Abstract
There is growing evidence suggesting that abnormalities in cortical-basal ganglia circuitry may play a significant role in determining outcomes in schizophrenia. The globus pallidus (GP), a critical structure within this circuitry, unique in its role as a mediator of competing inputs through the striatum, has not been well characterized in schizophrenia. The following study examined functional interactions of the GP in individuals with first-episode schizophrenia (FES). To probe the large-scale intrinsic connectivity of the GP, resting-state fMRI scans were obtained from patients with FES and sex and age-matched healthy controls. Participants with FES were also evaluated after 6 months via the Strauss–Carpenter Outcomes Scale to assess overall functional trajectory. The GP was parcellated to generate seeds within its substructures, and connectivity maps were generated. Our FES cohort showed significantly lower functional connectivity between the left GP interna and a network of regions including the dorsolateral prefrontal cortex, caudate, and cerebellum at baseline. In addition, FES participants with lower overall scores of functioning at 6 months showed significantly decreased connectivity between the GP interna and the dorsal anterior cingulate and bilateral insula, all regions important for motivational salience. These results provide novel evidence for unique abnormalities in functional interactions of the GP with key prefrontal cortical regions in FES. Our findings also suggest that reduced prefrontal-pallidal connectivity may serve as a predictor of early functional outcome.
Keywords: globus pallidus, fMRI, functional connectivity, schizophrenia, functional outcomes
Introduction
Functional outcomes of schizophrenia are heterogeneous, with illness trajectories ranging from recovery status in a small percentage of individuals to persistent and severe functional disability.1–4 Poor illness outcome, which accounts for much of the morbidity associated with the disorder,5–8 cannot be predicted early in a patient’s illness due to a lack of reliable prognostic markers and a limited understanding of the neurobiology underlying disease progression. Furthermore, treatment strategies for functional deficits in schizophrenia are limited and represent a critical unmet need in psychiatry.
Recent work has adopted functional neuroimaging approaches to examine the neural circuitry underlying clinical outcomes in schizophrenia. Much of this work centers around abnormalities of the basal ganglia and its connections with the cortex.9–14 Changes in functional circuitry linking the striatum and midbrain to cortical regions important for executive cognition are associated with the efficacy of antipsychotic drugs.15–17 Striatal deficits are also associated with dysfunctional reward processing and motivation in schizophrenia,18 which contribute to negative symptoms of the disorder that underlie poor functional outcomes.19,20 However, this line of inquiry has not yet made a significant impact in understanding the variability in functional outcomes.
Although more recent work focuses on motivation within the context of broader cortico-striatal-pallido-thalamo-cortical (CSPTC) circuits,21,22 less work has focused on the globus pallidus (GP), a critical component of this pathway and the main output nucleus of the basal ganglia. Here, we focused on the GP in part due to its distinct role within the basal ganglia in generating action from competing striatal inputs. Cortical pathways extend through the striatum and into the internal (GPi) segments of the GP via excitatory direct, or inhibitory indirect connections through the external segments (GPe).23–26 The GPi plays a crucial role in aggregating information from both pathways and serves as the primary output of the basal ganglia to the thalamus, giving it control over motoric and cognitive functions, as well as dopaminergic signaling via the subthalamic nucleus.25 Aberrant GP functioning in lesion studies, independent of the striatum, is linked to deficits in motivational salience and goal-directed behavior, which are core components of the negative and cognitive symptoms of schizophrenia associated with functional deficits of the disorder.27–30 Supporting these findings, work in an animal model demonstrates that excessive D2 receptor activity, a hallmark finding in schizophrenia, causes alterations in anatomical connections between the striatum and the GP.31 In addition, the GP is an important interventional target in neuropsychiatric illnesses. Deep brain stimulation of pallidal substructures may hold potential as a therapeutic strategy for individuals with schizophrenia who display severe functional deficits.32
Overall, the role of the GP, and to what degree it is damaged in schizophrenia, remains largely unknown. Abnormalities in GP functioning potentially contribute to the impairments underlying poor functional outcomes in schizophrenia, though its substructures and their connectivity have not been examined. In this study, we used a prospective and longitudinal approach to examine the functional interactions of the GP in a cohort of individuals with first-episode schizophrenia (FES) with limited exposure to antipsychotic treatment. We parcellated the GP and examined whether baseline differences exist in its connectivity in FES relative to matched healthy controls (HC), and whether abnormal connections predict poor 6-month outcomes. We hypothesized that (1) individuals with FES would show abnormal functional connectivity of the GP, echoing CSPTC circuitry, and (2) that individuals with poorer longitudinal outcomes will demonstrate lower connectivity between the GP and regions important for motivation and cognition. To test for specificity of GP-related impairments, we also examined whether our GP-related findings correlated with striatal connectivity.
Methods
Participants
A total of 67 participants were included in this study. Patients (N = 43) were recruited from clinical services at the University of Pittsburgh Medical Center. Patients were between the ages of 16 and 28 and experiencing first-episode psychosis with a diagnosis of schizophrenia (n = 27), schizoaffective disorder (n = 7), schizophreniform disorder (n = 2), or psychotic disorder not otherwise specified (n = 7). Diagnoses were determined using the Structured Clinical Interview for DSM-IV, completed by trained clinicians and supplemented by information from family members. A consensus conference review was used to finalize the diagnosis using all available data and review by senior diagnosticians including 2 authors (D.K.S. and G.L.H.). Patients were excluded if psychosis was determined to be substance induced. Patients were minimally treated with antipsychotic drugs (<2 months). At the time of the scan, 27 patients were receiving treatment via a second-generation antipsychotic drug, including risperidone (n = 18), olanzapine (n = 4), aripiprazole (n = 3), quetiapine (n = 1), and ziprasidone (n = 1). Sixteen patients were not taking antipsychotic drugs at time of scan. Fourteen patients were antipsychotic naive, and 1 patient was not naive and not currently taking antipsychotic medication. Patients were assessed at baseline, while entering treatment for their first episode of psychosis and were re-evaluated 6 months later to establish global functioning.
Our HC group (N = 24) included participants between the ages of 12 and 35 that were recruited from the greater Pittsburgh area and had no history of a major psychiatric disorder in themselves or their first-degree family members. Participants were excluded if they were diagnosed with a medical illness that affects the functioning of the central nervous system, scored lower than 75 on the Weschler Abbreviated Scale of Intelligence (WASI),33 or experienced contraindications to magnetic resonance imaging (MRI) scanning. Written informed consent was obtained from all participants, and all study procedures were approved by the University of Pittsburgh Institutional Review Board.
Clinical Ratings
Patients were assessed with a range of tools including a demographic evaluation and the Brief Psychiatric Rating Scale (BPRS).34 Outcome status was measured using the Strauss–Carpenter Outcome Scale (SCOS)35 at a 6-month follow-up appointment to assess global function. Nine FES participants did not complete follow-up assessments and were not included in our analysis of functional outcomes.
MRI Acquisition
MRI scanning was completed at baseline using a Siemens 3.0 Tesla TIM Trio scanner and 32-channel coil at the University of Pittsburgh Medical Center Magnetic Resonance Research Center. Participants completed a 5-minute resting-state scan during which they were asked to keep their eyes open and look at a fixation cross at the center of the screen. The resting-state scan was completed as part of a larger, 60-minute scan sequence.36 Details of our scan sequences are provided in supplementary methods.
MRI Preprocessing
Preprocessing was completed in AFNI (https://afni.nimh/nih.gov/) and FSL (http://www.fmrib.ox.ac.uk) using an established resting-state pipeline designed to minimize motion effects as described in Hallquist et al.37 Preprocessing steps included simultaneous 4D slice timing and motion correction with NIPy (http://nipy.org/), skull stripping, coregistration and warping to standard MNI space, field map unwarping with FSL FUGUE to correct for spatial distortion, spatial smoothing using a 5 mm full width at half maximum Gaussian kernel, high pass filtering at 100 volumes, and grand mean intensity normalization (10 000/global median).
Head motion during functional MRI (fMRI) scans influences both long-range and short-range functional connectivity measures.38,39 To denoise our data and account for motion, including movement-related “spikes,” we used wavelet despiking.40 In this approach, a data-driven method is used to identify movement-related artifacts and remove them without the loss of data that is typical of data “scrubbing.” An overview of this approach is described in supplementary methods. Following wavelet despiking, signal nuisance regression with motion, motion derivatives, cerebrospinal fluid, and white matter was performed, as well as bandpass filtering between 0.01875 and 0.08 Hz. To confirm that our results were not influenced by head motion, post hoc analyses were used to determine whether our functional connectivity results were related to measurements of framewise displacement (FD).
Functional Parcellation of the GP
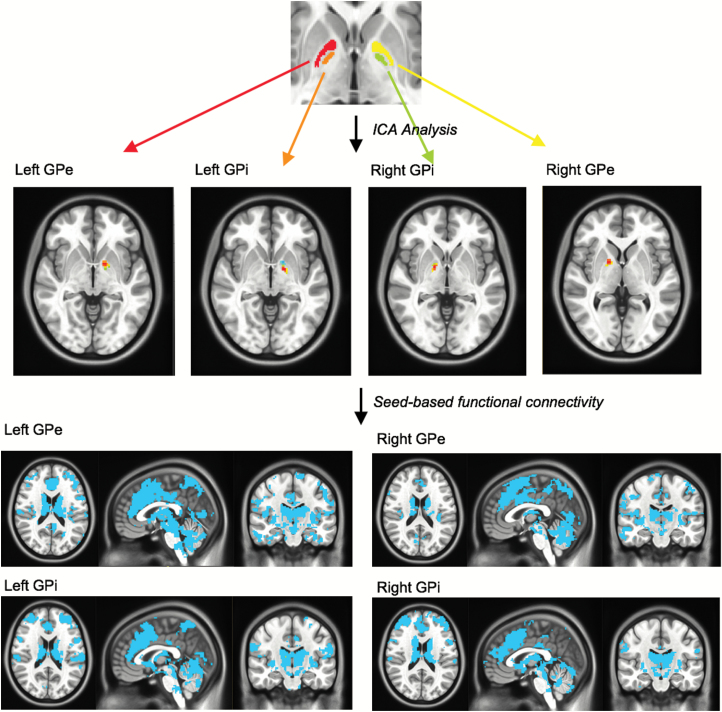
To explore differences in functional connectivity of the GP between HC and FES groups, we first parcellated the GP to identify functionally distinct seed regions of interest (ROIs) within its substructures. Parcellation of the GP was conducted in our HC cohort (figure 1). First, all 4 pallidal substructures (bilateral GPi and GPe segments) were hand drawn, based on anatomical location in the MNI template provided with FSL that was used for coregistration of our functional images. Then, to examine the intrinsic organization of fMRI signal within the GP, we used FSL MELODIC to perform an independent components analysis in each hemisphere, anatomically masked by hand-drawn GP segments. Each independent component analysis was restricted to 5 components to target peak regions for GP seeds and avoid redundancy. Results showed one independent component localized in each pallidal substructure in both left and right hemispheres (figure 1). Peak coordinates of these components were then used to generate spherical seed ROIs with a radius of 2 mm (left GPi: x = −17, y = −3, z = −4; right GPi: x = 15, y = −1, z = −2; left GPe: x = −15, y = 4, z = −4; right GPe: x = 17, i = 4, z = 1). The mean time courses were then extracted from all 4 GP seed ROIs, and corresponding whole-brain functional connectivity maps were generated for all HC and FES participants with the extracted waveforms as a reference. The resulting connectivity maps were z-transformed.
Fig. 1.
Outline of our globus pallidus (GP) parcellation method. Independent component analysis was performed on hand-drawn regions of interest in bilateral GPi and GPe. Peak coordinates from one component in each structure were used to perform seed-based whole-brain functional connectivity analyses and confirm successful parcellation.
To cross-validate that our GP ROIs functionally correlated with brain regions outside of the GP within distinct functional networks, group-level functional connectivity analyses were conducted in our HC cohort. One-sample t-tests were performed with functional connectivity maps for each GP ROI. Results were visually inspected and are displayed at a liberal threshold of P < .005, uncorrected (supplementary figure 1). Binary masks were created at this threshold to constrain subsequent between-group analyses (described in Between-Group Analyses). Differences in connectivity between GP ROIs in our HC group were also assessed and are reported in supplementary results and supplementary table 1.
Between-Group Analyses
Whole-brain connectivity differences between individuals with FES and HC were examined for each of the 4 GP ROIs using 3dttest++ in AFNI with cluster correction using the ClustSim option (compile date: April 4, 2018). Next, to assess the relationship between functional connectivity and outcome, participants in the patient group with follow-up SCOS data were split into high- and low-functioning groups based on the median split of the distribution of global functioning scores at 6 months. Connectivity differences were examined using analysis of variance (ANOVA) with each of the 3 groups entered as regressors into 3dMVM in AFNI. All between-group analyses were limited to a spatial extent defined by binary masks of each seed ROI’s corresponding functional network in our HC group, as described above (supplementary figure 1). For all analyses, significance was defined voxel-wise as P < .00 and with cluster correction at P < .05. Cluster correction was performed by using 3dClustSim in AFNI after the amount of smoothing present was estimated using a spatial autocorrelation function. The resulting values were entered into 3dClustSim along with 10 000 iterations, revealing a corrected cluster threshold of 128 contiguous voxels.
In post hoc analyses, average connectivity values from each participant’s resting-state scan were extracted from each significant cluster’s peak coordinates and correlated with FD, age, sex, IQ, handedness, antipsychotic medication usage, and functioning at baseline and follow-up as measured by the SCOS.
Other supplemental analyses were performed to further probe our GP connectivity findings. We assessed whether our connectivity findings were specific to the GP versus the striatum. In a supplemental analysis, significant functional connections related to outcome were examined in relation to striatal ROIs (see supplementary material for methods in detail). In addition, we performed volumetric analyses to evaluate whether group differences were observed in pallidal volume, and whether pallidal volume related our connectivity findings (see supplementary material for methods in detail).
Results
Participant Demographics
Demographics of our participants and clinical characteristics of our FES cohort are displayed in table 1. The HC and FES cohorts were matched for age, sex, and IQ. Of the 29 individuals with FES taking antipsychotic medication, the mean dose in chlorpromazine equivalents at time of scanning was 130.77 mg. Patients were entering treatment around the time of scanning and received treatment per routine clinical services. Patients were re-evaluated at 6 months for follow-up diagnostic evaluation and for an assessment of global functioning via the SCOS.
Table 1.
Participant Demographics and Clinical Information
| Characteristic | FES (N = 43) | HC (N = 24) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | t/χ2 | P value | |
| Age (y) | 22.92 | 5.04 | 22.29 | 2.86 | t = 0.66 | .51 | ||
| Sex | χ2 = 1.32 | .25 | ||||||
| Males | 31 | 14 | ||||||
| Females | 12 | 10 | ||||||
| IQ | 106.4 | 13.76 | 105.9 | 9.28 | t = 0.15 | .88 | ||
| Number of antipsychotic naive | 15 | |||||||
| BPRS (total) | 46.56 | 8.44 | NA | |||||
| BPRS (psychotic symptoms) | 13.28 | 3.53 | NA | |||||
| BPRS (negative symptoms) | 7.16 | 2.49 | NA | |||||
| Strauss–Carpenter follow-up total score | 12.3 | 2.53 | NA | |||||
| Mean antipsychotic dosage (chlorpromazine equivalent in mg) | 83.72 | 103.08 | NA |
Note: BPRS, Brief Psychiatric Rating Scale; FES, first-episode schizophrenia; HC, healthy control; NA, not available.
Group Differences in Functional Connectivity of the GP
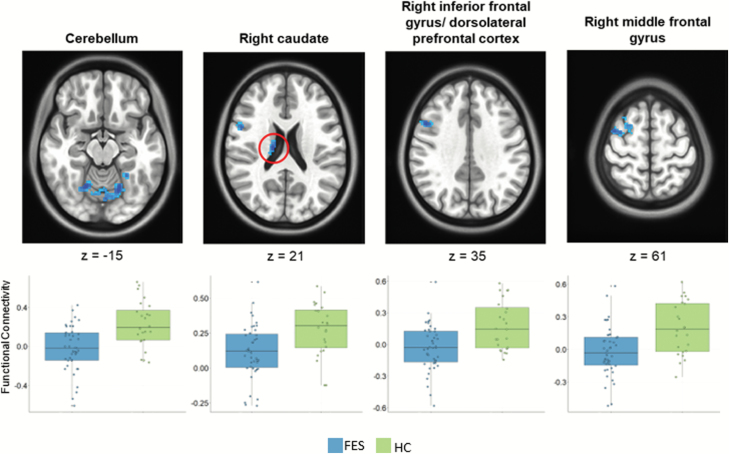
To examine the functional connectivity of the GP, we first parcellated its structures to determine seed regions within the GPi and GPe segments within each hemisphere in our HC group (figure 1). Then, to address our first hypothesis that our FES group will have lower overall connectivity, whole-brain connectivity maps of GP were examined between our FES and HC participants. Results indicated that patients demonstrated significantly lower functional connectivity between the left GPi and the contralateral dorsolateral prefrontal cortex, caudate, inferior frontal gyrus, caudate, and cerebellar lobules V and VI (P < .05, corrected; figure 2, supplementary table 2). In post hoc analyses, these findings were not associated with antipsychotic exposure or clinical measures at baseline (P > .05). No significant differences in connectivity between patients and controls were observed for seeds in left GPe, right GPe, or right GPi.
Fig. 2.
Group differences in functional connectivity of the globus pallidus (GP). Whole-brain maps show significant between-group differences in connectivity between left GPi and the right cerebellum, right middle frontal gyrus, right caudate, and right dorsolateral prefrontal cortex (P < .05, corrected). Boxplots show each participant’s average connectivity value around peak coordinates.
GP Functional Connectivity and Functional Outcomes
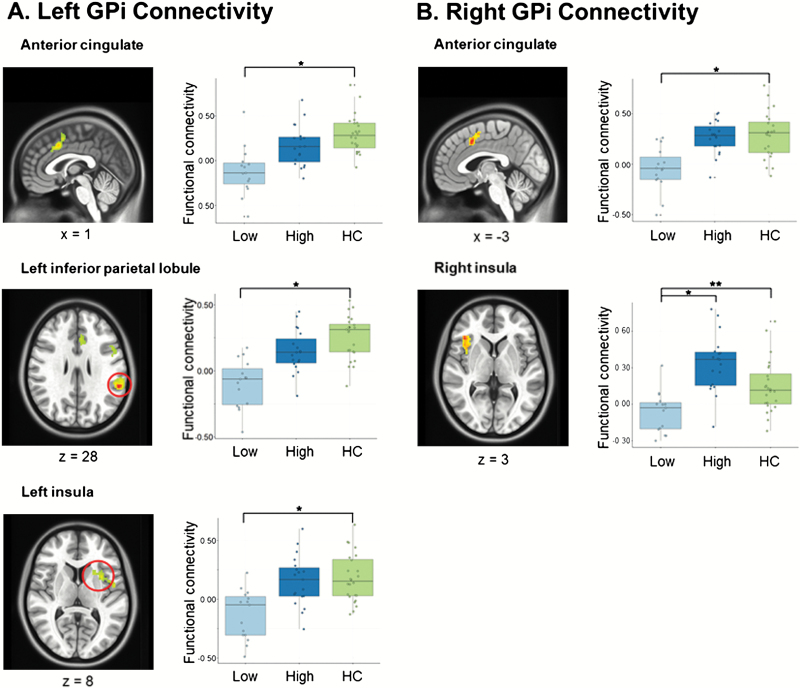
The second part of our analysis considered scores of global functioning 6 months following scanning. Patients were divided into low- and high-functioning groups based on our assessment of functional outcome with the SCOS. We performed a 3-group ANOVA with baseline GP maps between HC participants and low- and high-functioning FES individuals. Results revealed significant group differences in connectivity between the left GPi and the anterior cingulate, left and right insula, left and right parietal lobule, middle frontal gyrus, right thalamus, and left caudate (figure 3; supplementary table 3), regions of the salience network. Significantly lower connectivity was also seen between the right GPi and the anterior cingulate, right inferior frontal gyrus, and left putamen (P < .05, corrected; figure 3; supplementary table 3). Post hoc analyses showed that connectivity differences between controls and patients with lower overall functioning scores at 6 months were driven by the observed group differences for right GPi-cingulate and all left GPi connectivity results. No significant differences in connectivity between the high-functioning group and the low-functioning group were observed for these regions, except the high-functioning group did show significantly higher connectivity between the right GPi and right inferior frontal gyrus when compared with the low-functioning group. No significant connectivity differences for any seed were observed between patients in the upper follow-up functioning score group and controls. To ensure that our results were not related to motion artifact, findings were compared with measures of FD in post hoc analyses. Results were not significant. Supplemental analyses also supported that our findings are not related to pallidal volume or functional connectivity of the striatum (see supplementary material for further detail).
Fig. 3.
Globus pallidus (GP) and longitudinal functional outcomes. Whole-brain maps show significant between-group differences in connectivity between (a) left GPi and bilateral cingulate gyri, left inferior parietal lobule, and left insula, and (b) right GPi and bilateral cingulate gyri and right inferior frontal gyrus (P < .05, corrected). Plots display average functional connectivity around each cluster’s peak coordinate in lower-functioning (Low) and high-functioning (High) first-episode schizophrenia and healthy control participants.
Discussion
The GP is a crucial component of the basal ganglia that may play an important role in the functional deficits observed in schizophrenia. In this study, we parcellated the GP and examined its intrinsic connectivity in relation to functional outcomes in a cohort of minimally treated individuals with FES. Substructures of the GP revealed distinct whole-brain functional interactions. Individuals with FES demonstrated lower functional connectivity between GPi and brain regions critical for executive functioning. Those with poorer overall functioning at 6-month follow-up showed lower connectivity between the GPi and key regions of the salience network important for motivational drive, inhibitory control, and goal-oriented behavior. These connections were specific to the GPi, and not the striatum, based on post hoc confirmatory analyses. This study is the first to concentrate on functional connectivity of the GP in FES in relation to measures of outcome. These findings reveal unique pallidal impairments in individuals with FES, while contribute to our understanding of CSPTC circuits in schizophrenia, and the neurobiology of illness trajectories.
Our parcellation of the GP in HC participants, the first to our knowledge, uncovered functional seed regions in bilateral GPi and GPe segments. We cross-validated our GP ROIs with whole-brain connectivity maps that demonstrated a topography inclusive of regions in the striatum, thalamus, and cortex.24–26 Of note, functional connectivity between GP and the cortex emerges while there are no direct connections between these structures, further indicating that our findings tap into larger CSPTC circuitry. Connectivity of the right GPi was subsumed by the ipsilateral GPe, which may reflect activity of the indirect pathway via functional connections of the cortex through the thalamus. Interestingly, we did not observe this pattern in the left hemisphere, which may be related to intrinsic laterality of the GP.41 We also did not see differences in functional connectivity between the left and right GPi. This negative finding may reflect the similar bottlenecking of diffuse inputs flowing through these structures. Differences in GP connectivity probably exist in the topographic routes of neuronal input flowing into the GPi, via indirect or direct pathways, rather than the net amount of input converging onto either the left or right GPi. Supporting this idea, all of our functional connectivity differences between GP ROIs in our HC group involve the GPe, reflecting a greater amount of variance in how much information flows through the indirect pathway (via the GPe), rather than in how much converges onto the GPi. Though our GP parcellation, overall, reveals unique connectivity patterns, future work with larger cohorts is needed to replicate our findings and relate them to the precise wiring of the basal ganglia.23,24
Relative to the HC group, our FES cohort showed lower functional connectivity between the GP and corticostriatal structures important for goal-directed behavior, including the dorsolateral prefrontal cortex, inferior frontal gyrus, and the caudate.19 These findings are consistent with recent cross-sectional work in schizophrenia that describe overall dysconnectivity between intrinsic canonical attentional networks and basal ganglia structures, including the GP.22 Task-based imaging has linked cortico-basal ganglia pathways to working memory and inhibitory control.42,43 Findings of abnormal cortico-pallidal connectivity at rest may echo results from studies in schizophrenia patients that directly activate executive networks.44 Deficits have been observed in GP and prefrontal cortical activation during attentional control across an antipsychotic treatment course.45 Evidence from animal studies and lesion studies support the link between GP functioning and goal-oriented cognition.46–48 Of note, our results did not show significant group effects on connectivity between the GPi and ipsilateral striatal structures. Considering that GPi is dependent on descending striatal signals, our observation of contralateral results may reflect unique disease-related abnormalities of the GPi.
In addition to cross-sectional differences in connectivity, we were interested in the relationship between GP connectivity and clinical outcome. Individuals with FES were divided into high- and low-functioning groups based on SCOS score at 6-month follow-up. Baseline whole-brain connectivity maps revealed prominent differences between low-functioning FES and HC participants in bilateral GPi connectivity to key nodes within the salience network, including the anterior cingulate and insula. The salience network is involved in regulating environmental expectations and has been shown to be dysfunctional in schizophrenia.49–52 Our results show reduced connectivity between GPi and anterior cingulate in low-functioning patients and support prior work that demonstrated reduced GP and salience network connectivity in schizophrenia.22,49,50 These results show a notable resemblance to findings in patients with akinetic mutism. These patients exhibit poor functioning and phenotypic elements similar to the negative symptoms observed in schizophrenia.53 More broadly, the connectivity-related findings with the salience network may represent a transdiagnostic biomarker that globally captures impaired functioning, irrespective of DSM-based diagnosis.54 Lower connectivity between the GP and salience network may be the result of an imbalance between the inhibitory indirect pathway and the excitatory direct pathways of the basal ganglia, such that the direct pathway is less active and less able to incite goal-directed behavior.25 Our observed relationship between low-functioning patients and reduced GPi and salience network connectivity was not significantly associated with symptom severity at baseline, suggesting that this relationship is independent of acute psychosis. Furthermore, impacted striatal nodes did not show abnormal connections to striatal seeds in FES patients when compared with controls, supporting unique pallidal dysfunction in FES. Although more work is needed to characterize the link between the GP and the salience network, the present findings suggest that it may be crucial for motivation and goal-oriented behavior. Future work will also be necessary to characterize GP connectivity across development in the context of cognitive functions such as inhibitory control.55,56
Limitations of our study include our circumscribed clinical follow-up that focused on global functioning and did not include a second scan. Though our results were not associated with antipsychotic exposure at baseline, future work focused on antipsychotic response will be important to examine GP connectivity in relation to treatment. Future work with larger data sets and transdiagnostic approaches will be important for establishing whether the functional deficits and lower GP circuitry we observe is specific to schizophrenia. The present study offers a preliminary glimpse into the role of the GP in schizophrenia, and further work is necessary to characterize its place alongside other structures that make up the basal ganglia.
In summary, this study demonstrates the presence of abnormal functional interactions of the GP in FES and evidence of their association with longitudinal deficits observed relatively early in the course of the disorder. These results shed light on the neurobiological underpinnings of heterogeneous clinical trajectories observed in schizophrenia by anchoring them to network-based differences in connectivity within cortico-basal ganglia circuitry. Reduced connectivity between the GPi and large-scale cognitive networks in lower-functioning FES individuals suggests that GP connectivity has unique implications for functional and clinical outcomes that may affect the morbidity associated with this illness.7,8 Future work is necessary to determine whether these findings may serve as a target for novel therapeutic interventions or may be used as an indicator of long-term prognosis.
Funding
The project described was supported by the Brain & Behavior Research Foundation (NARSAD Young Investigator Grant) (to D.K.S.), National Institutes of Health through grants K23MH110661, P50MH103204, Conte Center for Translational Mental Health Research (David A. Lewis, Director), and UL1TR001857, Clinical and Translational Science Institute of the University of Pittsburgh (Steven E. Reis, PI).
Supplementary Material
Acknowledgments
We thank the faculty and staff of the WPIC Psychosis Recruitment and Assessment Core for their assistance in diagnostic and psychopathological assessments, Timothy Verstynen for scientific guidance, Benedicta Olonilua for research assistance, and our patients and their families. The authors report no conflicts of interest.
References
- 1. Huber G, Gross G, Schüttler R, Linz M. Longitudinal studies of schizophrenic patients. Schizophr Bull. 1980;6(4):592–605. [DOI] [PubMed] [Google Scholar]
- 2. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keefe RS, Mohs RC, Losonczy MF, et al. Characteristics of very poor outcome schizophrenia. Am J Psychiatry. 1987;144(7):889–895. [DOI] [PubMed] [Google Scholar]
- 4. Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30(2):279–293. [DOI] [PubMed] [Google Scholar]
- 6. Rajji TK, Miranda D, Mulsant BH. Cognition, function, and disability in patients with schizophrenia: a review of longitudinal studies. Can J Psychiatry. 2014;59(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. [DOI] [PubMed] [Google Scholar]
- 8. Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418–425. [DOI] [PubMed] [Google Scholar]
- 9. Duan M, Chen X, He H, et al. Altered basal ganglia network integration in schizophrenia. Front Hum Neurosci. 2015;9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16(3):391–402. [DOI] [PubMed] [Google Scholar]
- 11. Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5(3):267–271. [DOI] [PubMed] [Google Scholar]
- 12. Bernard JA, Russell CE, Newberry RE, Goen JR, Mittal VA. Patients with schizophrenia show aberrant patterns of basal ganglia activation: evidence from ALE meta-analysis. Neuroimage Clin. 2017;14:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitelman SA, Canfield EL, Chu KW, et al. Poor outcome in chronic schizophrenia is associated with progressive loss of volume of the putamen. Schizophr Res. 2009;113(2–3):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem. 2010;113(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hadley JA, Nenert R, Kraguljac NV, et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39(4):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarpal DK, Argyelan M, Robinson DG, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2016;173(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radua J, Schmidt A, Borgwardt S, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72(12):1243–1251. [DOI] [PubMed] [Google Scholar]
- 19. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(suppl 2):S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avram M, Brandl F, Bäuml J, Sorg C. Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology. 2018;43(11):2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beukema P, Yeh FC, Verstynen T. In vivo characterization of the connectivity and subcomponents of the human globus pallidus. Neuroimage. 2015;120:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 25. Dunovan K, Verstynen T. Believer-skeptic meets actor-critic: rethinking the role of basal ganglia pathways during decision-making and reinforcement learning. Front Neurosci. 2016;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. [DOI] [PubMed] [Google Scholar]
- 27. Miller JM, Vorel SR, Tranguch AJ, et al. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163(5):786–788. [DOI] [PubMed] [Google Scholar]
- 28. Arimura N, Nakayama Y, Yamagata T, Tanji J, Hoshi E. Involvement of the globus pallidus in behavioral goal determination and action specification. J Neurosci. 2013;33(34):13639–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piron C, Kase D, Topalidou M, et al. The globus pallidus pars interna in goal-oriented and routine behaviors: resolving a long-standing paradox. Mov Disord. 2016;31(8):1146–1154. [DOI] [PubMed] [Google Scholar]
- 30. Justin Rossi P, Peden C, Castellanos O, Foote KD, Gunduz A, Okun MS. The human subthalamic nucleus and globus pallidus internus differentially encode reward during action control. Hum Brain Mapp. 2017;38(4):1952–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cazorla M, de Carvalho FD, Chohan MO, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81(1):153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gault JM, Davis R, Cascella NG, et al. Approaches to neuromodulation for schizophrenia. J Neurol Neurosurg Psychiatry. 2018;89(7):777–787. [DOI] [PubMed] [Google Scholar]
- 33. Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- 34. Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 1988;24(1):112–117. [PubMed] [Google Scholar]
- 35. Strauss JS, Carpenter WT Jr. The prediction of outcome in schizophrenia. I. Characteristics of outcome. Arch Gen Psychiatry. 1972;27(6):739–746. [DOI] [PubMed] [Google Scholar]
- 36. Jalbrzikowski M, Murty VP, Stan PL, et al. Differentiating between clinical and behavioral phenotypes in first-episode psychosis during maintenance of visuospatial working memory. Schizophr Res. 2018;197:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel AX, Kundu P, Rubinov M, et al. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 2014;95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kooistra CA, Heilman KM. Motor dominance and lateral asymmetry of the globus pallidus. Neurology. 1988;38(3):388–390. [DOI] [PubMed] [Google Scholar]
- 42. Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1(2):137–160. [DOI] [PubMed] [Google Scholar]
- 43. Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry. 2006;60(3):235–241. [DOI] [PubMed] [Google Scholar]
- 45. Ikuta T, Robinson DG, Gallego JA, et al. Subcortical modulation of attentional control by second-generation antipsychotics in first-episode psychosis. Psychiatry Res. 2014;221(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saga Y, Hashimoto M, Tremblay L, Tanji J, Hoshi E. Representation of spatial- and object-specific behavioral goals in the dorsal globus pallidus of monkeys during reaching movement. J Neurosci. 2013;33(41):16360–16371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scott RB, Harrison J, Boulton C, et al. Global attentional-executive sequelae following surgical lesions to globus pallidus interna. Brain. 2002;125(Pt 3):562–574. [DOI] [PubMed] [Google Scholar]
- 48. Vijayaraghavan L, Vaidya JG, Humphreys CT, Beglinger LJ, Paradiso S. Emotional and motivational changes after bilateral lesions of the globus pallidus. Neuropsychology. 2008;22(3):412–418. [DOI] [PubMed] [Google Scholar]
- 49. White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123(2–3):105–115. [DOI] [PubMed] [Google Scholar]
- 50. Orliac F, Naveau M, Joliot M, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148(1–3):74–80. [DOI] [PubMed] [Google Scholar]
- 51. Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41(8):1701–1708. [DOI] [PubMed] [Google Scholar]
- 52. Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40(2):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci USA. 2018;115(42):10792–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174(7):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013;33(46):18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fryer SL, Roach BJ, Ford JM, et al. Should I stay or should I go? FMRI study of response inhibition in early illness schizophrenia and risk for psychosis. Schizophr Bull. 2019;45(1):158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.