Abstract
Rhythmic neural activity has been proposed to play a fundamental role in cognition. Both healthy and pathological aging are characterized by frequency-specific changes in oscillatory activity. However, the cognitive relevance of these changes across the spectrum from normal to pathological aging remains unknown. We examined electroencephalography (EEG) correlates of cognitive function in healthy aging and two of the most prominent and debilitating age-related disorders: Type-2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD). Relative to HC, AD patients were impaired on nearly every cognitive measure, while T2DM performed worse mainly on learning and memory tests. A continuum of alterations in resting-state EEG was associated with pathological aging, generally characterized by reduced alpha (α) and beta (β) power (AD<T2DM<HC) and increased delta (δ) and theta (θ) power (AD>T2DM>HC), with some variations across different brain regions. There were also reductions in the frequency and power density of the posterior dominant rhythm in AD. The ratio of (α+β)/(δ+θ) was specifically associated with cognitive function in a domain- and diagnosis-specific manner. The results thus captured both similarities and differences in the pathophysiology of cerebral oscillations in T2DM and AD. Overall, pathological brain aging is marked by a shift in oscillatory power from higher to lower frequencies, which can be captured by a single cognitively relevant measure of the ratio of (α+β) over (δ+θ) power.
Keywords: Cognitive aging, Type-2 diabetes mellitus, Alzheimer’s disease, EEG, Oscillations, Neuropsychology
Introduction
Some subtle neurocognitive changes occur with normal aging (Harada et al., 2013), while others are more severe and associated with specific pathophysiological processes. The most extreme example is dementia due to Alzheimer’s disease (AD). AD is associated with progressive alterations including the accumulation of beta-amyloid plaques and neurofibrillary tangles, cortical hypometabolism, and eventually widespread atrophy (Braak and Braak, 1998; Jack et al., 2013). Among AD risk factors (Burns and Iliffe, 2009), one of the most prominent is Type-2 Diabetes Mellitus (T2DM) (Biessels and Kappelle, 2005). T2DM is a chronic metabolic disorder characterized by abnormal glucose metabolism and insulin resistance, and is associated with myriad physiological complications, including in the central nervous system (CNS) (Alberti and Zimmet, 1998; Awad et al., 2004; Biessels and Kappelle, 2005; Gispen and Biessels, 2000; Koekkoek et al., 2014; Roberts et al., 2014; Saedi et al., 2016; Stewart and Liolitsa, 1999; Strachan et al., 2011). Mild deficits in memory, executive function and perceptual processing speed have been observed in T2DM (Cheng et al., 2012; Marseglia et al., 2016; Mooradian et al., 1988; Palta et al., 2014; Takeuchi et al., 2012; van den Berg et al., 2010). While the impact of T2DM on the CNS is likely multifactorial, microvascular damage and impaired insulin signaling have been identified as probable mediators in the higher risk for AD and vascular dementias (Biessels et al., 2014; Ohara, 2011; Toth, 2014). However, understanding of how T2DM fits into the spectrum from normal cognitive aging to AD remains incomplete (de la Monte, 2014).
Electroencephalography (EEG) permits noninvasive measurement of temporally synchronized (i.e., oscillatory) neural activity, a ubiquitous characteristic of the brain (Buzsaki et al., 2013) which has been proposed as a mechanism for encoding and transfer of information (Bonnefond et al., 2017; Fries, 2015). These proposals are based on reliable associations between frequency-specific oscillations and various cognitive functions (Ward, 2003), as well as their implication in various neuropsychiatric disorders (He et al., 2007; Oswal et al., 2013; Schnitzler and Gross, 2005; Uhlhaas and Singer, 2006). Systematic changes in neural oscillations occur with normal cognitive aging (Babiloni et al., 2006b; Marshall and Cooper, 2017; Rossini et al., 2007; Stomrud et al., 2010; Vlahou et al., 2014). For instance, alpha-band (8-13 Hz) activity decreases in both amplitude (Babiloni et al., 2006b; Marshall and Cooper, 2017) and peak frequency (Klimesch, 1999; Mierau et al., 2017; Knyazeva et al., 2018) throughout adulthood. However, changes in lower frequency (<8 Hz) activity, and the relationship with cognitive function, appear to be less consistent (Babiloni et al., 2006a; Cummins and Finnigan, 2007; Klass and Brenner, 1995; Leirer et al., 2011; Marshall and Cooper, 2017).
Oscillatory abnormalities have been consistently observed in pathological aging (Assenza et al., 2017; Babiloni et al., 2004, 2006a; Fraga et al., 2013; Neto et al., 2016; Voytek and Knight, 2015). In AD, the most prominent EEG finding is a shift in power from higher to lower frequencies: an increase in power in delta (δ; 1-4 Hz) and theta (θ; 4-8 Hz) frequency bands, and a concomitant decrease in power in alpha (α; 8-13 Hz) and beta (β; 13-30 Hz) bands, along with reduction of the individual peak α frequency (Babiloni et al., 2004; Bennys et al., 2001; Brenner et al., 1986; Coben et al., 1983; Moretti et al., 2004). The relationship between these oscillatory changes and cognitive dysfunction remains unclear, though some studies have reported correlations with individual tests of cognitive functions (Babiloni et al., 2007; Moretti et al., 2009; van der Hiele et al., 2007). While fewer studies have examined oscillatory changes in T2DM, there is some evidence of a similar shift in power from higher to lower frequencies (Bian et al., 2014; Cooray et al., 2011; Cui et al., 2014; Wen et al., 2016; Zeng et al., 2015).
The aim of the current study was to compare resting-state EEG oscillatory activity, and its relationship with neuropsychological function, across healthy and pathological aging (T2DM and AD). We hypothesized that neuropsychological testing and resting-state oscillatory activity would reveal a pattern of neurocognitive dysfunction from healthy controls (HC) to T2DM to AD. Additionally, we predicted that resting-state EEG measures (i.e. power density and peak frequencies) would be associated with domain-specific cognitive performance both within and across groups, with AD showing the strongest relationships (Babiloni et al., 2018, 2015).
Methods and Materials
Human Participants
This is an analysis of 72 adults who participated in research at the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center between 2012 and 2015. The local Institutional Review Board approved the study. All participants provided written informed consent prior to enrollment according to the Declaration of Helsinki. Participants were drawn from the following groups:
Alzheimer’s disease.
18 participants (11 females, aged 52-86) with a probable diagnosis of mild-to-moderate AD according to DSM-V/NINCDS-ADRDA criteria (McKhann et al., 2011), with a clinical dementia rating (CDR) of 1.0 and a mini-mental status exam (MMSE) (Folstein et al., 1975) score between 18-24. Six patients were medicated with Cholinesterase inhibitors, nine were on Cholinesterase inhibitors and Memantine, while 3 were not taking dementia-specific medications.
Type-2 diabetes mellitus.
27 participants (12 females, aged 50-78) had a clinical diagnosis of T2DM, and had normal cognition as indicated by a MMSE score ≥ 27 (Rosa et al., 2018), with no subjective cognitive complaints. All had their diabetes at least moderately controlled (hemoglobin A1c; HbA1c < 10) through some combination of diet, exercise, Metformin, insulin, or insulin homologues.
Healthy control.
27 participants (13 females, aged 50-77) had normal cognition (MMSE ≥ 27) and glucose metabolism (HbA1c < 6.5%).
General inclusion criteria included: age-adjusted score ≥ 80 on the 50-item Wechsler Test of Adult Reading (W-TAR; as a surrogate measure of premorbid IQ); no other unstable medical or neuropsychiatric conditions (apart from AD or T2DM). All participants underwent equivalent testing, including a structured neurological exam, medical history review, formal neuropsychological testing, and an EEG visit. Participant characteristics (Supplementary Table S1), including age, education, and premorbid IQ, were compared across groups using one-way analyses of variance (ANOVAs) with Tukey’s Honestly Significant Difference (HSD) post hoc comparisons. MMSE scores were compared using a non-parametric Kruskal-Wallis test. Gender proportions were compared using Fisher’s Exact Test. As the AD group was significantly older, Age was added as a covariate to all subsequent between-group analyses. Additionally, to verify that the main results were not confounded by between-group age differences, we reran several of the primary analyses on a cohort of 17 age-matched participants per group (see Supplementary Material for more details).
This and all subsequent analyses were performed in JMP Pro (v12.0, http://www.jmp.com) using a normal distribution and a two-tailed 95% confidence interval.
Neuropsychological testing
Neuropsychological testing was performed on a separate visit from the EEG recording by a trained psychometrist. Tests and inventories were drawn from the National Alzheimer’s Coordination Center’s Uniform Data Set version 1.1 (NACC-UDS) (Beekly et al., 2007). The following neuropsychological tests were employed: the 15-item Geriatric Depression Scale (GDS); a 23-item Activities of Daily Living inventory (ADLs); the Digit Symbol Substitution Test (DSST; number of correct substitutions in 90 sec); Digit Span Forward and Backward tasks (longest set length repeated); the Logical Memory, Story-A (number of items recalled immediately and after a 30-minute delay without cueing) from the Wechsler Memory Scale-Revised; the Trail Making Test (difference in time and in errors between parts B and A; TMTB-A) from the Halstead-Reitan Battery; the “animals” category of the Semantic Fluency Test (number of unique words generated in one min); and the 30-item Boston Naming Test (number of correctly named objects with semantic cue). In addition, the 70-item Cognitive subscale of the Alzheimer’s disease Assessment Scale (ADAS-Cog) (Mohs et al., 1983) was administered to measure global cognitive function, and a 10-item version of the Rey Auditory Verbal Learning Test (RAVLT; percent correct during learning, 20-min delayed recall, and delayed recognition trials) (Rosenberg et al., 1984) was administered to further probe verbal learning and memory ability (Calero and Navarro, 2004). All measures were Z-transformed by subtracting the overall mean (across all three populations) of all subjects from each individual’s score and dividing it by the overall standard deviation in order to equalize the scale across measures, and facilitate data visualization and statistical analysis. Z-scores for the ADAS-Cog, GDS, and TMT were inverted so that in all measures, higher scores reflect better performance. To investigate the relationship between the EEG Spectral Power Ratio and cognitive function, three composite scores were computed by averaging together Z-scores of tests that tap into similar cognitive processes or measures: Dementia severity (ADAS-Cog, ADLs; measuring general cognitive functioning and functional independence), Executive functions (Digit Span forward and backward, TMTB-A Semantic fluency, DSST; measuring attention, working memory, set-shifting, strategic thinking and psychomotor processing speed); and Learning and memory (RAVLT, Logical Memory; measuring the acute ability to learn and recall verbal information with and without context). This approach—modelled after one from the Alzheimer’s Disease Neuroimaging Initiative (Crane et al., 2012; Gibbons et al., 2012) and used in a prior neuroimaging study (Buss et al., 2018)— allowed oscillatory activity to be related to broad categories of cognitive processing rather than to specific tests.
Electroencephalography acquisition and preprocessing
Resting-state EEG was recorded using a 64-channel system (eXimia EEG, version 3.2, Nexstim Ltd, Finland) with a sampling rate of 1450Hz. EEG was acquired using an extended version of the “International 10-20 system” (Supplementary Figure S1). Ground and reference electrodes were placed on the forehead and two additional electrooculography electrodes (EOG) were placed below and at the outer canthi of the left eye to identify vertical and horizontal eye movements. Impedances for all electrodes were kept below 5 kΩ. A 5-minute resting-state EEG recording was obtained while subjects sat in a semi-reclined armchair with their eyes closed. During recordings, the participants were instructed to remain quiet with their face muscles relaxed. The participant and EEG were monitored for signs of drowsiness at which point the participant was asked to blink their eyes a few times before closing them again. EEG data preprocessing was performed offline using a combination of the EEGLab toolbox (Delorme and Makeig, 2004a) and custom scripts in Matlab 2016a (Mathworks, USA). Data were filtered for line noise using a 55-65 Hz notch filter. Additional low-pass (100 Hz) and high-pass (1 Hz) filters were applied using a zero-phase second-order Butterworth filter. Filtered recordings were divided into 3-second epochs for visualization. Faulty or excessively noisy channels were visually detected and removed (average ±SD channels removed = 3.9 ±2.3; range = 0-9) and the remaining data were re-referenced to the average of all channels. After re-referencing, noisy epochs were identified semi-automatically and those containing excessive artifacts were rejected after visual inspection (average ±SD epochs removed = 25.9 ±20.5; range = 2-88), resulting in 48-116 usable epochs per participant with an average (±SD) of 86.9 (±14.0). Independent components analysis (ICA) was performed on cleaned data using fastICA (Rogasch et al., 2014), and components corresponding to blink/oculomotor, muscle or transient electrode artifacts were subtracted from the data. After component rejection, previously rejected channels were interpolated using a spherical spline interpolation and the data were down-sampled to 1024Hz.
Experimental design and Statistical Analysis
Electroencephalography
After EEG preprocessing, mean absolute power spectral density across epochs was calculated for each frequency band (1-40 Hz, 0.5 Hz resolution) at all electrodes using the spectopo EEGlab function (window-size = 1024 samples, window-overlap = 512 samples) (Delorme and Makeig, 2004b). The power estimates for each frequency band were further divided by the sum of estimates across all frequencies in order to calculate the relative power of each frequency within the spectrum. To investigate group differences in EEG power, an analysis of covariance (ANCOVA) was performed at all electrode-frequency (1:40 Hz) points. The ANCOVA model included EEG power as the outcome measure, Diagnosis (HC, T2DM, AD) as a grouping variable, and Age as a continuous predictor to control for its effects on group differences in EEG power. Follow-up pairwise contrasts between groups were calculated using the Tukey-Kramer method. To control for the large number of multiple comparisons across electrode-frequency space, a non-parametric cluster based permutation approach was adopted (Maris and Oostenveld, 2007). Calculation of the test statistics involved the following: based on the initial ANCOVA’s and follow-up contrasts performed at all electrode-frequency points, data points corresponding to an uncorrected p-value < 0.05 were formed into clusters by grouping together adjacent significant electrode-frequency points. Note that for a sample to be included in a cluster it was required to have at least 1 neighboring significant sample in either frequency or space. The spatial neighborhood of each electrode was defined as all electrodes within 4 cm, resulting in a mean of 2.9 (min = 1, max = 4) and median of 3 neighbors per electrode. The F-values (overall ANCOVA) or t-values (follow-up contrasts) within each identified cluster were summed to produce a cluster-level statistic. For the follow-up contrasts, the cluster-building procedure was performed separately for data points with positive and negative t-values (two-tailed test). Subsequently, this cluster-building procedure was repeated across 2000 permutations of the data. On each iteration, diagnostic group labels were randomly shuffled, thereby cutting the hypothesized relationship between diagnostic group and EEG power. The most extreme cluster-level F- or t-score was retrieved on each iteration to build data-driven null hypothesis distributions, separately for both the overall model and for each of the follow-up contrasts. The location of an original real cluster statistic within the null hypothesis distribution indicates how probable such an observation would be if the null hypothesis were true (F-test: No difference in EEG power between any of the groups. Follow-up t-tests: No difference in EEG power between given two groups). For the overall model, if a given real cluster had a cluster-statistic > 95% of the respective null distribution cluster-statistics, then this was considered a significant effect (5% α level). For the follow-up contrasts, if a given negative/positive cluster had a cluster-statistic lower/higher than 97.5% (2.5% α per tail) of the respective null distribution cluster-statistics, then this was considered a significant effect (5% total α level). This entire analysis was performed separately for both absolute and relative EEG power.
EEG frequency bands and Spectral power ratio
For subsequent analyses of EEG power, including its relationship with cognitive function, relative and absolute power estimates were extracted for each classical frequency band: δ (1-4 Hz), θ (4-8 Hz), α (8-13 Hz), β (13-30 Hz), and gamma (γ; 30-40 Hz). Absolute power estimates were used to compute the Spectral Power Ratio, defined as the ratio of power in α and β to power in δ and θ: (α+β)/(δ+θ) (Supplementary Table S2). This approach has been utilized to assess alterations in the frequency distribution of EEG power, capturing in a single variable the pattern of a general shift in power from higher to lower frequencies that has been previously reported in AD (Babiloni et al., 2004; Bennys et al., 2001; Brenner et al., 1986; Coben et al., 1983; Moretti et al., 2004). In order to assess the spatial distribution of the effects, the average of the relative power estimates for each frequency band and the average of the Spectral power ratio values were calculated separately for four cortical regions of interest (ROIs): Frontal (incorporating electrodes FP1, FPz, FP2, AF1, AFz, AF2, F5, F1, Fz, F2, F6), Central (FC5, FC3, FC1, FCz, FC2, FC4, FC6, C5, C3, C1, Cz, C2, C4, C6, CP5, CP3, CP1, CPz, CP2, CP4, CP6), Temporal (F7, F8, FT9, FT7, FT8, FT10, T3, T4, TP9, TP7, TP8, TP10), and Posterior (P9, P7, P3, P1, Pz, P2, P4, P8, P10, PO3, POz, PO4, O1, Oz, O2, Iz).
The relative power estimates from each of the four frequency bands plus the Spectral power ratio values were assessed independently via five mixed-effects linear regression analyses, each with a full-factorial model comprised of the between-subjects factor Group and the within-subject factor Cortical ROI (crossed with the random factor Subject to control for variance associated with repeated observations within the same individual), plus Age as a covariate (for details on the linear regression analysis of Spectral power ratio in the age-matched subgroup cohort, see Supplementary materials). Each of the five analyses was followed by four fixed-effect linear regression analyses to test for group differences within each ROI separately. Significance values for these 20 follow-up analyses were adjusted for multiple comparisons using Holm-Bonferroni correction. Finally, post-hoc Tukey’s HSD tests were used to test for pairwise differences between groups.
Analysis of neuropsychological performance and its relationships with Spectral power ratio
Multivariate analyses of variance (MANOVAs) with a Wilk’s lambda (λ) distribution were used to compare neuropsychological performance across groups (MANOVA-1) and investigate its relationship with the Spectral power ratio across ROIs (MANOVA-2).
MANOVA-1 was performed on Z-scores for the individual neuropsychological tests with the main factor of Group (HC, T2DM, AD), and Age as a covariate (for details on the MANOVA-1 in the age-matched subgroup cohort, see Supplementary materials). Follow-up analyses consisted of separate linear regression models for each cognitive measure. Tukey’s HSD pairwise comparisons were performed for any regression model that survived a 5% false discovery rate (FDR) correction (Benjamini and Yekutieli, 2001).
To investigate relationships between the Spectral power ratio and cognitive functions, MANOVA-2 was performed on the three composite scores with the factors Group, Cortical ROI and Spectral power ratio in a full-factorial model, plus Age as a covariate. Follow-up linear regression analyses were performed for each domain (Learning and memory, Dementia severity, Executive functions), with the factors Group and Spectral power ratio in a full-factorial model with Age as a covariate (for details on the MANOVA-2 in the age-matched subgroup cohort, see Supplementary materials). As all effects that included the factor Cortical ROI were highly non-significant (see Results), it was excluded from post-hoc analyses. For Learning and memory, the Group*Spectral power ratio interaction was highly non-significant (see Results), so the model was rerun without that term. From these models, an overall correlation coefficient was calculated to express the relationship between the composite score and Spectral power ratio across all participants. Lastly, simple linear regression analyses were performed to assess the association of Spectral power ratio with each composite cognitive score in each group. Individual p-values for these 9 group-specific post-hoc analyses were adjusted for multiple comparisons with a 5% FDR.
Individual α and posterior dominant frequencies
During eyes-closed wakefulness, one of the most prominent features of the EEG signal is α-band (~8-13Hz) activity, leading to the characteristic α peak in the power spectrum (Klimesch, 2012; Keitel et al., 2019). We sought to investigate group differences in this dominant frequency, and whether these differences were related to cognitive function, using two independent metrics. First, in each participant we identified the individual frequency between 5-15 Hz with the highest power density across all posterior electrodes using an automated peak-finding algorithm based on smoothing of the 2nd order gradient of power spectral density (PSD) estimates with an 11-point, 3rd order polynomial Savitzky-Golay filter (Savitzky and Golay, 1964; Corcoran et al., 2018; Keitel et al., 2018; Benwell et al., 2019). The posterior electrodes included in the analysis were P9, P7, P3, P1, Pz, P2, P4, P8, P10, PO3, POz, PO4, O1, Oz, O2 and Iz. This approach incorporated a wider band of activity than the typical α range in order to capture potentially large shifts in the dominant frequency. Hence, we labelled this the Dominant frequency analysis. In parallel, to look specifically at frequency and power changes within the classic α-range (8-12 Hz), two clinical neurophysiologists trained to interpret EEG (authors PDP and MMS) manually estimated the individual α frequency (IAF) for each participant using visibly-identifiable alpha activity from the occipital and parieto-occipital electrodes. We labelled this the IAF analysis. For both the Dominant frequency and IAF analyses, we obtained both the peak frequency itself and the power density value averaged over the peak frequency ± 2.5 Hz. Hence, we were able to test simultaneously for group differences in both the peak frequency itself and the surrounding power density. These metrics were each entered into separate one-way ANOVAs (with Age as a covariate) to investigate group differences and were also correlated with the cognitive composite scores.
Results
Participant characteristics
By design, MMSE scores were lower in the AD group relative to both T2DM and HC. AD participants were also significantly older than HC, but not T2DM. The groups were equivalent in years of education, pre-morbid IQ, and proportions of men and women (for full details on participant characteristics across groups, see Supplementary Table S1).
EEG Power
The following details the results of the primary analysis of relative EEG power. For equivalent analyses of absolute EEG power and their results, see Supplementary Materials Section 1 (including Table S2 and Figure S2).
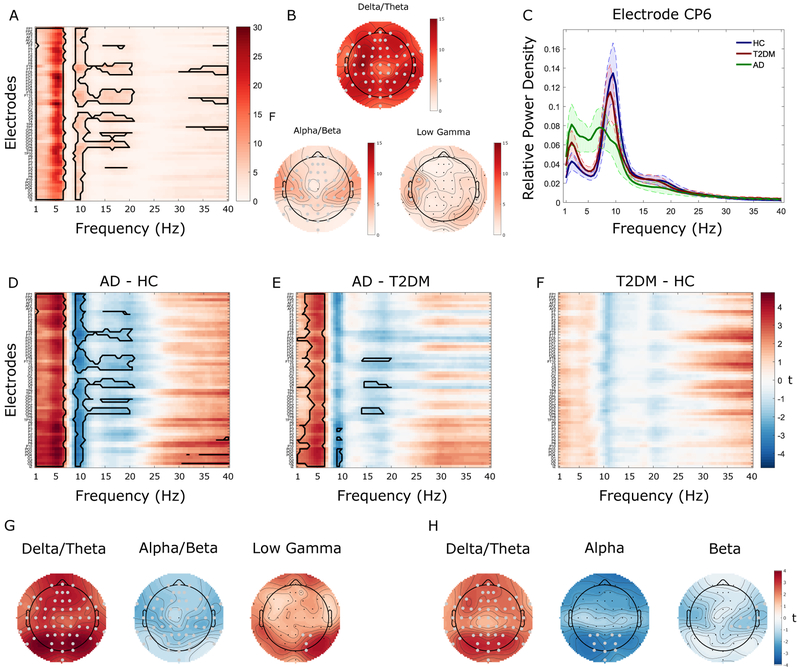
A main effect of Group, controlling for age, was identified in the δ+θ frequency bands (~1–7 Hz) and also in the α+β (~8.5–21 Hz) and low–γ bands (30–40 Hz, Figure 1A-B). Relative δ+θ power were higher for AD compared to T2DM and HC, whereas relative α+β power were lower for AD compared to T2DM and HC (Figure 1C). Pairwise contrasts (Figure 1D-F) demonstrated higher relative δ+θ power in AD than both HC and T2DM, and lower relative α+β power in AD compared to either HC or T2DM. Additionally, there was significantly higher relative power in the low–γ band in AD compared to HC. No clusters survived correction for the T2DM-HC contrast.
Figure 1. Whole-brain analysis of relative power.
A. F-ratios associated with between-group mass univariate analyses of variance (ANOVAs) comparing relative electroencephalography (EEG) power between Alzheimer’s disease (AD), Type-2 diabetes mellitus (T2DM), and healthy controls (HC) across all electrodes (y-axis) and frequencies (x-axis). The solid black contour represents data points surviving cluster-based multiple comparison correction. B. Topographic representation of the F-ratios averaged across the significant frequencies. C. Mean power spectra (with 95% confidence intervals; CI) for each group separately at the electrode (CP6) for which group differences were maximal. Alpha/beta power showed a linear decrease across groups, being highest for HC and lowest for AD with T2DM having intermediate values whereas delta/theta power showed a linear increase across groups. D-F. T-values associated with follow-up tests comparing relative EEG power between each pair of groups separately. Solid black contours indicate data points surviving cluster-correction. G-H. Topographic representation of the t-values associated with the respective significant effects. Significant electrodes are highlighted in gray.
Classic EEG frequency bands across ROIs
Delta:
There were significant main effects of Group (F2,68 = 8.7, p < .001) and Cortical ROI (F3,207 = 59.1, p < .001), but no Group*Cortical ROI interaction (F6,207 = 1.2, p = .292). Follow-up tests showed a similar pattern of group differences across the four Cortical ROIs (p values < .015, adjusted), with AD showing greater relative δ power than both HC and T2DM (p values < .05).
Theta:
There were significant main effects of Group (F2,68 = 12.7, p < .001) and Cortical ROI (F3,207 = 3.3, p = .023), but no Group*Cortical ROI interaction (F6,207 = 2.1, p = .060). Follow-up tests showed a similar pattern of group differences across the four ROIs (p values < .004, adjusted), with AD showing greater relative θ power than both HC and T2DM (p values < .05).
Alpha:
There were significant main effects of Group (F2,68 = 9.9, p < .001) and Cortical ROI (F3,207 = 61.7, p < .001), as well as a Group*Cortical ROI interaction (F6,207 = 4.9, p < .001). Follow-up tests showed somewhat different pattern of group differences across the four ROIs (p values < .013, adjusted). Relative α power was lower in AD than HC across all ROIs. Relative α power was also lower in AD than in T2DM in the Frontal, Temporal, and Posterior (but not Central) ROIs. T2DM had significantly lower α power than HC in the Temporal ROI only (all p values < .05).
Beta:
There was a significant main effect of Cortical ROI (F3,207 = 47.5, p < .001), while Group (F2,68 = 1.1, p = .337) and Group*Cortical ROI were not significant (F6,207 = 1.8, p = .094). Follow-up tests showed a similar pattern of equivalent β power across groups, regardless of the ROI (p values > .7, adjusted).
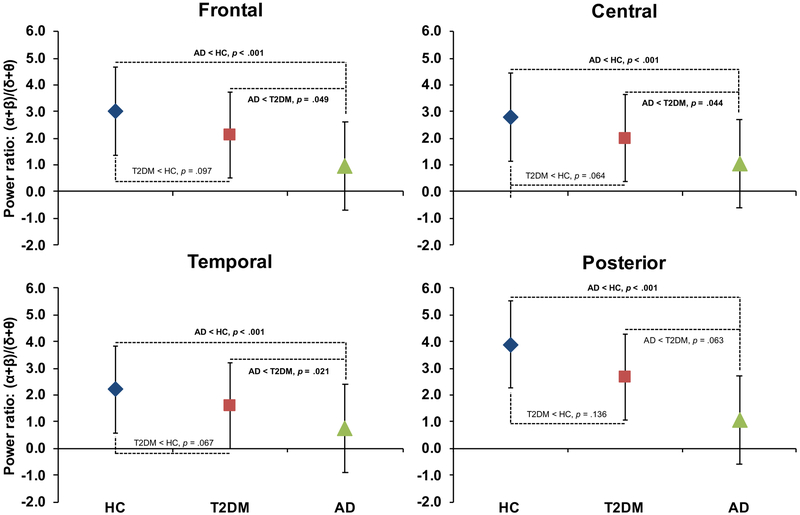
Spectral power ratio:
There were significant main effects of Group (F2,68 = 9.2, p < .001) and Cortical ROI (F3,207 = 20.8, p < .001), as well as a Group*Cortical ROI interaction (F6,207 = 3.3, p = .004). Follow-up analyses showed a pattern of group differences in Posterior ROI (HC > AD; p = .012, adjusted) that was distinct from the other ROIs (HC, T2DM > AD; p values < .008, adjusted) (Figure 2). These results indicate a shift of power from higher frequencies to lower frequencies in AD and suggest a similar pattern may be emerging in T2DM. Of note, an equivalent analysis in the age-matched sub-cohort demonstrated essentially identical findings (see Supplementary materials Section 2).
Figure 2. Spectral Power Ratio.
Figure shows the age-adjusted comparison across groups of the Spectral Power Ratio, (α+β)/(δ+θ), estimated from each cortical region of interest (ROI). Tukey’s Honestly Significant Difference post hoc tests demonstrated that (α+β)/(δ+θ) was lower in Alzheimer’s disease (AD) than in Healthy Controls (HC) across all ROIs (p values < 0.001) and lower than Type-2 Diabetes (T2DM) in all but the Posterior ROI (p values = 0.0499–0.063). T2DM was lower than HC across all ROIs though this difference did not reach significance (p values = 0.064–0.136). Data shown represent the least squared means and standard deviations derived from the linear regression models.
Neuropsychological function and relationship to EEG spectral power
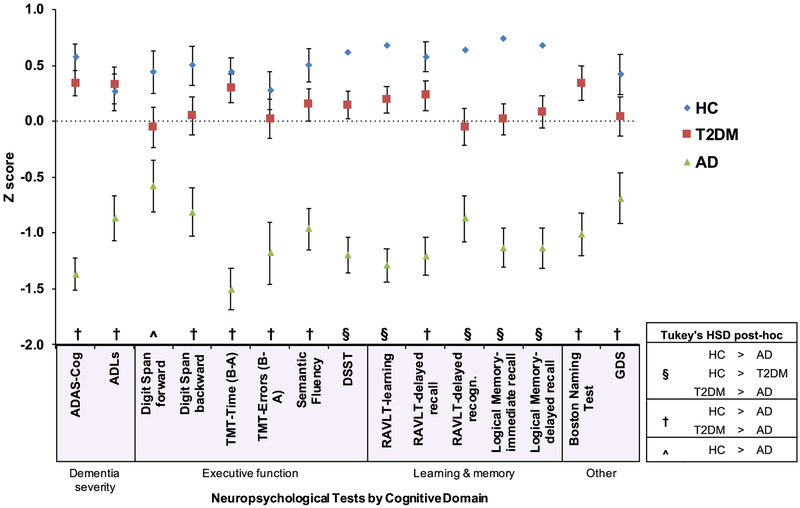
Group averaged neuropsychological test scores (z-scored) are displayed in Figure 3.
Figure 3. Group analysis and post-hoc comparisons of cognitive measures adjusted for age.
All data represent least squared means and standard error. Individual neuropsychological tests (x-axis) are shown grouped by cognitive domain. Scores (y-axis) were z-normalized and inverted (if necessary) so higher numbers reflect better performance/function. Following the first omnibus multivariate analysis of variance (MANOVA-1), group performance on individual tests was assessed using separate multiple linear regression analyses with age as a covariate. All results survived a 5% false discovery rate (FDR). In general, there was a continuum of deficits with healthy controls (HC) scoring higher than Type-2 diabetics (T2DM), who performed better than Alzheimer’s disease (AD). Post-hoc pairwise comparisons were conducted with Tukey’s honestly significant difference (HSD) tests. Three patterns were observed: (§) all three groups were significantly different; (†) AD scored significantly worse than both HC and T2DM, which were equivalent to each other; (^) HC were significantly better than AD, with T2DM not significantly different from either group. Additional abbreviations. Alzheimer’s disease Assessment Scale-Cognitive subscale (ADAS-Cog); Activities of Daily Living (ADLs); Digit Symbol Substitution Test (DSST); Trail Making Test (TMT); Rey Auditory Verbal Learning Test (RAVLT); Geriatric Depression Scale (GDS).
MANOVA-1 (Table 1) demonstrated that the variance in cognitive scores was different between the groups after controlling for Age, F(30, 86)=6.7, η2p=0.70, p<0.001, while Age itself was not a predictor of cognitive function, F(15,43)=1.7, η2p=0.37, p=0.096. Follow-up linear regression analyses yielded significant variance by Group for each neuropsychological measure after controlling for Age (F’s>5.7, p’s<0.006: see Supplementary Table S3). All measures survived a 5% FDR correction. For equivalent analyses in the age-matched sub-cohort with similar findings, see Supplementary materials Section 2.
Table 1.
Results of Multivariate Analyses of Variance (MANOVAs).
| MANOVA-1 | |||||
|---|---|---|---|---|---|
| Factor | Wilks' λ | df | F ratio | P value | Partial Eta2 |
| Group | 0.090 | 30,86 | 6.670 | <.001 | 0.699 |
| Age | 0.581 | 15,43 | 1.666 | 0.0958 | 0.368 |
| MANOVA-2 | |||||
| Factor | Wilks' λ | df | F ratio | P value | Partial Eta2 |
| Group | 0.550 | 6,522 | 30.200 | <.001 | 0.260 |
| Spectral Power Ratio | 0.399 | 3,261 | 36.100 | <.001 | 0.290 |
| Group*Spectral Power Ratio | 0.532 | 6,522 | 29.100 | <.001 | 0.250 |
| Age | 0.215 | 3,261 | 6.200 | <.001 | 0.070 |
In MANOVA-1, the dependent variables included z-normalized, rectified scores on the Alzheimer's disease Assessment Scale-Cognitive Subscale, Activities of Daily Living, Digit Symbol Substitution Test, Semantic Fluency Test, Trail Making Test time and errors (difference Part B-Part A), Digit Span length forward and backward, Rey Auditory Verbal Learning Test (learning, delayed recall, delayed recognition), Logical Memory story (immediate and delayed recall), Boston Naming Test, and Geriatric Depression Scale. In MANOVA-2, the dependent variables include the averaged Z-scores of the three cognitive domains (Learning & memory, Dementia severity, Executive function). Spectral Power Ratio refers to a whole-brain averaged power ratio [(alpha + beta)/(delta + theta)] obtained from eyes-closed resting-state electroencephalography.
Following Tukey’s HSD comparisons, two major patterns emerged (Figure 3): For scores on the DSST, RAVLT learning and delayed recognition trials, Logical Memory immediate and delayed recall trials, there were significant differences between all three groups with AD < T2DM < HC (p’s<0.03). By comparison, on the ADAS-Cog, ADLs, Semantic fluency, TMT time, TMT errors, Digit Span backward, RAVLT delayed recall, Boston Naming Test, and GDS, the AD group performed worse than either the HC or T2DM groups (p’s<0.04), while the latter two groups did not differ from each other (p’s>0.2). Lastly, on the Digit Span forward test was there a difference only between HC and AD (p=0.004) with T2DM not different from either HC or AD (p>0.1).
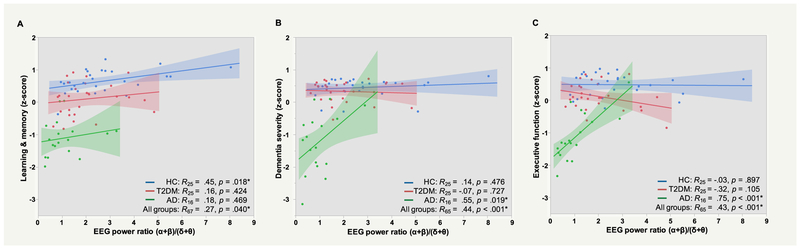
Concerning the association of cognitive function with the Spectral power ratio, MANOVA-2 (Table 1) indicated a main effect of Group, Wilks’ λ=0.55, F(6,522)=30.2, η2p=0.26, p<0.001, and an overall relationship between the composite neuropsychological scores and the Spectral power ratio, F(3,261)=36.1, η2p=0.29, p<0.001. In addition, there was a Group*Spectral power ratio interaction, F(6,522)=29.1, η2p=0.25, p<0.001, indicating that the overall relationship between cognition function and (α+β)/(δ+θ) differed between groups. Importantly, none of the effects that included Cortical ROI as a factor were significant (F ratios < .7, p values > .78), indicating that the overall relationship between the (α+β)/(δ+θ) and cognitive function did not vary as a function of cortical region. In contrast to MANOVA-1, Age was a predictor of cognitive function after controlling for Group, Cortical ROI, and Spectral power ratio, F(3,261)=6.2, p<.001. Post-hoc linear regression analyses showed that across all participants, Spectral power ratio had significant positive associations with Learning and memory (R67=0.27, p=0.040), Dementia severity (R65=0.44, p<0.001), and Executive functions (R65=0.43, p<0.001) (Figure 4); partial correlation coefficients were calculated from a model that included Group, Age, and the Group*Spectral power ratio interaction (except Learning and memory, for which the interaction term was highly non-significant, p=0.954).
Figure 4. Relationship between electroencephalography (EEG) Spectral power ratio and cognitive function.
Z-normalized scores (higher score indicates better performance) from individual neuropsychological tests were averaged together to form three domains: A. Learning & memory (Rey Auditory Verbal Learning Test, Logical Memory Story); B. Dementia severity (Alzheimer’s disease Assessment Scale-Cognitive subscale, Activities of Daily Living); C. Executive function (Digit Symbol Substitution Test, Semantic fluency, Trail Making, Digit Span forward and backward). Computed averages were related to the Spectral Power Ratio (α+β)/(δ+θ) and plotted separately for the three groups. In healthy controls (HC), higher (α+β)/(δ+θ) was significantly associated with better Learning & memory performance (p = 0.018, uncorrected). In Alzheimer’s disease (AD), higher (α+β)/(δ+θ) was significantly associated with better Dementia severity and Executive function (p’s < 0.05, uncorrected). By contrast, no significant relationships were observed in the Type-2 diabetes mellitus (T2DM) group (p’s > 0.1).
Considering cognition-EEG relationships within each group separately, higher Spectral power ratio was associated with better Learning and memory performance in HC (Figure 4A; p=0.018, uncorrected). In AD, higher (α+β)/(δ+θ) was associated with lower Dementia severity (Figure 4B) and better Executive function performance (Figure 4C), p’s<0.05, uncorrected). In contrast to HC and AD, no significant relationships were observed for T2DM (p’s>0.1). After subjecting p-values to a 5% FDR, the relationship between Spectral power ratio and Executive function in AD remained significant (p’s<0.05).
Individual alpha and posterior dominant frequencies
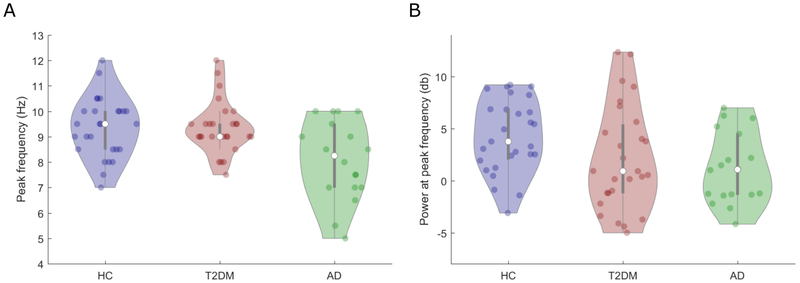
Dominant frequency (see Figure 5A):
Figure 5. Group analysis of Dominant posterior frequencies.
A. Individual frequency between 5-15 Hz with the highest power density across all posterior electrodes (Dominant posterior frequency) as a function of group (Healthy controls (HC), Type-2 diabetes mellitus (T2DM) and Alzheimer’s disease (AD)). B. Power density at the Dominant posterior frequency (averaged over the peak frequency ± 2 Hz) as a function of group. Colored dots denote individual participants, white dots denote group medians and background fills represent kernel density estimates.
A main effect of Group, controlling for Age, was identified (F(2,68) = 6.26, η2p = 0.22, p = 0.001). The Dominant frequency was significantly lower in AD (mean = 8.2 Hz) compared to both T2DM (9.4 Hz: p = 0.002) and HC (9.3 Hz: p = 0.003). There was no significant difference between T2DM and HC (p = 0.99).
Power density at dominant frequency (see Figure 5B):
A main effect of Group was identified (F(2,68) = 3.41, η2p = 0.09, p = 0.039). Power density in the Dominant frequency band was significantly lower in AD compared to HC (p = 0.05) but not compared to T2DM (p = 0.47). There was no significant difference between T2DM and HC (p = 0.08).
Similar results were found for the IAF analysis (see supplementary Section 3 and Figure S3). Hence, there was a shift of the dominant rhythm towards lower frequencies in AD relative to both T2DM and healthy controls. However there is also a reduction in power at both the Dominant frequency and the IAF in AD. Intriguingly, T2DM showed significantly higher Dominant frequency and IAF values compared to AD (in line with HC), but did not show any significant difference in terms of power density at either the Dominant frequency or the IAF (in contrast to HC). This suggests that, unlike in AD, the frequency of the dominant posterior rhythm in T2DM is indistinguishable to that observed in HC. However, in terms of power density at the dominant rhythm, T2DM resembled AD more closely than HC.
In contrast to the Spectral power ratio, there was no significant relationship between any of the composite cognitive measures and either the Dominant Frequency or IAF.
Discussion
The present study compared oscillatory power and neuropsychological function (and their relationship) between HC, AD and T2DM in order to better understand pathophysiological signatures of cognitive aging. Cognitively, AD was associated with deficits across almost all neuropsychological tests, whereas T2DM was associated with selective deficits in verbal/episodic learning, memory and psychomotor processing speed. Neurophysiologically, there was a pattern of shifting EEG power from higher to lower frequencies in AD, and evidence that a similar shift is also apparent to a lesser degree in T2DM, particularly over temporal regions. Capturing this shift as a single measure (the ratio of α+β/δ+θ power) across participants allowed us to investigate the relevance of these oscillatory changes for cognitive aging. This Spectral power ratio was uniquely associated with executive functions and dementia severity in the AD group, and with learning and memory function in the HC group. The results suggest that a shift in EEG power from higher to lower frequencies represents a candidate biomarker for specific cognitive deficits associated with aging and brain-related diseases.
Some of the results replicate findings from previous studies which, particularly given recent concerns about the reproducibility of scientific findings in both neuroimaging (Poldrack et al., 2017) and psychology (Open Science Collaboration, 2015), is of great importance in establishing the reliability of the reported effects. Moreover, the current findings go beyond replication to extend prior work by collapsing the spectral power distribution into a single easily obtainable summary metric, and then examining how this metric relates to specific domains of cognitive function. We contribute several important novel insights into the pathophysiology of cerebral oscillations in AD and T2DM relative to normal cognitive aging. The novel aspects include (1) a direct comparison of EEG activity and neuropsychological performance between AD, T2DM and healthy controls, (2) extensive testing of group differences in both absolute and relative EEG power across all electrodes and a wide range of frequencies (1-40 Hz), (3) a parsing of the relationship between oscillatory abnormalities and specific cognitive domains (i.e. memory versus executive function) across the different groups, (4) evaluation of the distribution of frequency changes across different brain regions, and (5) analyses of shifts in the Dominant frequency, as well as in the power density at this individually-defined dominant frequency.
Differences in cognitive function associated with AD and T2DM
AD participants showed marked neuropsychological deficits relative to both HC and T2DM. The most prominent deficits were observed on learning, memory and executive function tests. AD participants also reported impaired function in activities of daily living and increased symptoms of depression compared to both HC and T2DM. These symptoms are well established in AD (Burns and Iliffe, 2009).
Additionally, a pattern of performance differences was observed from HC to T2DM to AD on verbal/episodic learning (RAVLT and Logical Memory) and psychomotor processing speed (DSST). These findings accord with previous reports of mild decrements in memory, motor function and attention and perceptual processing speed in T2DM relative to HC (Cheng et al., 2012; Marseglia et al., 2016; Mooradian et al., 1988; Palta et al., 2014; Takeuchi et al., 2012; van den Berg et al., 2010). Thus, T2DM may affect these cognitive domains first, and the effects are detectable using commonly employed neuropsychological tests. It is important to acknowledge that cognitive impairment in T2DM is likely modulated by many variables, including vascular risk factors (Marseglia et al., 2016), presence of the apolipoprotein ε4 allele (Ravona-Springer et al., 2014) and glycemic control (Yaffe et al., 2012). These factors were not controlled for here, and may have contributed to the observed cognitive deficits. However, the current results provide evidence that mild neuropsychological deficits are detectable in T2DM even when participants report no cognitive impairment.
Changes in oscillatory activity and relationship with cognition in AD
The present study suggests that both AD and T2DM are associated with abnormal neural oscillations, relative to HC. In AD, we observed reduction in α+β power and increase in δ+θ power, in line with previous findings (Babiloni et al., 2016; Bennys et al., 2001; Brenner et al., 1986; Coben et al., 1990, 1983; Dierks et al., 1995; Fraga et al., 2013; Jeong, 2004; Moretti et al., 2004; Neto et al., 2016). There was a similar pattern of higher δ+θ power (HC < AD), and a similar pattern of lower α+β power (HC > AD), across all ROIs. These oscillatory signatures, as captured by the ratio of (α+β)/(δ+θ) power, correlated with learning and memory function across all groups combined, though the correlation was relatively weak within each group and only significant in HC. In AD, the Spectral power ratio was strongly associated with executive function performance and dementia severity, with the degree of change being positively correlated with symptom severity. Previous studies have found a correlation between band-specific EEG power and the severity of cognitive deficits in AD (Babiloni et al., 2007, 2006a; Dierks et al., 1995; Helkala et al., 1991; Luckhaus et al., 2008; Moretti et al., 2009; van der Hiele et al., 2007). The current results confirm and expand on this literature, suggesting that the ratio of (α+β)/(δ+θ) power is a strong predictor specifically of executive function in AD (accounting for more than 55% of the variance). Notably, the Spectral power ratio was also associated with overall dementia severity, suggesting that deficits in executive functions (as opposed to learning and memory) may be more closely tied to global indicators of dementia. Intriguingly, similar neural changes are predictive of progression from MCI to dementia (Babiloni et al., 2011; Grunwald et al., 2001; Jelic et al., 2000, 1996; Rossini et al., 2006) and have been associated with cognitive deficits in disorders such as ADHD (Barry et al., 2003), dyslexia (Penolazzi et al., 2008), schizophrenia (Bates et al., 2009; Boutros et al., 2008) and Parkinson’s disease (Klassen et al., 2011; Olde Dubbelink et al., 2014).
In line with previous studies (Moretti et al., 2004; Poza et al., 2007; Babiloni et al., 2015), we found lower dominant posterior frequencies in AD (mean = 8.2 Hz) relative to both HC and T2DM, who showed typical mean dominant frequencies in the α-band (Klimesch, 1999; Mierau et al., 2017; Knyazeva et al., 2018). However, in contrast to the Spectral power ratio, we found no relationship between the posterior dominant frequency or IAF and performance on any of the composite cognitive scores. This suggests that pathophysiological changes in power density in AD are more cognitively relevant than changes in peak frequency.
Changes in oscillatory activity and relationship with cognition in T2DM
Interestingly, α+β power density in T2DM participants was intermediate between HC and AD participants. This finding replicates and extends the results of Cooray et al. (2011), who found that α+β power was reduced in T2DM compared to HC. We also found that T2DM is specifically associated with a reduction of α power in the temporal regions, with no significant differences observed in other brain regions relative to HC. This is notable insofar as deficits in temporal α power have previously been linked to impairments in learning and memory in AD (Babiloni et al., 2009). Interestingly, a subset of T2DM participants in the study of Cooray et al. (2011) who received a 2-month glycemic control treatment showed an increase in α power, associated with improvements in visuospatial and semantic memory performance. Collectively, these results highlight alterations in brain function and α power associated with T2DM (Fried et al., 2017; Strachan et al., 2011).
No difference was found between T2DM and HC in either the Dominant posterior frequency or the IAF. However, despite the peak frequency remaining intact, a tendency was observed for a reduction in power density at both the Dominant posterior frequency and the IAF, with the power density profile in T2DM more closely resembling AD than HC. To our knowledge, this represents the first analysis of peak frequencies in T2DM. Though we found no link between these power density changes and neuropsychological performance in the current sample, future longitudinal studies may investigate further whether they are cognitively relevant and potentially prodromal of later changes in peak frequency.
Differences in cognitive relevance of oscillatory signatures between AD and T2DM
Though T2DM confers an increased risk for developing AD (Biessels and Kappelle, 2005; Barbagallo and Dominguez, 2014), little is known about the mechanistic underpinnings that link the two disorders (Chatterjee and Mudher, 2018; Chornenkyy et al., 2019). In contrast to AD, we found no correlation between the Spectral Power Ratio and the degree of cognitive impairment in T2DM for any of the neuropsychological tests in our battery. It is possible that this highlights the domain-specific nature of the EEG–cognition link, as the T2DM group showed no marked deficits on the executive function tests, which were most strongly related to the Spectral Power Ratio in AD. A related possibility concerns the multifactorial nature of T2DM-related impact on the brain. Despite some similarities in the observed EEG changes associated with AD and T2DM, the electrophysiological signatures linked to cognitive deficits may not be the same due to differing neurodegeneration and cerebrovascular pathologies. This proposal could be tested in future studies by combing resting-state EEG recordings and comprehensive neuropsychological testing with structural magnetic resonance imaging (MRI) in both AD and T2DM samples. This may allow for the establishment of a physiological link between oscillatory activity, structural abnormalities and cognitive functions. Such an approach would shed further light on similarities and differences in the neuropathological processes underlying cognitive impairment in T2DM and AD.
EEG oscillations and cognition
Oscillatory EEG activity reliably co-varies with cognitive functions in a band- and domain-specific manner (Basar et al., 2001). For example, α-band activity has been associated with memory (Bonnefond and Jensen, 2012; Klimesch, 1999; Palva and Palva, 2007), attention (Benwell et al., 2017, 2018; Foxe and Snyder, 2011), and arousal (Benwell et al., 2018; Cantero et al., 1999; Sadaghiani et al., 2010), while β-activity is believed to play a role in sensorimotor functions (Pfurtscheller et al., 1996) and the maintenance of top-down attention (Buschman and Miller, 2007; Engel and Fries, 2010). These findings have led to suggestions that oscillations are computationally relevant for neuronal synchrony/communication and higher-order cognition (Canolty and Knight, 2010).
Hence, changes in EEG power associated with pathophysiology may reflect abnormal synchronization of large-scale networks of pyramidal cortical neurons and consequent impairment of information transfer required for cognitive functions. Recent studies employing both structural neuroimaging and EEG/MEG suggest that increases in δ+θ power (and reductions in α power) correlate with neurodegenerative processes associated with AD such as atrophy of sub-cortical white matter, cortical gray matter and hippocampus (Babiloni et al., 2013, 2006b; Fernandez et al., 2003; Helkala et al., 1996).
From a functional perspective, one theory linking frequency ratio changes with cognitive impairment suggests a possible reciprocal relationship between α-band and low-frequency (δ+θ) activity (Knyazev, 2012, 2007). Specifically, α-activity is implicated in controlling adaptive functional inhibition (Klimesch et al., 2007), facilitating goal-directed sensory and behavioral regulation. Accordingly, when this reciprocal relationship is unbalanced, through reductions in α-mediated inhibition and/or abnormal increases in low-frequency activity, pathological disinhibition occurs with consequent cognitive and behavioral impairments (Knyazev, 2012, 2007). Notably, differences in the spectral ratio between T2DM and HC were primarily driven by reduced power in higher (α+β) frequencies in T2DM, without a strong increase in low-frequency (δ+θ) power. If reduction in α-power indexes decreased functional inhibition relevant for cognitive performance, then this may be prodromal in T2DM of subsequent increase in low-frequency activity and accelerated cognitive decline. Unfortunately, due to the single time-point/cross-sectional nature of the current study, the results cannot provide evidence as to the existence of any causal link between T2DM and AD. It is crucial to acknowledge that the causal factors underlying cognitive impairments may not be shared across the disorders; hence, we cannot yet ascribe the EEG differences to a single underlying cause. Future longitudinal, prospective studies are however warranted given existing epidemiologic data and the reported cross-sectional findings here. Longitudinal measurements of EEG power and neuropsychological performance in individuals with T2DM could test the prognostic power of EEG changes in terms of subsequent cognitive decline, including progression to AD (Gispen and Biessels, 2000; Stewart and Liolitsa, 1999).
Additional limitations of the current study include a lack of older participants, particularly in the T2DM and HC groups. Future studies should look to recruit from a wider range of older adults. It is important to note that, despite no individuals scoring as clinically impaired on the MMSE, we were unable to fully rule out the possibility of pre-clinical AD being present in the HC and T2DM groups. It would also be of benefit to collect more potentially relevant demographic details which were not available here, including smoking status, comorbid psychiatric symptomology and time since diagnosis. Additional information regarding medication use might be of particular value given that T2DM treated with medications may not experience equivalent neurocognitive consequences to those controlling the disease through exercise and diet (Walker and Harrison, 2015; Ngandu et al., 2015). Furthermore, we did not consider the potential association between γ-band (~30-100 Hz) oscillations and cognitive function in either T2DM or AD, despite previous research suggesting γ-band activity to be cognitively relevant (van Deursen et al., 2008; Başar et al., 2016). We chose not to include EEG-measured γ because it is often contaminated by muscle (Whitham et al., 2007) and eye-movement artifacts (Yuval-Greenberg et al., 2008). An optimal approach to investigate pathophysiological signatures of γ activity in future studies would be to employ magnetoencephalography, in which cerebral γ activity can be more clearly and robustly identified than in EEG (Mandal et al., 2018).
Conclusions
Neuropsychological deficits are widespread in AD and selective in T2DM (with relative sparing of executive functions). Relative to HC, AD patients had higher EEG power in lower frequencies and lower power in higher frequencies across all brain regions. In contrast, patients with T2DM showed decreases in specifically α power relative to HC restricted to the temporal regions. The ratio, (α+β)/(δ+θ), showed a continuum of differences from HC to T2DM to AD. This Spectral power ratio correlated with dementia severity and executive functioning in AD and learning and memory performance in HC and across all groups combined. In contrast, no relationship was found between IAF and cognitive function in any of the three groups. Shift in the ratio of relative power (in favor of low frequencies) within the EEG power-spectrum represents a candidate neural signature of cognitive deficits associated with aging-related diseases including AD and T2DM.
Supplementary Material
Acknowledgements
This study was primarily funded by the National Institutes of Health (NIH R21 NS082870). A.P.-L. was also supported in part by the Sidney R. Baer Jr. Foundation, the NIH (R01HD069776, R01NS073601, R21 MH099196, R21 NS085491, R21 HD07616), Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758), the Football Players Health Study at Harvard University, and by the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. C.S.Y.B. was also supported by the Economic and Social Research Council (UK) (ES/I02395X/1), the Experimental Psychology Society (UK) and the Guarantors of Brain (UK). E.S. is partially supported by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014-13121700007, by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) grant 2017, and by the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030, and the NIH (P01 AG031720-06A1, R01 MH117063-01, R01 AG060981-01). M.M.S. is supported by the CURE (Citizens United for Research in Epilepsy) foundation, the Football Players Health Study (FPHS) at Harvard University, and the NIH (R01 MH115949, R01AG060987, R01 NS073601, P01 AG031720-06A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, DARPA, IARPA, ODNI, BIDMC, or the Sidney R. Baer Jr. Foundation.
The authors thank E. Seligson, N. Atkinson, and S. Saxena for their assistance in data collection, and A. Connor and J. Macone for regulatory and compliance oversight and assistance with evaluation of participant health and medical history.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Disclosures
A.P.-L. serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Cognito, Constant Therapy, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. E.S. serves on the scientific advisory boards for EBNeuro Ltd and Neuroelectrics, and is listed as an inventor on issued and pending patents on the integration of non-invasive brain stimulation with neuroimaging data for therapeutic applications in neurodegenerative disorders and brain tumors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberti KG, Zimmet PZ, 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. J. Br. Diabet. Assoc 15, 539–553. [DOI] [PubMed] [Google Scholar]
- Assenza G, Capone F, di Biase L, Ferreri F, Florio L, Guerra A, Marano M, Paolucci M, Ranieri F, Salomone G, Tombini M, Thut G, Di Lazzaro V, 2017. Oscillatory Activities in Neurological Disorders of Elderly: Biomarkers to Target for Neuromodulation. Front. Aging Neurosci 9, 189 10.3389/fnagi.2017.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C, 2004. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J. Clin. Exp. Neuropsychol 26, 1044–1080. 10.1080/13803390490514875 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Cerboneschi D, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual-Marqui RD, Rodriguez G, Romani GL, Salinari S, Tecchio F, Vitali P, Zanetti O, Zappasodi F, Rossini PM, 2004. Mapping distributed sources of cortical rhythms in mild Alzheimer’s disease. A multicentric EEG study. NeuroImage 22, 57–67. 10.1016/j.neuroimage.2003.09.028 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM, 2006a. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 117, 252–268. 10.1016/j.clinph.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Lizio R, Vecchio F, Baglieri A, Bernardini S, Cavedo E, Bozzao A, Buttinelli C, Esposito F, Giubilei F, Guizzaro A, Marino S, Montella P, Quattrocchi CC, Redolfi A, Soricelli A, Tedeschi G, Ferri R, Rossi-Fedele G, Ursini F, Scrascia F, Vernieri F, Pedersen TJ, Hardemark H-G, Rossini PM, Frisoni GB, 2013. Resting state cortical electroencephalographic rhythms are related to gray matter volume in subjects with mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 34, 1427–1446. 10.1002/hbm.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Cassetta E, Binetti G, Tombini M, Del Percio C, Ferreri F, Ferri R, Frisoni G, Lanuzza B, Nobili F, Parisi L, Rodriguez G, Frigerio L, Gurzi M, Prestia A, Vernieri F, Eusebi F, Rossini PM, 2007. Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer’s disease. Eur. J. Neurosci 25, 3742–3757. 10.1111/j.1460-9568.2007.05601.x [DOI] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Boccardi M, Lizio R, Lopez S, Carducci F, Marzano N, Soricelli A, Ferri R, Triggiani AI, Prestia A, Salinari S, Rasser PE, Basar E, Famà F, Nobili F, Yener G, Emek-Savas DD, Gesualdo L, Mundi C, Thompson PM, Rossini PM, Frisoni GB, 2015. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 36, 556–570. 10.1016/j.neurobiolaging.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Lizio R, Noce G, Lopez S, Soricelli A, Ferri R, Pascarelli MT, Catania V, Nobili F, Arnaldi D, Fama F, Aarsland D, Orzi F, Buttinelli C, Giubilei F, Onofrj M, Stocchi F, Vacca L, Stirpe P, Fuhr P, Gschwandtner U, Ransmayr G, Garn H, Fraioli L, Pievani M, Frisoni GB, D’Antonio F, De Lena C, Güntekin B, Hanoğlu L, Başar E, Yener G, Emek-Savaş DD, Triggiani AI, Franciotti R, Taylor JP, De Pandis MF, Bonanni L, 2018. Abnormalities of Resting State Cortical EEG Rhythms in Subjects with Mild Cognitive Impairment Due to Alzheimer’s and Lewy Body Diseases. J. Alzheimers Dis. JAD 62, 247–268. 10.3233/JAD-170703 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni G, Steriade M, Bresciani L, Binetti G, Del Percio C, Geroldi C, Miniussi C, Nobili F, Rodriguez G, Zappasodi F, Carfagna T, Rossini PM, 2006b. Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 117, 1113–1129. 10.1016/j.clinph.2006.01.020 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni GB, Pievani M, Vecchio F, Lizio R, Buttiglione M, Geroldi C, Fracassi C, Eusebi F, Ferri R, Rossini PM, 2009. Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. Neuroimage 44, 123–135. 10.1016/j.neuroimage.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni GB, Vecchio F, Lizio R, Pievani M, Cristina G, Fracassi C, Vernieri F, Rodriguez G, Nobili F, Ferri R, Rossini PM, 2011. Stability of clinical condition in mild cognitive impairment is related to cortical sources of alpha rhythms: an electroencephalographic study. Hum. Brain Mapp 32, 1916–1931. 10.1002/hbm.21157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, Cordone S, Gesualdo L, Del Percio C, 2016. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 103, 88–102. 10.1016/j.ijpsycho.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Baker M, Akrofi K, Schiffer R, Boyle MWO, 2008. EEG Patterns in Mild Cognitive Impairment (MCI) Patients. Open Neuroimaging J 2, 52–55. 10.2174/1874440000802010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo M, Dominguez LJ, 2014. Type 2 diabetes mellitus and Alzheimer’s disease. World J. Diabetes 5, 889–893. 10.4239/wjd.v5.i6.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, 2003. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 114, 171–183. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M, 2001. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 39, 241–248. [DOI] [PubMed] [Google Scholar]
- Başar E, Emek-Savaş DD, Güntekin B, Yener GG, 2016. Delay of cognitive gamma responses in Alzheimer’s disease. NeuroImage Clin 11, 106–115. 10.1016/j.nicl.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF, 2009. Low-frequency EEG oscillations associated with information processing in schizophrenia. Schizophr. Res 115, 222–230. 10.1016/j.schres.2009.09.036 [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA, NIA Alzheimer’s Disease Centers, 2007. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis. Assoc. Disord 21, 249–258. 10.1097/WAD.0b013e318142774e [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Ann. Stat 1165–1188. [Google Scholar]
- Bennys K, Rondouin G, Vergnes C, Touchon J, 2001. Diagnostic value of quantitative EEG in Alzheimer’s disease. Neurophysiol. Clin. Clin. Neurophysiol 31, 153–160. [DOI] [PubMed] [Google Scholar]
- Benwell CSY, Keitel C, Harvey M, Gross J, Thut G, 2018. Trial-by-trial co-variation of pre-stimulus EEG alpha power and visuospatial bias reflects a mixture of stochastic and deterministic effects. Eur. J. Neurosci 48, 2566–2584. 10.1111/ejn.13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell CSY, London RE, Tagliabue CF, Veniero D, Gross J, Keitel C, Thut G, 2019. Frequency and power of human alpha oscillations drift systematically with time-on-task. Neuroimage 192, 101–114. 10.1016/j.neuroimage.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell CSY, Tagliabue CF, Veniero D, Cecere R, Savazzi S, Thut G, 2017. Prestimulus EEG Power Predicts Conscious Awareness But Not Objective Visual Performance. eNeuro 4 10.1523/ENEURO.0182-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z, Li Q, Wang L, Lu C, Yin S, Li X, 2014. Relative power and coherence of EEG series are related to amnestic mild cognitive impairment in diabetes. Front. Aging Neurosci 6, 11 10.3389/fnagi.2014.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Kappelle LJ, 2005. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem. Soc. Trans 33, 1041–1044. 10.1042/BST20051041 [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Strachan MWJ, Visseren FLJ, Kappelle LJ, Whitmer RA, 2014. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2, 246–255. 10.1016/S2213-8587(13)70088-3 [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O, 2012. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr. Biol. CB 22, 1969–1974. 10.1016/j.cub.2012.08.029 [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Kastner S, Jensen O, 2017. Communication between Brain Areas Based on Nested Oscillations. eNeuro 4 10.1523/ENEURO.0153-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, iacono W, 2008. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr. Res 99, 225–237. 10.1016/j.schres.2007.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1998. Evolution of neuronal changes in the course of Alzheimer’s disease. J. Neural Transm Suppl. 53, 127–140. [DOI] [PubMed] [Google Scholar]
- Brenner RP, Ulrich RF, Spiker DG, Sclabassi RJ, Reynolds CF 3rd, Marin RS, Boller F, 1986. Computerized EEG spectral analysis in elderly normal, demented and depressed subjects. Electroencephalogr. Clin. Neurophysiol 64, 483–492. [DOI] [PubMed] [Google Scholar]
- Burns A, Iliffe S, 2009. Alzheimer’s disease. BMJ 338, b158. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK, 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862. 10.1126/science.1138071 [DOI] [PubMed] [Google Scholar]
- Buss SS, Padmanabhan J, Saxena S, Pascual-Leone A, Fried PJ, 2018. Atrophy in Distributed Networks Predicts Cognition in Alzheimer’s Disease and Type 2 Diabetes. J. Alzheimers Dis. JAD 65, 1301–1312. 10.3233/JAD-180570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Logothetis N, Singer W, 2013. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80, 751–764. 10.1016/j.neuron.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero MD, Navarro E, 2004. Relationship between plasticity, mild cognitive impairment and cognitive decline. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol 19, 653–660. 10.1016/j.acn.2003.08.008 [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT, 2010. The functional role of cross-frequency coupling. Trends Cogn. Sci 14, 506–515. 10.1016/j.tics.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Gomez C, Salas RM, 1999. Spectral structure and brain mapping of human alpha activities in different arousal states. Neuropsychobiology 39, 110–116. 10.1159/000026569 [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Mudher A, 2018. Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front. Neurosci 12, 383–383. https://doi.Org/10.3389/fnins.2018.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Huang C, Deng H, Wang H, 2012. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J 42, 484–491. 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- Chornenkyy Y, Wang W-X, Wei A, Nelson PT, 2019. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. Zurich Switz 29, 3–17. 10.1111/bpa.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coben LA, Chi D, Snyder AZ, Storandt M, 1990. Replication of a study of frequency analysis of the resting awake EEG in mild probable Alzheimer’s disease. Electroencephalogr. Clin. Neurophysiol 75, 148–154. [DOI] [PubMed] [Google Scholar]
- Coben LA, Danziger WL, Berg L, 1983. Frequency analysis of the resting awake EEG in mild senile dementia of Alzheimer type. Electroencephalogr. Clin. Neurophysiol 55, 372–380. [DOI] [PubMed] [Google Scholar]
- Cooray G, Nilsson E, Wahlin A, Laukka EJ, Brismar K, Brismar T, 2011. Effects of intensified metabolic control on CNS function in type 2 diabetes. Psychoneuroendocrinology 36, 77–86. 10.1016/j.psyneuen.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Corcoran AW, Alday PM, Schlesewsky M, Bornkessel-Schlesewsky I, 2018. Toward a reliable, automated method of individual alpha frequency (IAF) quantification. Psychophysiology 55, e13064 10.1111/psyp.13064 [DOI] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, Alzheimer’s Disease Neuroimaging Initiative, 2012. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 6, 502–516. 10.1007/s11682-012-9186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Liu J, Bian Z, Li Q, Wang L, Li X, 2014. Cortical source multivariate EEG synchronization analysis on amnestic mild cognitive impairment in type 2 diabetes. ScientificWorldJournal 2014, 523216 10.1155/2014/523216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TDR, Finnigan S, 2007. Theta power is reduced in healthy cognitive aging. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 66, 10–17. 10.1016/j.ijpsycho.2007.05.008 [DOI] [PubMed] [Google Scholar]
- de la Monte SM, 2014. Relationships between diabetes and cognitive impairment. Endocrinol. Metab. Clin. North Am 43, 245–267. 10.1016/j.ecl.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004a. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004b. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dierks T, Frolich L, Ihl R, Maurer K, 1995. Correlation between cognitive brain function and electrical brain activity in dementia of Alzheimer type. J. Neural Transm. Gen. Sect 99, 55–62. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, 2010. Beta-band oscillations—signalling the status quo? Beta-Band Oscil. Status Quo 20, 156–165. 10.1016/j.conb.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Fernandez A, Arrazola J, Maestu F, Amo C, Gil-Gregorio P, Wienbruch C, Ortiz T, 2003. Correlations of hippocampal atrophy and focal low-frequency magnetic activity in Alzheimer disease: volumetric MR imaging-magnetoencephalographic study. AJNR Am. J. Neuroradiol 24, 481–487. [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. "Mini-mental state". A Practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC, 2011. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol 2, 154 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga FJ, Falk TH, Kanda PAM, Anghinah R, 2013. Characterizing Alzheimer’s disease severity via resting-awake EEG amplitude modulation analysis. PloS One 8, e72240 10.1371/journal.pone.0072240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Schilberg L, Brem A-K, Saxena S, Wong B, Cypess AM, Horton ES, Pascual-Leone A, 2017. Humans with Type-2 Diabetes Show Abnormal Long-Term Potentiation-Like Cortical Plasticity Associated with Verbal Learning Deficits. J. Alzheimers Dis. JAD 55, 89–100. 10.3233/JAD-160505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, 2015. Rhythms for Cognition: Communication through Coherence. Rhythms Cogn. Commun. Coherence 88, 220–235. 10.1016/j.neuron.2015.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK, Alzheimer’s Disease Neuroimaging Initiative, 2012. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 6, 517–527. 10.1007/s11682-012-9176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ, 2000. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 23, 542–549. 10.1016/S0166-2236(00)01656-8 [DOI] [PubMed] [Google Scholar]
- Grandy TH, Werkle-Bergner M, Chicherio C, Schmiedek F, Lovden M, Lindenberger U, 2013. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 50, 570–582. 10.1111/psyp.12043 [DOI] [PubMed] [Google Scholar]
- Grunwald M, Busse F, Hensel A, Kruggel F, Riedel-Heller S, Wolf H, Arendt T, Gertz HJ, 2001. Correlation between cortical theta activity and hippocampal volumes in health, mild cognitive impairment, and mild dementia. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 18, 178–184. [DOI] [PubMed] [Google Scholar]
- Harada CN, Natelson Love MC, Triebel K, 2013. Normal Cognitive Aging. Clin. Geriatr. Med 29, 737–752. 10.1016/j.cger.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T, 2007. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. NeuroImage 35, 488–500. 10.1016/j.neuroimage.2006.11.042 [DOI] [PubMed] [Google Scholar]
- Helkala EL, Hanninen T, Hallikainen M, Kononen M, Laakso MP, Hartikainen P, Soininen H, Partanen J, Partanen K, Vainio P, Riekkinen PS, 1996. Slow-wave activity in the spectral analysis of the electroencephalogram and volumes of hippocampus in subgroups of Alzheimer’s disease patients. Behav. Neurosci 110, 1235–1243. [DOI] [PubMed] [Google Scholar]
- Helkala EL, Laulumaa V, Soikkeli R, Partanen J, Soininen H, Riekkinen PJ, 1991. Slow-wave activity in the spectral analysis of the electroencephalogram is associated with cortical dysfunctions in patients with Alzheimer’s disease. Behav. Neurosci 105, 409–415. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216. 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Nordberg A, Winblad B, Wahlund LO, 2000. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol. Aging 21, 533–540. [DOI] [PubMed] [Google Scholar]
- Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO, 1996. Quantitative electroencephalography power and coherence in Alzheimer’s disease and mild cognitive impairment. Dement. Basel Switz 7, 314–323. [DOI] [PubMed] [Google Scholar]
- Jeong J, 2004. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 115, 1490–1505. 10.1016/j.clinph.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Keitel C, Benwell CSY, Thut G, Gross J, 2018. No changes in parieto-occipital alpha during neural phase locking to visual quasi-periodic theta-, alpha-, and beta-band stimulation. Eur. J. Neurosci 48, 2551–2565. 10.1111/ejn.13935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel C, Keitel A, Benwell CSY, Daube C, Thut G, Gross J, 2019. Stimulus-Driven Brain Rhythms within the Alpha Band: The Attentional-Modulation Conundrum. J. Neurosci. Off. J. Soc. Neurosci 39, 3119–3129. 10.1523/JNEUROSCI.1633-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass DW, Brenner RP, 1995. Electroencephalography of the elderly. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 12, 116–131. [DOI] [PubMed] [Google Scholar]
- Klassen BT, Hentz JG, Shill HA, Driver-Dunckley E, Evidente VGH, Sabbagh MN, Adler CH, Caviness JN, 2011. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology 77, 118–124. 10.1212/WNL.0b013e318224af8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, 2012. Alpha-band oscillations, attention, and controlled access to stored information. Alpha-Band Oscil. Atten. Control. Access Stored Inf 16, 606–617. 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, 1999. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev 29, 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, 2007. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev 53, 63–88. 10.1016/j.brainresrev.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Knyazev GG, 2012. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev 36, 677–695. 10.1016/j.neubiorev.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Knyazev GG, 2007. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev 31, 377–395. 10.1016/j.neubiorev.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Knyazeva MG, Barzegaran E, Vildavski VY, Demonet J-F, 2018. Aging of human alpha rhythm. Neurobiol. Aging 69, 261–273. 10.1016/j.neurobiolaging.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Koekkoek PS, Rutten GEHM, Biessels GJ, 2014. Handbook of Clinical Neurology. Handb. Clin. Neurol 126, 145–166. 10.1016/B978-0-444-53480-4.00011-4 [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, Jelic V, 2005. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 26, 165–171. 10.1016/j.neurobiolaging.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Leirer VM, Wienbruch C, Kolassa S, Schlee W, Elbert T, Kolassa I-T, 2011. Changes in cortical slow wave activity in healthy aging. Brain Imaging Behav. 5, 222–228. 10.1007/s11682-011-9126-3 [DOI] [PubMed] [Google Scholar]
- Luckhaus C, Grass-Kapanke B, Blaeser I, Ihl R, Supprian T, Winterer G, Zielasek J, Brinkmeyer J, 2008. Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): results of a 1-year follow-up study. Int. J. Geriatr. Psychiatry 23, 1148–1155. 10.1002/gps.2042 [DOI] [PubMed] [Google Scholar]
- Mandal PK, Banerjee A, Tripathi M, Sharma A, 2018. A Comprehensive Review of Magnetoencephalography (MEG) Studies for Brain Functionality in Healthy Aging and Alzheimer’s Disease (Ad). Front. Comput. Neurosci 12, 60–60. 10.3389/fncom.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Marseglia A, Fratiglioni L, Laukka EJ, Santoni G, Pedersen NL, Backman L, Xu W, 2016. Early Cognitive Deficits in Type 2 Diabetes: A Population-Based Study. J. Alzheimers Dis. JAD 53, 1069–1078. 10.3233/JAD-160266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AC, Cooper NR, 2017. The association between high levels of cumulative life stress and aberrant resting state EEG dynamics in old age. Biol. Psychol 127, 64–73. 10.1016/j.biopsycho.2017.05.005 [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc 7, 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau A, Klimesch W, Lefebvre J, 2017. State-dependent alpha peak frequency shifts: Experimental evidence, potential mechanisms and functional implications. Neuroscience 360, 146–154. 10.1016/j.neuroscience.2017.07.037 [DOI] [PubMed] [Google Scholar]
- Mohs RC, Rosen WG, Davis KL, 1983. The Alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol. Bull 19, 448–450. [PubMed] [Google Scholar]
- Mooradian AD, Perryman K, Fitten J, Kavonian GD, Morley JE, 1988. Cortical function in elderly non-insulin dependent diabetic patients. Behavioral and electrophysiologic studies. Arch. Intern. Med 148, 2369–2372. [PubMed] [Google Scholar]
- Moretti DV, Babiloni C, Binetti G, Cassetta E, Dal Forno G, Ferreric F, Ferri R, Lanuzza B, Miniussi C, Nobili F, Rodriguez G, Salinari S, Rossini PM, 2004. Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 115, 299–308. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Fracassi C, Pievani M, Geroldi C, Binetti G, Zanetti O, Sosta K, Rossini PM, Frisoni GB, 2009. Increase of theta/gamma ratio is associated with memory impairment. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 120, 295–303. 10.1016/j.clinph.2008.11.012 [DOI] [PubMed] [Google Scholar]
- Neto E, Biessmann F, Aurlien H, Nordby H, Eichele T, 2016. Regularized Linear Discriminant Analysis of EEG Features in Dementia Patients. Front. Aging Neurosci 8, 273 10.3389/fnagi.2016.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M, 2015. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet Lond. Engl 385, 2255–2263. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- Ohara T, 2011. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Psychiatr. Neurol. Jpn 115, 90–97. [PubMed] [Google Scholar]
- Olde Dubbelink KTE, Hillebrand A, Twisk JWR, Deijen JB, Stoffers D, Schmand BA, Stam CJ, Berendse HW, 2014. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology 82, 263–270. 10.1212/WNL.0000000000000034 [DOI] [PubMed] [Google Scholar]
- Open Science Collaboration, 2015. PSYCHOLOGY. Estimating the reproducibility of psychological science. Science 349, aac4716 10.1126/science.aac4716 [DOI] [PubMed] [Google Scholar]
- Oswal A, Brown P, Litvak V, 2013. Synchronized neural oscillations and the pathophysiology of Parkinson’s disease. Curr. Opin. Neurol 26, 662–670. 10.1097/WCO.0000000000000034 [DOI] [PubMed] [Google Scholar]
- Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F, 2014. Magnitude of Cognitive Dysfunction in Adults with Type 2 Diabetes: A Meta-analysis of Six Cognitive Domains and the Most Frequently Reported Neuropsychological Tests Within Domains. Magnit. Cogn. Dysfunct. Adults Type 2 Diabetes Meta-Anal. Six Cogn. Domains Most Freq. Rep. Neuropsychol. Tests Domains 20, 278–291. 10.1017/S1355617713001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Palva JM, 2007. New vistas for α-frequency band oscillations. New Vistas A-Freq. Band Oscil 30, 150–158. 10.1016/j.tins.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Penolazzi B, Spironelli C, Angrilli A, 2008. Delta EEG activity as a marker of dysfunctional linguistic processing in developmental dyslexia. Psychophysiology 45, 1025–1033. 10.1111/j.1469-8986.2008.00709.x [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Neuper C, 1996. Post-movement beta synchronization. A correlate of an idling motor area? Post-Mov. Beta Synchronization Correl. Idling Mot. Area 98, 281–293. 10.1016/0013-4694(95)00258-8 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline J-B, Vul E, Yarkoni T, 2017. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci 18, 115–126. 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poza J, Hornero R, Abasolo D, Fernandez A, Garcia M, 2007. Extraction of spectral based measures from MEG background oscillations in Alzheimer’s disease. Med. Eng. Phys 29, 1073–1083. 10.1016/j.medengphy.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Ravona-Springer R, Heymann A, Schmeidler J, Sano M, Preiss R, Koifman K, Hoffman H, Silverman JM, Beeri MS, 2014. The ApoE4 genotype modifies the relationship of long-term glycemic control with cognitive functioning in elderly with type 2 diabetes. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol 24, 1303–1308. 10.1016/j.euroneuro.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Cha RH, Mielke MM, Pankratz VS, Boeve BF, Kantarci K, Geda YE, Jack CRJ, Petersen RC, Lowe VJ, 2014. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med 55, 759–764. 10.2967/jnumed.113.132647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, C H-P, Julio, Daskalakis ZJ, Fitzgerald PB, 2014. Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage 101, 425–439. 10.1016/j.neuroimage.2014.07.037 [DOI] [PubMed] [Google Scholar]
- Rosa IM, Henriques AG, Wiltfang J, da Cruz E Silva OAB, 2018. Putative dementia cases fluctuate as a function of mini-mental exam state examination cut-off points. J. Alzheimers Dis 61, 157–167. [DOI] [PubMed] [Google Scholar]
- Rosenberg SJ, Ryan JJ, Prifitera A, 1984. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J. Clin. Psychol 40, 785–787. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Del Percio C, Pasqualetti P, Cassetta E, Binetti G, Dal Forno G, Ferreri F, Frisoni G, Chiovenda P, Miniussi C, Parisi L, Tombini M, Vecchio F, Babiloni C, 2006. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 143, 793–803. 10.1016/j.neuroscience.2006.08.049 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J, 2007. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Clin. Neurophysiol. Aging Brain Norm. Aging Neurodegener 83, 375–400. 10.1016/j.pneurobio.2007.07.010 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, Kleinschmidt A, 2010. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci. Off. J. Soc. Neurosci 30, 10243–10250. 10.1523/JNEUROSCI.1004-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedi E, Gheini MR, Faiz F, Arami MA, 2016. Diabetes mellitus and cognitive impairments. World J. Diabetes 7, 412–422. 10.4239/wjd.v7.i17.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitzky Abraham., Golay MJE, 1964. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 36, 1627–1639. 10.1021/ac60214a047 [DOI] [Google Scholar]
- Schnitzler A, Gross J, 2005. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci 6, 285–296. 10.1038/nrn1650 [DOI] [PubMed] [Google Scholar]
- Stewart R, Liolitsa D, 1999. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet. Med. J. Br. Diabet. Assoc 16, 93–112. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Minthon L, Blennow K, Rosen I, Londos E, 2010. Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years. Neurobiol. Aging 31, 215–223. 10.1016/j.neurobiolaging.2008.03.025 [DOI] [PubMed] [Google Scholar]
- Strachan MWJ, Reynolds RM, Marioni RE, Price JF, 2011. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat. Rev. Endocrinol 7, 108–114. 10.1038/nrendo.2010.228 [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Matsushima E, Kato M, Konishi M, Izumiyama H, Murata Y, Hirata Y, 2012. Characteristics of neuropsychological functions in inpatients with poorly-controlled type 2 diabetes mellitus. J. Diabetes Investig 3, 325–330. 10.1111/j.2040-1124.2011.00170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, 2014. Diabetes and neurodegeneration in the brain. Handb. Clin. Neurol 126, 489–511. 10.1016/B978-0-444-53480-4.00035-7 [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2006. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52, 155–168. 10.1016/j.neuron.2006.09.020 [DOI] [PubMed] [Google Scholar]
- van den Berg E, Reijmer YD, de Bresser J, Kessels RPC, Kappelle LJ, Biessels GJ, Utrecht Diabetic Encephalopathy Study Group, 2010. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 53, 58–65. 10.1007/s00125-009-1571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hiele K, Vein AA, Reijntjes RHAM, Westendorp RGJ, Bollen ELEM, van Buchem MA, van Dijk JG, Middelkoop HAM, 2007. EEG correlates in the spectrum of cognitive decline. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 118, 1931–1939. 10.1016/j.clinph.2007.05.070 [DOI] [PubMed] [Google Scholar]
- van Deursen JA, Vuurman EFPM, Verhey FRJ, van Kranen-Mastenbroek VHJM, Riedel WJ, 2008. Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J. Neural Transm. Vienna Austria 1996 115, 1301–1311. 10.1007/s00702-008-0083-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahou EL, Thurm F, Kolassa I-T, Schlee W, 2014. Resting-state slow wave power, healthy aging and cognitive performance. Sci. Rep 4, 5101 10.1038/srep05101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Knight RT, 2015. Dynamic Network Communication as a Unifying Neural Basis for Cognition, Development, Aging, and Disease. Dyn. Netw. Commun. Unifying Neural Basis Cogn. Dev. Aging Dis 77, 1089–1097. 10.1016/j.biopsych.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Harrison FE, 2015. Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer’s Disease: Impacts on Cognitive Decline. Nutrients 7, 7332–7357. 10.3390/nu7095341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LM, 2003. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci 7, 553–559. [DOI] [PubMed] [Google Scholar]
- Wen D, Bian Z, Li Q, Wang L, Lu C, Li X, 2016. Resting-state EEG coupling analysis of amnestic mild cognitive impairment with type 2 diabetes mellitus by using permutation conditional mutual information. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 127, 335–348. 10.1016/j.clinph.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO, 2007. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 118, 1877–1888. 10.1016/j.clinph.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Hamilton N, Schwartz AV, Simonsick EM, Satterfield S, Cauley JA, Rosano C, Launer LJ, Strotmeyer ES, Harris TB, 2012. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch. Neurol. 69, 1170–1175. 10.1001/archneurol.2012.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY, 2008. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron 58, 429–441. 10.1016/j.neuron.2008.03.027 [DOI] [PubMed] [Google Scholar]
- Zeng K, Wang Y, Ouyang G, Bian Z, Wang L, Li X, 2015. Complex network analysis of resting state EEG in amnestic mild cognitive impairment patients with type 2 diabetes. Front. Comput. Neurosci 9, 133 10.3389/fncom.2015.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.