Abstract
Background:
Preterm birth (PTB; gestational age <37 weeks), the leading cause of infant morbidity and mortality worldwide, is of particular concern in Puerto Rico. Rates of PTB in Puerto Rico peaked at 20% in 2006, which are historically some of the highest in the world. Oxidative stress and inflammation have been implicated as contributors to adverse birth outcomes, including PTB, and these associations have not been explored in Puerto Rico. Our objective was to examine associations between urinary oxidative stress biomarkers and PTB in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) pregnancy cohort (N=469).
Methods:
8-iso-prostaglandin F2α (8-iso-PGF2α), its primary metabolite, and prostaglandin F2α (PGF2α) were included as biomarkers of oxidative stress or inflammation. Biomarkers were measured in urine samples collected at up to 3 timepoints across pregnancy (mean 18, 24, 28 weeks gestation). We quantified the proportion of 8-iso-PGF2α originating from oxidative stress and inflammation pathways with a formula based on the ratio of 8-iso-PGF2α to PGF2α. Logistic regression models were used to calculate adjusted odds ratios (OR) for associations between average biomarker concentrations from each woman (visits 1-3) and PTB. Associations between biomarker concentrations at each study visit and PTB were analyzed in separate models.
Results:
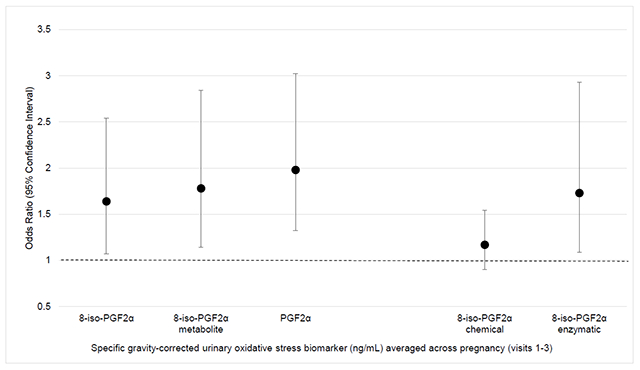
Averaged levels of 8-iso-PGF2α, its primary metabolite, and PGF2α were associated with increased odds of PTB (OR=1.64, 95% confidence interval [CI]=1.07-2.54; OR=1.79, 95% CI=1.14-2.84; OR=1.98, 95% CI=1.32-3.02, respectively). Odds ratios for PTB were greater in magnitude in association with oxidative stress biomarkers measured later in pregnancy. The fraction of 8-iso-PGF2α derived from inflammation was associated with PTB (OR=1.73, 95% CI=1.09, 2.93), while the fraction of 8-iso-PGF2α derived from oxidative stress was not associated with PTB (OR=1.17, 95% CI=0.90, 1.54).
Conclusions:
Our results suggest that oxidative stress and inflammation, as measured by these biomarkers, may be important contributors to PTB. Further research is needed to improve our understanding of the role these biomarkers may play in the causal pathway between environmental factors and PTB.
Keywords: oxidative stress, preterm birth, isoprostanes, repeated measures, Puerto Rico, epidemiology
Graphical Abstract
Introduction
Preterm birth (PTB), defined as delivery prior to 37 weeks gestation, is one of the leading causes of infant morbidity and mortality worldwide.1 Each year, roughly 15 million babies are born preterm and an estimated 35% of neonatal deaths are due to causes directly related to PTB.1 PTB is particularly problematic in Puerto Rico, where the rates of PTB are some of the highest in the U.S. and the world. Puerto Rico’s PTB rate was 19.9% in 20062 and although this rate has since declined to 11.4% in 2017, it remains high relative to the continental U.S.3
The biologic mechanisms leading to PTB are incompletely understood. Oxidative stress may represent one possible mechanism. Oxidative stress biomarkers have been associated with adverse pregnancy outcomes4,5 and several studies have shown that oxidative stress biomarkers are increased among women who go on to experience PTB or shortened gestation.6–8 However, the strength of association remains inconsistent across studies and discrepancies may in part be due to differences in biomarkers used to measure oxidative stress.
In the present study, we sought to examine associations between multiple biomarkers of oxidative stress and PTB in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) pregnancy cohort. 8-isoprostane-prostaglandin-F2α (8-iso-PGF2α), the major 8-isoprostane-PGF2α metabolite, and prostaglandin-F2α (PGF2α) were included as biomarkers of oxidative stress. 8-iso-PGF2α is widely studied with respect to pregnancy outcomes and is considered one of the best biomarkers of oxidative stress because of its reliability and stability during human pregnancy.9,10 However, the 8-iso-PGF2α metabolite may be a more sensitive and superior biomarker of oxidative stress than 8-iso-PGF2α11 and suggestive associations between the metabolite and spontaneous PTB have been observed.12 Lastly, we included PGF2α which is less studied within the context of human pregnancy but has been previously linked to the inflammation mechanisms underlying PTB.13 Previously, in the PROTECT study population, we found that indicators of lower socioeconomic status were associated with elevated levels of these biomarkers.14 Here, we build upon this work and examined longitudinal associations with these biomarkers and PTB, hypothesizing that women with increased oxidative stress biomarkers would be more likely to deliver preterm.
Materials and Methods
Study Population
Women included in the current analysis are a subset of women enrolled in the PROTECT cohort. PROTECT is an ongoing, prospective cohort study in Northern Puerto Rico and recruitment methods have been previously described.15,16 The 469 women included in this analysis delivered between 2011 and 2017 and were recruited between 14 and 20 weeks gestation from affiliated hospitals and prenatal clinics. Exclusion criteria for PROTECT included: maternal age less than 18 or greater than 40 years, maternal residence was outside of the Northern Karst aquifer region, use of oral contraceptives for 3 months prior to conception, and preexisting obstetric and medical complications (e.g., diabetes). All participants are invited to complete up to 3 study visits targeted at 16-20 weeks gestation, 20-24 weeks gestation, and 24-28 weeks gestation. Demographic information was collected by questionnaire at the first visit and spot urine samples were collected at all study visits. The study was approved by the Institutional Review Boards at the University of Puerto Rico, Northeastern University, University of Michigan, and University of Georgia. All women provided written, informed consent prior to participating in PROTECT.
Oxidative Stress Biomarker Assessment
Urine samples were collected in polypropylene containers, divided into aliquots, and frozen at −80 °C until analysis.17 Free 8-iso-PGF2α, its major metabolite 2,3-dinor-5,6-dihydro-15-F2t-isoprostane, and PGF2α were analyzed by the Eicosanoid Core Laboratory at Vanderbilt University Medical Center (Nashville, TN) as biomarkers of oxidative stress or inflammation. This was done using stable isotype dilution gas chromatography-negative ion chemical ionization-mass spectrometry in samples for 469 participants (N= 270 at visit 1, N= 349 at visit 2, N= 217 at visit 3). This method requires a C18 Sep-Pak column for solid-phase extraction, a thin-layer chromatography purification, and chemical derivation. During analyses, samples are thawed and 0.25 ml urine is diluted in 10 ml pH 3 water and acidified to pH 3 using 1N HCl prior to extraction. Further details describing measurement of oxidative stress biomarker concentrations are available elsewhere.18
8-iso-PGF2α is generated through both chemical free radical oxidation and enzymatic lipid peroxidation pathways. Thus, 8-iso-PGF2α may not be solely a biomarker of oxidative stress. To account for these separate mechanisms, we additionally examined the proportion of 8-iso-PGF2α that was derived from chemical free radical oxidation and enzymatic lipid peroxidation pathways.19 This was done utilizing the ratio of 8-iso-PGF2α to PGF2α which has been previously described in detail by van ’t Erve et al. and was calculated using a custom interface for the R package “Constrained Linear Mixed Effects (CLME)”.19 The chemical fraction captures non-enzymatic lipid peroxidation, and the enzymatic fraction is generated from prostaglandin-endoperoxide synthases and is more reflective of inflammation. As a sensitivity analysis, associations between PTB and the chemical and enzymatic fractions of 8-iso-PGF2α were analyzed separately to determine which pathway drove the associations between overall 8-iso-PGF2α and PTB.
To account for urine dilution, urinary specific gravity (SpG) was measured using a digital handheld refractometer. All urinary oxidative stress biomarker concentrations were corrected for SpG using the equation Oxc = Ox[(1.019-1)/(SpG-1)], where the median SpG was 1.019 in the PROTECT population.17,20 Ox is the measured oxidative stress concentration and Oxc is the SpG-corrected measure. All SpG-corrected oxidative stress biomarker concentrations were natural log transformed for normality.
Gestational Age
Gestational age was assessed based on American College of Obstetricians and Gynecologists guidelines using a combination of self-reported date of last menstrual period collected at the first study visit and first ultrasound estimates of gestational age.16,21 For analysis, we categorized gestational age into PTB (<37 weeks gestation) and full term birth (≥37 weeks gestation). PTB was classified as spontaneous if premature rupture of the membranes, spontaneous preterm labor, or both was present.22
Statistical Analysis
All statistical analyses were conducted in R Version 3.5.0 and SAS 9.4 (Cary, NC). The distributions of measured urinary oxidative stress biomarker concentrations were examined using geometric means, geometric standard deviations, and selected percentiles. Intraclass correlation coefficients (ICC) measurements from all study visits were calculated as an additional measure of individual variability in biomarker concentrations.23 ICC values range from 0 to 1 where ICC values of 1 indicate perfect reproducibility, values closer to 0 indicate greater variability, and values between 0.40 and 0.75 indicate good reliability.23
Geometric average biomarker concentrations were obtained by taking the geometric mean of the available SpG-corrected oxidative stress biomarker concentrations from all participants at visits 1-3. For example, if a participant had 8-iso-PGF2α measured at each visit, we took the geometric mean of SpG-corrected 8-iso-PGF2α at visits 1-3. If a participant had SpG-corrected 8-iso-PGF2α at visit 1 only, we used only that measure. No participants were missing oxidative stress biomarkers at visit 3 as a result of delivering preterm prior to the visit.
Logistic regression was used to calculate crude and adjusted odds ratios (OR) and 95% confidence intervals (CI) for the associations between averaged oxidative stress biomarkers and PTB. Maternal age, maternal education, employment status, marital status, alcohol use, smoking status, and insurance status were examined as potential covariates in adjusted models. Covariates retained in adjusted models were associated with oxidative stress biomarkers in bivariate analyses and have been previously associated with PTB. We additionally examined associations between PTB and SpG-corrected urinary oxidative stress biomarkers at each visit using individual logistic regression models. This was done to determine whether oxidative stress biomarkers at a particular timepoint were differentially associated with PTB. Gestational age at study visit and covariates from averaged models were retained in adjusted individual models. For consistency with previous research and to increase interpretability, ORs for PTB were standardized to reflect odds associated with an interquartile range (IQR) increase in SpG-corrected oxidative stress markers.12,24
Results
A detailed description of the demographics in the overall PROTECT cohort is available elsewhere.16 There were 469 women included in the present analysis who had urine samples analyzed for oxidative stress biomarkers. Within our analytic sample, 50 participants delivered preterm (11.2%), of this group 30 were classified as having a spontaneous PTB. The largest percentage of women included in the current analysis were between 18-24 years of age (40%), had a college degree or higher (41%), were employed (62%), and were married (54%) (Table 1). At the first study visit, few women reported ever smoking (4%) or consuming alcohol (5%) (Table 1). Demographic characteristics of participants included in our analytic sample were similar to that of the larger PROTECT cohort.16
Table 1.
Distributions of characteristics in the PROTECT analytic sample (N=469).
| Characteristic | N (%) |
|---|---|
| Preterm Birth | |
| Preterm | 50 (11.2) |
| Full Term | 396 (88.8) |
| Missing | 0 (0.00) |
| Maternal Age, years | |
| 18-24 | 188 (40.1) |
| 25-29 | 142 (30.3) |
| 30-34 | 85 (18.1) |
| ≥35 | 53 (11.3) |
| Missing | 1 (0.21) |
| Maternal Education | |
| <High school | 35 (7.46) |
| High school or equivalent | 70 (14.9) |
| Some college or technical school | 167 (35.6) |
| ≥College degree | 193 (41.5) |
| Missing | 4 (0.85) |
| Employment Status | |
| Unemployed | 176 (37.5) |
| Employed | 288 (62.4) |
| Missing | 5 (1.07) |
| Marital Status | |
| Single | 89 (19.0) |
| Married | 250 (53.3) |
| Living together | 128 (27.3) |
| Missing | 2 (0.43) |
| Alcohol Use | |
| Never | 204 (43.5) |
| Before pregnancy | 234 (49.9) |
| Currently | 25 (5.33) |
| Missing | 6 (1.28) |
| Smoking | |
| Never | 385 (82.1) |
| Before pregnancy | 63 (13.4) |
| Currently | 19 (4.05) |
| Missing | 2 (0.43) |
| Insurance Status | |
| Private | 281 (59.9) |
| Public | 165 (35.2) |
| Uninsured | 6 (1.28) |
| Missing | 17 (3.62) |
Note: percentages may not sum to 100% due to rounding; missing indicates the number and percent of participants for which information regarding the specific characteristic was not obtained.
Concentrations of 8-iso-PGF2α were higher among women who were between 18-24 compared to 25-29 years of age and who had public compared to private insurance (p-value <0.01).14 The geometric mean of the 8-iso-PGF2α metabolite was higher among women with a high school education or equivalent compared to women with a college education or greater and among women who were unemployed compared to employed (p-value <0.01).14 Associations between demographic characteristics and PGF2α were similar but less precise.
ICC values for 8-iso-PGF2α and the 8-iso-PGF2α metabolite demonstrated good temporal reliability (ICC=0.52, 95% CI=0.47-0.58 for both biomarkers). 8-iso-PGF2α and the 8-iso-PGF2α metabolite were also moderately correlated (Spearman R=0.67, p-value <0.01). ICC values for PGF2α were also moderate or good, but were somewhat less reliable (ICC=0.41, 95% CI=0.35-0.47).
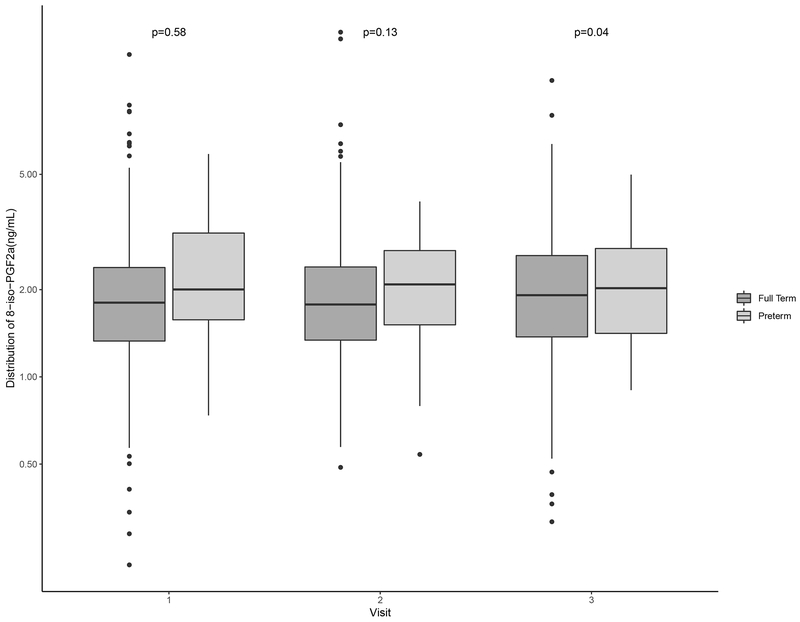
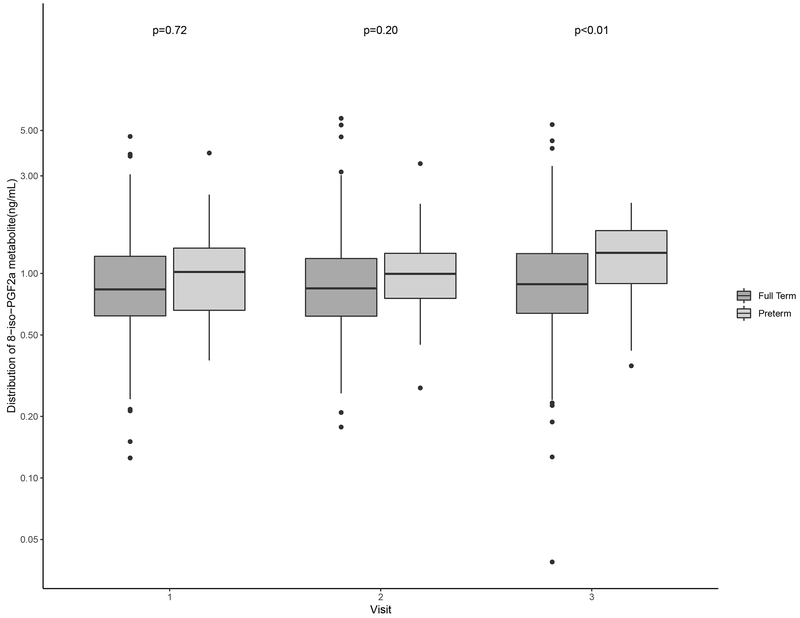
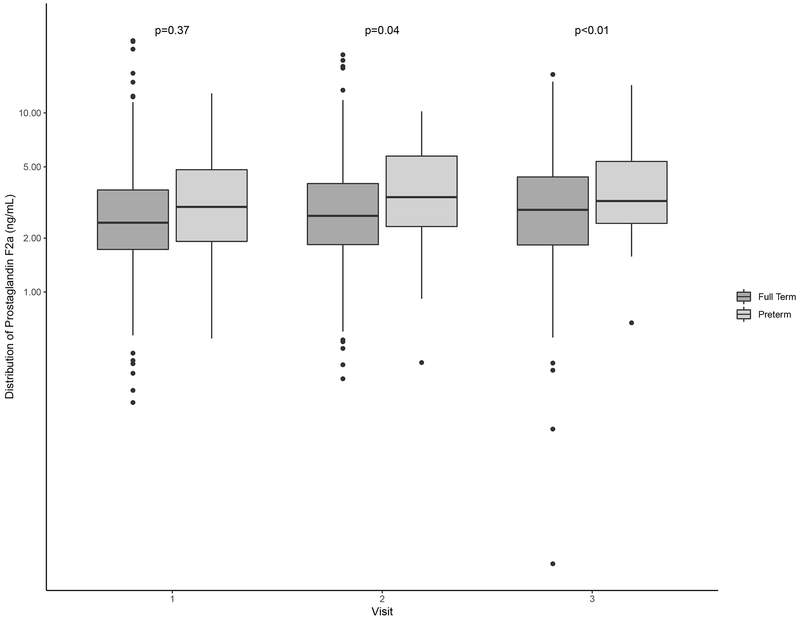
Distributions of oxidative stress biomarkers are shown in Table 2. Levels of 8-iso-PGF2α and the 8-iso-PGF2α metabolite across preterm and term births were similar at study visits 1 and 2 (Figure 1; Figure 2). PGF2α concentrations were elevated relative to those of 8-iso-PGF2α and the metabolite (Table 2). Additionally, PGF2α levels at visit 2 were significantly higher among women who delivered preterm compared to term (Figure 3). For all oxidative stress biomarkers, levels at visit 3 were significantly higher among women who delivered preterm compared to term (Figures 1–3).
Table 2.
Distribution of subject-specific urinary levels of oxidative stress biomarkers corrected with specific gravity (ng/mL).
| Percentile | |||||||
|---|---|---|---|---|---|---|---|
| N | Geometric Mean (Geometric SD) | 5 | 25 | 50 | 75 | 95 | |
| Measured | |||||||
| 8-iso-PGF2α | 1,146 | 1.84 (1.66) | 0.83 | 1.34 | 1.86 | 2.53 | 4.14 |
| 8-iso-PGF2α metabolite | 1,146 | 0.88 (1.72) | 0.38 | 0.63 | 0.87 | 1.26 | 2.10 |
| Prostaglandin-F2α | 1,146 | 2.71 (1.94) | 0.93 | 1.84 | 2.72 | 4.07 | 7.85 |
| Derived | |||||||
| 8-iso-PGF2α chemical | 1,146 | 1.43 (1.86) | 0.53 | 0.98 | 1.45 | 2.18 | 3.57 |
| 8-iso-PGF2α enzymatic | 1,146 | 0.10 (10.9) | 0.00 | 0.02 | 0.29 | 0.57 | 1.16 |
Abbreviations: SD, standard deviation.
Note: there were 270 samples from visit 1, 349 from visit 2, and 217 from visit 3. Measured biomarkers were analyzed in urine. Derived biomarkers were quantified using the ratio of 8-iso-PGF2α to PGF2α to determine the proportion of 8-iso-PGF2α that was derived from chemical and enzymatic lipid peroxidation pathways.
Figure 1.
Distribution of specific gravity-corrected urinary levels of 8-iso-PGF2α (ng/mL) at specific study visits stratified by preterm (gestational age <37 weeks) and full term birth (gestational age ≥37 weeks).
Note: The mean gestational age at study visit was 18, 24, and 28 weeks gestation, respectively. P-values were calculated using t-tests and p<0.05 indicates statistical significance.
Figure 2.
Distribution of specific gravity-corrected urinary levels of the 8-iso-PGF2α metabolite (ng/mL) at specific study visits stratified by preterm (gestational age <37 weeks) and full term birth (gestational age ≥37 weeks).
Note: The mean gestational age at study visit was 18, 24, and 28 weeks gestation, respectively. P-values were calculated using t-tests and p≥0.05 indicates statistical significance.
Figure 3.
Distribution of specific gravity-corrected urinary levels of PGF2α (ng/mL) by study visits stratified by preterm (gestational age <37 weeks) and full term birth (gestational age ≥37 weeks).
Note: The mean gestational age at study visit was 18, 24, and 28 weeks gestation, respectively. P-values were calculated using t-tests and p<0.05 indicates statistical significance.
Maternal age, marital status, and maternal education were retained as covariates in adjusted logistic regression models. Adjusted ORs of PTB in association with an IQR increase in oxidative stress biomarkers are shown in Table 3. An IQR increase in 8-iso-PGF2α (OR=1.64, 95% CI=1.07-2.54), the 8-iso-PGF2α metabolite (OR=1.79, 95% CI=1.14-2.84), and PGF2α (OR=1.98, 95% CI=1.32-3.02) were all associated with increased odds of PTB in models of pregnancy averages of these markers. Individual logistic regression models stratified by study visit indicated that odds of PTB in relation to oxidative stress biomarkers increased moderately throughout pregnancy. For example, an IQR increase in PGF2α was associated with a 1.24 (95% CI=0.78, 1.96), 1.60 (95% CI=1.01, 2.58), and 2.01 (95% CI=1.03, 4.17) increase in odds of PTB at visits 1, 2, and 3, respectively. Associations between oxidative stress biomarkers and PTB were similar in crude analyses.
Table 3.
Crude and adjusted odds ratios (95% confidence interval) for preterm birth with an interquartile range increase in specific gravity-corrected urinary oxidative stress biomarker (ng/mL) averaged across pregnancy (visits 1-3) and by study visit.
| Crude | Adjusted1 | |||||
|---|---|---|---|---|---|---|
| Biomarker | N (PTB, FTB) | OR (95% CI) | p-value | N (PTB, FTB) | OR (95% CI) | p-value |
| Measured | ||||||
| 8-iso-PGF2α | ||||||
| Average | (50, 396) | 1.60 (1.06, 2.43) | 0.03 | (50, 393) | 1.64 (1.07, 2.54) | 0.02 |
| Visit 1 | (31, 220) | 1.16 (0.70, 1.91) | 0.57 | (30, 214) | 1.11 (0.65, 1.88) | 0.71 |
| Visit 2 | (34, 305) | 1.49 (0.93, 2.41) | 0.10 | (34, 298) | 1.55 (0.95, 2.55) | 0.08 |
| Visit 3 | (24, 187) | 1.92 (0.98, 3.93) | 0.06 | (24, 179) | 1.77 (0.87, 3.80) | 0.13 |
| 8-iso-PGF2α metabolite | ||||||
| Average | (50, 396) | 1.76 (1.15, 2.71) | <0.01 | (50, 393) | 1.79 (1.14, 2.84) | 0.01 |
| Visit 1 | (31, 220) | 1.10 (0.64, 1.89) | 0.72 | (30, 214) | 1.19 (0.66, 2.14) | 0.57 |
| Visit 2 | (34, 305) | 1.33 (0.83, 2.14) | 0.23 | (34, 298) | 1.43 (0.88, 2.34) | 0.15 |
| Visit 3 | (24, 187) | 3.73 (1.96, 7.66) | <0.01 | (24, 179) | 3.56 (1.78, 7.69) | <0.01 |
| Prostaglandin-F2α | ||||||
| Average | (50, 396) | 1.88 (1.28, 2.80) | <0.01 | (50, 393) | 1.98 (1.32, 3.02) | <0.01 |
| Visit 1 | (31, 217) | 1.24 (0.79, 1.92) | 0.35 | (30, 211) | 1.24 (0.78, 1.96) | 0.36 |
| Visit 2 | (34, 303) | 1.57 (1.01, 2.48) | 0.05 | (34, 296) | 1.60 (1.01, 2.58) | 0.05 |
| Visit 3 | (24, 187) | 2.27 (1.18, 4.64) | 0.02 | (24, 179) | 2.01 (1.03, 4.17) | 0.05 |
| Derived | ||||||
| 8-iso-PGF2α chemical | ||||||
| Average | (50, 396) | 1.17 (0.91, 1.52) | 0.24 | (50, 393) | 1.17 (0.90, 1.54) | 0.25 |
| Visit 1 | (31, 217) | 1.07 (0.81, 1.45) | 0.65 | (30, 211) | 1.03 (0.76, 1.43) | 0.85 |
| Visit 2 | (34, 303) | 1.15 (0.86, 1.56) | 0.37 | (34, 296) | 1.18 (0.87, 1.62) | 0.29 |
| Visit 3 | (24, 187) | 1.22 (0.84, 1.82) | 0.31 | (24, 179) | 1.19 (0.81, 1.82) | 0.39 |
| 8-iso-PGF2α enzymatic | ||||||
| Average | (50, 396) | 1.69 (1.07, 2.84) | 0.04 | (50, 393) | 1.73 (1.09, 2.93) | 0.03 |
| Visit 1 | (31, 217) | 1.63 (0.68, 4.29) | 0.30 | (30, 211) | 1.62 (0.67, 4.33) | 0.30 |
| Visit 2 | (34, 303) | 1.45 (1.00, 2.28) | 0.08 | (34, 296) | 1.45 (1.00, 2.29) | 0.07 |
| Visit 3 | (24, 187) | 1.43 (0.89 2.60) | 0.18 | (24, 179) | 1.42 (0.88, 2.54) | 0.19 |
Average models adjusted for maternal age, education, and marital status. Visit 1, 2, and 3 models adjusted for maternal age, education, marital status, and gestational age at visit.
Abbreviations: OR, odds ratio; CI, confidence interval.
Note: average indicates biomarker concentrations that were obtained by taking the geometric mean of the available specific gravity-corrected oxidative stress biomarker concentrations from all participants at visits 1-3.
In our sensitivity analyses distinguishing between the chemical and enzymatic fractions of 8-iso-PGF2α, we observed that the association between averaged 8-iso-PGF2α and PTB appeared to be driven by associations with inflammation (Table 3). An IQR increase in the enzymatic fraction of 8-iso-PGF2α was associated with increased odds of PTB (OR=1.73, 95% CI=1.09, 2.93). The corresponding OR for the fraction of 8-iso-PGF2α derived from oxidative stress was null (OR=1.17, 95% CI=0.90, 1.54).
Discussion
In the present study, we examined the associations between multiple biomarkers of oxidative stress and PTB using data from a prospective pregnancy cohort in Puerto Rico. Findings from our study showed that levels of 8-iso-PGF2α, the 8-iso-PGF2α metabolite, and PGF2α are elevated among women who deliver preterm. Additionally, our sensitivity analysis with the chemical and enzymatic fractions of 8-iso-PGF2α revealed that the association between 8-iso-PGF2α and PTB may be attributable to elevated inflammation. In this study, oxidative stress biomarker concentrations measured later in pregnancy were more strongly associated with PTB relative to levels measured earlier in pregnancy. Results from our study contribute to the growing body of literature suggesting that oxidative stress is on the causal pathway to PTB.
The positive association between increasing 8-iso-PGF2α and PTB that we identified is consistent with previous research.6–8 For example, a case-control study in Boston found that an increase in 8-iso-PGF2α was associated with increased odds of spontaneous PTB and that associations were greater with 8-iso-PGF2α levels measured later in pregnancy.6 A second study of 237 premature infants showed that median 8-iso-PGF2α in cord blood was higher among infants born at less than 28 weeks gestation compared to those born between 34-36 weeks gestation.25 Two additional studies have found that levels of 8-iso-PGF2α measured in amniotic fluid were higher among pregnancies complicated by preterm premature rupture of the membranes (pPROM)26 and term PROM.27 Higher median levels of plasma 8-iso-PGF2α have also been observed among women who go on to develop preeclampsia and deliver small for gestational age infants,28 although that study did not observe an association between 8-iso-PGF2α and PTB.
An important strength of our study is that we included multiple biomarkers of oxidative stress. In addition to 8-iso-PGF2α, we also measured the 8-iso-PGF2α metabolite. The effect estimates and statistical associations we observed between the 8-iso-PGF2α metabolite and PTB were larger and stronger, both in averaged models and models specific to study visits, compared to associations with 8-iso-PGF2α and PTB. Our positive findings between the metabolite and PTB are confirmed by a recent study showing that an IQR increase in the 8-iso-PGF2α metabolite was associated with a 44% increase in odds of PTB.12 The 8-iso-PGF2α metabolite is hypothesized to be more sensitive than 8-iso-PGF2α in urine11 and eicosanoid production is thought to be best assessed by measurement of urinary metabolites,29,30 which may be one reason why the associations we observed were greater in magnitude compared to 8-iso-PGF2α itself.
It is plausible that oxidative stress may be one mechanism by which environmental exposures impact PTB. Previous research has identified associations between increases in environmental contaminants and increased oxidative stress levels.10,17 For example, elevated levels of oxidative stress have been observed among pregnant women with elevated concentrations of certain polycyclic aromatic hydrocarbons, phthalates, phenols, and parabens.24,31,32 Increases in oxidative stress levels have also been observed among individuals who smoke, are obese, and experience psychological stress,20,33 all of which have been previously associated with an increased risk of PTB.16,34,35 Smoking, obesity, and psychological stress have the potential to cause redox imbalance, which could lead to an increased consumption of antioxidant defenses and trigger uterine contractions.33 Inflammatory cytokines, such as interleukin-6 and C-reactive protein, also have been associated with an increased risk of PTB36 and may represent an additional pathway through which these exposures may influence PTB risk. Future research in this cohort will determine the host of contributing factors to elevated levels of these biomarkers.
Oxidative stress could be contributing to PTB and shortened gestation through multiple pathways. One plausible mechanism is that oxidative stress is acting as a precursor to spontaneous labor.33 Increased oxidative stress could lead to accelerated senescence of the maternal-fetal membranes, myometrial activation, and cervical ripening.33 Oxidative damage could also lead to apoptosis and telomere damage, leading to pPROM and spontaneous PTB.33 Finally, increased oxidative stress could lead to an increased risk of infection as a result of reduced immune function.33
Our sensitivity analyses used a novel approach to differentiate between the chemical and enzymatic fractions of 8-iso-PGF2α that has not been previously explored in this context. Previously, it was thought that 8-iso-PGF2α was only generated through chemical lipid peroxidation. However, more recent studies have shown that 8-iso-PGF2α can also be generated from enzymatic pathways, which capture cyclooxygenase activity and is responsive to inflammation.19 In this study, we observed that the association between urinary 8-iso-PGF2α and PTB was largely driven by the portion of 8-iso-PGF2α derived from the enzymatic pathway. Thus, the relationship between 8-iso-PGF2α and PTB we identified may be a result of increases in inflammation. Inflammation may be leading to PTB through increased inflammatory cytokines, which are associated with decreased membrane structural integration, myometrial activation, and cervical ripening.33 We also observed a strong association between urinary PGF2α and PTB. Proinflammatory cytokines induce prostaglandin production, which promotes cervical ripening and PGF2α to stimulate uterine contractions.13 Prostaglandins are generally metabolized in less than five minutes, thus urinary prostaglandins levels are more indicative of systemic production than plasma levels. Overall, our results provide strong evidence that the associations we observed between biomarkers of oxidative stress and PTB may in part be due to the upregulation of inflammation pathways.
Our study is not without limitations, as we did not have a large enough sample size to examine subtypes of PTB. Previous literature has shown that increased 8-iso-PGF2α is more strongly linked to PTB with spontaneous presentation.6 Despite our limitations, our study has many strengths. First, PROTECT employs a prospective cohort design and we had multiple measures of oxidative stress throughout pregnancy. This allowed us to create stable, average measures of oxidative stress, which may be crucial since we observed that oxidative stress levels were somewhat variable over gestation in this study population. In addition, these repeated measures allowed us to examine associations between oxidative stress levels and PTB at different timepoints. Susceptibility to oxidative stress may vary during different exposure windows, which is supported by the findings presented here. Second, we included multiple biomarkers of oxidative stress, which allowed us to examine many biologically relevant associations. These biomarkers were also measured using a highly sensitive and specific mass spectrometry method as opposed to immunoassay-based methods commonly used in other studies. Third, isoprostanes are some of the best biomarkers of oxidative stress because they are reliable, unaffected by lipids in the diet, and stable, including during human pregnancy.9 Lastly, biomarkers used in this study were measured in urine, which is thought to be better than plasma, as they are not susceptible to autooxidation during storage.37
Conclusions
In our study, increased 8-iso-PGF2α, the primary 8-iso-PGF2α metabolite, and PGF2α were associated with increased odds of PTB. Furthermore, our additional integration of the 8-iso-PGF2α and PTB relationship revealed that these associations may be more reflective of increased inflammation. Results from our study are consistent with previous research showing that oxidative stress is associated with adverse pregnancy outcomes. Future research should focus on the role of oxidative stress in inflammation pathways and is needed to better understand the role oxidative stress may play on the causal pathway between environmental factors and PTB.
Highlights:
8-iso-PGF2α, the 8-iso-PGF2α metabolite, and PGF2α were associated with preterm birth
Associations between 8-iso-PGF2α and preterm birth may be attributable to inflammation
Biomarkers measured later in pregnancy were strongly associated with preterm birth than those measured earlier
Acknowledgements:
This work was supported by the National Institute of Environmental Health Sciences grants P42ES017198 and P50ES026049 and the National Institutes of Health Environmental influence on Children’s Health Outcomes (ECHO) program grants UG3OD023251 and UH3OD023251. This research was funded in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health.
Abbreviations:
- PTB
preterm birth
- PROTECT
Puerto Rico Testsite for Exploring Contamination Threats
- OR
odds ratio
- CI
confidence interval
- 8-iso-PGF2α
8-isoprostane-prostaglandin-F2α
- PGF2α
prostaglandin-F2α
- LOD
limit of detection
- CLME
Constrained Linear Mixed Effects
- SpG
specific gravity
- ICC
intraclass correlation coefficient
- IQR
interquartile range
- pPROM
preterm premature rupture of the membranes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and PreventionAssuring Healthy, Outcomes In: Behrman RE, Butler AS, eds. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcome Washington (DC): National Academies Press (US) National Academy of Sciences.; 2007. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, et al. Births: Final Data for 2006. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 2009;57(7):1. [PubMed] [Google Scholar]
- 3.March of Dimes. 2018 Premature Birth Report Cards. 2019; https://www.marchofdimes.org/mission/prematurity-reportcard.aspx. Accessed Febuary 6, 2019.
- 4.Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. American journal of obstetrics and gynecology 2017;216(5):527.e521–527.e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YJ, Hong YC, Lee KH, et al. Oxidative stress in pregnant women and birth weight reduction. Reproductive toxicology (Elmsford, NY) 2005;19(4):487–492. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. American journal of obstetrics and gynecology 2015;212(2):208.e201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longini M, Perrone S, Vezzosi P, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clinical biochemistry 2007;40(11):793–797. [DOI] [PubMed] [Google Scholar]
- 8.Peter Stein T, Scholl TO, Schluter MD, et al. Oxidative stress early in pregnancy and pregnancy outcome. Free radical research 2008;42(10):841–848. [DOI] [PubMed] [Google Scholar]
- 9.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free radical biology & medicine 2000;28(4):505–513. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy. Environmental health perspectives 2017;125(3):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorjgochoo T, Gao YT, Chow WH, et al. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. The American journal of clinical nutrition 2012;96(2):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen EM, van ’t Erve TJ, Boss J, et al. Urinary oxidative stress biomarkers and accelerated time to spontaneous delivery. Free radical biology & medicine 2019;130:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler M, Rath W. The role of cytokines in the induction of labor, cervical ripening and rupture of the fetal membranes. Zeitschrift fur Geburtshilfe und Neonatologie 1996;200 Suppl 1:1–12. [PubMed] [Google Scholar]
- 14.Eick SM, Meeker JD, Brown P, et al. Associations between socioeconomic status, psychosocial stress, and urinary levels of 8-iso-prostaglandin-F2α during pregnancy in Puerto Rico. Free radical biology & medicine 2019;143:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology 2013;47(7):3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson KK, Rosario Z, McElrath TF, et al. Demographic risk factors for adverse birth outcomes in Puerto Rico in the PROTECT cohort. PloS one 2019;14(6):e0217770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. International journal of hygiene and environmental health 2015;218(2):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nature protocols 2007;2(1):221–226. [DOI] [PubMed] [Google Scholar]
- 19.van ’t Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2alpha)/PGF(2alpha) ratio distinguishes chemical from enzymatic lipid peroxidation. Free radical biology & medicine 2015;83:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eick SM, Barrett ES, van ’t Erve TJ, et al. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatric and perinatal epidemiology 2018;32(4):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists. Committee opinion no 611: method for estimating due date. Obstetrics and gynecology 2014;124(4):863–866. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr 2014;168(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosner B Fundamentals of biostatistics. Boston, MA: Brooks/Cole. [Google Scholar]
- 24.Ferguson KK, Lan Z, Yu Y, Mukherjee B, McElrath TF, Meeker JD. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates. Environment International 2019;131:104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mestan K, Matoba N, Arguelles L, et al. Cord blood 8-isoprostane in the preterm infant. Early human development 2012;88(8):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longini M, Perrone S, Vezzosi P, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clinical biochemistry 2007;40(11):793–797. [DOI] [PubMed] [Google Scholar]
- 27.Peter Stein T, Scholl TO, Schluter MD, et al. Oxidative stress early in pregnancy and pregnancy outcome. Free radical research 2008;42(10):841–848. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh TT, Chen SF, Lo LM, Li MJ, Yeh YL, Hung TH. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reproductive sciences (Thousand Oaks, Calif) 2012;19(5):505–512. [DOI] [PubMed] [Google Scholar]
- 29.Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ 2nd. Clinical implications of prostaglandin and thromboxane A2 formation (2). The New England journal of medicine 1988;319(12):761–767. [DOI] [PubMed] [Google Scholar]
- 30.Catella F, Nowak J, Fitzgerald GA. Measurement of renal and non-renal eicosanoid synthesis. The American Journal of Medicine 1986;81(2):23–29. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson KK, Cantonwine DE, Rivera-Gonzalez LO, et al. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environmental science & technology 2014;48(12):7018–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson KK, McElrath TF, Pace GG, et al. Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women. Environmental science & technology 2017;51(8):4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon R Oxidative stress damage as a detrimental factor in preterm birth pathology. Frontiers in immunology 2014;5:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal Stress and Preterm Birth. American Journal of Epidemiology 2003;157(1):14–24. [DOI] [PubMed] [Google Scholar]
- 35.Ion R, Bernal AL. Smoking and Preterm Birth. Reproductive Sciences 2015;22(8):918–926. [DOI] [PubMed] [Google Scholar]
- 36.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstetrics and gynecology 2010;116(2 Pt 1):393–401. [DOI] [PubMed] [Google Scholar]
- 37.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proceedings of the National Academy of Sciences of the United States of America 1990;87(23):9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]