Abstract
Catatonia is a nosologically unspecific syndrome, which subsumes a plethora of mostly complex affective, motor, and behavioral phenomena. Although catatonia frequently occurs in schizophrenia spectrum disorders (SSD), specific patterns of abnormal brain structure and function underlying catatonia are unclear at present. Here, we used a multivariate data fusion technique for multimodal magnetic resonance imaging (MRI) data to investigate patterns of aberrant intrinsic neural activity (INA) and gray matter volume (GMV) in SSD patients with and without catatonia. Resting-state functional MRI and structural MRI data were collected from 87 right-handed SSD patients. Catatonic symptoms were examined on the Northoff Catatonia Rating Scale (NCRS). A multivariate analysis approach was used to examine co-altered patterns of INA and GMV. Following a categorical approach, we found predominantly frontothalamic and corticostriatal abnormalities in SSD patients with catatonia (NCRS total score ≥ 3; n = 24) when compared to SSD patients without catatonia (NCRS total score = 0; n = 22) matched for age, gender, education, and medication. Corticostriatal network was associated with NCRS affective scores. Following a dimensional approach, 33 SSD patients with catatonia according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision were identified. NCRS behavioral scores were associated with a joint structural and functional system that predominantly included cerebellar and prefrontal/cortical motor regions. NCRS affective scores were associated with frontoparietal INA. This study provides novel neuromechanistic insights into catatonia in SSD suggesting co-altered structure/function-interactions in neural systems subserving coordinated visuospatial functions and motor behavior.
Keywords: catatonia, mCCA+jICA, MRI, motor symptoms, schizophrenia spectrum disorders
Introduction
Catatonia occurs in 9%–17% of patients with acute mental disorders.1 Catatonic (CT) symptoms include flamboyant motor phenomena (rigor, dyskinesia, counteracting [resistance to passive movements, “Gegenhalten”], posturing, catalepsy, stereotypies, etc.) as much as affective symptoms (anxiety, flat affect, or affect incontinence) and behavioral abnormalities (autism, mutism, echolalia, etc.).2–8 Catatonia can be associated with schizophrenia spectrum disorders (SSD) and major mood disorders, as well as with various neurological and medical conditions.2,9,10
Although catatonia has been known in clinical practice for more than 140 years, the underlying pathogenesis of CT symptoms in SSD is largely unknown.11 To date, a small number of magnetic resonance imaging (MRI) studies have highlighted structural12,13 and functional alterations9,10,14,15 of orbitofrontal/frontoparietal and basal ganglia networks underlying catatonia in SSD. Previous studies have been unimodal, mostly providing information on neural activity, with very few exceptions highlighting both abnormal structure and function.14,16 The relationships between structural and functional abnormalities in catatonia, however, remain unclear. Here, we used a multimodal data fusion approach, ie, multiset canonical correlation analysis + joint independent component analysis (mCCA + jICA)17–20 to identify networks with unique and shared covariance across structural (sMRI) and resting-state functional MRI (rs-fMRI) data underlying catatonia in SSD. This analysis incorporates neuroimaging data from multiple modalities, ie, gray matter volume (GMV) and intrinsic neural activity (INA) of the same participants to extract maximally independent components spanning across modalities to identify patterns of structural and functional changes that covary across participants.17–19 Delineating the interrelationship between structure and function associated with CT symptoms will provide a more comprehensive understanding of brain mechanism in SSD patients with catatonia.
To expand the extant knowledge on abnormalities spanning across multiple imaging modalities in terms of joint function–structure alterations, which might lead to catatonia in SSD, this study had 2 major objectives: First, conducting a categorical approach, we predicted that there will be a difference in both modality-specific (ie, brain structure or function) and transmodal (ie, structure and function) systems comprising frontoparietal and frontostriatal networks between SSD patients with and without catatonia. Second, acknowledging a dimensional perspective on catatonia21,22, we supposed that distinct dimensions of catatonia—ie, motor, behavioral, and affective domains—will be significantly associated with transmodal components in distinct cortico–subcortical networks, as involved in motor control, affective processing, and integration of spatiotemporal information.
Methods
Participants
We examined a total of 87 right-handed23 patients satisfying Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)24 criteria for schizophrenia (n = 84) or schizoaffective disorder (n = 3). Patients in this sample have been also included elsewhere.13 The inclusion and exclusion criteria are listed in supplementary material. From the sample of 87 SSD patients, we excluded 1 subject due to excessive head movements (>3 mm) during rs-fMRI. The local ethics committee (Medical Faculty at Heidelberg University, Germany) approved the study. Written informed consent was obtained from all SSD patients after all aims and procedures of the study had been fully explained.
Clinical Assessment
All patients were recruited and examined during inpatient treatment within 1 week after partial remission of psychotic symptoms. The duration between psychometric testing, motor assessment, and MRI examination was less than 3 days. At the time of the psychometric testing, motor assessment, and MRI examination, none of the SSD patients had taken benzodiazepines and all but 5 (5/87 = 5.7% antipsychotic-free) patients were on stable antipsychotic medication for at least 2 weeks. Benzodiazepines were discontinued at least 72 hours before the psychometric testing, motor assessment and MRI examination to avoid potential interactions between benzodiazepines, CT symptoms, and MRI scanning. All patients were electroconvulsive-therapy-naive at the time of scanning. Daily doses of antipsychotic medication were converted to olanzapine equivalents (OLZ) according to the classical mean dose method.25 For a detailed assessment of CT symptoms, we used the German version of the Northoff Catatonia Rating Scale (NCRS).26 The scale measures the presence and severity of 40 CT signs, considering 3 distinct dimensions of catatonia: motor (13 items), affective (12 items), and behavioral (15 items) symptoms.
This study used both a categorical approach and a dimensional (correlational) approach to investigate interrelationships between INA and GMV in catatonia in a total of 87 SSD patients. This enabled us to avoid a number of methodological obstacles when recruiting SSD patients selected for their severe CT symptoms (ie, CT subtype of schizophrenia).27 The NCRS criteria26 were used to cover all 3 categories of catatonia and to identify a clear cutoff to distinguish subjects with (NCRS total score ≥ 3; at least 1 point in the 3 different symptom categories; ie, motor, behavioral, and affective) and without (NCRS = 0) catatonia (presence vs absence). The control group included SSD patients without catatonia who were matched for age, gender, education, and OLZ. This approach allowed us to investigate neural underpinnings of catatonia controlling for the effects of the disorder.27,28 In a next step, we followed a dimensional (correlative) approach, assuming a neurobiological continuum between SSD patients with low and high severity of catatonia according to DSM-IV-TR (1 motor and at least 1 other symptom [behavioral or affective]).24 Thirty-two SSD patients who did not meet the NCRS or DSM-IV-TR criteria for catatonia or could not have been included in the matched control group were excluded from further analyses.
MRI Data Acquisition
MRI scans were acquired at Central Institute of Mental Health on a 3.0 Tesla MAGNETOM TIM Trio MR scanner (Siemens Medical Systems, Erlangen, Germany) equipped with a 32 channel multi-array head-coil. Technical details on MRI sequences are provided in supplementary material.
MRI Data Analysis
First Level Preprocessing
Voxel-based morphometry of T1-weighted structural images was calculated for gray matter density in each voxel using CAT12 (http://dbm.neuro.uni-jena.de/cat/), which is an extension to SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). The process included (a) segmentation of images into gray matter, white matter, and cerebrospinal fluid; (b) normalization using the DARTEL approach29; and (c) smoothing the gray matter probability values using an 8-mm full-width half-maximum (FWHM) isotropic Gaussian kernel. The fractional amplitude of low-frequency fluctuations (fALFFs) method was applied to rs-fMRI images using the Data Processing Assistant for Resting-State fMRI30. In contrast to task-based fMRI, examination of INA does not require participation in a task. Of note, INA does not refer to regional cerebral blood flow (eg, as measured by MRI-based arterial spin labeling) but rather explores the intrinsically (functionally) segregation or specialization of brain regions/networks.4,5 We chose to investigate resting-state fMRI and particularly fALFF because fALFF captures the relative magnitude of blood oxygen level dependent signal changes on INA in specific brain regions. fALFF represents the contribution of low-frequency oscillations (0.01–0.08 Hz frequency band) to the entire detectable range of spatial frequencies and is considered as a local intensity estimate (within-voxel time course) of spontaneous brain activity.6,7 In contrast to functional connectivity of specific brain networks, fALFF measures the amplitude of INA and might help to identify brain regions/networks with aberrant local functioning.8,9 Furthermore, different studies have shown that INA at rest is abnormal in SSD patients with catatonia10,11 and there are several fMRI studies that have shown abnormal fALFF in healthy and SSD samples.9,12,14–16,31 Although the examination of healthy individuals may provide interesting insights on the relationship between fALFF and functional connectivity, the study of SSD patients provides insight into abnormal local function within selective networks.16 One of the major benefits of using fALFF and combining both modalities is getting the information about both GMV and INA as well as increasing the power of data fusion analysis. The fALFF processing pipeline consisted of (a) slice timing with the middle slice as the reference frame, (b) head motion correction, (c) spatial normalization in Montreal Neurological Institute (MNI) space, (d) spatial smoothing with a 4-mm FWHM isotropic Gaussian kernel, and (e) regressing out nuisance covariates including mean signals from white matter and cerebrospinal fluid as well as the Friston 24-parameter model.32
Second Level Preprocessing
Before running the mCCA + jICA model, each modality was first reduced to a “feature” for each subject, providing a simpler, more tractable space to link the high-dimensional MRI data.12,17 In this study, for each subject, we extracted 2 imaging features, GMV and INA based on fALFF. The 2 features’ matrices were normalized within modality by variance to have the same average sum-of-squares (computed across all subjects and all voxels/locus for each modality) to ensure both features contribute equally in fusion and that no modality dominates the other.12,17 mCCA as a dimensionality reduction method and jICA33,34 were performed on both sMRI and rs-fMRI data using the Fusion ICA Toolbox (http://mialab.mrn.org/software/fit) in MATLAB 9.0.0 (R2016a). The number of components for each modality was estimated using both the minimum description length and the Akaike information criterion, as described in Calhoun et al.35. Five independent components (ICs) for each modality were estimated in the categorical analysis, and 3 ICs in the dimensional analysis. The quality index (Iq) from the ICASSO36 toolbox was used to assess the reliability of the results after running the approach 20 times to ensure consistency of the ICs. We used a threshold of Iq = 0.80 to exclude unstable ICs.37 All ICs in categorical and dimensional analyses had Iq’s above this threshold and were included in further analyses. For component visualization, the source matrix was reshaped back to a 3D-image, scaled to unit standard deviations (z), and thresholded at |z| > 2.5. The spatial maps from the components described in the results section were overlaid onto a MNI template. Anatomical labels and stereotaxic coordinates were derived from clusters above a threshold of |z| = 2.5 by linking the ICA output images (ie, the chosen components of interest) to the Talairach Daemon database (http://www.talairach.org/daemon.html). Significant clusters within ICs are reported using a spatial extent threshold of >0.5 cubic centimeters per at least 1 hemisphere.
Statistical Analysis
The procedure was similar to that described by Sui et al.38 and Lottman et al.33 For the “categorical” approach, we used cutoffs suggested by the NCRS to define the presence or absence of catatonia within the entire SSD patient group, assuming that between-group differences (CT vs non-catatonic [Non_CT]) would reflect a neural signature of catatonia within SSD. Analyses of covariance (n = 10 tests, ie, 5 ICs × 2 modalities) were conducted to compare the ICs’ loading coefficients of CT and Non_CT groups while controlling for both OLZ and Positive and Negative Syndrome Scale (PANSS) total score. A nominal significance threshold of P < .05 (uncorrected for multiple comparisons) was chosen. Acknowledging a dimensional view on catatonia, 2-tailed partial correlations (P < .05, uncorrected) adjusted for both OLZ and PANSS total score were used to investigate the relationship between the loading coefficients of ICs in an extended sample of SSD patients with catatonia according to DSM-IV-TR and NCRS scores.
Results
Categorical Approach
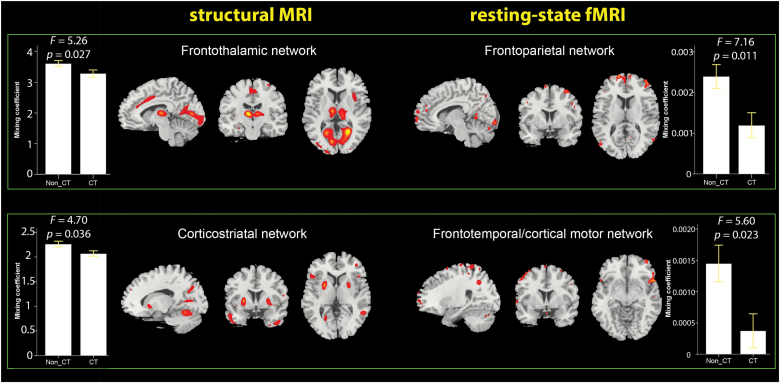
Following the categorical approach, 24 from 86 SSD patients (24/86 = 27.9%) were classified as catatonic according to NCRS criteria (NCRS total score ≥ 3; at least 1 point in the 3 different symptom categories; ie, motor, behavioral, and affective). Further, the group of 24 CT SSD patients (CT) was matched to the group of SSD patients (Non_CT) without CT symptoms (NCRS = 0; n = 22) based on age, gender, education, and OLZ equivalents (table 1). Following this approach, 2 (of 5) IC pairs showed statistically significant differences (adjusted for OLZ and PANSS total score) between patients with and without catatonia in both sMRI and rs-fMRI modalities (figure 1). Significantly lower mixing coefficients in CT patients indicated that these 4 components were less expressed in the CT group than in the Non_CT group. The first sMRI IC (frontothalamic network) predominantly comprised the posterior cingulate cortex, thalamus, cuneus, and middle frontal gyrus (supplementary table 1). The second sMRI IC (corticostriatal network) predominantly comprised the middle frontal gyrus, precentral gyrus (M1), postcentral gyrus, temporal gyrus and inferior parietal lobule (supplementary table 1). The first rs-fMRI component (frontoparietal network) had predominance in the superior, middle, and medial frontal cortices, as well as precuneus, postcentral, and inferior parietal cortex. The second rs-fMRI IC (frontotemporal/cortical motor network) had predominance in middle frontal and superior temporal gyri, as well as precentral gyrus (supplementary table 2).
Table 1.
Clinical and Demographic Variables in Schizophrenia Spectrum Disorders Patients With (n = 24) and Without (n = 22) Catatonia According to NCRS
| Patients with catatonia (n = 24) | Patients without catatonia (n = 22) | T a | Df a | Sig. (2-tailed) a | |
|---|---|---|---|---|---|
| Age | 38.92 ± 11.62 | 40.18 ± 11.83 | –.366 | 44 | .716 |
| Gender (m/f)b | 14/10 | 11/11 | — | 1 | .571 |
| Education (years) | 13.42 ± 2.41 | 13.32 ± 3.3 | .116 | 44 | .908 |
| Olanzapine equivalents | 17.17 ± 8.18 | 16.38 ± 10.54 | .285 | 44 | .777 |
| Duration of illness (years) | 13.38 ± 12.15 | 8.5 ± 9.54 | 1.503 | 44 | .14 |
| PANSS total score | 80.88 ± 21.87 | 60.09 ± 20.34 | 3.328 | 44 | .002 |
| PANSS positive score | 18.75 ± 8.62 | 13.95 ± 6.7 | 2.091 | 44 | .04 |
| PANSS negative score | 21.17 ± 9.32 | 14.59 ± 7.26 | 2.651 | 44 | .011 |
| PANSS global score | 40.96 ± 12.57 | 31.55 ± 10.22 | 2.77 | 44 | .008 |
| BPRS | 43.71 ± 14.8 | 33.73 ± 12.17 | 2.484 | 44 | .017 |
| GAF | 55.42 ± 14.73 | 74.55 ± 17.38 | –4.037 | 44 | <.001 |
| CGI-S | 4.54 ± .88 | 3.5 ± .74 | 4.313 | 44 | <.001 |
| NCRS motor score | 1.79 ± 1.31 | 0 | 6.37 | 44 | <.001 |
| NCRS affective score | 3.0 ± 1.74 | 0 | 8.058 | 44 | <.001 |
| NCRS behavior score | 2.13 ± 1.07 | 0 | 9.255 | 44 | <.001 |
| NCRS total score | 6.88 ± 2.38 | 0 | 13.518 | 44 | <.001 |
| SAS total score | 3.25 ± 2.23 | 1.95 ± 1.88 | 2.115 | 44 | .04 |
| AIMS total score | 2.21 ± 3.29 | .77 ± 2.2 | 1.72 | 44 | .092 |
| BARS global score | 1.38 ± 1.61 | .45 ± .96 | 2.326 | 44 | .025 |
Note: Data are mean ± standard deviation. Significant results (P < .05) are displayed in bold font. PANSS, Positive and Negative Syndrome Scale (p = positive, n = negative, g = global); BPRS, Brief Psychiatric Rating Scale; GAF, Global Assessment of Functioning; CGI-S, Clinical Global Impression Scale (Severity); SAS, Simpson and Angus Scale; AIMS, Abnormal Involuntary Movement Scale; BARS, Barnes Akathisia Rating Scale; NCRS, Northoff Catatonia Rating Scale.
aThe F and P values were obtained using an independent samples t-test;
bThe P values for distribution of gender were obtained by chi-square test.
Fig. 1.
Structural and functional networks exhibiting significant differences between catatonic (n = 24) and non-catatonic (n = 22) patients. Bar graphs depict the mean of mixing coefficients for each independent component (IC) in each group. The error bars represent ± 1 standard error of the mean. Analyses of covariance were used to test the effects of group on the mixing coefficients of each IC in structural magnetic resonance imaging (sMRI) and resting-state functional MRI (rs-fMRI) modalities controlled for olanzapine equivalent and Positive and Negative Syndrome Scale total score. The corresponding F and P values for each test are placed above the bar graphs. The regions in both modalities, with a threshold of |z| > 2.5, are shown in sagittal, coronal, and axial planes.
Dimensional Approach
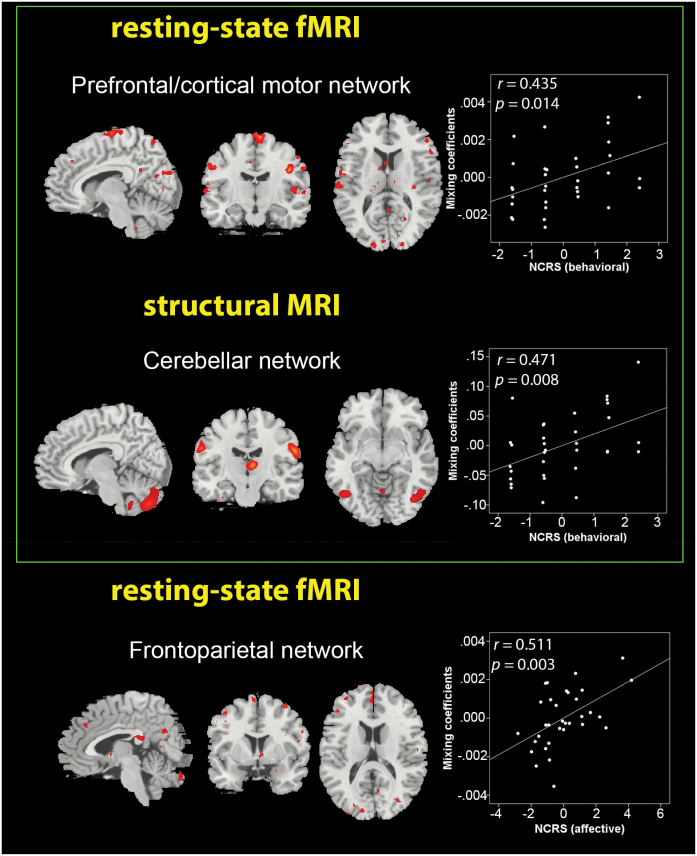
Following the dimensional (correlational) approach, 33 of 86 patients (33/86 = 38.3%) with 1 motor and at least 1 other CT symptom (behavioral or affective) were identified, resulting in a total of 33 SSD patients with catatonia according to DSM-IV-TR. Following this approach, we tested if the 3 identified IC pairs correlated with the NCRS scores when adjusted for OLZ and PANSS total score. The first sMRI IC (cerebellar network) predominantly comprised the cerebellum and the superior temporal gyrus. The first rs-fMRI IC (prefrontal/cortical motor network) had predominance in the precentral gyrus, cuneus, lingual, and middle frontal gyrus. This IC pair showed a significant correlation with NCRS behavioral scores (figure 2). The second rs-fMRI component (frontoparietal network) had predominance in the medial frontal gyrus and superior parietal lobule. This modality-specific IC showed a significant correlation with NCRS affective scores (figure 2).
Fig. 2.
Scatter plots depicting the partial correlations (adjusted for olanzapine equivalent and Positive and Negative Syndrome Scale total score) between Northoff Catatonia Rating Scale (NCRS) behavioral and affective scores in schizophrenia spectrum disorders patients with catatonia according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (n = 33) and the mixing coefficients of each independent component in each modality. Corresponding r and P values are placed above each scatter plot. Brain regions in structural magnetic resonance imaging (sMRI) and resting-state functional MRI (rs-fMRI) modalities are shown in sagittal, coronal, and axial slices, with a threshold of |z| > 2.5.
Discussion
This study aimed at investigating the direct link between intrinsic brain function and GMV in SSD patients with and without catatonia. Four main findings emerged: First, following a categorical approach, the sMRI source identified regions within the frontothalamic and corticostriatal network where patients with catatonia showed reduced GMV compared to patients without catatonia. Second, the rs-fMRI source localized regions within the frontoparietal and frontotemporal/cortical motor network where patients with catatonia showed reduced INA compared to patients without catatonia. Third, following a dimensional approach, linked INA and GMV alterations in cerebellar and prefrontal/cortical motor networks might contribute to behavioral CT symptoms. Fourth, linked INA and GMV alterations in frontotemporal and frontoparietal networks were associated with affective CT symptoms.
At present, there are different nosological definitions and diagnostic criteria of catatonia as well as unresolved conceptual issues with respect to specific catatonia dimensions, eg, the affective domain. This led to different prevalence of catatonia and inconsistent study results. A recent study showed large definition-dependent differences in the prevalence of catatonia according to Bush-Francis Catatonia Rating Scale, DSM-IV-TR and DSM-5.39 Here, we used the NCRS, because it represents a clinically acceptable compromise between conservative and liberal thresholds. We identified a higher number of SSD patients with catatonia according to DSM-IV-TR (39%) rather than according to NCRS (28.7%) criteria. This difference lies in the more strict and conservative cutoff criterion for catatonia according to NCRS compared to DSM-IV-TR.13
Co-altered INA and GMV Components in CT Patients
We detected frontothalamic and frontoparietal networks that represent joint, co-altered INA-GMV components that differ between SSD patients with and without catatonia. It is likely that GMV reduction in the frontothalamic network may affect the INA of frontoparietal circuit resulting in impaired processing of negative emotional stimuli and cessation of body movements, which can lead to affective and behavioral CT symptoms. The frontoparietal network includes the superior, middle, medial, and inferior frontal cortices, which also contain the orbitofrontal cortex (OFC). The OFC is crucial for cognitive control of emotional processing and highly interconnected with cingulate/medial prefrontal, premotor, and parietal cortical areas.10 Aberrant orbitofrontal-prefrontal/parietal cortical connectivity reflects disrupted “horizontal modulation” of cortico-cortical relation, which might lead to disturbed control and regulation of emotional stimuli and motor behavior.40,41 The second main finding reflects linked GMV and INA alterations of corticostriatal and frontotemporal/cortical motor networks suggesting that anatomical circuits between frontal cortex and M1 as well as basal ganglia (eg, a failure of striato-thalamic inhibition) are involved in catatonia.16 These networks once again illuminate the alteration in “top-down modulation” reflecting “vertical modulation” of lentiform nucleus and other basal ganglia by GABA-ergic-mediated OFC deficits. Further, we also identified a large cluster of pronounced difference between SSD patients with and without catatonia in the occipital cortex consisting of primary visual cortex and precuneus, suggesting disturbances in the processing of external spatial-related stimuli.40
Taken together, these results are highly suggestive of a potential mechanism by which the OFC cortex cannot perform its gating function on cortical and subcortical regions leading to an entirely disrupted horizontal and vertical modulation.40 With regard to clinical context, a patient with aberrant prefrontal activity might experience negative emotions and be unable to behave in a manner appropriate to the external situation.
Our findings build on previous fMRI studies on catatonia9,10,14,40,42–44 and are very much in line with a previous study of Walther et al., who found that increased functional connectivity between thalamus and M1 is associated with catatonia in SSD.16 A significant association between thalamo-sensorimotor connectivity and psychopathology was also found by Martino et al.45 The latter discussed the possibility that aberrant thalamo-sensorimotor connectivity may lead to spatiotemporal alterations of sensory input and motor output, thus contributing to the development of what has been described as “spatiotemporal psychopathology”.45–47 Similarly, our findings reflect linked INA and GMV alterations of corticostriatal and frontotemporal/cortical motor networks, suggesting that spatiotemporal disturbances of anatomical circuits between frontal cortex and M1 as well as basal ganglia are involved in catatonia.16
Co-altered INA and GMV Components Underlying Specific Catatonia Dimensions
Behavioral CT symptoms were associated with linked GMV and INA alterations in cerebellar and prefrontal/cortical motor networks. Lobules V and VI of the anterior cerebellum are crucial for fine motor and visuomotor adaptation skills.48–54 Remarkably, there might be some clinical overlap between catatonia in SSD and the cerebellar cognitive affective syndrome.55,56 In particular, the identified GMV alterations of the cerebellar network might lead to mutism, negativism, echolalia, “mitmachen,” and “mitgehen”, perseverative-compulsive behavior, all symptoms characteristic of catatonia. This is further supported by aberrant INA in the prefrontal/cortical motor network because impaired cerebellar modulation of neural circuits (cerebello-thalamic-cortical projections) that link prefrontal and parietal cortices can lead to impaired sensorimotor, cognitive, and affective processing. Finally, we found that frontoparietal INA alterations might lead to disturbed processing of negative stimuli and subsequent affective CT symptoms.57–59
Interestingly, we observed a positive correlation between coefficient loadings and catatonia symptom severity. Statistically, coefficient loading comparisons and correlations with catatonia project two different variables, the first one is a diagnostic category, the latter is parametric. It is possible to have reduced category loading, and thus the more the categorical loading is reduced, the more severe the parametrically rated CT symptoms might get. Therefore, the positive correlation should not be interpreted as a correlation between the degrees of the categories themselves (in which the positive correlation is indeed paradoxical) but as the degree of their loading (in which case the positive correlation matches perfectly with stronger loading entailing stronger CT symptoms). Taking into account the neurobiological perspective, positive correlations may reflect compensatory mechanism for the disturbed sensory input and motor output. Aberrant INA of frontoparietal circuit may lead to alterations of neuronal structure and thus cause an increase of GMV in frontothalamic and corticostriatal network.60 This is consistent with studies that showed positive relationship between symptoms and regional GMV in SSD.60,61 Taken together, disturbances of brain function and structure in SSD patients catatonia occur in conjunction with changes in the clinical state (akinetic vs excited catatonia) and illuminate that separate neurobiological mechanisms (particularly abnormal neural function and aberrant neurodevelopment) can cause CT symptoms.
Strengths and Limitations
The main strength of this study is the multimodal fusion analysis of rs-fMRI and sMRI data in SSD patients. Because of the rarity of CT symptoms in general and the even greater rarity of fMRI-eligible CT patients, we consider our sample size large enough to test our hypotheses. However, our work is not without limitations. First, the lack of healthy controls may be a potential limitation of this study. Second, our study did not include patients with CT schizophrenia according to ICD-10, who had a previous, but now remitted, CT episode. Including such individuals could be a potential advantage, at least with respect to symptom stability, ie, prominent CT symptoms being present for at least 2 weeks. At the same time, such patients are very frequently individuals who are in need of immediate treatment, predominantly including electroconvulsive therapy (ECT) and/or with higher dosages of benzodiazepines. Investigating such patients by means of MRI is particularly challenging, because written informed consent cannot be obtained in a substantial proportion of cases. Moreover, a high degree of positive symptom load can introduce further bias, as much as benzodiazepines or ECT can do with respect to brain function in these patients. Therefore, the results of this study can be interpreted as “state” anomalies (neurobiological underpinnings) of catatonia in SSD. Third, the lack of correction for multiple comparisons in the categorical analyses is an important limitation. A conservative correction (ie, using the Bonferroni method) for multiple comparisons would have rendered our findings nonsignificant, at the cost of an increased likelihood of false-negative findings. We fully acknowledge the difficulties of this trade-off. Yet, at the same time, we also believe that the neurobiological plausibility of our components and the regional overlap between our study and other MRI studies on catatonia are a further indication of the importance of the identified components.33
Conclusion
Our results provide a novel mechanistic insight on catatonia in SSD. The data-driven fusion method called mCCA + jICA highlights the importance of co-altered structure/function interactions in neural systems subserving coordinated visuospatial functions, emotional processing, and motor behavior. Our observed network-level effects illuminate catatonia as a multidimensional construct in which the dysfunction of motor, affective and behavioral processes occurs in parallel is subserved by distinct neural systems.
Funding
German Research Foundation (DFG; grant number DFG HI 1928/2-1 to D.H. and WO 1883/6-1 to R.C.W.). The DFG had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Supplementary Material
Acknowledgments
We are grateful to all the participants and their families for their time and interest in this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Northoff G, Koch A, Wenke J, et al. . Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14(3):404–416. [DOI] [PubMed] [Google Scholar]
- 3. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;127(441):1–47. [DOI] [PubMed] [Google Scholar]
- 4. Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36(2):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Francis A, Fink M, Appiani F, et al. . Catatonia in diagnostic and statistical manual of mental disorders, fifth edition. J ECT Dec. 2010;26(4):246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1–3):256–262. [DOI] [PubMed] [Google Scholar]
- 7. Kirby GH. The catatonic syndrome and its relation to manic-depressive insanity. J Nerv Ment Dis. 1913;40:694–704. [Google Scholar]
- 8. Lange J. Katatonische Erscheinungen im Rahmen manisch-depressiver Erkrankungen. Berlin: Springer; 1922. [Google Scholar]
- 9. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555–577; discussion 578. [DOI] [PubMed] [Google Scholar]
- 10. Northoff G, Kötter R, Baumgart F, et al. . Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull. 2004;30(2):405–427. [DOI] [PubMed] [Google Scholar]
- 11. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Verlag August Hirschwald; 1874. [Google Scholar]
- 12. Scozzafava J, Aladdin Y, Jickling G, Asdaghi N, Hussain M, Giuliani F. Stuporous catatonia and white matter lesions. J Clin Neurosci. 2009;16(10):1328, 1386. [PubMed] [Google Scholar]
- 13. Hirjak D, Kubera KM, Northoff G, et al. . Cortical contributions to distinct symptom dimensions of catatonia [published online ahead of print February 7, 2019]. Schizophr Bull. 2019. doi:10.1093/schbul/sby192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walther S, Schäppi L, Federspiel A, et al. . Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43(5):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janova H, Arinrad S, Balmuth E, et al. . Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest. 2018;128(2):734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sui J, He H, Liu J, et al. . Three-way FMRI-DTI-methylation data fusion based on mCCA+jICA and its application to schizophrenia. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:2692–2695. [DOI] [PubMed] [Google Scholar]
- 18. Sui J, He H, Pearlson GD, et al. . Three-way (N-way) fusion of brain imaging data based on mCCA+jICA and its application to discriminating schizophrenia. Neuroimage. 2013;66:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He H, Sui J, Du Y, et al. . Co-altered functional networks and brain structure in unmedicated patients with bipolar and major depressive disorders. Brain Struct Funct. 2017;222(9):4051–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugranyes G, Kyriakopoulos M, Dima D, et al. . Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophr Res. 2012;138(2–3):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ungvari GS, Caroff SN, Gerevich J. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophr Bull. 2010;36(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heckers S, Tandon R, Bustillo J. Catatonia in the DSM–shall we move or not? Schizophr Bull. 2010;36(2):205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 24. Sass H, Wittchen HU, Zaudig M, Houben I. Diagnostisches und Statistisches Manual Psychischer Störungen DSM-IV-TR: Textrevision. Göttingen: Hogrefe Verlag; Auflage: 1 (1. Januar 2003); 2003. [Google Scholar]
- 25. Leucht S, Samara M, Heres S, et al. . Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirjak D, Thomann PA, Northoff G, Kubera KM, Wolf RC. NCR-Skala—Deutsche version der Northoff Catatonia Rating Scale (NCRS-dv)—ein validiertes messinstrument zur erfassung katatoner symptome. Nervenarzt. 2017; 88(7):787–796. [DOI] [PubMed] [Google Scholar]
- 27. Dazzan P, Morgan KD, Orr KG, et al. . The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127(Pt 1):143–153. [DOI] [PubMed] [Google Scholar]
- 28. Gay O, Plaze M, Oppenheim C, et al. . Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. 2013;39(4):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 30. Yan C, Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Y, Zhuo C, Qin W, Zhu J, Yu C. Altered spontaneous brain activity in schizophrenia: a meta-analysis and a large-sample study. Biomed Res Int. 2015;2015:204628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friston KJ, , Williams S,, Howard R, , Frackowiak RS,, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–355. [DOI] [PubMed] [Google Scholar]
- 33. Lottman KK, White DM, Kraguljac NV, et al. . Four-way multimodal fusion of 7 T imaging data using an mCCA+jICA model in first-episode schizophrenia. Hum Brain Mapp. 2018;39(4):1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li YO, Adalı T, Wang W, Calhoun VD. Joint blind source separation by multi-set canonical correlation analysis. IEEE Trans Signal Process. 2009;57(10):3918–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–1222. [DOI] [PubMed] [Google Scholar]
- 37. Sambataro F, Visintin E, Doerig N, et al. . Altered dynamics of brain connectivity in major depressive disorder at-rest and during task performance. Psychiatry Res Neuroimaging. 2017;259:1–9. [DOI] [PubMed] [Google Scholar]
- 38. Sui J, He H, Yu Q, et al. . Combination of resting state fMRI, DTI, and sMRI data to discriminate schizophrenia by N-way MCCA + jICA. Front Hum Neurosci. 2013;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stuivenga M, Morrens M. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry. 2014;5:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Northoff G. Brain imaging in catatonia: current findings and a pathophysiologic model. CNS Spectr. 2000;5(7):34–46. [DOI] [PubMed] [Google Scholar]
- 41. Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. 2015;169(1–3):217–233. [DOI] [PubMed] [Google Scholar]
- 42. Northoff G, Nagel D, Danos P, Leschinger A, Lerche J, Bogerts B. Impairment in visual-spatial function in catatonia: a neuropsychological investigation. Schizophr Res. 1999;37(2):133–147. [DOI] [PubMed] [Google Scholar]
- 43. Northoff G, Steinke R, Czcervenka C, et al. . Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Northoff G, Steinke R, Nagel DCzerwenka C, et al. . Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol Med. 2000;30(3):583–596. [DOI] [PubMed] [Google Scholar]
- 45. Martino M, Magioncalda P, Yu H, et al. . Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naïve patients with schizophrenia. Schizophr Bull. 2018;44(2):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Northoff G. Spatiotemporal psychopathology II: how does a psychopathology of the brain’s resting state look like? Spatiotemporal approach and the history of psychopathology. J Affect Disord. 2016;190:867–879. [DOI] [PubMed] [Google Scholar]
- 47. Northoff G. Spatiotemporal psychopathology I: no rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. J Affect Disord. 2016;190:854–866. [DOI] [PubMed] [Google Scholar]
- 48. Bernard JA, Seidler RD. Cerebellar contributions to visuomotor adaptation and motor sequence learning: an ALE meta-analysis. Front Hum Neurosci. 2013;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernard JA, Seidler RD. Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum. 2013;12(5):721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. [DOI] [PubMed] [Google Scholar]
- 51. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–378. [DOI] [PubMed] [Google Scholar]
- 53. Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(Pt 2):290–292. [DOI] [PubMed] [Google Scholar]
- 54. Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23(1-2):65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998;2(9):362–371. [DOI] [PubMed] [Google Scholar]
- 56. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121 (Pt 4):561–579. [DOI] [PubMed] [Google Scholar]
- 57. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62(4): 404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Northoff G, Waters H, Mooren I, et al. . Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry Res. 1999;91(1):45–54. [DOI] [PubMed] [Google Scholar]
- 59. Ebert D, Feistel H, Kaschka W. Left temporal hypoperfusion in catatonic syndromes: a SPECT study. Psychiatry Res. 1992;45(4):239–241. [DOI] [PubMed] [Google Scholar]
- 60. Nesvåg R, Saetre P, Lawyer G, Jönsson EG, Agartz I. The relationship between symptom severity and regional cortical and grey matter volumes in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):482–490. [DOI] [PubMed] [Google Scholar]
- 61. Szeszko PR, Bilder RM, Lencz T, et al. . Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90(1):1–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.