Abstract
Integrating evidence that contradicts a belief is a fundamental aspect of belief revision and is closely linked to delusions in schizophrenia. In a previous functional magnetic resonance imaging (fMRI) study on healthy individuals, we identified functional brain networks underlying evidence integration as visual attention network (VsAN; dorsal anterior cingulate cortex, insula, occipital regions), default-mode network (DMN), and cognitive evaluation network (CEN; orbitofrontal cortex, inferior frontal gyrus, parietal cortex). In the current clinical fMRI study, we compared network-based activity during evidence integration between healthy controls (n = 41), nondelusional (n = 37), and delusional (n = 33) patients with schizophrenia, and related this activity to cognitive processing involved in evidence integration measured outside the scanner. Task-induced coordinated activation was measured using group-constrained principal component analysis for fMRI. Increased VsAN activation, reduced DMN deactivation, and reduced CEN activation were observed for schizophrenia, with this pattern being most pronounced for the delusional group. Importantly, poor evidence integration comprehensively measured outside the scanner was significantly associated with increased VsAN activation and reduced DMN deactivation when processing confirmatory evidence, and with reduced CEN activation when processing disconfirmatory evidence. This is the first comprehensive study of the functional brain networks associated with evidence integration in schizophrenia and highlights how an imbalance of functional brain networks responding to confirmatory and disconfirmatory evidence may underlie delusions in schizophrenia.
Keywords: psychosis, fMRI, connectivity, fMRI-CPCA, bias against disconfirmatory evidence, hypersalience of evidence-hypothesis matches
Introduction
There is increasing evidence for the role of cognitive biases in delusions in schizophrenia.1,2 Delusion maintenance is driven by both a focus on obtaining confirmatory evidence, which reinforces the delusional belief, and avoidance of disconfirmatory evidence, which prevents rejection of the belief.1,3 One account of delusions1 puts forward that delusional individuals show a hypersalience toward evidence that aligns with their hypotheses4–6 (viz., evidence–hypothesis [EVH] matches), possibly leading to the jumping to conclusions bias consistently associated with delusions.2,4,7,8 In addition, hypersalience of EVH matches encountered on an initial piece of evidence may persist when this initial information remains somewhat plausible. This may contribute to a bias against disconfirmatory evidence (BADE) also exacerbated in individuals with delusions.2,9–11 Initial hypothesis-matching evidence may, therefore, be more salient than novel nonmatching evidence for those with delusions during complex decision-making.1 Thus, delusion maintenance may be underpinned by both hypersalience of EVH matches (tendency to endorse hypotheses for which supporting evidence is provided1) and BADE (unwillingness to revise a belief in light of evidence that contradicts it12). These cognitive biases are associated with not only delusions in schizophrenia but also delusional ideation in healthy individuals13–16 and are separable from neuropsychological functioning.13,17 However, there has been little research into their neurobiological mechanisms, which we aimed to address in this study.
We previously identified 3 sequentially active functional brain networks associated with evidence integration in healthy individuals: a volitional visual attention network (VsAN; including a version of the salience network18—dorsal anterior cingulate cortex [dACC], insula), the traditional default-mode network (DMN), and a cognitive evaluation network (CEN; rostrolateral/orbitofrontal cortex, inferior frontal gyrus (IFG), inferior parietal lobule).19 In the current clinical research, we expand this work to a study of schizophrenia and delusions, comparing delusional and nondelusional patients with schizophrenia to healthy controls. We also investigate the extent to which individual differences in measured brain activity during an evidence integration task relate to a well-established behavioral evidence integration task measured outside the scanner. In terms of brain networks, we expected to replicate our previous finding of initial VsAN activation/DMN deactivation when evidence is encountered, followed by CEN activation, initiated once evidence integration is required. In line with our previous study, we expected that all networks would show greater activity (deactivity for DMN) during disconfirmatory vs confirmatory evidence integration. Moreover, we hypothesized that (1) patients with schizophrenia would show aberrant activity in all 3 networks, particularly during disconfirmatory evidence integration; (2) this effect would be increased for delusional patients with schizophrenia; and (3) activity in these networks would be associated with comprehensive evidence integration measures collected outside the scanner.
Methods
Participants
A total of 41 healthy controls and 70 patients with schizophrenia completed an assessment and functional magnetic resonance imaging (fMRI) evidence integration task (supplementary material). Delusional and nondelusional schizophrenia groups were based on a median split of the Psychotic Symptoms Rating Scales (PSYRATS)20 delusions total score, resulting in 37 nondelusional patients (score < 11) and 33 delusional patients (score > 10). Participants provided informed consent, were cleared for MRI compatibility, and remunerated for their time. The study was approved by the University of British Columbia Clinical Research Ethics Board.
Measures
Psychotic symptoms were assessed in patients with the Scales for the Assessment of Positive/Negative Symptoms21,22 and PSYRATS20 auditory hallucinations and delusions subscales. All participants were administered the Beck Depression Inventory II,23 Test of Premorbid Functioning,24 and Wechsler Abbreviated Scale for Intelligence.25
Participants completed the behavioral evidence integration BADE task used extensively in previous work9,10,13 outside the scanner, involving rating interpretations of a delusion neutral story that unfolds over 3 sequentially presented sentences. Composite BADE evidence integration (degree to which disconfirmatory evidence is integrated) and conservatism (reduced willingness to rate high when justified) scores were computed by summing the relevant items as recommended in previous research.9,10 Eleven patients did not complete the task due to technical issues or time constraints.
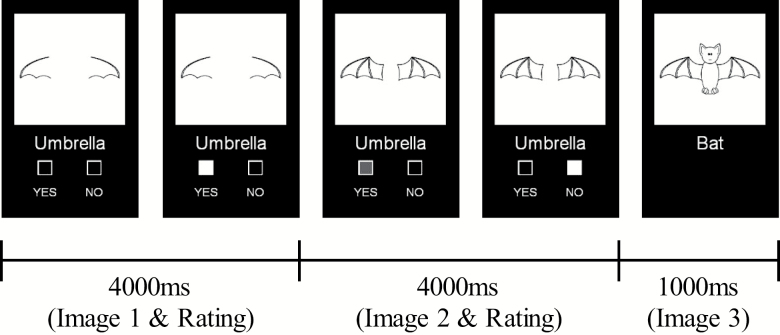
The fMRI evidence integration task was designed to capture the cognitive operations involved in integrating confirmatory and disconfirmatory evidence. Participants were presented (4000 ms) with a partial line drawing of common objects, food, or animals and responded (binary yes/no) whether they believed the full picture would be accurately described by a word displayed below the image. They were then presented (4000 ms) with a second more complete partial image and responded a second time, either changing or keeping their original response. Finally, the full picture was presented (1000 ms), which did not require a response but simply completed the trial. Figure 1 depicts experimental timing for a disconfirm trial with the picture “bat” and prompt word “umbrella” (see supplementary figure S1 for more examples). The first 2 images and response options were displayed for 4000 ms regardless of response timing, and the participant’s last response was selected as the final response for that image. Conditions were based on expected response patterns (2 responses per trial; supplementary figure S1): Yes–Yes (YY), No–No (NN), No–Yes (NY), and Yes–No (YN). YY and NN are “confirm” conditions (initial rating supported by second rating), whereas NY and YN are “disconfirm” conditions (second rating contradicts initial rating). Task development, fMRI acquisition, and preprocessing are described in detail in supplementary material.
Fig. 1.
Timing of the functional magnetic resonance imaging (fMRI) evidence integration task. Participants were presented with a partial line drawing and responded (yes/no) whether they believed the full picture would be accurately described by a prompt word. They were then presented with a second more complete image, and responded a second time, either changing or keeping their original response. Finally, the full picture was presented, which did not require a response.
Statistical Analysis
Group differences on behavioral BADE,9,10 fMRI accuracy (correct responses to both images per trial), and reaction time (RT) were examined using ANOVA and correlations were also computed. fMRI data were analyzed using group constrained principal component analysis for fMRI (group fMRI-CPCA),26,27 which identifies task-specific networks through multivariate regression of fMRI blood-oxygen-level-dependent (BOLD) signal onto a task timing model, followed by PCA on the resulting predicted scores (see supplementary material, pp. 4–5). The number of networks was determined by a scree plot28,29 and confirmed by significant poststimulus time effects using repeated-measures ANOVA (see supplementary material, p. 6). Group differences on estimated hemodynamic response (HDR) in extracted networks were examined by submitting the predictor weights (subject- and condition-specific estimates of network-based BOLD signal) to a repeated-measures ANOVA per network identified. We combined confirm (YY/NN) and disconfirm (NY/YN) conditions due to the absence of significant group × condition type (YY/NN and NY/YN) interactions.
Different cognitive processes can underlie the magnitude of the baseline-to-peak (increases) vs postpeak-to-baseline (decreases) trajectories of the task-related HDR shape reflected by the predictor weights.30,31 Therefore, for each component, using the peak averaged over all subjects as a reference, we computed 2 measures per network: one baseline-to-peak (mean estimated HDR from baseline to peak of activation, including peak) and a second post-peak measure (mean estimated HDR from the time point following peak activation to the end of trial). These computed scores were intercorrelated to study positive and negative interactions within and between functional brain networks (see supplementary material) and were correlated with symptoms and behavior (behavioral BADE scores, fMRI accuracy, and RT). Statistical tests for differences between correlations32 within and between groups33 were used to compare these relationships in conditions of interest. For the hypothesized association between reduced baseline-to-peak CEN activity and poor evidence integration measured outside the scanner, P < .05 one-tailed was used. For all other explored associations, P < .01 two-tailed was used to achieve a balance between overcorrection (eg, Bonferroni correction), and undercorrection (P = .05).
Results
Behavioural
Supplementary tables S1–S3 display demographic information, symptoms, and evidence integration performance, respectively. Significant group differences were observed on behavioral BADE evidence integration, F(2,97) = 6.42, P < .005, with delusional patients showing poorer evidence integration relative to nondelusional patients and controls, in line with previous research.9,10 BADE evidence integration was also significantly associated with PSYRATS delusions, r(58) = 0.34, P < .01, with no other symptoms reaching significance at P < .01. Finally, BADE evidence integration was significantly associated with fMRI accuracy, r(100) = –0.28, P < .01, and RT, r(58) = 0.30, P < .005, suggesting that the fMRI evidence integration task is a valid neuroimaging measure of BADE.
Poorer fMRI accuracy, F(2,108) = 4.28, P < .05, was observed in delusional (but not nondelusional, P > .06) patients vs controls (P < .05), and longer RTs, F(2,107) = 11.77, P < .001, in both delusional and nondelusional patients vs controls (P < .001 and P < .005, respectively). RT group differences were observed in confirm and disconfirm conditions (both Ps < .005); however, group differences in accuracy were only significant for disconfirm, with delusional and nondelusional patients performing more poorly than controls (both Ps < .05).
Neuroimaging
Three components (16.48%, 12.52%, 4.07% of the timing-predictable variance) showed significant main effects of poststimulus time (Ps < .001) and displayed biologically plausible and reliable HDR shapes,19,34 confirming they detected BOLD signal elicited by network-level neural task timing-related activity (see figures 2D, 3D, and 4C).
Component 1: VsAN
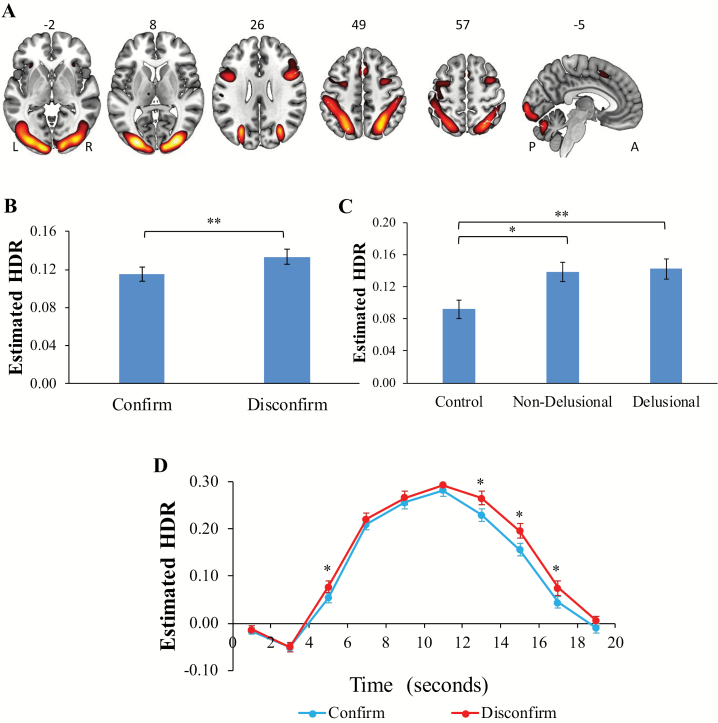
Component 1 (figure 2A/ supplementary table S4) included task-induced activations in bilateral occipital cortex (Brodmann Areas [BAs] 18,19), supramarginal gyrus (BA 40), middle frontal gyrus (BAs 6,45), dACC (BA 32), left motor cortex (BA 4), bilateral insula, thalamus, and cerebellum. Owing to the dominance of visual processing, attention, vigilance and response preparation regions,35,36 and replication of our previous work,19,37 we identified this network as VsAN.
Fig. 2.
(A) Dominant 10% of component loadings for volitional visual attention network (VsAN; Component 1: positive loadings, threshold = 0.24, max = 0.47; no negative loadings passed threshold). Montreal Neurological Institute Z-axis coordinates are displayed; left = left. (B) Estimated VsAN hemodynamic response (HDR)-main effect of condition. (C) Main effect of group. (D) Condition × time interaction. *P < .05; **P < .005; A = anterior; L = left; P = posterior; R = right. Error bars = standard errors.
There was a significant main effect of condition, F(1,108) = 8.74, P < .005, ηp2 = 0.08 (figure 2B: disconfirm > confirm), and a significant condition × poststimulus time interaction, F(9,972) = 2.30, P < .05, ηp2 = 0.02 (figure 2D: disconfirm > confirm at 5 s, 13 s, 15 s, and 17 s poststimulus, Ps < .05, ηp2s = 0.01–0.11). There was also a significant main effect of group, F(2,108) = 5.82, P < .005, ηp2 = 0.10. Delusional (P < .005, ηp2 = 0.08) and nondelusional (P < .01, ηp2 = 0.07) patients showed increased task-induced activation in this network relative to controls in both conditions (figure 2C). Although activation for delusional patients did not differ significantly from nondelusional patients, significant linear (P < .005, ηp2 = 0.08) and nonsignificant quadratic (P > .14) contrasts suggest that the nondelusional group did not deviate from a linear progression (controls < nondelusional < delusional) in terms of intensity of activation in this network.
Component 2: DMN
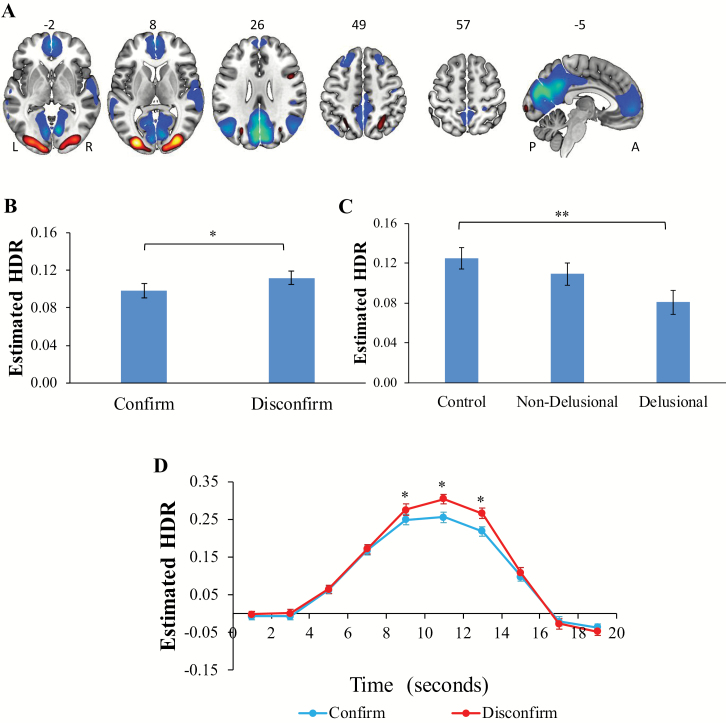
Component 2 was dominated by task-induced deactivations in bilateral precuneus (BAs 18,23), cingulate cortex (BAs 23,32), lateral temporal cortex (BAs 21,22,41,42), and ventromedial prefrontal cortex (BAs 9,10; figure 3A/supplementary table S5). It also included task-induced activations in bilateral occipital cortex (BAs 17,18,19) and superior parietal lobule (BA 7), similar to those reported previously in Component 1 (VsAN). These deactivating regions have previously been described as the DMN and show a coordinated decrease in activity in response to the performance of effortful cognitive tasks.36,38,39
Fig. 3.
(A) Dominant 10% of component loadings for default-mode network (DMN; Component 2: positive loadings, threshold = 0.19, max = 0.35; negative loadings, threshold = –0.19, min = –0.41). Montreal Neurological Institute Z-axis coordinates are displayed; left = left. (B) Estimated DMN hemodynamic response (HDR)-main effect of condition. (C) Main effect of group. (D) Condition × time interaction. *P < .05; **P < .01. A = anterior; L = left; P = posterior; R = right. Error bars = standard errors.
As with Component 1, there was a significant main effect of condition, F(1,108) = 4.43, P < .05, ηp2 = 0.04 (figure 3B: disconfirm > confirm), and a significant condition × poststimulus time interaction, F(9,972) = 5.41, P < .001, ηp2 = 0.05 (figure 3D: disconfirm > confirm at 9 s, 11 s, and 13 s poststimulus, Ps < .05, ηp2s = 0.06–0.16). A significant main effect of group was also observed (figure 3C), F(1,108) = 3.90, P < .05, ηp2 = 0.07, due to less intensity (reduced task-induced activations and deactivations) in delusional patients vs controls (P < .01, ηp2 = 0.07); nondelusional patients did not differ significantly from controls. As with the VsAN, although delusional patients did not differ significantly from nondelusional patients, significant linear (P < .01, ηp2 = 0.07), and nonsignificant quadratic (P > .6) contrasts suggest that nondelusional patients did not deviate from the linear progression (controls > nondelusional > delusional) in terms of intensity in this network. In summary, there was increased intensity in this network during disconfirmatory relative to confirmatory evidence integration at HDR peak, and delusional patients showed less intensity (reduced task-induced deactivation in DMN regions and reduced task-induced activation in visual/parietal regions) relative to controls, averaged over condition.
Component 3: CEN
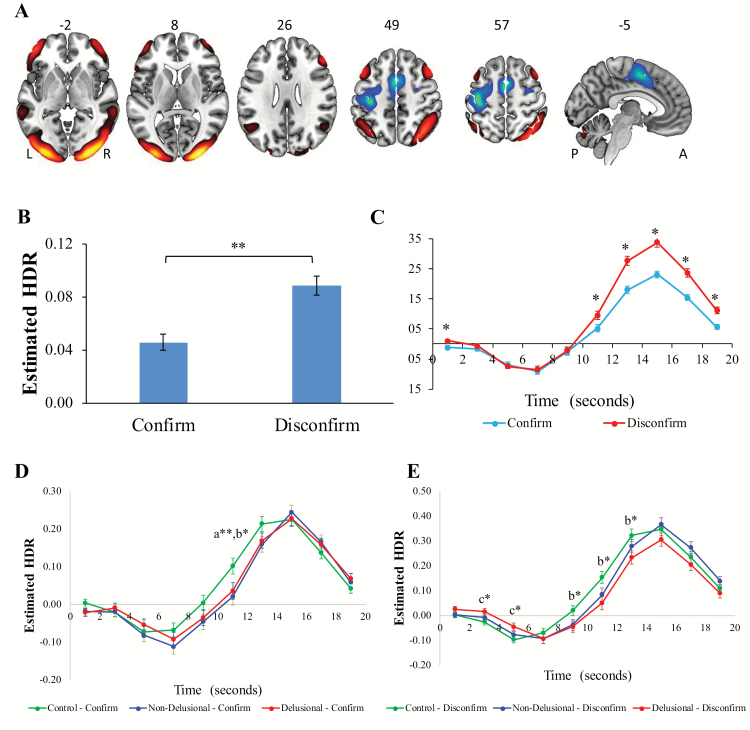
Component 3 (figure 4A/ supplementary table S6) included task-induced activations in bilateral orbitofrontal cortex (BA 38), dorsolateral prefrontal cortex (PFC; BAs 46,47), IFG, pars triangularis (BA 45), angular gyrus (BAs 39,40), middle temporal gyrus (BA 21), occipital cortex (BAs 18,19), and cerebellum. Task-induced deactivations were present in left precentral gyrus (BA 4) and bilateral supplementary motor area (BA 6). Owing to the emphasis of regions involved in higher-level cognitive processes such as evaluation,40,41 and replication of our previous work,19 we identified this network as the CEN.
Fig. 4.
(A) Dominant 10% of component loadings for cognitive evaluation network (CEN; Component 3: positive loadings, threshold = 0.11, max = 0.25; negative loadings, threshold = –0.11, min = –0.24). Montreal Neurological Institute Z-axis coordinates are displayed; left = left. (B) Estimated CEN hemodynamic response (HDR)-main effect of condition. (C) Condition × time interaction. (D) Time × group × condition interaction-confirm. (E): Time × group × condition interaction-disconfirm. a = non-delusional < controls, b = delusional < controls; c = delusional > controls; *P < .05; *P < .01. A = anterior; L = left; P = posterior; R = right. Error bars = standard errors.
There was a significant main effect of condition, F(1,108) = 69.09, P < .001, ηp2 = 0.39 (figure 4B: disconfirm > confirm), and a significant condition × poststimulus time interaction, F(9, 972) = 28.97, P < .001, ηp2 = 0.21 (figure 4C; disconfirm > confirm at 1 s, 11 s, 13 s, 15 s, 17 s, and 19 s poststimulus, Ps < .01, ηp2s = 0.07–0.45). Intensity was again higher for disconfirm vs confirm, but the effect size for condition was much greater (ηp2 = 0.39) than for the VsAN or DMN (ηp2 = 0.08, 0.04, respectively). A significant poststimulus time × condition × group interaction (figure 4D and E), F(18,972) = 2.36, P < .05, ηp2 = 0.04, was also observed, due to (1) significantly reduced intensity for nondelusional and delusional patients vs controls during confirm at 11 s (Ps < .05, ηp2 = 0.06 and 0.04) and (2) significantly reduced intensity for delusional patients vs controls during disconfirm at 3 s, 5 s, 9 s, 11 s, and 13 s (all Ps < .05, ηp2 = 0.04–0.07). Overall, these findings show that disconfirmatory vs confirmatory evidence integration led to increased task-induced activation in this network late in the trial and that patients with schizophrenia showed reduced task-induced activation relative to controls (only delusional patients differed from controls in the disconfirm condition).
Relationship to Behavioral Evidence Integration and Symptoms
Mean pre- and post-peak predictor weight values were correlated with behavioral BADE scores; increased post-peak VsAN/DMN activity in the confirm condition was associated with poorer behavioral BADE evidence integration, r(98) = 0.27, –0.27, respectively (Ps < .01). These correlations were significant for patients (VsAN: r(57) = 0.32 and DMN: r(57) = –0.33) but not controls (VsAN: r(39) = 0.01, DMN: r(39) = –0.09; group comparison P > .1). In addition, poor evidence integration was associated with reduced CEN baseline-to-peak activation in the disconfirm condition, r(98) = –0.22, P < .05. This correlation was significant in patients (r(57) = –0.27) but not controls (r(37) = –0.05; group comparison P = .29). Thus, those displaying good evidence integration showed higher CEN peak activity when faced with disconfirmatory evidence, and lower post-peak VsAN and DMN activity when processing confirmatory evidence. These associations were more strongly influenced by patients than controls, but group differences between correlations were not significant at these sample sizes. No correlations involving behavioral BADE conservatism reached significance.
For fMRI performance, increased disconfirm accuracy was associated with increased CEN disconfirm activity post-peak, r(109) = 0.28, P < .01, for both controls and patients [r(39) = 0.37, r(57) = 0.26, respectively; group comparison P = 0.28]. In addition, increased disconfirm accuracy was associated with more complete DMN confirm deactivation post-peak, r(109) = 0.25, P < .01 (the analogous association did not reach corrected significance for the VsAN, r(109) = –0.19, P < .05, but suggested that decreases in both DMN and VsAN during confirmatory evidence integration might be correlated with better accuracy during disconfirmatory evidence integration). This relationship did not differ significantly between patients and controls (r(57) = 0.23, r(39) = 0.16, respectively; group comparison, P = 73). No additional correlations with accuracy/RT or symptoms were significant (all Ps > .01).
Discussion
In this study, we observed task-induced increases in VsAN and decreases in DMN, followed by task-induced CEN increases, during evidence integration. This pattern was heightened during disconfirmatory evidence integration and was associated with effective evidence integration measured both inside and outside the scanner. Delusional patients with schizophrenia demonstrated increased VsAN activation, reduced DMN deactivation, and reduced CEN activation relative to controls, a pattern not observed in nondelusional patients. However, direct comparison of patient groups did not result in significant differences on any network, and network activity did not correlate with symptom severity. Finally, patients with poor evidence integration showed reduced CEN peak activity when processing disconfirmatory evidence and increased post-peak VsAN activity/decreased DMN deactivity when processing confirmatory evidence. Although these associations were significant for patients and not controls, the group comparison of correlation coefficients was not significant. Thus, this study demonstrates that increased VsAN activity and reduced DMN deactivity, followed by reduced CEN activity, underlie poor evidence integration in patients with schizophrenia, and this maps onto the hypersalience of EVH matches and BADE effects commonly reported in delusions.4,9,10,42 The completed and direct link between these network alterations, cognitive biases, and delusion severity in schizophrenia remains to be conclusively demonstrated, due to the absence of significant effects when directly comparing delusional to nondelusional patients.
Visual Attention Network
The VsAN (comprising a version of the salience network18) is activated when attending to salient stimuli, including the initial image and evidence presented. This is apparent in this network’s early and sustained peak (~9–13 s), and by its heightened activity during disconfirmatory evidence, due to the increased salience of evidence that contradicts a belief. This interpretation aligns with our previous study,19 the broader literature on evidence integration43,44 and functions of overlapping task-positive networks.18,45 The increased post-peak activity when confirmatory evidence is presented also accords with research on the role of hypersalience of EVH matches in delusions1; however, these findings should be considered preliminary due to the absence of significant differences between the delusional and nondelusional patient groups. Nonetheless, the VsAN overlaps with previous nonclinical research on EVH matches,46 and may underlie the hypersalience of EVH matches associated with delusions in schizophrenia.
Default-Mode Network
The DMN included activations in the VsAN and vice versa (clearer when viewing subthreshold activations), with the VsAN dominated by task-positive regions, and the DMN task-negative regions. The networks were still separable due to slightly different HDR shapes. Previous research has consistently reported reduced task-induced DMN deactivation in schizophrenia and related this to both impaired task performance and positive symptoms,38,47 as observed here. Given its involvement in stimulus-independent thought and self-referential processing,38 reduced task-induced DMN deactivation could reflect poor task engagement (consistent with impaired performance), or an impaired ability to distinguish between self/other or internal/external representations, which has been associated with positive symptoms.38,47 However, given that this is observed across various tasks and at rest in schizophrenia,46 further research is required to distinguish whether the current DMN findings reflect hypersalience of EVH matches or a more generalized impairment.
Cognitive Evaluation Network
Following VsAN salience detection, the CEN serves to evaluate the evidence and integrate it into the belief system, demonstrated by the network’s late-peaking HDR (following evidence presentation) and increased activity during disconfirmatory vs confirmatory evidence, which would be expected for a network involved in evaluation/integration processes. These findings accord with our previous study,19 despite the use of a clinical sample and different evidence integration task. They are also in line with region of interest-based research on evidence integration47 and network-based studies describing the functions of the frontoparietal network,48,49 which overlaps substantially with the CEN (eg, PFC, and IFG). Our novel findings that delusional patients with schizophrenia show reduced task-induced CEN activity relative to controls during processing of disconfirmatory evidence, and that reduced activity in this network (particularly during disconfirmatory evidence) is associated with poorer evidence integration behaviorally, suggest that reduced CEN activity may underlie the BADE associated with delusions.
Limitations
Although this study included adequately sized delusional and nondelusional groups, the influence of additional clinical characteristics (eg, delusion type, medications,50 illness onset and duration,17 and other symptoms) could not be determined. Though chlorpromazine equivalent dosage was not associated with evidence integration in the current study (see supplementary material), there is mixed evidence regarding the role of antipsychotics on cognitive biases,1 and so warrants further investigation. The absence of significant correlations between brain activity and symptoms may have been affected by low power. In addition, the VsAN included task demand and response-related brain regions (likely due to these processes occurring simultaneously) and (along with the CEN) showed early (3–5 s) HDR differences between conditions (possibly due to initial stimulus differences between conditions19; see supplementary figure S1). Careful consideration of task design and inclusion of neuroimaging techniques with high temporal resolution (eg, electroencephalography) may help distinguish between networks related to detection and integration of evidence and those related to responding or task demand.
Conclusion
Taken together, these results demonstrate that brain networks underlying evidence integration and cognitive biases show aberrant activation in schizophrenia and that these effects are stronger in delusional patients. Hypersalience of EVH matches, underpinned by increased VsAN activity/reduced DMN deactivity, might result in the initial hypothesis/evidence becoming hypersalient. Poor integration of new (disconfirmatory) evidence, underpinned by reduced CEN activity, might result in an unwillingness to reject the initial hypothesis/evidence. This corresponds to theoretical accounts of delusion maintenance in schizophrenia, which suggest important roles for both hypersalience of confirmatory evidence and avoidance of disconfirmatory evidence.1,3 Thus, this research shows, for the first time, aberrant activation of 3 functional brain networks (increased VsAN activity/reduced DMN deactivity, and reduced CEN activity) in schizophrenia that underlies cognitive biases related to delusions (hypersalience of EVH matches, BADE). These findings provide directions for future research on cognitive interventions in schizophrenia, as treatments for delusions (eg, cognitive-behavioural therapy and metacognitive training51) may exert their impact by normalizing functional brain activity in one or more of these networks.
Supplementary Material
Acknowledgments
This research was supported by the Canadian Institutes for Health Research and British Columbia Schizophrenia Society. The authors acknowledge the University of British Columbia High Field Magnetic Resonance Imaging Centre, and Devon Anderson, Kelsey Block, Sarah Flann, Jennifer Riley, Jessica Khangura, and Meighen Roes for their assistance with data collection.
References
- 1. Broyd A, Balzan RP, Woodward TS, Allen P. Dopamine, cognitive biases and assessment of certainty: a neurocognitive model of delusions. Clin Psychol Rev. 2017;54:96–106. [DOI] [PubMed] [Google Scholar]
- 2. McLean BF, Mattiske JK, Balzan RP. Association of the jumping to conclusions and evidence integration biases with delusions in psychosis: a detailed meta-analysis. Schizophr Bull. 2017;43(2):344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol. 2002;41(Pt 4):331–347. [DOI] [PubMed] [Google Scholar]
- 4. Speechley WJ, Whitman JC, Woodward TS. The contribution of hypersalience to the “jumping to conclusions” bias associated with delusions in schizophrenia. J Psychiatry Neurosci. 2010;35(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balzan R, Delfabbro P, Galletly C, Woodward T. Confirmation biases across the psychosis continuum: the contribution of hypersalient evidence-hypothesis matches. Br J Clin Psychol. 2013;52(1):53–69. [DOI] [PubMed] [Google Scholar]
- 6. Whitman JC, Menon M, Kuo SS, Woodward TS. Bias in favour of self-selected hypotheses is associated with delusion severity in schizophrenia. Cogn Neuropsychiatry. 2013;18(5):376–389. [DOI] [PubMed] [Google Scholar]
- 7. Moritz S, Woodward TS. Jumping to conclusions in delusional and non-delusional schizophrenic patients. Br J Clin Psychol. 2005;44(Pt 2):193–207. [DOI] [PubMed] [Google Scholar]
- 8. Fine C, Gardner M, Craigie J, Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007;12(1):46–77. [DOI] [PubMed] [Google Scholar]
- 9. Speechley WJ, Moritz S, Ngan ETC, Woodward TS. Impaired evidence integration and delusions in schizophrenia. J Exp Psychopathol 2012;3(4):688–701. [Google Scholar]
- 10. Sanford N, Veckenstedt R, Moritz S, Balzan RP, Woodward TS. Impaired integration of disambiguating evidence in delusional schizophrenia patients. Psychol Med. 2014;44(13):2729–2738. [DOI] [PubMed] [Google Scholar]
- 11. Luk J, Underhill K, Woodward TS. Psychotic symptoms predicting evidence integration in schizophrenia. Z Psychol. 2018;226(3):174–181. [Google Scholar]
- 12. Woodward TS, Moritz S, Cuttler C, Whitman JC. The contribution of a cognitive bias against disconfirmatory evidence (BADE) to delusions in schizophrenia. J Clin Exp Neuropsychol. 2006;28(4):605–617. [DOI] [PubMed] [Google Scholar]
- 13. Woodward TS, Buchy L, Moritz S, Liotti M. A bias against disconfirmatory evidence is associated with delusion proneness in a nonclinical sample. Schizophr Bull. 2007;33(4):1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchy L, Woodward TS, Liotti M. A cognitive bias against disconfirmatory evidence (BADE) is associated with schizotypy. Schizophr Res. 2007;90(1–3):334–337. [DOI] [PubMed] [Google Scholar]
- 15. Freeman D, Pugh K, Garety P. Jumping to conclusions and paranoid ideation in the general population. Schizophr Res. 2008;102(1–3):254–260. [DOI] [PubMed] [Google Scholar]
- 16. Lavigne KM, Menon M, Woodward TS. Functional brain networks underlying evidence integration and delusional ideation. Manuscript under review. [DOI] [PubMed]
- 17. Eisenacher S, Zink M. Holding on to false beliefs: the bias against disconfirmatory evidence over the course of psychosis. J Behav Ther Exp Psychiatry. 2017;56:79–89. [DOI] [PubMed] [Google Scholar]
- 18. Seeley WW, Menon V, Schatzberg AF, et al. . Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavigne KM, Metzak PD, Woodward TS. Functional brain networks underlying detection and integration of disconfirmatory evidence. Neuroimage. 2015;112:138–151. [DOI] [PubMed] [Google Scholar]
- 20. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29(4):879–889. [DOI] [PubMed] [Google Scholar]
- 21. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 22. Andreasen NC. Scale for the Assesment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 23. Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 24. Wechsler D. Test of Premorbid Functioning. San Antonio, TX: The Psychological Corporation; 2009. [Google Scholar]
- 25. Wechsler D. Wechsler Abbreviated Scale of Intelligence–Second Edition. San Antonio, TX: The Psychological Corporation; 2011. [Google Scholar]
- 26. Lavigne KM, Woodward TS. Hallucination- and speech-specific hypercoupling in frontotemporal auditory and language networks in schizophrenia using combined task-based fMRI data: an fBIRN study. Hum Brain Mapp. 2018;39(4):1582–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woodward TS, Tipper CM, Leung AL, Lavigne KM, Sanford N, Metzak PD. Reduced functional connectivity during controlled semantic integration in schizophrenia: a multivariate approach. Hum Brain Mapp. 2015;36(8):2948–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1(2):245–276. [DOI] [PubMed] [Google Scholar]
- 29. Cattell RB, Vogelmann S. A comprehensive trial of the scree and kg criteria for determining the number of factors. Multivariate Behav Res. 1977;12(3):289–325. [DOI] [PubMed] [Google Scholar]
- 30. Woodward TS, Leong K, Sanford N, Tipper CM, Lavigne KM. Altered balance of functional brain networks in Schizophrenia. Psychiatry Res Neuroimaging. 2016;248:94–104. [DOI] [PubMed] [Google Scholar]
- 31. Lavigne KM, Menon M, Woodward TS. Impairment in subcortical suppression in schizophrenia: evidence from the fBIRN Oddball Task. Hum Brain Mapp. 2016;37(12):4640–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raghunathan TE, Rosenthal R, Rubin DB. Comparing correlated but nonoverlapping correlations. Psychol Methods 1996;1(2):178–183. [Google Scholar]
- 33. Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron 1921;1:3–32. [Google Scholar]
- 34. Metzak P, Feredoes E, Takane Y, et al. . Constrained principal component analysis reveals functionally connected load-dependent networks involved in multiple stages of working memory. Hum Brain Mapp. 2011;32(6):856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–179. [DOI] [PubMed] [Google Scholar]
- 36. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Metzak PD. Multimodal examination of brain networks involved in attentional biasing in schizophrenia [dissertation]; 2017. [Google Scholar]
- 38. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 39. Raichle ME, MacLeod AM. A default mode of brain function. Proc Natl Acad Sci USA 2001;98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11(7):290–298. [DOI] [PubMed] [Google Scholar]
- 41. Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117(6):1161–1168. [DOI] [PubMed] [Google Scholar]
- 42. Balzan R, Delfabbro P, Galletly C, Woodward T. Reasoning heuristics across the psychosis continuum: the contribution of hypersalient evidence-hypothesis matches. Cogn Neuropsychiatry. 2012;17(5):431–450. [DOI] [PubMed] [Google Scholar]
- 43. Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27(44):11912–11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu T, Pleskac TJ. Neural correlates of evidence accumulation in a perceptual decision task. J Neurophysiol. 2011;106(5):2383–2398. [DOI] [PubMed] [Google Scholar]
- 45. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. [DOI] [PubMed] [Google Scholar]
- 46. Whitman JC, Metzak PD, Lavigne KM, Woodward TS. Functional connectivity in a frontoparietal network involving the dorsal anterior cingulate cortex underlies decisions to accept a hypothesis. Neuropsychologia. 2013;51(6): 1132–1141. [DOI] [PubMed] [Google Scholar]
- 47. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
- 48. Dosenbach NUF, Fair DA, Miezin FM, et al. . Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007;104(26):11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andreou C, Schneider BC, Braun V, Kolbeck K, Gallinat J, Moritz S. Dopamine effects on evidence gathering and integration. J Psychiatry Neurosci. 2015;40(6):422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moritz S, Woodward TS. Metacognitive training in schizophrenia: from basic research to knowledge translation and intervention. Curr Opin Psychiatry. 2007;20(6): 619–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.