Abstract
Although meta-analyses suggest that schizophrenia (SZ) is associated with a more severe neurocognitive phenotype than mood disorders such as bipolar disorder, considerable between-subject heterogeneity exists in the phenotypic presentation of these deficits across mental illnesses. Indeed, it is unclear whether the processes that underlie cognitive dysfunction in these disorders are unique to each disease or represent a common neurobiological process that varies in severity. Here we used latent profile analysis (LPA) across 3 distinct cognitive domains (cognitive control, episodic memory, and visual integration; using data from the CNTRACS consortium) to identify distinct profiles of patients across psychotic illnesses. LPA was performed on a sample of 223 psychosis patients (59 with Type I bipolar disorder, 88 with SZ, and 76 with schizoaffective disorder). Seventy-three healthy control participants were included for comparison but were not included in sample LPA. Three latent profiles (“Low,” “Moderate,” and “High” ability) were identified as the underlying covariance across the 3 domains. The 3-profile solution provided highly similar fit to a single continuous factor extracted by confirmatory factor analysis, supporting a unidimensional structure. Diagnostic ratios did not significantly differ between profiles, suggesting that these profiles cross diagnostic boundaries (an exception being the Low ability profile, which had only one bipolar patient). Profile membership predicted Brief Psychiatric Rating Scale and Young Mania Rating Scale symptom severity as well as everyday communication skills independent of diagnosis. Biological, clinical and methodological implications of these findings are discussed.
Keywords: schizophrenia, bipolar disorder, cluster analysis, schizoaffective disorder
Introduction
The comorbidity, heterogeneity, and potentially shared mechanisms that characterize psychiatric illnesses complicate efforts to understand their etiology. Cognitive deficits, eg, are characteristic of many psychiatric disorders including schizophrenia (SZ), schizoaffective disorder (SZ-A), and bipolar disorder (BD). These deficits vary in severity depending on the disease, with SZ patients often presenting with the worst dysfunction.1,2 Nonetheless, considerable heterogeneity and diagnostic overlap exist in these deficits. One study, eg, found that the majority of SZ and BD patients in a middle aged (~35 years) sample were neurocognitively indistinct from one another.3 Related to this point, a key question in psychiatry is the degree to which cognitive deficits in mental illness can be characterized as dysfunction in shared processes that vary in severity depending on the disorder. In support of this idea, the relative severity of cognitive impairment is often similar across domains in different disorders (reviewed by Barch, Sheffield4; eg, study by Reichenberg, Harvey, Bowie, Mojtabai, Rabinowitz, Heaton, Bromet5 showing the most impairment in verbal memory and least impairment in visual processing across illnesses). Conclusive evidence for a shared neurocognitive process would have major clinical implications as it would suggest a common treatment for cognitive symptoms independent of primary diagnosis. Alternatively, patterns of cognitive performance may be domain-independent and/or diagnosis-specific, suggesting a treatment targeting one domain may not effectively modulate another (depending on the disorder).
If they exist, how can shared neurocognitive processes be identified? One fruitful approach is use data-driven analyses to partition datasets into “clusters,” “classes,” or “profiles” based on the distance of each data point to the cluster mean (among other metrics). If these profiles share similar levels of performance across all features examined in the analysis, it suggests common dysfunctional mechanism that varies in severity. On the other hand, if a profile is abnormal in one feature but normal (or even enhanced) in another, it may suggest a unique mechanism that underlies pathology in that particular subset of patients.
As an illustrative example, a recent study Bora, Veznedaroglu, Vahip3 used latent class analysis (ie, latent profile analysis [LPA] as applied to continuous data) across a sample of patients with SZ or BD using data from 2 executive function tasks and 2 theory of mind (ToM) tasks as features. A 4-profile solution best fit the data, in which one profile performed at healthy levels, a second profile showed moderately impaired ToM but intact executive function, a third profile showed moderately impaired ToM and executive function, and a fourth profile showed severely impaired ToM and executive function. Substantial diagnostic overlap was observed between profiles. The finding that impaired ToM is dissociable from executive function in one profile suggests a separate mechanism for ToM deficits in this group that may persist independent of primary DSM diagnosis. The finding, however, that a dimensional structure was observed in 2 of the profiles (with moderate or severe deficits in both domains) suggests a common mechanism that may also contribute across domains in cases of moderate or greater executive dysfunction. Nonetheless, latent group-based analyses that incorporate cognitive data from across psychiatric disorders remain underutilized tools, and the extent to which dimensional and dissociable deficits exist across cognitive domains is still poorly understood.
To that end, the goal of this study was to perform LPA across a range of psychotic disorders using data from 3 cognitive domains: cognitive control, episodic memory, and visual integration (visual perception). These data were collected as part of a 5-site consortium using psychometrically optimized tasks from validated experimental cognitive paradigms.6 Healthy controls (HCs) were analyzed for comparison but not included in the clustering as we were exclusively concerned with heterogeneity in patient populations. Clinical between-profile comparisons were also performed. We also performed confirmatory factor analysis (CFA) to evaluate fit of a purely dimensional, single-factor solution relative to that of the multi-profile solutions extracted by LPA. As LPA and CFA are data-driven techniques, we did not hypothesize any particular clustering solution.
Materials and Methods
Participants
Participants were recruited as part of the CNTRACS consortium (http://cntracs.ucdavis.edu). The consortium consists of 5 sites: The University of California—Davis, The Maryland Psychiatric Research Center at the University of Maryland, Rutgers University –Robert Wood Johnson Medical School, The University of Minnesota—Twin Cities, and Washington University. The respective institutional review boards for each site approved study procedures, and informed consent was acquired for all subjects. Additional details regarding the consortium are provided in refs.6,7.
Two hundred ninety-six participants (ages 18–56) were included in the study sample—59 patients with Type I BD with a history of psychosis, 88 patients with SZ, 76 patients with SZ-A, and 73 HC participants. Exclusion criteria included head trauma, diagnosis of mental retardation or a pervasive developmental disorder, substance dependence in the past 6 months or substance abuse in the past month, and failed drug and alcohol urine screen on the day of testing. All patients were medication stable in the month leading up to test day and were either stable outpatients or had partial hospital status. All HCs had no history of SZ, SZ-A, BD, depression, or any other psychotic disorder and were not taking psychotropic or cognitive-enhancing drugs. Clinical assessments were performed or supervised by Master’s level clinicians and all raters achieved agreement with “gold” standard ratings (see ref.7 for details). Clinical ratings scales included the Schizo-Bipolar Scale (a continuous scale that measures the extent to which symptoms are BD-like [low scores] or SZ-like [high scores]).8 Young Mania Rating Scale (YMRS),9 the 24-item Brief Psychiatric Rating Scale (BPRS) (which includes subscales for negative, positive, disorganization, mania, and depressed mood symptoms10) and the Bipolar Depression Rating Scale (BDRS).11 Functional capacity was measured using the University of California San Diego Performance Skills Assessment-B (UPSA-B), which includes Financial Skills and Communications Skills subscales.12 Symptom data were not collected for HC individuals. Chlorpromazine (CPZ) equivalent antipsychotic doses were calculated using published guidelines for conventional13 and atypical14 antipsychotics.
Cognitive Domains Analyzed
Participant data from 3 tasks covering 3 different cognitive domains were used in latent profile analyses. These tasks were the Dot Probe Expectancy (DPX) task (used to measure cognitive control), the Relational and Item-Specific Encoding (RISE) task (used to measure episodic memory), and the Jittered Orientation Visual Integration (JOVI) task (a perceptual task used to measure visual integration). These tasks have been psychometrically “optimized” (http://cntracs.ucdavis.edu). Specifically, they are designed to (1) minimize task length; (2) simplify task administration across multiple sites; (3) maximize sensitivity and selectivity in assessing the specific cognitive mechanisms of interest; and (4) enhance reliability and minimize floor and ceiling effects. Furthermore, optimization of each task is designed to enhance its psychometric properties while preserving high construct validity. Details of these tasks have been described in detail elsewhere7,15–20 and are provided in brief in supplementary materials. The extracted DPX performance measure was d-prime context (a function of AX hits minus BX false alarms). The extracted RISE performance measure was a combination of d-prime (a function of hits minus false alarms) values for all 3 task trial types (associative recognition, relational encoding recognition, and item-specific encoding recognition). The extracted JOVI performance measure was a combination of accuracies from trials with 7–8, 9–10, 11–12, 13–14, and 15–16 degrees of orientational jitter. More specifically, the RISE and JOVI combined measures were defined by loadings from factors extracted by principle component analysis (SPSS v. 25 [Armonk, NY]). Only components with eigenvalues greater than one were retained, resulting in one component being extracted for the RISE and one component for the JOVI. These components explained 78% and 68% of the variances in RISE and JOVI, respectively.
LPA and CFA
LPA (a special case of cluster analysis, within the family of mixture cluster analysis) was performed in Mplus vs 821 using DPX d-prime context, RISE d-prime factor score, and JOVI factor score as features. A CFA was also performed in Mplus to evaluate the fit of a purely dimensional, one-factor solution using these features. Only patient data were used for LPA and CFA. For LPA, a one-profile solution was initially fitted, and the number of classes was systematically increased by one until the fit was no longer significantly improved by further increasing the number of profiles. For all evaluated LPA models, 10 000 random sets of starting values were specified, and the best log likelihood values were replicated. The optimal number of LPA profiles and CFA fit was evaluated using included the Akaike Information Criterion (AIC)22 and Bayesian Information Criterion (BIC).23 Lower AIC/BIC values indicated better fit. Comparing AIC between models, a model with difference < 2 (from the model with the lowest AIC) had substantial support, between 4 and 7 considerably less support, and > 10 virtually no support.24 Comparing BIC between models, a difference < 2 was considered inconsequential (“weak”), 2 to 6 as “positive,” 6 to 10 as “strong,” and greater than 10 as “very strong”.25 Vuong-Lo-Mendell-Rubin and Lo-Mendell-Rubin adjusted LR tests26 were also performed to compare LPA solutions. These tests compare the fit of a specified (“X”) profile solution to models with one fewer profile (“X” - 1); a significant P value indicates the former solution (“X”) should be preferred. Patients were assigned to the latent profile to which they had the highest probability of membership. The magnitudes of profile differences for each feature were further evaluated by ANOVA F and P values with profile membership as a fixed factor. An additional exploratory ANOVA comparing profiles was also performed after adjusting for diagnosis. It should be noted that significant between-profile differences were expected between profiles as they were constructed to be maximally differentiated.
Clinical Comparisons
Between-diagnosis and between-profile demographic, behavioral, and clinical comparisons as well as analysis of medication effects were conducted by ANOVA or chi-square analysis (for categorical data) in SPSS v. 25. Measures showing significant (P < .05) between-group differences were followed up by exploratory post hoc tests (threshold P < .05) as well as an a priori linear contrast (see Results) to discern the nature of the effects. Between-profile behavioral and clinical comparisons (ie, main effects of profile) were also performed using Diagnostic and Statistical Manual of Mental Disorders (DSM)-diagnosis (BD/SZ/SZ-A) and/or parental education (years) as additional factors/continuous covariates in univariate ANCOVAs. These additional analyses were performed to determine if clinical differences between profiles were affected by differences in the ratio of BD/SZ/SZ-A patients or educational attainment potential within each profile. Complete parental education data was missing for 38 patients.
Results
Demographics
Demographic and clinical information for participants segregated by DSM-based diagnosis are shown in table 1. Significant group differences were observed on gender ratio and the majority of clinical measures.
Table 1.
Demographic and Clinical Information
| HC | BD | SZ | SZ-A | F or χ2 (P) | |
|---|---|---|---|---|---|
| N | 73 | 59 | 88 | 76 | − |
| Age | 37.2 (11.3) | 38.4 (10.8) | 35.6 (10.7) | 36.6 (10.9) | 0.8 (.47) |
| % Male | 54.8 | 35.6 | 62.5 | 53.9 | 10.5 (.02) |
| % per Site (1/2/3/4/5) | 29/14/16/27/14 | 8/24/32/7/29 | 28/17/13/18/24 | 32/11/24/11/24 | 34.4 (<.01) |
| Education level (y) | 14.8 (2.3) | 14.7 (2.6) | 12.8 (2.1) | 13.6 (2.5) | 12.0 (<.01) |
| Parental education level (y) | 13.9 (2.7) | 14.3 (2.5) | 13.4 (3.3) | 14.2 (2.7) | 1.5 (.22) |
| SBS total | − | 1.5 (0.9) | 7.7 (1.2) | 4.7 (1.3) | 497.3 (<.01) |
| BDRS total | − | 5.5 (5.3) | 4.3 (4.6) | 7.0 (6.5) | 5.0 (.01) |
| Mood lability score | 0.5 (0.1) | 0.2 (0.1) | 0.4 (0.1) | 3.8 (.02) | |
| YMRS total | 10.0 (7.7) | 9.9 (6.2) | 13.1 (7.4) | 5.1 (<.01) | |
| BPRS total | 40.2 (8.8) | 43.2 (12.4) | 49.7 (14.6) | 10.8 (<.01) | |
| CAINS anhedonia | 8.4 (5.6) | 12.3 (6.9) | 14.4 (7.1) | 7.8 (<.01) | |
| CAINS blunting | 0.8 (1.7) | 3.8 (3.0) | 3.3 (3.1) | 22.0 (<.01) | |
| UPSA-B total | 83.9 (11.3) | 84.6 (9.0) | 75.0 (13.8) | 77.8 (13.0) | 10.7 (<.01) |
| Financial skills | 43.7 (6.1) | 42.9 (5.5) | 39.1 (8.1) | 40.9 (8.2) | 6.2 (<.01) |
| Communication skills | 40.3 (7.4) | 41.5 (6.4) | 35.8 (8.3) | 37.0 (8.0) | 8.7 (<.01) |
| % Taking antipsychotic | − | 49.2 | 76.1 | 71.1 | 110.5 (<.01) |
| Antipsychotic dose (mg CPZ equivalent) | 391.6 (522.7) | 371.9 (301.0) | 577.4 (778.2) | 2.03 (.14) | |
| % Taking mood stabilizer | 69.5 | 13.6 | 28.9 | 92.5 (<.01) | |
| % Taking antidepressant | 59.3 | 26.1 | 56.6 | 74.0 (<.01) | |
| % Taking anxiolytic | 20.3 | 17.0 | 19.7 | 16.4 (<.01) |
Note: BD, bipolar disorder; BDRS, Bipolar Depression Rating Scale; BPRS, Brief Psychiatric Rating Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; CPZ, chlorpromazine; HC, healthy control; SBS, Schizo-Bipolar Scale; SZ, schizophrenia; SZ-A, schizoaffective disorder; UPSA-B, University of California San Diego Performance-Based Skills Assessment; YMRS, Young Mania Rating Scale. Numbers in parentheses represent the standard error.
DSM-Based Behavioral Comparison
Behavioral data segregated by diagnosis are presented in table 2 and supplementary table 1. HCs performed significantly more poorly than all 3 patient groups (BD, SZ-A, SZ) for all 3 primary behavioral measures of interest (P < .05). SZ patients performed significantly worse on the RISE than BD patients (P < .05). No other significant between-group differences were observed.
Table 2.
Primary Behavioral Data Segregated by Diagnosis
| HC | BD | SZ | SZ-A | ANOVA Model F or χ2 (P) | |
|---|---|---|---|---|---|
| Cognitive control (Z Score) | 0.45 (0.86) | 0.04 (0.82) | −0.19 (1.03) | −0.25 (1.08) | 8.1 (<.001) |
| Episodic memory (RISE Factor Score) | 0.50 (0.77) | 0.09 (0.83) | −0.17 (1.06) | −0.36 (1.06) | 11.3 (<.001) |
| Visual integration (JOVI Factor Score) | 0.44 (0.90) | −0.03 (1.02) | −0.12 (0.96) | −0.26 (1.00) | 7.2 (<.001) |
Note: BD, bipolar disorder; HC, healthy control; SZ, schizophrenia; SZ-A, schizoaffective disorder. Numbers in parentheses represent the standard deviation.
LPA and CFA Results
LPA was performed in patient participants using DPX d-prime context z-score (cognitive control), RISE factor score (episodic memory), and JOVI factor score (visual integration) as features (see Methods; fit measures presented in table 3). The two-profile solution suggested better fit than the one-profile solution for all metrics. The 3-profile solution suggested better fit than the two-profile solution based on AIC; likelihood ratio tests also suggested better fit for the 3-profile solution. The 4-profile solution also suggested better fit than the 3-profile solution based on AIC; likelihood ratio tests, however, suggested no significant improvement over the 3-profile solution. The 3-profile solution, therefore, was considered the optimal model. For this solution, average latent profile probabilities were 88%, 92%, and 82%.
Table 3.
Model Fit Statistics for Latent Profile (LPA) and Confirmatory (single) Factor Analysis (CFA)
| Number of Profiles | LL | AIC | ΔAIC (vs Previous Unless Specified) | BIC | ΔBIC (vs Previous Unless Specified) | VLMR LR Test P-value (vs Previous) | LMR Adjusted LR Test P-value (vs Previous) |
|---|---|---|---|---|---|---|---|
| 1 | −949.1 | 1910.3 | – | 1930.7 | – | – | – |
| 2 | −907.8 | 1835.6 | −74.7 | 1869.6 | −61.1 | <.01 | <.01 |
| 3 | −900.4 | 1828.8 | −6.8 | 1876.5 | 6.9 | .03 | .04 |
| 4 | −892.4 | 1820.9 | −7.9 | 1882.2 | 5.7 | .10 | .11 |
| One-factor CFA | −914.5 | 1847.1 | 18.3 (vs 3-profile LPA) | 1877.8 | 1.3 (vs 3- profile LPA) | – | – |
Note: AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; LL, log-likelihood. Optimal LPA result (3 profiles) is italicized. Smaller minus LL, AIC, and BIC values suggest better fit. A significant result using the Vuong-Lo-Mendell-Rubin likelihood ratio (VLMR LR) test (threshold P < .05) to compare nested models also suggests better fit.
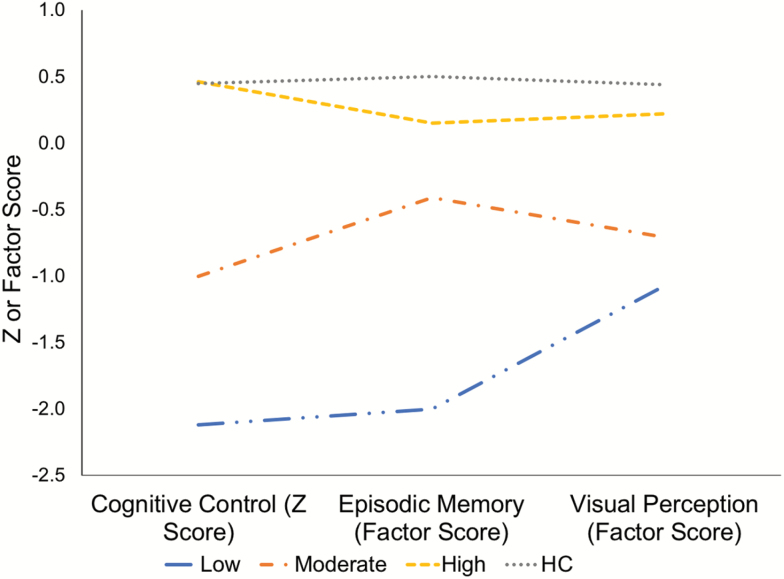
We then more closely examined the composition of the 3-profile solution. The solution was best described as a “High” ability profile (n = 142) with high scores on all 3 domains, a “Moderate” ability profile (n = 66) with moderate scores, and a “Low” ability profile (n = 15) with low scores (table 4, figure 1). As expected by clustering, profiles significantly differed on d-prime context (F(2,220) = 268.1, P < .001), RISE factor score (F(2,220) = 47.1, P < .001), and JOVI factor score (F(2,220) = 35.1, P < .001). Post-hoc tests further showed that each profile was significantly different from each other for all domains (all P values < .001) with the exception of the JOVI, in which the difference between Moderate and Low profiles did not reach significance (P = .15). Weighted linear contrasts (testing the significance of the linear pattern High > Moderate > Low), however, were significant for all 3 domains (all P values < .001). Exploratory comparisons between patients in the High ability profile and HC subjects showed no differences on d-prime context (t = 0.05, P = .97) or JOVI (t = 1.73, P = .09). High ability patients, however, still performed significantly worse than HCs on the RISE (factor score t = 2.85, P = .01). Trial type-specific performance data for each task segregated by latent profile are presented in supplementary table 2.
Table 4.
Primary Behavioral and Clinical Data Segregated by Latent Profile (High, Moderate, and Low Ability, With Healthy Control Averages Included for Visual Comparison)
| HC | High Ability (Patients) | Moderate Ability (Patients) | Low Ability (Patients) | F or χ2 (P) | Linear Contrast Beta (P) | Dx Adjust F (P) | |
|---|---|---|---|---|---|---|---|
| N | 73 | 142 | 66 | 15 | − | − | − |
| Cognitive control (Z Score) | 0.45 (0.86) | 0.46 (0.55) | −1.00 (0.54) | −2.12 (0.43) | 268.1 (<.01) | −1.82 (<.01) | 261.0 (<.001) |
| Episodic memory (RISE Factor Score) | 0.50 (0.77) | 0.15 (0.88) | −0.41 (0.80) | −2.00 (0.76) | 47.1 (<.01) | −1.52 (<.01) | 43.5 (<.001) |
| Visual integration (JOVI Factor Score) | 0.44 (0.90) | 0.22 (0.87) | −0.71 (0.85) | −1.07 (0.88) | 35.1 (<.01) | −0.91 (<.01) | 33.9 (<.001) |
| % BD/SZ/SZ-A | − | 30/38/32 | 23/44/33 | 7/33/60 | 7.11 (.13) | − | − |
| Age | 37.2 (11.3) | 35.7 (10.4) | 38.2 (11.6) | 39.1 (10.5) | 1.67 (.19) | ||
| % Male | 55 | 54 | 47 | 67 | 2.08 (.35) | ||
| Education level (y) | 14.8 (2.3) | 13.8 (2.6) | 13.0 (1.8) | 13.5 (3.4) | 2.39 (.09) | ||
| Parental education Level (y) | 13.9 (2.7) | 14.3 (2.7) | 13.2 (3.4) | 13.6 (2.3) | 2.69 (.07) | ||
| % per Site (1/2/3/4/5) | 29/14/16/27/14 | 26/18/22/11/23 | 23/12/18/18/29 | 13/20/33/7/27 | 6.56 (.59) | ||
| SBS total | − | 5.0 (2.9) | 5.1 (2.7) | 5.7 (1.9) | 0.41 (.67) | ||
| BDRS total | − | 5.4 (5.9) | 6.1 (4.9) | 4.3 (5.6) | 0.74 (.48) | ||
| YMRS total | 10.0 (6.8) | 13.0 (7.2) | 11.7 (71.8) | 4.10 (.02) | 1.18 (.38) | 4.08 (.02) | |
| BPRS total | 42.7 (11.4) | 47.5 (14.2) | 50.0 (17.2) | 4.60 (.01) | 5.12 (.04) | 3.52 (.03) | |
| CAINS anhedonia | 10.9 (6.8) | 11.9 (6.7) | 12.2 (8.7) | 0.62 (.54) | − | − | |
| CAINS blunting | 2.8 (3.1) | 2.7 (2.9) | 4.1 (3.0) | 1.40 (.25) | |||
| UPSA-B total | 83.9 (11.3) | 80.6 (12.1) | 74.3 (14.4) | 75.9 (10.0) | 5.74 (<.01) | −3.37 (.17) | 4.87 (.01) |
| Financial skills | 43.7 (6.1) | 41.6 (7.6) | 38.8 (7.7) | 40.3 (7.1) | 2.86 (.06) | − | − |
| Communication skills | 40.3 (7.4) | 38.9 (7.3) | 35.4 (9.4) | 35.6 (6.9) | 4.90 (.01) | −2.39 (.12) | 4.03 (.02) |
| % Taking antipsychotic | − | 65.5 | 68.2 | 80.0 | 1.33 (.51) | − | − |
| Antipsychotic dose (mg CPZ equivalent) | 455.9 (630.6) | 439.1 (422.1) | 407.4 (431.6) | 0.04 (.96) | |||
| % Taking mood stabilizer | 34.5 | 31.8 | 33.3 | 0.15 (.93) | |||
| % Taking antidepressant | 47.9 | 37.9 | 53.3 | 2.24 (.33) | |||
| % Taking anxiolytic | 18.3 | 22.7 | 6.7 | 2.13 (.34) |
Note: BDRS, Bipolar Depression Rating Scale; BPRS, Brief Psychiatric Rating Scale; CPZ, Chlorpromazine; CMH, Cochran-Mantel-Haenszel; GAF, Global Assessment of Function; HC, healthy control; PAS, Premorbid Adjustment Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale of the Assessment of Positive Symptoms; SBS, Schizo-Bipolar Scale. ANCOVA model F tests were conducted using only patient data. Linear contrasts and (DSM) diagnosis-adjusted F tests on continuous variables were only conducted if the initial ANOVA F value was significant (indicating a significant between-class group difference). “Linear contrast” was High > Moderate > Low or Low > Moderate > High. Numbers in parentheses represent the standard deviation. “Dx Adjust” = main effect of profile in ANCOVA model with diagnosis (bipolar disorder [BD]/schizophrenia [SZ]/schizoaffective disorder [SZ-A]) as an additional covariate.
Fig. 1.
Latent profile analysis (LPA) results. Three profiles were observed in patients: A “High” ability profile, a “Moderate” ability profile, and a “Low” ability profile. “Cognitive Control” was based on DPX d-prime context value, “Episodic Memory” based on RISE factor score, and Visual Perception based on JOVI factor score (see Methods). Healthy controls (HCs; top, dotted line) were included for comparison but not in the sample LPA.
Because the 3-profile solution suggested a one-dimensional pattern (with patients with Low, Moderate, and High scores clustering together) we next examined the fit of a one-factor (ie, one-dimensional/continuous) solution using CFA (table 3) and compared it to the 3-profile LPA solution. Fit comparisons were inconclusive. Specifically, although AIC suggested poorer fit (vs 3 LPA profiles), the difference in BIC (<2) was inconsequential (see Methods). In combination with results from the between-profile ANOVA, therefore, cluster results were best described as having a one-dimensional structure.
Between-Profile Demographic and Clinical Comparison
Demographic, clinical, and medication data for each latent profile are shown in table 4. No differences were observed in the ratio of BD/SZ/SZ-A patients or Schizo-Bipolar Scale scores between profiles.
ANOVA analyses revealed that profiles significantly differed in YMRS and BPRS total score as well as UPSA-B total score. Trend-level differences were also observed on education and parental education level. The UPSA-B total score was driven by significant differences in Communication subscore. Post hoc tests further demonstrated significant differences between High and Moderate profiles for YMRS score (P = .005), High and Moderate (P = .012) and High and Low (P = .037) profiles for BPRS score, and High and Moderate for UPSA-B Total score (P = .001) and Communication subscore (P = .004). No differences were observed between profiles on any medication-related measure.
As the observed latent profile pattern was dimensional across domains, we hypothesized that symptom severity would increase as profile varied from High to Low ability. Consistent with our hypothesis, linear contrasts demonstrated the BPRS difference mirrored profile dimensions (table 4, “Linear Contrast” column). Linear contrasts were not significant for any other measure.
Although no difference was observed in the ratio of BD/SZ/SZ-A patients between profiles, only one patient with BD was included in the Low ability profile, and the overall proportion of BD patients decreased as a function of ability. It was conceivable, therefore, that profiles varied clinically due in part to differences in symptom severity between DSM-based diagnostic groups (table 1). To test this possibility, measures that showed significant differences in the first set of tests were used as dependent variables in an ANCOVA analysis with profile and diagnosis as fixed factors. Main effects of profile remained significant after this adjustment for all behavioral and clinical measures (table 4, last column).
An exploratory analysis was also conducted to determine if differences between profiles on each task persisted after accounting for educational attainment potential (ie, parental education years). Using ANCOVA with profile as a fixed factor and parental education years as a continuous covariate, main effects of profile remained highly significant (P < .001) for all 3 measures.
Discussion
The present study used LPA across 3 distinct cognitive domains (cognitive control, episodic memory, and visual integration) to identify distinct profiles across psychotic disorders. The optimal solution was composed of 3 profiles: A “High” ability profile that consisted of patients with high performance (mostly indistinct from HC levels) on all 3 tasks, a “Moderate” ability profile that consisted of patients with moderate performance, and a “Low” ability profile that consisted of patients with low performance. As a comparison of these profiles suggested a dimensional structure across all 3 domains, a continuous, single-factor solution using CFA was estimated. BIC values suggested fit was nearly identical for the single-factor solution compared to the 3-profile solution suggesting an underlying one-dimensional structure across all cognitive variables. Examining diagnostic ratios between profiles, no significant differences were observed in the ratio of BD/SZ/SZ-A patients or Schizo-Bipolar Scale scores between profiles, suggesting that having a prevalence of BD or SZ-like symptoms did not determine profile membership (other than the “Low” ability profile which had only one BD patient) and that deficits in the 3 domains examined were not specific to one disorder. Profile differences in performance remained significant after adjusting for parental education. BPRS symptom severity and deficits in everyday communication skills varied inversely with increasing performance independent of diagnosis. Overall, these results suggest that cognitive deficits in psychosis tested across disparate domains may be characterized as a single continuum of ability (ranging from poor to near-normal) that also correlates with symptom severity.
Meta-analyses have demonstrated that cognitive dysfunction in SZ may be a “global” condition, affecting most cognitive domains with varying degrees of severity.27–29 A similar profile of deficits has also been reported in SZ-A and BD, with less severe deficits in BD and mixed degrees of severity in SZ-A (reviewed by Barch and Sheffield4). Consistent with the results of our study, as a whole these findings suggest a domain-overlapping, one-dimensional structure to cognitive dysfunction across these illnesses. What might be the core neuronal process(es) that can underlie such global impairment? It has been argued that one potential mechanism is dysfunction of the dorsolateral prefrontal cortex (DLPFC). Although the 3 tasks examined in the present study recruit distinct neuronal circuits,15,30,31 the common region activated by all 3 is the DLPFC, which (along with the superior parietal cortex) makes up the frontoparietal network most associated with cognitive control. As cognitive control is defined as the ability to use goals and plans to orchestrate behavior, on a neuronal level the DLPFC may be equivalent to the “conductor” ensuring the correct groups of orchestral instruments are playing on time and in tune depending on the demands of the task. Illustrating this point, top-down influences have been frequently observed during perceptual tasks in order to facilitate stimulus processing, interpretation, encoding, and recall.32 It follows that the degree to which the prefrontal cortex and cognitive control is disrupted may be directly related to the overall level of cognitive impairment in psychiatric populations independent of DSM diagnosis. Additional mechanisms may, of course, contribute to dysfunction across domains, eg, abnormalities in “bottom-up” perceptual (eg, visual or auditory) processing.33 Notably, however, previous work (in an independent sample collected prior to the present dataset) by the CNTRACS consortium using another perceptual task (the contrast-contrast task) suggests that deficits in contrast gain control in SZ may be accounted for by reduced attentional control (ie, attention “lapsing”),34 an index related to frontoparietal cognitive control.35 While our cognitive control-based interpretation is therefore, in our view, the most parsimonious given the present and previous findings, additional research using perceptual tasks (with experimental control over attention lapsing) together with the measures used in the present paper could be used to formally test whether another mechanism (such as early visual perception) could reproduce the single factor dimensional pattern of cognitive deficits observed in this study.
The dimensional structure observed here is conceptually consistent with previous findings from an LPA of executive function in SZ and BD,3 which also reported profiles segregated by performance deficit severity across diagnoses. Critically, the dimensional structure observed here also does not conceptually conflict with the clusters (“biotypes”) identified by the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) consortium from a k-means cluster analysis of cognitive control and sensorimotor reactivity in BD, SZ, and SZ-A patients.36 Although the 3 clusters identified by B-SNIP did not demonstrate parallel deficits in control and reactivity (with one biotype showing the highest reactivity but only moderate loss of cognitive control), performance deficits (vs HCs) for each biotype across the 3 cognitive control measures examined were similar. One may also argue that our results are not directly comparable with the B-SNIP findings as we did not include measures of sensorimotor reactivity. Nonetheless, our results combined with these previous studies suggest that cognitive deficits in these illnesses are best characterized as a having a dimensional structure, with a potential common mechanism underlying these deficits across disorders.
Surprisingly, the largest group of patients (“High” performers) was not significantly different from HCs except in episodic memory, suggesting that RISE ability features more prominently than DPX or JOVI task performance in psychotic illnesses. Related to this point, previous work has also found that episodic memory performance is one the most impaired cognitive deficits in SZ.29 It is also possible that severity in performance deficits is influenced by differences in task sensitivity. As the present sample (particularly BD patients) had relatively high functional capacity (based on UPSA-B scores), it is possible deficits in these domains would have only been apparent using very difficult tasks with high discriminating power (that said, we believe these tasks were sufficiently sensitive for this study given the significant differences in performance between HC and patient groups; furthermore, all tasks utilized by the CNTRACS consortium were designed to optimize their psychometric properties including sensitivity and selectivity). As a more chronically ill, lower functioning sample may show a different distribution, we caution against overinterpreting our results as suggesting the majority of patients with psychotic illnesses are cognitively “normal.” Finally, the lack of differences on the DPX and JOVI tasks in the high performing groups does not also imply that cognition in these individuals has not been disrupted by their psychotic illness. Kendler and colleagues have reported that higher cognitive ability individuals with SZ perform worse than would be predicted by their parents’ IQ scores.37 Future studies examining additional tasks in these domains will be necessary in order to determine the generalizability of this finding.
As frequently occurs in studies involving patients with psychosis, the potential confounding effects of medication on the observed findings cannot be completely disentangled. Although we did not observe any relationship between medication status, type, or dose and the likelihood a patient belonged to a particular profile, the present study was naturalistic and medication effects were therefore not tightly controlled. It thus remains possible that medication history is related to the observed profiles. Related to this point, previous cross-sectional work in an independent sample found that unmedicated patients showed lower DLPFC activation and worse performance during the AX-CPT (the task the DPX is based on) relative to medicated patients.38 As this study was based on a univariate dimensional approach, however, it does not necessarily contradict the present findings.
Overall, our results suggest that cognitive deficits in psychotic illness can be described as having cross-diagnostic, one-dimensional structure across the domains of cognitive control, episodic memory, and visual perception. Clinically, this result also suggests an effective treatment may be found that can broadly improve cognition across multiple illnesses. Ultimately, however, to prove clinically useful future studies of these profiles should include replication in a larger sample, comparison of longitudinal outcomes, analysis of controlled treatment effects, and links to systems-level dysfunction in neuronal circuits. Latent class/profile analyses may also be performed on young adults and adolescents at high risk for psychotic illness in order to determine if similar profiles exist in these participants, if they persist into illness, and/or are predictive of illness. Finally, we stress that this method may be readily applied to a variety of (or in combination with) other neurocognitive processes (eg, sensorimotor reactivity) to generate profiles of patients which may be evaluated in the same manner and provide greater insight into the cross-diagnostic mechanisms of cognitive dysfunction.
Funding
This research was supported by National Institutes of Health (NIH) grants MH084840, MH084826, MH084828, MH084861, MH08482, MH059883, and MH114325.
Supplementary Material
Acknowledgments
We thank the staff at each of the CNTRACS sites for their hard work and our subjects for their time, energy, and cooperation. J.D.R. has received research grants from the National Institutes of Health (NIH), the Brain & Behavior Research Foundation, the EJLB Foundation, and the Robert Wood Johnson Foundation. D.M.B. has received grants from the Brain & Behavior Research Foundation and the National Institutes of Health (NIH), and is a consultant for Pfizer, Amgen, Upsher-Smith and Takeda on studies related to the treatment of negative symptoms in schizophrenia. J.M.G. has received grants from National Institutes of Health (NIH), receives royalty payments from Brief Assessment of Cognition in Schizophrenia, and has acted as a consultant to Amgen, AstraZeneca, GlaxoSmithKline, Hoffman LaRoche, Merck, Pfizer, and Solvay. A.W.M. has received research grants from the National Institutes of Health (NIH) and the Brain & Behavior Research Foundation. S.M.S. has received research grants from the National Institutes of Mental Health (NIMH), The Brain & Behavior Research Foundation, the van Ameringen Foundation, the Jacob and Valeria Langaloth Foundation, the New England Research Institutes, the New York State Office of Mental Health, the New Jersey Division of Mental Health and Addiction Services, Janssen Pharmaceuticals, AstraZeneca, and Pfizer. CSC has received research grants from the National Institutes of Health (NIH), the Brain and Behavior Foundation, the Burroughs Wellcome foundation, GlaxoSmithKline, and the Robert Wood Johnson Foundation and has been an external consultant for Lilly, Merck, Pfizer, Roche, and Servier. The other authors have no conflicts of interest to report.
References
- 1. Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–241. [DOI] [PubMed] [Google Scholar]
- 2. Hill SK, Keshavan MS, Thase ME, Sweeney JA. Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatry. 2004;161:996–1003. [DOI] [PubMed] [Google Scholar]
- 3. Bora E, Veznedaroğlu B, Vahip S. Theory of mind and executive functions in schizophrenia and bipolar disorder: a cross-diagnostic latent class analysis for identification of neuropsychological subtypes. Schizophr Res. 2016;176:500–505. [DOI] [PubMed] [Google Scholar]
- 4. Barch DM, Sheffield JM. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reichenberg A, Harvey PD, Bowie CR, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gold JM, Barch DM, Carter CS, et al. Clinical, functional, and intertask correlations of measures developed by the cognitive neuroscience test reliability and clinical applications for schizophrenia consortium. Schizophr Bull. 2012;38:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson D, Poppe AB, Barch DM, et al. Optimization of a goal maintenance task for use in clinical applications. Schizophr Bull. 2012;38:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keshavan MS, Morris DW, Sweeney JA, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 10. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 11. Berk M, Malhi GS, Cahill C, et al. The bipolar depression rating scale (BDRS): its development, validation and utility. Bipolar Disord. 2007;9:571–579. [DOI] [PubMed] [Google Scholar]
- 12. Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 1997;154(4 Suppl):1–63. [DOI] [PubMed] [Google Scholar]
- 14. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 15. Lopez-Garcia P, Lesh TA, Salo T, et al. The neural circuitry supporting goal maintenance during cognitive control: a comparison of expectancy AX-CPT and dot probe expectancy paradigms. Cogn Affect Behav Neurosci. 2016;16:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ragland JD, Ranganath C, Barch DM, et al. Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophr Bull. 2012;38:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silverstein SM, Keane BP, Barch DM, et al. Optimization and validation of a visual integration test for schizophrenia research. Schizophr Bull. 2012;38:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. [DOI] [PubMed] [Google Scholar]
- 19. Strauss ME, McLouth CJ, Barch DM, et al. Temporal stability and moderating effects of age and sex on CNTRaCS task performance. Schizophr Bull. 2014;40:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones JA, Sponheim SR, MacDonald AW III. The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess. 2010;22:131–141. [DOI] [PubMed] [Google Scholar]
- 21. Muthén LK, Muthén BO.. Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 1998–2011. [Google Scholar]
- 22. Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 23. Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6(2):461–464. [Google Scholar]
- 24. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Method Res. 2004;33(2):261–304. [Google Scholar]
- 25. Raftery AE. Bayesian model selection in social research. In: Marsden PV, ed. Sociological methodology. London, UK: Tavistock; 1995. [Google Scholar]
- 26. Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 27. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gold JM, Dickinson D. “Generalized cognitive deficit” in schizophrenia: overused or underappreciated? Schizophr Bull. 2013;39:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. [DOI] [PubMed] [Google Scholar]
- 30. Ragland JD, Ranganath C, Harms MP, et al. Functional and neuroanatomic specificity of episodic memory dysfunction in schizophrenia: a functional magnetic resonance imaging study of the relational and item-specific encoding task. JAMA Psychiatry. 2015;72:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silverstein SM, Harms MP, Carter CS, et al. Cortical contributions to impaired contour integration in schizophrenia. Neuropsychologia. 2015;75:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013;14:350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barch DM, Carter CS, Dakin SC, et al. The clinical translation of a measure of gain control: the contrast-contrast effect task. Schizophr Bull. 2012;38:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phillips RC, Salo T, Carter CS. Distinct neural correlates for attention lapses in patients with schizophrenia and healthy participants. Front Hum Neurosci. 2015;9:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kendler KS, Ohlsson H, Mezuk B, Sundquist JO, Sundquist K. Observed cognitive performance and deviation from familial cognitive aptitude at age 16 years and ages 18 to 20 years and risk for schizophrenia and bipolar illness in a swedish national sample. JAMA Psychiatry. 2016;73:465–471. [DOI] [PubMed] [Google Scholar]
- 38. Lesh TA, Tanase C, Geib BR, et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.