Abstract
Background
Relapse risk during the early years of first-episode psychosis (FEP) considerably increases the risk of chronicity. The effectiveness of family intervention for psychosis (FIp) for preventing relapse after FEP remains unknown. We assessed the effectiveness of FIp until 24 months of follow-up for preventing relapse and other relapse-related outcomes in patients following FEP.
Methods
We searched the Cochrane, PubMed, PsycINFO, and ProQuest databases in June 2018. A systematic review with meta-analysis of randomized controlled trials (RCTs), sensitivity analyses, and publication bias were performed, comparing to treatment as usual (TAU) or TAU plus other psychosocial interventions. Outcomes assessed were relapse rates, duration of hospitalization, psychotic symptoms, and functionality. Risk ratios (RRs) and (standardized) mean differences (SMD; MD) were calculated.
Results
Of the 2109 records retrieved, 14 (11 RCTs) were included. Pooled results showed that FIp was effective for preventing relapse (RR = 0.42; 95% CI = 0.29 to 0.61) compared to TAU and/or other psychosocial interventions. It also proved effective when compared to TAU alone (RR = 0.36) and TAU plus other psychosocial interventions (RR = 0.48). FIp showed benefits in reducing duration of hospitalization (TAU, MD = −3.31; other interventions, MD = −4.57) and psychotic symptoms (TAU, SMD = −0.68), and increased functionality (TAU, SMD = 1.36; other interventions, SMD = 1.41).
Conclusions
These findings suggest that FIp is effective for reducing relapse rates, duration of hospitalization, and psychotic symptoms, and for increasing functionality in FEP patients up to 24 months. The study’s main limitations were the inclusion of published research only; authors were not contacted for missing/additional data; and high heterogeneity regarding relapse definition was observed.
Keywords: family intervention, psychosis, relapse, randomized controlled trial, systematic review, meta- analysis
Introduction
Schizophrenia is a severe mental disorder, characterized by profound disruptions in thinking, affective language, perception, and the sense of self. This condition can impair functioning through the loss of an acquired capability to earn a livelihood, or the disruption of studies.1 Schizophrenia affects more than 21 million people worldwide.1 This mental disorder has a negative impact at a personal, family, and social level, resulting in neurological decline2 accompanied by a range of cognitive deficits,3 neurological soft signs,4 and functional5 and structural6 brain alterations, which translate into a huge economic burden associated with disability and indirect costs due to lost productivity.7
Although antipsychotics have shown to significantly reduce positive symptoms of schizophrenia,8 no substantial improvements in negative symptoms, cognitive deficits, or social functioning have been reported in earlier literature.9 Furthermore, medication has some secondary effects which can cause low treatment adherence, thus increasing the risk of relapse.10 Psychosocial interventions play a critical role in enhancing the patient’s overall level of functioning, quality of life, and compliance with prescribed treatments that can help reduce the risk of relapse.11,12
A number of psychosocial interventions developed specifically for schizophrenia have received considerable empirical support. The most widely recommended psychosocial interventions are cognitive behavioral therapy (CBT) and family intervention for psychosis (FIp).13 Psychosocial interventions have shown to be an essential complement to pharmacological treatment in reducing psychotic symptoms8; in improving coping skills and psychosocial, emotional and behavioral adjustment14; and even in lessening the burden of family-related caregivers.15
The chronic course of schizophrenia emphasizes the need to implement interventions early on in this disorder.16 First-episode psychosis (FEP) frequently occurs during adolescence and young adulthood; it is also associated with high stress levels, affective disorders, and suicide.2 The first 5 years following FEP is seen as a critical period which entails increased cognitive decline,17,18 and the risk of relapse is a predictive factor for the disease’s trajectory.17 It is estimated that 85% of patients relapse during the first few years of FEP, resulting in increased risk of chronicity and suicidal behaviors as well as impaired functional ability.19 Hence, intervention soon after FEP is a useful strategy for preventing relapse and reducing disability due to schizophrenia.2
The role played by the relatives of patients with FEP is a topic of growing interest. The onset of FEP often manifests itself while the patient is still living at home with their parents.20 Previous research has shown that FIp is effective for reducing relapse, improving social functioning, and mitigating the severity of psychotic symptoms and levels of expressed emotion in relatives at a more advanced stage of illness.21,22 However, FIp’s efficacy in preventing future relapses in the early stages remains unknown, and current evidence reports some inconsistencies23 which preclude us from drawing firm conclusions.10,24 Three previously published reviews assessed the efficacy of FIp when it comes to preventing future relapses: in a systematic review with no meta-analysis, the authors found FIp to be effective24; in a meta-analysis which included only one trial, efficacy was nonsignificant10; and in a review published in 2007, the authors observed how FIp may even be iatrogenic for patients with FEP.23 However, a limited number of trials were included and identified in the aforementioned published reviews. Thus, further efficacy research is needed which includes more trials with meta-analysis to assesses FIp among patients with FEP in order to obtain evidence-based results on effectiveness.
The aim of this systematic review with meta-analysis is to assess the effectiveness of FIp for preventing second-episode psychosis (SEP) and other relapse-related outcomes until 24 months of follow-up in FEP patients within the first 5 years of onset compared to treatment as usual (TAU) and/or TAU plus other active psychosocial interventions including randomized controlled trials (RCTs) only.
Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.25 Please see supplementary material 1 for the PRISMA checklist.
Eligibility Criteria
We included studies that (a) published RCTs; (b) compared FIp delivered ≥6 months (The Schizophrenia Patient Outcomes Research Team [PORT] recommends that FIp should be delivered for at least 6 to 9 mo26) plus a pharmacotherapy condition according to individual study criteria (TAU) to either TAU or TAU plus non-FIp psychosocial interventions (eg, psychoeducation); (c) encompassed individuals with FEP <5 years; and (d) reported any post-intervention relapse (primary measure) or other relapse-related outcomes up to 24 months of follow-up. Secondary outcomes were duration of hospitalization, severity of psychotic symptoms, and functionality. TAU and relapse were defined as per criteria established in individual studies. Specifically, relapse was defined by authors when trials adopted pre-specified criteria or through rehospitalization due to exacerbated psychotic symptoms.
Search Strategy
Our primary search strategy was to conduct a database search of the Cochrane Library, PubMed, PsycINFO, and ProQuest; this was carried out on June 15, 2018. The following terms were searched and combined using the Boolean AND operator: ((“recent-onset” OR “early-onset” OR “first-episode” OR “early onset” OR “initial phase of” OR “early”) AND (“psychosis” OR “schizophrenia”) AND (“family intervention” OR “family therapy”) AND (“randomized controlled trial” OR “randomised controlled trial” OR “randomised-control trial” OR RCT)). The secondary strategy involved searching the reference list of included studies and relevant reviews. We only took into account articles published in English or Spanish.
Study Selection
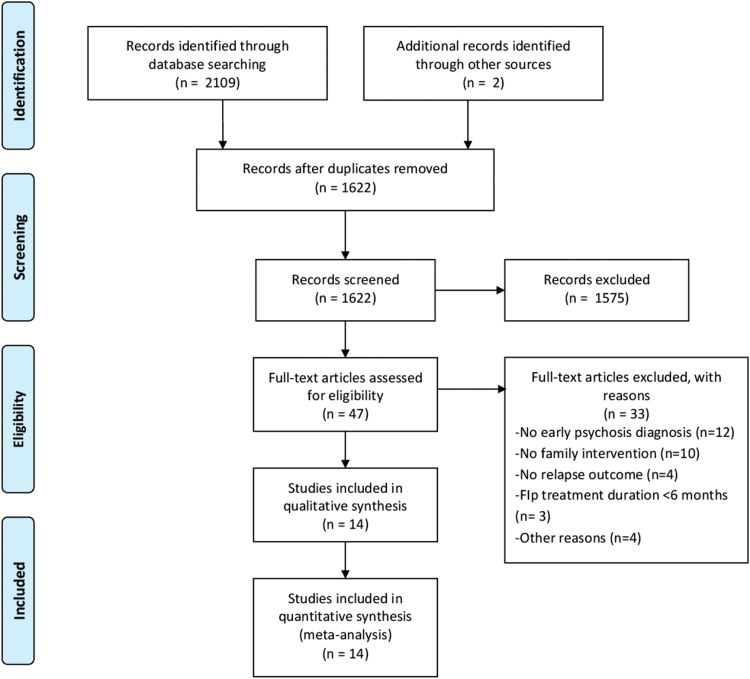
The first author (M.C-G.) conducted the search. Once the database outputs were combined, all duplicate records were removed, and the titles and abstracts were screened (by M.C-G.). During the title and abstract review, we did not exclude any trials based on the outcome of interest (relapse or other relapse-related outcomes). Thus, we maximized the identification of relevant articles. This was because measures of relapse and other relapse-related outcomes are typically a secondary measure reported in primary studies, and are seldom mentioned in the title or abstract. Both authors (M.C-G. and P.C.) independently reviewed the full text and extracted data. They then came together to discuss all trials with study inclusion and data extraction inconsistencies. Decisions were made on these trials after consensus was reached. In total, 14 articles (11 RCTs) met full inclusion criteria. They were taken from 1622 initially screened records after excluding duplicates, of which 1575 were discarded. Forty-seven articles were then full-text reviewed, resulting in 33 exclusions. Reasons for exclusion were as follows: 12 trials did not include patients with FEP; 10 trials did not deliver FIp; 4 trials did not assess relapse; 2 trials (3 articles) delivered FIp intervention <6 months; and 4 trials were excluded for other reasons (figure 1). Reasons for exclusion of full-text review references are presented in supplementary material 2.
Fig. 1.
PRISMA flowchart of literature search.
Data Collection
The following data were collected: (a) sample size; (b) clinical and sociodemographic characteristics (age, patient type, diagnostic criteria); (c) type of FIp and comparison group delivered; (d) qualitative and quantitative results of primary and secondary measures; (e) quality assessment indicators; and (f) duration of follow-up.
Quality Assessment
Trial validity was measured using criteria from the Cochrane Collaboration’s tool for assessing risk of bias.27 This tool assesses potential sources of bias in RCTs, including (a) the adequate generation of allocation sequence; (b) the concealment of allocation to treatment conditions; (c) blinding of participants and personnel; (d) blinding of outcome assessors; (e) handling of incomplete data; (f) selective outcome reporting; and (g) other potential sources of bias. Participant and personnel blinding was assessed; however, it was not included in the general bias assessment given the difficulty in masking any condition groups for participants and personnel. FIp is inherently different from pharmacological conditions, and results may be biased for this domain. When it comes to multi-component therapies, relatives and even patients must receive training in intervention group assignment. Furthermore, the authors argue that they can easily identify the allocated arm. Both MCG and PC carried out the assessments. Ratings were cross-checked and any discrepancies was discussed and resolved.
Meta-analysis
Meta-analyses were performed for the 2 main comparisons: (a) FIp versus TAU and (b) FIp versus TAU plus alternative psychotherapy approaches. For the dichotomous variables, combined risk ratios (RRs) were estimated with 95% CIs. For the continuous variables, the mean difference (MD) was estimated when outcome measurements across all studies were made using the same scale. The standardized mean difference (SMD) was used when an outcome was measured using different quantitative scales.28 Pooled estimates were assessed up to 24 months of follow-up.
We used random-effects meta-analyses to obtain pooled estimates, given that we observed between-study differences in several aspects, eg, (a) FIp content and methodology delivered to relatives; (b) duration of FIp delivered; (c) outcome measures and definition criteria; (d) follow-up assessments; and (e) clinical and sociodemographic characteristics (age, country, diagnostic criteria, and patient type).
To avoid unit of analysis problems, data from the Chien and Chan29 (which delivered a design-two FIp arm and one TAU) were combined into a single group using each formula to obtain pooled means and SDs into a single sample size, respectively:
Where Nx is the sample size, Mx is the mean, and SDx is the standard deviation of each group 1 and 2, respectively.
Heterogeneity was assessed using the I2 statistic. This test assesses the degree of heterogeneity, where a value of 0% to 40% indicates no observed heterogeneity; 30% to 60% shows moderate heterogeneity; 50% to 90% indicates substantial heterogeneity; and 75% to 100% shows considerable heterogeneity.30
We performed sensitivity analyses when substantial or considerable heterogeneity was detected for trials included in the meta-analyses. The following variables, except for the third criterion, were pre-specified in advance. They were considered moderating factors and, consequently, possible sources of heterogeneity. We excluded trials which were rated poor quality according to Agency for Healthcare Research and Quality (AHRQ) standards.27,31 Poor quality was defined as one unmet criterion (eg, high risk of bias for one domain) or 2 unclear criteria, whose assessment was likely to have biased the outcome and pose important limitations that could invalidate the results, or 2 or more criteria with high or unclear risk of bias. We further excluded trials covering other psychosocial therapies in addition to FIp in the experimental group (multi-component integrated therapies). Finally, we discarded trials that adopted an instrument for assessing psychotic symptom severity and functionality that differs from most other trials. Studies classified as poor quality or at high risk of bias were included to improve understanding of any quality domain and transparency. However, we excluded them from our sensitivity analyses to determine whether or not methodological quality represented a source of heterogeneity.
Publication bias was assessed by inspecting the included trials through a funnel plot (scatterplot of treatment effect against a measure of study size).32 In the absence of bias, the plot should resemble a symmetrical inverted funnel. An asymmetric funnel indicates a relationship between treatment effect and study size. This suggests the possibility of either publication bias or a systematic difference between smaller and larger studies. Namely, if publication bias exists, the largest published studies would likely report the smallest effects.32
Review Manager (RevMan) software was used to conduct all statistical analyses.33
Results
Characteristics of Included Studies
Eleven trials (14 articles)29,34–49 met full inclusion criteria, encompassing a total of 1360 patients with FEP (table 1). Five trials were developed in Asia, 5 in Europe, and 1 in Australia. Patient ages across studies ranged from 16 to 40 years. Eight trials diagnosed mental disorder using DSM-IV criteria. Parents who participated in the study were the patients’ main caregivers. The sample size of participating family members ranged from 1 to 15 per patient, the majority aged between 30 and 50 years. Both parents took part in most trials.
Table 1.
Characteristics of Included Studies
| Study (y), Country | Patient Type | Diagnostic Criteria | Age (Mean) | Sample Size | Therapy Type | Comparison Group | Duration of Therapy | Follow-up | Adherence Rates |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. (1994), China | FEP with schizophrenia | CMA | 23.8 | 78 | FIp focused on psychoeducation, mutual support and problem-solving + TAU | TAU (standard hospital out-patient service) | 18 mo | 18 mo | 100% |
| Linzsen et al. (1996), The Netherlands | FEP with schizophrenia | DSM-IV | 20.6 | 76 | FIp based on manual (Falloon et al50): psychoeducation, communication skills and problem- solving + TAU | TAU + psychiatric management, individual education for patients and training in recognizing EWS of relapse | 12 mo | 12 mo | 100% |
| Leavey et al. (2004), United Kingdom | FEP | ICD-10 | No data | 106 | FIp based on manual (Falloon et al50): psychoeducation, communication skills and problem- solving + TAU | TAU (standard treatment from psychiatric services) | 9 mo | 9 mo | 43% |
| Petersen et al. (2005); (Secher et al. 2015), Denmark | FEP | ICD-10 | 26.6 | 547 | Assertive community treatment provided by multidisciplinary teams (1:10 caseload): social skill training, FIp (psychoeducational multi- family groups focusing on problem-solving procedures) and low-dose SGA | TAU (standard treatment at community mental health services, including contact with physician, nurse and social worker) | 24 mo (FIp, 18 mo) | 10 y | 63% |
| Chien et al. (2006), Hong Kong | FEP with schizophrenia | DSM-IV | 27.3 | 96 | FIp focused on psychoeducation, mutual support and problem-solving + TAU | 2 groups: TAU (hospital out- patient service); TAU + group psychoeducation for patients | 6 mo | 18 mo | 78% |
| Chien & Wong (2007), Hong Kong | FEP with schizophrenia | DSM-IV | 28.1 | 84 | FIp based on manual (McFarlane et al51): structured psychoeducation and problem- solving + TAU | TAU (standard hospital out-patient service) | 6 mo | 12 mo | 100% |
| Chien (2008), Hong Kong | FEP with schizophrenia | DSM-IV | 25 | 68 | FIp focused on psychoeducation and mutual support + TAU | TAU (standard hospital out-patient service) | 8 mo | 12 mo | 94% |
| Gleeson et al. (2009, 2013), Australia | FEP | DSM-IV | 20.1 | 81 | Relapse prevention therapy: individual therapy and FIp (psychoeducation, problem- solving and relapse prevention) + TAU | TAU (standard treatment from EPPIC program) | 9 mo | 30 mo | 72% |
| Palma- Sevillano et al. (2011), Spain | FEP with schizophrenia | DSM-IV | 24 | 34 | Motivational therapy: CBT and FIp (psychoeducation, communication skills and problem-solving) + TAU | TAU (standard treatment for the initial phase of schizophrenia) | 12 mo | 24 mo | 94% |
| Chien & Chan (2013), Hong Kong | FEP with schizophrenia | DSM-IV | 24.3 | 135 | 2 FIp groups: (a) Family-led mutual support groups and (b) Group psychoeducation for caregivers (based on McFarlane et al51) +TAU | TAU (standard hospital out-patient service) | 9 mo | 24 mo | 94% |
| Calvo et al. (2014, 2015), Spain | Adolescents with FEP | DSM-IV | 16.1 | 55 | FIp based on manual (McFarlane et al51): structured psychoeducation and problem- solving + TAU | TAU (individual psychiatric management) + non- structured group intervention for caregivers and patients | 6mo | 24mo | 93% |
Note: CBT, Cognitive behavioral therapy; CMA, Chinese Medical Association; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; EWS, early warning signs; FEP, first-episode psychosis; FI, family intervention; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th revision; TAU, treatment as usual.
Of these 11 trials, 5 delivered FIp to relatives with the patient present. In 6 trials,29,36,37,39,44,48,49 FIp was an adaptation of the manual published by other authors50,51; and in 3 trials FIp was administered by including individual and family multi-component integrated treatment, eg, cognitive behavioral therapy (CBT) and relapse prevention therapy (RPT).41–43,48,49 Zhang et al35 focused on educational and family group sessions plus antipsychotic medication. Chien et al38 delivered a mutual support group centered around emotional expression, education, coping skills, and problem-solving; Chien40 focused on psychoeducation and support group approaches; and Chien and Chan29 held 2 FIp groups: (a) family-led mutual support groups; and (b) group psychoeducation for caregivers, both comprising 14 two-hour long group sessions. Seven trials compared FIp to TAU, and 3 trials compared FIp to TAU plus active psychotherapies other than FIp. In all trials that administered TAU plus other active psychotherapies as the comparison group, one trial administered TAU plus group psychoeducation for patients38; and 2 trials administered TAU plus individual or group education for patients or caregivers (the first delivered TAU plus individual education for patients36 and the second TAU plus non-structured group intervention for caregivers and patients44).
In one trial, 2 comparison groups (TAU and TAU plus psychoeducation)42 were extracted from the same sample, and no overall meta-analysis combining these groups was conducted when these trials were included in the meta-analysis. This applied to duration of hospitalization. A study should contribute several independent comparisons, and the inclusion of multiple groups from a single study can create a unit-of-analysis error due to the unaddressed correlation between the estimated intervention effects from multiple comparisons.28 As such, not including multiple groups from one study in a given meta-analysis is recommended.
Duration of FIp intervention ranged from 6 to 18 months. Post-treatment follow-up lasted between 6 and 24 months. Adherence, defined as the percentage of the sample who completed the FIp intervention, was ≥63% in most trials; adherence was <50% in only one trial.37 Duration of hospitalization was measured as the number of days admitted to hospital following SEP. Patient functioning was assessed using the Specific Levels of Functioning Scale (SLOF)52 and one trial adopted the Global Assessment of Functioning (GAF). Severity of psychotic symptoms was scored according to the Brief Psychiatric Rating Scale (BPRS)53 and the Positive and Negative Syndrome Scale (PANSS).54
The authors’ definitions of relapse were taken from 6 trials that considered relapse when assessing FIp efficacy. They were: (a) hospitalization in 4 trials; (b) ratings from 3 (mild) or below increasing to 6 or 7 (severe and very severe) on any of the following 3 BPRS items ((1) unusual thought content, (2) hallucinations, and (3) conceptual disorganization, meeting 1-wk duration criterion) in one trial41,43; and (c) the presence of all these 3 criteria: (1) recurrent or exacerbated psychotic symptoms explicitly recorded in psychiatric notes; (2) a significant increase in antipsychotic medication; and (3) psychotic symptoms persisting for at least 1 week in one trial36 (table 2).
Table 2.
Relapse Rates of Studies Included in the Meta-analysis
| Study | Nº (FIG/CG) | Relapse Criteria | Relapse Rates n/N (%) | Decrease in Relapse |
|---|---|---|---|---|
| Zhang et al. (1994) | FIG: 32 CG: 31 |
Hospitalization | FIp: 5/32 (15.4%) CG: 17/31 (53.8%) |
Significant |
| Linszent et al. (1996) | FIG: 37 CG: 39 |
Relapse defined as: (1) recurrent or exacerbated psychotic symptoms explicitly recorded in psychiatric notes; (2) a significant increase in antipsychotic medication; and (3) psychotic symptoms persisting for at least 1 wk. All 3 criteria must be present | FIp: 10/37 (27%) CG: 18/39 (50%) |
Not significant |
| Leavey et al. (2004) | FIG: 57 CG: 49 |
Hospitalization | FIp: 6/57 (10.52%) CG: 6/49 (12.24%) |
Not significant |
| Gleeson et al. (2010, 2013) | FIG: 41 CG: 40 |
Ratings from 3 (mild) or below increasing to 6 or 7 (severe and very severe) on any of the following 3 BPRS* items: (1) unusual thought content, (2) hallucinations, and (3) conceptual disorganization, meeting 1-wk duration criterion | 7 mo, FIp: 2/34 (5.9%) CG: 8/37 (21.6%) 30 mo, FIp: 9/30 (30%) CG: 13/30 (43.3%) |
Significant Not significant |
| Palma-Sevillano et al (2011) | FIG: 21 CG:13 |
Hospitalization | FIp: 4/21 (19%) CG: 9/13 (69.2%) |
Not significant |
| Calvo et al (2014, 2015) | FIG: 27 CG: 25 |
Hospitalization | FIp: 4/27 (13%) CG: 12/25(50%) |
Not significant |
Note: BPRS, Brief Psychiatric Rating Scale; CG, comparison group; FIF, family intervention group; FIp, family intervention for psychosis.
Risk of Bias Assessment
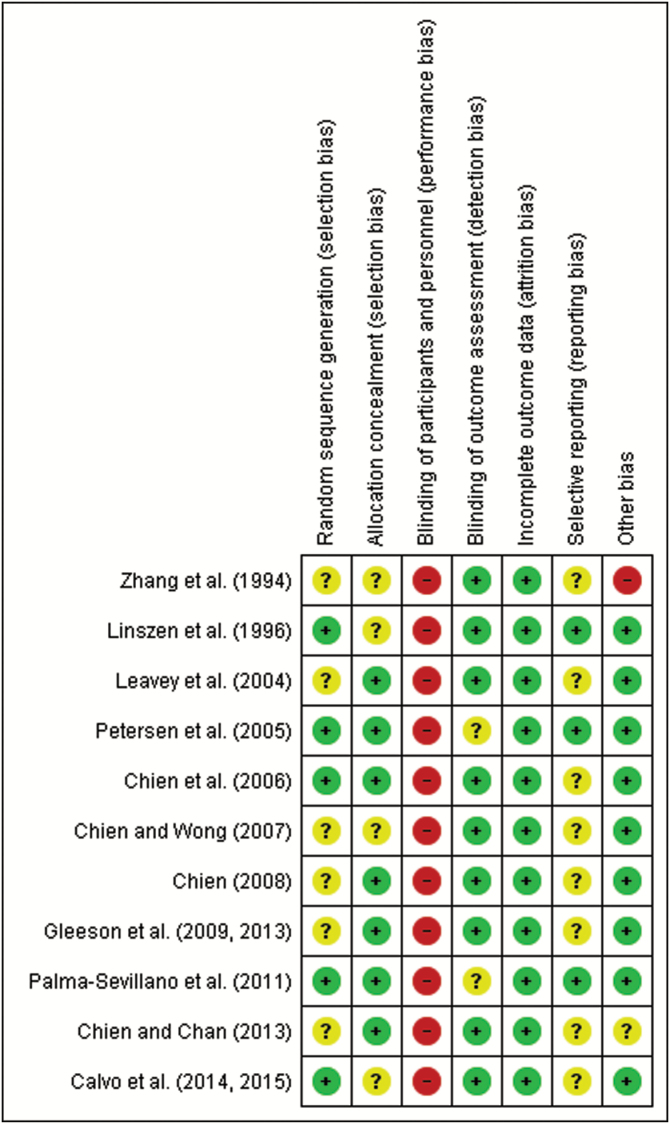
We assessed risk of bias across all domains of the Cochrane Collaboration’s tool,31 except for performance bias which has an inherent inability to mask individuals. Following assessment, the quality of included trials varied. Five trials reported adequate sequence generation; 7 trials reported adequate allocation concealment; 9 trials blinded relapse assessment; all trials reported attrition rates; only 2 trials reported enough outcomes; only one trial showed high risk of bias of other bias because the authors only included male subjects; and one trial reported an unclear risk of bias as the authors did not declare any funding sources (figure 2). Four trials met 5 good quality criteria, 4 trials met 4 criteria, 2 trials met 3 criteria, and just one trial met 2 criteria. According to AHRQ standards, 4 trials were rated good quality, 4 fair quality, and 3 poor quality.
Fig. 2.
Assessment of risk of bias in included studies.
Efficacy of Family Intervention for Preventing Relapses
Six comparisons were included in the meta-analysis (table 2 shows relapse rates during follow-up across all trials included in the meta-analysis). In global terms, the meta-analysis showed significant reductions in relapse during follow-up in favor of FIp intervention up to 24 months of follow-up (RR = 0.42; 95% CI = 0.29 to 0.61) with no observed heterogeneity (I2 = 1%). Sub-group analysis showed significant relapse reduction rates for FIp compared to TAU (RR = 0.36; 95% CI = 0.21 to 0.63) and other active interventions (RR = 0.48; 95% CI = 0.27 to 0.86), both with no observed heterogeneity (figure 3). Sensitivity analysis was not performed.
Fig. 3.
Relapse-risk differences in first-episode psychosis (FEP) patients in studies comparing family intervention for psychosis (FIp) with treatment as usual (TAU) and/or TAU plus other active interventions.
Efficacy of Family Intervention for Preventing Other Outcomes Related to Relapse
Duration of Hospitalization (Days).
Eight FIp versus TAU comparisons on days of hospital readmission were included. Meta-analysis showed a significant mean reduction of 3.31 days in patients and relatives administered FIp compared to TAU at 24 months of follow-up (MD = −3.31; 95% CI = −6.48 to −0.14), with substantial heterogeneity (I2 = 71%). When compared to other active interventions, a reduction of 4.57 days in favor of FIp (MD = −4.57; 95% CI = −7.49 to −1.65) was observed, indicating an absence of heterogeneity (supplementary material 3).
After conducting sensitivity analyses on trials excluding those that administered multi-component therapy,41,43,48,49 we found reduced heterogeneity (I2 = 39%), effective for fewer days of hospitalization when comparing FIp to TAU (MD = −4.80; 95% CI = −7.15 to −2.45). No reduction in heterogeneity was observed when we excluded low-quality studies29,39,40 (supplementary material 6).
Psychotic Symptoms.
Six comparisons were included in the meta-analysis. Patients with FEP subject to FIp intervention experienced a statistically significant reduction in psychotic symptoms compared to TAU at 24 months of follow-up (SMD = −0.68; 95% CI = −1.14 to −0.22), with substantial/considerable heterogeneity (I2 = 76%). Sensitivity analyses excluding low-quality and high-risk-of-bias trials (I2 = 76%),29,35,39 trials that included other psychosocial therapies in addition to FIp (I2 = 78%),41–43 and the trial that assessed psychotic symptoms using the PANSS (I2 = 80%)42 showed no reductions in heterogeneity (supplementary material 6). Patients with FEP experienced nonsignificant reductions in psychotic symptoms compared to TAU plus other active interventions (SMD = −0.27; 95% CI = −0.82 to 0.28). Heterogeneity was not applicable (supplementary material 4).
Functionality
Six comparisons were included in the meta-analysis. FIp showed a statistically significant improvement in functionality level during follow-up compared to TAU at 24 months of follow-up (SMD = 1.36; 95% CI = 0.59 to 2.12) with considerable heterogeneity (I2 = 94%), and to other active psychosocial interventions (SMD = 1.41; 95% CI = 0.87 to 1.96). Heterogeneity was not applicable (supplementary material 5).
Heterogeneity was not reduced after having performed sensitivity analyses on the functionality meta-analysis comparing FIp to TAU, when we excluded low-quality trials (I2 = 96%),29,39 excluding those trials that delivered multi-component therapy (I2 = 73%)48,49 and those that assessed functionality using the GAF scale (I2 = 73%).48,49 (supplementary material 6).
Publication Bias Analysis
There was no indication of asymmetry on the funnel plot for relapse rates, duration of hospitalization, or psychotic symptoms. For functionality, visual inspection suggests the presence of publication bias. This funnel plot suggest that positive results have more probability to be published than negative results (supplementary material 7). However, these results must be interpreted with caution given the limited number of trials included on the funnel plot.
Discussion
This meta-analysis examined the effectiveness of FIp for FEP on relapse, and on secondary relapse-related outcomes such as duration of hospitalization, severity of psychotic symptoms, and functionality up to 24 months. FIp was found to be more effective than TAU and TAU plus other psychosocial interventions. Psychoeducation and individual psychiatric management with education for patients and group education for caregivers and patients were compared at post-treatment in reducing relapse and duration of hospitalization, severity of psychotic symptoms, and in improving functionality in individuals with FEP up to 24 months post-treatment. Up to 24 months, estimated relative risk showed a 58% reduction in relapse risk; less days of hospitalization and reduced psychotic symptoms; and a significant improvement in functionality. These reductions were similar compared to TAU and TAU plus other active psychotherapies (in our meta-analysis, we identified trials that only delivered psychoeducation and individual or group psychiatric management with education for patients or caregivers plus TAU; no other interventions were compared), excepting for psychotic symptoms with nonsignificant results but only one comparison was included.
Our study offers new and useful insight into the effectiveness of FIp in patients with FEP for relapse and other relapse-related outcomes. For relapse, the effect size observed (RR = 0.42) compared to TAU or TAU plus psychoeducation was significant, encompassing 6 trials. A meta-analysis conducted in December 2008 and published in 2011 included only 2 trials and showed mixed results, concluding that further research is needed to determine FIp effectiveness among FEP patients.10 Our study provides more evidence on the effectiveness of FIp for FEP compared to the aforementioned meta-analysis. Furthermore, although FIp has proved significantly effective for FEP, CBT has proved ineffective in patients with FEP10 and schizophrenia55 for reducing relapses, yet it is effective at mitigating the severity of psychotic symptoms according to 2 previous meta-analyses.10,55 Thus, evidence suggests that FIp is a useful strategy for preventing relapse; however, other psychosocial interventions, including CBT, have shown to be effective at reducing psychotic symptoms. A likely useful prognosis-based approach for these patients would be multi-component integrated therapy which includes specific interventions that have demonstrated effectiveness for several outcomes. Regarding other relapse-related outcomes, no previous meta-analyses with pooled effect sizes in patients with FEP have been published. Yet authors have reported mixed results for reducing psychotic symptom severity in previous qualitative systematic reviews,24,55,56 and argue that family intervention may be effective at improving social and interpersonal functionality.21 In terms of duration of hospitalization, a lack of data provided little evidence of its effectiveness according to a previous systematic review.10 Regardless, we found a clear benefit of FIp over pharmacology alone or alongside psychoeducation for individuals with FEP when it comes to duration of hospitalization, psychotic symptoms, and functionality, which previous systematic reviews did not report.
However, these results must be interpreted with caution. Despite the clinical benefits of FIp for FEP, these results only appear effective in the short to medium term (24 mo), and not in the long term (eg, 10 y) according to individual studies.43,49 Furthermore, only 2 trials adopted a long-term approach, with one assessment at 30, 60, and 120 months, respectively. We identified only 3 trials that compared FIp to other psychosocial interventions; the key intervention component across all trials was educating about the illness. We found no trials comparing FIp to CBT. As such, further research is needed to compare other psychosocial interventions and their effectiveness. Lastly, and with the exception of relapse assessment, other meta-analyses showed substantial or considerable heterogeneity. Our results suggest that only whether the intervention encompasses multi-component integrated therapy or not, may constitute sources of heterogeneity only for days of hospitalization. According to our results, study quality and the outcome assessment tool used were not a source of heterogeneity. Other non-assessed sources may include differences in availability and admission criteria for psychiatric hospitals across different regions and countries, and several TAU conditions have been observed in the included trials.
According to our study, FIp proved effective at reducing relapses in patients with FEP, which is also consistent with the effects observed in patients with schizophrenia.21,22 Therefore, we recommend FIp intervention not only in schizophrenia but also at FEP. Evidence points to actively involving the relatives of patients with both recent psychosis onset and schizophrenia, thus contributing toward reducing relapse risk considerably. FIp may help relatives better understand this disorder and its impact on personal, social and interpersonal functioning, identify exacerbated psychotic symptoms, acquire problem-solving techniques during acute episodes, and gain awareness of the importance of treatment adherence.
Limitations
This study poses some limitations. First, the trials included in the meta-analysis varied substantially in terms of design and follow-up, the participants’ clinical characteristics, the relapse criteria employed, and the outcome assessment instruments used. However, the pooled treatment effects across comparisons showed that all estimates were in the same direction before 24 months of follow-up; only one comparison in the duration of hospitalization meta-analysis took the opposite direction. This suggests that the RCT subgroups (eg, FIp programs alone or combined with other therapies) were clinically meaningful, and that comparisons were sufficiently homogeneous for obtaining summary effect estimates across subgroups.28 Most relapse definitions included hospitalization only, whereas others covered additional specific criteria such as a significant increase in antipsychotic medication.36 Some authors argue that other factors besides just hospitalization need to be taken into account to successfully define relapse; these include family functioning, support when leaving hospital, and adherence to treatment.55 Thus, clinical consensus on defining relapse should be developed by clinicians and mental health professionals. Second, and as commented above, the duration of follow-up also varied across trials. While all trials included a follow-up of 6 to 24 months, studies differed with regard to the timing between baseline assessment and FIp intervention initiation, which may have influenced the relapse rates obtained. Moreover, given that previous research has found increased relapse rates over longer periods of time,57 the findings from the present meta-analysis can only be generalized to the first 2/3 years following treatment initiation. Third, only English and Spanish published trials were considered, meaning that relevant, other-language published trials were ruled out. Fourth, only a small number of trials were entered into the funnel plot to find evidence of publication bias. As such, we did not perform adequate formal testing for asymmetry, and our results for evidence of publication bias lacked robustness. Only in functionality assessment, results suggest that positive and significant and positive results are more likely to be published than nonsignificant and/or negative results. However, very few studies have been included in the funnel plot. Fifth, no previously published protocol of this systematic review was available. Sixth, only published studies were included in this systematic review. Missing data may have inflated our results, although having statistically significant results did not improve the chances of a study being published with leading biomedical journals.58 Finally, the authors of the studies were not contacted to provide missing or additional data on patients with FEP or subsamples of schizophrenic patients who met inclusion criteria.
Clinical Implications
Evidence suggests that the first 5 years of FEP are crucial for the prognosis of psychotic disorder where deterioration occurs, doing so aggressively in the early years with more relapses, and that critical psychosocial influences, including family and psychological reactions to psychosis and psychiatric services, develop during this period.17 Essentially, this period is marked by high relapse rates within 5 years of recovery, and this risk may decline with the maintenance of antipsychotic drug treatment57 as well as with FIp, as our pooled results suggest.
Relapse in early phase psychosis increases chronicity59 and suicide risk60 and worsens psychosocial functioning61 and family relationships.62 Furthermore, economic analyses have indicated that the cost associated with treating psychosis relapse is 4 times that of stable psychosis.63 Adolescents and youth who experience FEP usually still live at home, and family-based intervention can have positive effects on patients when it comes to preventing relapse and improving the course and development of psychotic disorder,64 thus rendering it more cost-effective.65
Although intervention in early phase psychosis is considered clinically relevant, our systematic review is the only study to specifically assess FIp effectiveness early on. Our results echo the promising results from previous reviews10,23,24 and replicate the obtained results at the more advanced stages of illness.21,22
Future Research
As commented above, FIp is effective in the short term to medium term (≤24 mo). As such, RCT trials with FIp delivered more than 6 months26 and with relapse definition consensus reached by clinicians, reporting good, solid clinical psychometric properties50,51,66 for improving comparability across studies and/or with more than 2 years’ follow-up, are needed to establish FIp effectiveness. Sending reminders to the relatives of patients with FEP after treatment would likely be an appropriate strategy for preventing future relapses.
Furthermore, future FIp interventions should seek to identify symptoms relative to the period before a psychotic episode occurs and prevent risk factors to enhance their effectiveness. Before a psychotic episode, most patients experience impaired functional capacity alongside increasing negative symptoms, followed by positive symptoms which build in intensity, severity, and frequency as the psychotic episode approaches.8 The overriding risk factors associated with a psychotic episode which should be prevented are non-adherence with medication, persistent substance use disorder, carers’ criticism, and poorer premorbid adjustment.67 Additionally, there is no evidence to suggest that FIp is more effective than CBT for relapses in patients with FEP given the lack of primary studies comparing the effectiveness of these interventions, and considering that no network meta-analyses featuring indirect comparisons have been published to date.
Finally, multi-component integrated therapies which encompass FIp plus other psychosocial therapies with evidence-based results on outcome efficacy are needed to improve the prognosis of patients with FEP. For example, CBT therapy has shown to be effective in improving psychotic symptoms,24 whereas early intervention services are successful for tackling psychotic symptoms, adherence to treatment, relapse reduction, and school and work involvement.68
To conclude, the current study has shown that FIp for FEP is effective at reducing not only relapse but also episode severity in terms of duration of hospitalization, psychotic symptoms, and functionality up to 24 months after treatment initiation. The current findings also indicate that FIp is more effective than TAU and TAU plus other psychosocial interventions, as our meta-analysis suggests.
Funding
This work was supported by the European Regional Development Fund (ERDF), the European Commission, and the University of Jaén, Spain (R08-06-2018 to P.C.).
Supplementary Material
Acknowledgments
The authors declare that there are no conflicts of interest in relation to the subject of this study. The authors report no additional financial or other affiliation relevant to the subject of this article. The authors thank Gustavo Reyes del Paso of the University of Jaén for his helpful comments on an earlier draft of this article.
References
- 1. World Health Organization (WHO). Schizophrenia World Health Organisation (WHO); 2016. http://www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed March 22, 2018. [Google Scholar]
- 2. Mueser KT, Cook JA. Rising to the challenge of first episode psychosis: the NIMH Recovery After Initial Schizophrenia Episode (RAISE) initiative. Psychiatr Rehabil J. 2014;37(4):267–269. [DOI] [PubMed] [Google Scholar]
- 3. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. [DOI] [PubMed] [Google Scholar]
- 4. Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. 1997;23(3):437–458. [DOI] [PubMed] [Google Scholar]
- 6. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36(4):1342–1356. [DOI] [PubMed] [Google Scholar]
- 7. Whiteford HA, Degenhardt L, Rehm J, et al. . Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. [DOI] [PubMed] [Google Scholar]
- 8. Larson MK, Walker EF, Compton MT. Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Expert Rev Neurother. 2010;10(8):1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Psychological Association (APA). Psychological treatments 2013. http://www.div12.org/psychological-treatments/treatments/. Accessed September 10, 2018.
- 10. Alvarez-Jiménez M, Parker AG, Hetrick SE, McGorry PD, Gleeson JF. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mueser KT, Deavers F, Penn DL, Cassisi JE. Psychosocial treatments for schizophrenia. Annu Rev Clin Psychol. 2013;9:465–497. [DOI] [PubMed] [Google Scholar]
- 12. Olivares JM, Sermon J, Hemels M, Schreiner A. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry. 2013;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Collaborating Centre for Mental Health. Psychosis and schizophrenia in adults: treatment and management. Nice. 2014;208–300. (February):Feb 54 Clinical Guidelines n° 178. doi: 10.1002/14651858.CD010823.pub2.Copyright [DOI] [Google Scholar]
- 14. Perona-Garcelán S, Cuevas-Yust C, Vallina-Fernandez O, Lemos-Giraldez S.. Cognitive-Behavioral Therapy for Schizophrenia. Clinal Guide. 1st ed Madrid, Spain: Minerva Ediciones; 2003. [Google Scholar]
- 15. Leal M, Sales R, Ibáñez E, Giner J, Leal C. Evaluation of the effect of a psychoeducational program on the burden in informal caregivers of patients with schizophrenia. Actas Esp Psiquiatr. 2008;36(2):63–69. [PubMed] [Google Scholar]
- 16. Alvarez-Segura M, Llorente C, Arango C. Estado actual de la detección e intervención temprana en psicosis. Jano. 2009;1723:27–30. [Google Scholar]
- 17. Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33):53–59. [PubMed] [Google Scholar]
- 18. Lieberman JA, Perkins D, Belger A, et al. . The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. [DOI] [PubMed] [Google Scholar]
- 19. Stephenson J. Delay in treating schizophrenia may narrow therapeutic window of opportunity. JAMA. 2000;283(16):2091–2092. [DOI] [PubMed] [Google Scholar]
- 20. Jansen JE, Gleeson J, Cotton S. Towards a better understanding of caregiver distress in early psychosis: a systematic review of the psychological factors involved. Clin Psychol Rev. 2015;35:56–66. [DOI] [PubMed] [Google Scholar]
- 21. Pharoah F, Mari J, Rathbone J, Wong W. Family intervention for schizophrenia. Cochrane Database Syst Rev. 2010;( 12):CD000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pitschel-Walz G, Leucht S, Bäuml J, Kissling W, Engel RR. The effect of family interventions on relapse and rehospitalization in schizophrenia–a meta-analysis. Schizophr Bull. 2001;27(1):73–92. [DOI] [PubMed] [Google Scholar]
- 23. Askey R, Gamble C, Gray R. Family work in first-onset psychosis: a literature review. J Psychiatr Ment Health Nurs. 2007;14(4):356–365. [DOI] [PubMed] [Google Scholar]
- 24. Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. Early intervention services, cognitive-behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry. 2010;197(5):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB; Schizophrenia Patient Outcomes Research Team (PORT) The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Green S.. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, UK: Wiley-Blackwell; 2008. doi: 10.1002/9780470712184.ch5 [DOI] [Google Scholar]
- 29. Chien WT, Chan SW. The effectiveness of mutual support group intervention for Chinese families of people with schizophrenia: a randomised controlled trial with 24-month follow-up. Int J Nurs Stud. 2013;50(10):1326–1340. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Chapter 8: assessing risk of bias in included studies The Cochrane Collaboration, 2011. http://handbook.cochrane.org. Accessed April 3, 2018. [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. https://community.cochrane.org/help/tools-and-software/revman-5. [Google Scholar]
- 34. Goldstein MJ, Rodnick EH, Evans JR, May PR, Steinberg MR. Drug and family therapy in the aftercare of acute schizophrenics. Arch Gen Psychiatry. 1978;35(10):1169–1177. [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Wang M, Li J, Phillips MR. Randomised-control trial of family intervention for 78 first-episode male schizophrenic patients. An 18-month study in Suzhou, Jiangsu. Br J Psychiatry Suppl. 1994;( 24):96–102. [PubMed] [Google Scholar]
- 36. Linszen D, Dingemans P, Van der Does JW, et al. . Treatment, expressed emotion and relapse in recent onset schizophrenic disorders. Psychol Med. 1996;26(2):333–342. [DOI] [PubMed] [Google Scholar]
- 37. Leavey G, Gulamhussein S, Papadopoulos C, Johnson-Sabine E, Blizard B, King M. A randomized controlled trial of a brief intervention for families of patients with a first episode of psychosis. Psychol Med. 2004;34(3):423–431. [DOI] [PubMed] [Google Scholar]
- 38. Chien WT, Chan SW, Thompson DR. Effects of a mutual support group for families of Chinese people with schizophrenia: 18-month follow-up. Br J Psychiatry. 2006;189:41–49. [DOI] [PubMed] [Google Scholar]
- 39. Chien WT, Wong KF. A family psychoeducation group program for chinese people with schizophrenia in Hong Kong. Psychiatr Serv. 2007;58(7):1003–1006. [DOI] [PubMed] [Google Scholar]
- 40. Chien WT. Effectiveness of psychoeducation and mutual support group program for family caregivers of Chinese people with schizophrenia. Open Nurs J. 2008;2:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gleeson JF, Cotton SM, Alvarez-Jimenez M, et al. . A randomized controlled trial of relapse prevention therapy for first-episode psychosis patients. J Clin Psychiatry. 2009;70(4):477–486. [DOI] [PubMed] [Google Scholar]
- 42. Palma-Sevillano C, Cañete-Crespillo J, Farriols-Hernando N, et al. . Randomised controlled trial of cognitive-motivational therapy program for the initial phase of schizophrenia: a 6-month assessment. Eur J Psychiatry. 2011;25(2):68–80. https://search.proquest.com/docview/898673301?accountid=14555. Accessed June 15, 2018. [Google Scholar]
- 43. Gleeson JF, Cotton SM, Alvarez-Jimenez M, et al. . A randomized controlled trial of relapse prevention therapy for first-episode psychosis patients: outcome at 30-month follow-up. Schizophr Bull. 2013;39(2):436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Calvo A, Moreno M, Ruiz-Sancho A, et al. . Intervention for adolescents with early-onset psychosis and their families: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53(6):688–696. [DOI] [PubMed] [Google Scholar]
- 45. Calvo A, Moreno M, Ruiz-Sancho A, et al. . Psychoeducational group intervention for adolescents with psychosis and their families: a two-year follow-up. J Am Acad Child Adolesc Psychiatry. 2015;54(12):984–990. [DOI] [PubMed] [Google Scholar]
- 46. Chien WT, Thompson DR, Lubman DI, McCann TV. A randomized controlled trial of clinician-supported problem-solving bibliotherapy for family caregivers of people with first-episode psychosis. Schizophr Bull. 2016;42(6):1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chien WT, Yip AL, Liu JY, McMaster TW. The effectiveness of manual-guided, problem-solving-based self-learning programme for family caregivers of people with recent-onset psychosis: a randomised controlled trial with 6-month follow-up. Int J Nurs Stud. 2016;59:141–155. [DOI] [PubMed] [Google Scholar]
- 48. Petersen L, Jeppesen P, Thorup A, et al. . A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ. 2005;331(7517):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Secher RG, Hjorthøj CR, Austin SF, et al. . Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull. 2015;41(3):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Falloon IR, Boyd JL, McGill CW, Razani J, Moss HB, Gilderman AM. Family management in the prevention of exacerbations of schizophrenia: a controlled study. N Engl J Med. 1982;306(24):1437–1440. [DOI] [PubMed] [Google Scholar]
- 51. McFarlane W. Multifamily Groups for the Treatment of Severe Psychiatric Disorders. New York & London: Guilford Press; 2002. [Google Scholar]
- 52. Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. 1983;19(3):9–21. [DOI] [PubMed] [Google Scholar]
- 53. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10(3):799–812. [Google Scholar]
- 54. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 55. Lynch D, Laws KR, McKenna PJ. Cognitive behavioural therapy for major psychiatric disorder: does it really work? A meta-analytical review of well-controlled trials. Psychol Med. 2010;40(1):9–24. [DOI] [PubMed] [Google Scholar]
- 56. Sadath A, Muralidhar D, Varambally S, Jose JP, Gangadhar BN. Family intervention in first-episode psychosis: a qualitative systematic review. SAGE Open. 2015;5(4):1–11. [Google Scholar]
- 57. Robinson D, Woerner MG, Alvir JM, et al. . Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–247. [DOI] [PubMed] [Google Scholar]
- 58. Lee KP, Boyd EA, Holroyd-Leduc JM, Bacchetti P, Bero LA. Predictors of publication: characteristics of submitted manuscripts associated with acceptance at major biomedical journals. Med J Aust. 2006;184(12):621–626. [DOI] [PubMed] [Google Scholar]
- 59. Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year follow-up of a Dutch incidence cohort. Schizophr Bull. 1998;24(1):75–85. [DOI] [PubMed] [Google Scholar]
- 60. Mueser KT, Lu W, Rosenberg SD, Wolfe R. The trauma of psychosis: posttraumatic stress disorder and recent onset psychosis. Schizophr Res. 2010;116(2–3):217–227. [DOI] [PubMed] [Google Scholar]
- 61. Penn DL, Waldheter EJ, Perkins DO, Mueser KT, Lieberman JA. Psychosocial treatment for first-episode psychosis: a research update. Am J Psychiatry. 2005;162(12):2220–2232. [DOI] [PubMed] [Google Scholar]
- 62. Addington J, Coldham EL, Jones B, Ko T, Addington D. The first episode of psychosis: the experience of relatives. Acta Psychiatr Scand. 2003;108(4):285–289. [DOI] [PubMed] [Google Scholar]
- 63. Almond S, Knapp M, Francois C, Toumi M, Brugha T. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184:346–351. [DOI] [PubMed] [Google Scholar]
- 64. Muela-Martinez JA, Godoy-García JF. Programa de intervención familiar en esquizofrenia: Dos años de seguimiento del estudio de Andalucía. Apunt Psicol. 2001;19(3):421–430. [Google Scholar]
- 65. Serretti A, Mandelli L, Bajo E, et al. . The socio-economical burden of schizophrenia: a simulation of cost-offset of early intervention program in Italy. Eur Psychiatry. 2009;24(1):11–16. [DOI] [PubMed] [Google Scholar]
- 66. Schooler NR, Keith SJ, Severe JB, et al. . Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54(5):453–463. [DOI] [PubMed] [Google Scholar]
- 67. Alvarez-Jimenez M, Priede A, Hetrick SE, et al. . Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1–3):116–128. [DOI] [PubMed] [Google Scholar]
- 68. Correll CU, Galling B, Pawar A, et al. . Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.