a genome-wide association study analysis.
In conclusion, these findings in a large, community-based cohort provide evidence of the role of childhood adversity in cognitive development for a specific subgroup of people with schizophrenia. Family history of psychosis was associated with schizophrenia diagnosis but not with cognitive subgroup. Factors such as lack of parental involvement, neglect, emotional deprivation, sexual abuse, family breakdown, and hardship were more strongly associated with early compromised cognitive functioning in schizophrenia in the present cohort. Furthermore, prenatal exposure to alcohol, externalizing behavior, and living in care or in guardianship were strongly associated with a greater likelihood of early compromised cognitive functioning in schizophrenia. These findings suggest that greater attention to the prevention of early adverse environmental experiences is warranted to mitigate cognitive impairment associated with schizophrenia in a substantial subset of people with schizophrenia. They also suggest that some of the more severe impairments in cognitive function observed in schizophrenia may be related to social factors which should be included in future research examining cognitive function in schizophrenia.
Funding
This work was supported by the University of New South Wales School of Psychiatry, Neuroscience Research Australia, the Schizophrenia Research Laboratory utilizing infrastructure funding from NSW Ministry of Health Office of Health and Medical Research and the Macquarie Group Foundation, and the Australian Schizophrenia Research Bank which was supported by the Australian National Health and Medical Research Council (NHMRC), the Pratt Foundation, Ramsay Health Care, and the Viertel Charitable Foundation. An Australian NHMRC Principal Research Fellowship (#1021970) supported C. S. Weickert. An Australian NHMRC Senior Principal Research Fellowship (ID: 1117079) supported Pantelis. An Australian NHMRC Career Development Fellowship (#1127700) supported Bousman.
Supplementary Material
Acknowledgments
We thank ASRB Investigators: V. Carr, U. Schall, R. Scott, A. Jablensky, B. Mowry, P. Michie, C. Pantelis, S. Catts, F. Henskens, and C. Loughland for collecting this data set. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Authors Contributions:
Wells and Jacomb contributed to analysis design, performed the analyses, and prepared the article. C. S. Weickert, Zalesky, Weinberg, and Swaminathan contributed to analysis design, interpretation, and article preparation. Bousman, Pereira, Lenroot, Cropley, Bruggemann, and Sundram contributed to interpretation and article preparation. C. S. Weickert, T. W. Weickert and Pantelis contributed to analysis design, interpretation, and article preparation.
References
- 1. Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubino IA, Nanni RC, Pozzi DM, Siracusano A. Early adverse experiences in schizophrenia and unipolar depression. J Nerv Ment Dis. 2009;197:65–68. [DOI] [PubMed] [Google Scholar]
- 3. Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol Med. 2013;43:225–238. [DOI] [PubMed] [Google Scholar]
- 4. McCabe KL, Maloney EA, Stain HJ, Loughland CM, Carr VJ. Relationship between childhood adversity and clinical and cognitive features in schizophrenia. J Psychiatr Res. 2012;46(5):600–607. [DOI] [PubMed] [Google Scholar]
- 5. De Bellis MD, Hooper SR, Spratt EG, Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J Int Neuropsychol Soc. 2009;15(6):868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Read J, Os Jv, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112:330–350. [DOI] [PubMed] [Google Scholar]
- 7. Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. 2010;197(5):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szymanski K, Sapanski L, Conway F. Trauma and ADHD—association or diagnostic confusion? A clinical perspective. J Infant Child Adolesc Psychother. 2011;10(1):51–59. [Google Scholar]
- 9. Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–913. [DOI] [PubMed] [Google Scholar]
- 10. Wells R, Swaminathan V, Sundram S, et al. The impact of premorbid and current intellect in schizophrenia: cognitive, symptom, and functional outcomes. NPJ Schizophr. 2015;1:15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janssen I, Krabbendam L, Bak M, et al. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand. 2004;109:38–45. [DOI] [PubMed] [Google Scholar]
- 12. Bebbington PE, Bhugra D, Brugha T, et al. Psychosis, victimisation and childhood disadvantage: evidence from the Second British National Survey of Psychiatric Morbidity. Br J Psychiatry. 2004;185:220–226. [DOI] [PubMed] [Google Scholar]
- 13. Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. [DOI] [PubMed] [Google Scholar]
- 14. Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol. 2003;15:297–311. [DOI] [PubMed] [Google Scholar]
- 15. Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. 2012;46:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. [DOI] [PubMed] [Google Scholar]
- 17. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vercammen A, Weickert CS, Skilleter AJ, Lenroot R, Schofield PR, Weickert TW. Common polymorphisms in dopamine-related genes combine to produce a ‘schizophrenia-like’ prefrontal hypoactivity. Transl Psychiatry. 2014;4:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noble KG, Tottenham N, Casey B. Neuroscience perspectives on disparities in school readiness and cognitive achievement. Future Child. 2005;15: 71–89. [DOI] [PubMed] [Google Scholar]
- 20. Mueller SC, Maheu FS, Dozier M, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris RW, Vercammen A, Lenroot R, et al. Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol Psychiatry. 2012;17:235, 280–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weickert TW, Goldberg TE, Callicott JH, et al. Neural correlates of probabilistic category learning in patients with schizophrenia. J Neurosci. 2009;29:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. [DOI] [PubMed] [Google Scholar]
- 24. Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. [DOI] [PubMed] [Google Scholar]
- 25. Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2011;129:149–155. [DOI] [PubMed] [Google Scholar]
- 26. Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. [DOI] [PubMed] [Google Scholar]
- 27. Rüsch N, Spoletini I, Wilke M, et al. Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 2007;93:79–89. [DOI] [PubMed] [Google Scholar]
- 28. McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Read J, Perry BD, Moskowitz A, Connolly J. The contribution of early traumatic events to schizophrenia in some patients: a traumagenic neurodevelopmental model. Psychiatry. 2001;64:319–345. [DOI] [PubMed] [Google Scholar]
- 30. Catts VS, Fung SJ, Long LE, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Os J, Marsman A, van Dam D, Simons CJ; GROUP Investigators Evidence that the impact of childhood trauma on IQ is substantial in controls, moderate in siblings, and absent in patients with psychotic disorder. Schizophr Bull. 2017;43:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loughland C, Draganic D, McCabe K, et al. Australian schizophrenia research bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Aust N Z J Psychiatry. 2010;44:1029–1035. [DOI] [PubMed] [Google Scholar]
- 33. Castle DJ, Jablensky A, McGrath JJ, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006;36:69–80. [DOI] [PubMed] [Google Scholar]
- 34. Dalby JT, Williams R. Preserved reading and spelling ability in psychotic disorders. Psychol Med. 1986;16:171–175. [DOI] [PubMed] [Google Scholar]
- 35. Wechsler D. Wechsler Adult Intelligence Scale—Third Edition and Wechsler Memory Scale—Third Edition Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 36. Green RE, Melo B, Christensen B, Ngo LA, Monette G, Bradbury C. Measuring premorbid IQ in traumatic brain injury: an examination of the validity of the Wechsler Test of Adult Reading (WTAR). J Clin Exp Neuropsychol. 2008;30:163–172. [DOI] [PubMed] [Google Scholar]
- 37. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 38. Wilk CM, Gold JM, Humber K, Dickerson F, Fenton WS, Buchanan RW. Brief cognitive assessment in schizophrenia: normative data for the Repeatable Battery for the Assessment of Neuropsychological Status. Schizophr Res. 2004;70:175–186. [DOI] [PubMed] [Google Scholar]
- 39. Rosenman S, Rodgers B. Childhood adversity in an Australian population. Soc Psychiatry Psychiatr Epidemiol. 2004;39:695–702. [DOI] [PubMed] [Google Scholar]
- 40. Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 41. Leeson VC, Barnes TR, Harrison M, et al. The relationship between IQ, memory, executive function, and processing speed in recent-onset psychosis: 1-year stability and clinical outcome. Schizophr Bull. 2010;36:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Badcock JC, Dragović M, Waters FA, Jablensky A. Dimensions of intelligence in schizophrenia: evidence from patients with preserved, deteriorated and compromised intellect. J Psychiatr Res. 2005;39:11–19. [DOI] [PubMed] [Google Scholar]
- 43. Donohoe G, Clarke S, Morris D, et al. Are deficits in executive sub-processes simply reflecting more general cognitive decline in schizophrenia? Schizophr Res. 2006;85: 168–173. [DOI] [PubMed] [Google Scholar]
- 44. Joyce EM, Hutton SB, Mutsatsa SH, Barnes TR. Cognitive heterogeneity in first-episode schizophrenia. Br J Psychiatry. 2005;187:516–522. [DOI] [PubMed] [Google Scholar]
- 45. Finkelhor D, Hotaling G, Lewis IA, Smith C. Sexual abuse in a national survey of adult men and women: prevalence, characteristics, and risk factors. Child Abuse Negl. 1990;14:19–28. [DOI] [PubMed] [Google Scholar]
- 46. Marshall WL, Maric A. Cognitive and emotional components of generalized empathy deficits in child molesters. J Child Sex Abus. 1996;5:101–110. [Google Scholar]
- 47. Amato PR. Children of divorce in the 1990s: an update of the Amato and Keith (1991) meta-analysis. J Fam Psychol. 2001;15:355–370. [DOI] [PubMed] [Google Scholar]
- 48. Keith VM, Finlay B. The impact of parental divorce on children’s educational attainment, marital timing, and likelihood of divorce. J Marriage Fam. 1988;50: 797–809. [Google Scholar]
- 49. Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. [DOI] [PubMed] [Google Scholar]
- 50. Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31:116–126. [DOI] [PubMed] [Google Scholar]
- 52. Weinstein D, Staffelbach D, Biaggio M. Attention-deficit hyperactivity disorder and posttraumatic stress disorder: differential diagnosis in childhood sexual abuse. Clin Psychol Rev. 2000;20:359–378. [DOI] [PubMed] [Google Scholar]
- 53. Dalteg A, Zandelin A, Tuninger E, Levander S. Psychosis in adulthood is associated with high rates of ADHD and CD problems during childhood. Nord J Psychiatry. 2014;68:560–566. [DOI] [PubMed] [Google Scholar]
- 54. Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. [DOI] [PubMed] [Google Scholar]
- 55. Tarzia L, Humphreys C, Hegarty K. Translating research about domestic and family violence into practice in Australia: possibilities and prospects. Evidence & Policy: A Journal of Research, Debate and Practice. 2017;13:709–722. [Google Scholar]
- 56. McDonald R, Jouriles EN, Skopp NA. Reducing conduct problems among children brought to women’s shelters: intervention effects 24 months following termination of services. J Fam Psychol. 2006;20:127–136. [DOI] [PubMed] [Google Scholar]
- 57. Ball JD, Hart RP, Stutts ML, Turf E, Barth JT. Comparative utility of Barona Formulae, Wtar demographic algorithms, and WRAT-3 reading for estimating premorbid ability in a diverse research sample. Clin Neuropsychol. 2007;21:422–433. [DOI] [PubMed] [Google Scholar]
- 58. Widom CS, Morris S. Accuracy of adult recollections of childhood victimization, part 2: childhood sexual abuse. Psychol Assess. 1997;9:34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.